Abstract
Background:
Regorafenib is a small molecule multikinase inhibitor that inhibits multiple kinases including BRAF, KIT, PDGFRB, RAF, RET, and VEGFR1–3.
Procedures:
The in vivo anticancer effects of regorafenib were assessed in a panel of six osteosarcoma models, three rhabdomyosarcoma models, and one Ewing sarcoma model.
Results:
Regorafenib induced modest inhibition of tumor growth in the models evaluated.
Conclusion:
The overall pattern of response to regorafenib appears similar to that of the kinase inhibitor sorafenib, with pronounced slowing of tumor growth in some models, limited to the period of agent administration, being the primary treatment effect.
Keywords: Ewing sarcoma, multitarget kinase inhibitor, osteosarcoma, patient derived xenograft, pediatric preclinical testing consortium, rhabdomyosarcoma
1 |. INTRODUCTION
The outcome for pediatric patients diagnosed with refractory sarcoma has not changed in several decades despite extensive clinical investigation. The 5-year event-free survival (EFS) for pediatric patients with metastatic osteosarcoma (OS), rhabdomyosarcoma (RMS), and Ewing sarcoma (ES) is approximately 20%, while the outcomes for patients who sustain recurrent disease is worse, in some cases less than 10%, despite extensive treatment.1–5 While the outcomes for pediatric patients with localized sarcoma have improved significantly, the outcomes for patients with refractory or metastatic disease remain poor, attesting to the urgent need for novel therapies moving forward.
Regorafenib is a small-molecule multitarget kinase inhibitor (MKI) developed by adding a fluorine atom to the phenyl ring of the receptor TKI sorafenib.6 Regorafenib inhibits several kinases including BRAF, KIT, PDGFRB, RAF, RET, and VEGFR1–3 with higher potency than sorafenib.6 It is approved by the FDA for the treatment of patients with metastatic colorectal cancer, gastrointestinal stromal tumors, and hepatocellular carcinoma.
The in vivo activity of sorafenib was reported by the Pediatric Preclinical Testing Program (PPTP), with its primary effect being tumor growth inhibition without regression.7 Tumor growth inhibition was seen across a range of histotypes, with four of five OS xenografts studied showing more than a twofold extension in median time to event for sorafenib compared to control treatment. In this study, the in vivo effects of regorafenib were assessed in OS, RMS, and ES xenograft patient-derived xenograft (PDX) models.
2 |. MATERIALS AND METHODS
The in vivo anticancer effects of regorafenib were assessed in a panel of six OS models (OS2, OS9, OS31, OS33, OS36, OS60), two alveolar RMS models with PAX3-FOXO1 (Rh30, Rh41), an embryonal RMS model (Rh18), and one ES model with EWSR1-FLI1 (EW5). PDX models were heterotopically injected into the flanks of CB17SC scid−/− female mice (Taconic Farms, Germantown, NY). Regorafenib solution (5 mg/mL) was administered by oral gavage at a dose of 30 mg/kg/day given daily for 21 consecutive days to cohorts (n = 10). Tumor volumes were measured as previously described, and a control cohort of mice (n=10) that was administered vehicle was utilized for each PDX model.8 Full molecular and pathologic descriptions of the models used have been previously published.9,10 During testing, all mice were maintained under barrier conditions, and experiments were conducted using protocols approved by the Institutional Animal Care and Use Committees at MD Anderson Cancer Center and Greehey Children’s Cancer Research Institute. Regorafenib’s activity was evaluated using standard Pediatric Preclinical Testing Consortium (PPTC) measures (see Supporting Information Materials for details), including the ratio of median time to event for the treated and control groups (EFS T/C), minimum relative tumor volume (minRTV), and objective response categories. The exact long-rank test (Proc StatXaxt for SASR®) was used to compare EFS distributions between treatment and control groups. P-values were two-sided and were considered statistically significant if <.05.
3 |. RESULTS
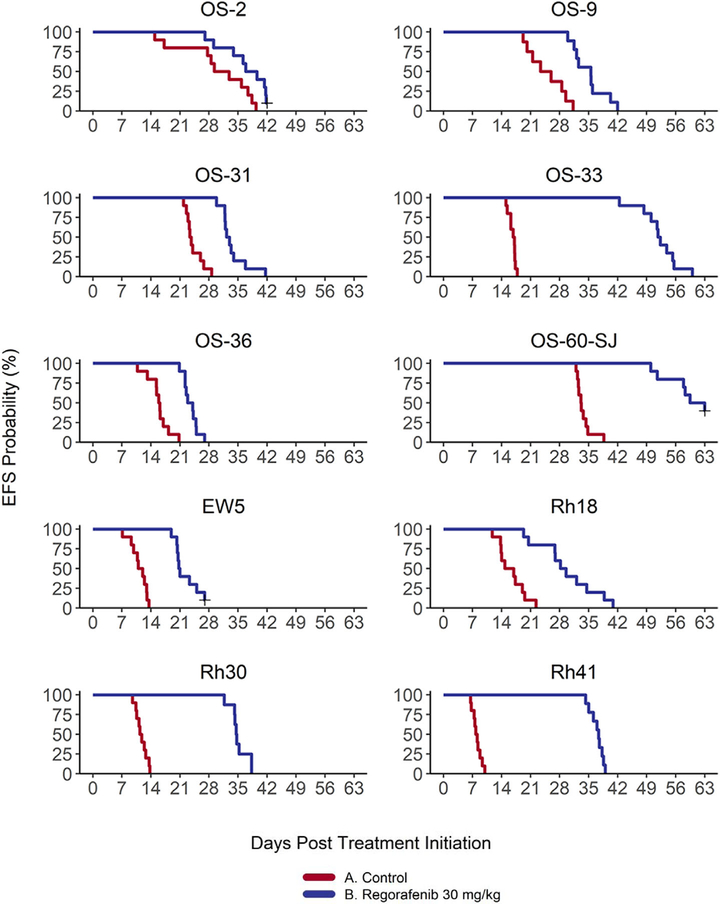
Regorafenib at a dose of 30 mg/kg/day was tested against six OS, three RMS, and one ES xenografts. Regorafenib was well tolerated across both the OS models (0–9.8% avg. weight lost) and the other sarcoma models (0–13.9% avg. weight lost). Across 120 tested mice, three experienced toxic death. Regorafenib significantly prolonged time to event in all 10 sarcoma models tested (P < .05) (Table 1 and Figure 1). There were no objective responses observed. Three out of 10 models demonstrated EFS T/C values > 2 (1/6 OS, 2/3 RMS, and 0/1 ES) (Table 1). These models (OS33, Rh30, and Rh41) were thus classified as having a progressive disease 2 (PD2) response. All other models experienced a response of PD1 (Table 1).
TABLE 1.
Regorafenib as single agent versus control for all PDX models
| Model | KM med (days) | EFS T-C (days) | EFS T/C | P-value Gehan-Wilcoxon | minRTV mean ± SD | minRTV, P-value | Objective response measure |
|---|---|---|---|---|---|---|---|
| OS-2 | 38.2 | 7.1 | 1.23 | .046 | 1.175 ± 0.221 | .075 | PD1 |
| OS-9 | 35.5 | 10.8 | 1.44 | <.001 | 1.040 ± 0.047 | <.001 | PD1 |
| OS-31 | 32.6 | 9.0 | 1.38 | <.001 | 1.062 ± 0.108 | <.001 | PD1 |
| OS-33 | 52.0 | 35.1 | 3.07 | <.001 | 1.065 ± 0.064 | <.001 | PD2 |
| OS-36 | 23.5 | 7.4 | 1.46 | <.001 | 1.557 ± 0.149 | .002 | PD1 |
| OS-60-SJ | 61.2 | 28.0 | 1.85 | <.001 | 0.840 ± 0.279 | <.001 | PD1 |
| EW-5 | 20.9 | 9.4 | 1.82 | <.001 | 1.597 ± 0.355 | <.001 | PD1 |
| Rh-18 | 28.9 | 13.0 | 1.82 | <.001 | 1.491 ± 0.225 | .035 | PD1 |
| Rh-30 | 34.6 | 23.1 | 2.99 | <.001 | 0.949 ± 0.253 | <.001 | PD2 |
| Rh-41 | 37.4 | 29.4 | 4.69 | <.001 | 0.739 ± 0.226 | <.001 | PD2 |
Three out of 10 sarcoma models demonstrated EFS T/C values > 2 (1/6 OS, 2/3 Rh, 0/1 EW). Treated mice from models OS33, Rh30, and Rh41 experienced an objective response of PD2 (progressive disease 2), and treated mice from all other models experienced an objective response of PD1. for sorafenib compared to control treatment. In this study, the in vivo effects of regorafenib were assessed in OS, RMS, and ES xenograft patient-derived xenograft (PDX) models.
FIGURE 1.
Event-free survival to regorafenib across all PDX models. Regorafenib induced significant improvements in event-free survival (EFS) compared to control in 100% (10/10) of the sarcoma models tested with significantly prolonged time to event in all models (P < .05). Mice still alive at the conclusion of the experiment, but whose tumors had not yet quadrupled are considered right-censored and are indicated by a plus sign (+)
Most models showed pronounced slowing of tumor growth compared to control during the 21 days of regorafenib treatment, with tumor growth generally approximating control rates soon after completion of regorafenib treatment (Figure 1). minRTV in treated mice was significantly lower than that in control mice for all models except for OS2 (Table 1/Supporting Information Figure 1). minRTVs ranged from 0.74 to 1.60, with OS60-SJ, Rh30, and Rh41 exhibiting some evidence of minor tumor regression (minRTV < 1) and with all other models exhibiting minRTV > 1 (indicating an absence of tumor regression in these models).
4 |. DISCUSSION
Multitargeted kinase inhibitors have undergone evaluation in multiple in vitro and in vivo sarcoma models. The PPTP demonstrated sorafenib to have tumor growth inhibition activity across a range of sarcoma models, including osteosarcoma.7 Slowing of tumor growth as a primary treatment effect was also observed by the PPTP for sarcoma xenografts for sunitinib,11 pazopanib,12 and cabozantinib.13 The higher potency with which regorafenib inhibits several kinase targets as compared to sorafenib rendered it an intriguing molecule for further study within the PPTC.6
Regorafenib induced inhibition of tumor growth in the pediatric sarcoma models evaluated. The overall pattern of response to the regorafenib against the PPTC sarcoma models appears similar to that of sorafenib, with pronounced slowing of tumor growth in some models that is generally limited to the period of agent administration. These results are similar to those previously reported by Daudigeos-Dubus, et al for regorafenib for ES, RMS, and neuroblastoma xenograft models,14 and they are similar to preclinical results reported for other MKIs tested against pediatric preclinical models.11–13
Regorafenib has shown activity in multiple adult cancers, including colorectal cancer,15 gastrointestinal stromal tumor,16 and hepatocellular carcinoma.17 Three recently published clinical trials documented modest activity for regorafenib in adults with refractory bone and soft tissue sarcomas. Regorafenib improved disease stabilization as compared to placebo in a phase 2, randomized, double-blind, placebo-controlled study of adult patients with metastatic progressive OS.18 A second randomized double-blind phase 2 study found that regorafenib improved time to progression compared to placebo (3.6 months vs 1.7 months, respectively) in adults with progressive, metastatic OS.19 In a clinical trial of adults with doxorubicin refractory soft tissue sarcoma, regorafenib improved progression-free survival as compared to placebo for the nonadipocytic sarcoma subtypes studied.20 These data for regorafenib are consistent with reports of slowing of tumor progression in patients with OS treated with other MKIs including sorafenib,21 pazopanib,22 cabozantinib,23 and lenvatinib.24
One challenge for moving regorafenib into the upfront treatment of OS (particularly for pediatric patients for whom methotrexate is typically given) is that there is a risk of increased methotrexate-related adverse reactions with concurrent treatment with the two agents as methotrexate is a BCRP substrate and regorafenib is a BCRP inhibitor. The preclinical activity seen in pediatric sarcoma xenografts, in conjunction with the modest activity seen in large randomized clinical trials of adult patients with refractory carcinomas as well as the activity seen in smaller studies of adults with bone or soft tissue sarcomas, support consideration of the evaluation of MKIs like regorafenib in OS.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Cancer Institute (Grant Nos. CA199221 and CA199222), Swim Across America, and the Foster Foundation. Regorafenib was supplied by Bayer.
Funding information
National Cancer Institute; Swim Across America; Foster Foundation
Abbreviations:
- ES
Ewing sarcoma
- MKI
multitarget kinase inhibitor
- OS
osteosarcoma
- PDX
patient-derived xenograft
- PPTC
Pediatric Preclinical Testing Consortium
- RMS
rhabdomyosarcoma
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18(1):39–50. [DOI] [PubMed] [Google Scholar]
- 3.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. [DOI] [PubMed] [Google Scholar]
- 4.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26(14):2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigel BJ, Lyden E, Anderson JR, et al. Intensive multiagent therapy, including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation, in patients with high-risk rhabdomyosarcoma: a Report From the Children’s Oncology Group. J Clin Oncol. 2016;34(2):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. [DOI] [PubMed] [Google Scholar]
- 7.Keir ST, Maris JM, Lock R, et al. Initial testing (stage 1) of the multitargeted kinase inhibitor sorafenib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55(6):1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton PJ, Morton CL, Tucker C, et al. The Pediatric Preclinical Testing Program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. [DOI] [PubMed] [Google Scholar]
- 9.Rokita JL, Rathi KS, Cardenas MF, et al. Genomic profiling of childhood tumor patient-derived xenograft models to enable rational clinical trial design. Cell Rep. 2019;29(6):1675–1689, e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neale G, Su X, Morton CL, et al. Molecular characterization of the pediatric preclinical testing panel. Clin Cancer Res. 2008;14(14):4572–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maris JM, Courtright J, Houghton PJ, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51(1):42–48. [DOI] [PubMed] [Google Scholar]
- 12.Keir ST, Morton CL, Wu J, Kurmasheva RT, Houghton PJ, Smith MA. Initial testing of the multitargeted kinase inhibitor pazopanib by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2012;59(3):586–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MA, Kang M, Reynolds P, et al. Pediatric Preclinical Testing Program (PPTP) stage 1 evaluation of cabozantinib. Proceedings of the 104th Annual Meeting of the American Association for Cancer Research. Washington DC. 2013. [Google Scholar]
- 14.Daudigeos-Dubus E, Le Dret L, Lanvers-Kaminsky C, et al. Regorafenib: antitumor activity upon mono and combination therapy in preclinical pediatric malignancy models. PLoS One. 2015;10(11):e0142612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 18.Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20(1):120–133. [DOI] [PubMed] [Google Scholar]
- 19.Davis LE, Bolejack V, Ryan CW, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol. 2019;37(16):1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry V, Basson L, Bogart E, et al. REGOSARC: regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma—a quality-adjusted time without symptoms of progression or toxicity analysis. Cancer. 2017;123(12):2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23(2):508–516. [DOI] [PubMed] [Google Scholar]
- 22.Umeda K, Kato I, Saida S, Okamoto T, Adachi S. Pazopanib for second recurrence of osteosarcoma in pediatric patients. Pediatr Int. 2017;59(8):937–938. [DOI] [PubMed] [Google Scholar]
- 23.Italiano A, Penel N, Bompas E, et al. Cabozantinib in patients with advanced osteosarcomas and Ewing sarcomas: a French Sarcoma Group (FSG)/US National Cancer Institute phase II collaborative study. CTOS Annual Meeting, November 14–17, Rome, Italy. 2018. [Google Scholar]
- 24.Gaspar N, Casanova M, Sirvent FJB, et al. Single-agent expansion cohort of lenvatinib (LEN) and combination dose-finding cohort of LEN + etoposide (ETP) + ifosfamide (IFM) in patients (pts) aged 2 to ≤25 years with relapsed/refractory osteosarcoma (OS). J Clin Oncol. 2018;36(15_suppl):11527–11527. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.