Abstract
Objectives:
We investigated whether the risk of death among noncoronavirus disease 2019 critically ill patients increased when numerous coronavirus disease 2019 cases were admitted concomitantly to the same hospital units.
Design:
We performed a nationwide observational study based on the medical information system from all public and private hospitals in France.
Setting:
Information pertaining to every adult admitted to ICUs or intermediate care units from 641 hospitals between January 1, 2020, and June 30, 2020 was analyzed.
Patients:
A total of 454,502 patients (428,687 noncoronavirus disease 2019 and 25,815 coronavirus disease 2019 patients) were included.
Interventions:
For each noncoronavirus disease 2019 patient, pandemic exposure during their stay was calculated per day using the proportion of coronavirus disease 2019 patients among all patients treated in ICU.
Measurements and Main Results:
We computed a multivariable logistic regression model to estimate the influence of pandemic exposure (low, moderate, and high exposure) on noncoronavirus disease 2019 patient mortality during ICU stay. We adjusted on patient and hospital confounders. The risk of death among noncoronavirus disease 2019 critically ill patients increased in case of moderate (adjusted odds ratio, 1.12; 95% CI, 1.05–1.19; p < 0.001) and high pandemic exposures (1.52; 95% CI, 1.33–1.74; p < 0.001).
Conclusions:
In hospital units with moderate or high levels of coronavirus disease 2019 critically ill patients, noncoronavirus disease deaths were at higher levels.
Keywords: coronavirus disease 2019, intensive care units, mortality, pandemic
Increasing numbers of coronavirus disease 2019 (COVID-19) cases seen in waves of the pandemic can rapidly overwhelm hospital capacities due to the high volume of cases mobilizing most of the available resources in healthcare staff, beds, and equipment (1, 2). Maintaining safe care for non-COVID-19 patients in this context is of major importance because excessive workload and resource prioritization toward COVID-19 cases may disorganize usual care for other patient and cause poor outcomes. We investigated whether the risk of death among non-COVID-19 critically ill patients increased when numerous COVID-19 cases were admitted concomitantly to the same ICUs or intermediate hospital units.
METHODS
Study Design and Data Source
We performed a nationwide study based on the medical information system from all public and private hospitals in France (Programme de Médicalisation des Systèmes d’Information. Source: Agence Technique de l'Information sur l'Hospitalisation). This database is routinely used for the purpose of care reimbursement, based on accurate and exhaustive data collection process. We extracted from the hospitalization stay: patient demographics (i.e., sex and age), comorbidities according to Elixhauser algorithms (3), admission context (i.e., surgical or medical care) and reason for managing certain diseases or disorders (nervous, respiratory, circulatory, digestive, musculoskeletal, urinary and reproductive, blood, or other systems), care delivery type (i.e., ICU or intermediate care unit), the Simplified Acute Physiology Score (SAPS) II measured within the first 24 hours of admission and a selection of life-sustaining medical procedures related to organ failures (i.e., invasive ventilation, hemodynamic support, and kidney replacement therapy) at ICU admission or during the ICU stay. To define patients’ socioeconomic status, the median household income of the patients’ city of residence (or one of the hundred department of residence in France if the city of residence was missing in dataset) provided by the National Institute of Statistics and Economic Studies was used. The ICU beds occupancy was defined daily as the number of ICU patients over the number of ICU beds to account for change in bed capacity over time. We also considered hospital status.
This observational study was based on anonymous data and declared for ethical considerations to the National Data Protection Commission (MR-4423250520).
Study Population, Measures of Pandemic Exposure, and Outcome
All adults (patients ≥ 18 yr) admitted to ICU between January 1, 2020, and June 30, 2020, were included.
Pandemic exposure was measured daily and defined at hospital level as the proportion of COVID-19 patients among all patients treated in ICU per day (Fig. E1, http://links.lww.com/CCM/G613). From this definition of pandemic exposure, we defined two different variables: peak of pandemic exposure and average pandemic exposure for each non-COVID-19 patient. First, for each hospital, peak of pandemic exposure corresponded to the highest daily proportion of COVID-19 patients among all patients treated in ICU over the study period and was categorized into a three-modality variable (< 25%, between 25% and 50%, and ≥ 50%). Second, for each non-COVID-19 patient, an average pandemic exposure was specifically calculated as the mean of the daily proportion of COVID-19 patients among all patients treated in ICU during their stay and categorized into three groups according to the prespecified variable distribution: low exposure less than 25%, moderate exposure 25–50%, and high exposure greater than or equal to 50%. Therefore this average pandemic exposure definition took into account change in exposure day by day during the study period and by distinguishing that from one hospital to another.
The primary outcome was all-cause mortality during the patient stay in ICU units. COVID-19 deaths were not included in the all-cause mortality outcome measure. Inhospital mortality was also considered as a secondary outcome in sensitivity analysis.
Statistical Analysis
First, we described characteristics of non-COVID-19 patient stays according to pandemic exposure and care delivery type. Categorical variables were presented using absolute and relative frequencies, and continuous variables were presented using median, 25th and 75th percentile.
Second, we mapped the peak of pandemic exposure for each hospital in France to describe the exposure distribution across hospitals.
Finally, we computed a multivariable logistic regression model to estimate the influence of pandemic exposure on non-COVID-19 patient mortality during ICU stay. We took into account the clustering effect of patients within hospitals with the robust variance estimator (i.e., patients treated and outcomes within a particular hospital tended to be more similar than those in another hospital). The model was adjusted for potential patient confounders, including: age, sex, household income, SAPS II, Elixhauser comorbidity index, a selection of life-sustaining medical procedures related to organ failures, their hospital admission type and reason, and their stay in ICU or intermediate care unit. Additionally, we adjusted non-COVID-19 patient mortality for hospital status and ICU beds occupancy. We tested and included any significant interactions between variables in the model. The results were presented as adjusted odds ratios (ORs) with corresponding 95% CIs. Using estimated parameters obtained from this model, we determined deaths attributable to the pandemic along with corresponding 95% CIs computed from nonparametric bootstrap based on 1,000 replications (4). We also analyzed separately admissions to ICUs and intermediate care units and performed sensitivity analyses on hospital admission related to the management of certain diseases or disorders.
All reported p values were two-sided, and we considered a value of less than 0.05 to be significant. Data were analyzed using SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
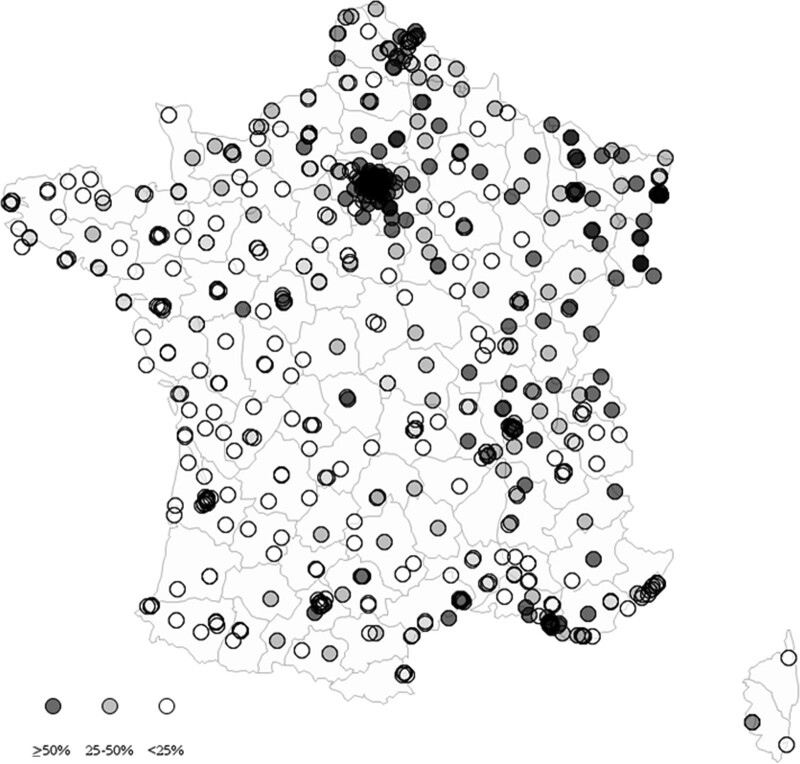
A population of 454,502 patients were admitted to ICUs from 641 hospitals, including 428,687 non-COVID-19 patients (27,055 deaths). Over the study period, 186 hospitals (29.0%) experienced a peak pandemic exposure greater than 50% of patients being COVID-19 cases in their ICU beds, 174 hospitals (27.2%) a peak comprised between 25% and 50%, and 281 hospitals (43.8%) a peak not exceeding 25% (Fig. 1). Overall, 395,089 patients (92.2%) experienced low pandemic exposure during their ICU stay, 27,145 (6.3%) moderate exposure, and 6,453 (1.5%) high exposure (Table E1, http://links.lww.com/CCM/G613).
Figure 1.
Hospital exposure to pandemic peak in France. We analyzed 641 hospitals in France. Every hospital’s exposure to the first pandemic wave was defined as the peak proportion of coronavirus disease 2019 cases among all patients concomitantly treated in ICUs and intermediate care units. Peak pandemic exposure was less than 25% in 281 hospitals (white), between 25% and 50% in 174 hospitals (light gray), and greater than or equal to 50% in 186 hospitals (dark gray).
Mortality rate was 6.2% among non-COVID-19 patients with low pandemic exposure, 7.2% for patients with moderate exposure, and 7.7% for patients with high exposure. After adjusting for potential confounders, the risk of death among non-COVID-19 critically ill patients increased in case of moderate (adjusted OR, 1.12; 95% CI, 1.05–1.19; p ≤ 0.001) and high pandemic exposures (1.52; 95% CI, 1.33–1.74; p < 0.001) (Table 1). This reflected a total of 378 (95% CI, 216–520) potentially deaths among non-COVID-19 patients, attributable to the pandemic. Results were consistent when analyzing separately admissions to ICUs and intermediate care units (Table E2, http://links.lww.com/CCM/G613), but death risk tended to be greater for patients highly exposed to pandemic in intermediate care units (1.57; 95% CI, 1.32–1.88; p < 0.001) than in ICUs (1.29; 95% CI, 1.08–1.55; p = 0.006). Similar results were found for inhospital mortality and according to different hospital admission reasons, in particular for diseases and disorders of the nervous (1.41; 95% CI, 1.17–1.69; p < 0.001), circulatory (1.29; 95% CI, 1.09–1.53; p = 0.003), and digestive (1.41; 95% CI, 1.14–1.75; p = 0.002) systems (Table E3, http://links.lww.com/CCM/G613).
TABLE 1.
Factors Associated With Mortality Among Noncoronavirus Disease 2019 Critically Ill Patients
| Factors | n = 428,687 (%) | Mortality Adjusted ORs (% CI) | p |
|---|---|---|---|
| Pandemic exposure | |||
| Low (< 25%) | 395,089 (92.2) | — | |
| Moderate (25–50%) | 27,145 (6.3) | 1.12 (1.05–1.19) | < 0.001 |
| High (≥ 50%) | 6,453 (1.5) | 1.52 (1.33–1.74) | < 0.001 |
| Patient sex | |||
| Female | 169,197 (39.5) | — | |
| Male | 259,490 (60.5) | 1.03 (0.99–1.06) | 0.150 |
| Patient agea (by 10 yr increase) | 69.0 (57.0–79.0) | 1.37 (1.34–1.40) | < 0.001 |
| Patient SAPS IIa,b (by 10 units increase) | 19.0 (0.0–33.0) | 1.89 (1.82–1.97) | < 0.001 |
| Elixhauser comorbidity indexa | 2.0 (1.0–4.0) | 1.11 (1.07–1.15) | < 0.001 |
| Patient median household incomea, € | 20.0 (18.7–21.9) | 0.99 (0.99–1.00) | 0.093 |
| Hospital admission type | |||
| Surgical care | 140,988 (32.9) | — | |
| Medical care | 287,699 (67.1) | 2.19 (2.03–2.36) | < 0.001 |
| Hospital admission reason | |||
| Diseases and disorders of the nervous system | 64,852 (15.1) | — | |
| Diseases and disorders of the respiratory system | 53,561 (12.5) | 0.76 (0.70–0.84) | < 0.001 |
| Diseases and disorders of the circulatory system | 167,170 (39.0) | 0.66 (0.60–0.70) | < 0.001 |
| Diseases and disorders of the digestive, hepatobiliary system, and pancreas | 46,949 (11.0) | 0.64 (0.59–0.73) | < 0.001 |
| Diseases and disorders of the musculoskeletal system, skin, injuries, poisonings, and burns | 31,145 (7.3) | 0.53 (0.48–0.59) | < 0.001 |
| Diseases and disorders of the kidney and urinary tract, reproductive systems | 23,385 (5.5) | 0.30 (0.26–0.34) | < 0.001 |
| Diseases and disorders of the blood and myeloproliferative tumors | 10,134 (2.4) | 0.56 (0.48–0.66) | < 0.001 |
| Others diseases and disorders | 31,491 (7.3) | 0.42 (0.37–0.49) | < 0.001 |
| Care delivery type | |||
| Intermediate care | 358,251 (83.6) | — | |
| Intensive care | 70,436 (16.4) | 1.80 (1.64–1.97) | < 0.001 |
| Invasive ventilationc | |||
| No | 372,122 (86.8) | — | |
| Yes | 56,565 (13.2) | 1.66 (1.54–1.80) | < 0.001 |
| Hemodynamic supportc | |||
| No | 364,539 (85.0) | — | |
| Yes | 64,148 (15.0) | 1.90 (1.78–2.02) | < 0.001 |
| Kidney replacement therapyc | |||
| No | 418,455 (97.6) | — | |
| Yes | 10,232 (2.4) | 2.48 (2.25–2.74) | < 0.001 |
| Hospital status | |||
| Teaching hospital | 141,770 (33.1) | — | |
| Public | 168,458 (39.3) | 1.07 (0.95–1.22) | 0.268 |
| Private | 118,459 (27.6) | 1.24 (1.04–1.46) | 0.015 |
| ICU beds occupancya (%) | 59.2 (49.6–73.8) | 1.00 (0.99–1.00) | 0.327 |
| Interaction term: SAPS II × Elixhauser comorbidity index | 0.96 (0.95–0.97) | < 0.001 | |
OR = odds ratio, SAPS II = Simplified Acute Physiology Score II.
aMedian (Q1–Q3).
bThe SAPS II was recorded at ICU admission.
cLife-sustaining medical procedures related to organ failures were recorded at ICU admission or during the ICU stay.
A total of 428,687 noncoronavirus disease 2019 patients from 641 hospitals were analyzed. Using multivariable logistic regression model (with a robust error variance) accounting for patient clustering within hospitals and other confounders, we estimated adjusted ORs with their 95% CIs.
DISCUSSION
Hospitals exposure to increasing numbers of COVID-19 cases observed during the first wave pandemic in France was associated with an increased risk of death among non-COVID-19 patients in need of critical care, in particular for patients admitted to intermediate care units.
A plausible explanation relates to overwhelming workload, resource prioritization, and the unanticipated creation of additional beds for managing the massive influx of COVID-19 cases. As a consequence, standard care provided to other patients may have been affected, potentially leading to safety issues with adverse events occurrence and failures to rescue (5–7). This result complement observation in the U.S. context that patients with COVID-19 in ICU had increased mortality during times of surge (8). The higher risk of death among patients admitted to intermediate units could explain because in case of overflow within ICUs, severe patients were by default admitted to intermediate care units and could not receive optimal care. During times of surge, the threshold to be admitted into ICU beds could change toward patients who were most likely to survive and benefit from ICU resources and the most deteriorated patients could be managed upstream of ICU (9).
Available data did not allow to fully control for patient severity including the eventuality that some patients simply avoided the hospital for elective or semi-elective care with plausible concentration of complex cases which can vary across hospitals. Nevertheless, we adjusted mortality on several patient characteristics with corresponding critical care delivery, and in particular, the SAPS II to control for cases severity. We also considered hospital status and ICU beds occupancy in adjustment. However, the daily number of beds seemed to be an imperfect proxy of resources to manage patient as it did not reflect appropriately available equipment and staffing with experienced professionals. Because of potential inaccuracies inherent in medico-administrative data, we cannot rule out the possibility that residual confounders influenced our findings.
Another finding reveals noteworthy heterogeneity in pandemic exposure across the country, as half of hospitals received only a limited number of cases. Close collaborations during the wave’s peak between hospitals would allow to anticipate ICU saturation and a better matching between patient needs and available healthcare staff or equipment, thus reducing mortality among both COVID and non-COVID cases (10).
CONCLUSIONS
In hospital units with moderate or high levels of COVID-19 critically ill patients, non-COVID deaths were at higher levels. This situation could be mitigated by shared strategies between hospitals for smoothing pandemic burden nationwide and better organizing patient care.
Footnotes
Drs. Payet and Duclos collaborated in the concept and design of the study; were responsible for study governance and logistical support; and drafted the article, which then was critically revised by all the authors. All authors prepared this article; contributed substantially to analyzing and interpretation of data; and have read and approved the final article.
The authors have disclosed that they do not have any potential conflicts of interest.
This observational study was based on anonymous data and declared for ethical considerations to the National Data Protection Commission (MR-4423250520).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rimmelé T, Pascal L, Polazzi S, et al. Organizational aspects of care associated with mortality in critically ill COVID-19 patients. Intensive Care Med. 2021; 47:119–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haviari S, Chollet F, Polazzi S, et al. Effect of data validation audit on hospital mortality ranking and pay for performance. BMJ Qual Saf. 2019; 28:459–467 [DOI] [PubMed] [Google Scholar]
- 4.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol. 2010; 63:2–6 [DOI] [PubMed] [Google Scholar]
- 5.Taccone FS, Van Goethem N, De Pauw R, et al. The role of organizational characteristics on the outcome of COVID-19 patients admitted to the ICU in Belgium. Lancet Region Health. 2021; 2:100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuraz A, Guérin C, Payet C, et al. Patient mortality is associated with staff resources and workload in the ICU: A multicenter observational study. Crit Care Med. 2015; 43:1587–1594 [DOI] [PubMed] [Google Scholar]
- 7.Aiken LH, Clarke SP, Sloane DM, et al. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002; 288:1987–1993 [DOI] [PubMed] [Google Scholar]
- 8.Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz S, Arabi YM, Alhazzani W, et al. Managing ICU surge during the COVID-19 crisis: Rapid guidelines. Intensive Care Med. 2020; 46:1303–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelson KA, Rees CA, Jayshree Sarathy J, et al. Inter-region transfers for pandemic surges. Clin Infect Dis. 2020. Oct 10. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]