Abstract
Background
Acute kidney injury (AKI) is both a consequence and determinant of outcomes in COVID-19. The kidney is one of the major organs infected by the causative virus, SARS-CoV-2. Viral entry into cells requires the viral spike protein, and both the virus and its spike protein appear in the urine of COVID-19 patients with AKI. We examined the effects of transfecting the viral spike protein of SARS-CoV-2 in kidney cell lines.
Methods
HEK293, HEK293-ACE2+ (stably overexpressing ACE2), and Vero E6 cells having endogenous ACE2 were transfected with SARS-CoV-2 spike or control plasmid. Assessment of gene and protein expression, and syncytia formation was performed, and the effects of quercetin on syncytia formation examined.
Findings
Spike transfection in HEK293-ACE2+ cells caused syncytia formation, cellular sloughing, and focal denudation of the cell monolayer; transfection in Vero E6 cells also caused syncytia formation. Spike expression upregulated potentially nephrotoxic genes (TNF-α, MCP-1, and ICAM1). Spike upregulated the cytoprotective gene HO-1 and relevant signaling pathways (p-Akt, p-STAT3, and p-p38). Quercetin, an HO-1 inducer, reduced syncytia formation and spike protein expression.
Interpretation
The major conclusions of the study are: 1) Spike protein expression in kidney cells provides a relevant model for the study of maladaptive and adaptive responses germane to AKI in COVID-19; 2) such spike protein expression upregulates HO-1; and 3) quercetin, an HO-1 inducer, may provide a clinically relevant/feasible protective strategy in AKI occurring in the setting of COVID-19.
Funding
R01-DK119167 (KAN), R01-AI100911 (JPG), P30-DK079337; R01-DK059600 (AA).
Keywords: SARS-CoV-2, COVID-19, Spike protein, Angiotensin-converting enzyme 2, Acute kidney injury, Heme oxygenase-1, Syncytia
1. Introduction
The human host infected with the virus SARS-CoV-2 displays a heterogeneity of responses that range from an asymptomatic state to fulminant organ dysfunction and ensuing mortality [1], [2], [3]. Such organ dysfunction reflects viral infection and as well as regional and systemic inflammatory responses, all of which may involve the lungs, kidney, and other major organs and tissues [1], [2], [3].
SARS-CoV-2 invades cells through a process that is dependent upon the engagement of the spike S glycoprotein of the virus with the ACE2 receptor abundantly present on host cells, including those in the kidney [1], [2], [3], [4]. Following such engagement, the following steps sequentially occur: the spike protein is proteolytically cleaved and primed by TMPRSS2 (and other proteases) also present on host cells; ACE2 receptor-mediated viral transmission via the plasma membrane into the intracellular compartment occurs; viral replication ensues; and offspring virions are released to neighboring cells [1], [2], [3], [4], [5].
In addition to enabling viral cell entry through the ACE2 receptor, and as increasingly recognized, the spike protein may exert diverse effects, including interaction with innate immune receptors [6], activation of the inflammasome [7] and the alternative complement system [8], endothelial injury and increased endothelial permeability [9], endothelial dysfunction attended by oxidative stress and mitochondrial injury [10], procoagulant processes including the upregulation of PAI-1 [11], and exhaustion of NK cells [12].
As one of the main targets in COVID-19, the kidney, when acutely injured, significantly contributes to COVID-19-associated morbidity and mortality [1], [2]. Acute kidney injury (AKI) may occur in as many as 20% of hospitalized patients and 50% of intensive care unit (ICU) patients, with renal replacement therapy (RRT) necessitated in over 50% of ICU patients with AKI. Mortality rates may exceed 50% when AKI occurs, with higher rates in patients requiring RRT [1], [2].
Understanding the pathogenesis of AKI in COVID-19 is thus timely and important. However, such an understanding is largely speculative, with current constructs positing that AKI is multifactorial in origin, reflecting, to a variable extent, the effects of alterations in systemic hemodynamics and impaired renal perfusion, heightened systemic and renal inflammation, and viral tropism of the kidney [1], [2]. As regards the latter, SARS-CoV-2 is the latest in the expanding list of viruses that are known to directly infect kidney cells [13], [14], [15], [16]. Recent studies in humans with COVID-19 and AKI not only demonstrate the presence of SARS-CoV-2 in the kidney, but also that the virus, when present in the kidney, is infectious and can propagate itself in previously uninfected kidney cells [13], [15]. Remarkably, SARS-CoV-2 is detected in the urine in patients with COVID-19; the virus co-localizes with the ACE2 receptor in tubular epithelial cells as demonstrable on renal biopsy; and higher viral titers in the urine correlate with AKI and increased mortality [17]. Moreover, in over 25% of humans with COVID-19 and AKI, the spike protein is present in urine [17], [18]. How the spike protein reaches the urinary space is uncertain, but on a priori grounds, this may reflect filtration of SARS-CoV-2 and/or its spike protein from plasma across the glomerular filtration barrier; the sloughing of spike protein into the urinary space from the plasma membrane of kidney cells infected with SARS-CoV-2; or the sloughing of kidney cells infected with the virus into the urinary space and the subsequent detachment of the spike protein from cell membranes. Whatever the basis for such urinary appearance of the spike protein, these seminal clinical observations attest to the fact that the spike protein is in contact with kidney cells that line the urinary space in patients with COVID-19 and AKI [17], [18].
The present study draws upon this clinical finding regarding the urinary presence of the spike protein and introduces an in vitro model that may be used for the exploration of the injurious effects of the spike protein on kidney cells. In the course of such exploration, this study uncovered a strategy that may be considered as an approach in protecting against AKI in COVID-19.
2. Methods
2.1. Cell culture and chemicals
HEK293-IIIA native (HEK293) cells and HEK293-IIIA-ACE2 (HEK293-ACE2+) stably overexpressing ACE2 cells were generated and provided by Dr. Michael A. Barry's laboratory. Vero E6 cells were provided by Dr. Richard Vile's laboratory. These cell lines were maintained in DMEM containing 10% FBS with the selection antibiotic G418 (0.5 mg/ml) added to maintain HEK293-ACE2+ cells. Pharmaceutical grade quercetin (PHR1488, Sigma Aldrich) and Zn (II) protoporphyrin (Zn625-9, Frontier Specialty Chemicals) were used in this study. All other chemicals were from Sigma Aldrich.
2.2. Plasmids and transfections
Plasmids used in this study were provided by Dr. Michael A. Barry. Briefly, a codon optimized cDNA encoding the original wild-type spike protein from severe acute respiratory syndrome coronavirus 2 (pcDNA1-SARS-CoV-2 spike; 7788 bp) isolate 2019-nCoV_HKU-SZ-002a_2020, accession number MN938384.1 and empty vector (pcDNA1; 4033 bp) plasmids were synthesized by Genewiz. HEK293 cells, HEK293-ACE2+, and Vero E6 cells were transfected using Lipofectamine LTX and Plus reagent kit as per the manufacturer's instructions. Briefly, cells were plated and grown to ~60–75% confluence before transfection. DNA complexes were prepared in Opti-MEM reduced serum media (Cat # 31982-070, ThermoFisher Scientific) and added to cells pre-incubated with Opti-MEM reduced serum media. Full media was added at 4–6 h post transfection and cells were collected at the indicated times (8–48 h). For studies examining the effect of quercetin, full media containing either quercetin (10 μM) or vehicle was added at 4–6 h post transfection. In a subsequent experiment, Zn (II) protoporphyrin (10 μM) was added 30 min prior to quercetin treatment.
2.3. Western analysis
Assessment of protein expression by Western blot analysis was performed as we have described previously [19], [20]. Primary antibodies employed in overnight 4 °C incubations included: Spike S (GeneTex Cat# GTX632604, RRID:AB_2864418), ACE2 (Novus Cat# NBP1-76614, RRID:AB_11021647), HO-1 (Enzo Life Sciences Cat# ADI-SPA-895, RRID:AB_10618757), p-Akt (Cell Signaling Technology Cat# 9271, RRID:AB_329825), p-STAT3 (Cell Signaling Technology Cat# 9138, RRID:AB_331262), p-p38 (Cell Signaling Technology Cat# 4511, RRID:AB_2139682) and GAPDH (Cell Signaling Technology Cat# 2118, RRID:AB_561053). Following incubations with appropriate peroxidase-labeled secondary antibodies at room temperature, band visualization was achieved using chemiluminescence.
2.4. mRNA expression by quantitative real-time reverse transcriptase-PCR
mRNA expression levels of TNF-α, MCP-1, ICAM1, and HO-1 were measured by quantitative real-time RT-PCR, as we have previously described [19], [20] employing a 2-step method. RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) and purified using a RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcription was done with a Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Indianapolis, IN). Relative quantitation for each target was achieved with TaqMan Gene Expression Assay sets (Applied Biosystems, Thermo Fisher) and the expression of 18S rRNA was used for normalization of the expression of each target gene.
2.5. Microscopy and cell area analysis
Phase contrast images were acquired on an Olympus CK40 microscope using Plan FL2 10× objective (RI 0.08; 160/−). The area covered by syncytia was measured using ImageJ software (National Institute of Health). Briefly, after the scale was set for the image using the calibration scale feature with calibration image, each syncytium was demarcated using a free hand tool and the corresponding area was determined with the measure function in ImageJ. The average size of syncytia per image and percent of area covered by syncytia per image were calculated from 9 images from 3 different sets of experiments.
2.6. Statistics
Data are expressed as means ± SD and considered statistically significant for P < 0.05. The Student's t-test was used for parametric data and the Mann-Whitney U test was employed for nonparametric data.
3. Results
3.1. Spike protein expression in HEK293-ACE2+ cells caused syncytia formation and cell sloughing
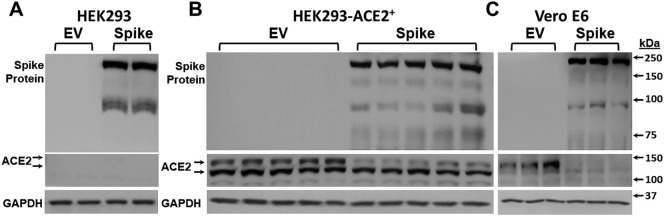
For these studies, we utilized HEK293, HEK293-ACE2+ cells, and Vero E6 cells that express endogenous ACE2 protein and binds to SARS-CoV-2 spike protein [5], [21] (see Methods). These cells were transfected with SARS-CoV-2 spike and empty vector plasmid using Lipofectamine LTX and Plus reagent kit. Spike protein and ACE-2 protein expression was assessed by Western blot analysis. Two major bands were observed with the 180 kDa band corresponding to full length SARS-CoV-2 spike protein and the 90 kDa band representing the cleaved SARS-CoV-2 spike protein [5]. Transfection of all the cell lines with SARS-CoV-2 spike plasmid resulted in expression of spike protein (Fig. 1A, B, and C). We confirmed robust expression of ACE2 in HEK293-ACE2+ cells whereas no such expression occurred in HEK293 cells. In addition, Vero E6 cells also showed endogenous ACE-2 expression (Fig. 1C). Interestingly, following spike protein expression in both HEK293-ACE2+ and Vero E6 cells, expression of ACE2 noticeably decreased (Fig. 1B and C).
Fig. 1.
Characterization of the effect of SARS-CoV-2 spike transfection in HEK293, HEK293-ACE2+, and Vero E6 cells. HEK293, HEK293-ACE2+, and Vero E6 cells were transfected with spike or empty vector plasmid, and cell lysates were prepared 24 h post transfection. A) Spike expression was observed in HEK293 cells transfected with spike plasmid. ACE-2 expression was not detected in these cells. Results are from 2 different samples each transfected with empty vector or spike plasmid. B) Spike protein expression was observed in HEK293-ACE2+ cells transfected with spike plasmid. ACE-2 expression decreased upon spike transfection when compared to empty vector. n = 5 in each group (spike or empty vector) from 2 different sets of experiments. C) Spike protein expression was observed in Vero E6 cells transfected with spike plasmid. ACE-2 expression decreased upon spike transfection when compared to empty vector. n = 3 in each group (spike or empty vector).
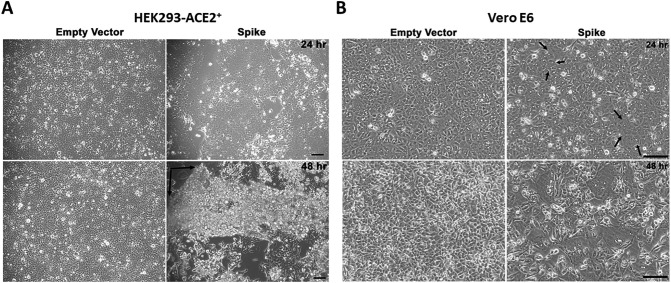
Phase contrast images of HEK293-ACE2+ cells upon spike transfection resulted in the formation of large syncytia at 24 h post transfection (Fig. 2A, upper right panel), whereas no syncytia were observed in HEK293 cells upon spike transfection (Supplementary Fig. 1); this indicates that ACE2 expression is required for the formation of syncytia. In addition, phase contrast images of Vero E6 cells at 24 h revealed syncytia formation (Fig. 2B, top right panel), albeit smaller than HEK293-ACE2+ cells at this time point. This may be due to the fact that ACE2 expression in Vero E6 cells is not as robust as in HEK293-ACE2+ cells that stably overexpress ACE2. However, at 48 h, we observed significantly increased size of syncytia in Vero E6 cells (Fig. 2B, lower right panel).
Fig. 2.
Effect of spike protein expression on syncytia formation and cell sloughing. HEK293-ACE2+ cells were transfected with spike or empty vector plasmid and phase contrast images were acquired. A) Expression of spike protein induced cellular fusion and the formation of large syncytia compared to empty vector at 24 h post transfection (upper right panel). At 48 h, spike transfection resulted in sloughing of cells in cellular sheets from the monolayer (lower right panel). The black arrows indicate areas of detachment of the sheet consisting of fused cells and syncytia. Scale bar = 500 μm. B) Spike protein expression in Vero E6 cells induced syncytia at 24 h (upper right panel, black arrows) that became larger at 48 h (lower right panel) post transfection. Scale bar = 500 μm.
Supplementary Fig. 1.
Effect of spike protein expression on syncytia formation in HEK293 cells. HEK293 cells were transfected with spike or EV plasmid and phase contrast images were acquired. Upper panels show the images of cells at 24 h post transfection while lower panels show the images of cells at 48 h post transfection. No syncytia were observed in HEK293 cells upon spike transfection at either of these time points. Scale bar = 500 μm.
At 48 h post transfection, we observed in spike-transfected HEK293-ACE2+ cells the sloughing of cells in sheets with ensuing detachment from the monolayer (Fig. 2A, lower right panel).
3.2. Spike protein expression in HEK293-ACE2+ cells induced genes recognized as nephrotoxic in acute kidney injury (AKI)
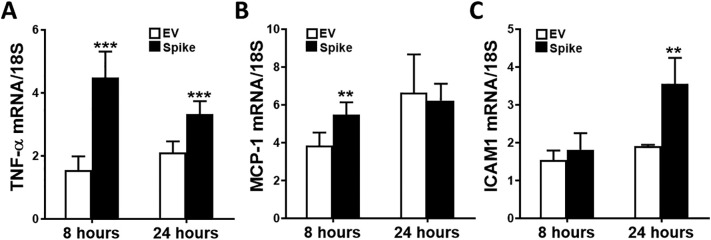
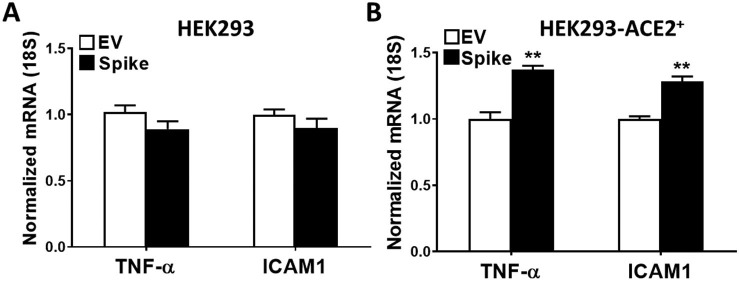
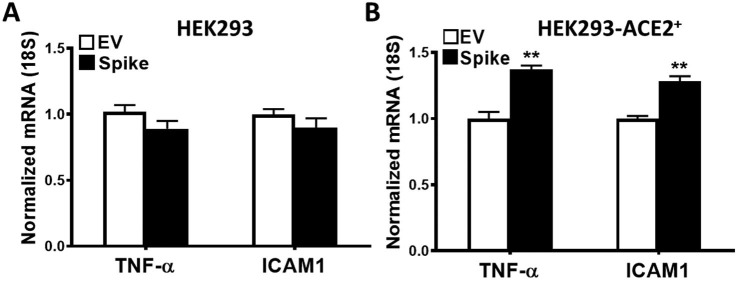
Spike protein expression in these cells led to upregulation of certain genes that are known to contribute to the pathogenesis of AKI. TNF-α, MCP-1, and ICAM1 mRNA expression was assessed by quantitative RT-PCR in HEK293-ACE2+ cells upon spike and EV transfections at 8 and 24 h post transfection. TNF-α mRNA levels were significantly increased both at 8 and 24 h post transfection (Fig. 3A), whereas MCP-1 mRNA expression was significantly higher at 8 h, and ICAM1 mRNA expression at 24 h after transfection with spike protein (Fig. 3B and C, respectively). In additional studies, we assessed the nephrotoxic gene expression in HEK293 cells (Supplementary Fig. 2A) and HEK293-ACE2+ cells (Supplementary Fig. 2B) with 24-hour spike or EV transfection. We again observed significantly higher TNF-α and ICAM1 mRNA expression upon spike transfection in HEK293-ACE2+ cells, while no significant differences were observed in HEK293 cells.
Fig. 3.
The effect of spike protein expression on potentially nephrotoxic genes. TNF-α, MCP-1 and ICAM1 mRNA expression was assessed in HEK293-ACE2+ using real-time RT-PCR in cells transfected with spike or empty vector plasmid at 8 and 24 h post transfection. A) TNF-α mRNA was significantly induced at 8 and 24 h (n = 6 in each condition, ***p < 0.001). B and C) MCP-1 mRNA was significantly induced at 8 h, while ICAM1 mRNA was significantly upregulated at 24 h (n = 6 in each condition, **p < 0.01).
Supplementary Fig. 2.
The effect of spike protein expression on gene expression in HEK293 and HEK293-ACE2+ cells. TNF-α and ICAM1 mRNA expression was assessed in HEK293 and HEK293-ACE2+ using real-time RT-PCR in cells transfected with spike or EV plasmid at 24 h post transfection. A) No changes in TNF-α and ICAM1 mRNA expression were observed in HEK293 cells upon EV and spike transfections at 24 h. B) Both TNF-α and ICAM1 mRNA expression was significantly induced at 24 h (n = 5 in each condition, **p < 0.01).
We considered the possibility that cells expressing ACE2 may shed extracellular vesicles containing spike, and that spike protein from such vesicles upon binding to cellular ACE2, becomes internalized and may thereby lead to nephrotoxic gene expression. We, therefore, collected media from HEK293 and HEK293-ACE2+ cells 24 h post transfection with spike or EV plasmid and analyzed for the presence of spike protein. Notably, we observed the cleaved spike proteins (90 kDa) in media from HEK293-ACE2+ cells indicating that spike protein was processed and cleaved before secretion into the media. However, spike transfection in HEK293 cells did not result in secretion of spike protein (Supplementary Fig. 3).
Supplementary Fig. 3.
Spike protein assessment in media from HEK293 and HEK293-ACE2+ cells upon spike transfection. HEK293 and HEK293-ACE2+ cells were transfected with spike or EV plasmid and media was collected at 24 h post transfection. Media was concentrated 3-fold and Western blot analysis for spike protein was performed for both cell lines. A) Spike protein expression was observed only in the media from HEK293-ACE2+ cells upon spike transfection; however, spike transfection in HEK293 cells did not result in spike protein secretion. B) Amido black staining of the same blot exhibits similar protein loading in media collected from HEK293 and HEK293-ACE2+ cells.
3.3. Spike protein expression induced heme oxygenase-1 (HO-1), a gene that is nephroprotective in AKI
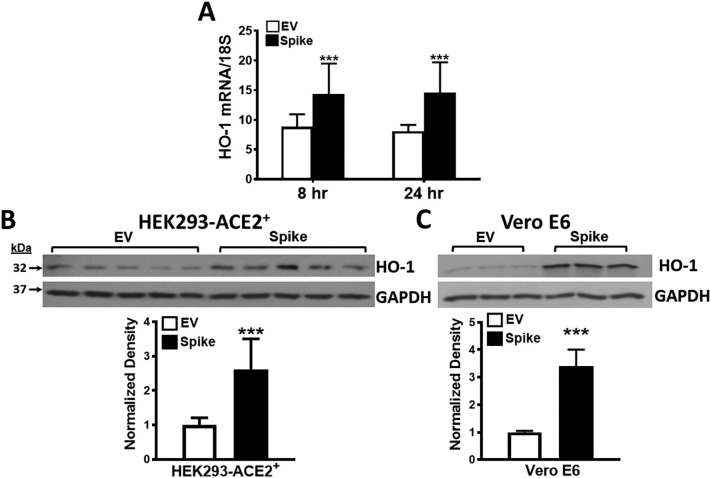
HO-1 mRNA expression was significantly increased in HEK293-ACE2+ cells after spike transfection (as compared with EV transfection) at both 8 and 24 h post transfection (Fig. 4A). Notably, spike transfection induced significantly higher HO-1 protein expression in both HEK293-ACE2+ cells and Vero E6 cells as assessed by Western blot analysis (Fig. 4B).
Fig. 4.
The effect of spike protein on heme oxygenase-1 (HO-1) expression. HO-1 mRNA and protein expression was assessed in HEK293-ACE2+ cells transfected with spike or empty vector plasmids. A) HO-1 mRNA was significantly induced at 8 and 24 h (n = 6 in each condition, ***p < 0.001). B) Western analysis revealed 2.5-fold induction in HO-1 protein levels (***p < 0.001) upon 24 h of spike transfection when compared to empty vector. n = 5 in each group (spike or empty vector) from 2 different sets of experiments. C) Spike transfection in Vero E6 cells induced >3-fold HO-1 protein expression (***p < 0.001) at 24 h post spike transfection when compared to empty vector. n = 3 in each group (spike or empty vector) from 3 different sets of experiments.
3.4. Spike protein expression induced activation of signaling molecules that may promote HO-1 transcription
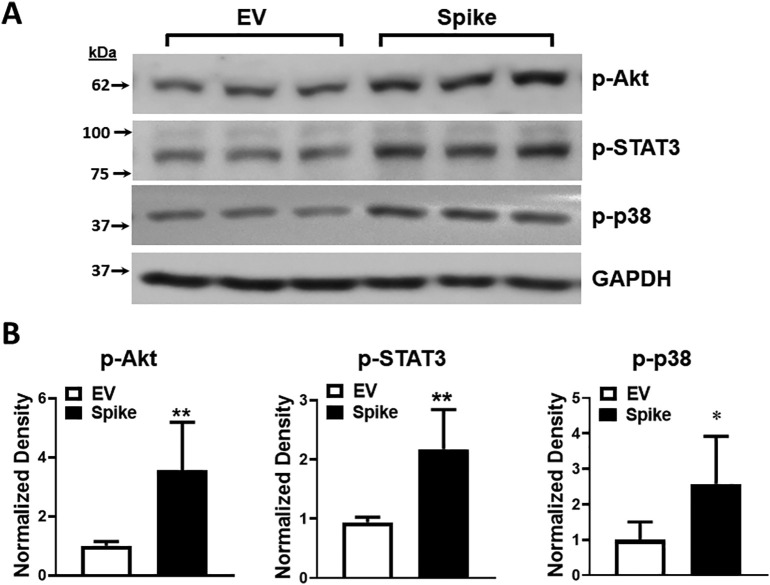
We then examined the activation of signaling molecules upstream of HO-1 which can promote HO-1 gene transcription. Following spike transfection, and as compared with EV transfection, expression levels of p-Akt, p-STAT3 and p-p38 were all increased as assessed by Western blot analysis at 24 h post transfection (Fig. 5A and B).
Fig. 5.
The effect of spike protein expression on signaling molecule activation that may promote HO-1 transcription. Expression of p-Akt, p-STAT3, and p-p38 protein was assessed in HEK293-ACE2+ cells after spike transfection as compared with empty vector at 24 h post transfection. A) Western blots of p-Akt, p-STAT3 and p-p38. B) Quantitation demonstrated significantly higher p-Akt (**p < 0.01), p-STAT3 (**p < 0.01), and p-p38 (*p < 0.05) protein expression. n = 5 in each group (spike or empty vector) from 2 different sets of experiments.
3.5. Quercetin reduced syncytia formation and induced HO-1 expression in spike protein-expressing HEK293-ACE2+ cells
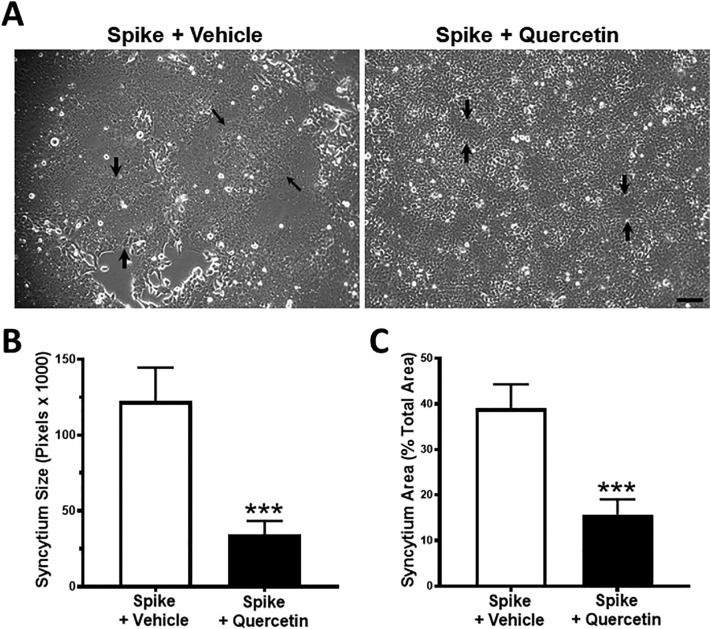
Since HO-1 expression is induced at both the mRNA and protein levels upon spike protein expression in HEK293-ACE2+ cells, we questioned whether inducing HO-1 in these cells would influence syncytia formation. To this end we employed quercetin, a recognized inducer of HO-1 that is widely used in preclinical studies in vitro and in vivo [22]. Cells were transfected with spike and empty vector plasmids and then exposed to quercetin or vehicle 4 h post transfection. Such exposure to quercetin significantly decreased syncytia formation (see arrows indicating syncytium size) (Fig. 6A). To determine if we had attained efficient transfection within 4 h of transfections, we assessed GFP and spike protein expression by Western blot analysis within transfected cells; GFP and spike protein expression was confirmed at 4 h post transfection (data not shown).
Fig. 6.
The effect of quercetin on syncytia formation. HEK293-ACE2+ cells were transfected with spike or empty vector plasmid. Cells were exposed to quercetin (10 μM) or vehicle which were added 4 h post transfection. Phase contrast images were acquired at 24 h post transfection. A) Expression of spike protein induced formation of large syncytia in control cells, whereas much smaller syncytial platforms were observed upon exposure to quercetin. Black arrows demarcate the syncytial platform in A. B) Quantitation of syncytia using ImageJ software revealed significantly decreased mean syncytia size (left panel) and syncytia area (percent of total area, right panel) upon quercetin treatment. n = 9 from 3 different sets of experiments; ***p < 0.001. Scale bar = 500 μm.
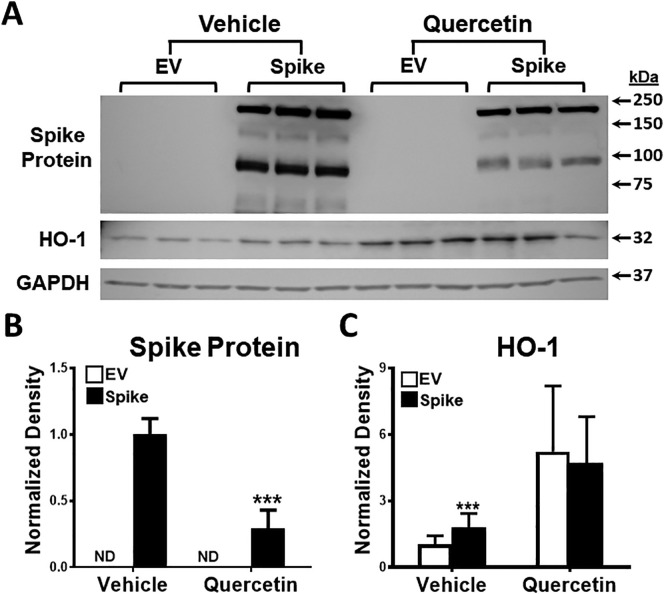
Quantitation of the size and area covered by syncytia revealed that both were significantly and markedly decreased by quercetin (Fig. 6B and C). Notably, we also observed significantly decreased spike protein expression (Fig. 7A and B), and as expected, HO-1 expression was prominently increased in quercetin-exposed cells (Fig. 7A and C).
Fig. 7.
The effect of quercetin on spike and HO-1 protein expression. A) Cells exposed to quercetin when compared to vehicle exhibited markedly decreased spike protein and increased HO-1 protein expression on Western blot analysis. B) Quantitation of Western blot analysis revealed decreased spike protein expression (***p < 0.001) and C) increased HO-1 protein expression (***p < 0.001), n = 6 in each condition from 2 different set of experiments.
We attempted to determine whether HO-1 mediated the anti-syncytial effects of quercetin by examining the effects of quercetin in the presence of the competitive inhibitor of HO activity, zinc protoporphyrin. However, zinc protoporphyrin exerted cytotoxic effects in these cells, even in cells transfected with the empty vector. Such toxic and confounding effects of zinc protoporphyrin also precluded determining whether HO-1, as induced in these cells when they express the spike protein (and in the absence of exposure to quercetin), represented an adaptive, cytoprotective response.
4. Discussion
The present study demonstrates that expression of the spike protein in HEK293-ACE2+ cells leads to cell fusion, progressive syncytia formation, and the lifting off and sloughing of sheets of fused cells from the monolayer, the latter giving rise to focal areas of denudation. These findings are reminiscent of what occurs during acute tubular injury/necrosis in AKI wherein injured renal epithelial cells, adhering to one another, detach from the tubular basement membrane and slough into the urinary space. The present study, to the best of our knowledge, is the first to call attention to this sloughing phenomenon in spike protein-expressing kidney cells and its mimicry of what may occur in the acute tubular necrosis variant of AKI. The relevance of syncytia to AKI is supported by a quite recent postmortem study in patients who died from COVID-19; in 28% of autopsy cases the presence of syncytia was reported in the kidney [23].
The formation of cellular syncytia when cells are infected with SARS-CoV-2 is well recognized [24], [25]. Cells infected with SARS-CoV-2 express the spike protein on their surface, and through the interaction of their surface spike protein with the ACE2 receptor on adjacent cells, these cells adhere to neighboring cells; in this way progressive cellular attachment leads to syncytia formation in cells infected with SARS-CoV-2. Remarkably, as quite recently shown, spike protein expression per se - without the concomitant expression of any other viral protein - causes these cells to adhere to neighboring ACE2-expressing cells [24]. The importance of ACE2 expression in syncytia formation is underscored by the fact that syncytia formation also occurred in Vero E6 cells (which express ACE2), when Vero E6 cells are transfected with spike plasmid; in contrast, HEK293 cells, which do not express ACE2 protein, do not form syncytia when transfected with spike plasmid. The clinical relevance of the current approach in investigating spike protein-dependent kidney disease in COVID-19 is thus validated because of the following salient findings in the recent literature: first, cell spike protein expression per se causes cell adherence to neighboring ACE2-expressing cells [24]; second, SARS-CoV-2 is present in the urine of patients with COVID-19, and the viral titer correlates with AKI and mortality [17]; third, the spike protein is present in urine in patients with COVID-19 and AKI [18].
We found that cleaved spike protein was readily and abundantly detected in the extracellular media of HEK293-ACE2+ cells transfected with spike plasmid, but not in the extracellular media of similarly transfected HEK293 cells. Such presence of spike protein in the media, likely reflecting the shedding of extracellular vesicles, may itself promote syncytia formation in these cells. Additionally, such appearance of spike protein in the extracellular media, as a likely consequence of shedding of extracellular vesicles from spike-expressing renal epithelial cells, may explain, at least in part, the appearance of spike protein in the urine of patients with COVID-19 and AKI.
Spike protein expression led to the upregulation in HEK293-ACE2+ cells of certain genes that are of special interest in and contribute to the pathogenesis of AKI. In these cells, TNF-α was significantly induced at 8 and 24 h, MCP-1 at 8 h, and ICAM1 at 24 h after transfection with spike protein. All three genes are upregulated in rodent models of AKI. In such models, and when each gene is individually studied, the pathogenetic contributions of TNF-α [26], MCP-1 [27], [28], and ICAM1 [29], [30] to AKI have all been confirmed by approaches employing either genetically deficient murine strains or by inhibitory antibodies, peptides, or relevant chemical inhibitors. We focused on these three genes because of their established role in nephrotoxicity in rodent models of AKI. ICAM1 may be of particular interest in view of its capacity to promote adhesion of cells, especially those in the kidney with AKI. We speculate that the upregulation of these genes in HEK293-ACE2+ cells expressing the spike protein reflects an injurious effect of the spike protein in these cells relevant to AKI. Studies that examine the pathophysiologic effects of expression of these specific genes are of interest but are beyond the scope of the present work. We also speculate that the failure of HEK293 cells to evince such gene expression (TNF-α and ICAM1) and syncytia formation when these cells are transfected with spike plasmid suggest a linkage between these two cellular responses.
In addition to potentially nephrotoxic genes, we also examined expression of a nephroprotective gene, HO-1. There is abundant literature that supports induction of HO-1 in models of ischemic and nephrotoxic AKI and that such induction confers protection against diverse forms of AKI [31], [32]. In the present studies we observed that HO-1 mRNA and HO-1 protein as well as upstream signaling species which elicit HO-1 expression - p-Akt, p-STAT3, and p-p38, depending upon the experimental context [31], [32] - were all induced. Thus, the HO-1 system - from signaling pathways that may elicit HO-1 gene transcription to HO-1 mRNA and HO-1 protein - is upregulated in kidney cells that express the spike protein. There is considerable interest in HO-1 as a protectant in COVID-19, but such demonstration of HO-1 induction, as provided by the current study, is yet to be reported in any study to date. Moreover, such induction of HO-1 also robustly occurred in another cell type, Vero E6 cells (which also endogenously express ACE2 protein), when these cells are transfected with the spike plasmid.
There are multiple reasons for this interest in HO-1 in COVID-19, which include at least four considerations [33], [34], [35], [36], [37]. First, it has been postulated that increased amounts of free heme exist in plasma or tissues in patients with COVID-19, a hypothesis that clearly merits examination. There is at least one study demonstrating that heme levels are increased in COVID-19 patients with reduced oxygen saturation [38]. Increased amounts of free heme may originate from destabilized hemoglobin released from lysed RBCs (as occurs in hemolysis) or destabilized myoglobin released from necrotic muscle (as occurs in rhabdomyolysis), both types of cell lysis having been described in COVID-19. Additionally, cytochrome P450 proteins, which reside in microsomes of all cells, are inherently unstable heme proteins, and when cells are injured from whatever cause - be it hypoxia, inflammation, or toxin-induced - these heme proteins are prone to release their heme prosthetic group [37]. It is also suggested that heme levels may increase as a consequence of the postulated high affinity binding of SARS-CoV-2 to porphyrins, the latter being essential moieties in the synthesis of heme proteins [34]. Increased levels of heme, postulated to occur from these mechanisms, are relevant to tissue injury in COVID-19 because heme is a potent prooxidant, proapoptotic, and proinflammatory species. In light of these considerations, it is theorized that induction of HO-1 is beneficial in COVID-19 because, by virtue of its catabolism of heme, HO-1 would remove this potentially cytotoxic, proinflammatory species. Second, the products of HO-1, namely, bile pigments and carbon monoxide, are recognized cytoprotectants by virtue of their antioxidant, anti-inflammatory, anti-thrombotic, and anti-apoptotic action; in essence, HO-1 would replace a toxic species (heme) by cytoprotective ones (bile pigments and carbon monoxide). Third, the SARS-CoV-2 open reading frame 3 can bind to the HO-1 protein thereby vitiating its potential protective effects [33]. Fourth, for a number of viruses other than SARS-CoV-2, HO-1 exerts anti-viral effects, an effect that, putatively, may extend to SARS-CoV-2 [33], [34], [35], [36], [37].
In light of these considerations and our finding that HO-1 is induced in kidney cells expressing the spike protein, we sought to determine whether induction of HO-1 is functionally significant. We thus questioned whether a known and widely employed inducer of HO-1, quercetin [22], would exert functional effects in these cells after transfection with the spike protein. We observed that such treatment with quercetin markedly reduced syncytia formation that otherwise occurred. Quercetin also reduced spike protein expression in these cells. We suggest that the reduction in spike protein expression with quercetin occurs because of the following considerations. Adhesion of the plasma membranes of spike protein-expressing cells to other cells that express spike protein, either in lesser amounts or not at all, may promote transcellular movement of the spike vector to these cells with lesser or no spike protein expression. These cells now with increased amounts of the spike vector will then augment their expression of spike protein. When syncytia formation is inhibited, as it occurs with quercetin, spike protein expression will accordingly and concomitantly decrease.
We confirmed, as expected, that quercetin markedly increased HO-1 expression. However, we were unable to resolve whether HO-1 indeed mediated the anti-syncytial effects of quercetin because the inhibitor of HO activity, zinc protoporphyrin, exerted cytotoxic effects on these cells, even in cells transfected with the empty vector. Such toxic and confounding effects of zinc protoporphyrin also precluded determining whether HO-1, as induced in these cells when they express the spike protein (and in the absence of exposure to quercetin), exerted countervailing cytoprotective effects. This toxicity of the HO inhibitor zinc protoporphyrin on these cells even when transfected with an empty vector (and thus, in essence, under basal conditions) raises the possibility that basal HO activity may be needed for cell survival since inhibition of such activity is attended by cytotoxic effects. Studies of HO-1 knockdown are thus of interest and are planned for the future.
Quercetin has numerous cellular effects besides the induction of HO-1. As for other naturally occurring compounds, especially those with anti-oxidant and anti-inflammatory effects, the suggestion has been made that quercetin may provide a therapeutic approach in COVID-19 [39]. Several studies have indicated that quercetin may be protective in models of AKI in settings not related to COVID-19 [40]. Based on such protective effects in AKI in these settings, it has been suggested that quercetin would be protective against AKI occurring specifically in COVID-19 [41]. Quite remarkably, the possibility that quercetin may be protective in AKI occurring in COVID-19 is supported by theoretic analyses based on network pharmacology and molecular docking studies [42]. Our present findings provide, to the best of our knowledge, the first experimental data in support of these speculations and analyses.
In summary, we introduce an in vitro model for the study of AKI in COVID-19 based on expressing the spike protein in kidney cells. This “reductionist” approach is supported by the burgeoning biology that attests to cellular effects of the spike protein per se relevant to the pathobiology of COVID-19; by the fact that SARS-CoV-2 infects the kidney in COVID-19; and by the fact that the spike protein is present in the urine in patients with AKI. In the course of the present studies, we uncover the induction of the HO-1 system in spike protein-expressing ACE2+ kidney cells, and that quercetin, which induces HO-1 and other molecular species and pathways, can reduce syncytia formation caused by spike protein expression.
The following are the supplementary data related to this article.
CRediT authorship contribution statement
Raman Deep Singh: Conceptualisation, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualisation, writing – original draft, and writing – review & editing.
Michael A. Barry: Conceptualisation, Methodology, Resources, Validation, writing – review & editing.
Anthony J. Croatt: Conceptualisation, data curation, methodology, resources, software, supervision, validation, visualisation, writing – original draft, and writing – review & editing.
Allan W. Ackerman: Conceptualisation, data curation, formal analysis, methodology, software, validation, visualisation, and writing – review & editing.
Joseph P. Grande: Conceptualisation, Formal analysis, Validation, and writing – review & editing.
Rosa M Diaz: Resources, writing - review & editing.
Richard G. Vile: Conceptualisation, Validation, and writing – review & editing.
Anupam Agarwal: Conceptualisation, Validation, and writing – review & editing.
Karl A. Nath: Conceptualisation, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualisation, writing – original draft, and writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadim M.K., Forni L.G., Mehta R.L., Connor M.J., Jr., Liu K.D., Ostermann M., et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat. Rev. Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparks M.A., South A.M., Badley A.D., Baker-Smith C.M., Batlle D., Bozkurt B., et al. Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: pressing needs and best research practices. Hypertension. 2020;76(5):1350–1367. doi: 10.1161/HYPERTENSIONAHA.120.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao C., Zeng J., Jia N., Stavenhagen K., Matsumoto Y., Zhang H., et al. SARS-CoV-2 spike protein interacts with multiple innate immune receptors. bioRxiv. 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7402034/ [Google Scholar]
- 7.Ratajczak M.Z., Bujko K., Ciechanowicz A., Sielatycka K., Cymer M., Marlicz W., et al. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45(-) precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Rev. Rep. 2020:1–12. doi: 10.1007/s12015-020-10010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020;146 doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ. Res. 2021;128(9):1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han M., Pandey D. ZMPSTE24 regulates SARS-CoV-2 spike protein-enhanced expression of endothelial plasminogen activator Inhibitor-1. Am. J. Respir. Cell Mol. Biol. 2021;65(3):300–308. doi: 10.1165/rcmb.2020-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bortolotti D., Gentili V., Rizzo S., Rotola A., Rizzo R. SARS-CoV-2 spike 1 protein controls natural killer cell activation via the HLA-E/NKG2A pathway. Cells. 2020;9(9):15. doi: 10.3390/cells9091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun F., Lutgehetmann M., Pfefferle S., Wong M.N., Carsten A., Lindenmeyer M.T., et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396(10251):597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan S., Chen L., Yang C.R., Raghuram V., Khundmiri S.J., Knepper M.A. Does SARS-CoV-2 infect the Kidney? J. Am. Soc. Nephrol. 2020;31(12):2746–2748. doi: 10.1681/ASN.2020081229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijayan A., Humphreys B.D. SARS-CoV-2 in the kidney: bystander or culprit? Nat. Rev. Nephrol. 2020;16(12):703–704. doi: 10.1038/s41581-020-00354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caceres P., Savickas G., Murray S., Umanath K., Uduman J., Yee J., et al. High SARS-CoV-2 viral load in urine sediment correlates with acute kidney injury and poor COVID-19 outcome. J. Am. Soc. Nephrol. 2021;32(10):2517–2528. doi: 10.1681/ASN.2021010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George S., Pal A.C., Gagnon J., Timalsina S., Singh P., Vydyam P., et al. Evidence for SARS-CoV-2 spike protein in the urine of COVID-19 patients. Kidney. 2021;360 doi: 10.34067/KID.0002172021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath K.A., Grande J.P., Belcher J.D., Garovic V.D., Croatt A.J., Hillestad M.L., et al. Antithrombotic effects of heme-degrading and heme-binding proteins. Am J Physiol Heart Circ Physiol. 2020;318(3) doi: 10.1152/ajpheart.00280.2019. H671-h81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath K.A., Garovic V.D., Grande J.P., Croatt A.J., Ackerman A.W., Farrugia G., et al. Heme oxygenase-2 protects against ischemic acute kidney injury: influence of age and sex. Am. J. Physiol. Renal. Physiol. 2019;317(3):F695–f704. doi: 10.1152/ajprenal.00085.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia B., Shen X., He Y., Pan X., Liu F.L., Wang Y., et al. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res. 2021;31(8):847–860. doi: 10.1038/s41422-021-00519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funes S.C., Rios M., Fernández-Fierro A., Covián C., Bueno S.M., Riedel C.A., et al. Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020;11:1467. doi: 10.3389/fimmu.2020.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivero J., Merino-López M., Olmedo R., Garrido-Roldan R., Moguel B., Rojas G., et al. Association between postmortem kidney biopsy findings and acute kidney injury from patients with SARS-CoV-2 (COVID-19) Clin. J. Am. Soc. Nephrol. 2021;16(5):685–693. doi: 10.2215/CJN.16281020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchrieser J., Dufloo J., Hubert M., Monel B., Planas D., Rajah M.M., et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2020;39:1–12. doi: 10.15252/embj.2020106267. e106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musarrat F., Chouljenko V., Dahal A., Nabi R., Chouljenko T., Jois S.D., et al. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. J. Med. Virol. 2020;92(10):2087–2095. doi: 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Paola R., Genovese T., Impellizzeri D., Ahmad A., Cuzzocrea S., Esposito E. The renal injury and inflammation caused by ischemia-reperfusion are reduced by genetic inhibition of TNF-αR1: a comparison with infliximab treatment. Eur. J. Pharmacol. 2013;700(1–3):134–146. doi: 10.1016/j.ejphar.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 27.Furuichi K., Wada T., Iwata Y., Kitagawa K., Kobayashi K., Hashimoto H., et al. Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2003;14(4):1066–1071. doi: 10.1097/01.asn.0000059339.14780.e4. [DOI] [PubMed] [Google Scholar]
- 28.Furuichi K., Wada T., Iwata Y., Kitagawa K., Kobayashi K., Hashimoto H., et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J. Am. Soc. Nephrol. 2003;14(10):2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- 29.Kelly K.J., Williams W.W., Jr., Colvin R.B., Bonventre J.V. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc. Natl. Acad. Sci. U. S. A. 1994;91(2):812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly K.J., Williams W.W., Jr., Colvin R.B., Meehan S.M., Springer T.A., Gutierrez-Ramos J.C., et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Invest. 1996;97(4):1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A., Bolisetty S. Adaptive responses to tissue injury: role of heme oxygenase-1. Trans. Am. Clin. Climatol. Assoc. 2013;124:111–122. [PMC free article] [PubMed] [Google Scholar]
- 32.Nath K.A. Heme oxygenase-1 and acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2014;23(1):17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batra N., De Souza C., Batra J., Raetz A.G., Yu A.M. The HMOX1 pathway as a promising target for the treatment and prevention of SARS-CoV-2 of 2019 (COVID-19) Int. J. Mol. Sci. 2020;21(17) doi: 10.3390/ijms21176412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakhouri E.W., Peterson S.J., Kothari J., Alex R., Shapiro J.I., Abraham N.G. Genetic polymorphisms complicate COVID-19 therapy: pivotal role of HO-1 in cytokine storm. Antioxidants (Basel) 2020;9(7) doi: 10.3390/antiox9070636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi M., Piagnerelli M., Van Meerhaeghe A., Zouaoui Boudjeltia K. Heme oxygenase-1 (HO-1) cytoprotective pathway: a potential treatment strategy against coronavirus disease 2019 (COVID-19)-induced cytokine storm syndrome. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh D., Wasan H., Reeta K.H. Heme oxygenase-1 modulation: a potential therapeutic target for COVID-19 and associated complications. Free Radic. Biol. Med. 2020;161:263–271. doi: 10.1016/j.freeradbiomed.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagener F., Pickkers P., Peterson S.J., Immenschuh S., Abraham N.G. Targeting the heme-heme oxygenase system to prevent severe complications following COVID-19 infections. Antioxidants (Basel) 2020;9(6) doi: 10.3390/antiox9060540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su W.L., Lin C.P., Hang H.C., Wu P.S., Cheng C.F., Chao Y.C. Desaturation and heme elevation during COVID-19 infection: a potential prognostic factor of heme oxygenase-1. J. Microbiol. Immunol. Infect. 2021;54(1):113–116. doi: 10.1016/j.jmii.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeedi-Boroujeni A., Mahmoudian-Sani M.R. Anti-inflammatory potential of quercetin in COVID-19 treatment. J. Inflamm. (Lond). 2021;18(1):3. doi: 10.1186/s12950-021-00268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoskes D.A. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation. 1998;66(2):147–152. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- 41.Diniz L.R.L., Souza M.T.S., Duarte A.B.S., Sousa D.P. Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury. Molecules. 2020;25(23) doi: 10.3390/molecules25235772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Y.Y., Zhang M., Cen H., Wu Y.F., Lu Z., Lu F., et al. Quercetin as a potential treatment for COVID-19-induced acute kidney injury: based on network pharmacology and molecular docking study. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0245209. [DOI] [PMC free article] [PubMed] [Google Scholar]