Abstract
Rationale
The impact of palliative care consultation on end-of-life care has not previously been evaluated in a multi-center study.
Objectives
To evaluate the impact of palliative care consultation on the incidence of cardiopulmonary resuscitation (CPR) performed and comfort care received at the end-of-life in hospitalized patients with COVID-19.
Methods
We used the Society of Critical Care Medicine’s COVID-19 registry to extract clinical data on patients hospitalized with COVID-19 between March 31st, 2020 to March 17th, 2021 and died during their hospitalization. The proportion of patients who received palliative care consultation was assessed in patients who did and did not receive CPR (primary outcome) and comfort care (secondary outcome). Propensity matching was used to account for potential confounding variables.
Measurements and Main Results
3,227 patients were included in the analysis. There was no significant difference in the incidence of palliative care consultation between the CPR and no-CPR groups (19.9% vs. 19.4%, p = 0.8334). Patients who received comfort care at the end-of-life were significantly more likely to have received palliative care consultation (43.3% vs. 7.7%, p < 0.0001). After propensity matching for comfort care on demographic characteristics and comorbidities, this relationship was still significant (43.2% vs. 8.5%; p < 0.0001).
Conclusion
Palliative care consultation was not associated with CPR performed at the end-of-life but was associated with increased incidence of comfort care being utilized. These results suggest that utilizing palliative care consultation at the end-of-life may better align the needs and values of patients with the care they receive.
Keywords: Palliative care, Cardiopulmonary resuscitation, Comfort care, End of life, COVID-19
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated disease (COVID-19) was first reported in Wuhan, China in 2019 before it spread to cause a worldwide pandemic. COVID-19 has resulted in significant morbidity and mortality. Large epidemiologic studies of COVID-19 patients report case fatality rates as high as 15%-19% among hospitalized patients.1., 2. These case fatality rates have been reported to be higher in older patients and in those with comorbidities such as cardiovascular disease and chronic pulmonary disease.1., 3. The Viral Infection and Respiratory Illness Universal Study (VIRUS)’s registry cohort was used to assess 20,608 patients who were hospitalized for COVID-19 infection between February 15, 2020 and November 30, 2020 at 168 medical centers and reported 49.8% in-hospital mortality in patients requiring mechanical ventilation which increased to 71.6% in-hospital mortality in patients receiving mechanical ventilation, vasoactive medication infusions, and new renal replacement therapy.4
Hospital palliative care teams are designed to enhance holistic and compassionate care to patients at all stages of potentially life-limiting diseases.5 During the COVID-19 pandemic, hospital systems have leveraged the expertise of palliative care specialists in assisting with symptom management, in emotionally supporting medically isolated, and, thus, also socially isolated patients, and in facilitating conversations between patients with COVID-19 infection, their families, and the medical teams caring for them.6., 7. Palliative care team consultation has been associated with a decreased incidence of CPR at the end of life in cancer patients8 and in medical intensive care unit (ICU) patients,9 and is also associated with increased incidence of implementation of comfort care measures in these patient groups.10 A systematic review of heterogeneous palliative care interventions found that palliative care team involvement increases transition to do-not-resuscitate (DNR) code status and speeds transition to comfort-focused measures without impacting overall mortality in ICU patients.11 Two small retrospective studies conducted at New York hospitals early in the COVID-19 pandemic found that palliative care team consultation resulted in an increased number of patients opting for DNR code status and decreased the incidence of CPR.12., 13.
The NIH’s National Institute on Aging describes the primary goal of comfort care measures to be to “prevent or relieve suffering as much as possible and to improve quality of life while respecting the dying person's wishes.14 Comfort care plans for critically ill patients may vary institution-to-institution, but generally these plans include protocols to reduce pain, fever, dyspnea, xerostomia, nausea, constipation, and anxiety15 as well as to plan in advance and to then provide the social, psychological, and spiritual care that is tailored to the individual patient in order to mitigate distress at the end of life. For these reasons, routine consultation of the palliative care service has been advocated for hospitalized COVID-19 patients by several reviews published during the COVID-19 pandemic.16., 17., 18.
There is a need for multi-center assessment of the association between palliative care team consultation and occurrence of end-of-life approaches such as initiation of CPR in patients who are hospitalized and critically ill with COVID-19 infection. We therefore use the VIRUS study’s large multi-center registry of hospitalized COVID-19 patients to assess patients who died during primary COVID-19 hospitalization at hospitals within the United States. We use this resource to test the primary study hypothesis that palliative care team consultation provided to hospitalized COVID-19 patients who die in-hospital is associated with significantly decreased incidence of CPR. We also use the VIRUS COVID-19 registry data to assess an exploratory secondary hypothesis that palliative care team consultation during patient hospitalization for COVID-19 infection is associated with significantly increased implementation of comfort care measures prior to in-hospital death.
Methods
VIRUS registry
The SCCM Discovery VIRUS COVID-19 registry was established as an international, multi-center registry of COVID-19 patients in March 2020.19., 20. From March 31, 2020 to March 17th, 2021, the registry included 29,768 patients with complete data, originating from 168 hospitals in 18 countries. The VIRUS COVID-19 registry study was reviewed by the Mayo Clinic (Rochester, MN)’s institutional review board (IRB) and was granted a waiver of need for patients’ informed consents because of its observational chart review design and its de-identified neutralized database structure. The study is registered on Clinicaltrials.gov: NCT04323787. Participating study sites additionally obtained site specific institutional review board (IRB) approvals and implemented data use agreements with the Mayo Clinic coordinating study site before data were collected. Each participating site enters de-identified data into the registry’s centralized study database maintained at Mayo Clinic Rochester, MN. Study data were stored and managed using Research Electronic Data Capture System (REDCap) tools hosted at the Mayo Clinic. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.21., 22.
The VIRUS COVID-19 registry contains data regarding hospitalized COVID-19 patients’ demographics, presenting comorbidities, hospitalization information regarding medical management, and in-hospital outcomes in patients admitted with COVID-19 infection. Furthermore, for COVID-19 patients who died during primary hospitalization, the database includes whether CPR measures were undertaken in-hospital before death and whether comfort care measures were provided before in-hospital death. These end-of-life intervention data were not collected for patients who did not die during primary hospitalization for COVID-19 infection.
Study cohort
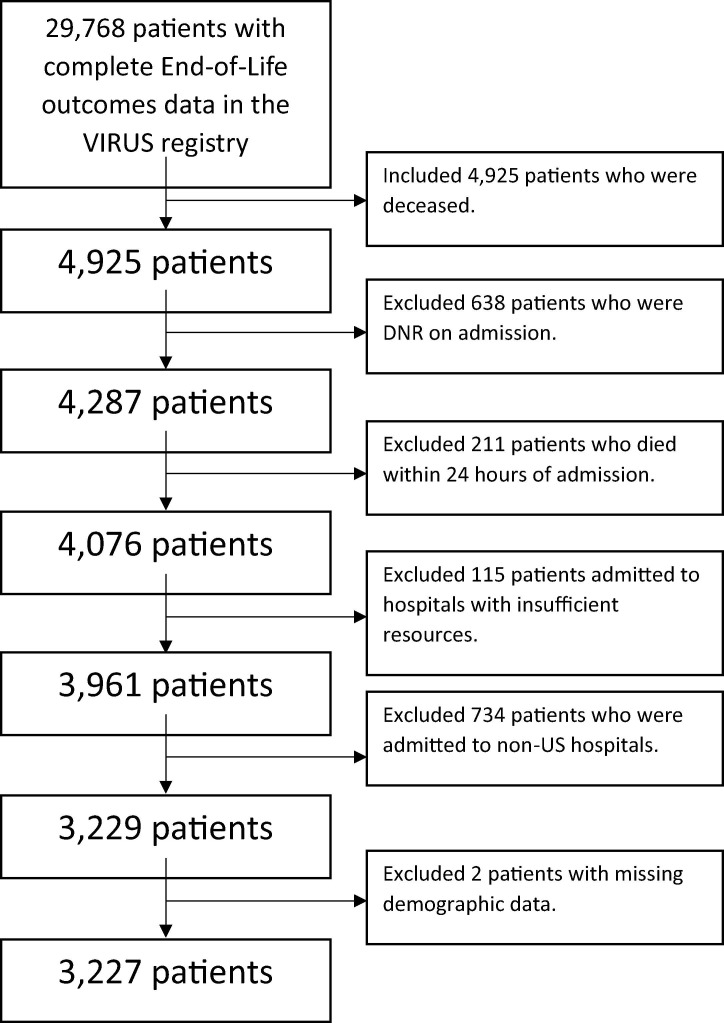
Of the 29,768 unique patients included in the VIRUS COVID-19 registry’s database as of March 17th, 2021, we identified a cohort of 3,227 (16.5% of overall registry) patients who were ≥18 years old, who were admitted to hospitals within the United States, who died during their primary hospitalization, and who did not meet the exclusion criteria. The registry did not collect data regarding discharge of patients to in-patient hospice. Patients were excluded from study analyses if they had do-not-resuscitate (DNR) code status existing on primary COVID-19 hospital admission, died within 24 hours of primary hospital admission, or were admitted to hospitals that reported in the registry database that they had insufficient bed capacity, ventilators, personnel, or personal protective equipment (PPE) during the time of a patient’s primary COVID-19 hospitalization (Fig. 1 ). Patients admitted to hospitals which utilized co-ventilation (i.e., the practice of ventilating multiple patients using a single ventilator) were also excluded as per the criteria of insufficient ventilator availability. We excluded patients hospitalized outside the United States because of variability in the availability, funding and cultural views affecting palliative care utilization.
Fig. 1.
Flow Chart Describing the Application of Inclusion and Exclusion Criteria.
Patient data
Data including demographics (e.g., age, gender, race), presenting co-morbidities (e.g., hypertension, diabetes), in-hospital clinical events (e.g., need for new dialysis for renal failure, need for intubation and mechanical ventilation), critical care interventions, and intensive care unit and hospital lengths of stay were collected at all study sites using a standardized case report form.
The primary study outcome was defined a priori as initiation of CPR in response to cardiac or pulmonary arrest at the end of life. This primary outcome definition included provision of intubation, chest compressions, electrical cardioversion therapies, and/or ACLS drug administration performed in code setting. The study’s secondary outcome was defined a priori as implementation of comfort care measures. The comfort care outcome included in the registry’s case report form was entered by each site as per that site’s interpretation.
Statistical analyses
Data analyses were performed using SAS 9.4 (SAS Institute, USA). Statistical significance was defined a priori as a two-sided p value < 0.05. Variables that involve continuous data are reported as means and standard deviations and were compared using the Student t-test. Categorical variables are presented as frequencies and proportions and compared using a Chi-square test.
For both the study’s primary and secondary outcomes, separate propensity score matching was used to 1:1 case: control match patients who did and did not receive CPR and who did and did not receive comfort care measures, respectively. Propensity score matched analyses were performed in order to account for potential confounding variables in the association between the primary study predictor of palliative care consultations and the study outcomes.
Propensity score matching included demographic and clinical characteristics that prior studies, or the consensus opinion of the clinical critical care and palliative care authors thought influence the association between palliative care consultation and the two study outcomes. For CPR, the variables included in propensity score matching included age by year, race (categorized into white, black and other), sex, hypertension, diabetes, chronic kidney disease and cancer. Among these, age was found to be an independent risk factor for CPR in a multivariable analysis of 5,019 critically ill COVID-19 patients.23 The study also found a significantly higher incidence of CPR in patients with hypertension in univariate analysis. The remaining variables: race, sex, chronic kidney disease and cancer were selected for propensity score matching based upon the opinion of the palliative care physician (AB) author on this manuscript.
For comfort care, the variables used for propensity score matching included age by year, sex, race (white, black, other), pre-admission congestive heart failure, chronic pulmonary disease, dialysis-dependent kidney failure and cancer, and the presence of two or more of the pre-admission comorbidities of congestive heart failure, pulmonary disease, dialysis dependent kidney disease and cancer. Because risk factors for comfort care implementation have not previously been reported, we queried our palliative care investigator (AB) to determine these factors. To check the success of the propensity score matching, univariate analyses were repeated in the propensity score matched cohort, and no measure used in propensity score matching was significantly different between groups at the p < 0.01 threshold.
Results
Overall study cohort
As outlined in Fig. 1, after implementing inclusion and exclusion criteria, there were 3,229 registry patients who died after 24 hours into primary hospitalization for COVID-19. Two patients were excluded for missing demographic variables used for propensity matching, resulting in 3,227 patients being included in the study analysis. The average age for the study cohort was 70 ± 13 years old. 61.6% of the cohort were men and 38.4 % were women. 59.7% were white or Caucasian, 25.9% were black or African American, and 14.5% percent of the study cohort were reported to be of other races.
Cardiopulmonary resuscitation (non-propensity matched comparisons)
Table 1 shows demographic, clinical and hospitalization characteristics for the patients who did and who did not undergo CPR at the end of life. 317 of these patients underwent CPR (9.8%) at the end of life, and 2,910 (90.2%) of these patients did not undergo CPR at the end of life (Table 1). There was no significant difference in the incidence of palliative care consultation between the CPR and no-CPR groups (19.9% of patients who underwent CPR at the EOL received a palliative care consultation, compared to 19.4% of patients who did not receive CPR; p = 0.8334). There was no significant difference in sex distribution between the groups. Patients who underwent CPR were significantly younger than those who did not undergo CPR (mean age 64 years versus 71 years, p < 0.0001). There were also significant differences in the racial distribution between the CPR and no-CPR groups, with a greater proportion of black patients in the CPR group than the non-CPR group. The percentages of presenting comorbidities for the patients in the CPR and no-CPR groups are also shown in Table 1. Patients who underwent CPR were significantly more likely to have hypertension, diabetes, and dialysis dependent kidney failure and cancer.
Table 1.
Patient and Hospitalization Characteristics of COVID-19 Patients Who Died During Primary Hospitalization Who Did and Not Receive CPR (Non-Propensity Score Matched and Propensity Score Matched). Chronic Pulmonary Disease does not include Asthma. * Indicates a variable used for propensity matching. SD: standard deviation, y: years.
| Non-Propensity Matched (n = 3227) |
Propensity Matched (n = 490) |
||||||
|---|---|---|---|---|---|---|---|
| CPR (n = 317) | No CPR (n = 2910) | p-value | CPR (n = 245) | No CPR (n = 245) | p-value | ||
| Demographics | Demographics | ||||||
| Age, mean ± SD, y* | 64.3 ± 14 | 71.0 ± 13 | < 0.0001 | *Age, mean ± SD, y* | 65.6 ± 13 | 65.9.0 ± 13 | 0.7657 |
| Sex | *Sex | ||||||
| Male | 206 (65.0%) | 1751 (61.2%) | 0.1915 | Male | 163 (66.5%) | 170 (69.4%) | 0.4980 |
| Female | 111 (35.0%) | 1109 (38.8%) | Female | 82 (33.5%) | 75 (30.6%) | ||
| Race | *Race | ||||||
| Black | 125 (39.4%) | 711 (24.4%) | <0.0001 | Black | 84 (34.3%) | 89 (36.3%) | 0.6006 |
| White | 157 (49.5%) | 1768 (60.8%) | White | 129 (52.7%) | 131 (53.5%) | ||
| Other | 35 (11.0%) | 431 (14.8%) | Other | 32 (13.1%) | 25 (10.2%) | ||
| Comorbidities | Comorbidities | ||||||
| Hypertension | 222 (70.0%) | 1784 (61.3%) | 0.0024 | *Hypertension | 174 (71.0%) | 166 (67.8%) | 0.4329 |
| Diabetes | 161 (50.8%) | 1158 (39.8%) | 0.0002 | *Diabetes | 124 (50.6%) | 115 (46.9%) | 0.4160 |
| Chronic Pulmonary Disease | 53 (16.7%) | 605 (20.8%) | 0.0876 | *Chronic Pulmonary Disease | 33 (13.5%) | 33 (13.5%) | >0.9999 |
| Congestive Heart Failure | 47 (14.8%) | 472 (16.2%) | 0.5213 | *Congestive Heart Failure | 29 (11.8%) | 20 (8.2%) | 0.1753 |
| Chronic Kidney Disease | 67 (21.1%) | 640 (22.0%) | 0.7260 | *Chronic Kidney Disease | 40 (16.3%) | 31 (12.7 %) | 0.2481 |
| Dialysis-Dependent Kidney Failure | 23 (7.3%) | 118 (4.1%) | 0.0081 | Dialysis-Dependent Kidney Failure | 14 (5.7%) | 12 (4.9%) | 0.6869 |
| Stroke | 30 (9.5%) | 395 (13.6%) | 0.0399 | Stroke | 24 (9.8%) | 28 (11.4%) | 0.5574 |
| Cancer | 22 (6.9%) | 337 (11.6%) | 0.0126 | *Cancer | 14 (5.7%) | 8 (3.3%) | 0.1906 |
| Outcomes | Outcomes | ||||||
| Palliative Care Consultation | 63 (19.9%) | 564 (19.4%) | 0.8334 | Palliative Care Consultation | 45 (18.4%) | 53 (21.6%) | 0.3663 |
Cardiopulmonary resuscitation (propensity matched comparisons)
There were 245 patients in the study cohort who were analyzed after using propensity score matching to achieve one to one matching between the groups that received CPR and those who did not. As with the non-propensity score matched cohort, there was no significant difference in the incidence of palliative care consultation between the CPR and no CPR groups. 18.4% of patients (45/245) in the CPR group underwent palliative care consultation versus 21.6% of patients (53/245) in the group that did not receive CPR (p = 0.3663).
Comfort care (non-propensity matched comparisons)
Table 2 compares demographic, clinical and hospitalization parameters between the groups of patients who did and did not receive comfort care at end of life. Among our 3227 study patients, 1063 patients received comfort care measures (33.1%) and 2164 patients did not (67.1%). Patients who received comfort care at the EOL were significantly more likely to have received palliative care consultation (43.4% vs. 7.7%, p < 0.0001). There was no significant difference in the age or sex distribution between the groups of patients who did and did not receive comfort care measures. There were significant differences in racial distribution between the comfort care and non-comfort care groups, with a smaller proportion of White patients in the comfort care group compared to the non-comfort care group. Patients who received comfort care measures were also significantly more likely to have pre-hospitalization hypertension, diabetes, stroke or cancer than those who did not.
Table 2.
Patient and Hospitalization Characteristics of COVID-19 Patients Who Died During Primary Hospitalization Who Did and Not Receive Comfort Care (Non-Propensity Score Matched and Propensity Score Matched). Chronic Pulmonary Disease does not include Asthma. * Indicates a variable used for propensity matching; SD: standard deviation, y: years, CHF: Congestive Heart Failure, CPD: Chronic Pulmonary Disease, ESRD: Dialysis-Dependent Kidney Failure.
| Non-Propensity Matched (n = 3227) |
Propensity Matched (n = 1790) |
||||||
|---|---|---|---|---|---|---|---|
| Comfort Care (n = 1063) | No Comfort Care (n = 2164) | p-value | Comfort Care (n = 895) | No Comfort Care (n = 895) | p-value | ||
| Demographics | Demographics | ||||||
| Age, mean ± SD, y* | 70.7 ± 12 | 70.1 ± 14 | 0.2133 | *Age, mean ± SD, y* | 70.7 ± 12 | 70.5 ± 12 | 0.6301 |
| Sex | *Sex | ||||||
| Male | 652 (61.4%) | 1305 (61.7%) | 0.8661 | Male | 552 (61.7%) | 575 (64.3%) | 0.2603 |
| Female | 410 (38.6%) | 810 (38.3%) | Female | 343 (38.3%) | 320 (35.8%) | ||
| Race | *Race | ||||||
| Black | 282(26.5%) | 554 (25.6%) | <0.0001 | Black | 217 (24.3%) | 226 (25.3%) | 0.7152 |
| White | 586 (55.1%) | 1339 (61.9%) | White | 530 (59.2%) | 513 (57.3%) | ||
| Other | 195 (18.3%) | 271 (12.5%) | Other | 148 (16.5%) | 156 (17.4%) | ||
| Comorbidities | Comorbidities | ||||||
| Hypertension | 755 (71.0%) | 1251 (57.8%) | <0.0001 | Hypertension | 627 (70.1%) | 528 (59.0%) | <0.0001 |
| Diabetes | 505 (47.5%) | 814 (37.6%) | <0.0001 | Diabetes | 418 (46.7%) | 349 (39.0%) | 0.0010 |
| Chronic Pulmonary Disease | 230 (21.6%) | 428 (19.8%) | 0.2181 | *Chronic Pulmonary Disease | 170 (19.0%) | 156 (17.4%) | 0.3912 |
| Congestive Heart Failure | 173 (16.3%) | 346 (16.0%) | 0.8355 | *Congestive Heart Failure | 121 (13.5%) | 102 (11.4%) | 0.1739 |
| Chronic Kidney Disease | 248 (23.3%) | 459 (21.2%) | 0.1713 | Chronic Kidney Disease | 198 (22.1%) | 159 (17.8%) | 0.0211 |
| Dialysis-Dependent Kidney Failure | 43 (4.1%) | 98 (4.5%) | 0.5277 | *Dialysis-Dependent Kidney Failure | 20 (2.2%) | 19 (2.1%) | 0.8714 |
| Stroke | 166 (15.6%) | 259 (12.0%) | 0.0040 | Stroke | 132 (14.8%) | 95 (10.6%) | 0.0086 |
| Cancer | 136 (12.8%) | 223 (10.3%) | 0.0346 | *Cancer | 92 (10.3%) | 75 (8.7%) | 0.1671 |
| ≥ 2 of CHF, CPD, Cancer, Dialysis | 143 (13.5%) | 256 (11.8%) | 0.1882 | *≥ 2 of CHF, CPD, Cancer, ESRD | 94 (10.5%) | 78 (8.7%) | 0.1994 |
| Outcomes | Outcomes | ||||||
| Palliative Care Consultation | 461 (43.4%) | 166 (7.7%) | <0.0001 | Palliative Care Consultation | 387 (43.2%) | 76 (8.5%) | <0.0001 |
Comfort care (propensity matched comparisons)
There were 1,790 patients in the study cohort who were analyzed after using propensity score matching. Propensity score matching was utilized to achieve one to one matching between the group of patients who received comfort care measures and the group of patients who that did not receive comfort care measures. As with the non-propensity score matched cohort, patients who received comfort care at the EOL were significantly more likely to have received palliative care consultation. (Table 2; 43.2% of the patients (387/895) who received comfort care at the EOL received a palliative care consultation versus 8.5% (76/895) in the non-comfort care group; p < 0.0001).
Discussion
Our analysis of data submitted to the VIRUS COVID-19 Registry by US centers found that palliative care consultation does not reduce the incidence of CPR performed at the EOL. Previous studies reported the association between palliative consultation and reduced frequency of CPR.8., 9., 12., 13. These studies have tended to be single-center studies focused on a specific subset of patients or have been systematic reviews comparing heterogenous study designs and interventions. Our study provides evidence against this association using a large, multi-center cohort and standardized data collection.
Our study also confirmed the association between palliative care service consultation and a higher incidence of comfort care initiation, which has been previously reported in other critically ill medical cohorts, but not in hospitalized patients with COVID-19.10., 24. Comfort care is widely accepted as an alternative to aggressive therapies in critically ill patients. While most physicians are well equipped to discuss life-sustaining treatments for critical illnesses, alternatives to these treatments, including comfort-oriented options, are less frequently discussed.25 Palliative care physicians are trained to ascertain the patient’s goals and create a comfort care plan that align these goals with the expected prognosis and treatments that are provided. A comfort care plan is individualized to each patient and may include a time-limited trial of medical treatments or may focus entirely on the alleviation of distressing symptoms. Implementation of comfort care plans has been associated with better patient and family satisfaction as well as significantly lower health care costs.24., 26.
Propensity matching was used to ensure that patients did not receive comfort care because of decisions made prior to their hospitalization with COVID-19. The variables used in the propensity model included demographics as well as severe comorbidities – congestive heart failure, non-asthmatic chronic pulmonary disease, dialysis-dependent kidney failure and cancer. We also ensured that the groups were matched for patients with two or more of the above comorbidities, as these patients may be more likely to have considered comfort care options prior to admission.
Our study revealed racial differences in the provision of CPR, with black patients comprising a higher proportion of the CPR group than the no-CPR group. While racial differences in care was not the focus of this study, this finding is consistent with previous epidemiologic studies27., 28., 29. and may be explained by lower rates of DNR orders30., 31. and greater utilization of life-sustaining treatments32 by black patients. While these discrepancies maybe explained at least partially by socioeconomic, cultural and religious differences, studies have also shown that black patients are also less likely to receive full prognostic information when discussing end-of-life care from physicians.33., 34. These findings suggest the need for future studies that are designed to clearly determine the causes and consequences of racial differences in CPR provision.
Strengths and limitations
Our study’s greatest strength is its large, multi-center dataset containing end-of-life outcomes in deceased COVID-19 patients. The data available in the registry was collected in real time throughout the pandemic, contributing to its credibility. Our large sample size allowed us to exclude patients who had a do-not-resuscitate code status at the time of hospital admission as well as those who died within 24 hours of hospital admission. The latter criteria were used because we wanted to ensure that our included patients had a reasonable chance of receiving a palliative care consultation after hospital admission. Because the VIRUS COVID-19 registry captured detailed information on resource shortages which were common during the pandemic, we were able to exclude patients admitted to hospitals while they were experiencing shortages in beds, PPE, medical personnel, or ventilators. This is because ethical guidelines published during the pandemic recommended that resource constraints should be taken into consideration when discussing EOL decisions with patients and against the provision of CPR when PPE, ventilators, and critical care beds are unavailable.35., 36.
Our study was limited by the data that was available in the VIRUS COVID-19 registry. Data regarding whether patients received palliative care consultation and data for the study’s outcomes of whether CPR was performed and whether comfort care was provided was only collected for patients who died during primary hospitalization. As a result, we could not evaluate the impact of palliative care consultation on patients who were discharged alive from the hospital and received palliative therapies at home, such as home hospice. The registry also did not collect information on whether patients were discharged to in-patient hospice. Because the registry did not prioritize the collection of the timing of palliative care consultation, we were unable to analyze the impact of palliative care timing and frequency on our outcomes of interest. Another limitation of our study was that the comfort care outcome was not explicitly defined by the registry and left to the interpretation of the clinical site providing the data. This is particularly important because there are no universally accepted definitions for the provision of comfort care.
In conclusion, while our study did not find a significant association between palliative care team consultation and implementation of CPR, our study did find a strong association between palliative care consultation and implementation of comfort care measures. Palliative care consultation in patients hospitalized with COVID-19 appears to increase implementation of individualized comfort care measures in hospitalized COVID-19 patients who died during primary hospitalization for severe COVID-19 infection.
Funding
This publication was supported by NIH/NCRR/NCATS CTSA Grant Number UL1 TR002377. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The registry is funded in part by the Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC. They had no influence on analysis, interpretation and reporting of pooled data.
CRediT authorship contribution statement
Sreekanth R. Cheruku: Conceptualization, Methodology, Investigation, Writing – original draft, Visualization. Alexis Barina: Conceptualization, Methodology, Writing – review & editing. Corey D. Kershaw: Writing – review & editing. Kristina Goff: Writing – review & editing. Joan Reisch: Formal analysis. Linda S. Hynan: Formal analysis. Farzin Ahmed: Data curation, Project administration. Donna Lee Armaignac: Resources, Data curation. Love Patel: Resources, Data curation. Katherine A. Belden: Resources, Data curation. Margit Kaufman: Resources, Data curation. Amy B. Christie: Resources, Data curation. Neha Deo: Project administration, Data curation, Resources. Vikas Bansal: Software, Project administration, Data curation, Resources. Karen Boman: Project administration, Data curation, Resources. Vishakha K. Kumar: Software, Project administration, Data curation, Resources. Allan Walkey: Project administration, Data curation, Resources. Rahul Kashyap: Project administration, Data curation, Resources, Funding acquisition. Ognjen Gajic: Project administration, Data curation, Resources. Amanda A. Fox: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Kumar is currently funded by funding the Gordon and Betty Moore Foundation, Centers for Disease Control and Prevention Foundation through the University of Washington, and Janssen Research & Development, LLC.
Dr. Kashyap receives funding from the NIH/National Heart, Lung and Blood Institute: R01HL 130881, UG3/UH3HL 141722; Gordon and Betty Moore Foundation and Janssen Research & Development, LLC; and royalties from Ambient Clinical Analytics. Inc.
Dr. Gajic receives funding from the Agency of Healthcare Research and Quality R18HS 26609-2, NIH/National Heart, Lung and Blood Institute: R01HL 130881, UG3/UH3HL 141722; Department of Defense DOD W81XWH; American Heart Association Rapid Response Grant—COVID-19; and royalties from Ambient Clinical Analytics. Inc.
Dr. Walkey currently receives funding from the NIH/National Heart, Lung and Blood Institute grants R01HL151607, R01HL139751, R01HL136660, Agency of Healthcare Research and Quality, R01HS026485, Boston Biomedical Innovation Center/NIH/NHLBI 5U54HL119145-07, and royalties from UpToDate.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resuscitation.2021.12.011.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Cates J., Lucero-Obusan C., Dahl R.M., et al. Risk for In-Hospital Complications Associated with COVID-19 and Influenza—Veterans Health Administration, United States, October 1, 2018–May 31, 2020. Morbid Mortal Week Rep. 2020;69:1528. doi: 10.15585/mmwr.mm6942e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 4.Domecq J.P., Lal A., Sheldrick C.R., et al. Outcomes of Patients With Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med. 2021;49:437–448. doi: 10.1097/CCM.0000000000004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ting R., Edmonds P., Higginson I.J., Sleeman K.E. Palliative care for patients with severe covid-19. bmj. 2020;370 doi: 10.1136/bmj.m2710. [DOI] [PubMed] [Google Scholar]
- 6.Ferrell B.R., Handzo G., Picchi T., Puchalski C., Rosa W.E. The urgency of spiritual care: COVID-19 and the critical need for whole-person palliation. J Pain Symptom Manage. 2020;60:e7–e11. doi: 10.1016/j.jpainsymman.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etkind S.N., Bone A.E., Lovell N., et al. The role and response of palliative care and hospice services in epidemics and pandemics: a rapid review to inform practice during the COVID-19 pandemic. J Pain Symptom Manage. 2020;60:e31–e40. doi: 10.1016/j.jpainsymman.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao C.-Y., Wang H.-M., Tang S.-C., et al. Predictive factors for do-not-resuscitate designation among terminally ill cancer patients receiving care from a palliative care consultation service. J Pain Symptom Manage. 2014;47:271–282. doi: 10.1016/j.jpainsymman.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Ma J., Chi S., Buettner B., et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019;47:1707–1715. doi: 10.1097/CCM.0000000000004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierucci R.L., Kirby R.S., Leuthner S.R. End-of-life care for neonates and infants: the experience and effects of a palliative care consultation service. Pediatrics. 2001;108:653–660. doi: 10.1542/peds.108.3.653. [DOI] [PubMed] [Google Scholar]
- 11.Aslakson R., Cheng J., Vollenweider D., Galusca D., Smith T.J., Pronovost P.J. Evidence-based palliative care in the intensive care unit: a systematic review of interventions. J Palliat Medcine. 2014;17:219–235. doi: 10.1089/jpm.2013.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., Abrukin L., Flores S., et al. Early Intervention of Palliative Care in the Emergency Department During the COVID-19 Pandemic. JAMA Intern Med. 2020;180(9):1252–1254. doi: 10.1001/jamainternmed.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obata R., Maeda T., Rizk D., Kuno T. Palliative care team involvement in patients with COVID-19 in New York city. Am J Hosp Palliat Med. 2020;37:869–872. doi: 10.1177/1049909120940986. [DOI] [PubMed] [Google Scholar]
- 14.NIA. Providing Care and Comfort at the End of Life. National Institute on Aging. (Accessed 10 March, 2021, at https://www.nia.nih.gov/health/providing-comfort-end-life. Updated 2017, May 17).
- 15.Blinderman C.D., Billings J.A. Comfort care for patients dying in the hospital. N Engl J Med. 2015;373:2549–2561. doi: 10.1056/NEJMra1411746. [DOI] [PubMed] [Google Scholar]
- 16.Fausto J., Hirano L., Lam D., et al. Creating a palliative care Inpatient Response plan for COVID19–the UW medicine experience. J Pain Symptom Manage. 2020;60(1):e21–e26. doi: 10.1016/j.jpainsymman.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercadante S., Adile C., Ferrera P., Giuliana F., Terruso L., Piccione T. Palliative care in the time of COVID-19. J Pain Symptom Manage. 2020;60(2):e79–e80. doi: 10.1016/j.jpainsymman.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen D.J., Ekström M., Currow D.C., et al. COVID-19: guidance on palliative care from a European Respiratory Society international task force. Eur Respir J. 2020;56 doi: 10.1183/13993003.02583-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walkey A.J., Kumar V.K., Harhay M.O., et al. The viral infection and respiratory illness universal study (VIRUS): an international registry of coronavirus 2019-related critical illness. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walkey A.J., Sheldrick R.C., Kashyap R., et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: The Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med. 2020;48:e1038. doi: 10.1097/CCM.0000000000004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayek S.S., Brenner S.K., Azam T.U., et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. bmj. 2020;371 doi: 10.1136/bmj.m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson L.C., Usher B., Spragens L., Bernard S. Clinical and economic impact of palliative care consultation. J Pain Symptom Manage. 2008;35:340–346. doi: 10.1016/j.jpainsymman.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Gattellari M., Voigt K.J., Butow P.N., Tattersall M.H. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol. 2002;20:503–513. doi: 10.1200/JCO.2002.20.2.503. [DOI] [PubMed] [Google Scholar]
- 26.Teno J.M., Fisher E.S., Hamel M.B., Coppola K., Dawson N.V. Medical care inconsistent with patients' treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc. 2002;50:496–500. doi: 10.1046/j.1532-5415.2002.50116.x. [DOI] [PubMed] [Google Scholar]
- 27.Ehlenbach W.J., Barnato A.E., Curtis J.R., et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361:22–31. doi: 10.1056/NEJMoa0810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazaure H.S., Roman S.A., Sosa J.A. Epidemiology and outcomes of in-hospital cardiopulmonary resuscitation in the United States, 2000–2009. Resuscitation. 2013;84:1255–1260. doi: 10.1016/j.resuscitation.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Wong S.P., Kreuter W., Curtis J.R., Hall Y.N., O’Hare A.M. Trends in in-hospital cardiopulmonary resuscitation and survival in adults receiving maintenance dialysis. JAMA Inter Med. 2015;175:1028–1035. doi: 10.1001/jamainternmed.2015.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy C.R., Fish R., Kramer A. Do-not-resuscitate and do-not-hospitalize directives of persons admitted to skilled nursing facilities under the Medicare benefit. J Am Geriatr Soc. 2005;53:2060–2068. doi: 10.1111/j.1532-5415.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- 31.Zweig S.C., Kruse R.L., Binder E.F., Szafara K.L., Mehr D.R. Effect of do-not-resuscitate orders on hospitalization of nursing home residents evaluated for lower respiratory infections. J Am Geriatr Soc. 2004;52:51–58. doi: 10.1111/j.1532-5415.2004.52010.x. [DOI] [PubMed] [Google Scholar]
- 32.Barnato A.E., Chang C.-C.-H., Saynina O., Garber A.M. Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med. 2007;22:338–345. doi: 10.1007/s11606-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry L.M., Walsh L.E., Horswell R., et al. Racial disparities in end-of-life care between black and white adults with metastatic cancer. J Pain Sympt Manage. 2021;61 doi: 10.1016/j.jpainsymman.2020.09.017. 342.e341–9.e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon H.S., Street R.L., Jr, Sharf B.F., Souchek J. Racial differences in doctors' information-giving and patients' participation. Cancer. 2006;107:1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 35.Kramer D.B., Lo B., Dickert N.W. CPR in the COVID-19 era—an ethical framework. N Engl J Med. 2020;383:e6. doi: 10.1056/NEJMp2010758. [DOI] [PubMed] [Google Scholar]
- 36.Sher T., Burger C.D., DeMartino E.S., de Moraes A.G., Sharp R.R. Paper presented at: Mayo Clin Proc. 2020. Resuscitation and COVID-19: Recalibrating Patient and Family Expectations During a Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.