Abstract
Background
The COVID-19 pandemic has put community pharmacists at the frontline of prevention, preparedness, response, and recovery efforts. Pharmacies had to reorganize and implement several different interventions and measures within a very short time frame.
Objectives
1) To map the current reported practice and trends and to review the literature on pharmacy-based interventions on COVID-19 provided in Europe; 2) To identify knowledge gaps and future avenues for pharmacy research, policy, and practice in response to public health emergencies.
Methods
We used a mixed methods approach combining country mapping of current practices of pharmacy interventions on COVID-19 reported by pharmacy associations in Europe with a scoping review of published literature.
Results
We mapped current practices on 31 pharmacy interventions on COVID-19 in 32 countries in Europe. Almost all preventive measures to reduce health risks have been provided in most countries. Other frequent interventions reflected preparedness for stockpiling, increased demand for services and products, and important patient care interventions exceeding dispensing role. Expanded powers granted to pharmacies and legislation passed in view of COVID-19 enabled services that improve access to medicines and relevant products, patient screening and referral including point-of-care antigen testing, support to vulnerable patients, and COVID-19 vaccination. We identified 9 studies conducted in pharmacies in 7 countries in Europe. Most studies are cross-sectional and/or descriptive. Pharmacy associations played an important supporting role by developing and updating guidance and emergency plans to assist community pharmacists.
Conclusions
A wide array of pharmacy interventions on COVID-19 was implemented in several countries within a very short time frame. Research on pharmacy interventions on COVID-19 is still in its infancy but confirmed the wide array of interventions provided and expanded powers granted to pharmacies. These findings may provide a significant impact to improve pharmacy research, policy, and practice in response to future public health emergencies in Europe and globally.
Keywords: Scoping review, Community pharmacy, COVID-19, Pandemic, Public health, Europe
1. Introduction
The World Health Organization (WHO) lists 10 Essential Public Health Operations (EPHOs) to deliver stronger Public Health Services and capacities in Europe.1 These include the delivery of health protection, disease prevention, and health promotion services informed by robust public health intelligence, including monitoring and response to health hazards and emergencies, and enhanced by enablers, including a sufficient and competent public health workforce, capable of contributing for supportive environments and resilient communities in a challenging social and economic environment. The aim is also to advance public health research to inform policy and practice.1
There are many definitions of the public health workforce. According to Tilson & Gebbie, the broadly defined public health workforce includes all those engaged in work that creates the conditions within which people can be healthy.2 It considers this workforce is composed of those who work for official government public health agencies, community-based and voluntary organizations with a health promotion focus, the public health staff of hospitals and health care systems and a range of others in private, industry, government, and the voluntary sector.2 Otok et al. considered a narrower definition comprising the providers who identify public health as being part of the primary part of their role, albeit acknowledging the wider public health workforce also includes those who contribute to public health only as part of their jobs, as well as other individuals whose work can have a positive impact on population health, including community pharmacists.3
Hence, although community pharmacists are not employed by public health government agencies or services and health promotion is not the primary core of their role, they can be viewed a part of a wider public health workforce.
Community pharmacists have been actively involved in earlier recent pandemic outbreaks, such as the 2009 Flu pandemic. Published literature reports roles in vaccination in US,4 , 5 UK and Portugal.6 , 7 Community pharmacists have also been included in pandemic prevention, preparedness, and response strategic guidance in the aftermath of the 2009 flu pandemic, although most examples are from US,8 Canada,9 and Australia.10
By April 2020, there seemed to be few research studies on interventions on COVID-19 provided by community pharmacists in Europe. In contrast, several reported news of relevant practice changes occurring almost every week emerged in early 2020 stemming from pharmacy associations. This prompted authors to perform this research.
A recent systematic review conducted in 2020 identified and outlined 15 studies published until July 2020 which addressed pharmacists' roles in disasters including COVID-19 pandemic.11 Pharmacists’ roles in the prevention of emergencies, including COVID-19, are focused on chronic disease medication supply and education. The roles in preparedness focused on health policy and population health planning. Roles in response included patient care and clinical roles. In addition, pharmacists have a key role in disaster recovery that involves several activities, such as restocking emergency kits and reestablishing normal stock. However, most of these roles were outside Europe including, but not restricted to community pharmacy.11
The COVID-19 pandemic has put community pharmacists at the frontline of prevention, preparedness, response, and recovery efforts. Pharmacies had to reorganize and adapt to the new context by implementing several different interventions and measures within a short time frame. Support was provided by national, European, and international pharmacy organizations and calls for granting extended powers emerged in 2020.
Watson et al. published a landmark perspective paper in 2019 which identified 43 pharmacists' roles in disasters throughout the four stages of response to a public health emergency.12 This paper evaluated the pharmacists’ role in disasters and the focus was on medicines supply, vaccination, and educating patients and consumers.
Two reviews attempted to identify services provided by community pharmacists during the COVID-19 pandemic. The scoping review performed until May 2020 included 11 studies, but most were found in the United States of America and China.13 Mendonça et al. performed the first systematic review which included 9 primary studies published between December 2019 and April 2020.14 As both searches were performed at the early onset of the pandemic, no information was available on actual interventions on COVID-19 provided in European pharmacies.13 , 14
Several pharmacy research papers at the onset of COVID-19 included scholarly commentaries, as expected in a pandemic, which offered an important reflection on additional new roles and challenges for pharmacy relevant in public health crisis including emergency supply of medications, increased demand/changes to repeat dispensing, extended prescribing roles, stock supply and management, dealing with shortages, home delivery of medications, drive-thru pharmacy services, managing minor ailments, managing chronic disease patients due to reduced primary care capacity, widespread use of technology for chronic disease patients, dealing with vulnerable patients including mental health, point-of-care antigen testing, antibody testing, COVID-19 vaccination, ensuring rapid access to approved antiviral treatment, and cross-country collaboration in guidance to assist pharmacists.15, 16, 17, 18, 19, 20, 21, 22, 23
Other research studies highlighted community pharmacists’ roles in COVID-19 pandemic prevention, preparedness, and response in other jurisdictions, such as India,24 Commonwealth countries,25 US,26 and Poland.27 Common features include disaster response and mitigation efforts to ensure continuing medicines supply, patient care, challenges and willingness or readiness to provide certain services.
A panel of experts convened by the International Pharmaceutical Federation (FIP) Pharmacy Practice Research Special Interest Group produced recommendations for pharmacy practice research priorities during the pandemic. This paper also acknowledged most research produced at earlier stages were scholarly commentaries. The panel recommended that future research is guided by proven theory and rigorous methods and identified 4 priority research areas. These areas include “medicines and vaccines related issues”, service focused, workforce issues, and the issues related to pharmacy education and training.28
Since published systematic reviews contained almost no information concerning pharmacy interventions on COVID-19 provided in Europe and despite the relevance of scholarly commentaries, it was important to identify whether there were published studies conducted on pharmacy interventions on COVID-19 provided in Europe. On the other hand, the detailed nature, extent of interventions and practice changes reported by pharmacy associations remained unknown. Not to mention how these fitted into the usual stages of response to a public health emergency.
Hence, the aim of this research was to identify and map current practices on COVID-19 reported by pharmacies. Another aim was to scope the body of literature, investigate research conduct, and identify knowledge gaps using a scoping review approach.29
The objectives of this research are:
1) To map the current reported practice and trends and to review the literature on pharmacy-based interventions on COVID-19 provided in Europe; 2) To identify knowledge gaps and future avenues for pharmacy research, policy, and practice in response to public health emergencies.
2. Methods
We used a mixed methods approach combining mapping of current practices of pharmacy interventions on COVID-19 in 32 countries in Europe with a scoping review of published literature. The scoping review aimed at identifying published research studies reporting on pharmacy interventions on COVID-19. Mapping consisted of applying a structured survey to pharmacy associations in 32 European countries to obtain reported ongoing or recently introduced pharmacy practices on COVID-19 in each country in response to the pandemic and it was used to complement the scoping review.
This research was carried in parallel with a second research project using similar methods but focusing on all pharmacy services provided in European pharmacies. This research will be reported in a separate paper.
2.1. Mapping practices on pharmacy interventions on COVID-19
We first mapped 30 pharmacy interventions on COVID-19 and further organized them under categories which correspond to the steps in response to public health emergencies. The categories were based on Watson et al. criteria.12 This was also used by Cadogan et al. including the mapping of some pharmacy interventions.15 We also used insights from informal reporting of interventions from pharmacy associations:
-
•
Prevention: measures to reduce health risks of COVID-19 pandemic.
-
•
Preparedness: measures to ensure timely and effective responses from the health care system.
-
•
Response: immediate actions in response to COVID-19 pandemic.
-
•
Recovery: measures to return to “normal” activities post-pandemic.
We defined key parameters to collect for each pharmacy intervention on COVID-19: 1) provided in most (≥80%) pharmacies; 2) expanded powers granted to pharmacies through amending regulations; 3) remunerated by Government/Payer; 4) extra legislation passed in view of COVID-19; 5) data sources. We were also interested in understanding the economic and social impact on pharmacies by adding two further questions on emerging temporary closures of pharmacies and deaths of pharmacy staff due to COVID-19.
This was the basis for the country survey design for mapping country practices and trends on pharmacy interventions on COVID-19. The survey was pretested and refined. The replies were obtained from Member Associations of the Pharmaceutical Group of the European Union (PGEU) in 32 countries in September 2020. We updated the information for point-of-care antigen testing and added one more intervention related to “COVID-19 vaccination in pharmacies” in a second round performed in March 2021 and updated in June 2021.
A template adapted from the survey was developed to assist in data extraction (SC). The data was extracted by one researcher (MM) and reviewed by two researchers (MRH, SC).
See “Country Survey Part 2: Pharmacy Interventions on COVID-19” (Appendix 1).
The “Country Survey Part 1” addresses all pharmacy services, and is part of the parallel research previously mentioned.
2.2. Scoping review of pharmacy interventions on COVID-19
We followed PRISMA Extension for Scoping Reviews (PRISMAScR) Checklist for reporting results.30
See Completed PRISMA-ScR Checklist (Appendix 2).
We performed a first review of primary studies published until 5 August 2020 and further updated this search until April 2021.
The studies were included if they met the following inclusion criteria: 1) Conducted in or including a European country; 2) Focusing on community pharmacy (not hospital setting, clinic nor ambulatory care); 3) Reporting on pharmacy interventions on COVID-19 provided; 4) Full research articles.
We excluded perspective studies, commentaries, and opinion surveys (e.g., not reporting pharmacy interventions on COVID-19 provided).
A first search was performed in MEDLINE® (via PubMed®) between 1 January and 5 August 2020, updated between 5 August 2020 and 1 February 2021, and again between 2 February and 14 April 2021 (MR). Searches were performed in Google® Scholar for recent studies (MR): using “pharmacy”, using “pharmacies”, using “pharmacist”, and using “pharmacists” in the mandatory keywords.
See search strategy (Appendix 3)
Citations that resulted from searches were downloaded, and duplications were removed. Screening of titles, abstracts and full papers was performed by one researcher (MM) against inclusion criteria. Full-text articles were reviewed by another author (SC). Disagreements were resolved through discussion.
A template was developed, piloted, and adapted to assist in data extraction (SC). The following data items were extracted: title; first author/date of publication; date range of data collection; journal; objectives; types of publications; country of origin; study design; population (if applicable); no. respondents; intervention category on COVID-19; interventions on COVID-19 (name as per country survey); interventions on COVID-19 (as described in paper); other key findings; expanded powers granted to pharmacists; extra legislation/regulation enforced; interventions on COVID-19 remunerated; source of funding; conflict of interest; comments. The data was extracted by one researcher (MM) and reviewed by another author (SC). Existing discrepancies were resolved through discussion. We did not assess the overall quality of evidence. However, we identified the design of each study and restricted to research studies reporting on actual pharmacy interventions provided.
In addition, we also scoped published as well as grey literature including reports, poster abstracts and conference abstracts of international pharmacy organizations on strategies to support or reporting examples of European community pharmacy interventions on COVID-19 (MRH, SC).
A narrative synthesis was performed, that is, relying primarily on a textual approach to summarize and explain the findings, in 3 steps: first, by synthesizing findings of survey responses on mapping practices; next, by synthesizing findings from retrieved studies; and, finally, by bringing evidence from these sources together.
3. Results
3.1. Mapping practices on pharmacy interventions on COVID-19
We have received replies to “Country Survey Part 2: Pharmacy Interventions on COVID-19” from 32 PGEU member countries for all 31 measures and interventions on COVID-19.
The number of countries with pharmacy interventions on COVID-19 in place varied according to each intervention. We have grouped these interventions into 5 tiers determined empirically by visualization of data distribution, that is by grouping pharmacy interventions on COVID-19 according to the number of countries providing them. Tier 1 comprises pharmacy interventions on COVID-19 in place in all 32 countries; tier 2 comprises interventions in 26–31 countries; tier 3 comprises intervention in 14–25 countries; tier 4 comprises interventions in 6–13 countries; and tier 5 in 1–5 countries. Hence, these tiers reflect the frequency of interventions – from tier 1 comprising interventions provided in all countries to tier 5 comprising interventions provided in less than 6 countries.
The most common pharmacy measures and interventions on COVID-19 in place in all 32 European countries were patient information and education on preventive measures; queue management in pharmacies; floor marking inside pharmacies; and barriers at counters in pharmacies.
The second tier was in place in 26–31 countries. This included protocols for disinfection of surfaces; use of masks by staff; stock and supply of hand sanitizers; stock and supply of protective masks; symptom-based referral pathways for suspected cases; increased demand/changes to home delivery of medicines; and reestablishing patient care services and stock levels.
The third tier was in place in 14–25 countries and included stock and supply of essential medicines; dealing with the supply of medicines shortages; preparing alcohol-based hand sanitizer formulations; and pharmacy telephone support to vulnerable patients during isolation and lockdown.
The fourth tier was in place in 6–13 countries: 1st and 2nd line pharmacy staff; quantity limits dispensed; increased demand/changes to repeat dispensing; emergency supply of medicines, supply of medicines usually supplied in the hospital setting; hotline numbers for home delivery of medicines; protocol for pharmacies for reporting on domestic violence during isolation/lockdown.
In this tier, we included extended powers and/or legislation passed for point-of-care antigen testing in pharmacies for 10 countries, of which 7 remunerate pharmacies through Governments or national/local Health Care Payers (Austria, France, Germany, Italy, Portugal, Spain, Sweden) and 3 have this service co-paid by citizens (Malta, Turkey, UK).
More recently, 8 countries had extended powers and/or legislation passed for COVID-19 vaccination in community pharmacies or by community pharmacists. In 6 of these countries (Belgium, France, Ireland, Italy, Norway, and UK) this service was already provided in pharmacies in June 2021, and 3 (Ireland, Italy, and UK) provide remuneration for pharmacies. COVID-19 vaccination in pharmacies has already been regulated in Poland and Turkey and is expected to start rolling out at pharmacies in the coming months.
The least frequent interventions on COVID-19 (practiced in fewer than 6 countries) include: use of other PPE by staff; restriction in opening hours; temporary suspension of patient care services; temporary waived prescription copayments for vulnerable patients; drive thru pharmacy services; and referral pathways of exposed patients to antibody testing for immunity assessment against COVID-19.
Eighteen interventions had expanded powers granted to pharmacists in 17 countries including: use of masks by staff (compulsory use); stock and supply of essential medicines; point-of-care antigen test-based referral pathways for suspected cases; increased demand/changes to repeat dispensing; emergency supply of medications; supply of medicines usually supplied in hospital; increased demand/changes to home delivery of medicines; dealing with the supply of medicines shortages; preparing alcohol-based hand sanitizers; pharmacy telephone support to vulnerable patients during isolation/lockdown; protocol for pharmacies for reporting on domestic violence during isolation/lockdown; temporary waived prescription copayments for vulnerable patients; dealing with new vulnerable patients; referral pathways of exposed patients to antibody testing; and COVID-19 vaccination in pharmacies or by community pharmacists.
Twenty-two countries passed legislation in view of COVID-19 for 24 interventions, including: stock and supply of essential medicines; stock and supply of hand sanitizers; point-of-care antigen test-based referral pathways for suspected cases; increased demand/changes to repeat dispensing; supply of medicines usually supplied in hospital; preparing alcohol hand sanitizers; and COVID-19 vaccination in pharmacies or by community pharmacists.
In total, 17 pharmacy measures and interventions on COVID-19 were remunerated by Governments or Health Care Payers in 29 countries. This included the use of masks by staff; stock and supply of essential medicines; stock and supply of hand sanitizers; stock and supply of protective masks; point-of-care antigen testing; increased demand/changes to repeat dispensing; emergency supply of medications; supply of medicines usually supplied in hospital; increased demand/changes to home delivery of medicines; dealing with the supply of medicines shortages; preparing alcohol-based hand sanitizers; hotline numbers/protocol for pharmacies for reporting on domestic violence during isolation/lockdown; temporary waived prescription copayments for vulnerable patients; dealing with new vulnerable patients; and COVID-19 vaccination in pharmacies or by community pharmacists.
At the time of reply (September 2020), emergency temporary closures of pharmacies had occurred in Germany (30 pharmacies), Spain (20), Sweden (10–20), Portugal (15), Croatia, Luxembourg, and Poland (2), Belgium (1). Czech Republic, Ireland, Norway, and Greece also reported temporary closures of pharmacies. These figures may have increased or changed.
The death of pharmacy staff due to COVID-19 were reported to have occurred in Spain (19), Turkey (15), Italy (16), UK (3), North Macedonia (1). However, not all countries were able to report on this parameter. These figures may have increased.
Fig. 1 provides a country mapping of pharmacy measures and interventions on COVID-19 in Europe.
Fig. 1.
Pharmacy interventions on COVID-19 in Europe – country mapping.
3.2. Scoping review on pharmacy interventions on COVID-19
The first search in MEDLINE® (via PubMed®) identified 58 potential records; the second update identified 96 potential records. The third update identified 25 records, which sums 179 potential records in MEDLINE® (via PubMed®).
Searches in Google® Scholar retrieved 438 potentially eligible records (94 in first search + 344 from second and third updates).
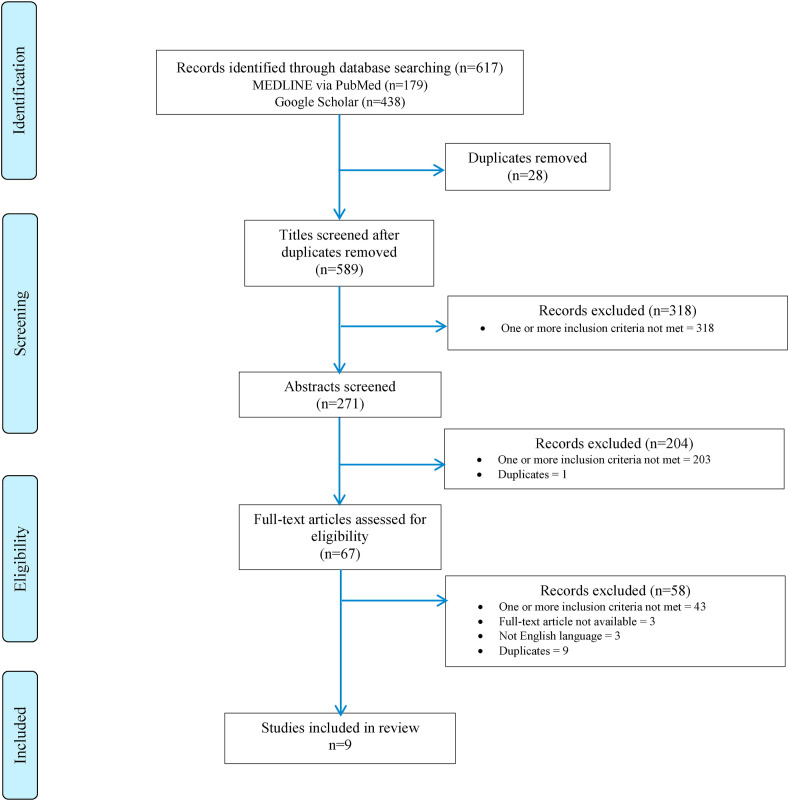
Hence, the first search in both databases until 5 August 2020 identified 152 potential records (58 in MEDLINE® + 94 in Google® Scholar). The initial title screening excluded 86 (28 duplicates and 58 not matching inclusion criteria or protocols) titles, leaving a total of 66 potentially relevant titles. Abstract assessment resulted in the further exclusion of 49 studies and 17 potentially relevant abstracts were retrieved. Of these, 14 were excluded because they did not meet the inclusion criteria. The full-text assessment process resulted in 3 articles being retrieved.31, 32, 33
The second and third searches performed between 5 August 2020 and 14 April 2021 identified 465 potential records (121 in MEDLINE® + 344 in Google® Scholar). The initial title screening excluded 260 (not matching inclusion criteria) titles, leaving a total of 205 potentially relevant titles. Abstract assessment resulted in the further exclusion of 155 studies and 50 potentially relevant abstracts were retrieved. Of these, 44 were excluded (29 not meeting the inclusion criteria; 3 papers in non-English language, 3 full papers not available, and 9 references duplicated from the first screening process), resulting in further 6 full-text articles being retrieved.34, 35, 36, 37, 38, 39
We, therefore, identified a total 9 studies meeting inclusion criteria published between June 2020 and March 2021.31, 32, 33, 34, 35, 36, 37, 38, 39
Fig. 2 illustrates the study selection process (figures report the combined result of all searches previously detailed).
Fig. 2.
Study selection process.
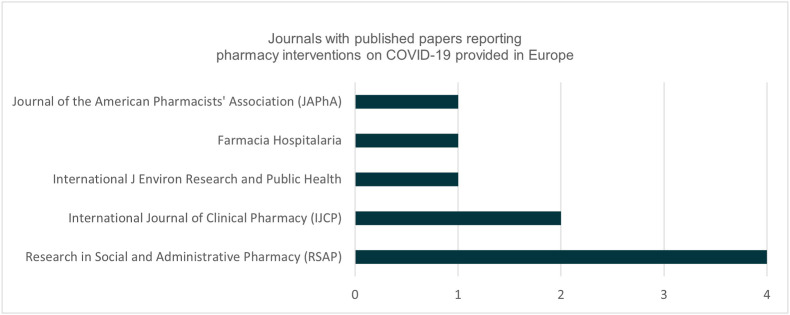
The studies were published in 5 different journals.
Fig. 3 provides an overview of journals for these published papers.
Fig. 3.
Journals with published papers reporting interventions on COVID-19 provided in pharmacies.
The study designs of these studies included: 1 literature review,31 7 cross-sectional studies using surveys or qualitative research in community pharmacies,32, 33, 34, 35, 36, 37, 38 and 1 paper from International Pharmaceutical Federation (FIP) authors describing examples of pharmacy interventions on COVID-19.39
Three papers had no funding,35 , 37 , 39 3 papers reported source of funding,31 , 34 , 36 while 3 other papers did not report any source of funding.32 , 33 , 38
Merks et al. reviewed the legal extension of the role of pharmacists in COVID-19 pandemic looking for published data in relevant country websites. This review published in June 2020 reported expanded powers granted to pharmacists or legislation passed in view of COVID-19 in 11 countries (Austria, Belgium, Croatia, Czech Republic, Germany, Italy, The Netherlands, Poland, Portugal, Spain, and UK) on several interventions, such as: relaxation of regulations on dispensing controlled medicines, some prescribing authority including extension of emergency supply of medications (without prescription), increased demand/changes to repeat dispensing, e-prescribing, substitution rights when in short supply due to shortages, supply of medicines usually supplied in hospitals, administration of oxygen to patients, preparing alcohol sanitizers, home delivery to vulnerable patients, protocol for reporting domestic violence, and access to patient electronic health care records.31
The 7 cross-sectional studies conducted in community pharmacies in 7 countries to collect data on interventions on COVID-19 provided during pandemic onset in 2020. These studies identified a wide range of pharmacy measures and interventions on COVID-19 that were put in place within a short time frame. These include: 1) prevention measures to reduce health risks, such as: patient information and education not only on preventive strategies but also dealing with the misinformation and questions on potential COVID-19 treatments; protocols for disinfection of pharmacy premises; use of masks and PPE; barriers at counters; queue management; temporary suspension of certain patient care activities; 2) preparedness measures to ensure timely and effective responses, such as: division of staff into teams, when possible; stock and supply of medicines; stock and supply of hand sanitizers and masks; limited quantity dispensed; 3) Immediate actions as response measures, such as: increased demand or changes to repeat dispensing; changes in home delivery of medicines; dealing with the shortages; preparing alcohol-based hand sanitizer; monitoring non-COVID patients; remote support to vulnerable patients.32, 33, 34, 35, 36, 37, 38
A more recent paper by FIP authors describes how pharmacy stepped up during the COVID-19 pandemic by providing examples reported by several countries, such as home delivery of medicines, increased changes to repeat dispensing in Portugal and Spain, testing in France, Spain, Switzerland and UK, and COVID-19 vaccination in the UK.39
Three papers reported difficulties experienced by pharmacies. This included price increases by the wholesalers and suppliers; frequent inspections from authorities; extended working hours; dealing with angered patients; financial loss in the pharmacy; reimbursement delays to pharmacies; and staff mental health as a result of prolonged stressful situations, increased workloads, and fear of infection and for the health of loved ones.32 , 33 , 38
In addition, we identified 4 sources which contain resources from the following international pharmacy organizations to support community pharmacies: FIP,40 , 41 World Pharmacy Council (WPC),42 and PGEU.43
A paper from FIP authors published in July 2020 also described the strategy adopted by FIP in collaboration with an international group of experts to support pharmacists and their teams throughout the pandemic.40 The paper also highlights examples of their contribution to health systems around the world, including Europe. The strategy adopted by FIP described in this paper included: 1) International guidance; 2) Call to action highlighting 23 measures to support pharmacists and pharmacies; 3) FIP COVID-19 Information Hub41; 4) Initiatives focusing on the impact on pharmaceutical education; 5) Initiatives by and for young pharmacists and pharmaceutical scientists.40
The WPC, an organization comprising pharmacy associations from 8 countries leading advanced pharmacy roles, of which 5 are European (Denmark, Ireland, Portugal, Spain, and UK), released a report in September 2020 highlighting the frontline role of community pharmacy during the COVID-19 pandemic based on responses from member organizations to its survey conducted in August 2020.42 The most common regulatory change across WPC member countries was to allow pharmacists to extend the duration of existing prescriptions and/or provide emergency supply. Preventive measures, preparing alcohol sanitizers, home delivery services, providing support to vulnerable patients were also reported. This report makes recommendations for embracing further roles of pharmacists in preparedness, response, and recovery.42
The PGEU also created a COVID-19 hub containing links to: 1) international resources of WHO, Organization for Economic Co-operation and Development (OECD) and FIP; 2) European resources of European Medicines Agency (EMA), European Centre for Disease Prevention and Control (ECDC), and European Commission; 3) national resources developed by pharmacy member associations from 30 European countries.43
Table 1 summarizes the findings of our scoping review of published studies reporting pharmacy interventions on COVID-19 provided in Europe.
Table 1.
Summary of findings of studies on pharmacy measures and interventions on COVID-19 provided in Europe.
| First author (month yr) [REF] | Objective | Country of origin | Study design | Pharmacy Interventions on COVID-19 provided | Other Findings |
|---|---|---|---|---|---|
| Merks P (June 2020)31 | To review the legal extension of the role of pharmacists in light of the COVID-19 pandemic | PL | Review | AT: e-prescribing, relaxation of regulations on dispensing controlled medicines; BE: preparing alcohol, exclusive right to sell PPE, masks, and alcohol gel. HR: Substitution of medicine in short supply, home delivery to vulnerable patients, increased quantity of hemophilia medication dispensed. CZ: Compounding antiseptic solutions, hand sanitizers, alcoholic gels, renewal of chronic treatment, protocol for reporting domestic violence. DE: preparation of alcoholic gel. IT: Administration of oxygen to patients, e-prescribing, home delivery to vulnerable patients, preparation of disinfectants. NL: video, telephone, email consultations, protocol for reporting domestic violence, preparation of disinfectant. PL: preparation of alcohol sanitizers, home delivery of medical devices, some prescribing authority. PT: Extension of emergency medicine delivery line to the whole country. ES: Home delivery to vulnerable and affected patients, dispensing hospital medicines in pharmacies. UK: extension of MAS and access to Emergency Care Summary Data, right to supply certain controlled drugs without prescription, home delivery to self-isolating patients | Several European countries adopted new legal solutions to mitigate drug shortages. Source of funding: Polish Pharmaceutical Group |
| Hoti K (June 2020)32 | To explore the experiences of community pharmacists in relation to provision of community pharmacy services during COVID-19 pandemic | XK | Cross-sectional | 1) Informing patients on medication currently being discussed for COVID-19. 2) monitoring patients for non-COVID health conditions. 3) Patient information on preventive measures. 4) Protocols in place for disinfection of surfaces. 5) Use of disposable masks by staff. 6) Use of PPE by staff. 7) Queue management. 8) Barriers at counters. 9) Increased demand of medication. |
Negative: 1) Price increases; 2) Patient panic, stockpiling; 3) Fear of getting infected; 4) Frequent pharmacy inspections; 5) Financial impact; 6) Extended working hours. Positive: 1) Moral, sense of duty; 2) Alignment with other providers Source of funding: NR |
| Zaidi STR (July 2020)33 | To understand the protective practices and well-being of pharmacists, and the delivery of pharmacy services during the COVID 19 pandemic. | UK | Cross-sectional | 1) Increased number of patients. 2) Patient information on potential medicines for COVID-19. 3) Pharmacy premises reorganization. 4) Use of mask or PPE by pharmacy staff. 5) Limiting quantity dispensed. 6) Stock management. 7) Symptom-based referral pathway for suspected cases. 8) Dealing with significant or critical drug shortages. 9) Dealing with inappropriate behavior from patients or carers | Anxiety issues reported by pharmacy staff Source of funding: NR |
| Koster E (July 2020)35 | To describe the impact of the COVID-19 epidemic on the provision of pharmaceutical care in the Netherlands. | NL | Cross-sectional | Patient information and education on preventive measures; Protocols in place for disinfection of pharmacy surfaces; use of disposable masks; barriers at counters; temporary suspension of pharmacy services; queue management; business continuity plan; stock and supply of essential medicines; stock and supply of hand sanitizers; increased demand to repeat dispensing; dealing with shortages, home delivery | Only a small number of pharmacies took part in pharmacotherapy consultation groups (regular meetings between groups of GPs and pharmacists to improve prescribing quality) or used video Source of funding: No funding |
| Cerbin-Koczorowska M (Sept 2020)34 | To evaluate the preparedness of Polish pharmacy employees for patient education on the new threat | PL | Cross-sectional (mystery shopper) | Most pharmacists and staff provided patients with evidence-based recommendation on prevention, symptoms, and management of SARS-CoV-2 | Source of funding: Poznan University of Medical Sciences |
| Lim RHM (Oct 2020)36 | To explore the experiences of the community pharmacy team in supporting people with dementia and their family carers with the management of medications during the COVID-19 pandemic | UK | Cross-sectional (qualitative) | Temporary suspension of dementia patient care face-to-face services NMS and MUR but pharmacy teams drew extensively from internal (pharmacy/personal) and external (government) resources and negotiated professional decision-making and personal values to provide essential medication services to people with dementia. | Source of funding: Undergraduate Research Opportunities Programme, University of Reading and Brian Revell Memorial Fund |
| Giua C (Jan 2021)37 | To describe procedures and critical logistical-organizational issues encountered by Italian community pharmacists, and to collect the main requests reported by patients to pharmacists | IT | Cross-sectional | The most frequently adopted measures were the use of gloves, surgical masks, and protective barriers at the drug counter. Most implemented services: booking of prescriptions, delivery of medications and implementation of phone consultations. In Red Zones (most affected), there was a higher use of FFP2 and FFP3 masks by pharmacists, home-delivery of medicines, and use of alcohol sanitizers prior to entering pharmacies. |
Source of funding: No funding |
| Novak H (Mar 2021)38 | To explore and compare community pharmacists' roles, practices, implemented safety measures, and psychological toll in Croatia and Serbia during the COVID-19 pandemic. | HR and RS | Cross-sectional | Patient information and education; use of disposable masks; barriers at counters; business continuity plan; stock and supply of hand sanitizers; quantity limits for patient for the supply of individual medicines; dealing with the supply of medicines shortages; patient care services in place. The study identified new pharmacists' roles: manufacturing hand sanitizers, online patient counseling, and home delivery of medicines. Most pharmacists continued to manage chronic diseases and patient consultations in addition to their new roles. |
This study also assessed psychological status of pharmacists: reports of prolonged stressful situations and increased workloads. Source of funding: NR |
| Jordan D (Feb 2021)39 | To describe how pharmacy has stepped up during the COVID-19 crisis by giving examples from several countries | FIP | Descriptive | Home delivery of medications; increased changes to repeat dispensing; vaccination; testing | Source of funding: No funding |
PPE: Personal Protective Equipment; MAS: Minor Ailment Services; GP: General Practitioner; NMS: New Medicines Service; MUR: Medication Use Review; NR: Not Reported.
AT: Austria; BE: Belgium; CZ: Czech Republic; DE: Germany; ES: Spain; HR: Croatia; IT: Italy; NL: The Netherlands; PL: Poland; PT: Portugal; RS: Serbia; UK: United Kingdom; XK: Kosovo.
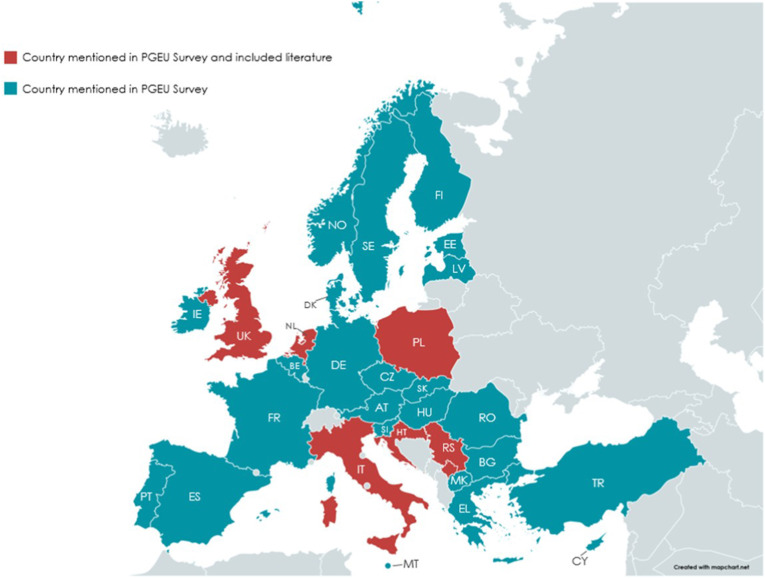
While mapping current practices of pharmacy interventions on COVID-19 was possible in 32 countries, published literature stemmed from 7 countries. The 8 studies reporting such interventions (excluding the paper of FIP authors) were from a small number of countries. There were 2 studies each from the UK and Poland. Serbia and Croatia, Italy, The Netherlands, and Kosovo, 1 study each.
It is, however, likely that the research and published papers on pharmacy interventions on COVID-19 has expanded since we conducted this research and will expand further in the coming months.
Fig. 4 illustrates the (still) scarce country research.
Fig. 4.
Map of countries with research vs. practices of pharmacy interventions on COVID-19.
4. Discussion
4.1. Summary of key findings
In this research, we mapped current practices on 31 pharmacy interventions on COVID-19 in 32 countries in Europe. We also performed a scoping review on these interventions stemming from 9 studies conducted in community pharmacies across Europe.
The European country reports portray a wide array of pharmacy interventions on COVID-19 implemented in most pharmacies. This was done in several countries within a short time frame. This reflects the highly reactive and adaptative character of pharmacies in response to the pandemic outbreak.
Research on pharmacy interventions on COVID-19 in Europe is still scarce with about 9 studies. Most studies are cross-sectional and descriptive and there are no published studies evaluating the interventions provided. This is expected, as community pharmacists faced multiple pressures during a pandemic in response to crisis which poses challenges to patient-level data collection to enable evaluation.
Despite the few research studies encountered (and the fact that most of them were published in 2020 with research conducted months before published date), these studies identified pharmacy measures and interventions on COVID-19 that match results stemming from the responses to the survey we conducted in 32 European countries for several pharmacy interventions on prevention, preparedness and response stages, as well as expanded powers granted to pharmacies or legislation passed to combat COVID-19.
The report on few use of PPE by staff, no restrictions in opening hours, and no temporary suspension of patient care services is coherent with difficulties in accessing PPE, extended operations in response to stockpiling and may reflect, to a certain degree, a shift of some primary care patient care services to pharmacies.
The results of mapping and scoping review are coherent in reflecting pharmacies preparedness for stockpiling and increased demand for services to ensure timely and effective access.
Even more interesting are the expanded powers granted to pharmacies and legislation passed in view of COVID-19. This reflects the relevance of the organized, reliable, and safe network of pharmacies in public health emergencies to provide fast access to medication, products and public health interventions.
Not surprisingly, perhaps, research studies did not capture other relevant interventions reported by pharmacy associations: symptom-based referral pathways for suspected cases; emergency supply of prescription medicines (without prescription); supply of medicines usually supplied in hospital setting; hotline numbers or protocols in pharmacies for reporting on domestic violence during isolation and lockdown; dealing with new vulnerable patients due to pandemic; and last but not least, point-of-care antigen testing and COVID-19 vaccination in pharmacies. These are all important patient care interventions in medication access, screening and referral, public health support to vulnerable patients and population-based disease prevention. This exceeds far beyond pharmacist's traditional dispensing role.
Emergency temporary closures of pharmacies affected patients’ access and patient care and had a negative economic impact. In small towns this also means that this could be a risk of reputation damage, when temporary closures occur.
International pharmacy organizations played an important supporting role, but it is fair to acknowledge that many national pharmacy organizations developed guidance and contingency plans to assist their community pharmacists early on which in turn were also used by FIP, WPC and PGEU in developing their own guidance, thereby fostering mutual learning and cross-country benefits.
Investing in pharmacy-based vaccination may be an important policy decision to accelerate and increase coverage. This is not only beneficial in COVID-19 pandemic but in future required mass vaccinations. This is particularly critical when part of the population has already been vaccinated in mass immunization centers, either because they fit into the high-risk groups and/or because they are the “innovators” and “early adopters”, but increased population coverage has not yet been reached. In addition, it becomes more difficult and inefficient to reach low-risk groups and/or “late adopters” and “laggards” who require other strategies that could make use of the proximity and trust of the network of pharmacies.
This is aligned with the existing evidence on the added value of pharmacists in vaccination. A systematic review and meta-analysis published in 2016 included 36 studies, of which 14 assessed pharmacists' role as administrators of vaccines, 7 of which were related to community pharmacies.44 All studies found an increase in vaccine coverage when pharmacists were involved compared with vaccine provision by traditional providers or without involving a pharmacist.44 In another important economic study published in 2017, authors developed a discrete event simulation model to forecast the potential effect of community pharmacy vaccine administration and its possible impact on pandemic influenza vaccine uptake.45 Results showed that weekly national US vaccine administration capacity increased to 25 million doses per week when community pharmacist vaccination capacity was included in the model. In addition, the time to achieve 80% vaccination coverage nationally was reduced by 7 weeks, assuming high public demand for vaccination. The results for individual states varied, but in 48 states the inclusion of pharmacies improved time to 80% coverage.45
The overall findings of this research are aligned with the 2020 Report from the OECD on primary health care which recognized that even before the COVID-19 pandemic, health systems in OECD countries faced significant challenges.46 The report identifies pharmacists as primary care providers in its definition of primary health care and outlines that there is ample scope for further developing the role of pharmacists and to develop more effective collaboration with the general practitioners and other healthcare professionals.
The report goes further in pointing out process changes that are key to improve care. This includes better use of digital technology, and ability to link datasets across primary care and other part of the health systems; payment instruments linked to outcomes or desired activities; better measurement of the inputs, outputs, and outcomes of the primary health care sector; patient access and interaction to their health records and accreditation of providers. The report highlights that these messages are as important as ever in the light of the COVID-19 pandemic which has, in many cases, accelerated the implementation of promising innovations in primary health care to achieve a system-wide transformation of care, such as expanding the role of pharmacists. Promoting the continuity of these practices and their wider adoption as health systems move into the pandemic recovery phase is critical for making health systems more resilient to health crisis.46
4.2. Strengths
This research is, to our best knowledge, is the first using a comprehensive mixed methods approach combining mapping of current practices and trends of pharmacy interventions on COVID-19 in 32 countries in Europe with a scoping review of evidence.
We generated a list of 31 pharmacy interventions on COVID-19 and further classified them under the categories which enabled to interpret findings on pharmacy interventions on COVID-19 in terms of stages used in response to public health emergencies.
We canvassed existing systematic reviews and primary studies of pharmacy interventions on COVID-19 using a scoping review which included grey literature.
4.3. Limitations
Mapping current practices relied on reported data from one or two respondents per country, hence, this could vary from the real practice. Since each country is represented by the responses of one or two individuals, this could result in selection bias (if respondent is not in full knowledge of the situation or is biased towards a self-preferred response pattern) or in social desirability bias (if the respondent tends to answer according to belief on what is desirable). However, it would have been impractical to perform surveys to a representative sample of pharmacies in all 32 countries and it is acknowledged that country pharmacy organizations tend to have a fair knowledge on current practices of most pharmacy services and, were particularly active in supporting and monitoring pharmacy interventions on COVID-19.
We did not use a systematic review approach as the aim was first to scope the existing body of literature on pharmacy-based COVID-19 interventions; examine types of research studies; and identify knowledge gaps. However, the scoping review approach also poses limitations. The absence of critical appraisal implies that discussion of implications for practice needs to be cautious.
We adopted a more inclusive perspective on study design acknowledging that conducting trials or performing longitudinal observational studies was difficult to conduct in pharmacies which had to face unexpected priorities and changes to their usual routine in a public health emergency.
Finally, we excluded 3 potential papers in non-English language which may have affected the synthesis of results stemming from just 9 studies.
5. Conclusions
5.1. Implications for research
There is room for improvement in future research to fill in gaps and provide additional evidence.
While mapping of practices of pharmacy services reflects data from 32 countries in Europe, the comprehensive review stemmed from 9 studies conducted in 7 countries. Almost all studies were cross-sectional and/or descriptive.
It is likely that the gap between practices and research on pharmacy interventions on COVID-19 will reduce, as more research would be published in near future.
It is important, though, that researchers are better prepared in future to respond with the same speed of practitioners by developing and conducting more studies. Acknowledging it is difficult to collect pharmacy-level or patient-level data amid pandemic as community pharmacists must respond to public health emergencies and hence have other priorities, efforts must be made to collect patient-level data in future to evaluate pharmacy interventions undertaken during public health emergencies. The use of patient-reported questionnaires or patient diaries using community pharmacies and the use of pharmacy dispensing software for database studies that capture dispensing patterns, adherence, and other interventions are important avenues to consider. Partnering with pharmacy organizations in such studies is recommended to improve relevance and policy usefulness of research findings.
We also recommend that future research on pharmacy interventions in response to public health emergencies adopts this framework of organizing interventions under the four steps used in public health to classify measures and interventions in response to public health emergencies (prevention, preparedness, response, and recovery).
5.2. Implications for policy and practice
Practice tends to precede research and moves forward at a much faster pace. So is the case with pharmacy interventions on COVID-19. Current practices portray a wide spectrum of interventions, some of them with expanded powers granted or legislation changes in response to the needs arising from the course of events.
Pharmacies have been able to implement a wide array of interventions on COVID-19, some of them beyond dispensing and which may have contributed to alleviate the burden on other health care services and provide valuable support to patients.
Expanded powers granted and legislation passed acknowledge that contribution.
Although this research does not aim to provide concrete guidance on practice and policy making, our findings pave the way for pharmacy associations to explore negotiations with governments for enhanced pharmacy roles in facilitating access to essential medication, medication usually supplied in hospitals, emergency supply, in point-of-care antigen-based test screening, structured referral pathways of exposed patients to antibody testing for immunity assessment and in vaccine administration.
Lessons learned from pharmacies’ involvement in response to this pandemic should also raise questions on the relevance of involving the network of pharmacies in future country preparedness plans for public health emergencies, namely in national contingency and emergency plans when a rapid response to massive population is required within a short time frame.
Availability of data and material
The datasets generated during and analyzed during the current study may be available from the corresponding author on reasonable request. Replies from PGEU Member Associations are subject to PGEU permission.
Ethics approval
This work does not contain studies with human participants performed by authors hence, it did not require ethics approval.
Funding
This research is supported by an unrestricted grant from the Pharmaceutical Group of the European Union (PGEU) and from the Associação Nacional das Farmácias (ANF) and developed by a Research Team from Portugal led by the Institute for Evidence-Based Health (ISBE), assisted by an Expert Panel of Researchers from the SDA Bocconi School of Management (Italy), London School of Economics and Political Science (UK), and the University of Huddersfield (UK).
The PGEU leadership and staff members were consulted to understand the context of the issue and collaborated on the development of the focus of this research, and country data collection.
PGEU and ANF had no role in the study design; data collection from literature review, analysis, interpretation of all collected data; writing of report; preparation of the manuscript; or decision to submit the article for publication.
CRediT authorship contribution statement
Suzete Costa: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition. Mariana Romão: Methodology, Software, Validation, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization. Maria Mendes: Validation, Formal analysis, Investigation, Writing – review & editing. Maria Rute Horta: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing. António Teixeira Rodrigues: Conceptualization, Methodology, Writing – review & editing. António Vaz Carneiro: Methodology, Writing – review & editing. Ana Paula Martins: Methodology, Writing – review & editing. Erika Mallarini: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision. Huseyin Naci: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision. Zaheer-Ud-Din Babar: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
ISBE (where SC, AVC, APM work) is supported by an unrestricted grant from the Pharmaceutical Group of the European Union (PGEU) and from the Associação Nacional das Farmácias (ANF) for this research. MR, MM, MRH, and ATR are employed by ANF. EM, HN, and ZB are supported by an unrestricted grant from PGEU for this research.
Acknowledgements
The authors would like to acknowledge the contribution of the following PGEU member associations in providing country replies and additional information used in this research (please see table below):
| Austria | Österreichische Apothekerkammer |
| Austria | Österreichischer Apothekerverband |
| Belgium | A.P.B - Association Pharmaceutique Belge/Algemene Pharmaceutische Bond |
| Belgium | Orde der Apothekers – Ordre des Pharmaciens |
| Bulgaria | Български фармацевтичен съюз |
| Croatia | Hrvatska Ljekarnička Komora |
| Croatia | Hrvatsko Farmaceutsko Društvo |
| Cyprus | Παγκύπριoς Φαρμακευτικός Σύλλoγoς (ΠΦΣ) |
| Czech Republic | Česká lékárnická komora |
| Denmark | Danmarks Apotekerforening |
| Estonia | Eesti Proviisorapteekide Liit |
| Finland | Suomen Apteekkariliitto/Finlands Apotekareförbund |
| France | Féderation des Syndicats Pharmaceutiques de France |
| France | Ordre National des Pharmaciens - Conseil Central A |
| France | USPO - Union des Syndicats de pharmaciens d'officine |
| Germany | ABDA - Bundesvereinigung Deutscher Apothekerverbände |
| Greece | Πανελλήνιος Φαρμακευτικός Σύλλογος |
| Hungary | Magyar Gyógyszerész Kamara |
| Ireland | Irish Pharmacy Union |
| Italy | Federazione Ordini Farmacisti Italiani (FOFI) |
| Italy | Federfarma |
| Kosovo | Oda e Farmacistëve të Kosovës |
| Latvia | Aptieku īpašnieku asociācija |
| Luxemburg | Syndicat des Pharmaciens Luxembourgeois a.s.b.l. |
| Malta | Kamra ta'l-Ispiżjara ta' Malta |
| Netherlands | KNMP - Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie |
| North Macedonia | Фармацевтска комора на Македонија |
| Norway | NAF – Apotekforeningen |
| Poland | Naczelna Izba Aptekarska |
| Portugal | Associação Nacional das Farmácias |
| Portugal | Ordem dos Farmacêuticos |
| Romania | Colegiul Farmacistilor din Romania |
| Serbia | Farmaceutska komora Srbije |
| Serbia | Savez farmaceutskih udruženja Srbije |
| Slovakia | Slovenská Lekárnická Komora |
| Slovenia | Lekarniška Zbornica Slovenije |
| Spain | Consejo General de Colegios Oficiales de Farmaceuticos España |
| Sweden | Sveriges Apoteksförening |
| Turkey | Türk Eczacıları Birliği |
| United Kingdom | National Pharmacy Association |
| United Kingdom | Pharmaceutical Society of Northern Ireland |
| United Kingdom | Royal Pharmaceutical Society |
The authors also wish to acknowledge Duarte Santos, President PGEU 2020 for challenging the team to develop this research, Dr. Ilaria Passarani, Secretary General of PGEU, and Jan de BELIE, Professional Affairs Advisor of PGEU, for their role in face validation and pretest of country surveys, assisting in the development of glossary of pharmacy services, and overseeing country response; Sónia Romano, senior researcher and Telma Escada, scientific information manager, for assisting authors in data extraction.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sapharm.2021.12.003.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.World Health Organization, Regional Office for Europe The 10 essential public health operations. https://www.euro.who.int/en/health-topics/Health-systems/public-health-services/policy/the-10-essential-public-health-operations
- 2.Tilson H., Gebbie K.M. The public health workforce. Annu Rev Publ Health. 2004;25:341–356. doi: 10.1146/annurev.publhealth.25.102802.124357. [DOI] [PubMed] [Google Scholar]
- 3.Otok R., Richardson E., Czabanowska K., et al. In: Organization and Financing of Public Health Services in Europe [Internet] Rechel B., Jakubowski E., McKee M., et al., editors. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2018. The public health workforce.https://www.ncbi.nlm.nih.gov/books/NBK535727/ (Health Policy Series, No. 50.) 5. Available from: [PubMed] [Google Scholar]
- 4.Rosenfeld L.A., Etkind P., Grasso A., Adams A.J., Rothholz M.C. Extending the reach: local health department collaboration with community pharmacies in Palm Beach County, Florida for H1N1 influenza pandemic response. J Publ Health Manag Pract. 2011;17:439–448. doi: 10.1097/PHH.0b013e31821138ae. [DOI] [PubMed] [Google Scholar]
- 5.Miller S., Patel N., Vadala T., Abrons J., Cerulli J. Defining the pharmacist role in the pandemic outbreak of novel H1N1 influenza. J Am Pharmaceut Assoc. 2012;52:763–767. doi: 10.1331/JAPhA.2012.110039. [DOI] [PubMed] [Google Scholar]
- 6.Czech M., Balcerzak M., Antczak A., et al. Flu vaccinations in pharmacies-A review of pharmacists fighting pandemics and infectious diseases. Int J Environ Res Publ Health. 2020;17:7945. doi: 10.3390/ijerph17217945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacinto I., Costa S., Horta M.R., et al. Serviço de Vacinação nas Farmácias Portuguesas [Vaccination Service in the Portuguese Pharmacies] Rev Port Farmacoter. 2015;7:160–166. doi: 10.25756/rpf.v7i3.13. [DOI] [Google Scholar]
- 8.Association of State and Territorial Health Officials (ASTHO) Public health and pharmacy collaboration in an influenza pandemic: summary of findings from an exploratory interview project; Arlington. 2014. https://www.astho.org/Infectious-Disease/Pandemic-Influenza/Public-Health-and-Pharmacy-Collaboration-in-an-Influenza-Pandemic/
- 9.Canadian Pharmacists Association Pandemic influenza. A pharmacist's guide to pandemic preparedness, Ottawa. 2009. http://www.pharmacists.ca/cpha-ca/assets/File/education-practice-resources/PandemicGuideEN.pdf
- 10.McCourt E. Deeble Institute of Health Policy Research, Australian Healthcare and Hospitals Association (AHHA), Canberra; 2018. Improving pharmacist involvement in pandemic influenza planning and response in Australia. Issues Brief.https://apo.org.au/sites/default/files/resource-files/2018-03/apo-nid136141.pdf Accessed June 21, 2021. [Google Scholar]
- 11.Aburas W., Alshammari T.M. Pharmacists' roles in emergency and disasters: COVID-19 as an example. Saudi Pharmaceut J. 2020;28:1797–1816. doi: 10.1016/j.jsps.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson K.E., Singleton J.A., Tippett V., Nissen L.M. Defining pharmacists' roles in disasters: a Delphi study. Murakami M, editor. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0227132. https://dx.plos.org/10.1371/journal.pone.0227132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visacri M.B., Figueiredo I.V., Lima T.M. Role of pharmacist during the COVID-19 pandemic: a scoping review. Res Soc Adm Pharm. 2021;17:1799–1806. doi: 10.1016/j.sapharm.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendonça A., Santos C., Pinto I.C. Community pharmacy services during the COVID-19 pandemic: a systematic review. INNOSC Theranostics and Pharmacological Sciences. 2020;3:18–26. doi: 10.36922/itps.v3i2.971. [DOI] [Google Scholar]
- 15.Cadogan C.A., Hughes C.M. On the frontline against COVID-19: community pharmacists' contribution during a public health crisis. Res Soc Adm Pharm. 2021;17:2032–2035. doi: 10.1016/j.sapharm.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden J.C., Parkin R. The challenges of COVID-19 for community pharmacists and opportunities for the future. Ir J Psychol Med. 2020;37(3):198–203. doi: 10.1017/ipm.2020.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkhurst C., Singh Purewal G., Donyai P. Community pharmacy and COVID-19-the unsung heroes on our high streets. J Patient Exp. 2020 Jun;7(3):282–284. doi: 10.1177/2374373520927638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain R., Dawoud D.M., Babar Z.U. Drive-thru pharmacy services: a way forward to combat COVID-19 pandemic. Res Soc Adm Pharm. 2021;17:1920–1924. doi: 10.1016/j.sapharm.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch M., O'Leary A.C. COVID-19 related regulatory change for pharmacists - the case for its retention post the pandemic. Res Soc Adm Pharm. 2021;17:1913–1919. doi: 10.1016/j.sapharm.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koçak F., Mrozovski J.M. La place du pharmacien dans la détection de la Covid-19 [The role of the pharmacist in the detection of Covid-19] Actual Pharmacol. 2020;59:41–43. doi: 10.1016/j.actpha.2020.10.023. French. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawoud D. Emerging from the other end: key measures for a successful COVID-19 lockdown exit strategy and the potential contribution of pharmacists. Res Soc Adm Pharm. 2021;17:1950–1953. doi: 10.1016/j.sapharm.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maidment I., Young E., MacPhee M., et al., A Rapid Realist Review of the Role of Community Pharmacy in the Public Health Response to COVID-19. medRxiv 2021.02.01.21250765. doi: https://doi.org/10.1101/2021.02.01.21250765. [DOI] [PMC free article] [PubMed]
- 23.Bukhari N., Rasheed H., Nayyer B., et al. Pharmacists at the frontline beating the COVID-19 pandemic. J Pharm Policy Pract. 2020;13:8. doi: 10.1186/s40545-020-00210-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meghana A., Aparna Y., Chandra S.M., Sanjeev S. Emergency preparedness and response (EP&R) by pharmacy professionals in India: lessons from the COVID-19 pandemic and the way forward. Res Soc Adm Pharm. 2021;17:2018–2022. doi: 10.1016/j.sapharm.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashiru-Oredope D., Chan A.H.Y., Olaoye O., Rutter V., Babar Z.U., C. P. A. COVID-19 Action Team Needs assessment and impact of COVID-19 on pharmacy professionals in 31 commonwealth countries. J Pharm Policy Pract. 2020;13:72. doi: 10.1186/s40545-020-00275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earl G., Cillessen L.M., Lyons-Burney H., et al. Pharmacists' role in infectious pandemics: illustration with COVID-19. Remington. 2021:849–876. doi: 10.1016/B978-0-12-820007-0.00064-7. [DOI] [Google Scholar]
- 27.Merks P., Religioni U., Bilmin K., et al. Readiness and willingness to provide immunization services after pilot vaccination training: a survey among community pharmacists trained and not trained in immunization during the COVID-19 pandemic in Poland. Int J Environ Res Publ Health. 2021;18:599. doi: 10.3390/ijerph18020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawoud D., Chen A.M.H., Rossing C.V., et al. Pharmacy practice research priorities during the COVID-19 pandemic: recommendations of a panel of experts convened by FIP pharmacy practice research special interest group. Res Soc Adm Pharm. 2021;17:1903–1907. doi: 10.1016/j.sapharm.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munn Z., Peters M.D.J., Stern C., et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMAScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 31.Merks P., Jakubowska M., Drelich E., et al. The legal extension of the role of pharmacists in light of the COVID-19 global pandemic. Res Soc Adm Pharm. 2021;17:1807–1812. doi: 10.1016/j.sapharm.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoti K., Jakupi A., Hetemi D., Raka D., Hughes J., Desselle S. Provision of community pharmacy services during COVID-19 pandemic: a cross sectional study of community pharmacists' experiences with preventative measures and sources of information. Int J Clin Pharm. 2020;42:1197–1206. doi: 10.1007/s11096-020-01078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaidi S.T.R., Hasan S.S. Res Soc Adm Pharm; 2020. Personal Protective Practices and Pharmacy Services Delivery by Community Pharmacists during COVID-19 Pandemic: Results from a National Survey. July). Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerbin-Koczorowska M., Waszyk-Nowaczyk M., Przymuszała P. Pharmacists' preparedness to patients education at the time of pandemic-A cross-sectional study with an example of SARS-CoV-2 outbreak in Poland. Int J Environ Res Publ Health. 2020;17:6659. doi: 10.3390/ijerph17186659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koster E.S., Philbert D., Bouvy M.L. Impact of the COVID-19 epidemic on the provision of pharmaceutical care in community pharmacies. Res Soc Adm Pharm. 2021;17:2002–2004. doi: 10.1016/j.sapharm.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim R.H.M., Shalhoub R., Sridharan B.K. The experiences of the community pharmacy team in supporting people with dementia and family carers with medication management during the COVID-19 pandemic. Res Soc Adm Pharm. 2021;17:1825–1831. doi: 10.1016/j.sapharm.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giua C., Paoletti G., Minerba L., et al. Community pharmacist's professional adaptation amid Covid-19 emergency: a national survey on Italian pharmacists. Int J Clin Pharm. 2021;43:708–715. doi: 10.1007/s11096-020-01228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak H., Tadić I., Falamić S., Ortner Hadžiabdić M. Pharmacists' role, work practices, and safety measures against COVID-19: a comparative study. J Am Pharmaceut Assoc. 2021;S1544–3191(21):104–107. doi: 10.1016/j.japh.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan D., Guiu-Segura J.M., Sousa-Pinto G., Wang L.N. How COVID-19 has impacted the role of pharmacists around the world. Farm Hosp. 2021;45:89–95. doi: 10.7399/fh.11652. English. [DOI] [PubMed] [Google Scholar]
- 40.Sousa Pinto G., Hung M., Okoya F., Uzman N. FIP's response to the COVID-19 pandemic: global pharmacy rises to the challenge. Res Soc Adm Pharm. 2021;17:1929–1933. doi: 10.1016/j.sapharm.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Pharmaceutical Federation FIP covid-19 information hub - FIP [internet] https://www.fip.org/coronavirus
- 42.World Pharmacy Council SectorAnalysis special edition: community pharmacy & COVID-19. https://d3r4tb575cotg3.cloudfront.net/static/2020 Sector Analysis Special Edition - COVID-19 - Final_compressed_web-v1.pdf
- 43.Pharmaceutical Group of the European Union COVID-19 information hub. https://www.pgeu.eu/covid-19-information-hub/
- 44.Isenor J.E., Edwards N.T., Alia T.A., et al. Impact of pharmacists as immunizers on vaccination rates: a systematic review and meta-analysis. Vaccine. 2016;34:5708–5723. doi: 10.1016/j.vaccine.2016.08.085. [DOI] [PubMed] [Google Scholar]
- 45.Schwerzmann J., Graitcer S.B., Jester B., et al. Evaluating the impact of pharmacies on pandemic influenza vaccine administration. Disaster Med Public Health Prep. 2017;11:587–593. doi: 10.1017/dmp.2017.1. [DOI] [PubMed] [Google Scholar]
- 46.OECD . Realising the Potential of Primary Health Care; 2020. OECD Health Policy Studies. Published. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study may be available from the corresponding author on reasonable request. Replies from PGEU Member Associations are subject to PGEU permission.