Abstract
Introduction
During the recent SARS-CoV-2 pandemic, circulating calprotectin (cCLP) gained interest as biomarker to predict the severity of COVID-19. We aimed to investigate the prognostic value of cCLP measured in serum, heparin, EDTA and citrate plasma.
Materials and methods
COVID-19 patients were prospectively included, in parallel with two SARS-CoV-2 negative control populations. The prognostic value of cCLP was compared with IL-6, CRP, LDH, procalcitonin, and the 4C-mortality score by AUROC analysis.
Results
For the 136 COVID-19 patients, cCLP levels were higher compared to the respective control populations, with significantly higher cCLP levels in serum and heparin than in EDTA or citrate. Higher cCLP levels were obtained for COVID-19 patients with i) severe/critical illness (n = 70), ii) ICU admission (n = 66) and iii) need for mechanical ventilation/ECMO (n = 25), but iv) not in patients who deceased within 30 days (n = 41). The highest discriminatory power (AUC [95% CI]) for each defined outcome was i) CRP (0.835 [0.755–0.914]); ii) EDTA cCLP (0.780 [0.688–0.873]); iii) EDTA cCLP (0.842 [0.758–0.925]) and iv) the 4C-mortality score (0.713 [0.608–0.818]).
Conclusion
Measuring cCLP in COVID-19 patients helps the clinician to predict the clinical course of COVID-19. The discriminatory power of EDTA and citrate plasma cCLP levels often outperforms heparin plasma cCLP levels.
Keywords: Calprotectin, COVID-19, Prognostic value, Serum, Plasma, Pre-analytics
1. Introduction
Since the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019, more than 240 million proven infections and nearly 5 million deaths of coronavirus disease 2019 (COVID-19) were reported (https://www.worldometers.info/coronavirus/). Although to date nearly half of the world population has received at least one dose of a COVID-19 vaccine (https://ourworldindata.org/covid-vaccinations), SARS-CoV-2 variants able to escape vaccine-induced immunity may emerge [1]. Several risk scores, diagnostic imaging and biomarkers have been evaluated and compared to help predict severe complications and outcome in COVID-19 patients [2]. Nevertheless, early prediction of COVID-19 severity remains difficult, emphasizing the need for additional biomarkers in daily practice.
Calprotectin (CLP) is a heterodimeric complex formed by two calcium-binding proteins S100A8 and S100A9, also known as myeloid-related protein (MRP)-8 and MRP-14. CLP is typically expressed and secreted by neutrophils, monocytes, and activated macrophages but can also be expressed and secreted by other cell lines including but not limited to dendritic cells, endothelial cells, keratinocytes and squamous mucosal epithelium [3]. CLP is part of the innate immune response and contributes to the inflammatory process through the recruitment of leucocytes, binding of arachidonic acid, and the expression of pro-inflammatory and anti-inflammatory mediators. CLP acts as an endogenous ligand of Toll-like receptor 4 (TLR4) and receptor for advanced glycation endproducts (RAGE) [4]. Furthermore, CLP has an antimicrobial activity and plays a role in cell proliferation, differentiation and apoptosis [3].
Fecal CLP measurement is already used as a reliable biomarker in the diagnosis of inflammatory bowel disease [5]. Circulating CLP (cCLP) has gained recent attention as a biomarker of neutrophil-related inflammation and chronic inflammatory disorders such as rheumatoid arthritis [6], systemic lupus erythematosus [7], but also in pneumonia patients - next to elevated CLP levels in bronchoalveolar lavage fluid and lung tissue [8], [9]. Recently, cCLP has been proposed in about a dozen independent studies as a promising serological biomarker to predict the severity of pathogen-associated tissue damage and the excessive cytokine storm in COVID-19 [10].
To date, the preferred matrix to measure cCLP remains topic of debate. Studies evaluating cCLP in and beyond the context of COVID-19 interchangeably used serum and plasma matrices, hampering the interpretation and comparison of the results obtained as serum and plasma cCLP concentrations differ significantly [11], [12], [13], [14]. The latter is mainly due to the in vitro lability of neutrophils and the platelet activation in serum, both enhancing the release of CLP into the extracellular matrix [10], whilst ethylene-diamine-tetra-acetic acid (EDTA) and citrate plasma, chelate calcium ions, inhibiting calcium dependent cCLP secretion and resulting in significantly reduced cCLP levels [11], [13], [14].
The aim of this monocentric study was to investigate if the prognostic value of cCLP on the clinical course of COVID-19 patients differed when cCLP was measured in various sample matrices. This was done by assessing the prognostic values of cCLP measured in both serum, lithium heparin, EDTA and citrate plasma in COVID-19 patients with confirmed SARS-CoV-2 infection presenting at the emergency department of the OLV Hospital Aalst, a secondary care hospital in Belgium. These values were compared to five other inflammatory biomarkers: C-reactive protein (CRP), interleukin-6 (IL-6), lactate dehydrogenase (LDH), procalcitonin (PCT) - and one disease severity scoring system (the 4C-mortality score).
2. Materials and methods
2.1. Study and control populations
Study patients were prospectively included between November 2020 and May 2021 at the OLV Hospital, Aalst, Belgium. Study populations included patients with primary diagnosis of SARS-CoV-2 (confirmed by real-time reverse transcription polymerase chain reaction (rRT-PCR)) who presented at the emergency department (ED) requiring hospitalization at i) a non-ICU ward or ii) ICU ward. Control populations were defined as i) patients presenting at the ED for whom a non-elective hospitalization was needed at a non-ICU ward and ii) patients who underwent cardiovascular (CV) surgery followed by hospitalization at the CV-ICU ward. All control patients had negative rRT PCR SARS-CoV-2 screening result.
2.2. Sample and data collection
The primary biomarkers of interest concerning the inflammatory response to COVID-19 were cCLP (measured in heparin, EDTA and citrate plasma and serum), CRP, IL-6, LDH and PCT.
After routine laboratory analysis, including CRP, LDH and PCT analysis, was performed on blood samples taken at the ED, aliquots of serum, heparin plasma, EDTA plasma and citrate plasma were stored at −20 °C, compliant with the pre-analytic requirements for analyzing cCLP (i.e. for serum samples: centrifugation within 2 h after blood draw; for EDTA samples: storage at −20 °C within 72 h after blood draw [12]). Batch analyses of cCLP (EliATM Calprotectin 2 assay on Phadia 200 instrument; serum/plasma protocol research use only, Thermo Fisher Scientific) and IL-6 (Elecsys IL-6 on cobas c801, Roche) were performed on stored aliquots.
Patient demographics, medical history including medication use and co-morbidities, vital signs at admission and clinical course were extracted from the electronic medical records. Data on the clinical outcomes of hospitalized patients were recorded until discharge or until 30 days after ED presentation. The 4C-mortality score (International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) World Health Organization (WHO) Clinical Characterization Protocol) [15], [16], [17] was calculated based on the collected clinical and biochemical data.
The study was performed with full respect for individuals’ rights to confidentiality and in accordance with procedures supervised by Local Authorities responsible for Ethical Research (Belgian registration number of ethical approval B1262021000002).
2.3. Outcomes
To evaluate the prognostic value of the included biomarkers for COVID-19 disease severity, following outcomes were defined: i) severe or critical disease vs. a-/pre-symptomatic or mild or moderate illness at ED presentation (definitions of disease severity are described in Supplemental Materials and Methods); ii) admission to the ICU vs. a non-ICU ward; iii) need for mechanical ventilation or extra corporeal membrane oxygenation (ECMO) vs. non-invasive ventilation and oxygenation therapy (i.e. no need for supplemental oxygen; supplemental oxygen by nasal cannula or oxygen mask; high flow nasal oxygen therapy (OptiflowTM) and non-invasive ventilation; iv) death after 30 days vs. discharged or still hospitalized after 30 days.
2.4. Statistical analysis
Categorical data were reported as absolute number (n) and relative frequency (%) and compared using chi-squared test, whilst continuous variables were reported as median and interquartile range (IQR) and compared using Mann-Whitney test (non-paired non-normally distributed data) or Wilcoxon (paired non-normally distributed data) as appropriate. Associations between biochemical parameters were examined with Spearman's coefficient of rank correlation (r). The discriminatory power of all biomarkers of interest were compared using Area Under the Receiver Operating Characteristic (AUROC) curve analysis for the different defined outcomes. In addition, a univariate analysis (including age, gender, BMI and total number of comorbidities) followed by multivariate analysis was performed including parameters from univariate analysis with p < 0.10. A stepwise approach was performed for final parameter selection by using p < 0.05. Odds ratios (ORs) with 95% confidence intervals (95% CI) were calculated as well. All data analyses were performed in MEDCALC® Statistical Software version 19.4 (MedCalc Software Ltd, Ostend, Belgium) and Analyse-it Software version 5.65.3 (Leeds, UK) with a p < 0.05 considered statistically significant.
3. Results
3.1. Patient cohorts
One hundred and thirty-six SARS-CoV-2 positive patients were included (70 hospitalized at a non-ICU ward; 66 at an ICU ward), next to 40 SARS-CoV-2 negative control patients (20 non-ICU; 20 CV-ICU). An overview of demographic data is presented in Table 1 ; an overview of co-morbidities, medication use before admission and clinical data during hospitalization in Supplemental Data Table S1-4. Remarkable was the significantly higher BMI of ICU patients (median [IQR] 29.4 [25.8–34.8]) compared to non-ICU patients (26.7 [24.2–29.4]) (p < 0.001) and the similar mortality rate between ICU patients (21/66 (31.8%)) and non-ICU patients (20/70 (28.6%)) (p = 0.681).
Table 1.
Demographic data of included study patients (n = 136) and control patients (n = 40).
| Study Population Non-ICU N = 70 |
Control Population Non-ICU N = 20 |
P-value | Study Population ICU N = 66 |
Control Population ICU N = 20 |
P-value | |
|---|---|---|---|---|---|---|
| Median Age [Range] |
79 [31–98] |
76 [38–86] |
0.058 | 65 [37–86] |
74 [56–88] |
0.008 |
| Female, N (%) | 26 (37.1%) | 9 (45.0%) | 0.527 | 28 (42.4%) | 2 (10.0%) | 0.008 |
| Ethnicity | ||||||
| Caucasian, N (%) | 69 (98.6%) | 20 (100.0%) | 59 (89.4%) | 20 (100.0%) | ||
| African, N (%) | 1 (1.4%) | 0 (0.0%) | 6 (9.1%) | 0 (0.0%) | ||
| Asian, N (%) | 0 (0.0%) | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) | ||
3.2. Calprotectin analyses
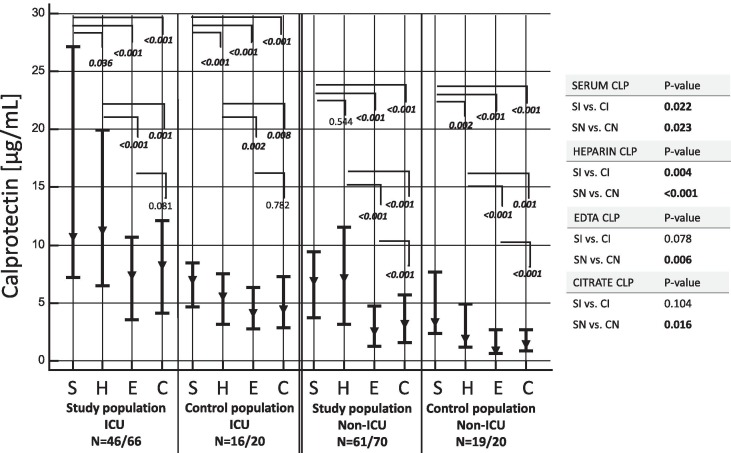
As shown in Fig. 1 , ICU- and non-ICU COVID-19 patients showed higher cCLP levels compared to their respective control groups. Furthermore, cCLP levels in serum and heparin were significantly higher compared to EDTA and citrate levels in all patient cohorts. A summary table of cCLP results in all populations and matrices is shown in Supplemental Data, Table S5.
Fig. 1.
Graphical plot (median, ▾; interquartile range, ▬) of calprotectin concentration in various included populations and matrices. To allow inter-matrices comparison (Wilcoxon test), only patients of whom the 4 matrices were available are shown in the figure. Statistical differences between populations (i.e. all patients; right column) are calculated using Mann-Whitney test. Abbreviations: C, citrate plasma; CI, control population ICU; CN, control population non-ICU; E, EDTA plasma; H, heparin plasma; S, serum; SI, study population ICU; SN, study population non-ICU.
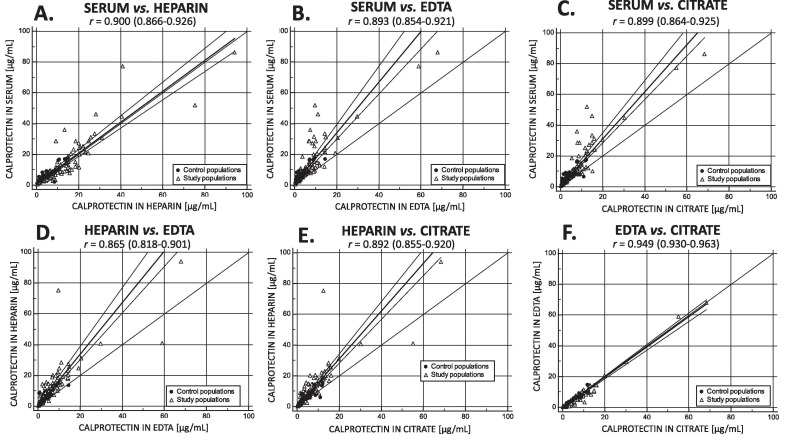
Spearman's coefficient of rank correlation revealed a very strong correlation of cCLP concentrations (r > 0.8, p < 0.05) between all matrices. However, Passing Bablok regression demonstrated a significant systematic bias between cCLP in serum vs. cCLP in heparin, cCLP in EDTA and in cCLP citrate in addition to a proportional bias between cCLP in serum vs. cCLP in EDTA and cCLP in citrate, and between cCLP in heparin vs. cCLP in EDTA and cCLP in citrate (Fig. 2 ). cCLP showed a strong positive correlation in all matrices with CRP (r = 0.595–0.625) and LDH (r = 0.623–0.707), a moderate positive correlation with IL-6 (r = 0.364–0.477) and a weak correlation with PCT (r = 0.280–0.396) (Supplemental Data, Table S6).
Fig. 2.
Passing and Bablok regression including Spearman's correlation r (95% CI) of cCLP measurement between A. serum and heparin; B. serum and EDTA; C. serum and citrate; D. heparin and EDTA; E. heparin and citrate; F. EDTA and citrate.
3.3. Covid-19 biomarkers and outcomes
Regarding the study population (n = 136), 70 patients (51.5%) presented with severe or critical COVID-19 symptoms, 66 (48.5%) needed ICU admission, 25 (18.4%) required mechanical ventilation or ECMO and 41 (30.1%) were deceased within 30 days. An overview of cCLP values complemented with age, CRP, IL-6, PCT and LDH in different subgroups are shown in Table 2 .
Table 2.
Comparison of age and biomarker concentration in the study population (n = 136) for the defined outcome variables. Data are presented as median (range). Statistical differences (Mann-Whitney test) between subgroups are highlighted. Abbreviations: cCLP, circulating calprotectin; ECMO, extra corporeal membrane oxygenation; ICU, intensive care unit; IL-6, interleukin-6; LDH, lactate dehydrogenase; PCT, procalcitonin.
| OUTCOME I disease severity |
OUTCOME II ICU admission |
OUTCOME III Need for mech vent/ECMO |
OUTCOME IV 30-day mortality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe Critical illness |
A-/pre-symptomatic Mild Moderate Illness |
P-value | ICU admission |
Non-ICU admission |
P-value | Mech vent ECMO |
Room air Nasal can Oxygen mask Optiflow |
P-value | Deceased | Discharged Still hospitalized |
P-value | |
| Age [years] |
69 (61–78) [n = 70] |
75 (62–84) [n = 66] |
0.043 | 65 (57–73) [n = 66] |
70 (68–85) [n = 70] |
<0.001 | 63 (55–72) [n = 25] |
74 (63–83) [n = 111] |
0.001 | 79 (71–85) [n = 41] |
68 (58–78) [n = 95] |
<0.001 |
| cCLP serum [µg/mL] |
9.593 (7.031–23.365) [n = 70] |
6.218 (3.372–8.904) [n = 58] |
<0.001 | 8.964 (6.374–21.366) [n = 65] |
6.854 (3.530–9.542) [n = 63] |
<0.001 | 9.575 (7.165–21.588) [n = 25] |
8.190 (4.453–11.516) [n = 103] |
0.026 | 9.200 (4.979–14.816) [n = 39] |
8.190 (5.111–11.389) [n = 89] |
0.506 |
| cCLP heparin [µg/mL] |
11.263 (6.216–18.680) [n = 66] |
6.212 (3.073–9.316) [n = 65] |
<0.001 | 9.905 (5.942–18.523) [n = 61] |
6.668 (3.071–10.360) [n = 70] |
0.001 | 10.130 (6.478–18.575) [n = 24] |
7.328 (3.759–13.325) [n = 107] |
0.021 | 8.741 (3.981–15.625) [n = 39] |
7.606 (4.555–13.240) [n = 92] |
0.620 |
| cCLP EDTA [µg/mL] |
7.111 (3.467–10.375) [n = 60] |
2.291 (1.010–3.767) [n = 57] |
<0.001 | 7.151 (3.528–10.685) [n = 50] |
2.552 (1.319–4.794) [n = 67] |
<0.001 | 9.006 (5.644–13.276) [n = 15] |
3.256 (1.787–6.924) [n = 102] |
<0.001 | 5.929 (2.552–9.296) [n = 34] |
3.322 (1.952–6.710) [n = 83] |
0.164 |
| cCLP citrate [µg/mL] |
7.546 (3.992–11.685) [n = 66] |
2.715 (1.493–4.628) [n = 53] |
<0.001 | 7.504 (3.996–11.680) [n = 56] |
3.318 (1.544–5.622) [n = 63] |
<0.001 | 8.538 (5.587–12.423) [n = 20] |
3.863 (2.064–7.357) [n = 99] |
<0.001 | 5.413 (2.306–9.074) [n = 37] |
4.160 (2.445–7.751) [n = 82] |
0.480 |
| CRP [mg/L] |
122.9 (73.3–189.5) [n = 69] |
33.7 (12.7–70.1) [n = 65] |
<0.001 | 119.8 (56.0–193.0) [n = 65] |
44.0 (15.4–95.9) [n = 69] |
<0.001 | 118.1 (54.3–213.3) [n = 25] |
64.4 (19.3–126.3) [n = 109] |
0.005 | 111.8 (36.1–144.9) [n = 39] |
64.4 (22.3–131.9) [n = 95] |
0.142 |
| IL-6 [pg/mL] |
80.1 (47.3–146.0) [n = 66] |
38.8 (20.8–65.5) [n = 65] |
<0.001 | 87.6 (42.2–146.8) [n = 61] |
46.8 (25.2–66.1) [n = 70] |
<0.001 | 118.0 (51.3–277.5) [n = 24] |
54.3 (26.3–83.4) [n = 107] |
0.003 | 70.8 (41.0–186.0) [n = 39] |
54.0 (25.6–93.2) [n = 92] |
0.011 |
| PCT [µg/L] |
0.158 (0.075–0.392) [n = 69] |
0.078 (0.046–0.164) [n = 65] |
0.001 | 0.173 (0.076–0.391) [n = 66] |
0.079 (0.039–0.149) [n = 68] |
0.001 | 0.180 (0.076–0.404) [n = 25] |
0.100 (0.049–0.265) [n = 109] |
0.047 | 0.180 (0.072–0.472) [n = 41] |
0.104 (0.049–0.198) [n = 93] |
0.020 |
| LDH [U/L] |
465 (380–578) [n = 67] |
337 (256–417) [n = 59] |
<0.001 | 441 (351–563) [n = 63] |
350 (267–452) [n = 63] |
<0.001 | 474 (406–576) [n = 25] |
382 (294–486) [n = 101] |
0.001 | 439 (398–548) [n = 36] |
378 (296–504) [n = 90] |
0.045 |
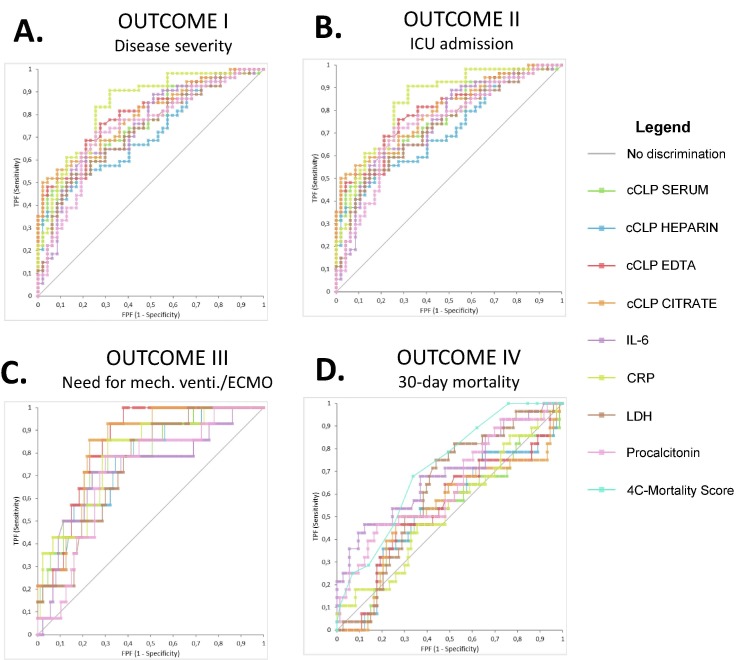
cCLP concentrations were significantly higher in patients presenting with severe or critical disease vs. patients with a-/pre-symptomatic or mild or moderate illness at ED admission (Table 2; Supplemental Data, Fig. S1). However, as shown in Fig. 3 and Table 3 , CRP showed the highest discriminatory power (AUC [95% CI] 0.835 [0.755–0.914]), which was significantly higher compared to cCLP in heparin (0.712 (0.621–0.811). To prevent collinearity issues, cCLP in different matrices was analyzed in separate multivariate logistic regressions. When adjusted for demographic confounders (age, gender, BMI and number of comorbidities), cCLP showed to be significantly associated with severe or critical disease in all matrices (Table 4).
Fig. 3.
Comparison of AUROC analyses between biomarkers (AUC [95 %CI]) for discriminating A. study patients with severe or critical illness vs. a-/pre-symptomatic, mild or moderate illness at ED presentation; B. the need for ICU admission vs. treatment at a non-ICU ward; C. the need for mechanical ventilation or ECMO vs. non-invasive oxygenation; D. study patients deceased within 30 days vs. patients discharged or still hospitalized after 30 days. Abbreviations: cCLP, circulating calprotectin; CRP, C-reactive protein; ECMO, extra corporeal membrane oxygenation; IL-6, interleukin-6; LDH, lactate dehydrogenase.
Table 3.
Comparison of area under the receiver operating characteristic curve (AUROC) (95% C.I.) of inflammatory biomarkers in the study population (N = 136) for the defined outcome variables. The biomarker with highest AUROC is underlined; significant differences in pairwise comparison of ROC curves are added in comment.
| Outcome I Disease severity |
Outcome II ICU admission |
Outcome 3 mech. venti./ECMO |
Outcome 4 30-day mortality |
|
|---|---|---|---|---|
| cCLP SERUM | 0.758 (0.665–0.850) |
0.728 (0.628–0.828) |
0.782 (0.655–0.908) |
0.520 (0.388–0.651) (4) |
| cCLP HEPARIN | 0.712 (0.621–0.811) (1) |
0.684 (0.577–0.791) (2) |
0.760 (0.632–0.888) (3) |
0.535 (0.404–0.665) (4) |
| cCLP EDTA | 0.797 (0.712–0.882) (1) |
0.780 (0.688–0.873)(2) |
0.842 (0.758–0.925)(3) |
0.537 (0.406–0.668) (4) |
| cCLP CITRATE | 0.794 (0.708–0.879) (1) |
0.765 (0.670–0.861) (2) |
0.828 (0.737–0.920) (3) |
0.527 (0.392–0.663) (4) |
| IL-6 | 0.744 (0.648–0.841) |
0.685 (0.577–0.793) |
0.724 (0.566–0.882) |
0.686 (0.562–0.811) (4) |
| CRP |
0.835 (0.755–0.914)(1) |
0.753 (0.655–0.850) |
0.790 (0.668–0.911) |
0.531 (0.403–0.659) (4) |
| LDH | 0.728 (0.630–0.826) |
0.732 (0.632–0.832) |
0.741 0.621–0.861) (3) |
0.616 (0.501–0.730) |
| Procalcitonin | 0.739 (0.641–0.837) (1) |
0.721 (0.618–0.824) |
0.714 (0.580–0.828) |
0.636 (0.510–0.762) |
| 4C-Mortality Score |
0.713 (0.608–0.818)(4) |
(1) CRP vs. cCLP heparin, p = 0.013; CRP vs. procalcitonin, p = 0.047; cCLP citrate vs. cCLP heparin, p = 0.001; cCLP EDTA vs. cCLP heparin, p = 0.004.
(2) cCLP EDTA vs. cCLP heparin, p < 0.001; cCLP citrate vs. cCLP heparin, p = 0.001.
(3) cCLP EDTA vs. cCLP heparin, p = 0.040; CLP EDTA vs. LDH, p = 0.007; cCLP citrate vs. LDH, p = 0.013; cCLP citrate vs. cCLP heparin, p = 0.048.
(4) 4C-Moratlity score vs. cCLP serum, p = 0.015; 4C-mortality score vs. cCLP heparin, p = 0.031; p = 0.021; 4C-mortality score vs. cCLP EDTA, p = 0.029; 4C-mortality score vs. cCLP citrate, 4C-mortality score vs. CRP, p = 0.010; IL-6 vs. cCLP serum, p = 0.033; IL-6 vs. cCLP citrate, p = 0.043; IL-6 vs. CRP, p = 0.015.
Table 4.
Univariate and multivariate logistic regression analysis for the various defined outcomes in all study patients (N = 136). Considering the stepwise approach, the variables ‘BMI’ and ‘number of comorbidities’ were finally not included in the multivariate model for any outcome (p > 0.05).
| OUTCOME 1 |
OUTCOME 2 |
OUTCOME 3 |
OUTCOME 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Univariate analysis |
Univariate analysis |
Univariate analysis |
|||||
| Variable | Odds ratio (95% C.I.) | P-value | Odds ratio (95% C.I.) | P-value | Odds ratio (95% C.I.) | P-value | Odds ratio (95% C.I.) | P-value |
| Age | 0.980 (0.956–1.001) |
0.125 | 0.942 (0.914–0.970) |
<0.001 | 0.953 (0.922–0.984) |
0.004 | 1.072 (1.035–1.110) |
<0.001 |
| Gender | 1.026 (0.516–2.039) |
0.942 | 1.247 (0.627–2.481) |
0.530 | 1.517 (0.633–3.632) |
0.350 | 1.283 (0.610–2.697) |
0.512 |
| BMI | 1.029 (0.980–1.081) |
0.251 | 1.088 (1.027–1.152) |
0.004 | 1.058 (1.000–1.118) |
0.049 | 0.970 (0.917–1.026) |
0.280 |
| Number of comorbidities* | 0.975 (0.792–1.200) |
0.810 | 0.959 (0.779–1.181) |
0.692 | 0.799 (0.600–1.065) |
0.125 | 1.378 (1.087–1.746) |
0.008 |
| Multivariate analysis | Multivariate analysis | Multivariate analysis | Multivariate analysis | |||||
| Variable | Odds ratio (95% C.I.) | P-value | Odds ratio (95% C.I.) | P-value | Odds ratio (95% C.I.) | P-value | Odds ratio (95% C.I.) | P-value |
| Age | – | – | 0.944 (0.915–0.973) |
<0.001 | 0.953 (0.920–0.986) |
0.006 | 1.071 (1.034–1.109) |
<0.001 |
| cCLP SERUM | 1.191 (1.090–1.302) | <0.001 | 1.082 (1.028–1.139) |
0.002 | 1.043 (1.008–1.080) |
0.017 | – | – |
| Age | – | – | 0.938 (0.910–0.967) |
<0.001 | 0.949 (0.916–0.983) |
0.004 | 1.071 (1.034–4.110) |
<0.001 |
| cCLP HEPARIN | 1.151 (1.076–1.232) |
<0.001 | 1.060 (1.010–1.113) |
0.018 | 1.046 (1.005–1.088) |
0.026 | – | – |
| Age | – | – | 0.954 (0.924–0.985) |
0.004 | – | – | 1.084 (1.040–1.131) |
<0.001 |
| cCLP EDTA | 1.368 (1.190–1.573) |
<0.001 | 1.251 (1.115–1.402) |
<0.001 | 1.167 (1.050–1.297) |
0.004 | – | – |
| Age | – | – | 0.955 (0.926–0.986) |
0.005 | 0.961 (0.923–1.000) |
0.049 | 1.062 (1.024–1.100) |
0.001 |
| cCLP CITRATE | 1.455 (1.245–1.701) |
<0.001 | 1.263 (1.125–1.418) |
<0.001 | 1.163 (1.046–1.292) |
0.005 | – | – |
* Included comorbidities were cardiac disease, hypertension, chronic obstructive pulmonary disease, cerebrovascular disease, chronic kidney disease, diabetes mellitus, immunodeficiency, cancer, smoking, auto-immune disease.
Subsequently, cCLP in all matrices was significantly higher in patients requiring ICU admission compared to those treated at a non-ICU ward (Table 2; Supplemental Data, Fig. S2). When comparing cCLP values between non-ICU study patients and ICU study patients who were transferred from a non-ICU ward to the ICU ward (N = 28/66 study ICU patients), cCLP was significantly elevated in the latter group when measured in EDTA and citrate, but not in serum or heparin (data not shown). Comparison of AUROC revealed a significantly higher discriminatory power for cCLP in EDTA (0.780 [0.688–0.873]) and cCLP in citrate (0.765 [0.670–0.861]) compared to cCLP in heparin (0.684 [0.577–0.791]) (Fig. 3, Table 3). Multivariate logistic regression adjusted for demographic confounders showed a significant association between cCLP and the need for ICU admission in all matrices (Table 4).
Similar results were obtained regarding the need for mechanical ventilation or ECMO: cCLP in all matrices was significantly higher in these patients compared to patients without need for mechanical ventiatlion (Table 2; Supplemental Data, Fig. S3). The discriminatory power of cCLP in EDTA (0.842 [0.758–0.925]) and cCLP in citrate (0.828 [0.737–0.920]) was higher compared to cCLP in heparin (0.760 [0.632–0.888]) (Fig. 3, Table 3). Multivariate analysis confirmed this significant association in all matrices (Table 4).
Finally, study patients who deceased within 30 days did not show a significant higher cCLP concentrations compared to patients who were discharged or still hospitalized within a 30-day follow-up period (Table 2; Supplemental Data, Fig. S4). The 4C-mortality score outperformed cCLP and all other inflammatory biomarkers in AUROC analysis (0.713 [0.608–0.818]) (Fig. 3, Table 3). Even when patients who were admitted to the geriatric ward were excluded (n = 36/136), cCLP was not significantly higher in patients deceased within 30 days (data not shown). However, baseline cCLP concentrations in patient discharged after 30 days were significantly lower compared to patients deceased or still hospitalized after 30 days when measured in citrate and EDTA, but not when measured in serum or heparin.
4. Discussion
Circulating calprotectin has been identified as one of the strongest predictors of COVID-19 disease severity by independent studies which analyzed thousands of expressed genes [10]. Comparative transcriptome analysis identified S100A8 and S100A9 as exclusively up-regulated genes in SARS-CoV-2 infection among human lung epithelial cells infected with respiratory viruses [8]. Next to gene expression studies, several small [18], [19], [20] and larger [21], [22], [23] patient cohort studies confirmed a prognostic role for this biomarker to predict disease severity and outcome.
In our study cohort of 136 COVID-19 positive patients, high cCLP concentrations at time of ED admission were significantly associated with severe or critical disease stage, the need for ICU admission and the need for mechanical ventilation or ECMO. Regarding the fourth defined outcome, cCLP concentrations at time of ED admission were not significantly higher in patients deceased within 30 days compared to patients discharged or still hospitalized after that time period. However, de Guadiana Ramuldo et al. [19] (n = 66 COVID-19 patients), Ducastel et al. [24] (n = 160 COVID-19 patients) and Chen et al. [21] (n = 121 COVID-19 patients) did find a good discriminatory capacity of cCLP to predict mortality (AUC: 0.801, 0.792, 0.875 respectively).
Given the high concentration of calprotectin in the cytoplasm of neutrophils and monocytes, our data seem to support the role of these leucocytes in severe COVID-19 cases. Predominantly, CLP is secreted through an active, calcium dependent Protein Kinase C (PKC) pathway. To a lesser extent however, cCLP passively leaks from necrotic cells and is also released in neutrophil extracellular traps (NETs) [25]. NETs are highly efficient in trapping, neutralizing and killing viruses and bacteria [26], but, when not properly regulated, are also known for its pathogenic role in various thrombo-inflammatory states including respiratory failure [27]. Interestingly, a recent study discovered NETs in postmortem lung specimens of COVID-19 patients, especially in the airway compartment and neutrophil-rich inflammatory areas of the interstitium [10], [28]. Thus, cCLP could act as a surrogate marker for NET formation associated with severe pulmonary complications in COVID-19.
Although the prognostic role of cCLP seems promising, its measurement in blood is hampered by crucial pre-analytical requirements [29], [30], sample matrix differences [12] and inter-assay variations [31]. First, comparing serum to plasma matrices, it is expected that in vitro coagulation induces release of intracellular CLP [32] which would result in higher cCLP concentrations in the former matrix. Indeed, as shown in studies on reference values [12] and in our own study cohort (Supplemental Data, S5), serum cCLP values were significantly higher compared to cCLP values when measured in EDTA or citrate plasma. In addition, cCLP values in heparin were significantly higher compared to cCLP values in EDTA and citrate. This indicates that the chelating properties of EDTA and the binding capacities of citrate to calcium prevent monocytes from PKC activation and therefore from further release of cCLP in vitro. Interestingly, cCLP values in heparin plasma were also significantly lower compared to serum in the control populations, but not in our study populations (Fig. 1). This indicates that at lower cCLP values, the role of in vitro coagulation is even more pronounced compared to in higher cCLP values.
Next to the nominal differences in cCLP concentration between matrices, AUROC analyses showed a marked difference in discriminating capacity of cCLP measured in different matrices. In all three outcomes with significant higher cCLP values (Table 2), cCLP in heparin showed lowest AUROC (Table 3), followed by serum and finally by EDTA/citrate. These data suggest that heparin plasma is not the preferred matrix to measure cCLP in this context.
The different concentrations of cCLP when measured in different matrices hampers the comparison of various studies on the prognostic value of cCLP. Udeh et al. [33] recently performed a systematic review and meta-analysis to evaluate cCLP differences between severe and non-severe COVID-19 cases. The authors included five studies that investigated the prognostic role of cCLP and combined them all as cCLP measured in serum (“Mean [serum] CLP in severe cases vs. non-severe cases was 7.425 µg/mL resp. 3.823 µg/mL”). However, a personal review of the included publications showed that only 2/5 used serum as sample matrix [18], [21], 1/5 EDTA plasma [23], 1/5 both serum and plasma [22] and 1/5 studies did not specify the matrix used [19]. As our study points out that cCLP results of different matrices cannot be used interchangeably, authors should specify the exact matrix used and refer to matrix specific reference values [12], [31] in order to allow and ease the comparison and interpretation of findings presented in the respective publications.
The data obtained in our study and control population can be useful in defining which matrix is most suitable for cCLP measurement, also outside the context of COVID-19. To the best of our knowledge, no other data of large patient cohorts in which different matrices are evaluated are available. Our data suggests that cCLP measurement in serum, EDTA and citrate plasma are most valuable, but results between serum and these plasma matrices cannot be interpreted interchangeably. To enable the introduction of cCLP in routine care, implementation on a random-access analyzer and reimbursement is warranted.
Some limitations of our study need to be highlighted. First, no viral or bacterial respiratory disease control group (SARS-CoV-2 negative) was included, which could have been useful to investigate if cCLP can also be used as a diagnostic tool too. Next, as our study lacks complete data on hematological parameters, we were not able to correlate neutrophil counts with cCLP levels.
In conclusion, inflammatory biomarkers can be useful tools in early triage and risk stratification of patients presenting with COVID-19 at the ED. As shown in our study cohort among others, cCLP has a high power to discriminate severe or critical COVID-19 cases vs. patients presenting with asymptomatic, mild or moderate disease, to predict the need for ICU admission and the need for mechanical ventilation or ECMO. However, this discriminatory capacity was lower when cCLP was measured in heparin plasma compared to cCLP measured in serum, EDTA or citrate plasma. Regarding the need for ICU admission and the need for mechanical ventilation/ECMO, cCLP measured in EDTA or citrate plasma showed a higher discriminatory capacity compared to CRP, IL-6, procalcitonin and LDH. Clinicians and researchers investigating cCLP should be aware of variations in cCLP levels when measured in different matrices, which may lead to divergent conclusions in predefined study objectives.
CRediT authorship contribution statement
Louis Nevejan: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft. Thomas Strypens: Conceptualization, Investigation, Resources, Writing – original draft. Mathias Van Nieuwenhove: Conceptualization, Investigation, Resources, Writing – review & editing. An Boel: Conceptualization, Methodology, Writing – review & editing. Lien Cattoir: Conceptualization, Methodology, Writing – review & editing. Peter Meeus: Conceptualization, Writing – review & editing. Xavier Bossuyt: Writing – review & editing, Supervision. Nikolaas De Neve: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision. Lieve Van Hoovels: Conceptualization, Methodology, Formal analysis, Resources, Data curation, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge SVDBB and LH for their technical assistance in performing the analyses, next to Thermo Fisher for sponsoring the calprotectin, IL-6 and PCT reagents. We also thank TN and GVB from Leuven Biostatistics and Statistical Bioinformatics Centre (L-BioStat) for their assistance and guidance in statistical analyses
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2021.12.011.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Krause P.R., Fleming T.R., Longini I.M., Peto R., Briand S., Heymann D.L., Beral V., Snape M.D., Rees H., Ropero A.-M., Balicer R.D., Cramer J.P., Muñoz-Fontela C., Gruber M., Gaspar R., Singh J.A., Subbarao K., Van Kerkhove M.D., Swaminathan S., Ryan M.J., Henao-Restrepo A.-M. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021;385(2):179–186. doi: 10.1056/NEJMsr2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.L. Wynants, B. Van Calster, G.S. Collins, R.D. Riley, G. Heinze, E. Schuit, M.M.J. Bonten, J.A.A. Damen, T.P.A. Debray, M. De Vos, P. Dhiman, M.C. Haller, M.O. Harhay, L. Henckaerts, N. Kreuzberger, A. Lohmann, K. Luijken, J. Ma, C.L. Andaur Navarro, J.B. Reitsma, J.C. Sergeant, C. Shi, N. Skoetz, L.J.M. Smits, K.I.E. Snell, M. Sperrin, R. Spijker, E.W. Steyerberg, T. Takada, S.M.J. Van Kuijk, F.S. Van Royen, C. Wallisch, L. Hooft, K.G.M. Moons, M. Van Smeden, Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal, BMJ. 369 (2020). https://doi.org/10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed]
- 3.Jukic A., Bakiri L., Wagner E.F., Tilg H., Adolph T.E. Calprotectin: From biomarker to biological function. Gut. 2021;70(10):1978–1988. doi: 10.1136/gutjnl-2021-324855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma L., Sun P., Zhang J.C., Zhang Q., Yao S.L. Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int. J. Mol. Med. 2017;40:31–38. doi: 10.3892/ijmm.2017.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb C.A., Kennedy N.A., Raine T., Hendy P.A., Smith P.J., Limdi J.K., Hayee B., Lomer M.C.E., Parkes G.C., Selinger C., Barrett K.J., Davies R.J., Bennett C., Gittens S., Dunlop M.G., Faiz O., Fraser A., Garrick V., Johnston P.D., Parkes M., Sanderson J., Terry H., Gaya D.R., Iqbal T.H., Taylor S.A., Smith M., Brookes M., Hansen R., Hawthorne A.B. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Chen W., Lin J. The role of calprotectin in rheumatoid arthritis. J. Transl. Intern. Med. 2020;7:126–131. doi: 10.2478/jtim-2019-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopeć-Mȩdrek M., Widuchowska M., Kucharz E.J. Calprotectin in rheumatic diseases: A review. Reumatologia. 2016;54:306–309. doi: 10.5114/reum.2016.64907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrashekar D.S., Athar M., Manne U., Varambally S. Comparative transcriptome analyses reveal genes associated with SARS-CoV-2 infection of human lung epithelial cells. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-95733-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotsiou O.S., Papagiannis D., Papadopoulou R., Gourgoulianis K.I. Calprotectin in lung diseases. Int. J. Mol. Sci. 2021;22:1–24. doi: 10.3390/ijms22041706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahler M., Meroni P.-L., Infantino M., Buhler K.A., Fritzler M.J. Circulating Calprotectin as a Biomarker of COVID-19 Severity. Expert Rev. Clin. Immunol. 2021;17(5):431–443. doi: 10.1080/1744666X.2021.1905526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen L., Birkemose E., Gils C., Safi S., Nybo M. Sample type and storage conditions affect calprotectin measurements in blood. J. Appl. Lab. Med. An AACC Publ. 2018;2(6):851–856. doi: 10.1373/jalm.2017.024778. [DOI] [PubMed] [Google Scholar]
- 12.M. Mylemans, L. Nevejan, S. Van Den Bremt, M. Stubbe, B. Vander Cruyssen, C. Moulakakis, H. Berthold, C. Konrad, X. Bossuyt, L. Van Hoovels, Circulating calprotectin as biomarker in neutrophil-related inflammation: Pre-analytical recommendations and reference values according to sample type, Clin. Chim. Acta. 517 (2021) 149–155. https://doi.org/10.1016/j.cca.2021.02.022. [DOI] [PubMed]
- 13.Nordal H.H., Fagerhol M.K., Halse A.-K., Hammer H.B. Calprotectin (S100A8/A9) should preferably be measured in EDTA-plasma; results from a longitudinal study of patients with rheumatoid arthritis. Scand. J. Clin Lab. Invest. 2018;78(1-2):102–108. doi: 10.1080/00365513.2017.1419371. [DOI] [PubMed] [Google Scholar]
- 14.Malham M., Carlsen K., Riis L., Paerregaard A., Vind I., Fenger M., Wewer V. Plasma calprotectin is superior to serum calprotectin as a biomarker of intestinal inflammation in ulcerative Colitis. Scand. J. Gastroenterol. 2019;54(10):1214–1219. doi: 10.1080/00365521.2019.1665097. [DOI] [PubMed] [Google Scholar]
- 15.WHO, Clinical Management of COVID-19, 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19.
- 16.NIH, Clinical Spectrum of SARS-CoV-2 Infection, December 17, 2020, 2020. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/#:∼:text=Patients with COVID-19 are, may experience rapid clinical deterioration (accessed January 28, 2021).
- 17.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., Dunning J., Fairfield C.J., Gamble C., Green C.A., Gupta R., Halpin S., Hardwick H.E., Holden K.A., Horby P.W., Jackson C., McLean K.A., Merson L., Nguyen-Van-Tam J.S., Norman L., Noursadeghi M., Olliaro P.L., Pritchard M.G., Russell C.D., Shaw C.A., Sheikh A., Solomon T., Sudlow C., Swann O.V., Turtle L.C.W., Openshaw P.J.M., Baillie J.K., Semple M.G., Docherty A.B., Harrison E.M. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:1–13. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer W., Diehl-Wiesenecker E., Ulke J., Galtung N., Havelka A., Hegel J.K., Tauber R., Somasundaram R., Kappert K. Outcome prediction by serum calprotectin in patients with COVID-19 in the emergency department. J. Infect. 2021;82(4):84–123. doi: 10.1016/j.jinf.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Guadiana Romualdo Luis García, Mulero M.D.R., Olivo M.H., Rojas C.R., Arenas V.R., Morales M.G., Abellán A.B., Conesa-Zamora P., García-García J., Hernández A.C., Morell-García D., Dolores Albaladejo-Otón M., Consuegra-Sánchez L. Circulating levels of GDF-15 and calprotectin for prediction of in-hospital mortality in COVID-19 patients: A case series. J. Infect. 2021;82(2):e40–e42. doi: 10.1016/j.jinf.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaya T., Yaylacı S., Nalbant A., Yıldırım İ., Kocayiğit H., Çokluk E., Şekeroğlu M.R., Köroğlu M., Güçlü E. Serum calprotectin as a novel biomarker for severity of COVID-19 disease. Ir. J. Med. Sci. 2021 doi: 10.1007/s11845-021-02565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y., Wei J., Luo H., Zhu H., Huang L., Meng F., Huang L., Wang N.a., Zhou X., Zhao L., Chen X., Mao Z., Chen C., Li Z., Sun Z., Zhao J., Wang D., Huang G., Wang W., Zhou J. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(9):992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H., Zuo Y.u., Yalavarthi S., Gockman K., Zuo M., Madison J.A., Blair C., Woodward W., Lezak S.P., Lugogo N.L., Woods R.J., Lood C., Knight J.S., Kanthi Y. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J. Leukoc. Biol. 2021;109(1):67–72. doi: 10.1002/JLB.3COVCRA0720-359R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvin A., Chapuis N., Dunsmore G., Goubet A.-G., Dubuisson A., Derosa L., Almire C., Hénon C., Kosmider O., Droin N., Rameau P., Catelain C., Alfaro A., Dussiau C., Friedrich C., Sourdeau E., Marin N., Szwebel T.-A., Cantin D., Mouthon L., Borderie D., Deloger M., Bredel D., Mouraud S., Drubay D., Andrieu M., Lhonneur A.-S., Saada V., Stoclin A., Willekens C., Pommeret F., Griscelli F., Ng L.G., Zhang Z., Bost P., Amit I., Barlesi F., Marabelle A., Pène F., Gachot B., André F., Zitvogel L., Ginhoux F., Fontenay M., Solary E. Elevated Calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182(6):1401–1418.e18. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducastel M., Chenevier-gobeaux C., Ballaa Y., Meritet J.F., Brack M., Chapuis N., Pene F., Carlier N., Szwebel T.A., Roche N., Terrier B., Borderie D. Oxidative stress and inflammatory biomarkers for the prediction of severity and icu admission in unselected patients hospitalized with covid-19. Int. J. Mol. Sci. 2021;22:1–12. doi: 10.3390/ijms22147462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ometto F., Friso L., Astorri D., Botsios C., Raffeiner B., Punzi L., Doria A. Calprotectin in rheumatic diseases. Exp. Biol. Med. 2017;242(8):859–873. doi: 10.1177/1535370216681551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuffrè M., Vetrugno L., Di Bella S., Moretti R., Berretti D., Crocè L.S. Calprotectin and SARS-CoV-2: A brief-report of the current literature. Healthc. 2021;9:1–8. doi: 10.3390/healthcare9080956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. Cells. 2020;9:1–11. doi: 10.3390/cells9061494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radermecker C., Detrembleur N., Guiot J., Cavalier E., Henket M., d’Emal C., Vanwinge C., Cataldo D., Oury C., Delvenne P., Marichal T. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020;217 doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.L. Van Hoovels, B. Vander Cruyssen, L. Bogaert, S. Van Den Bremt, X. Bossuyt, Pre-analytical and analytical confounders of serum calprotectin as a biomarker in rheumatoid arthritis, Clin. Chem. Lab. Med. 58 (2019) 40–49. https://doi.org/10.1515/cclm-2019-0508. [DOI] [PubMed]
- 30.Infantino M., Manfredi M., Albesa R., Grossi V., Lari B., Benucci M., Gobbi F.L., Matucci A., Sarra F., Mahler M. Critical role of pre-analytical aspects for the measurement of circulating calprotectin in serum or plasma as a biomarker for neutrophil-related inflammation. Clin. Chem. Lab. Med. 2021;59:E317–E321. doi: 10.1515/cclm-2021-0172. [DOI] [PubMed] [Google Scholar]
- 31.L. Nevejan, M. Mylemans, B. Vander Cruyssen, M. Stubbe, S. Van Den Bremt, L. Hofman, M. Infantino, M. Manfredi, Pre-analytical recommendations and reference values for circulating calprotection are sample type and assay dependent, Clin. Chem. Lab. Med. (2021), doi: 10.1515/cclm-2021-0998. [DOI] [PubMed]
- 32.I. Dale, Plasma levels of the calcium-binding LI leukocyte protein: Standardization of blood collection and evaluation of reference intervals in healthy controls, Scand. J. Clin. Lab. Invest. 50 (1990) 837–841. https://doi.org/10.3109/00365519009104950. [DOI] [PubMed]
- 33.Udeh R., Advani S., de Guadiana Romualdo L.G., Dolja-Gore X. Calprotectin, an emerging biomarker of interest in covid-19: A systematic review and meta-analysis. J Clin. Med. 2021;10(4):775. doi: 10.3390/jcm10040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.