Abstract
The transcriptional coactivator BOB.1/OBF.1 confers B-cell specificity on the transcription factors Oct1 and Oct2 at octamer site-containing promoters. A hallmark of the BOB.1/OBF.1 mutation in the mouse is the absence of germinal center development in secondary lymphoid organs, demonstrating the requirement for BOB.1/OBF.1 in antigen-dependent stages of B-cell differentiation. Here we analyzed earlier stages of B lymphopoiesis in BOB.1/OBF.1-deficient mice. Examination of B-cell development in the bone marrow revealed that the numbers of transitional immature (B220+ IgMhi) B cells were reduced and that B-cell apoptosis was increased. When in competition with wild-type cells, BOB.1/OBF.1−/− bone marrow cells exhibited defects in repopulating the bone marrow B-cell compartment and were unable to establish a presence in the periphery of host mice. The defective bone marrow populations in BOB.1/OBF.1−/− mice were rescued by conditional expression of a BOB.1/OBF.1 transgene controlled by the tetracycline gene expression system. However, the restored populations did not restore the numbers of IgDhi B cells in the periphery, where the BOB.1/OBF.1 transgene was not expressed. These results show that BOB.1/OBF.1−/− B cells exhibit multistage defects in B-cell development, including impaired production of transitional B cells and defective maturation of recirculating B cells.
The octamer motif is conserved in virtually all immunoglobulin (Ig) gene promoters and is essential for B-cell-specific promoter function. Transcriptional activity at octamer site-containing promoters requires the Oct1 or Oct2 transcription factor and a specific coactivator, BOB.1/OBF.1 (also called Bob1, OBF-1, or OCA-B), which functionally interacts with Oct1 and Oct2 (12, 20, 33). In addition to binding Oct1 and Oct2, BOB.1/OBF.1 contacts DNA and thereby increases the selectivity of Oct proteins for octamer motifs (5, 6, 11). Furthermore, recent analyses of octamer-dependent transcription in pre-B-cell lines derived from BOB.1/OBF.1-deficient mice demonstrate that BOB.1/OBF.1 is an essential and nonredundant component of transcription at octamer promoters (18). Expression of BOB.1/OBF.1 is largely restricted to B lymphocytes (30), with expression levels peaking in germinal center B cells and germinal center-derived B-cell lymphomas (10, 23). The expression of functional BOB.1/OBF.1 can also be induced in T lymphocytes by costimulation with phorbol ester and ionomycin (40).
Given the preponderance of octamer motifs in Ig gene promoters, the expression of BOB.1/OBF.1 in early B-cell populations and the essential role for BOB.1/OBF.1 in octamer-dependent transcription, BOB.1/OBF.1 could be expected to participate in B-cell development. Surprisingly however, mutation of BOB.1/OBF.1 in mice results in defects, which are largely restricted to late, antigen-dependent stages of B-cell development (15, 21, 28). Specifically, BOB.1/OBF.1−/− mice have greatly reduced expression levels of secondary Ig isotypes and a failure in germinal center development. Although BOB.1/OBF.1-deficient B cells are clearly defective in terminal B-cell differentiation and cannot participate in the germinal reaction, they still proliferate normally when stimulated with lipopolysaccharide, anti-Ig, or CD40 in the presence of interleukin 4 (IL-4). They also induce isotype switching at a normal rate when stimulated in vitro, indicating that they are very similar to normal resting B cells in the spleen despite the altered Ig cell surface phenotype.
No gross alterations of antigen-independent B-cell development have been observed, although several indications point to the existence of defects in BOB.1/OBF.1−/− B-cell maturation. BOB.1/OBF.1−/− mice have an overall two- to fourfold reduction in the number of splenic B cells. A greater reduction is seen in B cells in lymph nodes that typically contain a higher proportion of B cells in late stages of maturation (21, 28). Bone marrow B220hi IgMlo cells, representing mature recirculating or long-lived B cells, are also reduced in BOB.1/OBF.1−/− mice. A recent study showed that combining the BOB.1/OBF.1 mutation with the Btk mutation results in a significantly stronger phenotype (29). Mutation of the B-cell-specific kinase Btk in mice results in reduced numbers of conventional B cells and a complete absence of the B1 B-cell lineage (13, 26, 27, 38). Mice that are deficient for both BOB.1/OBF.1 and Btk show a nearly complete loss of B cells in the periphery, suggesting that BOB.1/OBF.1 and Btk have overlapping roles in B-cell development (29).
In this study, we analyzed the role of BOB.1/OBF.1 in the antigen-independent stages of B-cell development. We found that, indeed, BOB.1/OBF.1 is required for development of mature IgDhi B cells in the spleen and that, unexpectedly, additional defects were observed in immature and transitional immature B-cell development.
MATERIALS AND METHODS
Generation and genotyping of transgenic and knockout mice.
An AatII-ClaI fragment, derived from pUCβ-globin (2) containing the IgH enhancer (700 bp) and a minimal promoter with a synthetic octamer motif and pUHG15.1 (9) digested with AatII and EcoRI, was used to clone the μE-tTA vector. For the tetO-BOB.1/OBF.1 vector, the coding sequence of BOB.1/OBF.1 was cloned as an XhoI-BamHI fragment (22) into the bidirectional tetracycline-dependent expression vector pBi5 (3). In both cases, linearized AatII-AseI fragments were used for the injection of fertilized oocytes to generate transgenic animals. The genotype of transgenic animals was determined by Southern blot analysis using EcoRI-digested genomic DNA for μE-tTA and BamHI-digested genomic DNA for tetO-BOB.1/OBF.1 transgenic mice. For hybridization a tTA-specific probe derived from pUHG 15.1 or a luciferase-specific probe derived from pBi5 was used. Alternatively we analyzed the genomic DNA by PCR using the following primers: tTA3, GGCACCATACTCACTTTTGC; tTA4, CTTGTCGTAATAATGGCGGC; OCAB3.2, GATACTGCAGGCTGGAGGTG; and OCAB5.2, CGCATTGGCTCCATGGACAC. The genotype of BOB.1/OBF.1-deficient mice was determined as described previously (21).
Doxycycline treatment.
Administration of doxycycline was performed by adding 2 mg of doxycycline/ml to drinking water containing 5% sugar for 3 to 5 days. Pre-B cells were treated with 1 μg of doxcycline/ml for 1 to 3 days.
Competitive bone marrow transfers.
Bone marrow cells (107) from 6- to 8-week-old mice were injected into the tail veins of sublethally irradiated (600 rads) RAG-2−/− mice (strain BALB/c), and the lymphopoiesis in chimeric animals was analyzed 6 to 8 weeks after the transfer. To distinguish between wild-type and mutant donor cells, which were injected in a 1:1 ratio, the congenic C57BL/6a strain, bearing allotypic markers for IgM (IgMa) and Thy1 (Thy1.1), compared to IgMb and Thy1.2 in the C57BL/6 and BOB.1/OBF.1-deficient mice, was used.
FACS stainings and enzyme-linked immunosorbent assays.
Lymphoid tissues were isolated in fluorescence-activated cell sorter (FACS) buffer (1× phosphate-buffered saline [PBS], 1% bovine serum albumin [BSA], 0.1% sodium azide), single-cell suspensions were prepared, and 2 × 105 to 5 × 105 cells were used for FACS staining. Additionally, the lymphocytes of splenic preparations were enriched by a Lympholyte-M gradient (Cedarlane). Cell surface markers were stained using the following reagents: Fc-block (Pharmingen), anti-IgM-phycoerythrin (PE) (Dianova), anti-IgD-fluorescein isothiocyanate (FITC) (Pharmingen), anti-B220-FITC (Pharmingen), monoclonal antibody (MAb) 493-biotin (25), annexin V (Pharmingen), anti-IgMa-biotin (Pharmingen), anti-IgMb-FITC (Pharmingen), anti-Thy1.1-FITC (Pharmingen), anti-Thy1.2-PE (Pharmingen), and streptavidin-PE (Pharmingen). Enzyme-linked immunosorbent assays were performed as described previously (21).
Western immunoblots, luciferase assays, and pre-B-cell cultures.
Western immunoblots and luciferase assays were performed as described previously (22). Primary IL-7-dependent pre-B-cell cultures were established as described (16, 24).
BrdU labeling.
For bromodeoxyuridine (BrdU) labeling, mice were given drinking water supplemented with 700 μg of BrdU/ml ad libitum. At various time points, mice were sacrificed, cells were isolated from bone marrow and spleen, and BrdU incorporation was measured by staining with an anti-BrdU-FITC antibody (catalog no. 7583, Becton Dickinson). To do this, approximately 106 cells were washed twice in cold PBS–0.5% BSA, suspended in 0.5 ml of PBS, and fixed by dropwise addition of 1.2 ml of ice-cold 95% ethanol. After 30 min on ice, cells were washed twice in PBS-BSA, resuspended in 1 ml of PPT (1% paraformaldehyde, PBS, 0.05% Tween 20), and incubated for 30 min at room temperature. The cells were centrifuged and resuspended in 1 ml of MHD (PBS supplemented with 4.2 mM MgCl2, 10 μM HCl, 100 U of DNase I) (D-5025; Sigma). After incubation at 25°C for 30 min, the cells were washed once in PBS and stained with the anti-BrdU-FITC antibody. In all cases, the cells were also stained with additional antibodies recognizing B cells (anti-B220-PE, anti-IgM-biotin) or T cells (anti-Thy1-biotin). Cells were analyzed by flow cytometry as described above.
RESULTS
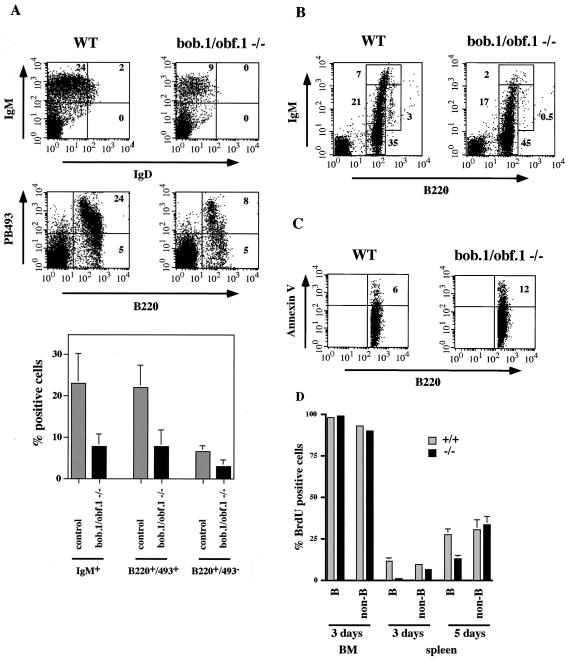
Previous analyses revealed a two- to fourfold reduction of splenic B cells in BOB.1/OBF.1-deficient mice (21, 28). However, only the more mature stages in the periphery appeared to be affected (21), suggesting that BOB.1/OBF.1 is required for B-cell maturation in the spleen. In the adult mouse, B cells are generated in the bone marrow. Immature B cells which leave the bone marrow and migrate to the periphery are called transitional B cells and are IgMhi IgDlo (1, 4, 19). In the spleen, transitional immature cells differentiate into mature IgMlo IgDhi B cells. Transitional immature cells express the recently described marker of immature B cells recognized by MAb 493 (25) (Fig. 1A). In 1-week-old mice, virtually all the B cells in the spleen are still transitional immature B cells (19). In order to determine whether loss of BOB.1/OBF.1 deleteriously affects these immature transitional or only more mature B cells, we analyzed B cells from the spleens of control and BOB.1/OBF.1-deficient mice. We observed a significant reduction (threefold) of B220+ and PB493+ cells in BOB.1/OBF.1−/− mice, indicating an important function for BOB.1/OBF.1 in B-cell development.
FIG. 1.
BOB.1/OBF.1-deficient mice show defects in early B-cell development. (A) Flow cytometry analysis of gated lymphocytes from spleens of 1-week-old control (WT) and BOB.1/OBF.1-deficient (bob.1/obf.1−/−) mice. Cells were stained with anti-IgM-PE and anti-IgD-FITC antibodies or with anti-B220-FITC and anti-PB493-biotin antibodies. The percentages of different B-cell populations are shown in the FACS quadrents. At the bottom, statistical analyses from at least three independent measurements are shown. (B) Flow cytometry analysis of bone marrow lymphocytes from 6- to 8-week-old control (WT) and BOB.1/OBF.1-deficient mice, which were stained with anti-B220-FITC and anti-IgM-PE antibodies. Percentages of pro- and pre-B cells (B220lo IgM−), immature B cells (B220lo IgM+), T1 B cells (B220lo IgMhi), and recirculating B cells (B220hi IgM+) are indicated in the figure. Statistical analyses from 13 independent measurements are shown in Fig. 4B and C. (C) Level of annexin V on the surface of control (WT) or BOB.1-deficient B220lo bone marrow B cells measured with an anti-annexin V-FITC antibody by flow cytometry. The cells were also stained with an anti-B220-PE antibody. The percentage of annexin V-positive cells is denoted in boldfaced numerals. Statistical analyses from three independent measurements are shown in Fig. 4E. (D) Percentage of BrdU-positive lymphocytes in bone marrow and spleen of control (grey bars) and BOB.1/OBF.1-deficient (black bars) mice. Labeling was performed for 3 or 5 days as indicated. Labeled cells were analyzed by flow cytometry after staining with anti-BrdU-FITC antibodies and anti-B220-PE for B cells.
The observed reduction in BOB.1/OBF.1−/− transitional immature B cells could be due to problems in the generation or migration of these cells from bone marrow to the spleen. To investigate these possibilities further, we analyzed the stages of pre-B and immature-B-cell development in the bone marrow. Pro-B- and pre-B-cell populations (B220lo IgM−) were normal or slightly increased in the absence of the coactivator. As previously described, mature recirculating B cells represented by B220hi IgM+ cells were greatly reduced in BOB.1/OBF.1−/− bone marrow (Fig. 1B) (15, 21, 28). Interestingly, BOB.1/OBF.1 knockout mice showed a decreased number of immature cells (B220lo IgM+) and, even more pronounced, a reduction of transitional immature B cells (B220lo IgMhi) compared to control littermates (Fig. 1B). We have analyzed at least 10 mice for each genotype and found that the average reduction of transitional immature B cells was two- to threefold. These data suggest that the reduction of transitional immature B cells in the spleens of BOB.1/OBF.1-deficient mice is due to inefficient development and/or survival within the bone marrow rather than defective migration and/or homing.
Transitional immature B cells represent the first checkpoint for deletion of self-reactive B cells (4). We therefore asked whether the decrease in this cell population in BOB.1/OBF.1−/− mice might be due to increased levels of apoptosis. The level of annexin V on the surface of B220lo bone marrow B cells was analyzed as an early marker for apoptosis. In the bone marrow of wild-type and heterozygous control mice (BOB.1/OBF.1+/−), 5 to 8% of the B220lo B cells were annexin V-positive (Fig. 1C and see Fig. 4B). The amount of annexin V-positive B cells in BOB.1 knockout littermates was increased to 10 to 15% (Fig. 1C and see Fig. 4B), indicating a higher rate of programmed cell death in the absence of the coactivator. We therefore conclude that the reduction in B cells appearing in the periphery is at least partly explained by the reduced survival of BOB.1/OBF.1-deficient transitional immature B cells in the bone marrow. These results demonstrate that BOB.1/OBF.1 not only is essential for the late steps in B-cell maturation and function but also plays an important role in early B-cell differentiation and survival.
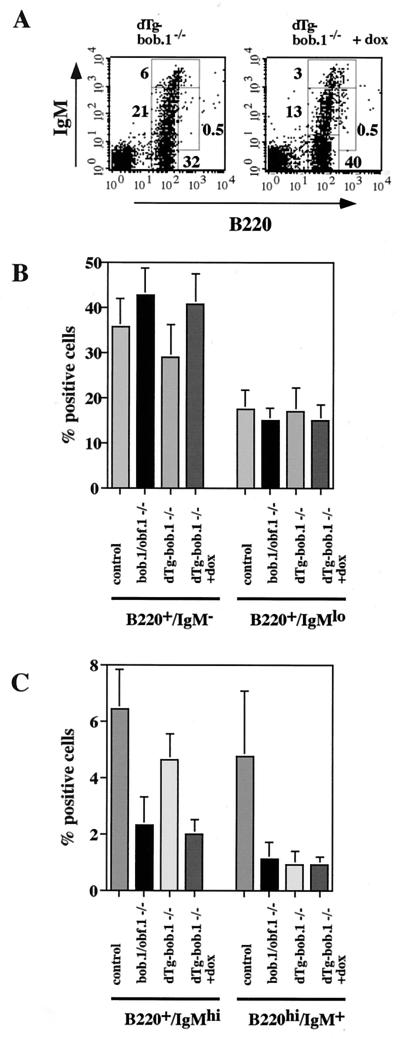
FIG. 4.
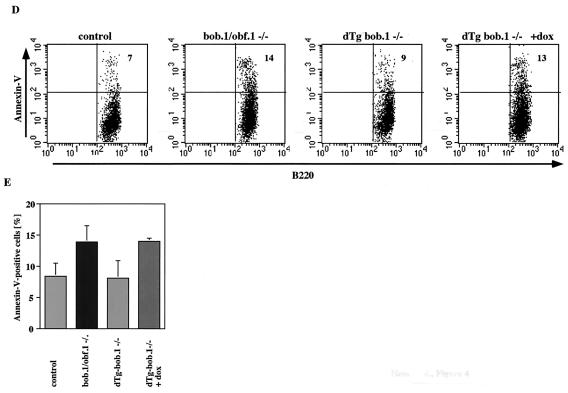
Rescue of the early B-cell phenotype by conditional expression of transgenic BOB.1/OBF.1. (A) Flow cytometry analysis of bone marrow cells from 6- to 8-week-old dTg BOB.1/OBF.1-deficient mice (dTg-bob.1−/−) stained with anti-B220-FITC and anti-IgM-PE antibodies. Percentages of pro- and pre-B cells (B220lo IgM−), immature B cells (B220lo IgM+), T1 B cells (B220lo IgMhi), and recirculating B cells (B220hi IgM+) are indicated. Transgene expression was turned off by administration of 2 mg of doxycycline (+dox)/ml to the drinking water for 5 days. (B and C) Statistical analysis of the percentages of various B-cell populations from at least four independent mice in each group. (D) Representative analysis of annexin V-positive B cells in bone marrow of mice with the indicated genotypes and treatments. The levels of annexin V on the surface B220+ bone marrow B cells of control mice, BOB.1/OBF.1-deficient mice, dTg BOB.1/OBF.1-deficient mice not treated with doxycycline, and dTg BOB.1/OBF.1-deficient mice treated with doxycycline (left to right) were measured with an anti-annexin V-FITC antibody by flow cytometry. Doxycycline had no effect on the percentage of annexin V staining cells in wild-type or BOB.1/OBF.1-deficient animals not bearing the tetracycline-regulated transgene. (E) Percentage of annexin V-positive bone marrow B cells. Data are shown as averages (± standard deviations) from three independent animals for each genotype.
The results described so far suggest that there is a partial block in early B-cell development, which results in a reduced rate of appearance of B cells in the periphery. To further examine this possibility, we analyzed production of B cells by in vivo BrdU labeling. Mice were treated with BrdU, and B cells from bone marrow and spleen were analyzed 3 and 5 days later. After 3 days of labeling, the mature recirculating B cells (B220hi IgM+) were unlabeled, and almost all B cells at earlier stages of development were labeled in both control and mutant mice (Fig. 1D). The same result was observed after 5 days of labeling. This suggests that the initial de novo generation of B precursors occurs normally in the absence of the BOB.1/OBF.1 coactivator. This result is consistent with earlier reports showing no gross change in precursor B-cell populations in the bone marrow of BOB.1/OBF.1-deficient mice. However, a striking difference between control and mutant mice was observed in the spleen. In control mice, the fraction of labeled splenic B cells after 3 and 5 days of BrdU administration was about 11 and 27%, respectively, whereas the proportion of BrdU-labeled B cells in BOB.1/OBF.1-deficient mice was only 1 and 13% for the same time points (Fig. 1D). This decrease is specific for B cells, since the fraction of BrdU-labeled non-B cells in the spleens of control and BOB.1/OBF.1-deficient mice was comparable (Fig. 1D). When expressed in absolute cell numbers, about 2.6 × 106 B cells/day appeared in the spleens of control animals. This agrees well with the B-cell production rate described in the literature (24, 39). This is in contrast to the almost 20-fold-lower production rate of 1.5 × 105 B cells/day in the BOB.1/OBF.1-deficient mice. These results are consistent with the FACS and annexin V stainings of bone marrow described above and show that the reduced production of transitional B cells in the bone marrow results in a reduced rate of appearance of B cells in the periphery.
In order to confirm the role for BOB.1/OBF.1 in B lymphopoiesis in the bone marrow, we tested the ability of BOB.1/OBF.1-deficient cells to repopulate B-cell compartments by adoptive transfer of bone marrow cells derived from control and BOB.1/OBF.1 mutant littermates into sublethally irradiated RAG-2−/− mice. The chimeric animals derived from injection of BOB.1/OBF.1-deficient donor cells were assessed at 6 to 8 weeks following transfer for B-cell development in bone marrow and splenic compartments. The reconstituted mice recapitulated the phenotype of BOB.1/OBF.1−/− mice with a decreased number of transitional immature B cells and mature recirculating B cells in the bone marrow, as well as a two- to threefold reduction of splenic B cells (data not shown).
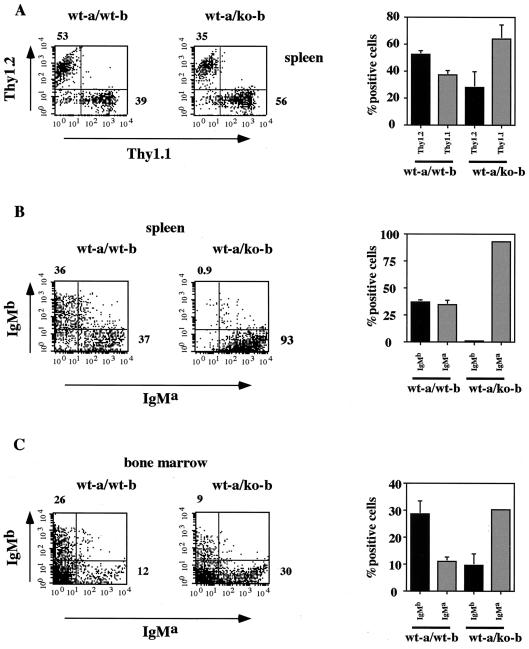
We next measured the performance of BOB.1/OBF.1-deficient cells when in the presence of competing wild-type cells. To distinguish between wild-type and mutant donor cells in a competitive transfer experiment, we used as a control the congenic C57BL/6a strain bearing allotypic markers for IgM (IgMa) and Thy1 (Thy1.1). In contrast, the corresponding allotypic markers in the C57BL/6 and BOB.1/OBF.1-deficient mice were IgMb and Thy1.2. Bone marrow cells from C57BL/6a mice and control (C57BL/6) or BOB.1/OBF.1-deficient mice were injected at a 1:1 ratio into sublethally irradiated RAG-2−/− mice. Lymphopoiesis in chimeric animals was analyzed 6 to 8 weeks after the transfer. The contribution of the two cell types was estimated by measuring the expression of the alleles for IgM and Thy1. Chimeric mice from injections with wild-type and control donor cells (wt-a/wt-b) or wild-type and BOB.1/OBF.1-deficient donor cells (wt-a/ko-b) had similar proportions of total splenic T versus non-T cells (Fig. 2A). In the bone marrow of wt-a/wt-b chimeric mice, both IgMa- and IgMb-positive B cells were detectable, but the proportion of IgMb-expressing cells was higher (Fig. 2B), indicating either a higher percentage of hematopoietic stem cells or a greater production of B cells within the wt-b donor cells. In contrast, the bone marrow of wt-a/ko-b chimeric mice displayed a reduction of IgMb-positive (BOB.1/OBF.1−/−) B cells compared to IgMa-positive B cells (Fig. 2B). This difference was, however, much more dramatic for splenic B cells. Whereas wt-a/wt-b chimeras developed similar amounts of IgMa- and IgMb-positive splenic B cells, no B cells in the spleens of wt-a/ko-b chimeras expressed IgMb and all were derived from the wild-type donor cells (Fig. 2B). These data demonstrate that in a competitive situation, the BOB.1/OBF.1-deficient B-cell precursors are at a strong disadvantage compared to the wild type and emphasize the essential role of BOB.1/OBF.1 in antigen-independent B-cell development.
FIG. 2.
Dramatic block of BOB.1-deficient B-cell development in the presence of wild-type cells. (A and B) Flow cytometry analysis of splenic lymphocytes 4 to 8 weeks after the transfer with bone marrow cells from wild-type C57BL/6a and control C57BL/6 mice (wt-a/wt-b) or wild-type C57BL/6a and BOB.1-deficient C57BL/6 mice (wt-a/ko-b). (A) The T-cell populations (T-cell-receptor-positive cells) in the spleen were analyzed with Thy1 antibodies recognizing the two alleles (anti-Thy1.1 and anti-Thy1.2). (B) The contributions of the two allotypes to the splenic B-cell population (B220-positive cells) were estimated by measuring the expression of the alleles for IgM (anti-IgMa and anti-IgMb). Statistical analyses from two independent transfers are shown on the right. (C) Analysis of B-cell populations in the bone marrow using the same antibodies as used for panel B. The diagram on right shows the statistical analysis of both transfers.
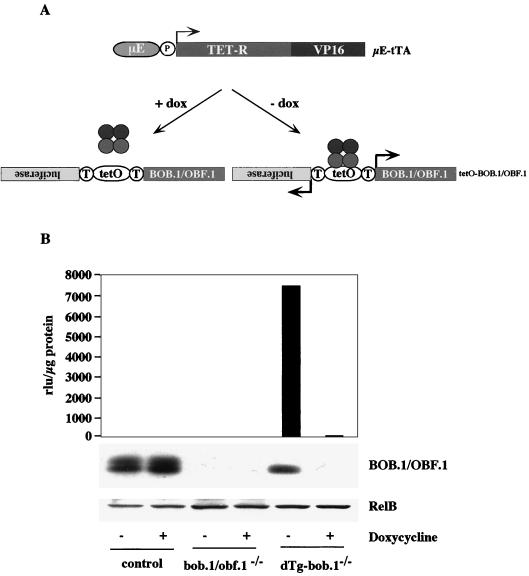
In order to determine the requirement for BOB.1/OBF.1 expression in B-cell development more directly, we used a conditional rescue system for BOB.1/OBF.1 in transgenic mice based on the Tet-Off system (9). Two transgenic mouse lines were generated for the conditional system. The first line (μE-tTA) contains a transgene that encodes a tetracycline-regulated transactivator (tTA) under the control of the intronic μ heavy chain enhancer (μE) and a minimal promoter (2). The second line (tetO-BOB.1/OBF.1) contains a bidirectional tetracycline operator sequence (tetO [3]), which simultaneously regulates the expression of transgenic BOB.1/OBF.1 and a luciferase reporter gene (Fig. 3A). The luciferase reporter gene was used to monitor the conditional activity of the Tet-Off system in vivo and in primary cell cultures. Several transgenic founders were obtained and tested. The combination showing the highest level of regulated BOB.1/OBF.1 expression was used in further studies.
FIG. 3.
Conditional BOB.1 transgene expression system. (A) Schematic representation of the Tet-Off system used. The doxycycline-controlled transactivator (tTA), composed of the tetracycline repressor (TetR) and the viral transactivator (VP16), is driven by a minimal promoter (P) containing a synthetic octamer motif and the 700-bp-fragment of the intronic IgH enhancer (μE). In the absence of doxycycline (−dox), tTA binds to the bidirectional tetracycline operator (tetO) and simultanously activates the expression of transgenic BOB.1 and the luciferase reporter gene. In the presence of doxycycline (+dox), binding of tTA and expression of the tTA-dependent transgenes are blocked. (B) Conditional transgene expression in pre-B cells. Primary IL-7-dependent pre-B-cell cultures were derived from control, BOB.1-deficient (bob.1−/−), and dTg BOB.1-deficient (dTg-bob.1−/−) mice were analyzed for luciferase activity and BOB.1 protein expression depending on doxycycline treatment (−, absence of doxycycline; +, presence of doxycycline). The luciferase activity is given in light units per microgram of protein. Expression of RelB was determined to prove the quality and quantity of protein extracts used for Western immunoblot analysis.
The μE-tTA and tetO-BOB.1/OBF.1 double transgenic (dTg) animals were crossed with BOB.1/OBF.1 mutant mice to finally obtain dTg animals in a BOB.1/OBF.1-deficient genetic background (dTg-bob.1−/−). Measurement of the luciferase activity in different tissues of dTg animals and Northern blot analysis for transgenic BOB.1/OBF.1 were used to determine the conditional expression of the tetracycline-dependent transgenes (data not shown). The experiments revealed a specific expression of the transgenes in thymus and bone marrow of dTg mice which was completely repressed by the administration of doxycycline. With the exception of skeletal muscle, all other analyzed tissues displayed no expression of transgenic BOB.1/OBF.1 and showed only basal levels of luciferase activity (data not shown). We next wanted to ensure that the Tet system was functioning properly in the pre-B-cell compartment. Therefore, pre-B cells derived from bone marrow of wild-type, BOB.1/OBF.1-deficient, and dTg-bob.1−/− mice were grown on stromal cells in the presence of IL-7 (24). We assayed luciferase activity and BOB.1/OBF.1 expression in cells grown either in the presence or absence of doxycycline. High luciferase activity was detected in dTg-bob.1−/− cells (Fig. 3B), and this activity could be completely repressed by the addition of doxycycline. Whereas expression of the endogenous BOB.1/OBF.1 protein in wild-type cells was unaffected by doxycycline treatment, transgenic BOB.1/OBF.1 was only seen in extracts from dTg-bob.1−/− pre-B cells growing in the absence of doxycycline (Fig. 3B). These data demonstrate that the conditional expression system functioned as expected in pre-B cells.
Interestingly, only low levels of transgene expression were detected in spleen or lymph nodes (data not shown). Several tTA and tetO-BOB.1/OBF.1 transgenic lines were generated to confirm the lack of transgene activity in the periphery with consistent results. Northern analyses of spleen and lymph nodes revealed that tTA-specific RNA was no longer detectable (data not shown). Therefore, the luciferase activity detected in spleen and lymph nodes most likely represents residual enzyme from earlier differentiation stages. Taken together, these data indicate that the activity of the Tet-Off system ceases during lymphocyte maturation. We conclude that tetracycline-dependent transgene expression occurs in a small window of lymphopoiesis and is restricted to early B- and T-cell development in bone marrow and thymus, respectively.
The efficient conditional expression of transgenic BOB.1/OBF.1 in the bone marrow allowed us to test the rescue of the decrease in B220+ IgMlo-hi cells in the bone marrow of transgenic BOB.1/OBF.1 knockout mice. When dTg-bob.1−/− mice were treated with doxycycline (transgene expression turned off), they exhibited a phenotype virtually indistinguishable from BOB.1/OBF.1-deficient mice. Numbers of pro- and pre-B cells were normal or slightly increased, but development of immature, transitional immature, and mature recirculating B cells was impaired (compare Fig. 1B and 4A). In contrast, untreated dTg-bob.1−/− littermates (transgenic BOB.1/OBF.1 expressed) had normal pro-, pre-, immature, and transitional immature B-cell populations compared to wild-type mice (compare Fig. 1B and 4A). However, the number of mature recirculating B cells in the bone marrow of untreated dTg-bob.1−/− mice was still reduced (Fig. 4A), indicating that a continued expression of the coactivator or expression at later stages of B-cell development is necessary for restoration of this population.
The rescue of the bone marrow B-cell developmental defects in BOB.1/OBF.1−/− mice by the BOB.1/OBF.1 transgene led us to investigate whether forced expression of transgenic BOB.1/OBF.1 would also correct the increased apoptosis in BOB.1/OBF.1−/− bone marrow B220lo cells. We compared the level of annexin V on the surface of B220lo bone marrow B cells derived from 6-week-old control, BOB.1/OBF.1-deficient, and dTg-bob.1−/− littermates. BOB.1/OBF.1-deficient and dTg-bob.1−/− mice treated with doxycycline showed a twofold increase in annexin V-positive cells (10 to 15%), whereas dTg-bob.1−/− mice without doxycycline treatment showed a normal ratio of apoptotic cells (6 to 8%) compared to that found in control littermates (Fig. 4B). Taken together, these data show that conditional expression of BOB.1/OBF.1 can completely rescue increased apoptosis and decreased numbers of BOB.1/OBF.1−/− B220+ bone marrow cells and confirm that these defects are due to the absence of BOB.1/OBF.1.
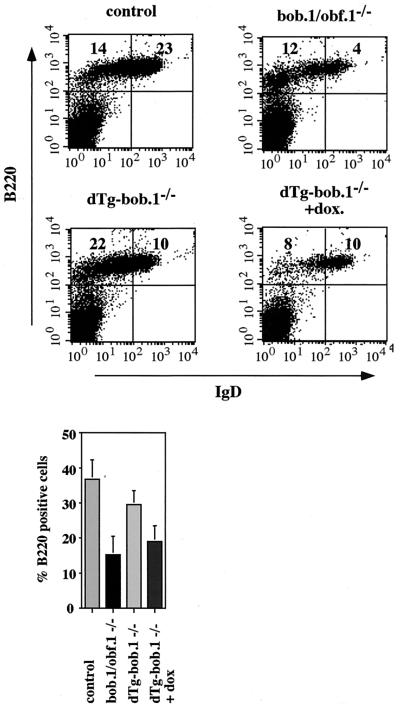
We next analyzed whether the conditional correction of transitional immature B-cell numbers in the bone marrow of dTg-bob.1−/− mice could rescue the developmental defects in BOB.1/OBF.1−/− splenic B cells. Transgenic BOB.1/OBF.1 is expressed in the bone marrow but not in the periphery; therefore, correction by the transgene of the reduced numbers of B220+ IgDhi B cells in the spleen would signify that this defect was due to reduced numbers of transitional immature cells exiting the bone marrow. B-cell populations in the spleens of 6-week-old dTg-bob.1−/− littermates were compared to those in wild-type and BOB.1/OBF.1-deficient mice. Consistent with the restored B-cell pools in the bone marrow, untreated dTg-bob.1−/− mice showed an increased number of B220+ IgDlo splenic B cells compared to BOB.1/OBF.1-deficient mice (Fig. 5). Strikingly, the B220+ IgDhi population was only slightly increased in dTg-bob.1−/− mice and was not restored to wild-type levels, indicating that the reduced numbers of splenic B cells represent an additional block in B-cell development in BOB.1/OBF.1−/− mice (Fig. 5). The moderate increase in IgDhi cells in the dTg doxycycline-treated mice compared to the number in BOB.1/OBF.1−/− mice may reflect the previous recruitment of BOB.1/OBF.1-containing cells from the dTg-bob.1−/− mice into the long-lived pool prior to treatment with doxycycline. Indeed, the B220+ IgDlo population in dTg-bob.1−/− mice was greater than in the wild type, possibly due to the effects on B-cell development of normal BOB.1/OBF.1 expression in the bone marrow and lack of BOB.1/OBF.1 expression in the spleen (Fig. 5). Additionally, levels in serum of secondary Igs were greatly reduced in BOB.1/OBF.1-deficient mice, and these were not restored in the untreated dTg-bob.1−/− mice (data not shown). Taken together, these data show that BOB.1/OBF.1 expression is necessary for the generation of mature IgDhi B cells in the spleen and that this represents a second, specific stage of antigen-independent B-cell development requiring BOB.1/OBF.1.
FIG. 5.
Conditional correction in the number of peripheral B cells in spleen. Flow cytometry analysis of splenocytes from 6- to 8-week-old control, BOB.1/OBF.1-deficient (bob.1/obf.1−/−) and dTg BOB.1/OBF.1-deficient (dTg-bob.1−/−) mice. Cells were stained in a combination of anti-B220-PE, anti-IgM-biotin, and anti-IgD-FITC antibodies. The percentage of different B-cell populations is denoted by boldfaced numerals. Transgene expression was turned off by administration of 2 mg/of doxycycline (+dox.)/ml to the animals' drinking water for 5 days. At the bottom is a bar diagram with the statistical analysis from at least three independent experiments.
DISCUSSION
The predominant defects in BOB.1/OBF.1−/− mice are the lack of germinal centers and the failure in isotype switching, both of which represent antigen-dependent stages in the terminal differentiation stages of B cells. Given that BOB.1/OBF.1 is a transcriptional coactivator whose transcriptional targets are still largely unknown, it is important to fully characterize the BOB.1/OBF.1−/− phenotype in order to define the role of any target proteins, once identified. We have now analyzed antigen-independent B-cell development in BOB.1/OBF.1−/− mice and used competitive reconstitution and transgene expression studies to demonstrate the existence of two additional blocks in maturation of these cells. Thus, our results and previously published reports together identify various stages of B-cell differentiation which require Bob to proceed normally: both early antigen-independent and later antigen-dependent stages.
All previous reports on BOB.1/OBF.1−/− bone marrow B-cell development have described it as normal. In addition, a recent study reiterated these findings and suggested that they imply a defect in the emigration or transit of BOB.1/OBF.1−/− B cells to the spleen, since numbers of splenic BOB.1/OBF.1−/− B cells are reduced two- to fourfold (29). The identification of defects in the production of B220+ IgMlo and B220+ Igmhi B cells in the bone marrow has been complicated by the fact that they are subtle and not complete and by the obvious reduction in mature recirculating B cells in the bone marrow. We performed extensive FACS analysis of the IgM+ cells in BOB.1/OBF.1−/− bone marrow and consistently saw a moderate (20 to 30% reduced) defect in the immature B cells and a more pronounced two- to threefold reduction in the transitional immature population. To extend these findings, we used bone marrow reconstitution assays and found that the immature and transitional B-cell defects were far more evident when BOB.1/OBF.1−/− bone marrow cells were in a competitive situation with wild-type cells. Moreover, we found an approximately 100% increase in apoptosis in BOB.1/OBF.1−/− bone marrow B220+ cells from that in the wild type, which could at least partly explain the reduction in cell numbers. How apoptosis is affected by BOB.1/OBF.1 is presently unknown. We have looked at expression of pro- and antiapoptotic genes on filter arrays and not detected any BOB.1/OBF.1-dependent differences in expression rates.
Competitive bone marrow transfer experiments were performed for Btk-deficient (xid) bone marrow B cells in the past. Those experiments demonstrated an initial presence of mutant B cells, which were lost over time, and virtually none were detectable after 6 months (31). We did not see evidence for even an initial presence of BOB.1/OBF.1-deficient B cells in the periphery when peripheral blood was sampled from the reconstituted mice between 4 and 5 weeks after reconstitution (data not shown). We therefore conclude that the disadvantage of BOB.1/OBF.1-deficient B cells, as compared to that for the Btk-deficient B cells, is even more pronounced.
One implication of the subtlety of BOB.1/OBF.1−/− developmental defects is that the severity of the BOB.1/OBF.1 phenotype may be ameliorated over the lifetime of an animal: i.e., the defects would be more evident in very young animals and would diminish with age. Along these lines, it was reported that numbers of mature recirculating B cells in BOB.1/OBF.1−/− animals' bone marrow increase over time (28). Our analyses of splenic B-cell populations at different ages do not confirm this possibility. Rather, we see the characteristic two- to fourfold reduction of splenic B cells in mice ranging in age from 17 days up to 1 year.
The reduction in the number of splenic B cells in BOB.1/OBF.1−/− mice could therefore be explained by the decreased influx from the bone marrow. In order to determine if this was the case, we took advantage of a conditional expression system for a BOB.1/OBF.1 transgene which is expressed only at early stages of B lymphopoiesis and not in the peripheral lymphoid organs, including the spleen. Expression of the BOB.1/OBF.1 transgene rescued the early immature and transitional B-cell defects in the bone marrow as well as the increased apoptosis in these populations. Normalization of BOB.1/OBF.1−/− bone marrow cell production did not, however, result in a splenic cell population resembling that in the wild type. Levels of IgDhi cells were nonetheless dramatically reduced, indicating an essential role for BOB.1/OBF.1 in terminal differentiation. This was also supported by measurement of the levels of switched Ig isotypes in the dTg-bob1−/− mice, which were as low as in the BOB.1/OBF.1-deficient animals. Thus, despite normal bone marrow B-cell production, BOB.1/OBF.1−/− splenic B cells are still unable to develop into recirculating, follicular B cells. The recent identification of the B-cell-specific chemokine, BLR1, as a potential target of BOB.1/OBF.1 suggests one potential explanation for this defect, since BLR1 is required for follicular entry of B cells (39).
The reasons for the lack of expression of the BOB.1/OBF.1 transgene in the periphery are currently unclear. The tTA transgene is driven by a μE-dependent cassette, which has worked efficiently in the past to drive transgene expression in peripheral lymphoid lymphoid organs (2). In addition, the μE is believed to function efficiently at all stages of B-cell development, including the mature B-cell and plasma cell stage. We were unable to detect any RNA for the transgene in the periphery, indicating that the stability of the tTA RNA might be a problem. Cryptic splice sites have been discovered in the tTA sequence, and it is known that B cells alter their splice program during differentiation. Interestingly, a similar tTA transgenic construct was used recently to regulate expression of the myc oncogene in mice. These mice had T-cell lymphomas and myeloid leukemias only, suggesting that this tTA transgene also did not work efficiently in the B-cell compartment (7).
One important result of our analysis was the finding that there are multiple stages in B-cell development where BOB.1/OBF.1 is required. Similar findings have been reported for Igα-, Btk-, P85α (phosphatidylinositol 3-kinase), Syk-, and BLNK/Slp-65-deficient mice (8, 13, 14, 19, 34, 36, 37). All of these molecules are required for productive B-cell receptor (BCR) signaling, and all these mice also exhibit defects in at least two separate stages of antigen-independent B-cell development. This includes impaired development of large pre-B cells from pro-B cells and reduced numbers of mature recirculating B cells in the periphery. The common blocks in bone marrow and splenic B lymphopoiesis in these mice raise the possibility that all molecules are required in a shared pathway. Indeed, it has been suggested that these blocks represent two BCR-dependent signaling thresholds, which must be met before further differentiation can take place. Moreover, expression of a functional BCR may be required to transduce a low-level survival signal, and its loss results in rapid, apoptotic death of B cells (17). As the decrease in mature B cells is usually more severe than the loss of bone marrow cells, it has been posited that this checkpoint requires higher levels of signaling through the BCR and may represent positive selection into the long-lived B-cell pool.
Therefore, a possible explanation for the defects in development in BOB.1/OBF.1−/− mice is that BOB.1/OBF.1 regulates expression of one or more molecules required for signaling through the BCR. Recent experiments identified the 3′ Ig enhancer as a potential target of BOB.1/OBF.1 function (32, 35). These findings may explain some of the late-stage phenotypes, such as the reduction of expression of secondary Ig isotypes, as it is believed that these isotypes are more dependent on the 3′ Ig enhancer element. However, this enhancer is not active at earlier stages of B-cell development, and it is questionable whether the defects described here can be attributed to this mechanism. The role of BOB.1/OBF.1 in activation of the variable heavy (Vh) promoter of the Ig heavy chain is not well understood. It is therefore possible that the absence of BOB.1/OBF.1 would cause subtle defects in transcription of either the heavy or light chain Ig genes and that the resulting, slightly lower levels of surface BCR may impede normal development of B cells. The defects in BOB.1/OBF.1−/− mice are not as severe as in the mice listed above, since the BOB.1/OBF.1−/− B1-cell and pro- and pre-B-cell populations appear normal, possibly reflecting the difference between abolition and a slight attenuation of a BCR signaling molecule. We are currently performing expression profiling of BOB.1/OBF.1-deficient B cells on filter arrays to clarify the role of BOB.1/OBF.1 at the promoter and enhancers of Ig. Moreover, any additional candidate genes for BOB.1/OBF.1 targets will be more readily assessed in the light of these analyses of the phenotype of BOB.1/OBF.1−/− mice.
ACKNOWLEDGMENTS
We thank A. Rolink for the 493 antibody, T. Hünig for help with the bone marrow chimeras, H. Haber for her excellent help regarding all aspects of animal work, M. Mitterer for excellent technical assistance, and K. Tedford for many important comments on the manuscript.
This work was supported by grants to T.W. (SFB 465-B7, SFB 497-C5, and Fonds der Chemischen Industrie).
REFERENCES
- 1.Allman D M, Ferguson S E, Lentz V M, Cancro M P. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 2.Annweiler A, Müller U, Wirth T. Functional analysis of defined mutations in the immunoglobulin heavy-chain enhancer in transgenic mice. Nucleic Acids Res. 1992;20:1503–1509. doi: 10.1093/nar/20.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron U, Freundlieb S, Gossen M, Bujard H. Co-regulation of two gene activities by tetracycline via a bidirectional promoter. Nucleic Acids Res. 1995;23:3605–3606. doi: 10.1093/nar/23.17.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carsetti R, Kohler G, Lamers M C. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cepek K L, Chasman D I, Sharp P A. Sequence-specific DNA binding of the B-cell-specific coactivator OCA-B. Genes Dev. 1996;10:2079–2088. doi: 10.1101/gad.10.16.2079. [DOI] [PubMed] [Google Scholar]
- 6.Chasman D, Cepek K, Sharp P A, Pabo C O. Crystal structure of an OCA-B peptide bound to an Oct-1 POU domain/octamer DNA complex: specific recognition of a protein-DNA interface. Genes Dev. 1999;13:2650–2657. doi: 10.1101/gad.13.20.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsher D W, Bishop J M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 8.Fruman D A, Snapper S B, Yballe C M, Davidson L, Yu J Y, Alt F W, Cantley L C. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 9.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greiner A, Müller K, Hess J, Pfeffer K, Müller-Hermelink K H, Wirth T. BOB.1/OBF.1 expression is upregulated in normal germinal center B cells and germinal center derived B cell lymphomas. Am J Pathol. 2000;156:501–507. doi: 10.1016/S0002-9440(10)64754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gstaiger M, Georgiev O, van Leeuwen H, van der Vliet P, Schaffner W. The B cell coactivator Bob1 shows DNA sequence-dependent complex formation with the Oct-1/Oct-2 factors, leading to differential promoter activation. EMBO J. 1996;15:2781–2790. [PMC free article] [PubMed] [Google Scholar]
- 12.Gstaiger M, Knoepfel L, Georgiev O, Schaffner W, Hovens C M. A B-cell coactivator of octamer-binding transcription factors. Nature. 1995;373:360–362. doi: 10.1038/373360a0. [DOI] [PubMed] [Google Scholar]
- 13.Hendriks R W, de Bruijn M F, Maas A, Dingjan G M, Karis A, Grosveld F. Inactivation of Btk by insertion of lacZ reveals defects in B cell development only past the pre-B cell stage. EMBO J. 1996;15:4862–4872. [PMC free article] [PubMed] [Google Scholar]
- 14.Jumaa H, Wollscheid B, Mitterer M, Wienands J, Reth M, Nielsen P J. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity. 1999;11:547–554. doi: 10.1016/s1074-7613(00)80130-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim U, Qin F-F, Gong S, Stevens S, Luo Y, Nussenzweig M, Roeder R G. The B-cell-specific transcription coactivator OCA-B/OBF-1/Bob-1 is essential for normal production of immunoglobulin isotypes. Nature. 1996;383:542–547. doi: 10.1038/383542a0. [DOI] [PubMed] [Google Scholar]
- 16.Kistler B, Rolink A, Marienfeld R, Neumann M, Wirth T. Induction of nuclear NF-κB during primary B cell differentiation. J Immunol. 1998;160:2308–2317. [PubMed] [Google Scholar]
- 17.Lam K P, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 18.Laumen H, Nielsen P J, Wirth T. The BOB.1/OBF.1 coactivator is essential for octamer-dependent transcription in B cells. Eur J Immunol. 2000;30:458–469. doi: 10.1002/1521-4141(200002)30:2<458::AID-IMMU458>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Loder F, Mutschler B, Ray R J, Paige C J, Sideras P, Torres R, Lamers M C, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Roeder R G. Cloning, functional characterization, and mechanism of action of the B-cell-specific transcriptional coactivator OCA-B. Mol Cell Biol. 1995;15:4115–4124. doi: 10.1128/mcb.15.8.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen P J, Georgiev O, Lorenz B, Schaffner W. B lymphocytes are impaired in mice lacking the transcriptional co-activator Bob1/OCA-B/OBF1. Eur J Immunol. 1996;26:3214–3218. doi: 10.1002/eji.1830261255. [DOI] [PubMed] [Google Scholar]
- 22.Pfisterer P, Zwilling S, Hess J, Wirth T. Functional characterization of the murine homolog of the B-cell-specific coactivator BOB.1/OBF.1. J Biol Chem. 1995;270:29870–29880. doi: 10.1074/jbc.270.50.29870. [DOI] [PubMed] [Google Scholar]
- 23.Qin X F, Reichlin A, Luo Y, Roeder R G, Nussenzweig M C. OCA-B integrates B cell antigen receptor-, CD40L- and IL 4-mediated signals for the germinal center pathway of B cell development. EMBO J. 1998;17:5066–5075. doi: 10.1093/emboj/17.17.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolink A, Kudo A, Karasuyama H, Kikuchi Y, Melchers F. Long-term proliferating early pre B cell lines and clones with the potential to develop to surface Ig-positive, mitogen reactive B cells in vitro and in vivo. EMBO J. 1991;10:327–336. doi: 10.1002/j.1460-2075.1991.tb07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolink A G, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Rolink A G, Brocker T, Bluethmann H, Kosco-Vilbois M H, Andersson J, Melchers F. Mutations affecting either generation or survival of cells influence the pool size of mature B cells. Immunity. 1999;10:619–628. doi: 10.1016/s1074-7613(00)80061-8. [DOI] [PubMed] [Google Scholar]
- 27.Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- 28.Schubart D B, Rolink A, Kosco-Vilbois M H, Botteri F, Matthias P. B-cell-specific coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature. 1996;383:538–542. doi: 10.1038/383538a0. [DOI] [PubMed] [Google Scholar]
- 29.Schubart D B, Rolink A, Schubart K, Matthias P. Cutting edge: lack of peripheral B cells and severe agammaglobulinemia in mice simultaneously lacking Bruton's tyrosine kinase and the B cell-specific transcriptional coactivator OBF-1. J Immunol. 2000;164:18–22. doi: 10.4049/jimmunol.164.1.18. [DOI] [PubMed] [Google Scholar]
- 30.Schubart D B, Sauter P, Massa S, Friedl E M, Schwarzenbach H, Matthias P. Gene structure and characterization of the murine homologue of the B cell-specific transcriptional coactivator OBF-1. Nucleic Acids Res. 1996;24:1913–1920. doi: 10.1093/nar/24.10.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprent J, Bruce J. Physiology of B cells in mice with X-linked immunodeficiency (xid). III. Disappearance of xid B cells in double bone marrow chimeras. J Exp Med. 1984;160:711–723. doi: 10.1084/jem.160.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens S, Ong J, Kim U, Eckhardt L A, Roeder R G. Role of OCA-B in 3′-IgH enhancer function. J Immunol. 2000;164:5306–5312. doi: 10.4049/jimmunol.164.10.5306. [DOI] [PubMed] [Google Scholar]
- 33.Strubin M, Newell J W, Matthias P. OBF-1, a novel B cell-specific coactivator that stimulates immunoglobulin promoter activity through association with octamer proteins. Cell. 1995;80:497–506. doi: 10.1016/0092-8674(95)90500-6. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 35.Tang H, Sharp P A. Transcriptional regulation of the murine 3′ IgH enhancer by OCT-2. Immunity. 1999;11:517–526. doi: 10.1016/s1074-7613(00)80127-2. [DOI] [PubMed] [Google Scholar]
- 36.Torres R M, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272:1804–1808. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 37.Turner M, Gulbranson-Judge A, Quinn M E, Walters A E, MacLennan I C, Tybulewicz V L. Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J Exp Med. 1997;186:2013–2021. doi: 10.1084/jem.186.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wicker L S, Scher I. X-linked immune deficiency (xid) of CBA/N mice. Curr Top Microbiol Immunol. 1986;124:87–101. doi: 10.1007/978-3-642-70986-9_6. [DOI] [PubMed] [Google Scholar]
- 39.Wolf I, Pevzner V, Kaiser E, Bernhardt G, Claudio E, Siebenlist U, Forster R, Lipp M. Downstream activation of a TATA-less promoter by Oct-2, Bob1, and NF-κB directs expression of the homing receptor BLR1 to mature B cells. J Biol Chem. 1998;273:28831–28836. doi: 10.1074/jbc.273.44.28831. [DOI] [PubMed] [Google Scholar]
- 40.Zwilling S, Dieckmann A, Pfisterer P, Angel P, Wirth T. Inducible expression and phosphorylation of coactivator BOB.1/OBF.1 in T cells. Science. 1997;277:221–225. doi: 10.1126/science.277.5323.221. [DOI] [PubMed] [Google Scholar]