Abstract
The receptor activator of nuclear factor kappa-B ligand (RANKL)-RANK-osteoprotegerin (OPG) system is critical to bone homeostasis, but genetically deficient mouse models have revealed important roles in the immune system as well. RANKL-RANK-OPG is particularly important to T cell biology because of its organogenic control of thymic development and secondary lymphoid tissues influence central T cell tolerance and peripheral T cell function. RANKL-RANK-OPG cytokine-receptor interactions are often controlled by regulation of expression of RANKL on developing T cells, which interacts with RANK expressed on some lymphoid tissue cells to stimulate key downstream signaling pathways that affect critical tuning functions of the T cell compartment, like cell survival and antigen presentation. Activation of peripheral T cells is regulated by RANKL-enhanced dendritic cell survival, and dysregulation of the RANKL-RANK-OPG system in this context is associated with loss of T cell tolerance and autoimmune disease. Given its broader implications for immune homeostasis and osteoimmunology, it is critical to further understand how the RANKL-RANK-OPG system operates in T cell biology.
Keywords: mTEC, AIRE, TRANCE, ODF, RANK-L, TNFSF11, TNFRSF11
Introduction
The RANKL-RANK-OPG system consists of the tumor necrosis factor superfamily (TNFSF) cytokine RANKL (receptor activator of nuclear factor kappa-B ligand; additionally identified as TRANCE, ODF, CD254 and TNFSF11), and the TNF receptor superfamily (TNFRSF) members RANK, a signaling receptor, and osteoprotegerin (OPG), a soluble decoy receptor for RANKL [1]. RANKL-RANK-OPG was discovered through parallel efforts beginning more than 20 years ago that were aimed at identifying elements of importance to immune and bone homeostasis. Genetically-deficient mouse models have revealed critical roles for RANKL-RANK-OPG in bone, and increasingly in the immune system as well. In particular, the roles of RANKL-RANK-OPG in T cell biology, which we aim to summarize in this review, intricately connect (1) organogenesis of the primary and secondary lymphoid tissues where T cells develop, reside, and function; and (2) peripheral activation, differentiation and tolerance of T cells.
T cells are critical mediators of cell-mediated immunity that derive from hematopoietic lineage lymphoid precursor cells that transit from the bone to the thymus where they undergo further differentiation and thymic education [2]. Thymic education, involving both positive and negative selection in response to self-antigens is essential to generating a repertoire of T cells with broad responsiveness while also establishing central tolerance to self. Upon leaving the thymus, T cells inhabit secondary lymphoid tissues where they are exposed, in the presence of other specialized immune cells, to antigens draining from the peripheral tissues that may induce productive immune responses or promote peripheral tolerance [3]. The nature of T cell activity in secondary lymphoid tissues is largely determined by interactions between T cells and professional antigen-presenting dendritic cells (DCs), which modulate their activating versus tolerizing impulses not only through pattern recognition receptor signaling but also through cytokine receptor signaling. If cytokine production is not properly regulated or if cytokine receptor signaling is defective, aberrant and possibly pathologic T cell differentiation and/or activation may occur. The RANKL-RANK-OPG system is critical at various steps of T cell selection and differentiation, primarily via regulation of critical cellular components of the lymphoid tissues that make possible the development, maintenance, and activation of a functional T cell compartment. These cytokine-receptor interactions are often controlled by regulation of expression of RANKL on developing T cells, which interacts with RANK expressed on key interacting lymphoid tissue cells to stimulate downstream signaling pathways that affect critical tuning functions of the T cell compartment, like cell survival and antigen presentation. Dysregulation of the RANKL-RANK-OPG system in this context is associated with loss of T cell tolerance and risk of autoimmune disease. Given its broader implications for immune homeostasis and osteoimmunology, it is critical to further understand how the RANKL-RANK-OPG system operates in T cell biology.
RANKL-RANK-OPG in the thymus
After leaving the bone marrow, hematopoietic stem cells migrate to the thymus to undergo positive and negative selection. First, CD4+CD8+ double-positive (DP) thymocytes in the thymic cortex that encounter low-affinity self-peptide–MHC complexes presented by cortical thymic epithelial cells (cTECs) are positively selected and differentiate into CD4+CD8− or CD4−CD8+ single-positive (SP) thymocytes [2, 4]. SP thymocytes migrate to the thymic medulla where they are presented self-antigens by various antigen-presenting cells (APCs), including medullary thymic epithelial cells (mTECs) [2, 4]. SP thymocytes with T cell receptors (TCRs) that exhibit high-affinity interactions with self-antigen are either negatively selected via apoptosis or induced to become regulatory T cells (Tregs) [2, 4]. These elements of thymic education are the basis for establishing central tolerance, which makes it possible to generate a T cell repertoire capable of recognizing a vast array of foreign antigens while lacking potentially pathological sensitivity to virtually all antigens produced by self-tissues, even tissues distal to the thymus. The fact that negative selection can be accomplished efficiently within a unitary primary lymphoid organ, the thymus, is owed in part to the specialized capacity of mTECs to express and present a uniquely broad array of self-antigens called tissue-restricted antigens (TRAs) [also called tissue-specific antigens (TSAs)]. DCs can facilitate negative selection of thymocytes by cross-presentation of extracellular TRAs initially expressed by AIRE+ mTECs [5]. The mechanism of promiscuous gene expression by which TRAs are produced requires expression of the critical transcription factors autoimmune regulator (AIRE) [6] and Fezf2 [7]. AIRE induction and maintenance in developing and maturing mTECs is dependent on the provision of RANKL, while the Fezf2 pathway appears to be RANKL-RANK-independent. Two related TNFSF members, lymphotoxin αβ (LTαβ) and CD40L, induce mTEC AIRE expression as well. The cells required for the expression of RANKL, LTαβ and CD40L, and the specific requirements for each cytokine, differ depending on the ontogenic stage of thymic development, with major shifts occurring from the embryonic to postnatal stages [5] (Fig. 1). During embryogenesis, at a phase before the emergence of SP thymocytes, RANKL-expressing C D3−CD4+IL-7Rα+ lymphoid tissue inducer (LTi) cells control formation of early AIRE+ mTECs through RANK activation [8]. Early mTECs and cTECs both derive from common progenitors during embryonic and postnatal thymic development, but only embryonic mTEC precursor cells that express cTEC markers, as well as UEA-1 ligand, require TRAF6-dependent RANKL-RANK-mediated activation of the non-canonical NF-κB pathway to induce AIRE induction [9]. Consistent with these findings, mice deficient for RANK or RANKL lack AIRE+ mTECs in the embryonic thymus [8, 10]. In addition to LTi cells, RANKL-expressing invariant Vγ5 TCR-expressing dendritic epidermal T cell (DETC) progenitors also contribute to the emergence of AIRE+ mTECs in the embryonic thymus. DETCs contribute to central T cell tolerance by providing RANKL that promotes the conversion of CD80−Aire−mTECs to CD80+Aire+ mTECs through RANK signaling [8, 11].
Fig. 1.
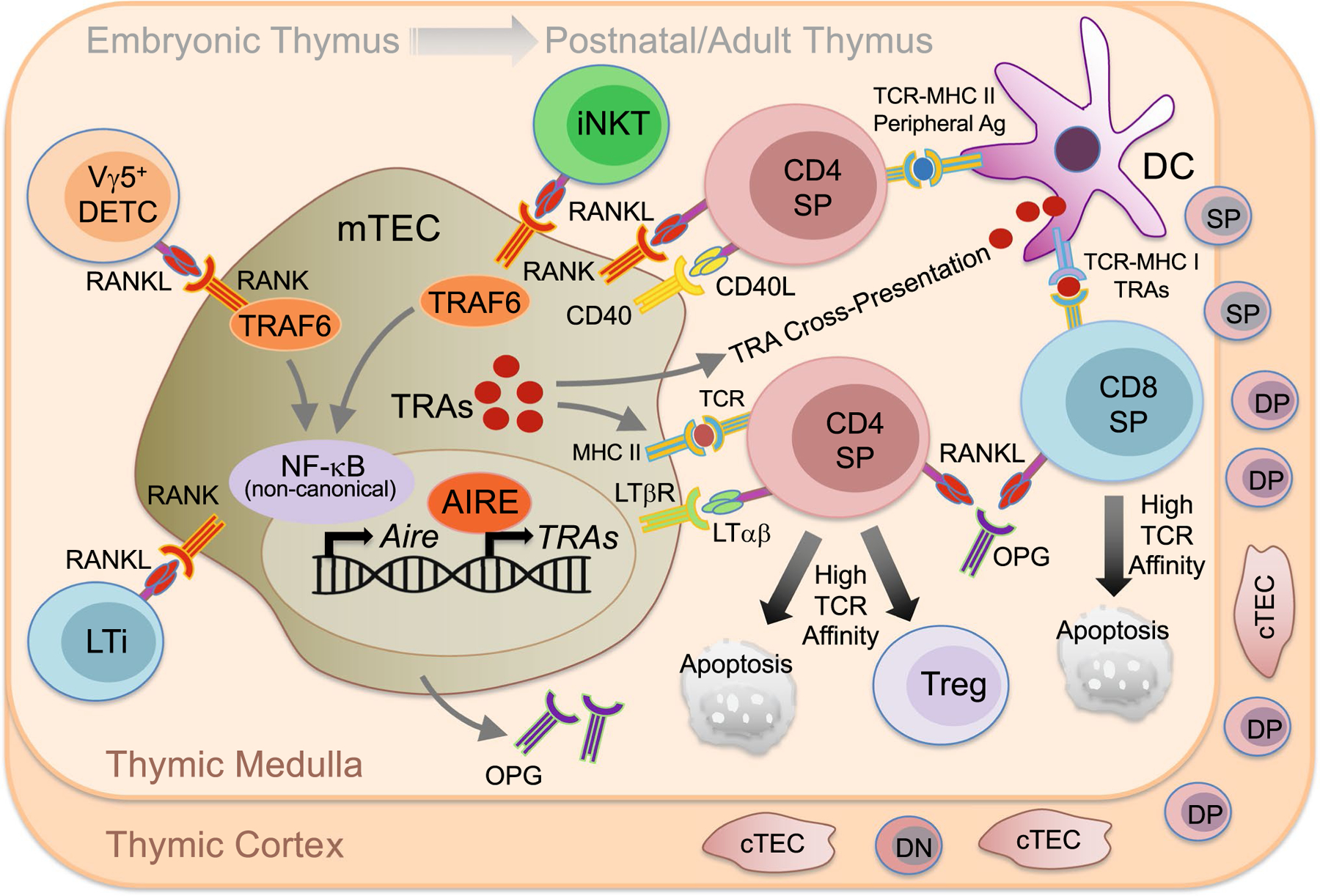
RANKL-RANK-OPG function in the thymus. Embryonic medullary thymic epithelial cell (mTEC) precursors require TRAF6-dependent RANKL-RANK-mediated activation of the non-canonical NF-κB pathway to induce expression of the mTEC transcription factor autoimmune regulator (AIRE). During embryogenesis, RANKL-expressing CD3−CD4+IL-7Rα+ lymphoid tissue inducer (LTi) cells and invariant Vγ5 TCR-expressing dendritic epidermal T cell (DETC) progenitors control early formation of AIRE+ mTECs. In the postnatal/adult thymus, double negative (DN) thymocytes acquire a CD4+CD8+ double positive (DP) phenotype in the thymic cortex, interact with cortical thymic epithelial cells (cTECs) during positive selection and migrate to the thymic medulla as CD4+CD8− or CD4−CD8+ single-positive (SP) thymocytes. In the postnatal/adult thymic medulla, mTEC AIRE is induced by RANKL, as well as the other TNFSF member CD40L and LTαβ, expressed by TCRαβ high CD4+ SP thymocytes. RANKL is expressed at lower levels by CD8+ SP thymocytes, and RANKL-expressing invariant NKT cells also contribute to AIRE+ mTEC differentiation. mTECs negatively regulate RANKL-mediated AIRE induction via expression and secretion of the inhibitor OPG. Negative selective of SP thymocytes is determined by presentation, through MHC-TCR interactions, of peripheral self-antigens (Ag) and tissue-restricted antigens (TRAs) by mTECs and DCs. TRA presentation requires promiscuous gene expression by AIRE+ mTECs and subsequent DCs cross-presentation of mTEC-expressed TRAs. SP thymocytes that experience high-affinity TCR interactions with peripheral self-antigen or TRAs are either negatively selected via apoptosis or induced to become regulatory T cells (Tregs)
While LTi- and invariant Vγ5+ DETC progenitor-expressed RANKL is exclusively required for embryonic AIRE induction, postnatal mTEC AIRE is induced by RANKL and CD40L expressed by TCRαβhighCD4+ thymocytes [12, 13]. Furthermore, while mice lacking RANK or CD40 alone exhibit only partial defects in adult AIRE+ mTECs, mice doubly deficient in both RANK and CD40 exhibit more severe defects, suggesting RANK and CD40 have overlapping requirements in the adult thymus [5]. In addition to roles for RANKL and CD40L, positively-selected thymocytes expressing LTαβ contribute to postnatal mTEC maturation associated with AIRE and involucrin expression by triggering LTβR on immature mTECs [14]. However, while the relative physiologic requirement for RANK-RANKL is reduced for postnatal versus embryonic mTEC development, antibody blocking experiments revealed a surprisingly important role for RANK signaling during repopulation of adult mTECs following short-term AIRE-DTR-mediated ablation [15].
It is of further interest to determine the cellular sources of RANKL and other cytokines relevant to postnatal mTEC differentiation. For example, RANKL is expressed by both CD4+ and CD8+ thymocytes, with higher expression by CD4+ thymocytes [12], while CD40L is expressed only by CD4+ thymocytes [16, 17]. Invariant natural killer T (iNKT) cells also express RANKL to promote AIRE+ mTEC differentiation in postnatal and adult mice and may function cooperatively with RANKL-expressing SP thymocytes in this context [18]. In addition to mature SP thymocytes, activated T cells can recirculate through the thymus and contribute RANKL to mTEC maturation [19]. It has also been shown that RANKL is upregulated in both CD4+ thymocytes and LTi cells during early thymic regeneration following cytoablative treatment, and that RANKL-GST treatment during bone marrow transplantation boosts production by LTi cells of LTαβ, which leads to enhanced mTEC and cTEC regeneration, thymus homing of lymphoid progenitors, and de novo thymopoiesis [20]. Expression of soluble RANKL has also been detected in the postnatal thymus under normal conditions, but mice with specific deficiency in soluble but not membrane-bound RANKL show no defect in AIRE+ mTECs, suggesting that soluble RANKL is dispensable during development [21]. However, there appears to be a physiologic role for a soluble negative regulator of RANKL function, as OPG is expressed by mTECs and mice deficient for OPG exhibit enlarged medulla with increased AIRE+ mTECs [16, 22].
Some studies have shown that mTECs contribute to the selection and survival of immunosuppressive Foxp3+ regulatory T cells (Tregs) [23, 24], though there still remains uncertainty about the degree to which mTEC-expressed TRAs drive thymic Treg (tTreg) differentiation [25]. In a model of autoimmune skin inflammation, it could be demonstrated that tTreg TCRs are self-reactive, and that generation of these tTregs could be enhanced using OPG-deficient mice or by adding exogenous RANKL to increase the mTEC population [26]. Other work elucidates additional mechanisms for establishing tolerance in the thymus. TGFβR-mediated MAPK signaling in developing thymocytes regulates RANKL expression during negative selection to limit potential autoimmunity. In the absence of TGFβR, potentially autoreactive CXCR3-expressing F oxp3−Helios+ thymocytes accumulate at the cortico-medullary junction and fail to express sufficient levels of RANKL to maintain medullary expression of AIRE and to promote negative selection [27]. These results represent a potential mechanism by which RANKL-RANK controls the development of pathologic autoimmunity, Conversely, because some TRAs expressed by AIRE+ mTECs are tumor antigens, negative selection may inadvertently delete anti-tumor T cells. It was recently shown that mTEC inhibition via blockade of RANKL may represent a viable approach to boosting anti-tumor T cell responses by temporarily disrupting thymic negative selection to TRAs expressed by tumors [28]. Together, these studies highlight the importance of RANKL-RANK signaling to thymic development and T cell selection.
RANKL-RANK-OPG, T cells, and secondary lymphoid tissues
Lymph nodes
Lymph nodes (LN) are secondary lymphoid organs spread throughout the body and connected by the lymphatic vasculature that are critical areas for both T cell activation and peripheral tolerance. Mice lacking RANK or RANKL exhibit an absence of LNs [29, 30]. Considerable work has established a model in which RANKL-RANK signaling and LTαβ-LTβ receptor (LTβR) signaling interact to drive a feedback loop that promotes lymph node organogenesis (Fig. 2a). Initiation of LN formation occurs during embryogenesis with the recruitment of hematopoietic lineage lymphoid tissue inducer (LTi) cells to LN anlagen made up of mesenchymal and endothelial stroma that include lymphoid tissue organizer (LTo) progenitor/precursor cells [31]. LTi cells expressing RANKL, RANK, and LTαβ then interact with LTβR-expressing LTo cells. Activation of LTβR induces LTo cells to mature, increasing expression of RANKL and the chemokine CXCL13, which increases the attraction of LTi cells. LTi then cluster with the LTo cells to initiate LN formation. Since LTo cells may also express RANK, a positive feedback loop can develop involving LTo and LTi cells with RANKL-RANK and LTαβ-LTβR signaling amplifying the recruitment-clustering process of lymph node development [31]. More recent work has further shown how RANKL-RANK signaling contributes to the specialized cells required for LN formation. LTo cells had been thought to be primarily of mesenchymal lineage, however, it has been shown that lymphatic endothelial cells (LECs) that line the LN subcapsular sinus express increased RANK compared to peripheral LECs, and appear to act as another type of LTo that are critical to LTi cell retention during LN organogenesis, as LEC-specific RANK deletion results in defective LTi retention and LN formation [32, 33]. The CCR6+ subset of group 3 innate lymphoid cells (ILC3s), which require the transcription factor RORγt, are termed “LTi-like” cells and are necessary for lymph node development [34]. RORγt-specific deletion of RANK, but not RANKL, resulted in defects in cervical, brachial, and inguinal lymph node development, suggesting that RANK expression by fetal ILC3s contributes to lymph node organogenesis, but that required RANKL may be provided by other cell types [35]. It is clear that RANKL-RANK-OPG signaling is critical to LN development, and future efforts may focus on better understanding how these signaling requirements interact with peripheral T cells function and differentiation.
Fig. 2.
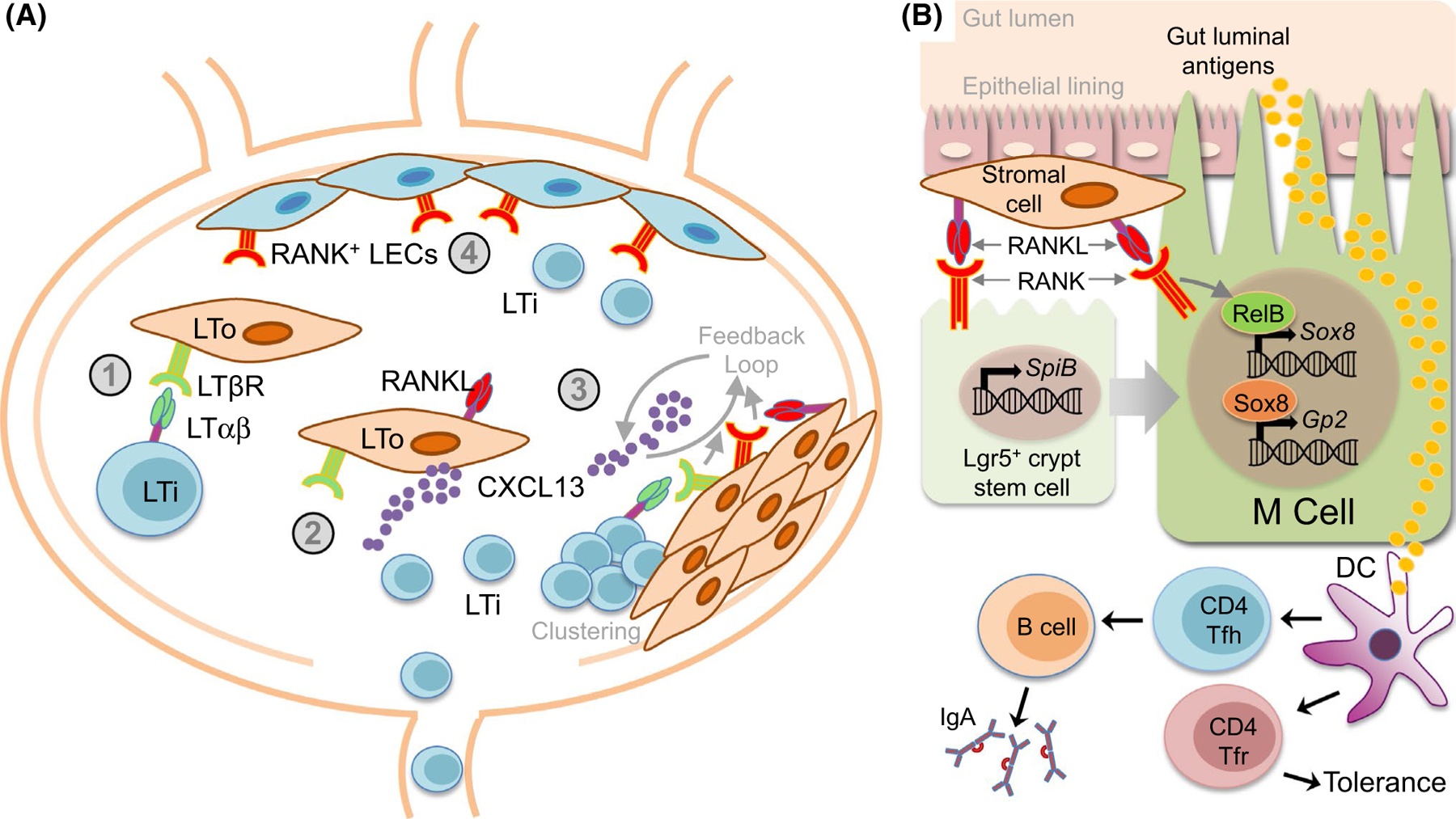
RANKL-RANK-OPG contributes to secondary lymphoid organogenesis. a RANKL-RANK-OPG contributions to lymph node organogenesis. Initiation of lymph node (LN) formation involves recruitment of hematopoietic lineage lymphoid tissue inducer (LTi) cells to LN anlagen that include lymphoid tissue organizer (LTo) progenitor/precursor cells. (1) LTi cells expressing RANKL, RANK, and LTαβ interact with LTβR-expressing LTo cells. (2) Activation of LTβR induces LTo cells to mature, increasing expression of RANKL and the chemokine CXCL13, which increases attraction of more LTi cells. (3) LTi cells then cluster with the LTo cells to initiate LN formation. Since LTo cells may also express RANK, a positive feedback loop develops involving LTo and LTi cells with RANKL-RANK and LTαβ-LTβR signaling amplifying the recruitment-clustering process of lymph node development. (4) RANK-expressing lymphatic endothelial cells (LECs) line the LN subcapsular sinus and act as another type of LTo that are critical to LTi cell retention, via RANKL-RANK interactions, during LN organogenesis. b Peyer’s patch microfold (M) cells. Peyer’s patches (PP) are a gut-associated lymphoid tissue (GALT) found along the small intestine where CD4+ follicular helper T cells (Tfh) are critical components of germinal centers necessary for gut-associated IgA responses. CD4+ follicular regulatory T cells (Tfr) also inhabit PPs to regulate peripheral tolerance. Microfold (M) cells are specialized epithelial cells that transport gut luminal antigens for presentation to PP T cells. RANK signaling is required for M cell differentiation and antigen uptake. RANKL expression by subepithelial stromal cells in PP domes drive M cell differentiation. RANK signaling contributes to the earliest stages of M cell differentiation by inducing expression of the transcription factor SpiB in M cell precursor Lgr5+ intestinal crypt stem cells. RANK-dependent activation of the NF-κB factor RelB is required for expression of the transcription factor Sox8, which then activates the promoter of the key M cell factor Gp2
Peyer’s patches and cryptopatches
Peyer’s patches (PP) are gut-associated lymphoid tissue (GALT) found along the small intestine where CD4+ follicular helper T cells (Tfh) are critical components of germinal centers necessary for gut-associated IgA responses [36]. CD4+ follicular regulatory T cells (Tfr) also inhabit PPs to regulate peripheral tolerance [37]. Microfold cells (M cells) of the PPs are specialized epithelial cells that transport gut luminal antigens, including both those derived from gut microorganisms and tolerance-inducing food antigens, for presentation to PP T cells [38]. Initial characterization of mice deficient for RANK or RANKL revealed severely reduced Peyer’s patch formation [29, 30]. It was further showed that RANK signaling is required for M cell differentiation and antigen uptake, and that while RANK is broadly expressed across small intestine epithelial cells, selective expression of RANKL by subepithelial stromal cells in PP domes drives M cell differentiation [39]. Targeted deletion of mesenchymal RANKL, including in M cell-inducing stromal cells, results in defective M cell formation and antigen sampling that is linked to decreases in IgA production and microbial diversity [40]. At the same time, M cells themselves negatively regulate differentiation of neighboring follicle-associated epithelial cells into M cells through expression of OPG, and OPG-deficient mice exhibit increased M cell numbers and attendant increases in immunoglobulin responses against commensal bacteria [41].
By inducing expression of the Ets family transcription factor SpiB, which is required for M cell development, in M cell precursor L gr5+ intestinal crypt stem cells, RANK signaling contributes to the earliest stages of M cell differentiation [42]. Recently, it has been shown that RANK-dependent activation of the NF-κB factor RelB is required for expression of the M cell-specific SRY-related HMG box transcription factor family member Sox8, which itself drives expression, through direct binding to the promoter, of the key M cell factor Gp2 (Fig. 2b) [43]. Determining local environmental factors that control the levels of RANKL-RANK signaling in the context of M cell differentiation has also been an area of interest. It has been reported that M cell density increases in the presence of Salmonella Typhimurium and the secreted S. Typhimurium type III effector protein SopB, which triggers Wnt/beta-catenin-mediated induction of both RANKL and RANK expression [44]. RANK-dependent RelB activation then promotes epithelial to M cell differentiation via induction of the transcription factor Slug [44]. More recently, it has been shown that the secreted calcium-binding protein S100A4 coordinates with RANKL to further regulate M cell maturation [45].
RANK signaling also affects GALT-associated T cells in cryptopatches (CPs), which are cellular clusters distributed throughout the lamina propria crypts of the small and large intestines that serve as sites of extra-thymic lymphocyte differentiation [46]. RANKL expression on intestinal stromal cells is required for postnatal development of CPs, with RANKL-deficient mice exhibiting severely reduced CP development [47]. Interestingly, the requirement for RANKL expression to drive CXCL13-dependent maturation of CPs is restricted only to CPs found in the small intestine [47]. Additional work will be required to further determine how RANKL-RANK-OPG cooperates with other factors to regulate T cells in the GALT and to more specifically determine how RANK signaling contributes to systemic T cell homeostasis via its role in PP and CP development.
Effects of RANKL-RANK-OPG ON T cell activation and differentiation
T cells that have migrated out of the thymus and to the secondary lymphoid tissues encounter antigens presented by various APCs, including DCs, which, as highly specialized professional APCs, are often the primary determinants of the quantitative and qualitative nature of T cell immune responses [48]. DCs control T cell activation and differentiation by modulating not only levels of TCR-antigen engagement via antigen processing and MHC expression, but also by expressing various costimulatory receptors and cytokines that act on T cells. Though immature DCs are continuously sampling antigens in their local environment, activation of T cells requires DC maturation by an inflammatory signal such as toll-like receptor (TLR) ligands [49] or CD40L [50]. Under inflammatory conditions, DCs undergo maturation that enables them to produce many of the factors that potentiate their capacity to activate T cells. However, once DCs undergo maturation, they may be more susceptible to apoptosis and require pro-survival signals if they are to maintain sufficiently long DC-T cell interactions to productively activate T cells. Some of the earliest characterizations of RANKL function showed that RANKL stimulation of RANK expressed on DCs prolongs DC survival and DC-T cell interactions in vitro [51, 52]. At the same time, RANKL treatment imparts no apparent direct pro-survival or activating effects on T cells [51]. While RANKL expression is not typically detected on resting T cells, anti-CD3 stimulation has been shown to induce surface RANKL expression for up to 4 days on both CD4+ and CD8+ T cells, and anti-CD28 costimulation further enhances RANKL expression, particularly on CD4+ T cells [53]. Therefore, in response to initial TCR activation, T cells may provide RANKL directly to DCs to promote prolonged interaction and enhance activation (Fig. 3). The effect of RANKL on DC stimulatory capacity was observed in adoptively-transferred antigen-pulsed DCs that drove increased T cell primary and memory responses in vivo [54]. The potential adjuvant effect of RANKL on DC-based immunotherapy was demonstrated by the enhanced efficacy of an experimental DNA vaccine when the gene for RANKL was incorporated [55]. Interestingly, this effect was specific to RANKL and did not occur when CD40L was used.
Fig. 3.
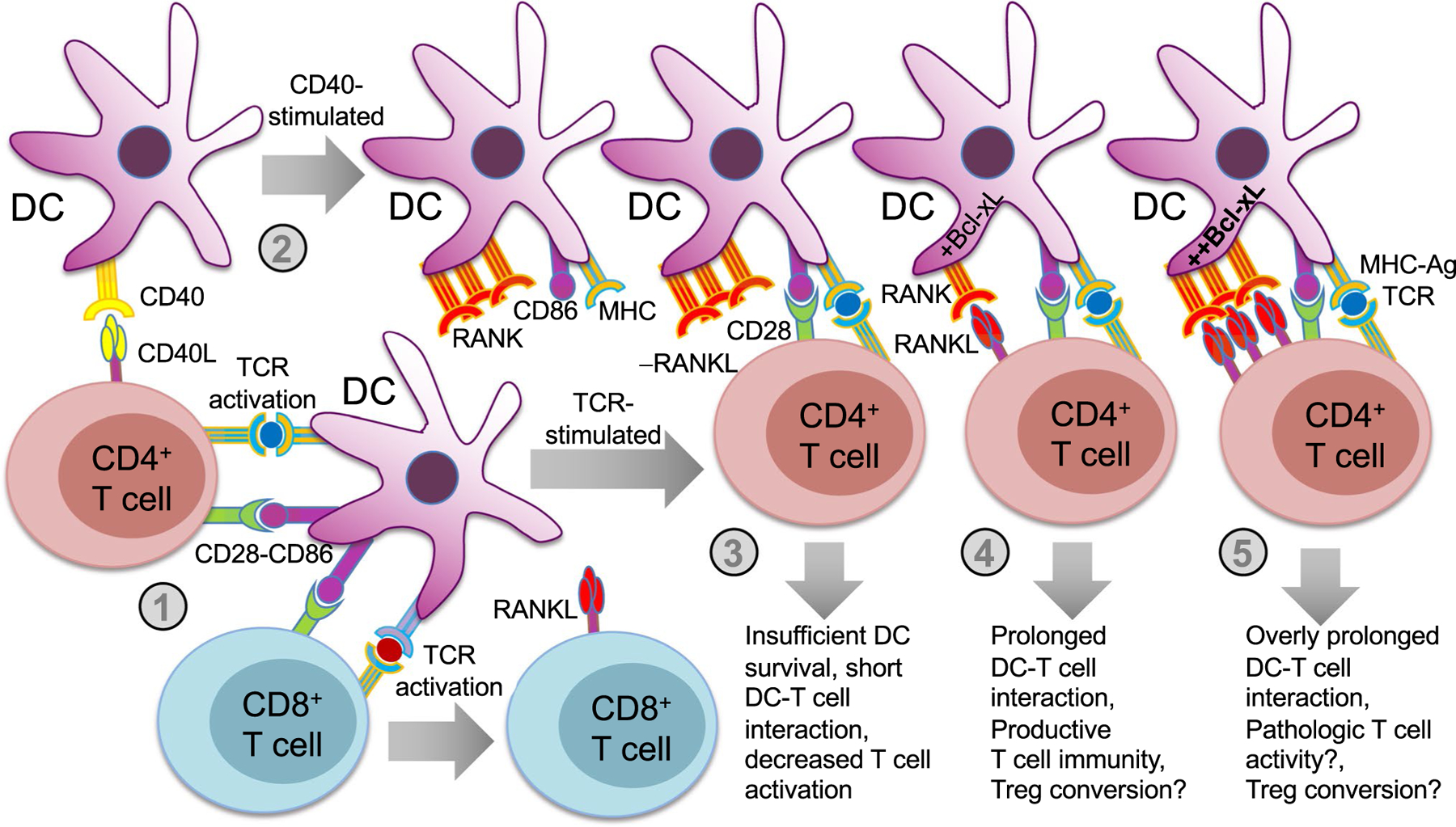
RANKL stimulation of DCs prolongs DC survival and DC-T cell interactions. T cell activation and development of T cell-mediated immune responses in the peripheral lymphoid tissues depends largely on presentation of MHC-antigen and costimulatory ligands directly by mature dendritic cells (DCs) to T cells. Upon stimulation by an inflammatory trigger, terminally differentiated mature DCs must survive long enough for DC-T cell interactions to be productive. A mechanism exists that enables T cells to enhance survival of DCs with which they are interacting: (1) TCR engagement induces surface RANKL expression on both CD4+ and CD8+ T cells, and CD28 costimulation enhances RANKL expression on CD4+ T cells further. (2) T cell-expressed CD40L activates CD40 expressed on DCs, which triggers DC maturation and activation events, including upregulation of MHC, costimulatory molecules (e.g., CD86), and RANK. The level of RANK activation triggered by RANKL-expressing T cells can impact the nature of the T cell response. (3) In the absence of RANKL-RANK engagement, DCs may be at increased risk of apoptosis, resulting in shortened DC-T cell interaction, and decreased T cell activation. (4) For optimal RANKL-RANK engagement, DC survival will be enhanced by the induction of Bcl-xL, and DC-T cell interactions will be prolonged such that productive T cell responses can develop. This level of engagement may also promote regulatory T cell (Treg) conversion under some conditions. 5) Excessive RANKL-RANK engagement results in overly prolonged DC-T cell interactions, which may contribute to overactivation/pathologic activation of T cells, but also possibly Treg conversion
CD40L and its receptor CD40 are closely related to RANKL and RANK, respectively, and utilize similar intracellular TRAF-mediated signaling mechanisms [50]. Both RANK and CD40 are expressed on DCs and play similar but not identical roles in DC maturation and DC-T cell interactions [50]. It is possible that CD40 plays a larger role in initial DC maturation and activation, while RANK, whose expression is actually induced by CD40L, is more involved in promoting subsequent Bcl-xL-dependent survival [50, 51]. There is evidence, however, that their functions overlap, as inhibiting RANKL-RANK interactions with RANK-Fc treatment suppresses activation of CD4+ T cell responses to viral [56] and parasitic infections [57], but only when given to mice lacking CD40. Conversely, mice with DCs lacking A20, which causes hypersensitive CD40 and RANK activity, exhibit excessive DC survival that promotes activation of self-reactive T cells [58]. Other studies have also implicated RANK signaling in peripheral DCs in loss of immune tolerance. For example, it was shown that transferring RANKL-stimulated DCs exacerbated autoimmunity in MRL/lpr recipient mice [59]. A study using an IL-2-deficient mouse model of colitis and bone loss showed that enhanced DC survival by RANKL-expressing T cells exacerbated disease, and that this effect could be inhibited with OPG treatment [60].
In the context of RANK-mediated effects on CD8+ T cell responses during infection, it was recently showed that while RANK expression on DCs is dispensable for early priming of C D8+ T cells in response to either Listeria monocytogenes or VSV, DC-expressed RANK is required for optimal recruitment and activation of pathogen-specific memory CTLs upon re-challenge [61]. Further, it was also showed that ablation of RANK expression on CD169+ marginal zone macrophages leads to impaired viral replication and promotes the acquisition of viral antigens by cross-presenting DCs [61].
RANKL-RANK signaling has also been implicated in Treg cell-mediated tolerance, as it has been shown that RANKL+ keratinocytes in skin trigger epidermal DCs to convert inflammatory infiltrating T cells to a Treg phenotype [62]. RANKL-RANK interactions are required for generation and activation of pancreatic Tregs that inhibit cytotoxic killing of beta islet cells in a mouse type-1 diabetes model [63], and for control of a mouse colitis model by RANKL-expressing Tregs [64]. However, fate mapping has also shown that acquisition of RANKL expression by Tregs can be associated with conversion to pathogenic Th17 cells that promote bone loss in rheumatoid arthritis in response to synovial fibroblast-derived IL-6 [65]. Together, these results highlight an emerging appreciation for the role of the RANKL-RANK-OPG system in modulating peripheral T cell responses. Additional efforts will be required to determine what signals downstream of RANK triggered on DCs may contribute to differential outcomes with respect to T cell phenotype.
Conclusions
The RANKL-RANK-OPG system plays such a significant biological role in bone homeostasis, with well-established therapeutic implications for bone-related diseases, that it can be easy to overlook the roles of RANKL-RANK-OPG in T cell biology. Tools that enable better tissue- and cell-specific investigations of how and when RANKL is expressed, and whether it is produced in soluble or membrane-bound form, will continue to improve prospects for teasing apart the outsized role RANKL-RANK signaling plays in bone homeostasis from its effects on T cell differentiation and immunity. These effects may, in some cases be subtle, but because central and peripheral T cell tolerance are critical to controlling autoimmune responses, it will be important to continue to focus on further elucidating the regulatory mechanisms the RANKL-system employs.
Acknowledgements
The authors acknowledge grant support in part from the NIH (AR069546, AI125284, AR077526).
Funding
This work was supported in part by grant support from the National Institutes of Health (NIH) (AR069546, AI125284, AR077526).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Walsh MC, Choi Y (2014) Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol 5:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takaba H, Takayanagi H (2017) The mechanisms of T cell selection in the thymus. Trends Immunol 38:805–816 [DOI] [PubMed] [Google Scholar]
- 3.Xing Y, Hogquist KA (2012) T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol 4:a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahama Y, Ohigashi I, Baik S, Anderson G (2017) Generation of diversity in thymic epithelial cells. Nat Rev Immunol 17:295–305 [DOI] [PubMed] [Google Scholar]
- 5.Lopes N, Serge A, Ferrier P, Irla M (2015) Thymic crosstalk coordinates medulla organization and T-cell tolerance induction. Front Immunol 6:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi RT, Anderson MS (2011) The role of Aire in clonal selection. Immunol Cell Biol 89:40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, Kodama T, Takayanagi H (2015) Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell 163:975–987 [DOI] [PubMed] [Google Scholar]
- 8.Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJ, Anderson G (2007) RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 204:1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama N, Takizawa N, Miyauchi M, Yanai H, Tateishi R, Shinzawa M, Yoshinaga R, Kurihara M, Demizu Y, Yasuda H, Yagi S, Wu G, Matsumoto M, Sakamoto R, Yoshida N, Penninger JM, Kobayashi Y, Inoue J, Akiyama T (2016) Identification of embryonic precursor cells that differentiate into thymic epithelial cells expressing autoimmune regulator. J Exp Med 213:1441–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J (2008) The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29:423–437 [DOI] [PubMed] [Google Scholar]
- 11.Akiyama N, Shinzawa M, Miyauchi M, Yanai H, Tateishi R, Shimo Y, Ohshima D, Matsuo K, Sasaki I, Hoshino K, Wu G, Yagi S, Inoue J, Kaisho T, Akiyama T (2014) Limitation of immune tolerance-inducing thymic epithelial cell development by Spi-B-mediated negative feedback regulation. J Exp Med 211:2425–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desanti GE, Cowan JE, Baik S, Parnell SM, White AJ, Penninger JM, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G (2012) Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J Immunol 189:5519–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, Cunningham AF, Paolino M, Penninger JM, Simon AK, Nitta T, Ohigashi I, Takahama Y, Caamano JH, Hayday AC, Lane PJ, Jenkinson EJ, Anderson G (2012) Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity 36:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, Narendran P, Pfeffer K, Nitta T, Takahama Y, Caamano JH, Lane PJ, Jenkinson EJ, Anderson G (2010) Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol 185:4769–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzger TC, Khan IS, Gardner JM, Mouchess ML, Johannes KP, Krawisz AK, Skrzypczynska KM, Anderson MS (2013) Lineage tracing and cell ablation identify a post-Aire-expressing thymic epithelial cell population. Cell Rep 5:166–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y (2008) The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29:438–450 [DOI] [PubMed] [Google Scholar]
- 17.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W (2008) Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29:451–463 [DOI] [PubMed] [Google Scholar]
- 18.White AJ, Lucas B, Jenkinson WE, Anderson G (2018) Invariant NKT cells and control of the thymus medulla. J Immunol 200:3333–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin C, Pei XY, Shen H, Gao YN, Sun XY, Wang W, Ge Q, Zhang Y (2017) Thymic homing of activated CD4(+) T cells induces degeneration of the thymic epithelium through excessive RANK signaling. Sci Rep 7:2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes N, Vachon H, Marie J, Irla M (2017) Administration of RANKL boosts thymic regeneration upon bone marrow transplantation. EMBO Mol Med 9:835–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asano T, Okamoto K, Nakai Y, Tsutsumi M, Muro R, Suematsu A, Hashimoto K, Okamura T, Ehata S, Nitta T, Takayanagi H (2019) Soluble RANKL is physiologically dispensable but accelerates tumour metastasis to bone. Nat Metab 1:868–875 [DOI] [PubMed] [Google Scholar]
- 22.McCarthy NI, Cowan JE, Nakamura K, Bacon A, Baik S, White AJ, Parnell SM, Jenkinson EJ, Jenkinson WE, Anderson G (2015) Osteoprotegerin-mediated homeostasis of rank+ thymic epithelial cells does not limit F oxp3+ regulatory T Cell development. J Immunol 195:2675–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiyama T, Shinzawa M, Qin J, Akiyama N (2013) Regulations of gene expression in medullary thymic epithelial cells required for preventing the onset of autoimmune diseases. Front Immunol 4:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G (2013) The thymic medulla is required for F oxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med 210:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L (2007) Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8:351–358 [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Yang L, Silva HM, Trzeciak A, Choi Y, Schwab SR, Dustin ML, Lafaille JJ (2016) Increased generation of Foxp3(+) regulatory T cells by manipulating antigen presentation in the thymus. Nat Commun 7:10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarron MJ, Irla M, Serge A, Soudja SM, Marie JC (2019) Transforming growth factor-beta signaling in alphabeta thymocytes promotes negative selection. Nat Commun 10:5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan IS, Mouchess ML, Zhu ML, Conley B, Fasano KJ, Hou Y, Fong L, Su MA, Anderson MS (2014) Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med 10.1084/jem.20131889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323 [DOI] [PubMed] [Google Scholar]
- 31.Mueller CG, Hess E (2012) Emerging functions of RANKL in lymphoid tissues. Front Immunol 3:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordeiro OG, Chypre M, Brouard N, Rauber S, Alloush F, Romera-Hernandez M, Benezech C, Li Z, Eckly A, Coles MC, Rot A, Yagita H, Leon C, Ludewig B, Cupedo T, Lanza F, Mueller CG (2016) Integrin-Alpha IIb identifies murine lymph node lymphatic endothelial cells responsive to RANKL. PLoS ONE 11:e0151848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onder L, Morbe U, Pikor N, Novkovic M, Cheng HW, Hehlgans T, Pfeffer K, Becher B, Waisman A, Rulicke T, Gommerman J, Mueller CG, Sawa S, Scandella E, Ludewig B (2017) Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity 47:80–92 [DOI] [PubMed] [Google Scholar]
- 34.Withers DR, Hepworth MR (2017) Group 3 innate lymphoid cells: communications hubs of the intestinal immune system. Front Immunol 8:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bando JK, Gilfillan S, Song C, McDonald KG, Huang SC, Newberry RD, Kobayashi Y, Allan DSJ, Carlyle JR, Cella M, Colonna M (2018) The tumor necrosis factor superfamily member RANKL suppresses effector cytokine production in group 3 innate lymphoid cells. Immunity 48:1208–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lycke NY, Bemark M (2012) The role of Peyer’s patches in synchronizing gut IgA responses. Front Immunol 3:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgiev H, Ravens I, Papadogianni G, Halle S, Malissen B, Loots GG, Forster R, Bernhardt G (2018) Shared and unique features distinguishing follicular T helper and regulatory cells of peripheral lymph node and Peyer’s patches. Front Immunol 9:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi N, Takahashi D, Takano S, Kimura S, Hase K (2019) The Roles of Peyer’s patches and microfold cells in the gut immune system: relevance to autoimmune diseases. Front Immunol 10:2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, Akiba H, Yagita H, Kiyono H, Williams IR (2009) RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol 183:5738–5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagashima K, Sawa S, Nitta T, Tsutsumi M, Okamura T, Penninger JM, Nakashima T, Takayanagi H (2017) Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol 18:675–682 [DOI] [PubMed] [Google Scholar]
- 41.Kimura S, Nakamura Y, Kobayashi N, Shiroguchi K, Kawakami E, Mutoh M, Takahashi-Iwanaga H, Yamada T, Hisamoto M, Nakamura M, Udagawa N, Sato S, Kaisho T, Iwanaga T, Hase K (2020) Osteoprotegerin-dependent M cell self-regulation balances gut infection and immunity. Nat Commun 11:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Lau W, Kujala P, Schneeberger K, Middendorp S, Li VS, Barker N, Martens A, Hofhuis F, DeKoter RP, Peters PJ, Nieuwenhuis E, Clevers H (2012) Peyer’s patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts.” Mol Cell Biol 32:3639–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura S, Kobayashi N, Nakamura Y, Kanaya T, Takahashi D, Fujiki R, Mutoh M, Obata Y, Iwanaga T, Nakagawa T, Kato N, Sato S, Kaisho T, Ohno H, Hase K (2019) Sox8 is essential for M cell maturation to accelerate IgA response at the early stage after weaning in mice. J Exp Med 216:831–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahoun A, Mahajan S, Paxton E, Malterer G, Donaldson DS, Wang D, Tan A, Gillespie TL, O’Shea M, Roe AJ, Shaw DJ, Gally DL, Lengeling A, Mabbott NA, Haas J, Mahajan A (2012) Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe 12:645–656 [DOI] [PubMed] [Google Scholar]
- 45.Kunimura K, Sakata D, Tun X, Uruno T, Ushijima M, Katakai T, Shiraishi A, Aihara R, Kamikaseda Y, Matsubara K, Kanegane H, Sawa S, Eberl G, Ohga S, Yoshikai Y, Fukui Y (2019) S100A4 protein is essential for the development of mature microfold cells in Peyer’s patches. Cell Rep 29:2823–2834 [DOI] [PubMed] [Google Scholar]
- 46.Suzuki K, Oida T, Hamada H, Hitotsumatsu O, Watanabe M, Hibi T, Yamamoto H, Kubota E, Kaminogawa S, Ishikawa H (2000) Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 13:691–702 [DOI] [PubMed] [Google Scholar]
- 47.Knoop KA, Butler BR, Kumar N, Newberry RD, Williams IR (2011) Distinct developmental requirements for isolated lymphoid follicle formation in the small and large intestine: RANKL is essential only in the small intestine. Am J Pathol 179:1861–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belz GT, Nutt SL (2012) Transcriptional programming of the dendritic cell network. Nat Rev Immunol 12:01–113 [DOI] [PubMed] [Google Scholar]
- 49.Hemmi H, Akira S (2005) TLR signalling and the function of dendritic cells. Chem Immunol Allergy 86:120–135 [DOI] [PubMed] [Google Scholar]
- 50.Ma DY, Clark EA (2009) The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 21:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y (1997) TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 186:2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179 [DOI] [PubMed] [Google Scholar]
- 53.Josien R, Wong BR, Li HL, Steinman RM, Choi Y (1999) TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol 162:2562–2568 [PubMed] [Google Scholar]
- 54.Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y (2000) TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med 191:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyahira Y, Akiba H, Katae M, Kubota K, Kobayashi S, Takeuchi T, Garcia-Sastre A, Fukuchi Y, Okumura K, Yagita H, Aoki T (2003) Cutting edge: a potent adjuvant effect of ligand to receptor activator of NF-kappa B gene for inducing antigen-specific C D8+ T cell response by DNA and viral vector vaccination. J Immunol 171:6344–6348 [DOI] [PubMed] [Google Scholar]
- 56.Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y (1999) TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med 189:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padigel UM, Kim N, Choi Y, Farrell JP (2003) TRANCE-RANK costimulation is required for IL-12 production and the initiation of a Th1-type response to Leishmania major infection in CD40L-deficient mice. J Immunol 171:5437–5441 [DOI] [PubMed] [Google Scholar]
- 58.Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, van Praet J, Branco-Madeira F, Janssens S, Reizis B, Elewaut D, Beyaert R, Hammad H, Lambrecht BN (2011) The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35:82–96 [DOI] [PubMed] [Google Scholar]
- 59.Izawa T, Ishimaru N, Moriyama K, Kohashi M, Arakaki R, Hayashi Y (2007) Crosstalk between RANKL and Fas signaling in dendritic cells controls immune tolerance. Blood 110:242–250 [DOI] [PubMed] [Google Scholar]
- 60.Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, Child JA, Dunstan C, Felsburg PJ, Morgan GJ, Carding SR (2003) Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity 19:849–861 [DOI] [PubMed] [Google Scholar]
- 61.Habbeddine M, Verthuy C, Rastoin O, Chasson L, Bebien M, Bajenoff M, Adriouch S, den Haan JMM, Penninger JM, Lawrence T (2017) Receptor activator of NF-kappaB orchestrates activation of antiviral memory CD8 T cells in the spleen marginal zone. Cell Rep 21:2515–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S (2006) Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med 12:1372–1379 [DOI] [PubMed] [Google Scholar]
- 63.Green EA, Choi Y, Flavell RA (2002) Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity 16:183–191 [DOI] [PubMed] [Google Scholar]
- 64.Totsuka T, Kanai T, Nemoto Y, Tomita T, Okamoto R, Tsuchiya K, Nakamura T, Sakamoto N, Akiba H, Okumura K, Yagita H, Watanabe M (2009) RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol 182:6079–6087 [DOI] [PubMed] [Google Scholar]
- 65.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H (2014) Pathogenic conversion of F oxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 20:62–68 [DOI] [PubMed] [Google Scholar]


