Abstract
Extracellular matrix signaling via integrin receptors is important for smooth muscle cell (SMC) differentiation during vasculogenesis and for phenotypic modulation of SMCs during atherosclerosis. We previously reported that the noncatalytic carboxyl-terminal protein binding domain of focal adhesion kinase (FAK) is expressed as a separate protein termed FAK-related nonkinase (FRNK) and that ectopic expression of FRNK can attenuate FAK activity and integrin-dependent signaling (A. Richardson and J. T. Parsons, Nature 380:538–540, 1996). Herein we report that in contrast to FAK, which is expressed ubiquitously, FRNK is expressed selectively in SMCs, with particularly high levels observed in conduit blood vessels. FRNK expression was low during embryonic development, was significantly upregulated in the postnatal period, and returned to low but detectable levels in adult tissues. FRNK expression was also dramatically upregulated following balloon-induced carotid artery injury. In cultured rat aortic smooth muscle cells, overexpression of FRNK attenuated platelet-derived growth factor (PDGF)-BB-induced migration and also dramatically inhibited [3H]thymidine incorporation upon stimulation with PDGF-BB or 10% serum. These effects were concomitant with a reduction in SMC proliferation. Taken together, these data indicate that FRNK acts as an endogenous inhibitor of FAK signaling in SMCs. Furthermore, increased FRNK expression following vascular injury or during development may alter the SMC phenotype by negatively regulating proliferative and migratory signals.
Smooth muscle cell (SMC) proliferation and migration are essential features of vasculogenesis and blood vessel maturation and clearly play a role in the pathophysiology of several prominent cardiovascular disease states, such as atherosclerosis, restenosis following balloon angioplasty, and hypertension (15, 24). These processes are regulated by a number of humoral factors, including growth factors (i.e., platelet-derived growth factor [PDGF] and fibroblast growth factor), contractile agonists (i.e., angiotensin II and thrombin), and cytokines (i.e., transforming growth factor β and interleukins) (1, 15, 24). Extracellular matrix (ECM) interactions are also important, and it is clear that deposition of ECM components within the blood vessel wall and the specific expression of SMC integrin receptors are regulated during vascular development and under pathologic conditions (27). Thus, it is important to identify the molecular mechanisms by which growth factor- and ECM-mediated signaling pathways in SMCs converge to regulate proliferation and migration of vascular SMCs.
Activation of the integrin families of transmembrane cell surface receptors is mediated by contact with ECM components such as fibronectin and collagen and leads to the formation of focal adhesion structures. Focal adhesions contain not only cytoskeletal components that physically tether the integrin cytoplasmic tail to the actin cytoskeleton but also a number of signaling molecules that are activated in an adhesion-dependent fashion (3). Recruitment of the protein tyrosine kinase focal adhesion kinase (FAK) to the integrin-rich complexes appears to be central to focal adhesion formation, remodeling, and subsequent downstream signaling (23). We and others have suggested that FAK activation is necessary for optimal growth factor-mediated stimulation of cell proliferation and that growth factor and ECM signaling pathways converge (17, 26).
FAK signaling is modulated by expression of an endogenous FAK inhibitor termed FRNK (FAK-related nonkinase), which is expressed as an independent protein and consists of the carboxyl-terminal noncatalytic domain of FAK (22). Although ectopically expressed FRNK is directed to focal adhesions upon overexpression in fibroblasts and inhibits FAK-mediated signaling events, the role of endogenously expressed FRNK in focal adhesion signaling is unclear (19, 20).
We report here that in contrast to FAK, which is ubiquitously expressed, FRNK is expressed selectively in SMCs, with particularly high levels observed in large arteries. We show that FRNK expression is regulated during development and disease in vivo and that increased FRNK expression modulates SMC growth and migration in vitro. These data indicate that FRNK may serve as a regulator of adhesion and growth factor signaling in SMCs and that changes in FRNK expression may be central to SMC phenotype modulation during vascular development and remodeling.
MATERIALS AND METHODS
Cell culture.
Rat aortic SMCs were obtained from adult rat thoracic aortas by enzymatic digestion as previously described (5). Cells were maintained in Dulbecco's modified Eagleś medium (DMEM)-F12 (1:1) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were used between passages 10 and 25. 9E11G cells were derived from retinoic acid-treated P19 cultures and cultured as previously described (2).
Immunoprecipitation and Western blot analysis.
Rat aortic SMCs were lysed by homogenization in a modified radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES, 0.15 M NaCl, 2 mM EDTA, 0.1% NP-40, 0.05% sodium deoxycholate, pH 7.2) containing 1 mM Na3VO4, 40 mM NaF, and 10 mM Na2 pyrophosphate. Paxillin and p130 Crk-associated substrate (CAS) were immunoprecipitated by incubation of 1 to 2 mg of cell extract with 5 μg of the appropriate antibody for 2 h at 4°C, followed by a 1-h incubation with protein A-Sepharose-conjugated beads (Pharmacia). For paxillin immunoprecipitation, the beads were precoupled with rabbit anti-mouse antibody (10 μg/ml; Jackson Laboratories). The immune complexes were collected by centrifugation, the beads were washed three times with RIPA buffer and once with Tris-buffered saline (0.2 M NaCl, 50 mM Tris-HCl, pH 7.4) and boiled in sample buffer. Proteins were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and were transferred to nitrocellulose. Western blots were performed using the appropriate primary antibody at a 1/1,000 dilution, followed by incubation with either horseradish peroxidase-conjugated rabbit anti-mouse antibody (Ab) or horseradish peroxidase-conjugated protein A-Sepharose (Amersham) at a 1/1,000 dilution. Blots were visualized after incubation with chemiluminescence reagents (ECL; Amersham). For the analysis of FAK and Pyk2 expression, cell lysates were subjected to SDS-PAGE as described above and Western blot assays were performed using the activation-specific antibodies for tyrosine-phosphorylated FAK (pY397) or Pyk2 (pY402) (Biosource International).
Northern blotting and RNase protection.
RNA was isolated from rodent tissues or cell lines using Qiagen RNA preparation columns, and mRNA was selected using oligo(dT)-Sepharose chromatography (Qiagen, Chatsworth, Calif.). For Northern analysis, RNA (10 μg of total RNA per lane) was resolved on a 1% agarose gel containing 2.2 M formaldehyde and transferred to nitrocellulose filters, and blots were probed with 32P-labeled FAK cDNA probes (nucleotides 1163 to 2088, 5′ probe; nucleotides 2296 to 3213, 3′ probe). The probes were labeled and hybridized, and the Northern blots were washed as described previously (25). For the RNase protection assay, FRNK- and FAK-specific RNA probes were generated by reverse transcription-PCR, hybridized to RNA (50 μg), and digested with RNase T1 as previously described (14). Protected fragments were resolved on a 6% denaturing polyacrylamide–urea gel. Autoradiograms were obtained by exposing the blots to Kodak XAR film for 1 to 7 days.
Expression constructs and adenovirus production.
The cDNA construct encoding the amino-terminal green fluorescent protein (GFP)-tagged variant of FRNK was generated by cloning chicken FRNK into the BglII/EcoRI sites of the mammalian expression vector pEGFP-C1. The amino-terminally Myc-tagged variant of FAK was generated by cloning chicken FAK into the BamHI/NotI sites of the mammalian expression vector pRK5myc. For adenovirus production, GFP and GFP-tagged FRNK were generated by PCR from pEGFP-C1 and pEGFP-C1-FRNK, respectively, using primers that added 5′ and 3′ BamHI restriction sites. The resultant PCR products were digested with BamHI and ligated with BamHI-digested pAd-lox (an adenovirus shuttle vector generously provided by Stephen Hardy, Somatix Therapy Corporation, Alameda, Calif.). Correct orientations of all reading frames were confirmed by sequencing and Western blot analysis of expressed proteins. The GFPAd-lox and GFP-FRNKAd-lox constructs were subsequently cotransfected with replication-defective ψ5 virus into HEK293 cells that produce stable overexpression of the Cre recombinase. After recombination, plaque-purified virus (1010 PFU/ml) was generated and purified by using cesium chloride gradients as described elsewhere (8).
Immunocytochemistry.
For immunofluorescent staining, cells were washed three times with phosphate-buffered saline (PBS; calcium and magnesium free) and fixed using 4% paraformaldehyde in PBS for 20 min at room temperature. Cells were washed three times in PBS and permeabilized with 0.4% Triton X-100 in PBS for 3 min at room temperature. Slides were then washed three times in PBS to remove the detergent and incubated with the either the primary anti-Myc monoclonal Ab 9E10 (1 μg/ml) or the polyclonal anti-pTyr Ab (1:500; from Santa Cruz and UBI, respectively) for 1 h. Cells were washed three times in PBS and incubated with either Texas Red-conjugated donkey anti-rabbit or donkey anti-mouse Ab (2 μg/ml) for 1 h.
Carotid artery-injury.
Balloon injury of the rat carotid artery was performed as previously described (5). Briefly, male Wistar rats weighing 300 to 350 g were anesthetized with ketamine (10 mg/kg) and xylazine (10 mg/kg) and the right common and external carotid arteries were exposed and isolated. A 2 French Fogarty balloon catheter was introduced through the external carotid into the common carotid artery, inflated to 2 atm, and pulled back and forth three times to induce endothelial damage. Following removal of the balloon, blood flow was restored and the wound was closed. Arterial segments were harvested at the indicated time points, three cross sections (5 μm) were obtained from each carotid artery for histological staining and identification of intimal hyperplasia, and the remainder of the material was used for Western blotting. All procedures followed guidelines for university laboratory animal practices.
Cell migration.
Rat aortic SMCs were infected with GFP or GFP-FRNK adenovirus (multiplicity of infection of 2) for 2 h. Cells were washed in PBS, trypsinized, and collected in PBS containing soybean trypsin inhibitor (1 mg/ml). Cells were pelleted, washed once, and resuspended in serum-free DMEM-F12 containing 0.1% crystalline bovine serum albumin. Cells (105/well) were plated in the upper well of fibronectin (1 μg/ml)-coated Boyden chambers. The bottom chambers were filled with serum-free DMEM-F12 containing 0.1% bovine serum albumin and vehicle or PDGF-BB (1 to 30 ng/ml). Chambers were incubated at 37°C for 8 h. Chambers were rinsed with PBS, cells were removed from the top chamber by scraping with a cotton swab, and the remaining cells (in the bottom chamber) were fixed in 4% paraformaldehyde for 20 min. Cells that had migrated to the bottom chamber were visualized by fluorescence assay using a Leica inverted fluorescence microscope. Data represent the total number of cells in four separate fields for each condition.
[3H]thymidine incorporation, cell number, and cell cycle analysis.
Rat aortic SMCs were cultured in 12-well plates at a density of 2 × 104 cells/ml in serum-containing DMEM-F12 (1:1). At 24 h, cells were incubated with either GFP or GFP-tagged FRNK adenovirus at a multiplicity of infection of 2 for 6 h. Cells were rinsed twice in serum-free medium and incubated in the absence of serum for 4 h. To asses the levels of [3H]thymidine incorporation, cells were treated with either 10% fetal calf serum (FCS) or PDGF-BB for 16 h with the last 4 h in the presence of [3H]thymidine (1 μCi/ml). Cells were then rinsed twice with PBS and three times with trichloroacetic acid (10%) and lysed with 400 μl of 1 M NaOH. After neutralization with 400 μl of 1 M HCl, 75% of the final volume was counted by liquid scintillation and 25% was used to determine protein content. To assess cell numbers, cells were treated with 10% FCS-containing medium for the times indicated following infection and serum withdrawal as described above. Both suspended and attached cells were collected, and the entire population was counted using a Coulter Counter (Z1 Dual) gated to count events between 7 and 20 μm. To examine cell cycle analysis, both suspended and attached cells from a confluent 100-mm-diameter dish were collected following a 72-h treatment with 10% FCS-containing medium or PDGF-BB as described above. The cells were washed and resuspended in 300 μl of a solution containing 0.3% NP-40, 0.1% Na citrate, 1 mg of RNase per ml, and 50 μg of propidium iodide per ml. After a 24-h incubation at 4°C, cells were sorted by fluorescence-activated cell sorter. A minimum of 10,000 events was counted for each sample.
RESULTS
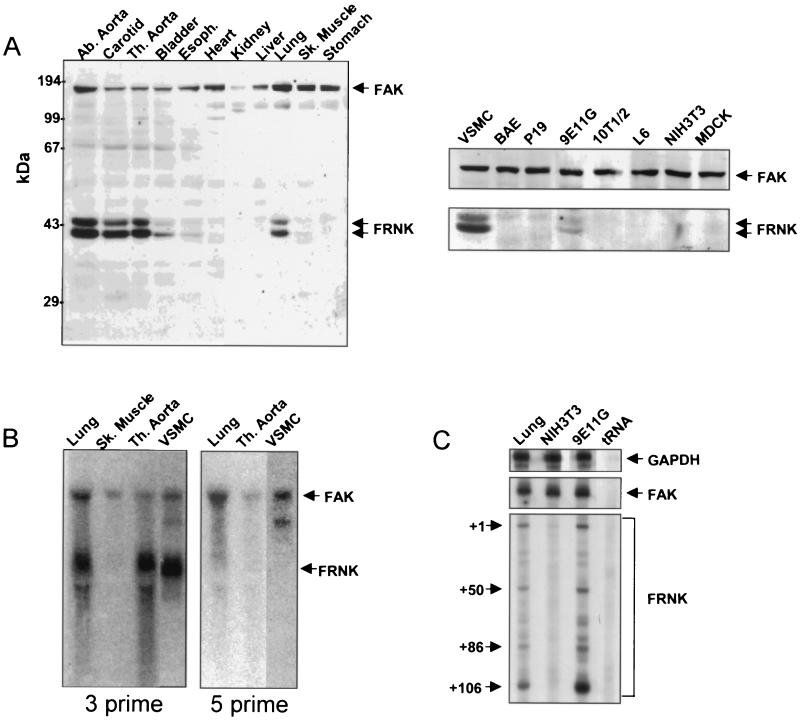
As shown in Fig. 1, Western blot analysis of lysates prepared from various rat tissues using an antibody to the carboxyl terminus of FAK revealed that FAK was ubiquitously expressed whereas FRNK expression was restricted to smooth muscle tissues. Readily detectable levels of FRNK were present in lysates from large arteries such as the carotid and the abdominal and thoracic aorta (Fig. 1A). In contrast, the level of FRNK in the smaller arterioles, such as the femoral and renal arteries, was low but detectable (data not shown). Significant levels of FRNK protein were also detected in the lung and the bladder, which contains a large smooth muscle component (Fig. 1A). FRNK was also readily detected in lysates from cultured rat aortic SMCs and in a clonal mouse cell line derived from retinoic acid-treated P19 cultures (9E11G) which stably express multiple smooth muscle differentiation marker genes (2). It should be noted that levels of FRNK expression in the cultured rat aortic SMC line varied somewhat (compare the levels in Fig. 1B and 3A), with higher levels usually observed in lower-passage cells. In contrast, FRNK was not detected in a variety of other cultured cells, including undifferentiated P19 cells, bovine aortic endothelial cells, 10T1/2 cells; L6 myoblasts, NIH 3T3 cells, or MDCK cells (Fig. 1A right).
FIG. 1.
Expression of FRNK in smooth muscle tissues and cultured cells. (A) Individual tissues from rats (4-day-old pups) were dissected, washed in PBS, and lysed in RIPA buffer. Protein extracts (100 μg/lane) from tissues (left) or cultured cells (right) were subjected to SDS-PAGE. Western blotting was performed using an anti-FAK carboxyl-terminal Ab. (B) RNA prepared from rat tissues (3-week-old pups) or vascular SMCs was subjected to Northern analysis using a 3′ or 5′ cDNA probe from the FAK coding region (corresponding to nucleotides 2296 to 3213 or 1163 to 2088, respectively). The arrows indicate the positions of the FAK and FRNK RNAs. (C) A sequencing gel showing the positions of labeled, RNase-resistant products obtained after incubation of total mouse lung, 9E11G RNA, or NIH 3T3 RNA with a probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), FAK, or FRNK. Abbreviations: Ab., abdominal; Th., thoracic; Sk., skeletal; VSMC, rat aortic SMC; BAE, bovine aortic endothelial cells.
FIG. 3.
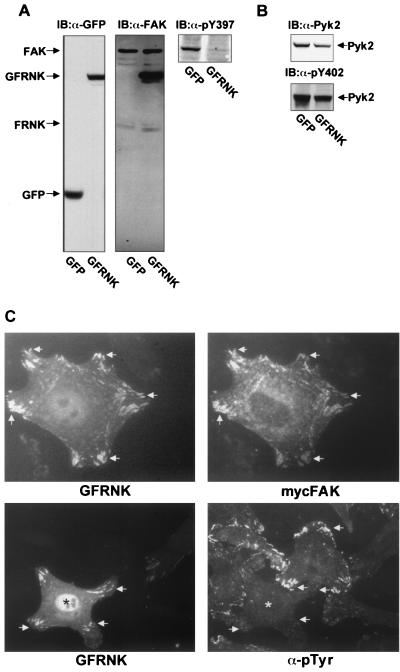
FRNK inhibits FAK autophosphorylation and colocalizes with FAK in SMCs. Rat aortic SMCs were infected with GFP or GFP-FRNK (GFRNK) adenovirus for 6 h. Extracts (100 μg) were analyzed by SDS-PAGE and immunoblotting (IB) using an Ab specific for GFP (A, left), an Ab for the C terminus of FAK (A, middle), a phosphorylation-specific Ab to Y397 of FAK (A, right), Pyk2 (B top), and a phosphorylation-specific Ab to Y402 of PYK2 (B, bottom). (C) Rat aortic SMCs were transfected with GFP-tagged FRNK cDNA with or without Myc-tagged FAK (mycFAK) for 18 h. Cells were trypsinized and plated on fibronectin-coated chamber slides for 3 h. Cells were fixed, permeabilized, and stained with the appropriate epitope tag Ab or an antiphosphotyrosine Ab. Arrows indicate focal-adhesion structures. Asterisks indicate the cell expressing GFRNK.
We previously demonstrated that in the chicken, FRNK transcription results from the utilization of an alternative promoter embedded between exons encoding the FAK-kinase domain and the beginning of the carboxyl -terminal region (FRNK). FRNK-specific mRNAs are initiated from multiple starts within a unique 5′ noncoding exon (14). However, recently reported data also indicate that FRNK-like peptides can be generated in cells by protease degradation of FAK (4, 28). To discriminate between these two possibilities, we isolated RNAs from various smooth and nonsmooth muscle tissues and subjected the RNAs to Northern analysis. As shown in Fig. 1B, hybridization with a cDNA probe from the 3′ end of the FAK coding region revealed an approximately 4.2-kb FAK RNA in rat lung, skeletal muscle, thoracic aorta, and cultured aortic SMCs and an ∼2.2-kb presumptive FRNK RNA in rat lung, thoracic aorta, and cultured aortic SMCs but not in rat skeletal muscle. These findings correlated well with FRNK expression patterns detected by Western blot analysis (Fig. 1A). A cDNA probe derived from the 5′ end of the FAK coding region hybridized to the 4.2-kb but not the 2.2-kb message, indicating that the 2.2-kb RNA coded for FRNK. To rule out the possibility that the 2.2-kb RNA species was an alternative FAK RNA splice variant, we performed an RNase protection assay using an RNA probe directed against the unique 5′-untranslated murine FRNK leader sequence. Figure 1C demonstrates that four RNA species from murine lung and SMCs (9E11G) were protected from RNase digestion following hybridization with the murine FRNK-specific probe. In contrast, hybridization of the same probe with RNA isolated from NIH 3T3 fibroblasts, a cell line that does not express detectable levels of FRNK protein, failed to generate these protected RNA species. The size of the protected RNA species in the murine SMC line is in agreement with our previous report indicating the presence of four putative transcriptional start sites within the chicken FRNK leader sequence (14). Taken together, these data show that FRNK is expressed selectively in SMCs and that FAK and FRNK expression is derived from differentially regulated RNA transcription.
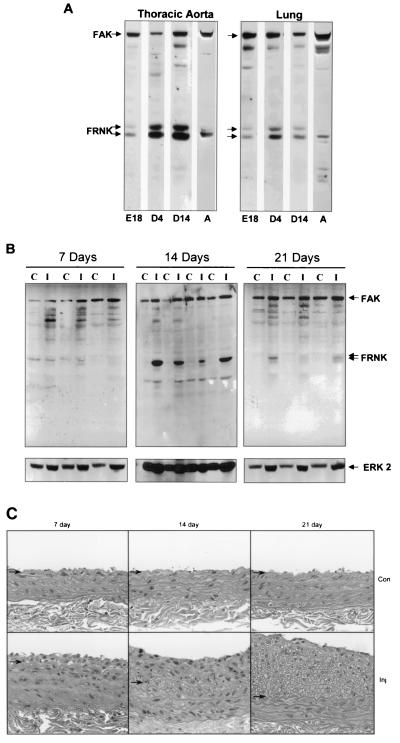
Several ECM components and integrin signaling molecules that affect SMC function are developmentally regulated; therefore, we examined whether FRNK expression was also regulated during smooth muscle development. Figure 2A demonstrates that aortic expression of FRNK was relatively low in the embryo (E18), increased during the neonatal period (4 to 14 days after birth), and returned to low but detectable levels in the adult (8 weeks). A similar developmental pattern of FRNK expression was observed in the lung. During development, two forms of FRNK (with apparent molecular masses of 41 and 43 kDa) appear to be present, with the lower-mass form being predominant in adult tissues. The origin of these two forms is unknown; however, they could arise from either alternative translational start sites or phosphorylation (21, 22)
FIG. 2.
Expression of FRNK in smooth muscle tissues during development and following vascular injury. (A) Thoracic aorta and lung tissues were dissected from animals during development. Protein extracts were prepared and analyzed by Western immunoblot analysis as described in the legend to Fig. 1. E, embryonic day; D, day after birth; A, adult. Tissues were pooled from four rat embryos and two neonates (day 4). Data are representative of three separate experiments. (B) Rat carotid arteries were injured by balloon-mediated endothelial denudation. Vessels were dissected either 7, 14, or 21 days postinjury from the injured left carotid (I) or control contralateral right carotid (C) artery. Extracts were prepared, subjected to SDS-PAGE, and immunoblotted with anti-FAK carboxyl-terminal Ab (top) or anti-extracellular-signal-regulated kinase 2 to serve as a loading control (bottom). No significant differences in FAK pTyr were observed as determined by blotting with a phosphorylation-specific Ab to Y397 of FAK (data not shown). Data are representative of seven animals per time point from two separate experiments. (C) Histological staining of uninjured control (Con) or injured (Inj) sections 7, 14, or 21 days following surgery. Arrows denote the position of the internal elastic lamina.
Since FRNK was highly expressed during the postnatal period, a time during which the vasculature is undergoing extensive growth and remodeling, we investigated whether FRNK expression was also altered during the SMC growth response that occurs following vascular injury. FRNK expression was determined by Western blot analysis of extracts prepared from rat carotid arteries subjected to balloon-induced injury and uninjured contralateral control vessels. Figure 2B demonstrates that compared to those in the uninjured contralateral control vessel, FRNK protein levels were unchanged at 7 days following endothelial denudation. At 14 days following injury, when SMC proliferation begins to decline, a significant increase in FRNK protein levels was observed in all injured vessels. By 21 days, when vessel remodeling is virtually complete, FRNK expression was low but increased slightly in injured vessels compared to that in the uninjured controls. The immunoblot for extracellular-signal-regulated kinase 2 shown in the bottom of Fig. 2B was used as a control for the relative amount of total protein present in each lysate. Vascular injury was examined histologically at each time point, and the extent of intimal hyperplasia observed was in agreement with that found by others (Fig. 2C; reference 5). These data indicate that FRNK is expressed during periods of vessel maturation and remodeling and may be important for control of SMC growth under these conditions.
In embryo fibroblasts, overexpression of FRNK results in the attenuation of matrix-stimulated FAK activation, indicating that FRNK may act as an endogenous inhibitor of FAK signaling (20). To determine whether FRNK down-regulates FAK activity in SMCs, GFP-tagged FRNK was expressed in rat aortic SMC cultures using replication-defective adenovirus constructs. GFP-FRNK and control GFP constructs were efficiently expressed in 90 to 100% of the SMC population, as determined by immunofluorescence assay (data not shown). As shown in Fig. 3A, both GFP and GFP-FRNK proteins were detectable in rat aortic SMC lysates within 6 h after infection. Moreover, infection of SMC with GFP-FRNK, but not GFP, inhibited FAK activation as assessed by immunoblotting with antibodies specific for the major site of FAK autophosphorylation, pTyr397 (Fig. 3A, top right). As previously reported for fibroblasts, we also observed that overexpression of FRNK also attenuated the tyrosine phosphorylation of two putative FAK substrates, paxillin and CAS, in rat aortic SMCs (data not shown). Interestingly, FRNK overexpression did not appear to attenuate activation of the structurally related protein tyrosine kinase Pyk2 as assessed by immunoblotting with antibodies specific for the major site of Pyk2 autophosphorylation, pTyr402 (Fig. 3B, bottom). As shown in Fig. 3C, GFP-FRNK colocalized with FAK in focal adhesions and decreased focal-adhesion-associated phosphotyrosine content following plating of rat aortic SMCs on fibronectin. These data indicate that overexpression of GFP-FRNK significantly inhibited integrin-mediated FAK signaling in SMCs.
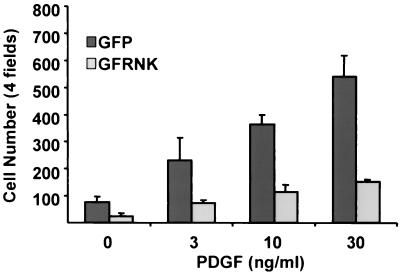
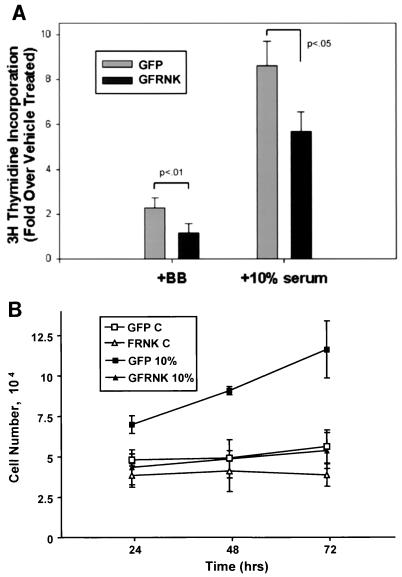
Because FRNK inhibited integrin-mediated FAK activation, we determined whether FRNK expression in SMCs also blocked integrin-dependent functions such as migration and proliferation. As shown in Fig. 4, analysis of cell migration using Boyden chamber assays demonstrated that PDGF-BB stimulated a dramatic chemotactic response in GFP-infected rat aortic SMCs plated on fibronectin (1 μg/ml). Overexpression of GFP-FRNK significantly inhibited SMC migration toward PDGF-BB by approximately 70% of that observed in GFP-infected cells. As shown in Fig. 5A, GFP-FRNK overexpression in rat aortic SMCs completely prevented the PDGF-BB-induced increase in [3H]thymidine incorporation and significantly inhibited the effects of 10% serum. Similarly, GFP-FRNK expression significantly attenuated 10% serum- or PDGF-BB-induced progression from G1 to S phase as analyzed by fluorescence-activated cell sorting (data not shown). The GFP-FRNK-mediated inhibition of DNA synthesis was concomitant with a decrease in cell number in serum-stimulated cultures over a 72-h period (Fig. 5B). Taken together, these data indicate that two very important SMC functions, proliferation and migration, can be attenuated by overexpression of FRNK.
FIG. 4.
FRNK inhibits ECM and PDGF-BB-stimulated SMC migration. Rat aortic SMCs were infected with GFP or GFP-FRNK adenovirus for 2 h. Cells were trypsinized and plated in serum-free medium on Boyden chambers coated with fibronectin (1 μg/ml) as described in Materials and Methods. PDGF-BB (0 to 30 ng/ml) was added to serum-free medium in the bottom chamber. Chambers were incubated at 37°C for 8 h before fixation with paraformaldehyde. Cells that migrated to the bottom of the membrane were counted using an inverted light or fluorescence microscope (Zeiss). The data are presented as the mean number of total cells (± the standard deviation) counted in four fields using a 20× objective in three separate experiments. P < 0.05 between GFP and GFP-FRNK (GFRNK) for each PDGF treatment group.
FIG. 5.
FRNK inhibits DNA synthesis and cell proliferation in SMCs. (A) Rat aortic SMCs were plated, serum starved, and infected with GFP or GFP-FRNK (GFRNK) virus as described in Materials and Methods. Cells were treated with vehicle, PDGF-BB, or 10% FCS for 16 h with the last 4 h in the presence of [3H]thymidine (1 μCi/ml). The extent of DNA labeling was determined as described in Materials and Methods. Data are presented as mean fold increases in the counts per minute per migrogram of protein over vehicle-treated controls in four separate experiments (± the standard deviation). Significant differences between groups are indicated. (B) To assess cell number, cells were treated with 10% FCS-containing medium following infection and serum withdrawal as described in Materials and Methods. The x axis reflects the time elapsed following addition of 10% FCS. Cells were collected and counted as described in Materials and Methods. Data represent the mean ± the standard error for three separate experiments. P < 0.05 between GFP and GFP-FRNK in 10% serum at 24 h, and P < 0.01 at 48 and 72 h. No significant differences were observed between the GFP and GFP-FRNK groups in serum-free medium. C, control.
DISCUSSION
Alterations in SMC migration and growth responsiveness play prominent roles during the pathophysiologic progression of atherosclerosis, hypertension, and restenosis (1, 15, 24). Substantial evidence links these processes in SMCs to ECM (integrin)-dependent signaling events (27). However, the cell type-specific mechanisms that differentially regulate integrin signaling pathways are poorly understood. In this report, we show for the first time that in SMCs both proliferative and migratory signals can be regulated by the selective expression of the negative regulatory domain of FAK, FRNK. We also demonstrate that FRNK is selectively expressed in SMCs with particularly high levels observed in conduit blood vessels, and that FRNK expression was dramatically altered during vascular development and following vessel injury. Expression of FRNK in SMCs inhibited migration and proliferation, suggesting that differential expression of FRNK is important in the regulation of these integrin-dependent functions. Taken together, these data indicate that FRNK may act as an endogenous inhibitor of FAK signaling in SMC and suggest that FRNK may modulate ECM-mediated signaling events in smooth muscle tissues.
The finding that FRNK is expressed relatively highly in lung tissue compared to other tissues that contain a much larger smooth muscle component, such as the stomach, liver, and uterus, suggests the possibility that FRNK is also expressed in other, as yet unidentified, cell types. Alternatively, expression in the lung could reflect high levels in the bronchial smooth muscle or the presence of large blood vessels within the tissue. Nevertheless, our findings that FRNK is highly expressed in SMCs and is regulated developmentally and following vascular injury suggest that promoter sequences within the FAK-FRNK gene contain smooth-muscle-specific regulatory elements that are responsive to changes in SMC phenotype. We have previously shown that a 2-kb DNA fragment derived from a region 5′ of the FRNK noncoding region efficiently directs expression of a luciferase reporter when transfected into cultured chicken fibroblasts but not chicken B cells (14). Although we have yet to describe the specific promoter regions that are responsible for SMC-selective expression of FRNK, identification of these regulatory elements will provide valuable information on the role of FRNK in SMCs, as well as on SMC-specific gene expression.
FRNK transcription results in the autonomous production of the C-terminal domain of FAK, which contains sequences required for focal-adhesion targeting, as well as binding sites for a number of signaling molecules, including the adapter proteins CAS and paxillin and the GTPase regulators GRAF and ASAP1 (3, 16, 16a; J.T.P., unpublished observations). Like FAK, FRNK interacts with the aforementioned binding partners and localizes in focal adhesions when overexpressed. The ability of ectopically expressed FRNK to inhibit FAK autophosphorylation and tyrosine phosphorylation of the putative substrates CAS and paxillin suggests that this domain acts as a competitive inhibitor of FAK (19, 20). The exact mechanism of FRNK-mediated inhibition of FAK is unclear. One possibility is a displacement model which argues that although FRNK and FAK can colocalize in focal adhesions, high levels of FRNK within a focal adhesion may diminish auto- or transphosphorylation of FAK at tyrosine 397. This could occur by alteration of the topology of FAK within the focal adhesion or by simple limitation of the amount of FAK recruited to focal adhesions following integrin clustering. Alternatively, if FAK activation proceeds through a dimerization and transphosphorylation mechanism (as is observed for many receptor tyrosine kinases), then an increase in the amount of FRNK in a focal complex might inhibit this process by forming inactive FRNK-FAK heterodimers. A second model suggests that increased FRNK expression may lead to the increased recruitment of negative regulators of FAK to focal complexes. For example, FRNK might increase the concentration of tyrosine phosphatases, GTPase-activating proteins such as GRAF and ASAP1, or other, unknown, proteins. The presence of any such proteins could alter focal adhesion turnover and result in a reduction in FAK tyrosine phosphorylation. Future studies will address the mechanisms by which endogenous FRNK may alter FAK-dependent signaling.
Proliferation and migration of SMCs are critical for normal vascular development and have been shown to play a role in the development of atherosclerotic disease. SMC proliferation plays a critical role in formation of the central medial layer that makes up the bulk of the wall in large vessels (15). After a dramatic increase in SMC proliferation that occurs during the later stages of vessel formation, SMCs typically modulate to a less proliferative and more contractile phenotype. However, even in adult animals, vascular SMCs maintain the ability to regulate their phenotype and under certain pathological conditions, such as atherosclerosis and vessel injury, SMCs are stimulated to migrate into and subsequently proliferate in the intima (12). Interestingly, FRNK expression levels were highest in developing vessels and in the later stages following vessel injury (e.g., 14 days), suggesting that FRNK contributes to the regulation of proliferative and/or migratory signals related to the transition from the proliferative to the contractile state.
Indeed, our data indicate that overexpression of FRNK in cultured SMCs significantly inhibits both proliferative and migratory signals. Furthermore, FRNK levels in adenovirus-infected SMC cultures compare favorably with FRNK expression in arterial SMCs. For example, as determined by densitometric analysis of Western blots probed with an anti-FAK antibody that recognizes both FAK and FRNK, adenovirus infection with a cytomegalovirus promoter driven FRNK expression construct in cultured SMCs resulted in an about 6- to 10-fold increase in FRNK protein. This resulted in a ratio of FRNK to FAK of around 3.5 to 1. Analysis of extracts derived from aortic SMCs isolated from several different newborn mice revealed comparable ratios of FRNK to FAK of approximately 3 to 1. In healthy adult carotid arteries, FRNK expression is very low. However, after balloon injury, FRNK levels are dramatically increased after 14 days, with FRNK-to-FAK ratios approximating 1.5 to 1. Since not all of the SMCs in the injured tissue likely make FRNK, the observed increase in FRNK levels in injured tissues appears to be in a range that could lead to decreases in SMC migration and proliferation, as observed in the adenovirus-infected SMC cultures.
The idea that specific matrix signals modulate mitogenic signaling in SMCs in vivo is supported by correlative evidence that matrix deposition is regulated during vascular development and during certain disease states. Notably, an enhanced level of medial fibronectin is produced early in the developing vasculature and in atherosclerotic lesions, whereas the basement membrane components collagen types I and IV and laminin are produced in the mature vessel (6, 7). These regulated shifts in ECM production correlate with the ability of these matrices to support proliferation in vitro. For example, cultured cells placed on fibronectin maintain a synthetic phenotype whereas those plated on collagen type IV and laminin are more contractile (9–11). Interestingly, plating of SMCs on fibronectin results in robust activation of FAK compared to the modest increases observed when they are plated on laminin and collagen (unpublished observations). Thus, it is tempting to speculate that enhanced fibronectin signaling through FAK is important for SMC migration and proliferation and that regulated FRNK expression may provide a mechanism by which to more precisely control these processes.
Adhesion-dependent signals provide the basis for numerous cell processes, including cell growth, migration, and proliferation. Due to the direct apposition of SMCs with matrix in the vasculature, this tissue is particularly sensitive to ECM-dependent signaling. Herein, we describe the smooth-muscle-specific expression of a putative inhibitor of FAK, termed FRNK, which may act as a critical mediator of integrin signaling. The capacity of FRNK to inhibit integrin-mediated migration and proliferation of cultured SMCs suggests the possibility that regulation of these events in the intact arteries could be achieved by altering levels of a single molecule. To our knowledge, this is the first example of SMC-specific expression of an ECM-signaling molecule and is a finding that suggests that FRNK signaling is important for SMC function.
ACKNOWLEDGMENTS
This work was supported in part by grants R01 CA29243 and P01 CA40042 from the DHHS to J.T.P. and grants RO1 HL-38854 and PO1 HL-19242 to G.K.O. J.M.T. was supported by an American Heart Scientist Development grant (0030188N) and a Jeffress research grant (J-555). C.P.M. was supported by an NIH fellowship (F32 HL-10038).
We thank Wen Xiong and Karen Martin for providing the Myc-FRNK and GFP-FRNK constructs, respectively, and Joshua D. Rovin for aid in generation of the GFP and GFP-FRNK adenoviruses. We also thank Liisa Sundberg for technical assistance.
The first two authors contributed equally to this work.
REFERENCES
- 1.Abedi H, Zachary I. Signalling mechanisms in the regulation of vascular cell migration. Cardiovasc Res. 1995;30:544–556. [PubMed] [Google Scholar]
- 2.Blank R S, Swartz E A, Thompson M M, Olson E N, Owens G K. A retinoic acid-induced clonal cell line derived from multipotential P19 embryonal carcinoma cells expresses smooth muscle characteristics. Circ Res. 1995;76:742–749. doi: 10.1161/01.res.76.5.742. [DOI] [PubMed] [Google Scholar]
- 3.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 4.Carragher N O, Levkau B, Ross R, Raines E W. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol. 1999;147:619–630. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clowes A W, Reidy M A, Clowes M M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Investig. 1983;49:327–333. [PubMed] [Google Scholar]
- 6.Glukhova M, Koteliansky V, Fondacci C, Marotte F, Rappaport L. Laminin variants and integrin laminin receptors in developing and adult human smooth muscle. Dev Biol. 1993;157:437–447. doi: 10.1006/dbio.1993.1147. [DOI] [PubMed] [Google Scholar]
- 7.Glukhova M A, Frid M G, Shekhonin B V, Balabanov Y V, Koteliansky V E. Expression of fibronectin variants in vascular and visceral smooth muscle cells in development. Dev Biol. 1990;141:193–202. doi: 10.1016/0012-1606(90)90114-x. [DOI] [PubMed] [Google Scholar]
- 8.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedin U, Bottger B A, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107:307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedin U, Bottger B A, Luthman J, Johansson S, Thyberg J. A substrate of the cell-attachment sequence of fibronectin (Arg-Gly-Asp-Ser) is sufficient to promote transition of arterial smooth muscle cells from a contractile to a synthetic phenotype. Dev Biol. 1989;133:489–501. doi: 10.1016/0012-1606(89)90052-3. [DOI] [PubMed] [Google Scholar]
- 11.Hedin U, Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33:239–246. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 12.Hungerford J E, Little C D. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36:2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- 13.Lin T H, Chen Q, Howe A, Juliano R L. Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J Biol Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- 14.Nolan K, Lacoste J, Parsons J T. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens G K. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 16.Parsons J T. Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- 16a.Randazzo P A, Andrade J, Miura K, Brown M T, Long Y Q, Stauffer S, Roller P, Cooper J A. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X D, Kiosses W B, Schwartz M A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renshaw M W, Ren X D, Schwartz M A. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson A, Malik R K, Hildebrand J D, Parsons J T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson A, Parsons J T. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 21.Richardson A, Shannon J D, Adams R B, Schaller M D, Parsons J T. Identification of integrin-stimulated sites of serine phosphorylation in FRNK, the separately expressed C-terminal domain of focal adhesion kinase: a potential role for protein kinase A. Biochem J. 1997;324:141–149. doi: 10.1042/bj3240141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaller M D, Borgman C A, Parsons J T. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz S M, Heimark R L, Majesky M W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70:1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- 25.Taylor J M, Hildebrand J D, Mack C P, Cox M E, Parsons J T. Characterization of graf, the GTPase-activating protein for rho associated with focal adhesion kinase. Phosphorylation and possible regulation by mitogen-activated protein kinase. J Biol Chem. 1998;273:8063–8070. doi: 10.1074/jbc.273.14.8063. [DOI] [PubMed] [Google Scholar]
- 26.Taylor J M, Rovin J D, Parsons J T. A role for focal adhesion kinase in phenylephrine-induced hypertrophy of rat ventricular cardiomyocytes. J Biol Chem. 2000;275:19250–19257. doi: 10.1074/jbc.M909099199. [DOI] [PubMed] [Google Scholar]
- 27.Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- 28.Wen L P, Fahrni J A, Troie S, Guan J L, Orth K, Rosen G D. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056–26061. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]