Supplemental Digital Content is available in the text.
Keywords: body mass index, critical illness, mortality, severe acute respiratory syndrome coronavirus 2
Abstract
OBJECTIVES:
Obesity is a risk factor for severe coronavirus disease 2019 and might play a role in its pathophysiology. It is unknown whether body mass index is related to clinical outcome following ICU admission, as observed in various other categories of critically ill patients. We investigated the relationship between body mass index and inhospital mortality in critically ill coronavirus disease 2019 patients and in cohorts of ICU patients with non-severe acute respiratory syndrome coronavirus 2 viral pneumonia, bacterial pneumonia, and multiple trauma.
DESIGN:
Multicenter observational cohort study.
SETTING:
Eighty-two Dutch ICUs participating in the Dutch National Intensive Care Evaluation quality registry.
PATIENTS:
Thirty-five–thousand five-hundred six critically ill patients.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Patient characteristics and clinical outcomes were compared between four cohorts (coronavirus disease 2019, nonsevere acute respiratory syndrome coronavirus 2 viral pneumonia, bacterial pneumonia, and multiple trauma patients) and between body mass index categories within cohorts. Adjusted analyses of the relationship between body mass index and inhospital mortality within each cohort were performed using multivariable logistic regression. Coronavirus disease 2019 patients were more likely male, had a higher body mass index, lower Pao2/Fio2 ratio, and were more likely mechanically ventilated during the first 24 hours in the ICU compared with the other cohorts. Coronavirus disease 2019 patients had longer ICU and hospital length of stay, and higher inhospital mortality. Odds ratios for inhospital mortality for patients with body mass index greater than or equal to 35 kg/m2 compared with normal weight in the coronavirus disease 2019, nonsevere acute respiratory syndrome coronavirus 2 viral pneumonia, bacterial pneumonia, and trauma cohorts were 1.15 (0.79–1.67), 0.64 (0.43–0.95), 0.73 (0.61–0.87), and 0.81 (0.57–1.15), respectively.
CONCLUSIONS:
The obesity paradox, which is the inverse association between body mass index and mortality in critically ill patients, is not present in ICU patients with coronavirus disease 2019–related respiratory failure, in contrast to nonsevere acute respiratory syndrome coronavirus 2 viral and bacterial respiratory infections.
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterized by a high variation in disease severity (1). In the most severe cases, patients develop acute respiratory distress syndrome (ARDS) requiring invasive mechanical ventilation, which is associated with high mortality. To date, several risk factors predicting an unfavorable disease course of COVID-19 are known, including higher age, male sex, type 2 diabetes mellitus, hypertension, coronary artery disease, and a higher body mass index (BMI) (2–4). In comparison with obese patients suffering from other acute respiratory pulmonary diseases, obese COVID-19 patients are at higher risk of requiring admission to the ICU and invasive mechanical ventilation (5, 6). This BMI-related increased risk for ICU admission is also observed in obese patients with influenza pneumonia, severe acute respiratory syndrome coronavirus 1 infections, and the Middle East respiratory syndrome (7–9).
In the general population, obesity is associated with multiple chronic diseases which are independently associated with increased mortality compared with nonobese patients. Also, in ICU patients, obesity may be a risk factor for developing ARDS and the need for mechanical ventilation (10). In contrast, multiple studies show reduced ICU and hospital mortality rates in overweight and obese critically ill patients compared with those with a normal BMI (11–13). This observation is known as the “obesity paradox” and several underlying mechanisms have been suggested, including a higher metabolic reserve in obese patients and differences in pulmonary mechanics and immunological aspects between obese and nonobese patients (14). It is currently unclear whether or not the obesity paradox is present in critically ill COVID-19 patients, as fat tissue might play a specific pathophysiological role in this disease, for instance through modulating expression of the angiotensin-converting enzyme 2 (ACE2) receptor which facilitates SARS-CoV-2 cell entry (15). Up to now, in smaller cohorts, either no association (16) or an inverse relationship between higher BMI and clinical outcomes after ICU admission (17–19) have been reported.
The aim of this study is to assess associations between BMI and mortality in critically COVID-19 patients using data of 82 Dutch ICUs. Associations between BMI and clinical outcomes of critically ill COVID-19 patients were compared with those of ICU patients with non-SARS-CoV-2 viral pneumonia, bacterial pneumonia, and trauma patients, the latter representing a nonpulmonary critically ill control group.
MATERIALS AND METHODS
Data Collection
Data of all patients admitted to 82 teaching and nonteaching in nonurban and urban hospitals in the Netherlands are collected in the Dutch National Intensive Care Evaluation (NICE) quality registry (http://www.stichting-nice.nl) (20). For this observational multicenter study on prospective collected data, four cohorts were defined: 1) all COVID-19 patients admitted to the ICU from January 2020 to July 2020, 2) all other non-SARS-CoV-2 viral pneumonia patients, 3) all bacterial pneumonia patients, and 4) all multiple trauma patients from June 2015 to June 2020. Data of patients who were readmitted to the ICU during the same hospitalization period were excluded, so that only data of the first ICU admission were used. Collected data, including patient characteristics (BMI, age, sex), medical history, respiratory function, disease severity scores of the first 24 hours following ICU admission, and clinical outcomes (length of ICU stay and hospital stay as well as inhospital mortality), were anonymized before use. All four cohorts were divided into BMI categories (< 18.5 kg/m2, 18.5–20 kg/m2, 20–25 kg/m2, 25–30 kg/m2, 30–35 kg/m2, ≥ 35 kg/m2) according to the classification of the World Health Organization (21). The primary endpoint of this study was the association between BMI and inhospital mortality in COVID-19 patients and the other three cohorts, particularly because severe COVID-19 is associated with a prolonged disease course. Also, differences in baseline demographic characteristics and physiologic data between the four cohorts and between different BMI categories within each cohort were investigated.
Data collection is completely standardized using strict definitions and subject to data quality checks (22). In accordance to Dutch legislation and compliant with the European General Data Protection Regulation, there is no need to obtain consent when anonymous data are used.
Statistical Analyses
Unadjusted analysis of differences inpatient characteristics and clinical outcomes between the four cohorts, and between different BMI categories within the cohorts was performed using chi-square tests or Kruskal-Wallis tests, followed by post hoc pairwise chi-square and Wilcoxon tests, respectively. To account for multiple testing, a p value of less than 0.001 was considered to indicate statistical significance in all analyses. To assess the association between BMI and inhospital mortality an etiological approach was used to calculate the adjusted odd ratios and corresponding 95% CIs within each cohort. We identified confounding factors that were not on the causal pathway between BMI and mortality based on expert opinion (intensivists and data scientist), literature, and availability in the database. The used multivariable logistic regression models included the following confounders: sex, age (categorized in 11 groups), chronic diagnoses (immunological insufficiency, renal insufficiency, chronic respiratory insufficiency, cardiovascular insufficiency, cirrhosis, malignancy, and diabetes mellitus), Acute Physiology and Chronic Health Evaluation (APACHE) III Acute Physiology Score (APS) (categorized in quintiles), need for mechanical ventilation, use of vasoactive medication, and lowest Pao2/Fio2 ratio (categorized in quintiles) in the first 24 hours following ICU admission. As one may argue that a chronic diagnosis like diabetes mellitus may be related to both BMI and survival, we also explored whether or not diabetes mellitus was independently associated with mortality. To illustrate the association between BMI and covariate-adjusted inhospital mortality, the relative mortality risks (RRs) according to BMI in all four cohorts is plotted using a smooth plot function and using the BMI category of 18.5–25.0 kg/m2 as reference.
All statistical analyses were performed using R Studio v1.2.1335 (Rstudio Team [2020], RStudio: Integrated Development for R. RStudio, PBC, Boston, MA).
RESULTS
Patient Characteristics
A total of 2,635 unique COVID-19 patients, 2,940 non-SARS-CoV-2 viral pneumonia patients, 14,250 bacterial pneumonia patients, and 15,681 trauma patients were included. Patient characteristics of the four cohorts are listed in Table 1. In short, compared with all other cohorts, COVID-19 patients were more likely male, had a higher BMI, lower Pao2/Fio2 ratio, and were more likely mechanically ventilated during the first 24 hours following ICU admission. Importantly, these differences do not translate into a higher disease severity score, as APACHE III scores of COVID-19 patients were lower compared with non-SARS-CoV-2 viral and bacterial pneumonia patients. The lower APACHE III score in COVID-19 patients admitted to the ICU was mainly driven by younger age and lower prevalence of chronic cardiovascular insufficiency, history of malignancies, immunological insufficiency, chronic obstructive pulmonary disease, chronic respiratory insufficiency, and chronic renal failure. In contrast, the percentage of patients with diabetes mellitus was higher in all respiratory infection cohorts, including COVID-19, compared with trauma patients.
TABLE 1.
Patient Characteristics of the Four Cohorts
| Characteristics | Coronavirus Disease 2019(n = 2,635) | Nonsevere Acute Respiratory Syndrome Coronavirus 2 Viral Pneumonia (n = 2,940) | Bacterial Pneumonia (n = 14,250) | Trauma (n = 15,681) | p |
|---|---|---|---|---|---|
| Age, yr | 65 (56–72) | 66 (57–74)a | 69 (60–77)a | 60 (42–76)a | < 0.001 |
| Body mass index, kg/m2 | 27.8 (25.2–31.1) | 25.7 (22.6–30.1)a | 25.3 (22.4–29.3)a | 24.8 (22.6–27.8)a | < 0.001 |
| Male sex, n (%) | 1,899 (72.1) | 1,484 (50.5)a | 8,414 (59.0)a | 10,282 (65.6)a | < 0.001 |
| Lowest Pao2/Fio2 ratio of the first 24 hr in the ICU, mm Hg | 118 (84–165) | 168 (112–234)a | 146 (97–217)a | 300 (210–382)a | < 0.001 |
| Mechanical ventilation on ICU admission | 1,272 (48.3) | 1,555 (52.9)a | 5,396 (37.9)a | 4,904 (31.3)a | < 0.001 |
| Mechanical ventilation in first 24 hr in the ICU | 2,064 (78.3) | 2,043 (69.5)a | 7,768 (54.5)a | 5,363 (34.2)a | < 0.001 |
| Use of vasoactive medication in first 24 hr in the ICU | 1,758 (66.7) | 1,227 (41.7)a | 5,991 (42.0)a | 4,409 (28.1)a | < 0.001 |
| APACHE III Acute Physiology Score | 46 (37–57) | 48 (37–62)a | 53 (40–67)a | 33 (24–49)a | < 0.001 |
| APACHE III score | 58 (46–71) | 62 (49–77)a | 68 (54–84)a | 45 (31–63)a | < 0.001 |
| Simplified Acute Physiology Score II score | 37 (29–45) | 37 (30–46) | 39 (31–49)a | 28 (20–39)a | < 0.001 |
| Medical history | |||||
| Malignancy | 60 (2.3) | 158 (5.4)a | 1,116 (7.8)a | 201 (1.3)a | < 0.001 |
| Immunological insufficiency | 195 (7.4) | 442 (15.0)a | 2,326 (16.3)a | 364 (2.3)a | < 0.001 |
| Chronic obstructive pulmonary disease | 217 (8.2) | 1,382 (47.0)a | 5,110 (35.9)a | 1,152 (7.3) | < 0.001 |
| Chronic respiratory insufficiency | 104 (3.9) | 503 (17.1)a | 1,846 (13.0)a | 235 (1.5)a | < 0.001 |
| Chronic renal failure | 73 (2.8) | 188 (6.4)a | 1,186 (8.3)a | 531 (3.4) | < 0.001 |
| Chronic cardiovascular insufficiency | 32 (1.2) | 101 (3.4)a | 549 (3.9)a | 357 (2.3) | < 0.001 |
| Diabetes mellitus | 500 (19.0) | 589 (20.0) | 2,998 (21.0) | 1,450 (9.2)a | < 0.001 |
APACHE III = Acute Physiology and Chronic Health Evaluation III.
ap < 0.001 compared with coronavirus disease 2019 using pairwise χ2 or Wilcoxon tests.
Data presented as median (interquartile range) or n (%). p values calculated using χ2 tests or Kruskal-Wallis tests across all four cohorts.
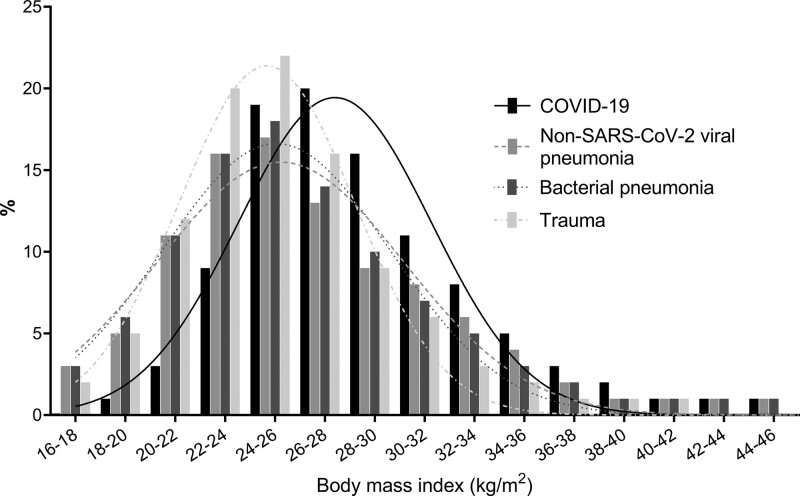
The distribution of BMI within the four cohorts is shown in Figure 1 and patient characteristics according to BMI categories within the COVID-19 cohort and other three cohorts are listed in Table 2 and Supplemental Digital Content 1–3 (http://links.lww.com/CCM/G614), respectively. Within the COVID-19 cohort, patients with a higher BMI were younger, less likely male, and more likely to have diabetes mellitus. Of interest, higher BMI was neither associated with a lower Pao2/Fio2 ratio or the likelihood to require mechanical ventilation during the first 24 hours following ICU admission (Table 2). A similar pattern concerning age, gender, and diabetes mellitus in relationship to a higher BMI was observed in patients with non-SARS-CoV-2 viral or bacterial pneumonia. In the bacterial pneumonia cohort, a higher BMI was also related to higher likelihood to require mechanical ventilation, both on ICU admission and during the first 24 hours of ICU admission.
Figure 1.
Distribution of body mass index for the coronavirus disease 2019 (COVID-19), nonsevere acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral pneumonia, bacterial pneumonia, and multiple trauma cohorts. The proportion of patients with overweight and obesity was notably higher in COVID-19 patients compared with the other cohorts.
TABLE 2.
Patient Characteristics of Body Mass Index Categories in the Coronavirus Disease 2019 Cohort
| Characteristics | BMI < 18.5 kg/m2 (n = 10) | BMI 18.5–25 kg/m2 (n = 592) | BMI 25–30 kg/m2 (n = 1,196) | BMI 30–35 kg/m2 (n = 565) | BMI ≥ 35 kg/m2 (n = 272) | p |
|---|---|---|---|---|---|---|
| Age, yr | 73 (66–75) | 67 (58–73) | 65 (58–72) | 63 (54–71)a | 59 (49–67)a | < 0.001 |
| Male sex, n (%) | 4 (40.0) | 444 (75.0) | 927 (77.5) | 381 (67.4) | 143 (52.6)a | < 0.001 |
| Lowest Pao2/Fio2 ratio of the first 24 hr in the ICU, mm Hg | 121 (73–190) | 126 (89–175) | 120 (86–165) | 112 (77–155) | 106 (76–150) | 0.30 |
| Mechanical ventilation on ICU admission | 3 (30.0) | 286 (48.3) | 599 (50.1) | 265 (46.9) | 119 (43.8) | 0.24 |
| Mechanical ventilation in first 24 hr in the ICU | 7 (70.0) | 453 (76.5) | 947 (79.2) | 440 (77.9) | 217 (79.8) | 0.65 |
| Use of vasoactive medication in first 24 hr in the ICU | 8 (80.0) | 407 (68.8) | 802 (67.1) | 373 (66.0) | 168 (61.8) | 0.28 |
| APACHE III Acute Physiology Score | 57 (43–75) | 48 (38–60) | 46 (36–56) | 46 (37–57) | 46 (37–55) | 0.15 |
| APACHE III score | 73 (59–86) | 61 (49–75) | 59 (47–71) | 57 (44–71) | 56 (43–68) | 0.03 |
| Simplified Acute Physiology Score II score | 50 (46–58) | 37 (30–46) | 37 (29–45) | 36 (30–45) | 34 (27–43) | 0.42 |
| Medical history | ||||||
| Malignancy | 0 (0) | 23 (3.9) | 27 (2.3) | 6 (1.1) | 4 (1.5) | 0.03 |
| Immunological insufficiency | 0 (0) | 55 (9.3) | 77 (6.4) | 40 (7.1) | 23 (8.5) | 0.18 |
| Chronic obstructive pulmonary disease | 0 (0) | 52 (8.8) | 83 (6.9) | 53 (9.4) | 29 (10.7) | 0.13 |
| Chronic respiratory insufficiency | 0 (0) | 22 (3.7) | 40 (3.3) | 25 (4.4) | 17 (6.2) | 0.21 |
| Chronic renal failure | 0 (0) | 14 (2.4) | 31 (2.6) | 23 (4.1) | 5 (1.8) | 0.24 |
| Chronic cardiovascular insufficiency | 0 (0) | 8 (1.4) | 16 (1.3) | 6 (1.1) | 2 (0.7) | 0.86 |
| Diabetes mellitus | 0 (0) | 86 (14.5) | 212 (17.7) | 131 (23.2)a | 71 (26.1)a | < 0.001 |
APACHE III = Acute Physiology and Chronic Health Evaluation III, BMI = body mass index.
ap < 0.001 compared with BMI 18.5–25 kg/m2 using pairwise χ2 or Wilcoxon tests.
Data presented as median (interquartile range) or n (%). p values calculated using χ2 tests or Kruskal-Wallis tests across all five categories.
Clinical Outcomes
Outcome variables of the four cohorts are listed in Table 3. In surviving patients, median ICU length of stay (LOS) of COVID-19 patients was five- to six-fold higher compared with the non-SARS-CoV-2 viral and bacterial pneumonia cohorts, and 18-fold higher than that of trauma patients (Table 3). Additionally, median hospital LOS was approximately threefold higher in COVID-19 survivors compared with the other three cohorts (Table 3). Comparable differences between cohorts were observed in nonsurvivors. The percentage of readmissions in the COVID-19 cohort was lower, whereas inhospital mortality (29.2%) was higher compared with all other cohorts (18.5% in non-SARS-CoV-2 viral pneumonia, 21.2% in bacterial pneumonia, and 9.3% in trauma; Table 3).
TABLE 3.
Clinical Outcomes of the Four Cohorts and Odds Ratios of Inhospital Mortality of Body Mass Index Categories in the Multivariable Logistic Regression Model, With Body Mass Index 18.5–25 kg/m2 Used As Reference Category
| Outcomes | Coronavirus Disease 2019(n = 2,635) | Nonsevere Acute Respiratory Syndrome Coronavirus 2 Viral Pneumonia (n = 2,940) | Bacterial Pneumonia (n = 14,250) | Trauma (n = 15,681) | p |
|---|---|---|---|---|---|
| LOS ICU of survived patients, d | 18 (10–33) | 4 (2–8)a | 3 (2–7)a | 1 (1–3)a | < 0.001 |
| LOS ICU deceased patients, d | 10 (5–18) | 5 (2–9)a | 4 (1–8)a | 2 (1–6)a | < 0.001 |
| LOS hospital of survived patients, d | 31 (19–46) | 11 (7–18)a | 12 (7–20)a | 9 (5–16)a | < 0.001 |
| LOS hospital of deceased patients, d | 12 (6–19) | 7 (3–13)a | 7 (3–13)a | 5 (2–10)a | < 0.001 |
| Number of readmissions | 11 (0.4) | 48 (1.6)a | 1,348 (8.6)a | 238 (1.5)a | < 0.001 |
| 28-d mortality, n (%) | 660 (25.0) | 507 (17.2)a | 2,858 (20.1)a | 1,394 (8.9)a | < 0.001 |
| Inhospital mortality, n (%) | 769 (29.2) | 545 (18.5)a | 3,019 (21.2)a | 1,456 (9.3)a | < 0.001 |
| Odds ratios, kg/m2 | |||||
| BMI < 18.5 | 1.92 (0.51–7.13) | 1.50 (0.95–2.37) | 1.88 (1.57–2.25) | 1.23 (0.86–1.78) | |
| BMI 18.5–25 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |
| BMI 25–30 | 0.95 (0.75–1.21) | 0.78 (0.61–0.99) | 0.78 (0.70–0.86) | 0.9 (0.78–1.03) | |
| BMI 30–35 | 0.87 (0.65–1.16) | 0.76 (0.55–1.04) | 0.81 (0.70–0.93) | 0.99 (0.79–1.23) | |
| BMI ≥ 35 | 1.15 (0.79–1.67) | 0.64 (0.43–0.95) | 0.73 (0.61–0.87) | 0.81 (0.57–1.15) | |
BMI = body mass index, LOS = length of stay.
ap < 0.001 compared with coronavirus disease 2019 using pairwise χ2 or Wilcoxon tests.
Covariates used for the multivariable logistic regression analyses included sex, age, medical history (chronic diagnoses), Acute Physiology and Chronic Health Evaluation III Acute Physiology Score, vasoactive medication, mechanical ventilation, and Pao2/Fio2 ratio on ICU admission.
Data presented as median (interquartile range) or n (%). p values calculated using χ2 tests or Kruskal-Wallis tests across all four cohorts.
No statistically significant differences in ICU/hospital LOS were present between the different BMI categories in both surviving and nonsurviving COVID-19 patients (Supplemental Digital Content 4, http://links.lww.com/CCM/G614). In the non-SARS-CoV-2 viral pneumonia and bacterial pneumonia cohorts, 28-day mortality was lower in higher BMI categories (Supplemental Digital Content 4, http://links.lww.com/CCM/G614). Inhospital mortality was also lower in patients with a higher BMI in the bacterial pneumonia cohort. In both the COVID-19 and trauma cohorts, no differences in 28-day mortality and inhospital mortality were present between different BMI categories. Survival curves of the different BMI categories in the four cohorts are illustrated in Supplemental Digital Content 5 (http://links.lww.com/CCM/G614).
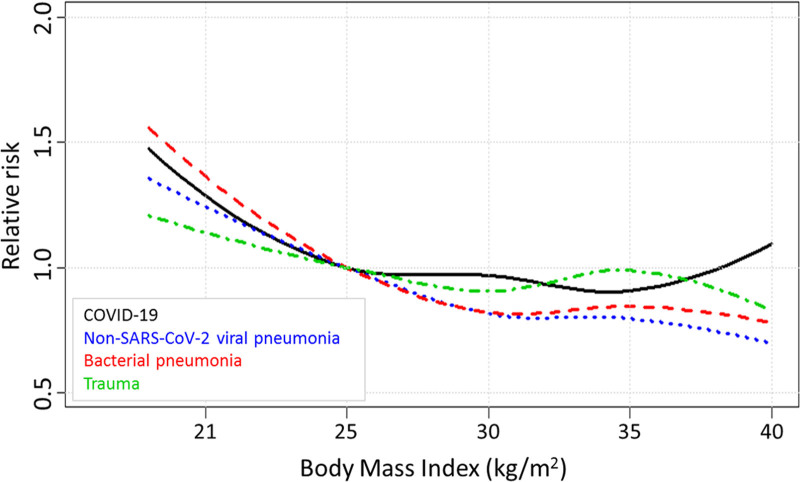
Multivariable analyses for inhospital mortality were performed to adjust for differences in baseline patient characteristics between cohorts. For these analyses with BMI as covariate of interest, sex, age, chronic diagnoses, APACHE III APS, and need for mechanical ventilation, use of vasoactive medication, and lowest Pao2/Fio2 ratio in the first 24 hours following ICU were entered as confounders. In these multivariable analyses, BMI remained unrelated to mortality risk in the COVID-19 cohort, while diabetes mellitus was independently associated with a higher inhospital mortality (Supplemental Digital Content 6, http://links.lww.com/CCM/G614). In contrast, following confounder adjustment, a higher BMI was still associated with lower mortality in the non-SARS-CoV-2 viral pneumonia and bacterial pneumonia cohorts compared with the normal BMI category (Table 3). Furthermore, in the bacterial pneumonia cohort, being underweight was associated with higher mortality (Table 3). Similar to the crude analysis, no association between BMI and mortality was found in the adjusted analysis of trauma patients (Table 3). Odds ratios of contribution to mortality for all other covariates are listed in Supplemental Digital Content 6–9 (http://links.lww.com/CCM/G614). Covariate-adjusted associations between BMI and relative risk of inhospital mortality in the four cohorts are illustrated in Figure 2. In contrast to the lower RR in the highest BMI in the other cohorts, the RR increases in patients with the highest BMI in the COVID-19 cohort.
Figure 2.
Relative inhospital mortality risks according to body mass index (BMI) in the four cohorts. BMI of 25.0 kg/m2 was used as reference. Relative risks were adjusted for sex, age, chronic diagnosis, Acute Physiology and Chronic Health Evaluation III Acute Physiology Score and need for mechanical ventilation, use of vasoactive medication, and lowest Pao2/Fio2 ratio in the first 24 hr following ICU admission. The mortality risk decreases with higher BMI in the nonsevere acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral pneumonia, bacterial pneumonia, and trauma cohorts, but not in coronavirus disease 2019 (COVID-19) patients.
DISCUSSION
In the present study, the association between BMI and inhospital mortality was investigated in critically ill COVID-19 patients, and compared with non-SARS-CoV-2 viral pneumonia, bacterial pneumonia, and trauma patients admitted to the ICU. While COVID-19 patients had a higher BMI compared with the other cohorts, no relationship between BMI and mortality was found in critically ill COVID-19 and trauma patients, while a higher BMI was associated with lower mortality in the other respiratory infection cohorts. Furthermore, being underweight was related to higher mortality in bacterial pneumonia patients.
Previous studies in nonselected patient populations reported increased susceptibility to SARS-CoV-2 in patients with higher BMI (23, 24). Also, obesity has been identified as important risk factors for an unfavorable disease course in patients with COVID-19 requiring admission to the ICU and mechanical ventilation, ultimately translating in an overall higher mortality in overweight patients (3–6). This may have led to the assumption that patients with a high BMI have a worse prognosis at any stage of COVID-19. For critically ill COVID-19 patients, conflicting results have emerged from relatively small studies (16–19). As such, it remained unclear whether obese and/or underweight patients are also at higher risk for poor clinical outcome once they are in the ICU compared with critically ill COVID-19 patients with a normal weight. This is of particular interest, because previous studies in non-COVID-19 critically ill patients rather demonstrated an inverse relationship between BMI and mortality (11–13), which was coined the obesity paradox. In this large multicenter study including several thousand COVID-19 patients, no increased mortality rates were observed for COVID-19 patients in the higher BMI categories. However, while we confirm the obesity paradox in the non-SARS-CoV-2 viral and bacterial pneumonia patients, we did not observe a lower mortality risk in overweight/obese critically ill COVID-19 patients either. In response to the absence of a protective role of a higher BMI in critically ill COVID-19 patients, one may argue that overweight/obese patients in the ICU are therefore at a relative disadvantage when suffering from COVID-19 compared with other respiratory infections.
Several explanations can be put forward for the discrepancy regarding the relation between BMI and mortality in nonselected COVID-19 patient populations in previous studies and critically ill COVID-19 patients in our study. First, it might be argued that differences in reasons for ICU admission between patients from different BMI categories play a role. Since it is known that obese patients are more likely to develop atelectasis than patients with a normal weight, obese COVID-19 patients may more likely be admitted to the ICU for mechanical respiratory support and possibly also earlier in their disease course, while the reason for ICU admission inpatient with normal weight may more often be severe systemic inflammation and/or failure of other organs. If so, this should translate into differences in disease severity on ICU admission, which is the most important prognostic factor for survival. However, this is not supported by our data, as disease severity scores were similar between the different BMI categories and were also included as confounder in the multivariable analyses. Furthermore, we recently demonstrated that inflammatory variables do not differ between obese and nonobese critically ill COVID-19 patients (25), arguing against BMI-related immunological differences that may explain discrepancies in ICU admission characteristics between BMI categories. Second, although multiple covariates were included in the multivariable logistic regression analysis, residual confounders might still be present, such as the prevalence of smoking, chronic use of immunomodulatory drugs, socioeconomic status, and ethnicity.
In keeping with previous reports describing a survival benefit in ICU patients with a higher BMI (11, 12), we confirm a relation between higher BMI and lower mortality in ICU patients suffering from non-SARS-CoV-2 viral pneumonia and bacterial pneumonia. It is tempting to speculate why this obesity paradox is not observed in COVID-19 patients. It has been hypothesized that obese patients display a more anti-inflammatory phenotype (26, 27). As briefly alluded to before, we previously investigated circulating levels of various inflammatory cytokines, including the anti-inflammatory mediators interleukin-10 and interleukin-1 receptor antagonist in critically ill COVID-19 patients, but found no differences between obese and nonobese patients (25). Therefore, this suggested underlying mechanism of the obesity paradox may not be present in critically ill COVID-19 patients but only in ICU patients with other etiologies. Furthermore, it has been postulated that the higher circulating cholesterol and lipid levels in patients with a higher BMI may result in more effective binding of endotoxin, thereby removing an important inflammatory trigger. This may provide an explanation for the survival benefit in obese patients infected with Gram-negative bacteria, which likely constitute a substantial part of our bacterial pneumonia cohort. Furthermore, endotoxin binding may also plays a role in case of translocation of bacteria and/or their products from the gut, which is commonly observed in sepsis patients, also in those not infected with Gram-negative bacteria. Although a recent small study reported the presence of circulating endotoxin in critically ill COVID-19 patients (28), it is unclear whether this is a widespread phenomenon in this disease and does not explain the difference between the COVID-19 and non-SARS-CoV-2 viral pneumonia cohorts in terms of the BMI-mortality relationship. Of interest, adipose tissue, especially visceral fat, may play a pathophysiological role in COVID-19 disease (15). Adipokines such as leptin may enhance pulmonary inflammation and exacerbate respiratory failure (29). Also, the ACE2 expression is higher in adipocytes of people with obesity and diabetes mellitus (30), suggesting that fat tissue may function as a reservoir for the virus. Although its relevance has yet to be elucidated, our findings may be a reflection of this pathophysiological role of adipose tissue.
A strength of this work is that it represents the largest study to date investigating the relation between BMI and mortality in critically ill COVID-19 patients. Furthermore, we included three different relevant comparison groups. This study also has limitations. First, the very small number of underweight COVID-19 patients illustrates that patients with a low BMI are less likely to become critically ill, but hampers assessment of the association between underweight and mortality, which was associated with higher mortality in the bacterial and non-SARS-CoV-2 viral (trend) pneumonia patients. Second, due to the larger sample size of the bacterial pneumonia cohort, the statistical power to detect the presence of an association between BMI and mortality is higher than in the COVID-19 cohort. However, the obesity paradox was also present in the non-SARS-CoV-2 viral pneumonia cohort, which is similar in size as the COVID-19 cohort. Furthermore, in both the non-SARS-CoV-2 viral and bacterial pneumonia cohorts, the lowest mortality was observed in the group with the highest BMI (> 35 kg/m2), whereas increased odds for mortality were observed for this BMI category in the COVID-19 cohort, although this did not reach statistical significance. Therefore, it seems unlikely that the absence of the obesity paradox in COVID-19 patients is the consequence of limited statistical power. Third, one may argue that surge capacity issues may have influenced our observations. In the Netherlands, we did not reach conditions in which COVID-19 patients could not be admitted to the ICU if required. However, ICUs did work hard and beyond their normal capacity. As a consequence, one would expect a selection of higher BMI COVID-19 patients with less comorbidities and lower age to be admitted to the ICU, and this would plausibly translate into a better prognosis of these patients. The opposite was observed: COVID-19 patients with a higher BMI did not have a better outcome, in contrast to non-SARS-CoV-2 ICU patients. Fourth, besides Pao2/Fio2 ratio, no statements can be made about the effect of individual clinical variables on mortality. Nonetheless, the most important clinical variables are included in APACHE III score, which was used as covariate in the multivariable analyses. Finally, clinical variables are only recorded during the first 24 hours following ICU admission in the NICE database. Therefore, possible differences between BMI categories and cohorts in the development of complications during ICU stay (e.g., the development of secondary infections or thromboembolic events) could not be assessed. However, such serially collected data would especially be valuable if a relationship between BMI and mortality was apparent in COVID-19 patients, which was not the case. Therefore, it appears unlikely that significant differences in relevant variables and complications between BMI groups would emerge from a longitudinal dataset.
CONCLUSIONS
In conclusion, the obesity paradox, which is the inverse J-shaped association between BMI and mortality in critically ill patients, is not present in critically ill patients with COVID-19–related respiratory failure in contrast to non-SARS-CoV-2 viral and bacterial respiratory infections. Nevertheless, once admitted to the ICU, obese COVID-19 patients also do not have a higher risk for mortality than patients with normal weight. As such, we argue that triage decisions for ICU admission of COVID-19 patients should not be based on BMI. Whether the lack of an association between BMI and mortality in COVID-19 patients is the result of a specific pathophysiological role of (visceral) fat or other factors related to BMI has yet to be elucidated.
ACKNOWLEDGMENTS
We would like to thank all Dutch ICUs for their contribution to this study by recording data in the Dutch National Intensive Care Evaluation (NICE) quality registry. We also thank all employees of NICE Research & Support and members of the NICE-Coronavirus Disease 2019 (COVID-19) Research Consortium for their contribution in data collection of all COVID-19 patients.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Kooistra, van der Voort, Kox, and Pickkers designed the study. Brinkman was responsible for collecting data from the National Intensive Care Evaluation database and performed statistical analyses. Dr. Kooistra drafted the article. Ms. Brinkman and Drs. van der Voort, de Keizer, Dongelmans, Kox, and Pickkers critically revised the article. All authors read and approved the final article.
Dr. Kooistra was funded by a Radboudumc Coronavirus Disease 2019 grant (Radboudfonds). Drs. Brinkman’s and de Keizer’s institutions received funding from the National Intensive Care Evaluation (NICE) Foundation. Dr. de Keizer disclosed that she is a member of the NICE Board. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This study was carried out in accordance to Dutch legislation and compliant with the European General Data Protection Regulation. Because anonymous data was used, there was no need to obtain consent.
REFERENCES
- 1.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging (Albany NY). 2020; 12:12493–12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020; 99:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Földi M, Farkas N, Kiss S, et al. ; KETLAK Study Group. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes Rev. 2020; 21:e13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonnet A, Chetboun M, Poissy J, et al. ; LICORN and the Lille COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020; 28:1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain A, Mahawar K, Xia Z, et al. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020; 14:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int J Infect Dis. 2016; 49:129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: A cohort study. Clin Infect Dis. 2011; 53:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honce R, Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019; 10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong MN, Bajwa EK, Thompson BT, et al. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010; 65:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickkers P, de Keizer N, Dusseljee J, et al. Body mass index is associated with hospital mortality in critically ill patients: An observational cohort study. Crit Care Med. 2013; 41:1878–1883 [DOI] [PubMed] [Google Scholar]
- 12.Hutagalung R, Marques J, Kobylka K, et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011; 37:1793–1799 [DOI] [PubMed] [Google Scholar]
- 13.Zhi G, Xin W, Ying W, et al. “Obesity paradox” in acute respiratory distress syndrome: Asystematic review and meta-analysis. PLoS One. 2016; 11:e0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jong A, Wrigge H, Hedenstierna G, et al. How to ventilate obese patients in the ICU. Intensive Care Med. 2020; 46:2423–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Zelst CM, Janssen ML, Pouw N, et al. Analyses of abdominal adiposity and metabolic syndrome as risk factors for respiratory distress in COVID-19. BMJ Open Respir Res. 2020; 7:e000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biscarini S, Colaneri M, Ludovisi S, et al. The obesity paradox: Analysis from the SMAtteo COvid-19 REgistry (SMACORE) cohort. Nutr Metab Cardiovasc Dis. 2020; 30:1920–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson RJ, Hunter J, Dutton J, et al. Clinical characteristics and outcomes of critically ill patients with COVID-19 admitted to an intensive care unit in London: A prospective observational cohort study. PLoS One. 2020; 15:e0243710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chand S, Kapoor S, Orsi D, et al. COVID-19-associated critical illness-report of the first 300 patients admitted to intensive care units at a New York City Medical Center. J Intensive Care Med. 2020; 35:963–970 [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020; 180:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Klundert N, Holman R, Dongelmans DA, et al. Data resource profile: The Dutch National Intensive Care Evaluation (NICE) registry of admissions to adult intensive care units. Int J Epidemiol. 2015; 44:1850–1850h [DOI] [PubMed] [Google Scholar]
- 21.WHO Consultation on Obesity, World Health Organization: Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva, Switzerland, World Health Organization, 2000 [PubMed] [Google Scholar]
- 22.Arts D, de Keizer N, Scheffer GJ, et al. Quality of data collected for severity of illness scores in the Dutch National Intensive Care Evaluation (NICE) registry. Intensive Care Med. 2002; 28:656–659 [DOI] [PubMed] [Google Scholar]
- 23.de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: A cross-sectional study. Lancet Infect Dis. 2020; 20:1034–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Yoo DM, Min C, et al. Analysis of mortality and morbidity in COVID-19 patients with obesity using clinical epidemiological data from the Korean Center for Disease Control & Prevention. Int J Environ Res Public Health. 2020; 17:E9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooistra EJ, de Nooijer AH, Claassen WJ, et al. ; RCI-COVID-19 study group. A higher BMI is not associated with a different immune response and disease course in critically ill COVID-19 patients. Int J Obes (Lond). 2021; 45:687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005; 115:911–919 [DOI] [PubMed] [Google Scholar]
- 27.Zampieri FG, Jacob V, Barbeiro HV, et al. Influence of body mass index on inflammatory profile at admission in critically ill septic patients. Int J Inflam. 2015; 2015:734857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirivongrangson P, Kulvichit W, Payungporn S, et al. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med Exp. 2020; 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Voort PHJ, Moser J, Zandstra DF, et al. Leptin levels in SARS-CoV-2 infection related respiratory failure: A cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon. 2020; 6:e04696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity (Silver Spring). 2020; 28:1187–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.