Supplemental Digital Content is available in the text.
Keywords: children, coronavirus disease 2019, intensive care unit, multisystem inflammatory syndrome in children, outcomes, severe acute respiratory syndrome coronavirus 2
Abstract
Objectives:
Multicenter data on the characteristics and outcomes of children hospitalized with coronavirus disease 2019 are limited. Our objective was to describe the characteristics, ICU admissions, and outcomes among children hospitalized with coronavirus disease 2019 using Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study: Coronavirus Disease 2019 registry.
Design:
Retrospective study.
Setting:
Society of Critical Care Medicine Viral Infection and Respiratory Illness Universal Study (Coronavirus Disease 2019) registry.
Patients:
Children (< 18 yr) hospitalized with coronavirus disease 2019 at participating hospitals from February 2020 to January 2021.
Interventions:
None.
Measurements and Main Results:
The primary outcome was ICU admission. Secondary outcomes included hospital and ICU duration of stay and ICU, hospital, and 28-day mortality. A total of 874 children with coronavirus disease 2019 were reported to Viral Infection and Respiratory Illness Universal Study registry from 51 participating centers, majority in the United States. Median age was 8 years (interquartile range, 1.25–14 yr) with a male:female ratio of 1:2. A majority were non-Hispanic (492/874; 62.9%). Median body mass index (n = 817) was 19.4 kg/m2 (16–25.8 kg/m2), with 110 (13.4%) overweight and 300 (36.6%) obese. A majority (67%) presented with fever, and 43.2% had comorbidities. A total of 238 of 838 (28.2%) met the Centers for Disease Control and Prevention criteria for multisystem inflammatory syndrome in children, and 404 of 874 (46.2%) were admitted to the ICU. In multivariate logistic regression, age, fever, multisystem inflammatory syndrome in children, and pre-existing seizure disorder were independently associated with a greater odds of ICU admission. Hospital mortality was 16 of 874 (1.8%). Median (interquartile range) duration of ICU (n = 379) and hospital (n = 857) stay were 3.9 days (2–7.7 d) and 4 days (1.9–7.5 d), respectively. For patients with 28-day data, survival was 679 of 787, 86.3% with 13.4% lost to follow-up, and 0.3% deceased.
Conclusions:
In this observational, multicenter registry of children with coronavirus disease 2019, ICU admission was common. Older age, fever, multisystem inflammatory syndrome in children, and seizure disorder were independently associated with ICU admission, and mortality was lower among children than mortality reported in adults.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, in December 2019 and was declared a worldwide pandemic by the World Health Organization (WHO) in March 2020 (1). SARS-CoV-2 is a highly contagious virus that causes coronavirus disease 2019 (COVID-19). In adults, severe COVID-19 is commonly characterized by severe pneumonia followed by severe systemic inflammation that can result in death (2–4). Older age, comorbidities, and multiple organ failure are associated with increased in-hospital mortality (5). The initial reports of SARS-CoV-2 in children described a spectrum of illness with a milder clinical course than adults (6, 7). Subsequently, an early report of a small cohort (n = 48) of critically ill children with COVID-19 admitted to American and Canadian PICUs suggested that severe disease is infrequent in children, and outcomes are better than in adults (8). A systematic review of pediatric COVID-19 concluded that a high proportion of children infected with SARS-CoV-2 are asymptomatic (9). Since the beginning of the pandemic, the pediatric community has observed varied presentations of COVID-19 in children over time, encompassing COVID-19 pneumonia with or without pediatric acute respiratory distress syndrome and multisystem inflammatory syndrome in children (MIS-C), ileitis, appendicitis, and meningoencephalitis (10–13). Currently, most data related to COVID-19 in children have been derived from case reports, case series, retrospective reviews of small cohorts of patients from local registries, systematic reviews, and meta-analyses (14–21). Although a recent multicenter European study described characteristics and risk factors for severe COVID-19 in children, the proportion of children with severe disease requiring ICU was small, only 8% (22). Recent reports of COVID-19 among children hospitalized in the U.S. hospitals described either outcomes among ICU cohort (23) or compared MIS-C with acute COVID-19 (24) but did not provide comparative analysis of ICU and non-ICU cohorts.
There is a paucity of data from large, multicenter studies of children hospitalized with COVID-19 that have been able to determine factors associated with severe disease requiring ICU care. In March 2020, the Society of Critical Care Medicine (SCCM) Discovery investigators designed and launched a multicenter registry called the Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 registry (ClinicalTrials.gov: NCT04323787) (25). The VIRUS case report form was customized to reflect pediatric-specific data elements. The prospective VIRUS COVID-19 registry was used to identify patients below 18 years old. The objectives of this retrospective study were to describe the characteristics, pre-existing conditions, and outcomes in children hospitalized with COVID-19 and identify risk factors for severe disease requiring ICU admission.
METHODS
Subjects, Virus Registry
We conducted a retrospective study of children hospitalized with COVID-19 at 51 participating sites from February 2020 to January 2021, with 28-day outcomes. Each site obtained institutional review board approval/exempt status, with a waiver of informed consent. Deidentified data were entered into a centralized Research Electronic Data Capture (REDCap) database (26). Children less than 18 years old hospitalized at a participating center with polymerase chain reaction (PCR)–confirmed (within 21 d from hospitalization) or clinically suspected COVID-19 were included. According to VIRUS registry, “clinically suspected” case was defined as any patient with signs and symptoms suggestive of COVID-19 and/or MIS-C. Children hospitalized for elective procedures and surgeries and tested positive for SARS-CoV-2 PCR/antigen on preprocedure/presurgical screening were excluded. We collected data related to demographics, comorbidities, presentation, illness severity, ICU admission, and outcomes. Data from the registry were subject to numerous data quality checks; errors and omissions were resolved at each site. Data were extracted at multiple intervals with updates of specific fields. The most recent update was in January 2021. Age was categorized based on WHO classification (27), and body mass index (BMI) was calculated and categorized based on percentiles according to the Centers for Disease Control and Prevention (CDC) definitions (28) and the World Health Organization (WHO) growth charts for children less than 24 months old (29) (Table 1). Presenting signs and symptoms were organized into system-based categories (SDC Table 2, http://links.lww.com/CCM/G618) and subclassified into categories of 1, 2, 3, 4, and greater than 4. The VIRUS registry included an option for free-text entry for signs and symptoms, admission diagnosis, and comorbidities not included in the registry. Free-text entries were manually reviewed by two investigators (K.M.G., S.T.) and coded accordingly, and discrepancies were managed through consensus. Comorbidities were further subclassified into none, 1, 2, or 3 or more comorbidities. The reporting of this study conforms to The Strengthening the Reporting of Observational Studies in Epidemiology statement (SDC Table 3, http://links.lww.com/CCM/G618) (30).
TABLE 1.
Demographics and Clinical Characteristics Among Pediatric Coronavirus Disease 2019 Patients and Comparison of ICU Versus Non-ICU Admissions
| Category and Subcategory | Total (N = 874) | Non-ICU (N = 470) | ICU (N = 404) | p |
|---|---|---|---|---|
| Age (yr), median (interquartile range) | 8 (1.25–14) | 5.67 (0.74–13.7) | 10.0 (3.0–15.0) | < 0.01 |
| Neonate, n (%) | 36 (4.1) | 25 (5.3) | 11 (2.7) | < 0.01 |
| Infant, n (%) | 217 (24.7) | 146 (31.0) | 71 (17.6) | |
| Child, n (%) | 296 (33.7) | 144 (30.6) | 152 (37.6) | |
| Adolescent, n (%) | 325 (37.2) | 155 (32.9) | 170 (42.1) | |
| Sex (males), n (%) | 473 (54.1) | 252 (53.6) | 221 (54.7) | 0.78 |
| Race, n (%) | ||||
| White | 379 (43.3) | 210 (45.2) | 169 (41.8) | 0.53 |
| Black | 212 (25.8) | 105 (22.6) | 107 (26.5) | |
| Othera | 230 (26.4) | 122 (26.2) | 108 (26.7) | |
| Unknown | 53 (6.1) | 27 (5.8) | 20 (4.9) | |
| Symptoms, n (%) | ||||
| Fever | 586 (67.0) | 307 (65.2) | 279 (69.1) | 0.25 |
| Nausea, vomiting | 296 (33.9) | 132 (28.0) | 164 (40.6) | < 0.01 |
| Cough | 264 (30.2) | 135 (28.7) | 129 (31.9) | 0.33 |
| Abdominal pain | 211 (24.1) | 93 (19.7) | 118 (29.1) | < 0.01 |
| Myalgia/fatigue | 186 (21.3) | 80 (17.0) | 106 (26.2) | < 0.01 |
| Dyspnea | 183 (20.9) | 67 (14.2) | 116 (28.7) | < 0.01 |
| One symptom | 144 (16.4) | 94 (20) | 50 (12.4) | < 0.01 |
| Two symptoms | 148 (16.9) | 92 (19.5) | 56 (13.9) | 0.03 |
| Three symptoms | 169 (19.3) | 89 (18.9) | 80 (19.8) | 0.73 |
| ≥4 symptoms | 413 (47.2) | 195 (41.4) | 218 (54.0) | < 0.01 |
| Any comorbidity, n (%) | 378 (43.2) | 194 (41.2) | 194 (48.0) | 0.04 |
| ≥2 comorbidities, n (%) | 209 (23.9) | 99 (21.0) | 110 (27.2) | 0.03 |
| Asthma | 101 (11.5) | 44 (9.3) | 57 (14.1) | 0.02 |
| Seizure disorder | 67 (7.6) | 26 (5.5) | 41 (10.2) | 0.01 |
| Obesity | 58 (6.6) | 18 (3.8) | 40 (9.9) | < 0.01 |
| Developmental delay | 57 (6.5) | 24 (5.1) | 33 (8.2) | 0.07 |
| Diabetes | 25 (2.8) | 6 (1.2) | 19 (4.7) | < 0.01 |
| Cerebral palsy | 23 (2.6) | 11 (2.3) | 12 (3.0) | 0.67 |
| Body mass index (N = 817), median (interquartile range) | 19.4 (16.0–25.8) | 18.9 (15.4–24.3) | 20.1 (16.4–28.0) | < 0.01 |
| Underweight, n (%) | 73 (8.9) | 41 (9.6) | 32 (8.2) | 0.05 |
| Normal, n (%) | 336 (41.0) | 186 (43.5) | 150 (38.5) | |
| Overweight, n (%) | 110 (13.4) | 63 (14.7) | 47 (12.1) | |
| Obese, n (%) | 298 (36.5) | 137 (32.0) | 161 (41.2) | |
| Ethnicity (N = 780), n (%) | ||||
| Non-Hispanic | 492 (62.9) | 255 (60.0) | 237 (66.8) | 0.05 |
| Hispanic | 288 (36.9) | 170 (40.0) | 118 (33.2) | |
| Multisystem inflammatory syndrome in children (N = 838), n (%) | 236 (28.2)b | 65 (14.6) | 173 (43.8) | < 0.01 |
aOther race is represented as Asian-American 13 (1.4%), East Asian 1 (0.1%), Mixed race 12 (1.3%), Native Hawaiian or Pacific Islander 4 (0.4%), other 161 (18.5%), South Asian 31 (3.5%), Southeast Asian 3 (0.3%), West Asian 5 (0.5%), unknown 47 (5.4%). Missing race in six patients. Body mass index missing in 57 patients. Multisystem inflammatory syndrome in children (MIS-C) categorization only available in 838 patients.
bOf 236 patients with MIS-C, 234 had coronavirus disease 2019 (COVID-19) immunoglobulin G (IgG) levels done, 138 of 234 were positive for COVID-19 IgG.
Six most common signs and symptoms and six most common comorbidities are presented. Obesity in the comorbidities section (physician defined only).
Illness Severity
Pediatric Risk of Mortality (PRISM) III score was calculated for all patients admitted to the ICU for assessment of illness severity (31). The participating sites entered either the actual PRISM-III score or data required for calculation of the score. PRISM-III score for sites that provided data were calculated by investigators (S.T., J.K.) based on calculators available online (32). We classified illness severity into mild (PRISM-III score < 10), moderate (PRISM-III score 10–19), and severe illness (PRISM-III score > 19) as suggested by the study by Gonçalves et al (33).
Outcomes
The primary outcome was ICU admission. Secondary outcomes included hospital and ICU length of stay (LOS) and ICU, hospital and 28-day mortality.
Statistical Analyses
Descriptive statistics for continuous and categorical variables are reported as median with interquartile range (IQR) and number with percent (%), respectively. For comparisons between those who did and did not receive ICU care, continuous variables were compared using nonparametric Wilcoxon rank-sum tests. Categorical variables were compared using chi-square or Fisher exact tests. It is likely that at certain participating sites, children with COVID-19–positive status got admitted to ICU for the purpose of isolating them rather than medical reasons. Therefore, a logistic regression with a random effect for site was performed to assess univariate associations with ICU admission. Multivariate logistic regression was performed to assess the factors associated with ICU admission. All variables with p value of less than 0.2 in univariate analysis were considered in a full multivariate regression model. Potential collinearity of covariates was assessed by calculating correlation coefficients and the variance inflation factors (VIFs) from the full model. When a correlation was greater than 0.8 and the VIF greater than 4, investigators determined how to consolidate or remove predictors for interest in the final model. For example, as various measures of BMI were correlated with both the outcome and each other, the investigators decided to use one measure (percent BMI) in the full model. After determining the full model and examining the extent of data with complete covariate information, investigators decided to implement multiple imputation techniques to assess the sensitivity of the odds ratio (OR) estimates using only the complete case information compared with multiple imputed datasets. Multiple imputation was conducted in SAS Version 9.4 (SAS Institute, Cary, NC) via PROC MI and PROC MI ANALYZE with 10 imputed datasets. As the fully conditional specification (FCS) method was amenable to the numerous categorical covariates and has been shown to be comparable with multivariate normality methods, FCS was applied to all variables in the full model list (34). No auxiliary variables were used in the imputation. A p value of less than 0.05 was considered statistically significant. All analyses were performed using JMP Pro 15 and SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
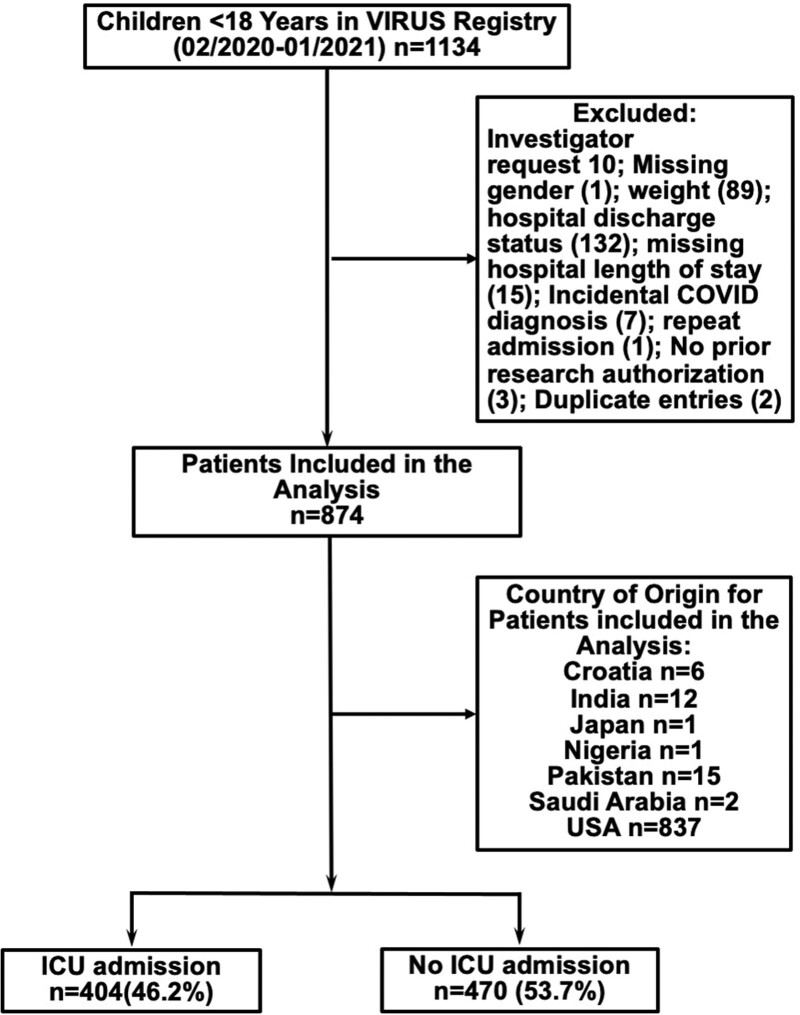
A total of 874 children were admitted with COVID-19 to 51 participating sites (Fig. 1), six sites located outside the United States, 19 mixed hospitals/ICUs, and remaining dedicated pediatric hospitals/ICUs. Of those included in this report, COVID-19 PCR was positive within 21 days of hospital admission in 766 of 874 (87.6%), and there was a high clinical suspicion in an additional 108 of 874 (12.3%). Of 849 total patients with COVID-19 test results available, 417 had testing prior to admission, 428 had testing on or after admission, and four were unknown; 232 patients had COVID tests performed on multiple days. Of 845 patients in whom sample acquisition was reported to the registry, most common was nasopharyngeal swab (807/845; 95.7%) with sputum/tracheal aspirate samples in eight of 845 (0.95%), bronchoalveolar lavage in two of 845 (0.24%), and other in 26 of 845 (3.08%).
Figure 1.
Consort diagram demonstrating the final sample size of children who were hospitalized with coronavirus disease (COVID) 2019 at participating sites and included in our analysis. VIRUS = Viral Infection and Respiratory Illness Universal Study.
Demographic Characteristics, Clinical Characteristics on Presentation and Comorbidities
Demographics, clinical characteristics, resource utilization, and outcomes are detailed in Tables 1–3 and SDC Table 1 (http://links.lww.com/CCM/G618) and SDC Table 2 (http://links.lww.com/CCM/G618).
Hospital Course
Based on PRISM-III score at ICU admission, among the ICU cohort, approximately 83% of children had mild, 15.2% had moderate, and 1.7% had severe illness (Table 3). Among hospitalized children, 200 of 874 (22.8%) received oxygen via nasal cannula. Of 858 children in whom data on respiratory support were available, 92 (10.7%) received high-flow nasal cannula (HFNC), whereas 78 (9%) received noninvasive ventilator (NIV) and 74 (8.6%) received invasive mechanical ventilatory support. Shock necessitating inotrope support was reported in 67 of 874 children (7.6%), whereas renal replacement and extracorporeal therapies were used in a minority, one of 874 children (0.1%) and nine of 874 children (1.03%), respectively.
TABLE 3.
Admission Characteristics Including Severity of Illness Among coronavirus disease 2019 PICU Patients
| Category | N | Value, n (%) |
|---|---|---|
| Source of admission | 872 | |
| Home | 87 (9.9) | |
| Hospital ED | 558 (64.0) | |
| Outside ED | 133 (15.2) | |
| Transfer | 62 (7.0) | |
| Nursing home | 1 (0.1) | |
| Other | 31 (3.5) | |
| Country | 874 | |
| United States | 837 (95.8) | |
| Others | 37 (4.2) | |
| ICU admission (yes)a | 874 | 404 (46.2) |
| Multisystem inflammatory syndrome in children (yes) | 838 | 236 (28.2) |
| Hospital day patient admitted to the ICUb, median (interquartile range) | 382 | 0 (0–1) |
| PRISM-III score, median (interquartile range) | 342 | 3 (0–8) |
| PRISM-III score categories | 342 | |
| Mild (< 10) | 284 (83.0) | |
| Moderate (10–19) | 52 (15.2) | |
| Severe (> 19) | 6 (1.7) | |
| Hospital mortality | 874 | 16 (1.8) |
ED = emergency department, PRISM-III = Pediatric Risk of Mortality-Third Edition.
aICU admission source: hospital ED 220 (59.3%), hospital floor/ward 63 (17.0%), operating room 3 (0.8%), other 6 (1.6%), outside ED/hospital (transfer) 65 (17.5%), outside ICU (transfer) 14 (3.8%) (total data available on 371 patients), number of patients admitted directly to ICU 232 (57.4%).
bHospital day patient admitted to the ICU represents duration of stay in hospital prior to ICU admission.
Other countries: Croatia 6 (0.6%), India 12 (1.3%), Japan 1 (0.1%), Nigeria 1 (0.1%), Pakistan 15 (1.7%), and Saudi Arabia 2 (0.2%).
Outcomes
Of 874 children hospitalized for COVID-19 in our study, 404 (46.2%) were admitted to the ICU. Hospital mortality was 16 of 874 (1.8%). Hospital and ICU LOS were 4 days (1.9–7.5 d) and 3.9 days (2–7.7 d), respectively (Table 2). Among 152 children who were ventilated, the duration of invasive and NIV was 4.7 days (1.5–7.9 d) and 2.0 days (0.9–4.3 d), respectively (Table 2). A majority (804/851 [94.5%]) of children were discharged home without assistance. Of 787 patients in whom 28-day outcome data were reported, 679 (86.4%) were reported as “alive,” 106 (13.4%) as “unknown” status, and two (0.2%) as “dead” (Table 2).
TABLE 2.
Outcomes Among All Patients, Non-ICU and ICU Patients and Hospital and ICU Length of Stay (Mean ± sd and Median [Interquartile Range]) Among All Patients, Survivors, and Nonsurvivors
| Category and Subcategory | Outcomes | |||
|---|---|---|---|---|
| Total (N = 874) | Non-ICU (N = 470) | ICU (N = 404) | p | |
| Hospital length of staya (n = 857), median (interquartile range) | 4 (1.9–7.5) | 2.2 (1.4–4.6) | 6.3 (3.7–11) | < 0.0001 |
| ICU length of staya (n = 379), median (interquartile range) | 3.9 (2–7.7) | NA | 3.9 (2–7.7) | |
| Invasive ventilator daysa (n = 74), median (interquartile range) | 4.7 (1.5–7.9) | 1.21 (0.02–3.05)e | 5 (1.74–8.81) | 0.06 |
| Noninvasive ventilator daysa (n = 78), median (interquartile range) | 2.0 (0.9–4.3) | 3.0 (2.07–7.36) | 1.94 (0.63–4.30) | 0.09 |
| High-flow nasal cannula daysa (n = 92), median (interquartile range) | 2.2 (0.9–3.8) | 1.32 (0.32, 7.88) | 2.22 (1.06, 3.81) | 0.55 |
| Hospital mortality (n = 874), n (%) | 16/874 (1.8) | 1 (0.21) | 15 (3.71) | < 0.01 |
| ICU mortalityb (n = 874), median (interquartile range) | 13/874 (1.4%) | NA | 13 | NA |
| 28-d vital statusc, n (%) | ||||
| Alive | 679 (86.3) | |||
| Loss to follow-up or unknown | 106 (13.4) | |||
| Deceased | 2 (0.3) | |||
| New O2 requirement at discharge (N = 812), n (%) | 14 (1.7) | 5 (1.13) | 9 (1.11) | 0.18 |
| Hospital discharge location (N = 851), n (%) | < 0.01 | |||
| Home with home healthd | 28 (3.3) | 5 (1.08) | 23 (5.96) | |
| Home without assistance | 804 (94.5) | 452 (97.20) | 352 (91.19) | |
| Long-term care facility | 2 (0.2) | 0 (0) | 2 (0.52) | |
| Subacute rehabilitation | 5 (0.6) | 0 (0) | 5 (1.3) | |
| Other hospital (overflow) | 1 (0.1) | 1 (0.22) | 0 (0) | |
| Other | 11 (1.3) | 7 (1.51) | 4 (1.04) | |
| Length of Stay Among Survivors and Nonsurvivors | ||||
| LOS (d) | All | Survivors | Nonsurvivors | |
| Hospital LOS | n = 874 | n = 857 | n = 17 | |
| Mean ± sd | 6.92 ± 11.69 | 6.87 ± 11.74 | 9.63 ± 8.95 | |
| Median (interquartile range) | 4 (1.9–7.59) | 4 (1.90–7.5) | 5.55 (2.26–18.75) | |
| ICU LOS | n = 404 | n = 379 | n = 25 | |
| Mean ± sd | 7.10 ± 13.33 | 7.01 ± 13.46 | 9.63 ± 8.95 | |
| Median (interquartile range) | 3.99 (2–8) | 3.91 (2–7.67) | 5.55 (2.26–18.75) | |
LOS = length of stay, NA = not available.
aSurvivors only.
bICU mortality = number of ICU deaths as a proportion of the whole cohort.
c28-d status—missing information in 72 patients.
dHome with home health = patient discharged home with a provision of a wide range of healthcare services given in his/her home for an illness or injury.
eThis is explained by tracheostomy patients not being admitted to ICU.
There were several differences between patient demographics and clinical characteristics among ICU admission versus general floor admission (Tables 1 and 2). On univariate analysis, patients admitted to ICU were more likely to be older (10.0 [3.0–15.0] vs 5.67 [0.74–13.7]; p < 0.01), have higher BMI (20.1 (16.4–28.0) vs 18.9 [15.4–24.3]; p < 0.01), and meet CDC MIS-C criteria (43.8% vs 14.6%; p < 0.01).
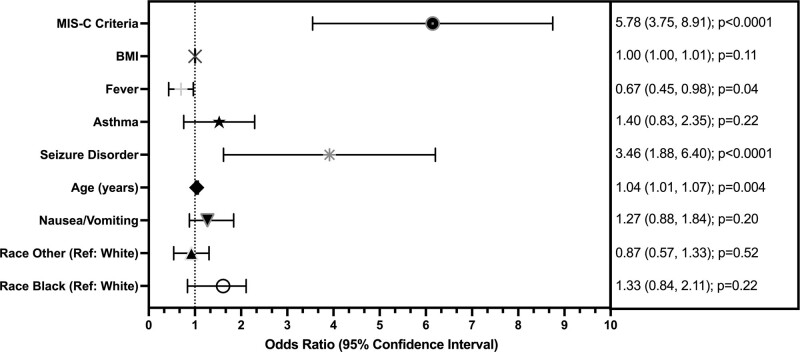
In the multivariate analysis, after adjusting for variables with p value of less than 0.2 in univariate assessment, the final full model included age, pre-existing seizure disorder, asthma, fever, BMI, MIS-C, race, and nausea. Examination of VIF and correlations indicated no concerns with collinearity. There were no more than 7% missing data for any single covariate (6.5% missing BMI percent, 4.1% missing CDC MIS-C criteria). Estimated adjusted ORs for the full multivariate model are presented in Figure 2. The full model included 782 of 874 individuals with complete covariate information (89%). Age, MIS-C criteria, seizure disorder, and nausea remained significant predictors in the model associated with ICU admission (p < 0.05). The complete case full model estimated a 5.9 (3.8–9.1) (p < 0.0001) greater odds of patients who met CDC MIS-C criteria being admitted to the ICU and a 3.4 (1.9–6.4) (p < 0.0001) greater odds for patients with seizure disorder as a pre-existing condition being admitted to ICU. Indication of fever was also associated with admission (OR, 0.67; 95% CI, 0.45–0.98; p = 0.04). For every 1-year increase in age, there was a 4% (1.01–1.08%) (p = 0.003) increase in odds of ICU admission (Fig. 2). The model estimates using multiple imputation remained consistent in direction with reduced standard errors (data not shown). MIS-C and pre-existing seizure disorder were also associated with ICU admission in sensitivity analyses comparing estimates from both univariate models and multivariate models with imputed data.
Figure 2.
Forest plot depicting multivariate analysis of risk factors for ICU admission with final model estimates (analysis of the full model).
DISCUSSION
Our study summarizes findings of a large, multicenter registry of children hospitalized with COVID-19 over the sampling period February 2020 to January 2021. Our report is the largest to characterize 28-day outcomes in hospitalized children with COVID-19. A good proportion of the patients were 6–18 years old (71%), White (43.3%), non-Hispanic (62.9%), and overweight/obese (50%) with a slight male preponderance (~1.2:1). Although 46.2% of children in our study received ICU-level care, 83% of those admitted to ICU had mild disease based on PRISM-III illness severity scores at ICU admission, and overall outcomes were favorable with hospital and ICU mortality, 1.8% and 1.4%, respectively.
As compared with previous, multicenter published reports (8, 22, 35–37), our study reported a comparatively larger number of critically ill children and examined hospital and ICU LOS, 28-day vital status, and factors associated with ICU admission. The median age of 8 years in our study was similar to that reported by Chinese studies (7, 34) but older than what has been reported among European cohorts (22, 36, 37) and younger than other American cohorts (8, 38). Our study did not include asymptomatic subjects. Although fever has been reported to be the most common presenting complaint among children with COVID-19 in many registries (7, 8, 22–24, 37–39), including the VIRUS registry, in our cohort, gastrointestinal manifestations were more common as compared to other studies. The greater proportion of children presenting with gastrointestinal manifestations may be confounded by the fact that MIS-C was diagnosed in a larger proportion (28.3%) as compared to the recent report from the United Kingdom (11%) (37).
In our study, 43.2% of children exhibited at least one comorbidity, similar to what has been reported by the CDC in the United States and recently published reports from the United Kingdom (24, 37, 38), as opposed to other prior studies (8, 22). Similar to the findings of Götzinger et al (22) and Sachdeva et al (23), chronic respiratory disease, especially asthma, was the most common comorbidity in our cohort. In contrast, Swann et al (37) reported neurologic comorbidity as the most frequently occurring comorbidity. Compared with the 83% of critically ill children with comorbidities reported by Shekerdemian et al (8), we noted 48% of critically ill children with similar characteristics. This could be related to overall differences in geographical distribution and patient mix at participating sites of the two registries and different definitions of comorbidities used between the studies.
We reported 83% of ICU patients with mild illness at ICU admission based on admission PRISM-III score compared with 51% of children with mild illness previously reported based on clinical and laboratory findings (35). In short, the majority of ICU patients (98.2%) in our study demonstrated mild-to-moderate disease on ICU admission and a corresponding low mortality rate. In a recent report of COVID-19 among hospitalized children in the United Kingdom, only 18% of children were admitted to ICU (37), as compared to 46.3% of children admitted to the ICU in our study. This could be related to the number of participating sites with ICU capabilities (20/138 sites in the U.K. study vs all 51 participating centers in our study), the difference in overall availability of ICU beds between the two countries (much higher in the United States), the number of MIS-C cases (11% in U.K. study vs 28.3% in our study), and profile of participating investigators (the majority being pediatric intensivists in our study, prioritized entering ICU patients in the registry).
Among critically ill children in our study, ICU interventions were similar to the U.K. study (37) but much higher when compared with other reports from China (7, 35). We expected that a larger proportion of critically ill children with respiratory failure would be intubated due to concern for aerosolization and viral exposure to healthcare workers associated with the use of HFNC and NIV. However, most critically ill children with respiratory failure were supported with HFNC and/or NIV.
In adults admitted with COVID-19, mortality is associated with specific risk factors such as older age and obesity (39). Mortality in our study was lower than in adults, and as such, the risk factors for death could not be assessed in multivariate regression. Instead, we sought to evaluate associations with ICU admission. In a multicenter report examining a European registry, multivariate analysis demonstrated that male sex, age less than 1 month, lower respiratory tract infection at presentation, and presence of comorbidities were associated with ICU admission (22). In this European study, only 48 of 582 COVID-19 patients (8.2%) were critically ill (22). In the study by Swann et al (37), African American race, age under 1 year, and between 10 and 14 years were independently associated with the risk of ICU need (37).
The strength of our study is that it reflects a multicenter registry involving 51 centers. To date, this is one of the largest reports of pediatric COVID-19. In our study, a much larger sample size of critically ill children (n = 404) allowed for appropriate logistic regression analysis of factors associated with severe disease, age, seizure disorder, and MIS-C diagnosis being the only factors that remained independently associated with ICU admission. Manual data collection and several iterations of data quality checks support our findings’ robustness and generalizability to facilitate the design of specific hypothesis-driven research studies.
There are several limitations of our study. Similar to all multicenter registries, analysis of the data may be limited by data integrity and validation issues at participating sites. It is unknown whether all patients with COVID from each site were entered into the database, and thus, there may be some bias on the results. The uniform data collection, consistent definitions, numerous data quality checks, and large sample size were intended to minimize these sources of bias. Due to retrospective nature of the study, it is possible that nonspecific symptoms may not have been documented, and this may have impacted the conclusions that can be made around symptoms and also the confidence with which the number of symptoms present can be categorized. Also, the retrospective design limited the ability of gathering the granular data at admission capabilities and any change in admission pattern over time. There is a possibility that there is an overlap of the patients reported in the SCCM VIRUS registry with the prior reported patients. Notably, 120 of 874 patients (13.7%) participated in COVID-19 clinical trials or prospective studies; we do not know how many were included in COVID-19 reports published in the literature. Another potential limitation of our study was sampling bias based on a general priority of including patients admitted to the ICU, regardless of indication. We also acknowledge that it is possible that the proportion of patients with mild illness admitted to the ICU decreased over time as knowledge of the disease increased. Unfortunately, given the constraints of the database, the study cannot confirm this. The VIRUS database did not allow identification of children with medical complexity (CMC); therefore, our study could not determine whether prevalence of CMC differed between ICU and non-ICU patients. Also, a significant proportion of the patients did not have baseline and follow-up Functional Status Scores in VIRUS database, and therefore, we could not conduct a meaningful analysis related to functional outcomes. Since a large majority of sites were in the United States, this report may not truly reflect the global landscape of characteristics and outcomes of children hospitalized with COVID-19.
CONCLUSIONS
This large, multicenter COVID-19 registry describes the characteristics and outcomes of hospitalized children with confirmed or suspected COVID-19 voluntarily reported to the VIRUS registry. In multivariate regression analysis, age, baseline seizure disorder, and MIS-C were independently associated with ICU admission. Hospital and 28-day mortality in children were lower than mortality reported in adults, and the overwhelming majority of children were discharged home, requiring no long-term assistance.
Supplementary Material
Footnotes
New affiliations for Dr. Bhalala: The Children’s Hospital of San Antonio, Baylor College of Medicine, San Antonio, TX; and. Driscoll Children’s Hospital, Corpus Christi, TX.
This work was performed at Multiple participating institutions (mentioned above); Society of Critical Care Medicine headquarters, Mount Prospect, IL, was Data Coordinating Center.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
All of the authors listed on this article had substantial contribution to the conception or design of the work or the acquisition, analysis, or interpretation of data for the article and drafting of the work or revising it critically for important intellectual content. The final article was approved, and all members were in agreement and accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supported, in part, by the Gordon and Betty Moore Foundation and Janssen Research & Development, LLC. Supported, in part, also by the National Institutes of Health/National Center for Research Resources/National Center for Clinical and Translational Science, Center for Translational Science Activity Grant Number UL1 TR002377.
Dr. Bhalala is currently funded by the National Institutes of Health (NIH) (Site Principal Investigator [PI] for Stress Hydrocortisone in Pediatric Septic Shock—R01HD096901), The Children’s Hospital of Philadelphia (Site-PI for Pediatric Resuscitation Quality Collaborative – PediResQ), Voelcker Pilot Grant (PI for project on prearrest electrocardiographic changes), The Children’s Hospital of San Antonio Endowed Chair Funds for ancillary projects related to Society of Critical Care Medicine (SCCM) Viral Infection and Respiratory Illness Universal Study (VIRUS) (coronavirus disease 2019 [COVID-19]) Registry and SCCM VIRUS electronic medical records, automation pilot. Ms. Boman’s institution received funding from The Gordon and Betty Moore Foundation. Ms. Boman and Drs. Kumar and Kashyap's institutions received funding from Janssen Research and Development, LLC. Dr. Kumar is currently funded by Gordon and Betty Moore Foundation, Centers for Disease Control and Prevention (CDC) Foundation through University of Washington and Janssen Research & Development, LLC. Dr. Chiotos funded by Agency for Healthcare Research and Quality(K12-HS026393). Dr. Bjornstad’s institution received funding from the International Society of Nephrology, The NIH-National Institute of Diabetes and Digestive and Kidney Diseases/T32, and Bioporto. Dr. Dapul disclosed that the deidentified data of some patients submitted to the VIRUS registry were also used in the Overcoming COVID-19 Virus Registry. Dr. Blatz funded by NIH grant T32GM-075766. Dr. Levy’s institution received funding from the CDC (No. 75D30120C07725) and the National Institute of Allergy and Infectious Diseases (AI 144301); she received funding from SinoUnited Health Shanghai Children’s Hospital. Drs. Kaufman’s, Abdelaty’s, and Briton’s institutions received funding from SCCM. Dr. Kaufman received funding from EngleWood Health for consulting work as medical director. Dr. Khanna’s institution received funding for COVID-19 trials Blood Volume Analyzer-Daxor and Chair Steering Committee for the Siltuximab in Selected Hospitalized Patients With Viral Acute Respiratory Distress Syndrome trial. Dr. Khanna is currently funded by a Clinical Translational Science Institute NIH/National Center for Advanced Translational Science KL2 TR001421 award for a trial on continuous postoperative hemodynamic and saturation monitoring and is a site PI (institutional funding) for a randomized trial of cytokine filtration in severe COVID-19 and a prospective observational trial of blood volume assessment in COVID-19. His institution also received funding for the SCCM VIRUS EMR automation pilot. Dr. Stulce received funding from the American Physician Institute. Dr. Anderson III disclosed that he is on the Advisory Board for the Gift of Life Michigan. Dr. Bello disclosed government work. Dr. Walkey currently funded the NIH/National Heart, Lung and Blood Institute (NHLBI) grants R01HL151607, R01HL139751, R01HL136660, Agency of Healthcare Research and Quality, R01HS026485, Boston Biomedical Innovation Center/NIH/NHLBI 5U54HL119145-07, and royalties from UptoDate. Dr. Bihorac received support for article research from the NIH. His institution received funding from the NIH (R01 GM110240 R21 EB 027344), Atox Bio, Astute Medical Research, Mallinckrodt Pharmaceuticals, and La Jolla Pharmaceuticals; she disclosed three institutional patents (20200161000, WO2020172607A12020, and 20190326013). Dr. Zimmerman’s institution received funding from the NIH-National Institute of Child Health and Human Development and Immunexpress; he received funding from Elsevier Publishing. Dr. Kashyap receives funding from the NIH/NHLBI: R01HL 130881, UG3/UH3HL 141722; Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC; and royalties from Ambient Clinical Analytics. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Members of the initiative are listed in the Appendix (http://links.lww.com/CCM/G617).
ClinicalTrials.gov Identifier: NCT04323787.
REFERENCES
- 1.Shah A, Kashyap R, Tosh P, et al. Guide to understanding the 2019 novel coronavirus. Mayo Clin Proc. 2020; 95:646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns Hopkins University of Medicine. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html. Accessed August 24, 2020
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med. 2020; 382:1663–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020; 174:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng B, Wang H, Yu C. An increasing public health burden arising from children infected with SARS-CoV2: A systematic review and meta-analysis. Pediatr Pulmonol. 2020; 55:3487–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuhara J, Kuno T, Takagi H, et al. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol. 2020; 55:2565–2575 [DOI] [PubMed] [Google Scholar]
- 11.Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020; 130:5967–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tullie L, Ford K, Bisharat M, et al. Gastrointestinal features in children with COVID-19: An observation of varied presentation in eight children. Lancet Child Adolesc Health. 2020; 4:e19–e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Li H, Fan R, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016; 59:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalyanaraman M, McQueen D, Morparia K, et al. ARDS in an ex-premature infant with bronchopulmonary dysplasia and COVID-19. Pediatr Pulmonol. 2020; 55:2506–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiehao C, Jin X, Daojiong L, et al. A case series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin Infect Dis. 2020; 71:1547–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Tang J, Xie R, et al. Clinical and epidemiological features of 46 children <1 year old with coronavirus disease 2019 in Wuhan, China: A descriptive study. J Infect Dis. 2020; 222:1293–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derespina KR, Kaushik S, Plichta A, et al. Clinical manifestations and outcomes of critically ill children and adolescents with COVID-19 in New York City. J Pediatr. 2020; 226:55–63.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui X, Zhao Z, Zhang T, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2020; 93:1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: A systematic review. EClinicalMedicine. 2020; 24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Rinaldi E, Zusi C, et al. Coronavirus disease 2019 (COVID-19) in children and/or adolescents: A meta-analysis. Pediatr Res. 2020; 89:733–737 [DOI] [PubMed] [Google Scholar]
- 21.Zare-Zardini H, Soltaninejad H, Ferdosian F, et al. Coronavirus disease 2019 (COVID-19) in children: Prevalence, diagnosis, clinical symptoms, and treatment. Int J Gen Med. 2020; 13:477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020; 4:653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sachdeva R, Rice TB, Reisner B, et al. The impact of coronavirus disease 2019 pandemic on U.S. and Canadian PICUs. Pediatr Crit Care Med. 2020; 21:e643–e650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021; 325:1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walkey AJ, Kumar VK, Harhay MO, et al. The viral infection and respiratory illness universal study (VIRUS): An international registry of coronavirus 2019-related critical illness. Crit Care Explor. 2020; 2:e0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Position Paper. Paediatric Age Categories to be Used in Differentiating Between Listing on a Model Essential Medicines List for Children. Available at http://archives.who.int/eml/expcom/children/Items/PositionPaperAgeGroups.pdf. Accessed August 24, 2020
- 28.Center for Disease Control National Center for Health Statistics. Clinical Growth Charts. Available at: https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed August 24, 2020
- 29.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC Growth Charts for Children Aged 0--59 Months in the United States. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5909a1.htm?s_cid=rr5909a1_w. Accessed February 1, 2021 [PubMed]
- 30.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014; 12:1495–1499 [DOI] [PubMed] [Google Scholar]
- 31.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 32.Pollack MM, Dean JM, Butler J, et al. The Collaborative Pediatric Critical Care Research Network (CPCCRN) Pediatric Risk of Mortality (PRISM) III Calculator. Available at: https://www.cpccrn.org/calculators/prismiiicalculator/. Accessed August 24, 2020
- 33.Gonçalves JP, Severo M, Rocha C, et al. Performance of PRISM III and PELOD-2 scores in a pediatric intensive care unit. Eur J Pediatr. 2015; 174:1305–1310 [DOI] [PubMed] [Google Scholar]
- 34.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007; 16:219–242 [DOI] [PubMed] [Google Scholar]
- 35.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020; 145:e20200702. [DOI] [PubMed] [Google Scholar]
- 36.Parri N, Lenge M, Buonsenso D; Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with COVID-19 in pediatric emergency departments in Italy. N Engl J Med. 2020; 383:187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swann OV, Holden KA, Turtle L, et al. ; ISARIC4C Investigators. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: Prospective multicentre observational cohort study. BMJ. 2020; 370:m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC COVID-19 Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:422–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020; 180:e203596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.