Abstract
Background:
Despite investigating coronavirus among respiratory tract infected cases is a top priority to prevent further transmission, severe acute respiratory syndrome coronavirus 2 positivity among this group of patients remains unexplored in resource-limited settings. Therefore, this study intended to assess the severe acute respiratory syndrome coronavirus 2 positivity among patients presenting with acute respiratory tract infection from 1 July to 31 December 2020 in Harar Region, Ethiopia, from 15 February to 10 March 2021.
Methods:
A facility-based cross-sectional study design was used. Severe acute respiratory syndrome coronavirus 2 was tested by assaying oropharyngeal swabs using reverse transcriptase–polymerase chain reaction among patients presenting with acute respiratory tract infection in Harari Public Hospitals. A binary logistic regression was used to identify factors associated with severe acute respiratory syndrome coronavirus 2 positivity with an adjusted odds ratio at a 95% confidence interval.
Results:
Out of a total of 1692 study participants, 388 (22.9%) of them tested positive for severe acute respiratory syndrome coronavirus 2. Of these severe acute respiratory syndrome coronavirus 2 positive patients, 364 (21.6%) patients presented with lower respiratory tract infection, while the rest only 24 (1.4%) presented with upper respiratory tract infection. Independent variables included separated/divorced in marital status (AOR = 0.53, 95% CI: 0.29–0.95), presenting with cough, fever, and difficulty of breathing (AOR = 2.5, 95% CI: 1.22–4.7), age group of 30–39 years (AOR = 0.35, 95% CI: 0.15–0.79), 40–49 years (AOR = 0.37, 95% CI: 0.14–0.94), and 50–59 years (AOR = 0.31, 95% CI: 0.13–0.76) compared to patients with the age of ⩾ 60 years, had statistically significant association with severe acute respiratory syndrome coronavirus 2 positivity.
Conclusion:
Severe acute respiratory syndrome coronavirus 2 was positive among 388 (22.9%) acute respiratory tract infected people. Elder age, particular symptoms, such as cough, fever, and difficulty of breathing, and married marital status were associated with a severe acute respiratory syndrome coronavirus 2 positive test. In resource-limited setups, where a shortage of testing equipment is common, these findings could contribute to boosting targeted symptom-oriented screening schemes. Moreover, this study could have paramount clinical importance for further studies in the country.
Keywords: Associated factors, SARS-COV-2, COVID-19, RT-PCR, Ethiopia
Introduction
Coronaviruses belong to a large family of single-stranded RNA (ssRNA) viruses that are causative agents of the common cold and severe respiratory infections, which include severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).1–5 On 31 December 2019, an outbreak of pneumonia caused by an unknown agent was reported in Wuhan, China, later named severe acute respiratory syndrome coronavirus 2 (SARS-COV-2).6,7 The outbreak of Coronavirus disease 2019 (COVID-19) has become a global most challenging health crisis. 8 Several assessments reported that because of the incident of this pandemic, there is the social, economic, and physiological damage to the society in both developed and developing countries. 9 According to the World Health Organization, reports starting from the first-day existence of coronavirus in both the number of deaths and the confirmed cases are increasing. 10 A study showed that the progression of the coronavirus could result in severe respiratory tract infection. 11 Even though countless efforts are being made, there are excess deaths and impacts on the life expectancy of the world’s population due to COVID-19. 12 In Ethiopia, before the virus reached the country, the government had designed directions to undertake preventive measures for the passengers and service providers. However, Ethiopia formally announced the first coronavirus case on 13 March 2020 and had increased to 2019 cases with 27 deaths as of 7 July 2020. 13 Besides, as of 10 April 2021, there were 225,516 confirmed COVID-19 cases and 3111 deaths recorded in Ethiopia. 14
Despite that a survey conducted in Addis Ababa suggested that the large majority of the people are susceptible to COVID-19, 15 no data revealed the magnitude of this disease at the community and facility level in the country, even in Africa, with adequate sample size. In addition, a resource-limited country, such as Ethiopia, might not afford to test all respiratory tract infected patients for SARS-COV-2. 16 Thus, prioritizing resources and knowing the people at high risk of getting SARS-COV-2 is crucial to contain the pandemic by policymakers, planners, and health facilities in the resource-poor setups. Even if inclusively identifying all possible SARS-COV-2 positive cases using PCR has public health benefits for the screening and providing immediate response through prevention and control measures against COVID-19, SARS-COV-2 positivity among acute respiratory tract infections (ARTI) remains unknown. Therefore, this study aimed to assess the positivity of SARS-COV-2 and its associated factors among patients presented with ARTI.
Methods and materials
Study area, period, and design
A facility-based cross-sectional study design was implemented in the public hospitals that found in Harari Regional State. Harari region is one of the 10 regional administrations in Ethiopia. The region is located 522 km away from Addis Ababa, the capital city of Ethiopia due east. The region contains two public hospitals (Hiwot Fana Specialized University Hospital and Jogul General Hospital), one Federal Police Hospital, one Fistula Center, Private Hospitals, and eight health centers. This study was conducted in both Hiwot Fana Specialized University Hospital and Jugol General Hospital, the public hospitals in the region. The two hospitals are the public health facilities owned by the government where everybody who seeks medical care could get service. Hiwot Fana Specialized University Hospital is the largest referral and teaching hospital in Eastern Ethiopia and receives tertiary referrals from the Harari region, Eastern Oromia, Somali region, and Dire Dawa City. During COVID-19 pandemic, the hospital is one of the 10 regional centers designated by the Ethiopia Ministry of Health to manage the COVID-19 cases as a center in Eastern Ethiopia.
Study population and eligibility criteria
The source population was all patients who presented with ARTI in Hiwot Fana Specialized University Hospital and Jugol Hospital. And the study population was all patients who presented with ARTI in Hiwot Fana Specialized University Hospital and Jugol Hospital from 1 July to 31 December 2020. All patients who presented with respiratory tract infections in the Hiwot Fana Specialized University Hospital and Jugol Hospital were retrospectively included in the study. However, a patient who had no documented SARS-COV-2 result (positive or negative) was excluded.
Sample size determination and sampling procedure
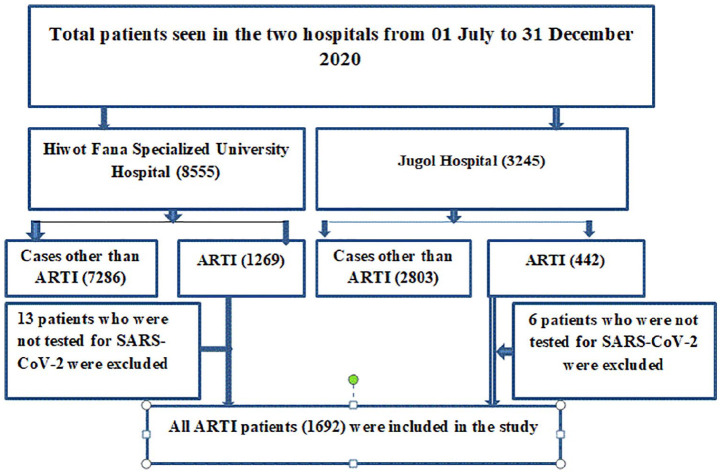
Initially, a minimum sample size was determined using single proportion population with p = 0.5 (no prior data from the study area), Z = 95% confidence interval, margin of error (d) = 0.05, n = (zα/2) 2 pq/d2 = 384. The final sample was determined to be 422 with 10% of non-response rate. However, we included all patients presenting with ARTI at both public hospitals. A total of 1256 and 436 study participants were included from Hiwot Fana Specialized University Hospital and Jugol Hospital, respectively. Finally, a total of 1692 study participants were involved in this study (Figure 1).
Figure 1.
Schematic diagrammatic presentation of sampling procedure.
Study variables
The dependent variable was the SARS-COV-2 positivity. The independent variables included socio-demographics, such as age, sex, residence, marital status, education status, and occupation. COVID-19-related risk factors, signs, and symptoms included chills, rigors, cough, sputum, malaise, myalgia, diarrhea, rhinorrhea, fever, travel history, contact history, smoking, medical history, history of medication, and SARS-COV-2 testing result. Other independent variables included underlying diseases, such as diabetes, hypertension, chronic lung disease, ischemic heart disease, chronic renal disease, thyroid disease, and neoplastic disease.
Operational definitions
Acute respiratory infection is an infection that may interfere with the normal breathing system that can be commonly presented with cough, fever, shortness of breathing, runny nose, sneezing, and chest pain for short period. 17 The classifications of the respiratory tract infections, lower and upper types of infection, were diagnosed by physicians depending on the anatomical sites involved by the infection and the symptoms that the patients presented with.
SARS-COV-2 positive is the proportion of positively tested SARS-COV-2 cases using reverse transcriptase–polymerase chain reaction (RT-PCR) detection from oropharyngeal swabs. 18
Contact history was defined as having a close or causal contact with COVID-19 confirmed/probable cases in the last 14 days prior to attending the health facilities.
Travel history was defined as having a history of travel to COVID-19 reporting areas in the past 14 days before attending the health facilities.
Comorbidity is any disease that co-exists with COVID-19.
History of medication was reviewed for chronic health conditions.
Equipment used and preparation made on the SARS-COV-2 assay using RT-PCR
Viral transport medium (VTM), swab sticks, ice-box, ice-pads, tongue depressor, marker, requisition form, and personal protective equipment (PPE) were prepared and used beforehand. The VTM that contains 3 mL fluids composed of gelatin and antimicrobial agents in a buffered salt solution was used. This VTM was used to prevent the sample collected from drying, maintains the viability of the virus, and avoids the growth of contaminants. The used swabs are made up of rayon with a plastic shaft. Ice-box and ice-pads are used for maintaining a cold chain during sample transportation from the sample collection area to the laboratory center. 19 The ice-pad was filled with water and stored in the freezer (−20°C) before and after use.
The sample contamination from the nasal vestibule was avoided by sterilely opening the outer case of the swab, inserting the swab into the mouth by slightly elevating the tip of the nose, and keeping the tip of the swab in the oropharynx for a few seconds, then rotated to achieve the highest absorption of oropharyngeal secretions.20,21
Data quality and procedures
Data were retrospectively extracted from the patients’ medical record by six BSc health professionals. Two public health professionals were involved in the data collection as supervisors. Before data collection, they took 2 days of training on the objective and relevance. Before actual data collection, 5% of the sample size was pre-tested on the patients presented with ARTI before 1 July 2020, in Hiwot Fana Specialized University Hospital. All patients who presented with ARTI were screened by RT-PCR for SARS-COV-2 in oropharyngeal. SARS-COV-2 loads were estimated with cycle threshold (Ct) values. SARS-COV-2 viral load is estimated with Ct values with the cutoff value of the mean value plus 3 standard deviations of the negative control. 22
Statistical analysis
The quality of the data was maintained by checking for consistency, completeness, and accuracy manually during data collection time. Data were entered using Epi-data 3.1 and exported to STATA 14.2 for analysis. Descriptive measures, such as mean with standard deviation, percentages, and frequencies, were used to characterize the study population. The binary logistic regression was used to identify the association between outcome variables (SARS-COV-2 positivity) and independent variables with an adjusted odds ratio (AOR) at a 95% confidence interval. Statistically, a significance level was declared at a p-value of less than 0.05.
Ethical statement
Haramaya University College of Health and Medical Sciences Institutional Health Research Ethical Review Committee (IHRERC) ethically cleared the paper (Ref. no: IHRERC/019/2021, with the approval date of 10 February 2021). A letter of permission was sent from the College of Health and Medical Sciences to Hiwot Fana Specialized University Hospital and Jugol Hospital. Due to difficulty to reach patients to take informed consent, written informed consent was obtained from legally authorized representatives of the facilities before the extraction of data. The data were obtained from patients’ medical records anonymously guaranteeing information confidentiality, and all data extraction processes were conducted as per the declaration of Helsinki. The collected data were used only for the intended purpose.
Results
Socio-demographic characteristics of the study participants
Out of a total of 1692 patients, 816 (48.2%) were males, while 876 (51.8%) were females. The mean age of the participants was 32.6 years, with standard deviation of 2 years. The majority (77.5%) of the study participants were Muslim, followed by 16.8% Orthodox religion. Among the total study participants, single, married, and separated/divorced were 13.4%, 55.6%, and 31%, respectively. Among the total study participants, 1163 (68.7%) of them had formal education, while the rest 529 (31.3%) had no formal education. Out of a total of 1692 study participants, 754 (44.6%) of them were Harari region residents, while the rest 938 (55.4%) were from the Oromia region. Among the total participants, only 14.4% and 1.6% of them had a contact history with suspected/confirmed COVID-19 cases and travel history to the pandemic areas, respectively (Table 1).
Table 1.
Socio-demographic characteristics of the study participants (N = 1692).
| Variables | Category | N | % |
|---|---|---|---|
| Sex | Male | 816 | 48.2 |
| Female | 876 | 51.8 | |
| Age (years) | Mean = 32.6 (± 2SD) | ||
| Age (years) | 0–9 | 375 | 22.2 |
| 10–19 | 112 | 6.6 | |
| 20–29 | 557 | 32.9 | |
| 30–39 | 265 | 15.6 | |
| 40–49 | 148 | 8.7 | |
| 50–59 | 112 | 6.6 | |
| ⩾60 | 123 | 7.3 | |
| Religion | Muslim | 1292 | 77.5 |
| Orthodox | 280 | 16.8 | |
| Protestant | 94 | 5.5 | |
| Others | 4 | 0.24 | |
| Marital status | Single | 224 | 13.4 |
| Married | 932 | 55.6 | |
| Separated/divorced | 520 | 31 | |
| Educational status | Unable to read and write | 529 | 31.3 |
| Able to read and write | 1163 | 68.7 | |
| Region | Harari | 754 | 44.6 |
| Oromia | 938 | 55.4 | |
| Contact history | Yes | 244 | 14.4 |
| No | 1448 | 85.6 | |
| Travel history | Yes | 28 | 1.6 |
| No | 1664 | 98.4 |
Clinical characteristics and comorbidities of study participants
Out of a total of 1692 study participants, 388 (22.9%) of them tested positive for SARS-COV-2. Out of a total of 1692 study participants, a majority (78.3%) of them presented with cough, fever, and shortness of breathing. The mean duration of COVID-19 symptoms presentation was 14.6 days (± 2.6SD). About 182 (10.8%) of the study participants had comorbidities at the time of visit. Of these comorbidities, hypertension, diabetes mellitus (DM), cardiac diseases, tumor, and HIV/AIDS diagnosed among 165 (9.8%), 63 (3.7%), 87 (5.1%), 8 (0.5%), and 24 (1.4%), respectively. A majority of the study participants, 220 (13%) and 64 (3.8%), had history of taking chronic case medication and smoking, respectively. Regarding the types of respiratory tract infection, 510 (30.1%) participants presented with lower respiratory tract infection, while the rest 1182 (69.9%) presented with upper respiratory tract infection (Table 2).
Table 2.
Clinical characteristics of the study participants (N = 1692).
| Variables | Category | N | % |
|---|---|---|---|
| COVID-19 symptoms on time of visit | Sore throat, headache, sneezing | 141 | 8.4 |
| Cough, fever, and SOB | 1325 | 78.3 | |
| Chest pain, diarrhea | 226 | 13.3 | |
| Mean duration of the symptoms | 14.6 days (± 2.6SD) | ||
| Existence of comorbidities on presentation | Yes | 182 | 10.8 |
| No | 1510 | 89.2 | |
| Hypertension | Yes | 165 | 9.8 |
| No | 1524 | 90.2 | |
| Diabetes mellitus | Yes | 63 | 3.7 |
| No | 1629 | 96.3 | |
| Cardiac disease | Yes | 87 | 5.1 |
| No | 1603 | 94.9 | |
| Malignant/tumors | Yes | 8 | 0.5 |
| No | 1684 | 99.5 | |
| HIV/AIDS | Yes | 24 | 1.4 |
| No | 1668 | 98.6 | |
| History of taking any medication | Yes | 220 | 13 |
| No | 1472 | 87 | |
| Smoking history | Yes | 64 | 3.8 |
| No | 1628 | 96.2 | |
| Types of respiratory tract infection | Lower | 510 | 30.1 |
| Upper | 1182 | 69.9 |
COVID-19: coronavirus disease 2019; SD: standard deviation; SOB: shortness of breathing.
Factors affecting SARS-COV-2 positivity
Variables that had a p-value less than 0.3 were transformed to multivariable logistic regression. A separated/divorced marital status, age above, and symptoms with the patients presented had statistically significant association with SARS-COV-2 infection (p < 0.05). The odd of SARS-COV-2 positivity was reduced by 47% among separated/divorced in marital status compared to married individuals (AOR = 0.53, 95% CI: 0.29–0.95). Besides, patients who were in the age group of 30–39 years became positive for SARS-COV-2 65% less likely than the patients with the age of 60 years and above (AOR = 0.35, 95% CI: 0.15–0.79). Moreover, patients who were in the age group of 40–49 years became positive for SARS-COV-2 63% less likely compared to patients with the age of 60 years and above (AOR = 0.37, 95% CI: 0.14–0.94). Furthermore, patients who were in the age group of 50–59 years were positive for SARS-COV-2 69% less likely than the patients with the age of ⩾ 60 years (AOR = 0.31, 95% CI: 0.13–0.76). The study subjects who presented with cough, fever, and difficulty of breathing had SARS-COV-2 2.5 times more likely compared to those who presented with symptoms, such as sore throat, headache, and sneezing (AOR = 2.5, 95% CI: 1.22–4.7) (Table 3).
Table 3.
Factors associated with the SARS-COV-2 positivity among study participants (N = 1692).
| Variables | Category | SARS-COV-2 status |
COR | AOR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Sex | Male | 183 | 633 | 1.1 (0.84–1.3) | 1.1 (0.7–1.6) | 0.6 |
| Female | 205 | 671 | 1 | |||
| Age (years) | <18 | 100 | 387 | 2.2 (1.4–3.5) | 0.54 (0.12–2.3) | 0.2 |
| 20–29 | 113 | 444 | 1.9 (1.3–3.1) | 0.5 (0.23–1.3) | 0.12 | |
| 30–39 | 56 | 209 | 1.9 (1.2–3.1) | 0.35 (0.15–0.79) | 0.01* | |
| 40–49 | 44 | 104 | 1.3 (0.7–2.1) | 0.44 (0.14–1.02) | 0.05 | |
| 50–59 | 33 | 79 | 1.1 (0.7–2.5) | 0.39 (0.12–1.04) | 0.06 | |
| >60 | 42 | 81 | 1 | 1 | ||
| Marital status | Married | 226 | 706 | 1 | 1 | |
| Not married | 36 | 188 | 1.67 (1.13–2.46 | 1.1 (0.6–2.1) | 0.4 | |
| Separated/divorced | 123 | 397 | 1.1 (0.8–1.3) | 0.53 (0.29–0.95) | 0.03* | |
| Educational status | Unable to read and write | 152 | 377 | 1 | 1 | |
| Able to read and write | 236 | 927 | 1.5 (1.2–2.1) | 0.86 (0.57–1.3) | 0.5 | |
| Region | Harari | 165 | 589 | 1 | 1 | |
| Oromia | 223 | 715 | 0.8 (0.7–1.3) | 0.7 (0.6–1.4) | 0.7 | |
| Contact history | Yes | 44 | 200 | 1.4 (0.9–2.1) | 0.86 (0.46–1.59) | 0.6 |
| No | 344 | 1104 | 1 | 1 | ||
| Travel history to areas with COVID-19 pandemic | Yes | 7 | 21 | 0.9 (0.4–2.1) | 0.58 (0.13–2.64) | 0.5 |
| No | 381 | 1283 | 1 | 1 | ||
| Existence of comorbidities | Yes | 50 | 132 | 0.7 (0.5–1.1) | 1.3 (0.53–3.1) | 0.6 |
| No | 338 | 1172 | 1 | 1 | ||
| History of medication taking | Yes | 63 | 157 | 0.7 (0.5–0.9) | 0.76 (0.33–1.6) | 0.4 |
| No | 323 | 1145 | 1 | 1 | ||
| Smoking history | Yes | 21 | 45 | 0.6 (0.36–1.1) | 1.1 (0.4–2.5) | 0.9 |
| No | 366 | 1528 | 1 | |||
| COVID-19 symptoms on time of visit | Sore throat, headache, and sneezing | 141 | 502 | 1 | 1 | |
| Cough, fever, and SOB | 21 | 151 | 2.1 (1.23–3.31) | 2.5 (1.22–4.7 | 0.01 | |
| Fever, chest pain, and diarrhea | 226 | 651 | 0.81 (0.63–1.2) | 0.7 (0.45–1.1 | 0.5 | |
AOR; adjusted odds ratio: CI: confidence interval; COVID-19: coronavirus disease 2019; SOB: shortness of breathing; COR: crude odds ratio.
Hosmer–Lemeshow χ2(8) = 4.15, Prob > χ2 = 0.8435.
Significant factors (p < 0.05).
Discussion
This study demonstrated that SARS-COV-2 was positive for 388 (22.9%) acute respiratory infected people. Majority (21.6%) of SARS-COV-2 positive patients presented with lower respiratory tract infection, (p < 0.0001). The factors, such as separated/divorced marital status, age, and particular symptoms, such as cough, fever, and shortness of breathing, had statistically significant association with SARS-COV-2 positivity.
This study revealed that the SARS-COV-2 positivity was 22.9% among acute respiratory tract infected people. The majority (21.6%) of the patients who were diagnosed with lower respiratory tract infections were positive for SARS-COV-2. This finding was higher than the studies detected SARS-COV-2 using RT-PCR. The SARS-COV-2 was positive among 13.2% in Thailand 23 and 13.1% in Iran. 24 The discrepancy might be due to the variation in socio-demographic characteristics and potential spatiotemporal variations in viral distribution. The discrepancy may support the importance of generating evidence from both developed and resource-limited setups like the study area of the present study. In addition, the current study’s higher finding is likely due to the increasing infectiousness (R0) of SARS-Cov-2 over time. However, this study finding was lower than the report from Iran that was 36.08% cases. 19 This can be because the probability of testing positive for SARS-COV-2 among patients who presented with severe pneumonia is highly expected due to the highest degree of manifestations overlapping of the two infections. In addition, the finding from the current study was lower than the SARS-COV-2 positives (30.4%) reported from Nicaragua, Central America. 20 This can be since healthcare workers are at high risk of getting SARS-COV-2 infection due to their working area than a general population studied in the present study.
The study participants with divorced/separated marital status had 47% less likely to be positive for SARS-Cov-2. The finding was in line with the report from Switzerland that revealed higher SARS-COV-2 positive tests (57·2%) among family members and those who had close contact (19%). 21 A divorced/separated/widowed man/woman may live with a smaller family size/alone compared to married individuals. The smaller family size could decrease familial transmissions of SARS-Cov-2. Thus, living with less family size decreases the probability of unsafe contact with suspected/confirmed COVID-19 patients that result in a lesser viral spread.
This study also revealed that people above the age of 30 years had SARS-COV-2 infection 63% less likely compared to patients with the age of 60 years and above. This study was supported by the finding from Wuhan, China, Iran, and the United Kingdom.22,24,25 In fact, due to aging and the risk of developing comorbidities, the older (> 60 years) population may have hampered immunity that exposes them to SARS-COV-2 infection. According to the survey conducted in Ethiopia, the older population had a 31% lesser probability of engagement in COVID-19 preparedness and response, 26 and this can be due to distinct psychosocial support they need during this critical period for their lives. 27 Therefore, because of their less involvement in the preparedness and response against COVID-19 and a distinct psychosocial need, older population might not fully adhere to SARS-COV-2 containing ways, such as wearing masks, maintaining the physical distance to optimum, frequent hand washing, and practicing safe contact with suspected/confirmed COVID-19 cases.
In addition, the study participants who presented with cough, fever, and difficulty of breathing infected with SARS-COV-2 2.5 times more likely compared to those who presented with symptoms, such as sore throat, headache, and sneezing. In contrary to the present finding, according to the study conducted in Sweden; 28 fever and loss of smell and taste and in Brazil; 29 anosmia and ocular pain were associated with positive SARS-COV-2. Regarding the strength of this study, the study could be the first report in Ethiopia, even for other developing countries. It could be also the study conducted with a larger sample size inclusion that would make the study representative. As a limitation, false-negativity of RT-PCR could underestimate the positive SARS-COV-2 test. In addition, due to resource constraints, some other laboratory investigations and radiologic investigations were not done for the study participants.
Conclusion and recommendations
The SARS-COV-2 tested positive among 388 (22.9%) acute respiratory infected people. The majority (21.6%) of SARS-COV-2 positive patients presented with lower respiratory tract infections. A divorced/separated, age, presenting with symptoms, such as cough, fever, and difficulty of breathing, had statistically significant associations with of SARS-COV-2 positive test. In resource-limited setups, where a shortage of testing equipment is frequent, these findings could contribute to boosting targeted symptom-oriented screening schemes. Moreover, strengthening the protection and control strategies for older adults is of priority to contain the spread of the virus.
Acknowledgments
The authors thank their study participants, study facilities staff, data collectors, and supervisors for their commitment throughout the data collection process.
Footnotes
Author contributions: A.B. was involved in conception and design of the research idea, analysis, and writing up of the paper. G.M.A., Y.D., A.M., and M.B. participated in the manuscript drafting and editing. Finally, all authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Haramaya University College of Health and Medical Sciences Institutional Health Research Ethical Review Committee (IHRERC) (Ref. no. IHRERC/019/2021, with the approval date of 10 February 2021).
Informed consent: Written informed consent was obtained from legally authorized representatives before the study.
Data availability: The data can be accessed from the corresponding author upon request.
ORCID iD: Abdi Birhanu  https://orcid.org/0000-0003-1312-0637
https://orcid.org/0000-0003-1312-0637
Galana Mamo Ayana  https://orcid.org/0000-0001-6082-0172
https://orcid.org/0000-0001-6082-0172
References
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395(10223): 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res 2020; 7(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Groot RJ, Baker SC, Baric RS, et al. Commentary: Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 2013; 87(14): 7790–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348(20): 1967–1976. [DOI] [PubMed] [Google Scholar]
- 5. Kuiken T, Fouchier RA, Schutten M, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 2003; 362(9380): 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Centre for Disease Prevention and Control (ECDC). Novel coronavirus disease 2019 (COVID—19) pandemic: increased transmission in the EU/EEA and the UK—sixth update 12 March 2020. Stockholm: ECDC, 2020. [Google Scholar]
- 8. Ma X-L, Chen Z, Zhu J-J, et al. Management strategies of neonatal jaundice during the coronavirus disease 2019 outbreak. World J Pediatr 2020; 16: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. United Nations (UN). Recovering better: economic and social challenges and opportunities. New York: UN, 2020. [Google Scholar]
- 10. World Health Organization (WHO). Global report of coronavirus. Geneva: WHO, 2020. [Google Scholar]
- 11. Sharifipour E, Shams S, Esmkhani M, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis 2020; 20(1): 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aburto JM, Kashyap R, Schöley J, et al. Estimating the burden of the COVID-19 pandemic on mortality, life expectancy and lifespan inequality in England and Wales: a population-level analysis. J Epidemiol Community Health 2021; 75(8): 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Federal Ministry of Health (FMOH). National comprehensive COVID19 management handbook, 2020, https://covidlawlab.org/wp-content/uploads/2020/06/National-Comprehensive-COVID19-Management-Handbook.pdf
- 14. Woldometer. Total Coronavirus cases in Ethiopia, 2021, https://www.worldometers.info/coronavirus/country/ethiopia/
- 15. Kempen JH, Abashawl A, Suga HK, et al. SARS-CoV-2 serosurvey in Addis Ababa, Ethiopia. Am J Trop Med Hyg 2020; 103(5): 2022–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bellos A, Mulholland K, O’Brien KL, et al. The burden of acute respiratory infections in crisis-affected populations: a systematic review. Confl Health 2010; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burrel S, Hausfater P, Dres M, et al. Co-infection of SARS-CoV-2 with other respiratory viruses and performance of lower respiratory tract samples for the diagnosis of COVID-19. Int J Infect Dis 2021; 102: 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung IF-N, Cheng VC-C, Li X, et al. SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: a case series. Lancet Infect Dis 2020; 20(9): 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abolnezhadian F, Makvandi M, Alavi SM, et al. Prevalence of SARS-CoV-2 in patients with severe pneumonia in Khuzestan Province, Iran. Iran J Allergy Asthma Immunol 2020; 19(5): 471–477. [DOI] [PubMed] [Google Scholar]
- 20. Huete-Pérez JA, Cabezas-Robelo C, Páiz-Medina L, et al. First report on prevalence of SARS-CoV-2 infection among health-care workers in Nicaragua. PLoS One 2021; 16(1): e0246084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dupraz J, Butty A, Duperrex O, et al. Prevalence of SARS-CoV-2 in household members and other close contacts of COVID-19 cases: a serologic study in canton of Vaud, Switzerland. Open Forum Infect Dis 2021; 8: ofab149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo Y, Liu X, Deng M, et al. Epidemiology of COVID-19 in older persons, Wuhan, China. Age Ageing 2020; 49(5): 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaimayo C, Kaewnaphan B, Tanlieng N, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J 2020; 17(1): 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalantari H, Tabrizi AHH, Foroohi F. Determination of COVID-19 prevalence with regards to age range of patients referring to the hospitals located in western Tehran, Iran. Gene Rep 2020; 21: 100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldstein E, Lipsitch M, Cevik M. On the effect of age on the transmission of SARS-CoV-2 in households, schools and the community. medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.07.19.20157362v2 [DOI] [PMC free article] [PubMed]
- 26. Addis Y, Abate D, Ferreira JB. Social work responses and household-level determinants of coronavirus preparedness in rural Ethiopia. Research Square 2020, https://www.researchsquare.com/article/rs-62813/v1 [DOI] [PubMed]
- 27. Al-Zahrani J. SARS-CoV-2 associated COVID-19 in geriatric population: a brief narrative review. Saudi J Biol Sci 2021; 28(1): 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindahl JF, Hoffman T, Esmaeilzadeh M, et al. High seroprevalence of SARS-CoV-2 in elderly care employees in Sweden. Infect Ecol Epidemiol 2020; 10(1): 1789036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buonafine CP, Paiatto BNM, Leal FB, et al. High prevalence of SARS-CoV-2 infection among symptomatic healthcare workers in a large university tertiary hospital in São Paulo, Brazil. BMC Infect Dis 2020; 20(1): 917. [DOI] [PMC free article] [PubMed] [Google Scholar]