Abstract
The cellular function of p53 is complex. It is well known that p53 plays a key role in cellular response to DNA damage. Moreover, p53 was implicated in cellular senescence, and it was demonstrated that p53 undergoes modification in senescent cells. However, it is not known how these modifications affect the ability of senescent cells to respond to DNA damage. To address this question, we studied the responses of cultured young and old normal diploid human fibroblasts to a variety of genotoxic stresses. Young fibroblasts were able to undergo p53-dependent and p53-independent apoptosis. In contrast, senescent fibroblasts were unable to undergo p53-dependent apoptosis, whereas p53-independent apoptosis was only slightly reduced. Interestingly, instead of undergoing p53-dependent apoptosis, senescent fibroblasts underwent necrosis. Furthermore, we found that old cells were unable to stabilize p53 in response to DNA damage. Exogenous expression or stabilization of p53 with proteasome inhibitors in old fibroblasts restored their ability to undergo apoptosis. Our results suggest that stabilization of p53 in response to DNA damage is impaired in old fibroblasts, resulting in induction of necrosis. The role of this phenomenon in normal aging and anticancer therapy is discussed.
Normal animal cells, with few exceptions, do not divide indefinitely. Eventually, cell divisions are arrested and cells enter cellular or replicative senescence (for a review, see reference 10). Replicative senescence is especially stringent in human cells, which almost never spontaneously immortalize (41)
Cellular senescence is a genetically controlled process (for a review, see references 56 and 57). There is strong support for the theory that telomere shortening limits the longevity of human cells in culture (9, 26, 27). It has been proposed that telomere shortening eventually causes chromosome instability, leading to the activation of the DNA damage response pathway followed by p53-dependent cell cycle arrest and senescence (59). Furthermore, the important role of p53 in cellular senescence is supported by the following observations. First, functional inactivation of p53 rescues cells from senescence-related growth arrest and instead they enter crisis at a delayed time point (7, 24, 48, 49, 54). Second, the p21WAF1 gene, which encodes an inhibitor of cyclin-dependent kinases (15, 28), is transcriptionally regulated by p53 (17, 18) and is overexpressed in senescent cells (45). Third, exposure of human diploid fibroblasts to γ-irradiation leads to a p53-dependent, prolonged G1 arrest and induction of p21WAF1 expression that is reminiscent of senescence (14). Fourth, the DNA binding and transcriptional activities of p53 increase with cell age, and in most cases this occurs in the absence of any marked increase in the level of p53 (1, 4, 36, 47, 60).
p53 is more widely recognized for its role as a tumor suppressor which mediates growth arrest and apoptosis in response to DNA damage (25, 37). Apoptosis is generally viewed as the “cleanest” way a cell can die. Apoptotic cell death does not have a negative effect on surrounding tissues and is not accompanied by inflammation. Analysis of p53 knockout mice showed that in the absence of p53, cells with damaged DNA do not enter cell cycle arrest and die by an alternative death pathway, necrosis (43). Necrosis is characterized by the loss of membrane integrity, cell swelling, and release of the cell contents, which may result in inflammation (20). Not much is known about the molecular mechanisms underlying the necrotic pathway.
Little is known of how cell aging affects the ability of the cells to respond to genotoxic stress. It has been observed that senescent cells are resistant to apoptotic stimuli (22, 62). However the mechanism by which senescent cells resist apoptotic death is not well understood. A different set of data indicates that p53 is modified in senescent cells (60). It has recently been demonstrated that senescence is associated with specific posttranslational modifications of p53 (63).
Based on these observations, we hypothesized that the inability of senescent cells to undergo apoptosis is caused by changes in the state of p53. Furthermore, as senescent cells are resistant to apoptosis, we were interested in understanding the fate of senescent cells subjected to apoptotic stimuli. For this purpose, we studied the effects of DNA-damaging agents, including actinomycin D, UV irradiation, cisplatin, and etoposide, that are known to induce different types of damage on cells at early and late passages in culture. As a model system, we chose the WI-38 human primary diploid fibroblasts, the best-characterized cells with respect to cellular senescence (4, 47).
We found that apoptosis induced by actinomycin D, UV, or a low dose of cisplatin was p53-dependent in WI-38 fibroblasts, whereas apoptosis induced by etoposide or a high dose of cisplatin was p53-independent. Senescent fibroblasts were unable to induce p53-dependent apoptosis, even though p53-independent apoptosis was only slightly reduced. Instead of undergoing p53-dependent apoptosis in response to DNA damage, senescent fibroblasts underwent necrosis. Artificial stabilization of p53 in old cells by proteasome inhibitors led to the induction of apoptosis. Moreover, transient expression of the wild-type p53 in the old cells fully restored their ability to undergo p53-dependent apoptosis, indicating that the absence of p53-dependent apoptosis in old cells was due to their inability to stabilize p53. Our results suggest that stabilization of p53 in response to DNA damage is impaired in old fibroblasts, resulting in a change of the death pathway from apoptosis to necrosis.
MATERIALS AND METHODS
Cell culture and transfection.
WI-38 human diploid fibroblasts (American Type Culture Collection) were cultured in minimal essential medium with all nonessential amino acids, 10% fetal bovine serum, and 1 mM pyruvate. Twice a week, 105 cells were passaged on a new plate using 0.25% trypsin–EDTA. Care was taken not to let the cells reach 70% confluency.
Based on previous observation (4, 47), cells were defined as young if they had completed 60% of their life span (32 to 40 population doublings; passage 21) and as old if they had completed 90% of their life span (54 to 56 population doublings; passage 29).
Genotoxic stress was induced by the addition of one of the following DNA-damaging agents to the culture medium of fibroblasts at 40 to 50% confluency: actinomycin D (25 ng/ml), cisplatin (1 or 10 μg/ml), or etoposide (30 μM). UV irradiation (4 J/m2) was performed by a UV cross-linker (Stratagene).
Transfection experiments were carried out using FuGENE-6 (Roche) according to the manufacturer's instructions. Wild-type p53 was expressed under a cytomegalovirus (CMV) promoter from the pC53-SN3 plasmid (5) provided by R. Vogelstein (The Johns Hopkins University School of Medicine). The dominant-negative p53 fragment (DD) was expressed under the CMV promoter from the pCMV-DD plasmid (53) provided by M. Oren (Weizmann Institute of Science). E6 was expressed under the simian virus 40 promoter from the pSG5-E6 plasmid (55) provided by L. Sherman (Tel Aviv University).
Enrichment of transfected cells was performed with the MACSorter Kk kit from Miltenyi Biotec according to the manufacturer's instructions. Briefly, the method is based on cotransfection with a fragment consisting of the extracellular loop of the Kk protein, followed by application of anti-Kk antibodies bound to magnetic beads for the selection of transfected cells.
Following the transfection, the cells were allowed to recover for 24 or 48 h and then were harvested and incubated with antibodies conjugated to magnetic beads, transfected cells were selected by passing them through a magnetic column. The efficiencies of transfection and sorting were estimated by fluorescence-activated cell sorter (FACS) analysis in control experiments using a plasmid containing green fluorescent protein (GFP) under the CMV promoter.
Detection of apoptosis by DNA ladder.
Detached and adherent fibroblasts were harvested, and equal numbers of cells (5 × 105) were suspended in 30 μl of sample buffer (10% glycerol, 10 mM Tris[pH 8], 0.1% [wt/vol] bromophenol blue) mixed at 1:1 ratio with 10-mg/ml RNase A solution. The cells were loaded on an agarose gel which contained two parts: the lower part (from the comb to the end of the gel) consisted of 0.8% agarose in Tris-borate-EDTA; the upper part (from the comb to the beginning of the gel) consisted of 0.8% agarose, 2% sodium dodecyl sulfate (SDS), and 64 μg of proteinase K/ml in Tris-borate-EDTA. The cells were electrophoresed for 10 h at 60 V at room temperature. The gel was stained with 2 mg of ethidium bromide/ml in water for 1 h and then destained with water (16).
Western blot analysis.
Detached and adherent fibroblasts were harvested, and equal numbers of cells (106) were lysed in protein sample buffer with 5% β-mercaptoethanol and 1 mM phenylmethylsulfonyl fluoride, boiled for 10 min, and loaded on an SDS-polyacrylamide gel. A 7.5 and a 10% running gel were used for the detection of poly(ADP-ribose) polymerase (PARP) and p53, respectively. The proteins were transferred to a nitrocellulose membrane using a semidry transfer cell (Bio-Rad). The blots were probed with either a human p53-specific monoclonal antibody (DO-1) or anti-PARP monoclonal antibody (Biomol). The bands were visualized with the aid of the Super Signal kit (Pierce).
Analysis of apoptosis and necrosis by acridine orange staining.
Cells were fixed in 3 ml of a solution containing 80% ethanol and 20% Hanks balanced salt solution (HBSS) and stored at −20°C for a period not exceeding 1 week. On the day of the assay, the cells were gently remixed and centrifuged at 800 rpm on a Sorval GLC-3 centrifuge. The pellets were gently resuspended, washed in 1 ml of HBSS, and centrifuged at 1,000 rpm on a Hettich microcentrifuge. The cells were resuspended in 1 ml of a 1:30 solution of RNase in HBSS and incubated at 37°C for 1.5 h. The cells were then centrifuged at 1,200 rpm in an Eppendorf centrifuge, gently resuspended in 200 μl of HBSS, and transferred to FACS tubes, and 0.5 ml of 0.1 M HCl in HBSS was added. After approximately 1 min, the acid denaturation was quenched by the addition of 2 ml of a solution of 90% citric acid, 10% Na2HPO4, and 0.06% acridine orange (Molecular Probes). The cells were analyzed in a FACS SORT flow cytometer (Becton Dickinson). An excitation wavelength of 488 nm was used, 575- and 650-nm emissions were collected, and the data were analyzed with CellQuest software (Becton Dickinson). Low-speed centrifugation and gentle handling of the fixed cells were critical to the success of this assay, as they prevented aggregation and breakage of the cells.
Detection of necrosis by release of DNA.
The cellular DNA fragmentation enzyme-linked immunosorbent assay kit from Boehringer Mannheim was used for the detection of bromodeoxyuridine (BrdU)-labeled DNA released from necrotic cells into the cell culture medium. Briefly, WI-38 human fibroblast cells were incubated in culture with the thymidine analogue BrdU, which is incorporated into the genomic DNA. BrdU was added to the medium 24 or 48 h before treatment. The cells were treated with various drugs, as described above, in the presence of BrdU, and the supernatants of the cell cultures were collected after different time intervals. BrdU did not affect the rate of growth, apoptosis, or necrosis of the fibroblasts in the time window used, as determined by FACS analysis of BrdU-treated and untreated cells. The DNA fragments were captured with an anti-DNA antibody bound to a Nunc-Immuno flat-bottom plate and were detected by an anti-BrdU antibody-peroxidase conjugate. The photometric measures were done at a wavelength of 450 nm with a reference wavelength of 690 nm.
RESULTS
Apoptosis in human primary fibroblasts.
To examine whether cellular aging affects the ability of cells to undergo apoptosis in response to genotoxic stress, we chose WI-38 primary human fibroblasts. Since the detection of apoptosis in fibroblasts is controversial (3, 14, 22, 29, 64), we first analyzed the abilities of various DNA-damaging agents to induce apoptosis in these cells.
Exponentially growing populations of young human fibroblasts were treated with the following DNA-damaging agents: actinomycin D, UV irradiation, etoposide, and low (0.5- to 2-μg/ml) and high (5- to 20-μg/ml) concentrations of cisplatin. Apoptosis was determined by the appearance of apoptotic features, such as DNA fragmentation, chromatin condensation, and cleavage of PARP.
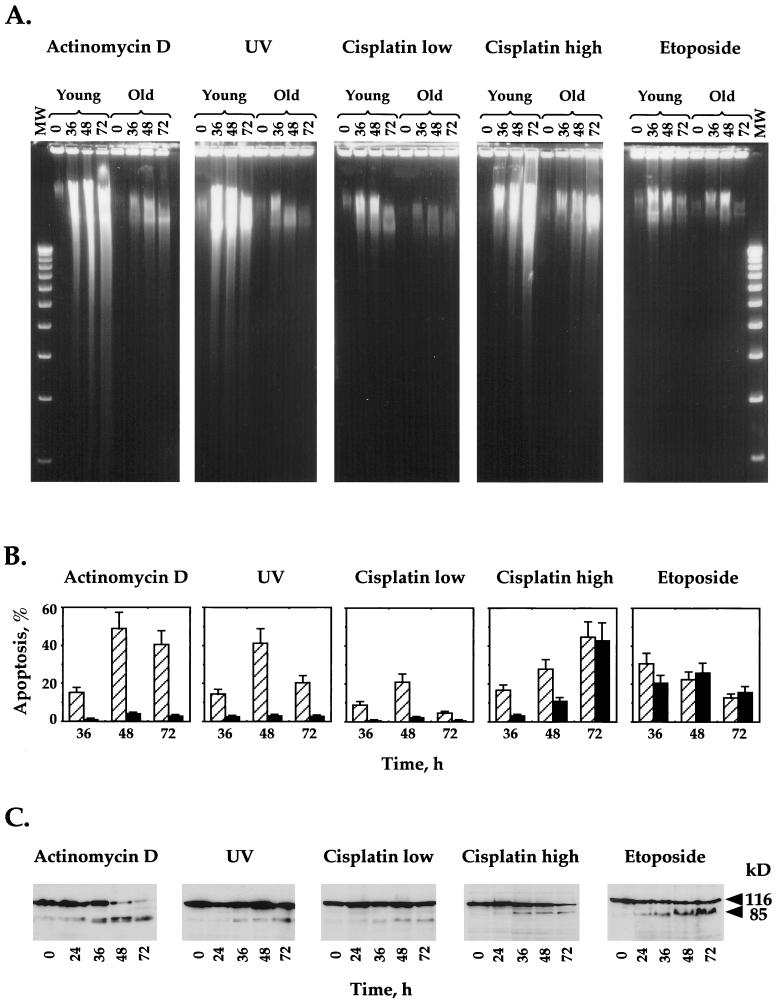
DNA fragmentation was analyzed by the DNA ladder technique. It was previously shown that fibroblasts produce mostly DNA fragments of high molecular weight not followed by the internucleosomal DNA cleavage typical of hematopoietic cells (8, 16, 46). Furthermore, it was suggested that the cleavage of DNA into high-molecular-weight, approximately 50- and 300-kb fragments is an essential early step in apoptosis for all cell types, in contrast to the later and nonessential internucleosomal DNA fragmentation (8, 46). Therefore, fibroblasts undergoing apoptosis yield a high-molecular-weight smear instead of the typical 180-bp DNA ladder. The optimal time and drug concentration for the induction of apoptosis were defined for every DNA-damaging agent (data not shown). Upon treatment of young cells with actinomycin D, UV irradiation, etoposide, and low and high concentrations of cisplatin, we detected the appearance of high-molecular-weight smears in a time-dependent manner (Fig. 1A). Different levels of DNA fragmentation were observed for the different agents.
FIG. 1.
Apoptosis in young and old normal human fibroblasts induced by DNA-damaging agents. Young and old fibroblasts were treated with various DNA-damaging agents (actinomycin D, UV, etoposide, and low [1-μg/ml] and high [10-μg/ml] concentrations of cisplatin), and induction of apoptosis was analyzed by DNA ladder (A), acridine orange staining for chromatin condensation (B), and PARP cleavage (C). (A) After 0, 36, 48, and 72 h of induction, all detached and adherent fibroblasts were collected, and equal numbers of the cells were directly subjected to gel electrophoresis as described in Materials and Methods. MW, 1-kb ladder standard. (B) Percent apoptosis was determined by FACS analysis after acridine orange staining of fibroblasts treated with genotoxic agents. The hatched bars represent young fibroblasts, and the solid bars represent old fibroblasts. All of the experiments were repeated at least three times, and standard errors are shown. (C) Induction of apoptosis in young human fibroblasts was further confirmed by Western blotting with anti-PARP antibodies. The full-length (113-kDa) and apoptosis-specific (89-kDa) fragments of PARP are indicated.
Chromatin condensation was analyzed by acridine orange DNA staining followed by FACS analysis. Figure 1B shows similar kinetics of apoptosis for young cells treated with actinomycin D, UV irradiation, and a low concentration of cisplatin. A peak of apoptosis (20 to 60%) was reached 48 h after treatment. The reduction in the percentage of apoptosis measured 72 h after treatment may be due to the disintegration of the apoptotic cells. The percentage of spontaneous apoptosis in untreated cells did not exceed 2%. Cells treated with high concentrations of cisplatin and etoposide exhibited different kinetics of apoptosis than cells treated with the other drugs. It should be noted that low and high concentrations of cisplatin display different patterns of apoptosis: a low concentration leads to a peak at 48 h, and a high concentration shows a linear increase up to 72 h. The apoptotic patterns obtained for the young cells by DNA condensation analysis measured by acridine orange staining followed by FACS are in good correlation with those obtained by the DNA fragmentation assay.
Finally, cleavage of PARP, which is indicative of apoptosis-induced caspase-3 and/or -8 activity, was analyzed by Western blotting with an anti-PARP antibody (Fig. 1C). A typical apoptotic pattern of the uncleaved form of PARP (113 kDa) with an increase in the level of the cleaved fragment of PARP (89 kDa) was detected between 24 and 36 h after all treatments. This occurred despite the differences in the kinetics of the drugs.
In conclusion, we have demonstrated by three different techniques that young human fibroblasts are able to undergo apoptosis in response to actinomycin D, UV irradiation, etoposide, and cisplatin.
Next, we analyzed the induction of apoptosis in old WI-38 human fibroblasts with the same set of DNA-damaging agents used for young cells. Apoptosis was determined by the DNA ladder and acridine orange techniques under the same conditions as for the young fibroblasts. According to the DNA ladder patterns obtained, it appears that young and old cells exhibit comparable fragmentation smears in response to high concentrations of cisplatin and etoposide (Fig. 1A). However, following treatment with actinomycin D, UV irradiation, or a low concentration of cisplatin, old cells show a much lower level of apoptosis than young cells. This difference is more pronounced in the acridine orange analysis. In this case, no significant levels of apoptosis are detected in old cells in response actinomycin D, UV irradiation, or a low concentration of cisplatin, whereas high levels of apoptosis are evident in young cells (Fig. 1B). In contrast, similar patterns of apoptosis were seen in young and old cells in response to etoposide. Treatment of old cells with a high concentration of cisplatin resulted in a low level of apoptosis at 36 h, followed by a marked increase at 48 h after treatment. By 72 h, the level of apoptosis in old cells was high and comparable to that of young cells.
To summarize, we found that the treatments with etoposide and high concentrations of cisplatin induced similar levels of apoptosis in both young and old cells whereas actinomycin D, UV irradiation, and a low concentration of cisplatin induced high levels of apoptosis in young cells and much lower levels of apoptosis in old cells. This possibly means that cellular response pathways induced by etoposide and high concentrations of cisplatin remain unchanged when cells enter senescence, while response pathways induced by actinomycin D, UV irradiation, and a low concentration of cisplatin are altered in senescent cells.
Human fibroblasts undergo p53-dependent or p53-independent apoptosis.
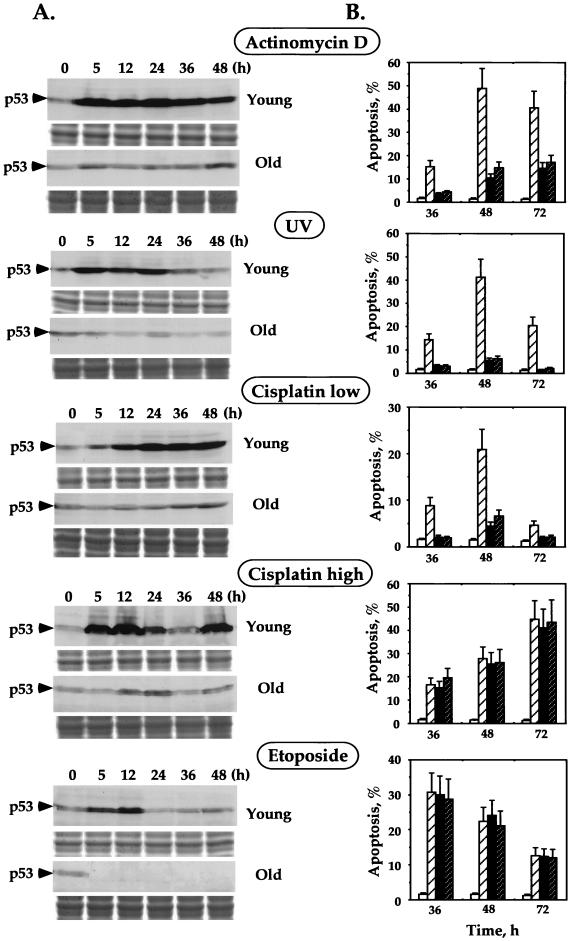
We were interested in studying whether there is a correlation between the differential apoptotic responses of the young and old cells and the tumor suppressor protein p53. Apoptosis is known to occur via p53-dependent and p53-independent pathways, depending on the treatment applied and the cell type. We first investigated the involvement of p53 in the induction of apoptosis by genotoxic stress in young human fibroblasts. Upon treatment of young fibroblasts with actinomycin D, cisplatin, and UV, we detected an increase in p53 levels by Western blotting with DO-1 anti-p53 antibodies (Fig. 2A). However, etoposide failed to induce accumulation of p53 (Fig. 2A), which suggests that etoposide, in contrast to actinomycin D, cisplatin, and UV, is unable to stabilize p53. In order to test whether stabilization of p53 is accompanied by its activation, we analyzed the induction of the p53 downstream gene Mdm2 upon genotoxic stress. Induction of Mdm2 was monitored by Western blotting with the anti-Mdm2 antibody (data not shown). We observed strong correlation between accumulation of p53 and induction of the Mdm2 gene in the cells treated with actinomycin D, UV, and low concentrations of cisplatin. Even though fibroblasts treated with high concentrations of cisplatin exhibit high levels of p53, we did not detect any induction of the Mdm2 gene. Etoposide-treated cells, which were found not to accumulate p53 (see above), were unable to induce Mdm2 in response to stress.
FIG. 2.
Role of p53 in the induction of apoptosis in young and old human fibroblasts. (A) Accumulation of p53 in young and old fibroblasts upon treatment with various DNA-damaging agents. After 0, 5, 12, 24, 36, and 48 h of induction, all detached and adherent fibroblasts were collected, and equal numbers of cells were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) followed by Western blotting with DO-1 anti-p53 antibodies. The blots were stained with India ink to check the equivalence of protein transfer. One-third of each sample was subjected to SDS-PAGE and stained with Coomassie blue to demonstrate equal loading of samples (shown below each Western blot). (B) Inactivation of p53-dependent apoptosis by transient expression of dominant-negative p53 fragment (DD) or human papillomavirus type 16 protein E6. Young fibroblasts transiently transfected with empty vector (hatched bars), plasmid expressing DD (solid bars), or E6 (dark hatched bars) were treated with various DNA-damaging agents for 36, 48, and 72 h. The level of apoptosis was determined by acridine orange staining followed by FACS analysis. The open bars represent the level of apoptosis in the transfected but untreated cells. All the experiments were repeated at least three times, and standard errors are shown.
We next examined whether the increase of p53 protein is associated with the induction of apoptosis in fibroblasts. For this purpose, we used functional depletion of p53 by a dominant-negative fragment and the viral E6 protein. The minimal requirement for the dominant-negative function of mutant p53 is its C terminus from amino acids 302 to 390. Transient expression of this minimal dominant-negative p53 fragment, designated DD, leads to strong functional inactivation of endogenous p53 (53). To exclude the possible gain-of-function effect of the DD fragment on suppression of apoptosis, we used a second method of p53 inactivation. To this end, we depleted p53 by the transient expression of the human papillomavirus type 16 protein E6, which binds to p53 and promotes its rapid proteolysis (21, 51).
Young fibroblasts were transiently transfected with plasmids harboring the dominant-negative DD or E6 under the strong constitutive CMV and simian virus 40 promoters, respectively. Empty vectors were used as a control. Inactivation of p53 was confirmed by cotransfection of DD or E6 with the plasmids carrying the reporter (luciferase) gene under a p53-inducible RGC or Bax promoter. In the cells transfected with the plasmids harboring DD or E6, the level of luciferase expression was strongly reduced compared to that in control cells transfected with empty vectors (data not shown). The efficiency of transfection was ∼15%, as determined by cotransfection with the plasmid containing GFP under the CMV promoter followed by FACS analysis. We enriched the population for up to 80% of transfected cells using the magnetic cell sorter (see Materials and Methods). After recovery from transfection and cell sorting, the cells were treated with actinomycin D, UV irradiation, etoposide, and low and high concentrations of cisplatin, and apoptosis was analyzed by the more quantitative acridine orange method. The functional depletion of p53 in fibroblasts markedly decreased their ability to undergo apoptosis in response to actinomycin D, UV irradiation, or a low concentration of cisplatin (Fig. 2B). However, apoptosis induced by etoposide or a high concentration of cisplatin was not affected. Functional depletion of p53 by the dominant-negative DD fragment and E6 gave similar results, indicating that only inactivation of p53 is responsible for the observed effect rather than other effects of the DD or E6 protein. These results indicate that young WI-38 human fibroblasts undergo p53-dependent apoptosis in response to treatment with actinomycin D, UV irradiation, and a low concentration of cisplatin. In contrast, etoposide and a high concentration of cisplatin induce p53-independent apoptosis.
As described above, old WI-38 human fibroblasts predominantly underwent apoptosis in response to high concentrations of cisplatin and etoposide and to a much lower extent in response to actinomycin D, UV irradiation, and a low concentration of cisplatin. In light of the observations that actinomycin D, UV, and a low concentration of cisplatin induced p53-dependent apoptosis in young fibroblasts, it appears that old fibroblasts are able to undergo p53-independent apoptosis but not p53-dependent apoptosis.
Old human fibroblasts are unable to stabilize p53 in response to genotoxic stress.
We have found that, unlike young cells, old fibroblasts exhibit negligible levels of p53-dependent apoptosis in response to genotoxic stress. It is well accepted that p53-dependent apoptosis induced by genotoxic stress requires p53 protein stabilization. Hence, we analyzed the stabilization of p53 in old fibroblasts after treatment with actinomycin D, UV irradiation, etoposide, and low and high concentrations of cisplatin, using Western blotting with the DO-1 anti-p53 antibody (Fig. 2A). We detected very low or no stabilization of the p53 protein in old cells compared to that in young cells. However, the basal levels of p53 in young and old fibroblasts were comparable (Fig. 2A), as shown previously (1, 4). This suggests that the reduction in p53-dependent apoptosis in old fibroblasts may be due to their inability to stabilize p53.
Old human fibroblasts that are unable to enter p53-dependent apoptosis undergo necrosis.
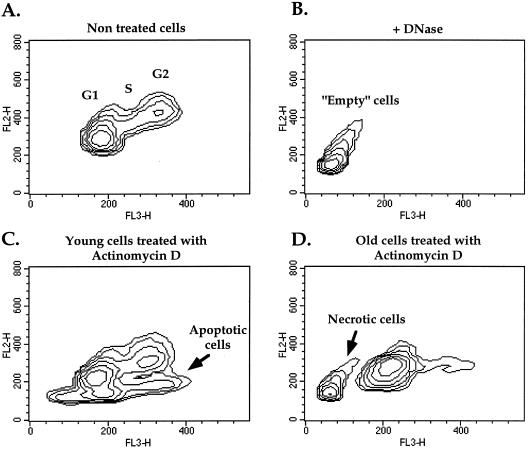
The above-mentioned results showed that old human fibroblasts are unable to undergo p53-dependent apoptosis in response to DNA damage. A question arises as to the fate of those cells with damaged DNA. During preliminary microscopic examination, we observed similar death rates in the young and old cells treated with all the tested DNA-damaging agents (data not shown). Hence, we suspected that necrosis was responsible for the death of DNA-damaged old cells. Release of the cellular matrix from the cell and the appearance of “empty” ghost cells, consisting of only cell membranes, are typical necrotic features (20, 31, 34). As mentioned previously, acridine orange staining followed by FACS analysis was effective in differentiating between viable and apoptotic WI-38 fibroblasts. Acridine orange binds to DNA and hence can detect sub-G1 DNA content. Moreover, it is dichromatic and undergoes a shift from green to red fluorescence when it binds to the condensed DNA typical of apoptosis. Therefore, it allows the detection of apoptosis even in cells that do not exhibit a sub-G1 content, like fibroblasts. Figure 3C shows an example of the fluorescence shift of acridine orange due to apoptosis induced by actinomycin D treatment.
FIG. 3.
Detection of necrotic and apoptotic cells by acridine orange staining followed by FACS analysis. (A) Density plot of normal cell cycle distribution of untreated fibroblasts. Cell populations at the G1, S, and G2 stages of the cell cycle are indicated. (C) Density plot of empty cells obtained from normal fibroblasts by DNase and RNase treatment for 1.5 h prior to acridine orange staining. This distinct population of empty cells (without DNA and RNA) was used to set the parameters for the identification of the population of necrotic cells. (B and D) Typical examples of young and old fibroblasts undergoing apoptosis or necrosis upon treatment with actinomycin D for 48 h.
To examine whether the acridine orange technique can also detect necrotic cells as a population separate from viable and apoptotic cells, we treated viable cells with DNase and RNase to produce the empty WI-38 cells typical of necrosis and assayed them by acridine orange. We obtained a clearly segregated population, with a fluorescence lower and greener than that of viable cells (Fig. 3B), which is probably the result of acridine orange staining of membrane-bound glycosaminoglycans and proteoglycans, as shown previously (13). We therefore designated this population necrotic cells.
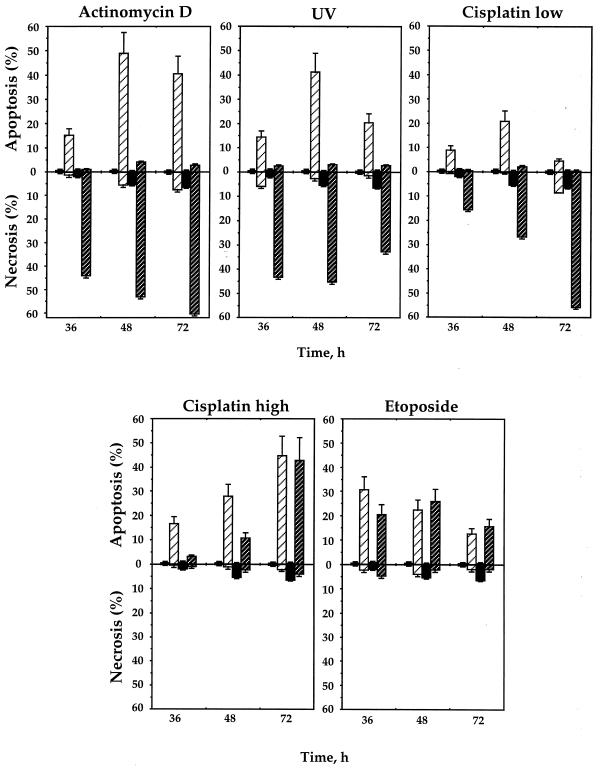
Old and young human fibroblasts treated with actinomycin D, UV irradiation, etoposide, and low and high concentrations of cisplatin were stained with acridine orange and analyzed by FACS. The accumulation of necrotic and/or apoptotic cells was observed following all treatments (Fig. 4). Strikingly, in response to actinomycin D, UV irradiation, and a low concentration of cisplatin, old fibroblasts exhibited a population that corresponded exactly to the population defined above as necrotic (compare Fig. 3B and D). Under the same conditions, young cells underwent apoptosis (Fig. 3C). A time-dependent increase in necrotic cells can be seen in Fig. 4, following treatment with actinomycin D, UV irradiation, and a low concentration of cisplatin. No significant levels of apoptosis were detected in the old cells in any of these treatments. Treatment of young and old cells with a high concentration of cisplatin and etoposide induced apoptosis to similar extents at most time points used, with no significant levels of necrosis.
FIG. 4.
Induction of apoptosis and necrosis in young and old human fibroblasts by various DNA-damaging agents. After 36, 48, and 72 h of treatment, the cells were stained with acridine orange and analyzed by FACS (see Materials and Methods), which allowed the measurement of both apoptosis and necrosis. The open and solid bars represent untreated young and old cells, respectively. The levels of apoptosis and necrosis in young untreated cells were too low to be seen on the graph. The hatched bars represent the level of apoptosis or necrosis in treated cells, and light and dark hatched bars represent young and old cells, respectively. All the experiments were repeated at least three times, and standard errors are shown.
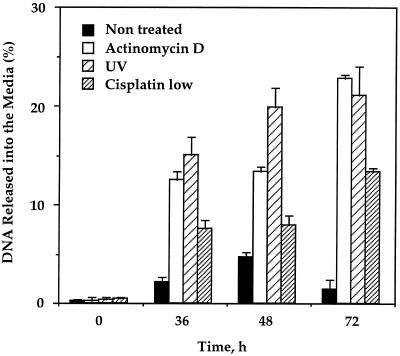
To further confirm that cell death in senescent cells occurred by necrosis, we used a different method for detection of necrosis which is based on the release of DNA from necrotic cells into the medium. Following treatment of senescent cells with actinomycin D, UV irradiation, and a low concentration of cisplatin, an increase in the DNA level detected in the medium was observed relative to that of untreated cells (Fig. 5). We have not detected any significant levels of DNA release for young cells. The DNA release increased in a time-dependent manner in all cases. This indicates that old fibroblasts treated with actinomycin D, UV irradiation, and a low concentration of cisplatin undergo necrosis in response to the treatments. The kinetics and relative extent of necrosis detected by DNA release were similar to those detected by acridine orange. However, in all the treatments the percentage of necrotic cells detected by DNA release was twice as small as that with acridine orange. This can be explained by the fact that free DNA released into the medium undergoes rapid degradation, while empty cells (membrane vesicles) detected by acridine orange are much more stable.
FIG. 5.
Induction of necrosis in old human fibroblasts by actinomycin D, UV, and low concentration of cisplatin. Necrosis was analyzed by the release of DNA from necrotic cells into the medium. Total DNA of the old fibroblasts was metabolically labeled with BrdU for 24 or 48 h prior to induction. DNA released into the medium upon induction of necrosis was quantified with a cellular DNA fragmentation enzyme-linked immunosorbent assay kit (see Materials and Methods). The percent of released DNA from the total labeled DNA is shown. Experiments were repeated five times, and standard deviations are indicated.
These findings suggest that towards senescence, human fibroblasts change their death pathway from p53-dependent apoptosis to necrosis in response to genotoxic stress. DNA-damaging agents that cause p53-dependent apoptosis in young cells induce necrosis in old cells.
Stabilization of p53 in old human fibroblasts restores their ability to undergo apoptosis.
To examine whether the reduction in p53-dependent apoptosis in old fibroblasts is due to their inability to stabilize p53, we used two approaches. One was aimed at stabilizing p53 in old cells by the inhibition of its proteolysis, and the other approach was to exogenously express the p53 protein by transient transfection of wild-type p53.
(i) Stabilization of p53 by proteasome inhibitors.
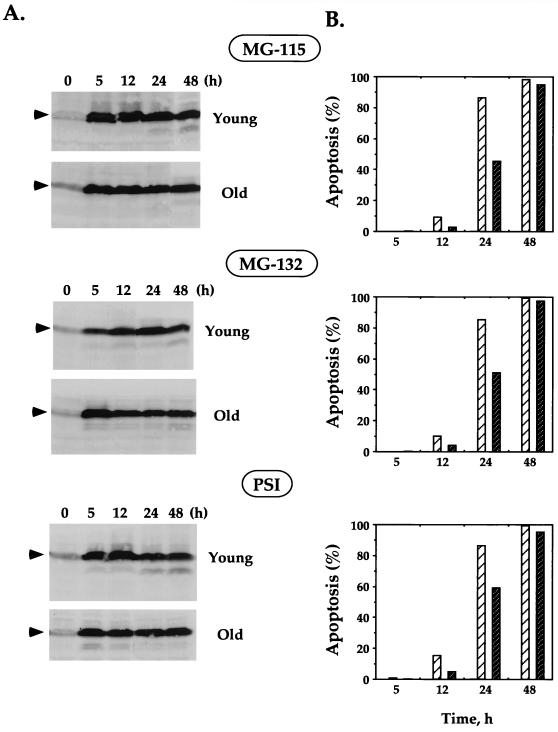
Degradation of p53 is mediated by the ubiquitin-proteasome pathway (40). In order to induce the accumulation of endogenous p53 in old fibroblasts, we used the proteasome inhibitors MG-115, MG-132, and PSI (proteasome inhibitor I), which were shown to stabilize p53 (11, 23, 39). Importantly, it was demonstrated that apoptosis of immortalized cells induced by MG-115 and PSI is p53 dependent, suggesting that stabilization of p53 plays a key role in apoptosis induced by proteasome inhibitors (39).
Young and old human fibroblasts were treated with proteasome inhibitors, and the accumulation of p53 was analyzed up to 48 h after the treatment. All the tested proteasome inhibitors induced rapid and strong accumulation of p53 in young and old fibroblasts (Fig. 6A). These results clearly demonstrate that even though p53 in the old fibroblasts is not stabilized in response to genotoxic stress, it could be stabilized by proteasome inhibitors. Furthermore, the kinetics of p53 accumulation confirmed previous observations that the rates of synthesis of p53 in young and old cells are similar (1, 4).
FIG. 6.
Stabilization of p53 and induction of apoptosis in young and old fibroblasts treated with proteasome inhibitors. Young and old fibroblasts were treated with the following proteasome inhibitors: MG-115 (30 μM), MG-132 (10 μM), and PSI (30 μM). (A) Stabilization of p53 at various time points after treatment was analyzed by Western blotting with DO-1 antibodies. The p53 protein is indicated by arrowheads. The blots were stained with India ink to check the equivalence of protein loading and transfer, and the blots showing equal loading and transfer within young and old cells are presented. (B) Induction of apoptosis was analyzed by acridine orange staining followed by FACS analysis. The percent apoptosis in young (light hatched bars) and old (dark hatched bars) cells treated with proteasome inhibitors is shown. The level of apoptosis in untreated cells (young and old) was too low to be seen on the graph. Experiments were repeated at least three times; standard errors were less than 3% of the average and are not shown on the graph.
In order to examine the induction of apoptosis in the cells treated by proteasome inhibitors, the cells were analyzed by acridine orange DNA staining. Rapid and strong induction of apoptosis was observed in both young and old fibroblasts treated with MG-115, MG-132, and PSI (Fig. 6B). It should be noted that up to 24 h following this treatment, the level of apoptosis in old cells was lower than that in young cells. However, both young and old cells reached ∼100% apoptosis 48 h after treatment. The fact that the proteasome inhibitors that are associated with p53 stabilization were able to induce apoptosis in old cells suggests that induction of apoptosis under these conditions was p53 dependent. Apoptosis induced by proteasome inhibitors was higher than apoptosis induced by other treatments. This can be explained by the fact that proteasome inhibitors lead to the accumulation of other regulatory molecules in addition to p53. However, it has been shown that modulation of p53 turnover is a key event in apoptosis induced by proteasome inhibitors (39).
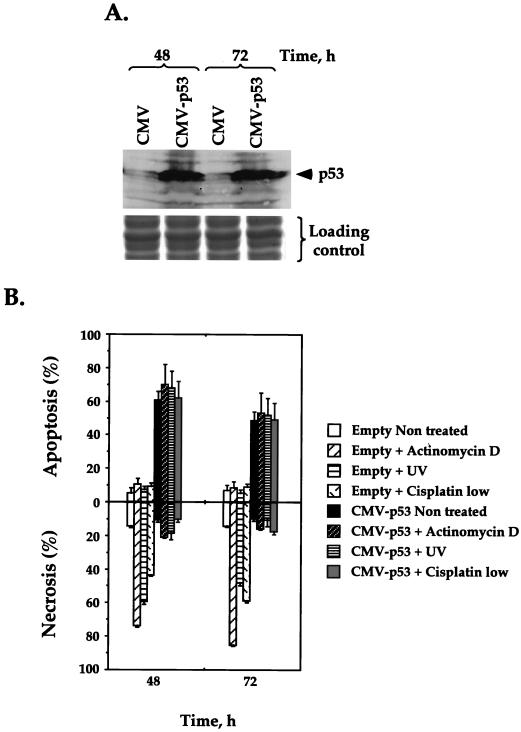
(ii) Exogenous expression of wild-type p53.
To confirm that the observed apoptosis induced by the proteasome inhibitors is indeed due to an increase in p53 levels rather than stabilization of other factors, we tested whether overexpression of exogenous p53 would force old cells to enter apoptosis instead of undergoing necrosis. To this end, old human fibroblasts were transfected with a plasmid carrying wild-type p53 under the CMV promoter. The efficiency of transfection was ∼15%, as monitored by cotransfection with a GFP-harboring plasmid. The population of transfected cells was enriched up to 80% by magnetic cell sorting (see Materials and Methods). The exogenous p53 was highly expressed in transfected old fibroblasts within 48 h after transfection, as shown by Western blotting (Fig. 7A). This high level of p53 in transfected cells induced massive apoptosis even without genotoxic stress (Fig. 7B). Similar massive apoptosis was observed with p53-transfected old fibroblasts that were treated with actinomycin D, UV irradiation, and a low concentration of cisplatin. This may indicate that following exogenous expression of p53, the cells reached a maximum level of p53-dependent apoptosis that could not be further increased by drug treatment. Importantly, the level of DNA damage-induced necrosis was significantly reduced by overexpression of p53 in the old cells in comparison to that observed for the old cells transfected with the control vector. Therefore, by overexpression of exogenous p53, we were able to override senescence-related changes in the old cells and switch them back from the necrotic to the apoptotic pathway of cell death.
FIG. 7.
Transient expression of p53 in old fibroblasts restores their ability to undergo apoptosis and inhibits their ability to undergo necrosis. Old fibroblasts were transiently transfected with a plasmid harboring the wild-type p53 gene under the CMV promoter or with the control plasmid containing the CMV promoter alone. The population of transfected cells was enriched using the MACSorter Kk kit. (A) The levels of p53 expression at 48 and 72 h after transfection were analyzed by Western blotting with DO-1 antibodies. The blots were stained with India ink to check the equivalence of protein transfer. One-third of each sample was subjected to SDS-polyacrylamide gel electrophoresis and stained with Coomassie blue to demonstrate equal loading of samples (shown below the Western blot). (B) Immediately following the transfection, cells were subjected to genotoxic stress (actinomycin D, UV, or a low concentration of cisplatin). The levels of induced apoptosis and necrosis 48 and 72 h after treatment were analyzed by acridine orange staining followed by FACS. Experiments were repeated at least three times, and standard errors are shown.
Taken together, these results demonstrate that the cellular milieu of old fibroblasts permits the expression of high levels of p53 sufficient for apoptosis. Furthermore, the apoptotic machinery downstream of p53 is fully functional in old cells.
DISCUSSION
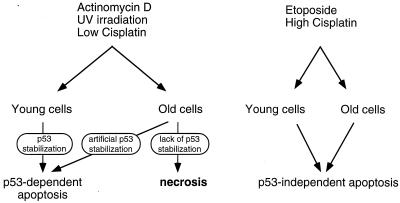
We were the first to analyze the response of senescent cells to genotoxic stress and the role of p53 in this process. Our main finding is that old cells do not induce p53-dependent apoptosis in response to genotoxic stress, which is likely the result of an inability to stabilize p53. Upon DNA damage, senescent cells that are unable to undergo apoptosis die by necrosis. A summary of our results is shown schematically in Fig. 8.
FIG. 8.
Model of the death pathways taken by young and old human fibroblasts in response to various genotoxic stresses.
Apoptosis in young human fibroblasts.
Based on the analysis of DNA fragmentation, chromatin condensation, and the cleavage of PARP, we demonstrated that young human fibroblasts are able to undergo apoptosis in response to DNA insults mediated by actinomycin D, UV irradiation, etoposide, and low and high concentrations of cisplatin. Assays of chromatin condensation and caspase-dependent cleavage of PARP in treated fibroblasts showed classical apoptosis. In the DNA fragmentation assay, high-molecular-weight smears were obtained following all treatments, which is in accordance with previous studies reporting that fibroblasts are unable to produce internucleosomal DNA fragmentation during apoptosis (8, 16, 46). This explains why the methods based on detection of extensive DNA fragmentation (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) or exclusion of the small DNA fragments from the nucleus (propidium iodide-based detection of a sub-G1 population) would not be sensitive enough for the analysis of apoptosis in normal human fibroblasts.
Using functional depletion of p53, we were able to determine that young fibroblasts undergo p53-dependent apoptosis in response to actinomycin D, UV irradiation, and a low concentration of cisplatin and undergo p53-independent apoptosis in response to etoposide and a high concentration of cisplatin. The choice to undergo either p53-dependent or p53-independent apoptosis may be a function of the type of DNA damage, the level of damage, or the cell cycle phase within the different cellular milieux (2, 33, 42).
Senescent human fibroblasts are resistant to p53-dependent apoptosis and instead undergo necrosis.
We have observed that old fibroblasts were unable to undergo p53-dependent apoptosis, while the ability to undergo p53-independent apoptosis was only slightly affected. It was previously reported that, unlike young cells, old fibroblasts were unable to undergo apoptosis in response to stress caused by serum deprivation or oxygen radicals (22, 62). In order to understand what happened with the damaged old cells which were unable to induce p53-dependent apoptosis, we performed detailed analysis of those cells. We found that senescent fibroblasts were dying via a different pathway—necrosis. The necrotic death that we observed was characterized by the loss of cellular content and a lack of chromatin condensation and membrane blebbing. Our observation that the inhibition of an apoptotic pathway results in necrotic cell death correlates well with data from recent studies that used broad-spectrum caspase inhibitors to block apoptosis (12, 30, 32, 61). Furthermore, data from an assessment of the effect of DNA damage on the limb development of wild-type and p53 knockout mice (43) showed that the damage induced apoptosis in the limbs of wild-type mice but not those of p53−/− mice. However, cell death which exhibited necrotic features was much higher in the limbs of homozygous p53−/− mice. Collectively, these observations suggest that apoptosis and necrosis may be alternative pathways in the process of cell death. The physiological role of necrosis is only starting to be understood. Future studies will show the role played by the change of the death pathway from apoptosis to necrosis in senescence.
Inability of senescent fibroblasts to undergo p53-dependent apoptosis is due to lack of p53 stabilization.
Our findings suggest that old cells are unable to undergo p53-dependent apoptosis due to an inability to stabilize p53. This was demonstrated by two independent methods. First, proteasome inhibitors forced the accumulation of p53 in old cells, which in turn induced apoptosis. This indicates that the apoptotic machinery downstream of p53 is functional in old cells. Second, similar results were obtained following overexpression of exogenous p53 in these cells. By the forced accumulation of p53, we were able to restore the inability of old cells to enter p53-dependent apoptosis.
Lack of p53-dependent apoptosis in senescent cells in response to genotoxic stress may be caused by impaired function of the upstream regulators of p53 involved in recognition of DNA damage, such as PARP, DNA-PK, or ATM. It has been reported that DNA-PK and PARP are down regulated in senescent fibroblasts (50). On the other hand, studies showing activation of p53 in senescent cells suggest that p53 itself is undergoing modifications (4, 60). It has been shown recently that the phosphorylation pattern of p53 in senescent cells overlaps but is distinct from that induced by DNA damage (63). It is possible that the senescence-specific phosphorylation pattern is also responsible for the lack of p53 stabilization upon DNA damage in senescent cells. We can speculate that the lack of apoptotic response to DNA damage in senescent cells has the following physiological rationale. Apoptosis is a protective mechanism that prevents cancer by killing potentially dangerous cells that have mutated DNA. Senescent cells are entering irreversible growth arrest and do not pose a threat of malignant transformation. Therefore, apoptotic response to DNA damage becomes unnecessary in senescent cells. Instead, the preservation of viable cells might be more important in aging tissues.
Implications of the change of death pathway from apoptosis to necrosis in senescent cells for anticancer therapy.
One of the problems in geriatric medicine is the increased sensitivity of old patients to stress induced by DNA damage and other types of cellular damage (19). This problem becomes crucial in oncology, since cancer is an age-associated disease and anticancer chemotherapy results in severe genotoxic stress to normal tissues. It was observed that some anticancer drugs have a greater toxicity in the elderly than in young patients (6, 38). Our finding of a senescence-related transition of the death pathway from apoptosis to necrosis in old cells provides a good explanation for this augmented toxicity. Analysis of studies concerning the application and toxicity of anticancer drugs in the elderly revealed good correlation between increased toxicity of the drug in the elderly and induction of necrosis in old human fibroblasts (Table 1). Thus, etoposide is widely used for anticancer chemotherapy in old patients (38, 44). Our results show that etoposide induces p53-independent apoptosis in old cells. Cisplatin is one of the most effective and widely used anticancer drugs, and it is considered to have acceptable toxicity for old patients (35, 38). No increased toxicity was observed in the elderly when the cisplatin dose was increased (52, 58), and it was suggested that its toxicity was inversely dependent on the dose administered. We found that a low dose of cisplatin causes necrosis in old cells whereas a high dose induces apoptosis. Actinomycin D is widely used in cancer therapy; however, its severe toxicity in old patients has been reported (52). We found that while in young cells actinomycin D induces apoptosis, in old cells it results in massive necrosis. As previously mentioned, necrosis can result in inflammation, and this can explain the increased toxicity of the necrosis-inducing drugs observed in the elderly. Thus, elucidation of the death pathways induced by genotoxic stress in the context of cellular senescence may contribute to optimization of the current chemotherapeutic regimens.
TABLE 1.
Toxicity of anticancer drugs to the elderly correlated with the death pathway they induce in senescent fibroblasts
| Anticancer druga | Recommendations of oncologists for use in elderly | Death pathway induced in fibroblasts
|
|
|---|---|---|---|
| Young cells | Old cells | ||
| Etoposide | Most often used | Apoptosis | Apoptosis |
| Cisplatin (high dose) | No increased toxicity reported | Apoptosis | Apoptosis |
| Cisplatin (low dose) | Used with acceptable toxicity | Apoptosis | Necrosis |
| Actinomycin D | Not used due to high toxicity | Apoptosis | Necrosis |
All the analyzed anticancer drugs are widely used for treatment of young patients.
ACKNOWLEDGMENTS
This study was supported by a grant from the Israel Cancer Association and in part by grants from the Israel-USA Binational Science Foundation, the German Israeli Foundation for Scientific Research and Development, and the Israel Cancer Research Fund (V.R.). A.S. was supported by a postdoctoral fellowship from the Weizmann Institute of Science. V.R. holds the Norman and Helen Asher Professorial Chair in Cancer Research at the Weizmann Institute.
REFERENCES
- 1.Afshari C A, Vojta P J, Annab L A, Futreal P A, Willard T B, Barrett J C. Investigation of the role of G1/S cell cycle mediators in cellular senescence. Exp Cell Res. 1993;209:231–237. doi: 10.1006/excr.1993.1306. [DOI] [PubMed] [Google Scholar]
- 2.Aladjem M I, Spike B T, Rodewald L W, Hope T J, Klemm M, Jaenisch R, Wahl G M. ES cells do not activate p53-dependent stress and undergo p53-independent apoptosis in response to DNA damage. Curr Biol. 1998;8:145–155. doi: 10.1016/s0960-9822(98)70061-2. [DOI] [PubMed] [Google Scholar]
- 3.Andera L, Wasylyk B. Transcription abnormalities potentiate apoptosis of normal human fibroblasts. Mol Med. 1997;3:852–863. [PMC free article] [PubMed] [Google Scholar]
- 4.Atadja P, Wong H, Garkavtsev I, Veillette C, Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker S J, Markowitz S, Fearon E R, Willson J K V, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 6.Balducci L, Extermann M. Cancer chemotherapy in the older patient: what the medical oncologist needs to know. Cancer. 1997;80:1317–1322. [PubMed] [Google Scholar]
- 7.Bond J A, Wyllie F S, Wynford-Thomas D. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene. 1994;9:1885–1889. [PubMed] [Google Scholar]
- 8.Brown D G, Sun X-M, Cohen G M. Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to intronucleosomal fragmentation. J Biol Chem. 1993;268:3037–3039. [PubMed] [Google Scholar]
- 9.Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 10.Campisi J. Replicative senescence: an old lives' tale? Cell. 1996;84:497–500. doi: 10.1016/s0092-8674(00)81023-5. [DOI] [PubMed] [Google Scholar]
- 11.Chang Y C, Lee Y S, Tejima T, Tanaka K, Omura S, Heintz N H, Mitsui Y, Magae J. mdm2 and bax, downstream mediators of the p53 response, are degraded by the ubiquitin-proteasome pathway. Cell Growth Differ. 1998;9:79–84. [PubMed] [Google Scholar]
- 12.Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol. 1999;9:967–970. doi: 10.1016/s0960-9822(99)80425-4. [DOI] [PubMed] [Google Scholar]
- 13.Darzynkiewicz Z. Acid-induced denaturation of DNA in situ as a probe of chromatin structure. Methods Cell Biol. 1994;41:527–541. doi: 10.1016/s0091-679x(08)61738-0. [DOI] [PubMed] [Google Scholar]
- 14.Di Leonardo A, Linke S P, Clarkin K, Wahl G M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 15.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;79:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 16.Eastman A. Assays for DNA fragmentation, endonucleases, and intracellular pH and Ca2+ associated with apoptosis. Methods Cell Biol. 1995;46:41–55. doi: 10.1016/s0091-679x(08)61923-8. [DOI] [PubMed] [Google Scholar]
- 17.El-Deiry W, Tokino T, Velculescu V, Levy D, Parsons R, Trent J, Lin D, Mercer W, Kinzler K, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 18.El-Deiry W S. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345–357. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 19.Ershler W B, Longo D L. The biology of aging: the current research agenda. Cancer. 1997;80:1284–1293. [PubMed] [Google Scholar]
- 20.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 21.Freedman D A, Levine A J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gansauge S, Gansauge F, Gause H, Poch B, Schoenberg M H, Beger H G. The induction of apoptosis in proliferating human fibroblasts by oxygen radicals is associated with a p53- and p21/WAF1/CIP1 induction. FEBS Lett. 1997;404:6–10. doi: 10.1016/s0014-5793(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 23.Glockzin S, von Knethen A, Scheffner M, Brune B. Activation of the cell death program by nitric oxide involves inhibition of the proteasome. J Biol Chem. 1999;274:19581–19586. doi: 10.1074/jbc.274.28.19581. [DOI] [PubMed] [Google Scholar]
- 24.Gollahon L S, Shay J W. Immortalization of human mammary epithelial cells transfected with mutant p53 (237his) Oncogene. 1996;12:715–725. [PubMed] [Google Scholar]
- 25.Hansen R, Oren M. p53: from inductive signal to cellular effect. Curr Opin Genet Dev. 1997;7:46–51. doi: 10.1016/s0959-437x(97)80108-6. [DOI] [PubMed] [Google Scholar]
- 26.Harley C B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 27.Harley C B, Futcher A B, Greider C W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 28.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a protein inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 29.Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53-and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr J F R, Gobe G C, Winterford C M, Harmon B V. Anatomical methods in cell death. Methods Cell Biol. 1995;46:1–27. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- 32.Kitanaka C, Kuchino Y. Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ. 1999;6:508–515. doi: 10.1038/sj.cdd.4400526. [DOI] [PubMed] [Google Scholar]
- 33.Komarova E A, Gudkov A V. Could p53 be a target for therapeutic suppression? Semin Cancer Biol. 1998;8:389–400. doi: 10.1006/scbi.1998.0101. [DOI] [PubMed] [Google Scholar]
- 34.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 35.Kubota K, Furuse K, Kawahara M, Kodama N, Ogawara M, Takada M, Masuda N, Negoro S, Matsui K, Takifuji N, Kudoh S, Kusunoki Y, Fukuoka M. Cisplatin-based combination chemotherapy for elderly patients with non-small-cell lung cancer. Cancer Chemother Pharmacol. 1997;40:469–474. doi: 10.1007/s002800050689. [DOI] [PubMed] [Google Scholar]
- 36.Kulju K S, Lehman J M. Increased p53 protein associated with aging in human diploid fibroblasts. Exp Cell Res. 1995;217:336–345. doi: 10.1006/excr.1995.1095. [DOI] [PubMed] [Google Scholar]
- 37.Lane D. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 38.Lichtman S M. Recent developments in the pharmacology of anticancer drugs in the elderly. Curr Opin Oncol. 1998;10:572–579. doi: 10.1097/00001622-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Lopes U G, Erhardt P, Yao R, Cooper G M. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 40.Maki C G, Huibregtse J M, Howley P M. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 1996;56:2649–2654. [PubMed] [Google Scholar]
- 41.McCormick J J, Maher V M. Towards an understanding of the malignant transformation of diploid human fibroblasts. Mutat Res. 1988;199:273–291. doi: 10.1016/0027-5107(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 42.Midgley C A, Owens B, Briscoe C V, Thomas D B, Lane D P, Hall P A. Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J Cell Sci. 1995;108:1843–1848. doi: 10.1242/jcs.108.5.1843. [DOI] [PubMed] [Google Scholar]
- 43.Moallem S A, Hales B F. The role of p53 and cell death by apoptosis and necrosis in 4-hydroperoxycyclophosphamid-induced limb malformations. Development. 1998;125:3225–3234. doi: 10.1242/dev.125.16.3225. [DOI] [PubMed] [Google Scholar]
- 44.Niitsu N, Umeda M. Evaluation of long-term daily administration of oral low-dose etoposide in elderly patients with relapsing or refractory non-Hodgkin's lymphoma. Am J Clin Oncol. 1997;20:311–314. doi: 10.1097/00000421-199706000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 46.Oberhammer F, Wilson J W, Dive C, Morris I D, Hickman J A, Wakeling A E, Walker P R, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rittling S R, Brooks K M, Cristofalo V J, Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci USA. 1986;83:3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogan E, Bryan T, Hukku B, Maclean K, Chang A, Moy E, Englezou A, Warneford S, Dalla-Pozza L, Reddel R. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol Cell Biol. 1995;15:4745–4753. doi: 10.1128/mcb.15.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rovinski B, Benchimol S. Immortalization of rat embryo fibroblasts by the cellular p53 oncogene. Oncogene. 1988;2:445–452. [PubMed] [Google Scholar]
- 50.Salminen A, Helenius M, Lahtinen T, Korhonen P, Tapiola T, Soininen H, Solovyan V. Down-regulation of Ku autoantigen, DNA-dependent protein kinase, and poly(ADP-ribose) polymerase during cellular senescence. Biochem Biophys Res Commun. 1997;238:712–716. doi: 10.1006/bbrc.1997.7371. [DOI] [PubMed] [Google Scholar]
- 51.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 52.Sekine I, Fukuda H, Kunitoh H, Saijo N. Cancer chemotherapy in the elderly. Jpn J Clin Oncol. 1998;28:463–473. doi: 10.1093/jjco/28.8.463. [DOI] [PubMed] [Google Scholar]
- 53.Shaulian E, Zauberman A, Ginsberg D, Oren M. Identification of minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol. 1992;12:5581–5592. doi: 10.1128/mcb.12.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shay J W, Wright W E, Brasiskyte D, Van der Haeger B A. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993;8:1407–1413. [PubMed] [Google Scholar]
- 55.Sherman L, Schlegel R. Serum- and calcium-induced differentiation of human keratinocytes is inhibited by the E6 oncoprotein of human papillomavirus type 16. J Virol. 1996;70:3269–3279. doi: 10.1128/jvi.70.5.3269-3279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smeal T, Guarente L. Mechanisms of cellular senescence. Curr Opin Genet Dev. 1997;7:281–287. doi: 10.1016/s0959-437x(97)80139-6. [DOI] [PubMed] [Google Scholar]
- 57.Smith J, Pereira-Smith O. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- 58.Thyss A, Saudes L, Otto J, Creisson A, Gaspard M H, Dassonville O, Schneider M. Renal tolerance of cisplatin in patients more than 80 years old. J Clin Oncol. 1994;12:2121–2125. doi: 10.1200/JCO.1994.12.10.2121. [DOI] [PubMed] [Google Scholar]
- 59.Vaziri H, Benchimol S. From telomere loss to p53 induction and activation of a DNA-damage pathway at senescence: the telomere loss/DNA damage model of cell aging. Exp Gerontol. 1996;31:295–301. doi: 10.1016/0531-5565(95)02025-x. [DOI] [PubMed] [Google Scholar]
- 60.Vaziri H, West M D, Allsopp R C, Davison T S, Wu Y, Arrowsmith C H, Poirier G G, Benchimol S. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 1997;16:6018–6033. doi: 10.1093/emboj/16.19.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995;55:2284–2292. [PubMed] [Google Scholar]
- 63.Webley K, Bond J, Jones C, Blaydes J, Craig A, Hupp T, Wynford-Thomas D. Posttranslational modification of p53 in replicative senescence overlapping but distinct from those induced by DNA damage. Mol Cell Biol. 2000;20:2803–2808. doi: 10.1128/mcb.20.8.2803-2808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeargin J, Haas M. Elevated levels of wild-type p53 induced by radiolabeling of cells leads to apoptosis or sustained growth arrest. Curr Biol. 1995;5:423–431. doi: 10.1016/s0960-9822(95)00083-2. [DOI] [PubMed] [Google Scholar]