Abstract
OBJECTIVES:
Vaccine-induced immune thrombotic thrombocytopenia is an unexpected consequence of the coronavirus disease 2019 pandemic era. We reviewed the pathogenesis, clinical presentation, diagnosis, and treatment of this rare side effect.
DATA SOURCES:
Online search of published medical literature through PubMed, Scopus, Web of Science, and Google Scholar using the terms “COVID-19,” “vaccine,” “thrombosis” was performed.
STUDY SELECTION:
Articles were chosen for inclusion based on their relevance to coronavirus disease 2019, vaccine, and thrombosis.
DATA SYNTHESIS:
Vaccine-induced immune thrombotic thrombocytopenia manifests most often as unusual thromboses (cerebral venous sinus thrombosis, splanchnic vein thrombosis) but sometimes also “usual” thromboses (arterial stroke, pulmonary embolism, deep-vein thrombosis), with oftentimes severe thrombocytopenia, that becomes clinically evident 5–30 days after adenovirus-vectored coronavirus disease 2019 vaccine administration. Most patients have disseminated intravascular coagulation. These features are the result of vaccine-triggered formation of anti-platelet factor 4 immunoglobulin G that activate platelets, clinically mimicking autoimmune heparin-induced thrombocytopenia. Early recognition based on thrombosis (sometimes, hemorrhage), thrombocytopenia, and d-dimer elevation within the day 5–30 postvaccine “window” is important given treatment with high-dose IV immunoglobulin plus nonheparin anticoagulation.
CONCLUSIONS:
Vaccine-induced immune thrombotic thrombocytopenia is a serious complication of vaccination that is not feasible to anticipate or prevent. When the patient presents with sustained headache, neurologic symptoms/signs, abdominal pain, dyspnea, or limb pain/swelling beginning 5–30 days post vaccination, platelet count and d-dimer must be measured, and imaging for thrombosis performed. Confirmation of vaccine-induced immune thrombotic thrombocytopenia diagnosis should be ordered (platelet factor 4/polyanion enzyme-linked immunosorbent assay; platelet factor 4–enhanced platelet activation testing) as treatment is initiated (nonheparin anticoagulation, IV immunoglobulin).
Keywords: autoimmune heparin-induced thrombocytopenia, coronavirus disease 2019, disseminated intravascular coagulation, thrombosis, vaccine
On March 19, 2021, the World Health Organization released a statement on the risk of thrombotic events that can occur after vaccination with the AstraZeneca coronavirus disease 2019 (COVID-19) vaccine (ChAdOx1 nCoV-19). According to the statement, “the available data do not suggest any overall increase in clotting conditions such as deep venous thrombosis or pulmonary embolism following administration of COVID-19 vaccines;” they concluded, “the AZ COVID-19 vaccine continues to have a positive benefit-risk profile, with tremendous potential to prevent infections and reduce deaths across the world.” However, they also stated: “very rare and unique thromboembolic events in combination with thrombocytopenia, such as cerebral venous sinus thrombosis (CVST), have also been reported following vaccination in Europe” (1). Thereafter, infrequent reports of multiple thromboses in unusual sites, bleeding, thrombocytopenia, and laboratory findings recognized in healthy individuals shortly after receiving the vaccine began to emerge (2). Since the onset of these thromboses was typically 1–2 weeks after the vaccination, and almost all were accompanied by thrombocytopenia, the involvement of vaccine-induced autoimmune responses resembling heparin-induced thrombocytopenia (HIT) was investigated. On April 13, 2021, the Centers for the Disease Control and Prevention and Food and Drug Administration in the United States recommended pausing the administration of Johnson & Johnson (J&J)/Janssen COVID-19 vaccine (Ad26.COV2.S) because of a similar side effect profile in a small number of patients (3, 4). On April 9, 2021, the first two articles (5, 6) regarding vaccine-induced immune thrombotic thrombocytopenia (VITT) were released, which provided a detailed explanation of this potentially life-threatening side effect of vaccination. A week later, a larger case-series was published (7). The United States subsequently lifted the pause, and so both vaccines implicated in VITT—ChAdOx1 nCoV-19 and Ad26.COV2.S—continue to be administered worldwide; thus, clinicians need to be able to recognize this rare but severe prothrombotic complication aware of these adenoviral vector vaccines. In this review, we discuss the pathogenesis and clinical evaluation of suspected VITT.
LITERATURE SEARCH
We reviewed related articles to the VITT in PubMed, Scopus, Web of Science, and Google Scholar. Two authors independently searched the articles using following subject headings: “COVID-19,” “vaccine,” and “thrombosis.” We also checked the related articles, press releases, announcements, and home pages of the medical societies on the websites. The search strategies were modified for each electronic database using database-specific search terms, field names, and syntax. We identified eight relevant articles published between March 2020 and April 20, 2021 (Table 1). In addition, relevant literature regarding “heparin-induced thrombocytopenia,” “cerebral vein,” “thrombus,” and “anticoagulants” were identified in the electronic resources. The authors reviewed the 24 relevant references identified.
TABLE 1.
Case-Series and Case Reports of Vaccine-Induced Immune Thrombotic Thrombocytopenia
| S. No. | References | Date Published | No. of Cases (Female/Male) | Site of Thrombus | Key Findings |
|---|---|---|---|---|---|
| 1 | Muir et al (4) | April 14, 2021 | 1 (1/0) | Splanchnic vein | The patient showed severe thrombocytopenia (13,000/mm3) with schistocytes, low fibrinogenemia, and DIC. |
| 2 | Greinacher et al (5) | April 9, 2021 | 11 (9/2) | CVT: 9, splanchnic vein: 3, PE: 3, other: 4 | Five cases had DIC and one presented fatal intracranial hemorrhage. |
| 3 | Schultz et al (6) | April 9, 2021 | 5 (4/1) | Cerebral vein: 4, splanchnic vein: 1 | Four cases had major cerebral hemorrhage. Platelet counts increased in all cases despite of the treatment with low-molecular-weight heparin. |
| 4 | Scully et al (7) | April 16, 2021 | 23 (14/9) | CVT: 13, PE: 4, splanchnic vein: 2, etc. | Secondary cerebral hemorrhage was recognized in some cases after CVT. Two cases had ischemic stroke. Seven (30%) died. |
| 5 | Sadoff et al (8) | April 16, 2021 | 1 (0/1) | CVT | Single case of CVT with thrombocytopenia occurred in a vaccine recipient during the clinical trial program for the Ad26.COV2.S vaccine (of which ∽50,000 received active vaccine). |
| 6 | Franchini et al (9) | April 12, 2021 | 1 (0/1) | CVT | CVT with multiple parenchymal hemorrhage. |
| 7 | Mehta et al (10) | April 20, 2021 | 2 (0/2) | CVT | Both two were males. |
| 8 | Thaler et al (11) | April 20, 2021 | 1 (1/0) | None | Petechiae and hematomas were the only symptoms. |
CVT = cerebral venous thrombosis, DIC = disseminated intravascular coagulation, PE = pulmonary embolism.
Epidemiology and Clinical Features
According to the European Medicines Agency (EMA), 30 cases of thromboembolic events had been reported by March 10, 2021, among the approximately 5 million recipients of the AstraZeneca COVID-19 vaccine in Europe (12). The EMA subsequently stated that “The number of thromboembolic events in vaccinated people is no higher than the number seen in the general population” (13). Østergaard et al (14) analyzed the data from the epidemiologic view and reported that the number of thromboembolic events does not seem to be increased relative to the expected number estimated from occurrence rates before the introduction of the vaccination program. However, the onset of thrombotic events with thrombocytopenia in several healthy people shortly after vaccination in unusual sites such as cerebral venous sinus and splanchnic veins indicated the possibility of a rare vaccine-induced prothrombotic disorder (15). As a result, Canada, Germany, and several European countries recommended against the use of the Oxford-AstraZeneca COVID-19 vaccine use in younger people. Following this announcement, the University of Oxford paused the dosing trial in children and teenagers (16). Furthermore, a similar side effect was reported after the administration of J&J/Janssen vaccine. As of April 12, 2021, more than 6.8 million doses have been injected in the United States, and six CVST cases were seen in combination with thrombocytopenia. All the cases are female between the ages of 18 and 48 (3, 4, 8).
Initial reports indicated certain features of VITT, including female predominance, relatively young age, and unusual sites of thrombosis. In the report by Greinacher et al (5), 11 cases of VITT were reported, of which nine were women; the median age was 36 years (range, 22–49 yr). Schultz et al (6) also reported five VITT cases (four female) with ages ranging from 32 to 54 years. At least 13 of the 16 patients reported in these two studies had CVST, and four patients had splanchnic vein thrombosis (indicating clotting of portal, mesenteric, splenic, or hepatic veins). However, the predominance of young women may have reflected the population of recipients of this particular vaccine in Germany and Austria, which was mainly healthcare workers (predominantly young women). Indeed, in the subsequent report of VITT from the United Kingdom, where the vaccine was given more broadly in the population, only 14 of 23 patients (60.9%) were females, and the median age was somewhat higher (46 yr; range, 21–77 yr). Although CVST was most common (at least 13/23 patients), and splanchnic vein thrombosis also observed (4/23), a more diverse spectrum of thrombotic events was evident, including pulmonary embolism (5/23), middle cerebral artery (MCA) stroke (2 cases), myocardial infarction (1 case), deep-vein thrombosis (3 cases, 1 upper limb and 2 lower limb), acute aortic thrombosis (1 case), and bilateral adrenal hemorrhage (1 case). One patient presented with “hemorrhagic symptoms only.” Remarkably, the two patients with MCA stroke were only 21 and 39 years old. Interestingly, if there is a true female predominance in VITT, this could mirror that seen in HIT (approximately two-fold increase in females) (17).
Early Recognition of VITT
VITT presents in a characteristic time period between 5 and 30 days post vaccination, usually with signs and symptoms indicating thrombosis. The name of the condition—VITT—provides a helpful mnemonic (VITT), as follows: 1) Vaccine, 2) “Interval” (5–30 d), 3) Thrombosis, and 4) Thrombocytopenia. We recommend in addition coagulation screening (d-dimer, prothrombin test/international normalized ratio, activated partial thromboplastin test, fibrinogen), as the combination of unexpected thrombocytopenia and elevated d-dimer (often with reduced fibrinogen) in an otherwise well individual who now has symptoms or signs of thrombosis 5 or more days post vaccination requires further urgent investigation (discussed subsequently). These additional coagulation tests are helpful to the diagnosis of VITT and necessary in distinguishing VITT from other entities. However, the cutoffs of each test remain to be elucidated.
A severe and persistent headache or abdominal pain are the most common symptoms, indicting potential CVST or splanchnic vein thrombosis, but abnormal neurologic symptoms/signs and fever can also occur. Dyspnea, tachypnea, and tachycardia can indicate pulmonary embolism. Signs of limb ischemia or swelling, chest pain, or even cardiovascular collapse (massive pulmonary embolism, bilateral adrenal hemorrhagic necrosis) are reported. A patient with VITT presenting with sudden unexplained death post vaccination can be shown to have pathogenic antibodies using a postmortem blood sample (5). Depending on clinical suspicion, imaging studies are required to diagnose thrombosis (MRI venography, CT brain, CT angiography, etc.).
The standard HIT platelet factor 4 (PF4)–dependent enzyme-linked immunosorbent assays are usually strongly positive (optical density is generally > 2.0) (5–7); however, rapid HIT immunoassays (e.g., latex-enhanced immunoassay, chemiluminescence immunoassay) are usually negative (4, 18). Platelet activation assays (performed using a variety of activation endpoints, including aggregation and serotonin release) show serum-/plasma-induced heparin-independent platelet aggregation (5–7, 18). Interestingly, standard washed platelet activation assays, usually performed using patient serum, are sometimes negative unless PF4 is supplemented (5, 18) (Table 2).
TABLE 2.
Comparison of Heparin-Induced Thrombocytopenia and Vaccine-Induced Immune Thrombotic Thrombocytopenia
| Variables | HIT | Vaccine-Induced Immune Thrombotic Thrombocytopenia |
|---|---|---|
| Age | Usually older | Usually younger? |
| Sex | Slight female predominance | Female predominance? |
| Prevalence | 0.2–3% after heparin use | 1/20,000 to 1/100,000 post vaccination |
| Recognition after exposure | 5 d to 2 wk | 5 d to 4 wk |
| Site of thrombosis | DVT/PE, arterial thrombosis | Cerebral venous sinus, splanchnic vein, DVT/PE, arterial |
| Bleeding | Usually none; if present, usually secondary to thrombosis (hemorrhagic infarction) or anticoagulation | Possible; usually secondary to thrombosis (e.g., cerebral venous sinus thrombosis) |
| HIT enzyme-linked immunosorbent assay | Anti-PF4/polyanion IgG positive | Anti-PF4/polyanionic IgG positive |
| HIT rapid screening assays (e.g., latex immunoassay, chemiluminescence immunoassay, particle gel immunoassay) | Usually positive | Usually negative |
| Washed platelet activation assay | Usually positive (heparin-dependent ± heparin-independent activation) | Often negative unless PF4 is supplemented |
| Platelet count | Median, ~50,000/mm3 (usual range, 10–150× ×103) | Median, ~20,000/mm3 (usual range, 10–100× ×103) |
| Prothrombin test | Normal/mildly prolonged | Normal/mildly prolonged |
| d-dimer | Greatly increased | Greatly increased |
| Worsened by heparin administration? | Sometimes | Uncertain |
DVT = deep-vein thrombosis, HIT = heparin-induced thrombocytopenia, IgG = immunoglobulin G, PE = pulmonary embolism, PF4 = platelet factor 4.
The optimal treatment of VITT is not established; however, anticoagulation using nonheparin agents, including direct thrombin inhibitors (argatroban, bivalirudin), indirect (antithrombin-dependent) Xa inhibitors (fondaparinux, danaparoid), or direct oral anticoagulants (DOACs), is currently suggested pending ruling out heparin-dependent increase in platelet activation or emerging data indicating heparin safety and efficacy. Referral of patient serum/plasmas to reference laboratories is usually required to perform high-quality functional (platelet activation) assays. In addition, IV immunoglobulin (IVIG, 1.0 g/kg/d for 2 consecutive days) therapy is recommended, as immunoglobulin inhibits VITT antibody-induced platelet activation and decreases hypercoagulability (18). IVIG is also known to be effective for treating autoimmune HIT (16). In the absence of IVIG, the severe platelet-activating process can last for several weeks (the natural history of platelet count recovery in VITT remains unknown). Plasma exchange is a potential option to reduce antibody levels. Unless bleeding, platelet transfusions and fibrinogen replacement should be considered relatively contraindicated.
The natural history of VITT is unknown, including the duration of thrombocytopenia and hypercoagulability. Patients usually have severe thrombosis, so at least 3–6 months of anticoagulation will usually be required, at which time thrombocytopenia will likely have recovered (by analogy with the usual natural history of autoimmune HIT) Also, by analogy with HIT complicated by thrombosis, longer term anticoagulation is usually managed with DOAC therapy after discharge from hospital.
PATHOPHYSIOLOGY
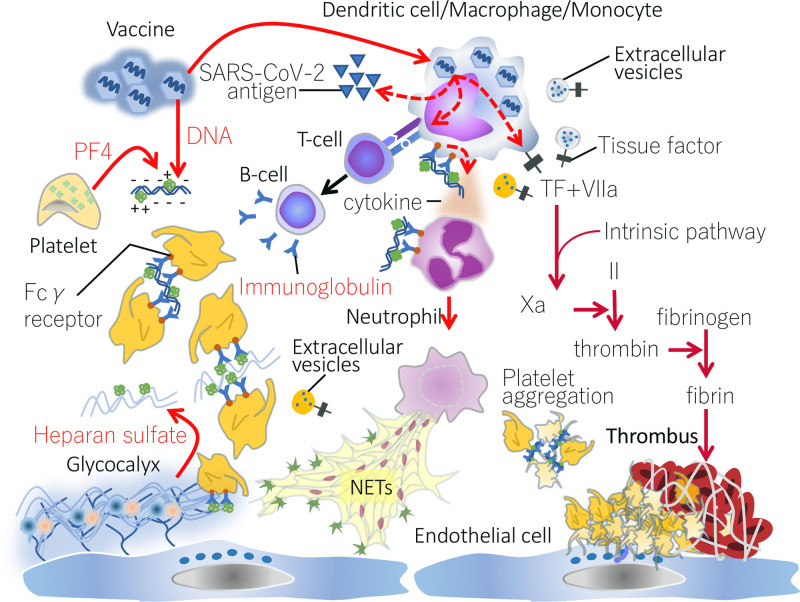
The most mysterious part of this new disease category is its initial trigger and subsequent pathogenesis. Since both AstraZeneca and J&J/Janssen vaccines are adenovirus-vectored vaccines, a vector-specific mechanism is suggested. However, the adenovirus-vectored vaccine has a long history of being used for making Ebola and AIDS vaccines, and the VITT-mimicking thrombosis-related side effect had not been reported. The key finding to date is that patients have platelet-activating antibodies that target PF4 even in the absence of heparin (5). This phenomenon of heparin-“independent” platelet-activating antibodies that target PF4 has been reported with “autoimmune HIT” (16), which refers to patients who have both heparin-dependent and heparin-independent platelet-activating properties and which explains unusual features of HIT, including onset after stopping heparin (delayed-onset HIT) (19), prolonged thrombocytopenia despite stopping heparin (persisting/refractory HIT) (20), and HIT that occurs only with heparin “flush” exposures (21). In addition, a rare disorder, called “spontaneous HIT,” also features heparin-independent platelet-activating antibodies that target PF4 (22). Some of these patients also present with CVST (23, 24). Presumably, both in autoimmune HIT as well as in VITT, the platelet-activating anti-PF4 antibodies interact with PF4 either in the absence of a polyanion or in association with platelet-associated polyanions, such as chondroitin sulfate or polyphosphates (16). It is currently hypothesized that one or more components within the vaccine is somehow triggering a strong anti-PF4 immune response that helps to explain the broad similarities between VITT and these autoimmune HIT disorders. If indeed VITT parallels severe autoimmune HIT, it is expected that its pathogenesis will include (besides platelet activation) NETs release, monocyte, and endothelial activation (25). Figure 1 indicates potential features of VITT pathogenesis, based on existing information as well as expected parallels with severe autoimmune HIT.
Figure 1.
Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia. Adenovirus, a vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, infects dendritic cell/macrophage/monocyte and induces the production of an antispike protein antibody. Since adenovirus is a DNA virus, the vaccine contains viral DNA as well as human proteins. Platelet factor 4 (PF4) released from platelets binds to negatively charged substances such as DNA and heparan sulfate and forms PF4-polyanionic component. After binding, PF4 changes its structure and is recognized as an antigen. If high-affinity anti-PF4 platelet-activating antibodies are generated, these can bind to PF4 (potentially requiring platelet-associated polyanions), inducing platelet activation and aggregation, as is observed in heparin-induced thrombocytopenia (HIT). Furthermore, the antibodies stimulate tissue factor (TF) expression on monocytes and provoke inflammation. These reactions maximize coagulation and inflammation by increasing proinflammatory cytokine production, up-regulating TF expression, and releasing TF-bearing extracellular vesicles. As a result of the innate immune system activation, neutrophils eject neutrophil extracellular traps (NETs) that amplify the coagulation cascade and disrupt antithrombogenicity of the endothelium by damaging glycocalyx. The above HIT-mimicking reaction is suspected to be the fundamental mechanism of vaccine-induced thrombotic thrombocytopenia. Both activated coagulation and platelet aggregation may collaboratively accelerate the clot formation in the unusual sites. Fcγ receptor = receptor for Fc portion of immunoglobulin G, Xa = factor Xa.
A striking unresolved question is why CVST is so common in VITT. CVST is rare, accounting for less than 1% of strokes (26). Non-VITT CVST is reported in the relatively younger patients, with 70% younger than 50 years old and more common in females (27). Further studies of VITT pathogenesis may help in a better understanding of CVST occurrence.
SUMMARY
VITT is a serious complication of vaccination which unfortunately cannot be anticipated or prevented. Clinicians need to have a high index of suspicion when patients develop any of a number of symptoms or signs (headache, neurologic, abdominal, chest, limb) within the day 5–30 window post vaccination. In this situation, check the platelet count and d-dimer. If thrombocytopenia is recognized, and especially if the d-dimer is elevated, urgent further investigation is mandatory.
Footnotes
Supported, in part, by a Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan and from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Dr. Iba has received a research grant from Japan Blood Products Organization and JIMRO. Dr. Levy disclosed that he is on the steering committees for Instrumentation Labs, Merck, and Octapharma. Dr. Warkentin reports receiving consulting fees from Aspen Global, Bayer, CSL Behring, Ergomed, Instrumentation Laboratory, and Octapharma; he received research support from Instrumentation Laboratory; royalties from Informa (Taylor & Francis); consulting fees related to medical-legal consulting and testimony; and disclosed the off-label product use of high-dose intravenous immunoglobulin to treat vaccine-induced immune thrombotic thrombocytopenia.
REFERENCES
- 1.WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID-19 subcommittee. Available at: https://www.who.int/news/item/19-03-2021-statement-of-the-who-global-advisory-committee-on-vaccine-safety-(gacvs)-covid-19-subcommittee-on-safety-signals-related-to-the-astrazeneca-covid-19-vaccine. Accessed April 17, 2021.
- 2.Pharmacovigilance Risk Assessment Committee of the European Medicines Agency. Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant]) – COVID-19 Vaccine AstraZeneca (Other viral vaccines). Available at: https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant-covid_en.pdf. Accessed April 11, 2021
- 3.Schuchat A, Marks P: Available at:https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html. Accessed April 17, 2021
- 4.Muir KL, Kallam A, Koepsell SA, et al. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021; 384:1964–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021; 384:2092–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384:2124–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384:2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination-response from the manufacturer. N Engl J Med. 2021; 384:1965–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M, Testa S, Pezzo M, et al. Cerebral venous thrombosis and thrombocytopenia post-COVID-19 vaccination. Thromb Res. 2021; 202:182–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta PR, Apap Mangion S, Benger M, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - A report of two UK cases. Brain Behav Immun. 2021; 95:514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaler J, Ay C, Gleixner KV, et al. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost. 2021; 19:1819-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021; 372:n699. [DOI] [PubMed] [Google Scholar]
- 13.Pharmacovigilance Risk Assessment Committee of the European Medicines Agency. COVID-19 Vaccine AstraZeneca: PRAC investigating cases of thromboembolic events - vaccine’s benefits currently still outweigh risks - Update. Available at: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-investigating-cases-thromboembolic-events-vaccines-benefits. Accessed April 17, 2021
- 14.Østergaard SD, Schmidt M, Horváth-Puhó E, et al. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: Side-effect or coincidence? Lancet. 2021; 397:1441–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel G, Kupferschmidt K. Side effect worry grows for AstraZeneca vaccine. Science. 2021; 372:14–15 [DOI] [PubMed] [Google Scholar]
- 16.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017; 15:2099–2114 [DOI] [PubMed] [Google Scholar]
- 17.Warkentin TE, Sheppard JA, Sigouin CS, et al. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006; 108:2937–2941 [DOI] [PubMed] [Google Scholar]
- 18.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced thrombotic thrombocytopenia. N Engl J Med. 2021. Jun 9. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001; 135: 502–506 [DOI] [PubMed] [Google Scholar]
- 20.Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: Update of hamilton experience and literature review. Blood. 2017; 130:1104–1113 [DOI] [PubMed] [Google Scholar]
- 21.Mian H, Warkentin TE, Sheppard JI, et al. Autoimmune HIT due to apheresis catheter heparin flushes for stem cell harvesting before autotransplantation for myeloma. Blood. 2017; 130:1679–1682 [DOI] [PubMed] [Google Scholar]
- 22.Warkentin TE, Makris M, Jay RM, et al. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am J Med. 2008; 121:632–636 [DOI] [PubMed] [Google Scholar]
- 23.Hwang SR, Wang Y, Weil EL, et al. Cerebral venous sinus thrombosis associated with spontaneous heparin-induced thrombocytopenia syndrome after total knee arthroplasty. Platelets. 2020. Oct 1. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Moores G, Warkentin TE, Farooqi MAM, et al. Spontaneous heparin-induced thrombocytopenia syndrome presenting as cerebral venous sinus thrombosis. Neurology: Clinical Practice. 2015; 26:602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong BH. Evolving concepts of pathogenesis of heparin-induced thrombocytopenia: Diagnostic and therapeutic implications. Int J Lab Hematol. 2020; 42(Suppl 1):25–32 [DOI] [PubMed] [Google Scholar]
- 26.Maali L, Khan S, Qeadan F, et al. Cerebral venous thrombosis: Continental disparities. Neurol Sci. 2017; 38:1963–1968 [DOI] [PubMed] [Google Scholar]
- 27.Alet M, Ciardi C, Alemán A, et al. ; Argentinian Stroke and Cerebrovascular Diseases Study Group - Argentine Neurological Society. Cerebral venous thrombosis in Argentina: Clinical presentation, predisposing factors, outcomes and literature review. J Stroke Cerebrovasc Dis. 2020; 29:105145. [DOI] [PubMed] [Google Scholar]