Abstract
Faithful chromosome segregation in mitosis and meiosis requires that chromosomes properly attach to spindle microtubules. Initial kinetochore-microtubule attachments are often incorrect and rely on error correction mechanisms to release improper attachments, allowing the formation of new attachments. Aurora B kinase, and in mammalian germ cells Aurora C kinase, function as the enzymatic component of the Chromosomal Passenger Complex (CPC), which localizes to the inner centromere/kinetochore and phosphorylates kinetochore proteins for microtubule release during error correction. In this review, we discuss recent findings of the molecular pathways that regulate the chromosomal localization of Aurora B and C kinases in human cell lines, mice, fission yeast, and budding yeast. We also discuss differences in the importance of localization pathways between mitosis and meiosis.
Keywords: meiosis, mitosis, biorientation, Aurora B, Aurora C, Aurora kinases, chromosome segregation
Introduction
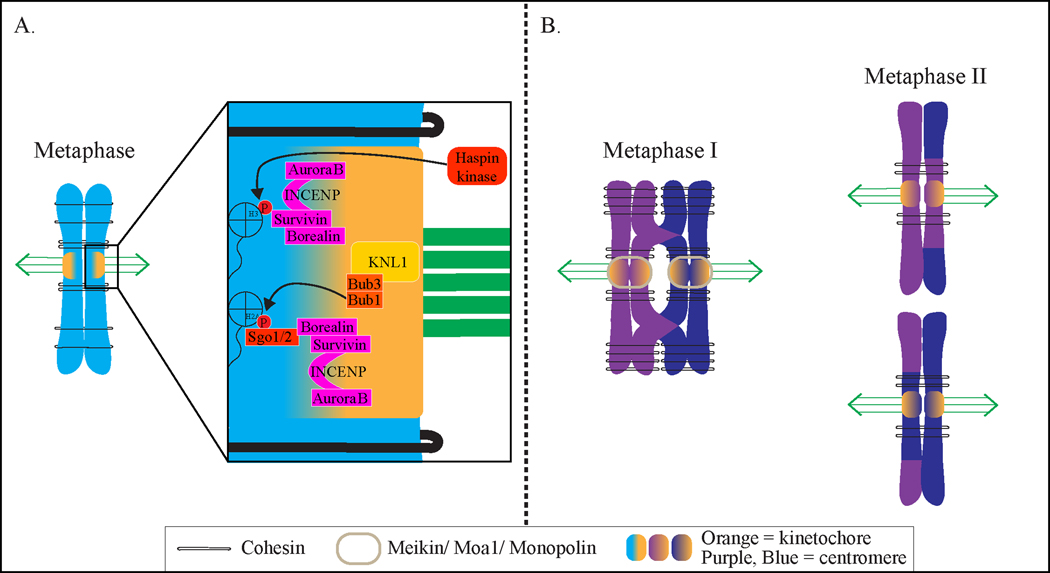
A requirement for faithful chromosome segregation is establishing proper attachments between chromosomes and spindle microtubules. During mitotic metaphase, biorientation occurs when the kinetochores on the two sister chromatids make end-on attachments to microtubules emanating from opposite spindle poles (Figure 1) (1). The poleward spindle forces that pull kinetochores in opposite directions are resisted by cohesins that encircle the two sister chromatids, holding them together. Biorientation is associated with tension within and between the kinetochores (2–5). Meiosis I has a unique chromosome configuration in which paired homologous chromosomes are physically linked together by crossovers. Biorientation in metaphase I occurs when the two sister chromatid kinetochores co-orient and the kinetochores of homologous chromosomes attach to microtubules from opposite spindle poles (Figure 2) (6). In meiosis I, the spindle forces are resisted by the crossover combined with the cohesins along the chromosome arms. In metaphase II, biorientation occurs similarly to mitosis in that sister chromatid kinetochores are attached to microtubules from opposite spindle poles (Figure 2).
Figure 1:
Biorientation in mitosis and meiosis. A) Chromosomes are bioriented in mitosis when sister kinetochores are properly attached to microtubules from opposite poles. Inset shows two Aurora B recruitment pathways. B) In meiosis I (left), chromosomes biorient when sister kinetochores are attached to microtubules from the same pole and homologous chromosome kinetochores attach to opposite spindle poles. In meiosis II, biorientation occurs when sister chromatid kinetochores attach to microtubules from opposite spindle poles.
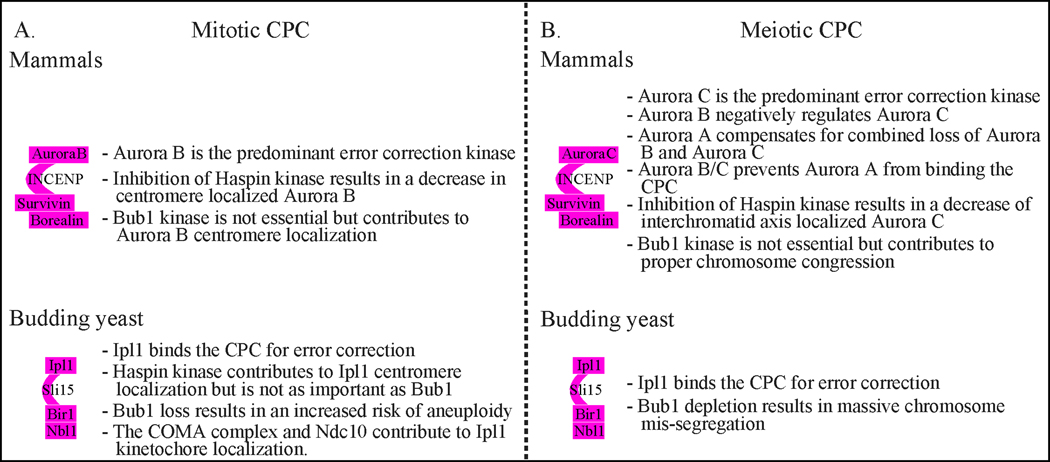
Figure 2:
Comparison of mitotic and meiotic CPC localization and function in mammals and budding yeast for error correction of kinetochore-microtubule attachments.
Most initial attachments are incorrect and must be released to make proper attachments. Correction of errors in kinetochore-microtubule attachments occurs through the activity of the evolutionarily conserved Aurora B kinase and its paralog expressed in mammalian germ cells Aurora C (6–8). Aurora B/C releases improper kinetochore-microtubule attachments, mediated partly by the phospho-regulation of kinetochore proteins that bind microtubules (9–16). Aurora B/C activity leads to the release of attachments that are not under tension, allowing another round of microtubule binding to establish proper attachments. For example, a lack of tension occurs when both sister kinetochores attach to the same pole in mitosis or meiosis II, or when homologous chromosomes attach to the same pole in meiosis I (2, 3).
A third Aurora kinase, Aurora A kinase, localizes to spindle poles and functions in bipolar spindle assembly in mitosis and meiosis and centrosome maturation in mitosis (17–19). Although the major role of Aurora A is in spindle assembly, several studies now suggest that Aurora A phosphorylates kinetochore proteins to contribute to error correction of kinetochore-microtubule attachments in mitosis and meiosis I (20–26). Aurora A can perform these roles through its localization both at the spindle poles and, surprisingly, at kinetochores (20–23). These findings were the subject of a recent review (see (27)), so we only highlight new work on Aurora A kinase localization in meiosis.
The mechanism of how Aurora B selectively destabilizes microtubule-kinetochore interactions is currently under debate and the topic of several recent reviews (see (1, 7, 28)). In this review, we discuss multiple molecular pathways that localize Aurora B and Aurora C to the inner centromere and kinetochore in both mitosis and meiosis. We highlight several recent papers suggesting new recruitment mechanisms and how the importance of different recruitment pathways differ between mitosis and meiosis and among different model organisms.
Localization of Aurora B kinase in mitosis
Localization of Aurora B kinase to either the inner centromere or the kinetochore is crucial for its function in error correction. Aurora B kinase assembles into a complex called the Chromosomal Passenger Complex (CPC), which includes Survivin (also known as Bir1 in yeast), Borealin (also known as Dasra in Xenopus and Nbl1 in yeast), and Inner Centromeric Protein (INCENP, also known as Sli15 in yeast) (29–38). Survivin and Borealin bind to the N-terminus of INCENP and regulate the localization of the CPC to the centromere (34, 39–48). Aurora B binds to a region of INCENP near the C-terminus called the IN box and phosphorylates INCENP (32, 36, 49–53). Full activation of Aurora B requires binding to INCENP, phosphorylation of INCENP, and autophosphorylation, which may occur in trans (32, 46, 49, 51, 52, 54).
Work from human cell lines, Xenopus egg extracts, and fission and budding yeast have revealed the mechanisms of inner centromere localization of the CPC. Two highly conserved pathways function through two histone kinases, Haspin and Bub1. First, Haspin kinase phosphorylates histone H3, which serves as a receptor for binding the Survivin subunit of the CPC (39, 43, 46, 47, 55). Second, Bub1 kinase phosphorylates histone H2A, which is bound by shugoshins (Sgo1 and Sgo2 in human cells) (47, 56–62). The Borealin subunit of the CPC then binds to human Sgo1 and Sgo2 (33, 37, 63). In fission yeast, Sgo2 but not Sgo1 is mainly responsible for the recruitment of the CPC (47, 63–65). Interestingly, fission yeast Sgo2 binds the Survivin subunit of the CPC (called Bir1) (63, 64). Budding yeast has only a single shugoshin, Sgo1, which binds phosphorylated histone H2A and also plays a role in maintaining Aurora B at the kinetochore (56, 66–69). However, whether Sgo1 directly binds Survivin (called Bir1) or Borealin (Nbl1) in budding yeast has not been established (70, 71). Therefore, although there are some organismal differences in subunit binding, the pathways of CPC inner centromere recruitment function similarly in different organisms.
Initially, Haspin and Bub1 pathways were proposed to cooperate to bring Aurora B to a chromatin region with overlapping histone H3T3ph and H2AT120ph marks (47). However, three recent studies from cell lines showed that tethering either Haspin or Bub1 individually to an ectopic chromatin location resulted in the recruitment of Aurora B to that location (72–74). Furthermore, these studies also show that each pathway individually recruits a distinct pool of Aurora B at the centromere (72–74). These results suggest that the two histone kinase pathways can function independently to bring Aurora B to the inner centromere.
Inhibition of either Haspin or Bub1 kinases causes decreased Aurora B inner centromere localization. In human cell lines and Xenopus egg extracts, the loss of Haspin kinase activity through depletion, drug inhibition, or mutation causes delayed chromosome alignment and some defective correction of erroneous attachments (43, 72, 75, 76). In contrast, drug inhibition of Bub1 kinase activity in cell lines does not result in alignment defects (61, 72). Mice with a deletion of the Bub1 kinase domain are viable, suggesting that the Bub1 kinase activity is not essential (77). However, mouse embryonic fibroblasts derived from a Bub1 kinase mutant mouse had delays in chromosome alignment and error correction. Aneuploidy rates in spleen, liver, and lung tissue were higher in the mutant mice than in wildtype. Additionally, inhibition of both the Haspin and the Bub1 kinase pathways in human cell lines causes more severe defects in chromosome alignment and segregation than mutating the individual pathways (72, 74). Therefore, although not essential, Bub1 kinase likely contributes to Aurora B localization for error correction in mammals.
In budding yeast, the Haspin kinase pathway is not as crucial for CPC recruitment as the Bub1 kinase pathway. Budding yeast cells with combined deletion of the two genes encoding Haspin kinase, ALK1 and ALK2, do not have a growth defect but do show decreased Ipl1/Aurora B levels at the kinetochore (55, 78). In contrast, cells with a deletion of SGO1 or BUB1 grow slowly and have an increased risk of aneuploidy (56, 67, 71, 79, 80). Similarly, in fission yeast, inhibition of Haspin and Bub1 pathways gave more severe defects than the loss of the individual pathways (47). Overall, these results suggest that the Haspin and Bub1 pathways are additive for error correction in an unperturbed mitosis.
There are likely other pathways that recruit Aurora B to the kinetochore in human cells. The inhibition of Aurora B with a small molecule inhibitor causes a more severe defect in chromosome alignment than the loss of both Haspin and Bub1 pathways (15, 16, 72, 81–84). Three recent studies showed that with loss of both pathways, Aurora B inner centromere localization is disrupted, but Aurora B kinetochore substrates are still phosphorylated (72–74). The phosphorylation of kinetochore substrates was not dependent on microtubules, eliminating the role of the microtubule-localized pool of Aurora B (72, 74, 85). A Haspin- and Bub1-independent pool of Aurora B was identified at the kinetochore and is proposed to phosphorylate substrates that regulate kinetochore-microtubule attachments (1, 7, 73, 86–90).
In budding yeast, there are two additional known pathways to recruit Ipl1/Aurora B to the centromere or kinetochore in addition to the Bub1 and Haspin kinase-dependent pathways. The third pathway also requires the Bir1 (Survivin) subunit of the CPC. Bir1 binds the inner centromere protein Ndc10, a component of the budding yeast centromeric DNA-binding Cbf3 complex (91, 92). Finally, in a recently discovered pathway, Ipl1/Aurora B localization is not dependent on the Bir1 component of the CPC. Instead, Sli15 (INCENP) directly binds the COMA kinetochore complex (containing Ctf19, Okp1, Mcm21, and Ame1, homologous to CENP-O,P,Q,U in mammals) (93, 94). Depletion of either Bir1 or Mcm21, a non-essential component of the COMA complex, reduces Ipl/Aurora B kinetochore localization (94). Double depletion of both BIR1 and MCM21 abolishes both biorientation and Ipl1/Aurora B localization. Overall, the results suggest that both Bir1-dependent and Bir1-independent pathways recruit Ipl1/Aurora B to the inner centromere and kinetochore.
An outstanding question in the field is how tension affects Aurora B substrate phosphorylation for correction of erroneous kinetochore-microtubule attachments. One of the prevailing models in the field, the spatial separation model, depends on the inner centromere localization of Aurora B, as this localization would allow Aurora B to discriminate between substrates that are spatially separated from the inner centromere (12, 95). In this model, a single pool of Aurora B at the inner centromere would phosphorylate substrates that are in close proximity to this pool but would be unable to phosphorylate substrates that are spatially separated when a kinetochore is under tension. However, studies in budding yeast, vertebrate cells, and human cells have shown that inner centromere localization of Aurora B is not essential for biorientation (45, 78, 96). In addition, the newly discovered kinetochore-localized pools of Aurora B are also important for phosphorylation of kinetochore proteins for error correction (72–74, 87, 88, 93, 94). Exciting future directions will be to test new models of how this pool could distinguish correct and incorrect attachments.
Establishing Biorientation in Meiosis
The unique chromosome configuration for biorientation in meiosis I requires several events leading up to metaphase I (Figure 2). Homologous chromosomes must pair and undergo crossovers, which physically tether the chromosomes together. The two sister kinetochores must co-orient to capture microtubules from the same pole. In mice, a protein called Meikin binds the kinetochore and recruits Polo kinase for co-orientation (97–99). In fission yeast, the meiosis-specific kinetochore protein Moa1 interacts with the meiosis-specific kleisin subunit of cohesin, Rec8, and recruits Polo-like kinase for co-orientation of sister kinetochores (100). The Aurora B kinase homolog, Ark1, is also needed for co-orientation but functions independently of Moa1, Polo, and Rec8 (101). In budding yeast, a complex called monopolin interacts with Spo13 and clamps sister chromatid kinetochores. Monopolin is composed of the subunits Mam1, Lrs4, and Hrr25 and forms a V-shaped structure with two kinetochore-binding domains (102–105). Spo13 interacts with Polo kinase and recruits or stabilizes monopolin binding to the kinetochore (106–109). Although the functional orthologs Meikin, Moa1, and Spo13 do not share significant sequence homology, they all recruit Polo kinase for co-orientation of sister kinetochores in meiosis I (98).
For accurate chromosome segregation in meiosis II, the cohesin around the pericentromere must be protected from cleavage in meiosis I. If cohesins are cleaved in meiosis I, the two sister chromatids do not remain connected, and no tension bearing attachments can be established at metaphase II. Protecting cohesin from cleavage ensures that the sister chromatids remain together and resist spindle forces in metaphase II (Figure 2). Shugoshins, along with Meikin (mouse), Moa1 (fission yeast), or Spo13 (budding yeast), protect cohesins (98). Mice, humans, and fission yeast have two shugoshins, Sgo1 and Sgo2. In mice, Sgo2 is needed for meiotic cohesin protection, chromosome congression, and chromosome biorientation in meiosis I (110–112). In fission yeast, Sgo1 has a crucial role in cohesin protection (66). Sgo2 is dispensable for meiotic cohesin protection but interacts with the CPC for Aurora kinase localization in both mitosis and meiosis (64, 66, 101). Budding yeast has one shugoshin, Sgo1, that protects pericentromeric cohesin in meiosis and is important for biorientation in mitosis and meiosis by recruiting the CPC (67–69, 71, 113). Shugoshins mediate protection of cohesin by recruiting PP2A, a phosphatase that dephosphorylates Rec8 so that it cannot be cleaved by separase at anaphase I (58, 62). In budding yeast, Ipl1/Aurora B is needed to maintain PP2A levels at the kinetochore for cohesin protection (70).
Localization and Function of Aurora Kinases in Mammalian Meiosis
As in mitosis, Aurora B kinase is important for chromosome biorientation through the correction of kinetochore-microtubule attachments in both metaphase I and metaphase II. Mammals also express another Aurora kinase in the germline, Aurora C kinase (AURKC), which is important for biorientation (114–120). In the mouse germline, AURKC is the prevalent CPC kinase suggested to have replaced the major function of Aurora B kinase (AURKB) at the centromere regulating error correction of kinetochore-microtubule attachments (121–125). AURKC localizes to the spindle poles, centromeres and at the interchromatid axis of bivalents during metaphase I (126–129). Although AURKB localization to centromeres has been undetectable, AURKB localizes to the spindle in metaphase I and metaphase II and may have a role in chromosome alignment (121, 130).
Mice lacking AURKB or AURKC during meiosis have viable offspring, although they are subfertile, suggesting that the kinases mostly compensate for one another, but perhaps not as effectively as having both kinases (23, 119, 121, 131, 132). AURKB and AURKC share a consensus phosphorylation sequence and likely have overlapping substrates (133). When compared to wildtype and Aurkc−/− mice, AURKB conditional knockout (cKO) female mice are less fertile and have an increase in aneuploid eggs; these phenotypes are likely due to too much AURKC activity (23). The increase in aneuploidy of AurkB−/− cKO mice is suppressed by adding a heterozygous deletion of AURKC. Therefore, AURKB negatively regulates and limits the activity of AURKC. In Aurkc−/− oocytes, AURKB is localized to the centromere, suggesting that AURKB corrects improper kinetochore-microtubule connections in the absence of AURKC (121).
Surprisingly, Aurora A kinase can compensate for the combined loss of both AURKB and AURKC to allow error correction of kinetochore-microtubule attachments. The Aurkc−/− AurkB−/− cKO double mutant female mice are fertile (23). Although Aurora A kinase (AURKA) normally functions in spindle assembly, AURKA localizes to the interchromatid axis in a CPC-dependent manner in the double mutant mice. The substrate INCENP is phosphorylated in the double mutant oocytes. In contrast, inhibition of AURKB and AURKC with the drug ZMM447439 shows no phosphorylation of INCENP, suggesting that the presence of AURKB/C prevents AURKA from binding to the CPC, likely by outcompeting AURKA. The AURKA compensation is meiosis-specific; deletion of AURKB in HeLa cells causes abnormal metaphase chromosome configurations and a lack of INCENP phosphorylation and AURKA at the interchromatid axis. An important future direction is to determine how AURKA compensation occurs in meiosis but not mitosis.
Haspin kinase phosphorylation of histone H3 is important for the localization of AURKC to the interchromatid axis in mouse oocytes (134, 135). Inhibition of Haspin kinase with the drug 5-Iodotubercidin (5-Itu) in late prometaphase I results in an increase in aneuploid eggs, an accelerated meiosis I, a weakened spindle assembly checkpoint response, and an approximately 60% decrease in AURKC and Survivin localization at the inter-chromatid axis (135, 136). There is also a decrease in inter-chromatid axis-localized phosphorylated INCENP, suggesting lower AURKC activity. Surprisingly, there was not a decrease of AURKC at the kinetochore, despite no detectable histone H3 phosphorylation. These results differ from those in mitosis in which inhibition of Haspin kinase causes a decrease in AURKB at the kinetochore (76). These results suggest that other recruitment pathways localize AURKC at the kinetochore. Furthermore, because the Haspin-inhibited oocytes have defects in chromosome alignment and segregation, the interchromatid axis-localized AURKC likely contributes to error correction and chromosome segregation.
Haspin kinase may work redundantly with other kinases for AURKC centromere recruitment. Bub1 kinase is a likely candidate due to its role in bringing Aurora B to the centromere in mitosis in human cells. However, loss of Bub1 kinase does not cause a major defect in chromosome segregation. Female mice lacking Bub1 kinase activity did not have a fertility defect, suggesting that correction of erroneous kinetochore-microtubule attachments still occurred (77). Oocytes lacking Bub1 kinase activity that were matured in vitro have a strong spindle checkpoint delay in metaphase I, likely because of chromosome congression defects. Eggs isolated in metaphase II do not show gross chromosomal aneuploidies suggesting that homologous chromosomes segregate normally. Therefore, oocytes lacking Bub1 kinase activity may have defects in chromosome alignment, but can segregate chromosomes properly after a spindle checkpoint delay (137). Another candidate is Mps1 kinase, which is involved in AURKC localization (138). If the Mps1 expressed in meiosis does not have the kinetochore binding domain, there are defects in chromosome alignment at metaphase I, the spindle checkpoint response, and the normal timing of meiosis I. In addition, AURKC levels at chromosomes and centromeres were diminished without Mps1 kinetochore localization. However, because not all oocytes are aneuploid, there are likely multiple AURKC recruitment pathways that could function redundantly to bring AURKC to the centromere. Future work is needed to identify the additional centromere/kinetochore recruitment pathways and to determine how the pathways compensate for one another.
Localization and Function of Aurora Kinases in Budding and Fission Yeast
Budding yeast and fission yeast have only one Aurora kinase, Ipl1, and Ark1, respectively. Loss of Aurora B in budding or fission yeast meiosis causes massive chromosome mis-segregation due to a failure to correct initial improper attachments (70, 101, 109, 139). In budding yeast meiosis, cells lacking Bub1 have greatly reduced levels of Ipl1/Aurora B at the centromere (70, 140). We recently found that meiotic depletion of Bub1 results in massive chromosome mis-segregation, in which most chromosomes travel to the old spindle pole body (the budding yeast equivalent to the centrosome) due to a failure to correct initial attachments (140). Interestingly, the defect in meiosis II is more severe than that in meiosis I. For comparison, loss of Ipl1/Aurora B had a more severe defect than loss of Bub1 in meiosis I but a similar defect in meiosis II. The meiosis II results are surprising because the meiosis II division is assumed to be similar to mitosis. However, loss of Bub1 or Bub3 in mitosis does not cause massive chromosome mis-segregation, only an increased risk of single chromosome aneuploidy in each mitotic division (79, 80, 140). Our results suggest that the recruitment of Ipl1/Aurora B through the Bub1 pathway is crucial for correcting attachment errors in meiosis. An important future direction is to determine whether the other Ipl1/Aurora B recruitment pathways are also important for maintaining Ipl1/Aurora B levels at the centromere/kinetochore.
Conclusions
Recent studies have identified new molecular mechanisms involved in centromere/kinetochore localization of the CPC. Exciting future directions will be to further understand how these pathways are interconnected for full localization and activity of Aurora B/C kinase. Future studies should also aim to identify the molecular mechanisms of these novel recruitment pathways. In addition, recent studies have revealed key differences between Aurora kinase localization and function between mitosis and meiosis. Further study of these differences are warranted and will be crucial to our understanding of chromosome segregation in mitosis and meiosis.
Summary:
We discuss the mechanisms of localization of Aurora B and Aurora C kinases, which have conserved roles in correcting improper kinetochore-microtubule attachments in mitosis and meiosis.
Recent studies have shown that in addition to the Haspin kinase and Bub1 kinase pathways for recruiting Aurora B/C to the centromere, other pathways also independently contribute to Aurora B/C kinase centromere and kinetochore localization.
We highlight several recent studies showing that the importance of specific Aurora B/C recruitment pathways differ between mitosis and meiosis and among model organisms
Acknowledgements
We thank members of the Lacefield Lab for their critical reading of the manuscript. This work was supported by a grant from the NIH to SL (GM105755).
Footnotes
Conflicts of Interest
The authors do not have any conflicts of interest to declare.
References
- 1.Krenn V, Musacchio A. The Aurora B Kinase in Chromosome Bi-Orientation and Spindle Checkpoint Signaling. Front Oncol. 2015;5:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicklas RB, Koch CA. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J Cell Biol. 1969;43(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ault JG, Nicklas RB. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma. 1989;98(1):33–9. [DOI] [PubMed] [Google Scholar]
- 4.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184(3):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184(3):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen AL, Schindler K. Specialize and Divide (Twice): Functions of Three Aurora Kinase Homologs in Mammalian Oocyte Meiotic Maturation. Trends Genet. 2017;33(5):349–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hindriksen S, Lens SMA, Hadders MA. The Ins and Outs of Aurora B Inner Centromere Localization. Front Cell Dev Biol. 2017;5:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JR, Koretke KK, Birkeland ML, Sanseau P, Patrick DR. Evolutionary relationships of Aurora kinases: implications for model organism studies and the development of anti-cancer drugs. BMC Evol Biol. 2004;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127(5):983–97. [DOI] [PubMed] [Google Scholar]
- 10.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133(3):427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, et al. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108(3):317–29. [DOI] [PubMed] [Google Scholar]
- 12.Welburn JP, Vleugel M, Liu D, Yates JR 3rd, Lampson MA, Fukagawa T, et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38(3):383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15(23):3118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127(5):969–82. [DOI] [PubMed] [Google Scholar]
- 15.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161(2):281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161(2):267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22(4):451–64. [DOI] [PubMed] [Google Scholar]
- 18.Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120(Pt 17):2987–96. [DOI] [PubMed] [Google Scholar]
- 19.Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV. Aurora A, meiosis and mitosis. Biol Cell. 2004;96(3):215–29. [DOI] [PubMed] [Google Scholar]
- 20.DeLuca KF, Meppelink A, Broad AJ, Mick JE, Peersen OB, Pektas S, et al. Aurora A kinase phosphorylates Hec1 to regulate metaphase kinetochore-microtubule dynamics. J Cell Biol. 2018;217(1):163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye AA, Deretic J, Hoel CM, Hinman AW, Cimini D, Welburn JP, et al. Aurora A Kinase Contributes to a Pole-Based Error Correction Pathway. Curr Biol. 2015;25(14):1842–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chmatal L, Yang K, Schultz RM, Lampson MA. Spatial Regulation of Kinetochore Microtubule Attachments by Destabilization at Spindle Poles in Meiosis I. Curr Biol. 2015;25(14):1835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen AL, Drutovic D, Vazquez BN, El Yakoubi W, Gentilello AS, Malumbres M, et al. Genetic Interactions between the Aurora Kinases Reveal New Requirements for AURKB and AURKC during Oocyte Meiosis. Curr Biol. 2018;28(21):3458–68 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunitoku N, Sasayama T, Marumoto T, Zhang D, Honda S, Kobayashi O, et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell. 2003;5(6):853–64. [DOI] [PubMed] [Google Scholar]
- 25.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, et al. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278(51):51786–95. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Holland AJ, Lan W, Cleveland DW. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell. 2010;142(3):444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLuca JG. Aurora A Kinase Function at Kinetochores. Cold Spring Harb Symp Quant Biol. 2017;82:91–9. [DOI] [PubMed] [Google Scholar]
- 28.Funabiki H. Correcting aberrant kinetochore microtubule attachments: a hidden regulation of Aurora B on microtubules. Curr Opin Cell Biol. 2019;58:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams RR, Eckley DM, Vagnarelli P, Wheatley SP, Gerloff DL, Mackay AM, et al. Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma. 2001;110(2):65–74. [DOI] [PubMed] [Google Scholar]
- 30.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153(4):865–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116(Pt 14):2987–98. [DOI] [PubMed] [Google Scholar]
- 32.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14(8):3325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166(2):179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17(6):2547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romano A, Guse A, Krascenicova I, Schnabel H, Schnabel R, Glotzer M. CSC-1: a subunit of the Aurora B kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J Cell Biol. 2003;161(2):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10(19):1172–81. [DOI] [PubMed] [Google Scholar]
- 37.Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118(2):187–202. [DOI] [PubMed] [Google Scholar]
- 38.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–21. [DOI] [PubMed] [Google Scholar]
- 39.Jeyaprakash AA, Basquin C, Jayachandran U, Conti E. Structural basis for the recognition of phosphorylated histone h3 by the survivin subunit of the chromosomal passenger complex. Structure. 2011;19(11):1625–34. [DOI] [PubMed] [Google Scholar]
- 40.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131(2):271–85. [DOI] [PubMed] [Google Scholar]
- 41.Ainsztein AM, Kandels-Lewis SE, Mackay AM, Earnshaw WC. INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J Cell Biol. 1998;143(7):1763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du J, Kelly AE, Funabiki H, Patel DJ. Structural basis for recognition of H3T3ph and Smac/DIABLO N-terminal peptides by human Survivin. Structure. 2012;20(1):185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330(6001):231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vader G, Kauw JJ, Medema RH, Lens SM. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 2006;7(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue Z, Carvalho A, Xu Z, Yuan X, Cardinale S, Ribeiro S, et al. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. J Cell Biol. 2008;183(2):279–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330(6001):235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330(6001):239–43. [DOI] [PubMed] [Google Scholar]
- 48.Niedzialkowska E, Wang F, Porebski PJ, Minor W, Higgins JM, Stukenberg PT. Molecular basis for phosphospecific recognition of histone H3 tails by Survivin paralogues at inner centromeres. Mol Biol Cell. 2012;23(8):1457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, et al. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18(3):379–91. [DOI] [PubMed] [Google Scholar]
- 50.Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, et al. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10(17):1075–8. [DOI] [PubMed] [Google Scholar]
- 51.Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell. 2002;13(9):3064–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277(31):27577–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang J, Cheeseman IM, Kallstrom G, Velmurugan S, Barnes G, Chan CS. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J Cell Biol. 2001;155(5):763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H. Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell. 2007;12(1):31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edgerton H, Johansson M, Keifenheim D, Mukherjee S, Chacon JM, Bachant J, et al. A noncatalytic function of the topoisomerase II CTD in Aurora B recruitment to inner centromeres during mitosis. J Cell Biol. 2016;213(6):651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327(5962):172–7. [DOI] [PubMed] [Google Scholar]
- 57.Kitajima TS, Hauf S, Ohsugi M, Yamamoto T, Watanabe Y. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr Biol. 2005;15(4):353–9. [DOI] [PubMed] [Google Scholar]
- 58.Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441(7089):46–52. [DOI] [PubMed] [Google Scholar]
- 59.Liu H, Jia L, Yu H. Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr Biol. 2013;23(19):1927–33. [DOI] [PubMed] [Google Scholar]
- 60.Liu H, Qu Q, Warrington R, Rice A, Cheng N, Yu H. Mitotic Transcription Installs Sgo1 at Centromeres to Coordinate Chromosome Segregation. Mol Cell. 2015;59(3):426–36. [DOI] [PubMed] [Google Scholar]
- 61.Baron AP, von Schubert C, Cubizolles F, Siemeister G, Hitchcock M, Mengel A, et al. Probing the catalytic functions of Bub1 kinase using the small molecule inhibitors BAY-320 and BAY-524. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Z, Cetin B, Anger M, Cho US, Helmhart W, Nasmyth K, et al. Structure and function of the PP2A-shugoshin interaction. Mol Cell. 2009;35(4):426–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467(7316):719–23. [DOI] [PubMed] [Google Scholar]
- 64.Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y. Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 2007;21(4):420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanoosthuyse V, Prykhozhij S, Hardwick KG. Shugoshin 2 regulates localization of the chromosomal passenger proteins in fission yeast mitosis. Mol Biol Cell. 2007;18(5):1657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427(6974):510–7. [DOI] [PubMed] [Google Scholar]
- 67.Indjeian VB, Stern BM, Murray AW. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science. 2005;307(5706):130–3. [DOI] [PubMed] [Google Scholar]
- 68.Marston AL, Tham WH, Shah H, Amon A. A genome-wide screen identifies genes required for centromeric cohesion. Science. 2004;303(5662):1367–70. [DOI] [PubMed] [Google Scholar]
- 69.Kiburz BM, Amon A, Marston AL. Shugoshin promotes sister kinetochore biorientation in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19(3):1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu HG, Koshland D. The Aurora kinase Ipl1 maintains the centromeric localization of PP2A to protect cohesin during meiosis. J Cell Biol. 2007;176(7):911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peplowska K, Wallek AU, Storchova Z. Sgo1 regulates both condensin and Ipl1/Aurora B to promote chromosome biorientation. PLoS Genet. 2014;10(6):e1004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hadders MA, Hindriksen S, Truong MA, Mhaskar AN, Wopken JP, Vromans MJM, et al. Untangling the contribution of Haspin and Bub1 to Aurora B function during mitosis. J Cell Biol. 2020;219(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broad AJ, DeLuca KF, DeLuca JG. Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells. J Cell Biol. 2020;219(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang C, Zhang Z, Chen Q, Yan H, Zhang M, Zhou L, et al. Centromere-localized Aurora B kinase is required for the fidelity of chromosome segregation. J Cell Biol. 2020;219(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F, Ulyanova NP, Daum JR, Patnaik D, Kateneva AV, Gorbsky GJ, et al. Haspin inhibitors reveal centromeric functions of Aurora B in chromosome segregation. J Cell Biol. 2012;199(2):251–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Antoni A, Maffini S, Knapp S, Musacchio A, Santaguida S. A small-molecule inhibitor of Haspin alters the kinetochore functions of Aurora B. J Cell Biol. 2012;199(2):269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ricke RM, Jeganathan KB, Malureanu L, Harrison AM, van Deursen JM. Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J Cell Biol. 2012;199(6):931–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campbell CS, Desai A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature. 2013;497(7447):118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoyt MA, Totis L, Roberts BT. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66(3):507–17. [DOI] [PubMed] [Google Scholar]
- 80.Warren CD, Brady DM, Johnston RC, Hanna JS, Hardwick KG, Spencer FA. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol Biol Cell. 2002;13(9):3029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16(17):1711–8. [DOI] [PubMed] [Google Scholar]
- 82.Girdler F, Sessa F, Patercoli S, Villa F, Musacchio A, Taylor S. Molecular basis of drug resistance in aurora kinases. Chem Biol. 2008;15(6):552–62. [DOI] [PubMed] [Google Scholar]
- 83.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–7. [DOI] [PubMed] [Google Scholar]
- 84.Tao Y, Zhang P, Girdler F, Frascogna V, Castedo M, Bourhis J, et al. Enhancement of radiation response in p53-deficient cancer cells by the Aurora-B kinase inhibitor AZD1152. Oncogene. 2008;27(23):3244–55. [DOI] [PubMed] [Google Scholar]
- 85.Trivedi P, Zaytsev AV, Godzi M, Ataullakhanov FI, Grishchuk EL, Stukenberg PT. The binding of Borealin to microtubules underlies a tension independent kinetochore-microtubule error correction pathway. Nat Commun. 2019;10(1):682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Posch M, Khoudoli GA, Swift S, King EM, Deluca JG, Swedlow JR. Sds22 regulates aurora B activity and microtubule-kinetochore interactions at mitosis. J Cell Biol. 2010;191(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DeLuca KF, Lens SM, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci. 2011;124(Pt 4):622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caldas GV, DeLuca KF, DeLuca JG. KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity. J Cell Biol. 2013;203(6):957–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoo TY, Choi JM, Conway W, Yu CH, Pappu RV, Needleman DJ. Measuring NDC80 binding reveals the molecular basis of tension-dependent kinetochore-microtubule attachments. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bekier ME, Mazur T, Rashid MS, Taylor WR. Borealin dimerization mediates optimal CPC checkpoint function by enhancing localization to centromeres and kinetochores. Nat Commun. 2015;6:6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoon HJ, Carbon J. Participation of Bir1p, a member of the inhibitor of apoptosis family, in yeast chromosome segregation events. Proc Natl Acad Sci U S A. 1999;96(23):13208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho US, Harrison SC. Ndc10 is a platform for inner kinetochore assembly in budding yeast. Nat Struct Mol Biol. 2011;19(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fischbock-Halwachs J, Singh S, Potocnjak M, Hagemann G, Solis-Mezarino V, Woike S, et al. The COMA complex interacts with Cse4 and positions Sli15/Ipl1 at the budding yeast inner kinetochore. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Rodriguez LJ, Kasciukovic T, Denninger V, Tanaka TU. Aurora B-INCENP Localization at Centromeres/Inner Kinetochores Is Required for Chromosome Bi-orientation in Budding Yeast. Curr Biol. 2019;29(9):1536–44 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323(5919):1350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hengeveld RCC, Vromans MJM, Vleugel M, Hadders MA, Lens SMA. Inner centromere localization of the CPC maintains centromere cohesion and allows mitotic checkpoint silencing. Nat Commun. 2017;8:15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim J, Ishiguro K, Nambu A, Akiyoshi B, Yokobayashi S, Kagami A, et al. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature. 2015;517(7535):466–71. [DOI] [PubMed] [Google Scholar]
- 98.Miyazaki S, Kim J, Sakuno T, Watanabe Y. Hierarchical Regulation of Centromeric Cohesion Protection by Meikin and Shugoshin during Meiosis I. Cold Spring Harb Symp Quant Biol. 2017;82:259–66. [DOI] [PubMed] [Google Scholar]
- 99.Miyazaki S, Kim J, Yamagishi Y, Ishiguro T, Okada Y, Tanno Y, et al. Meikin-associated polo-like kinase specifies Bub1 distribution in meiosis I. Genes Cells. 2017;22(6):552–67. [DOI] [PubMed] [Google Scholar]
- 100.Yokobayashi S, Watanabe Y. The kinetochore protein Moa1 enables cohesion-mediated monopolar attachment at meiosis I. Cell. 2005;123(5):803–17. [DOI] [PubMed] [Google Scholar]
- 101.Hauf S, Biswas A, Langegger M, Kawashima SA, Tsukahara T, Watanabe Y. Aurora controls sister kinetochore mono-orientation and homolog bi-orientation in meiosis-I. EMBO J. 2007;26(21):4475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toth A, Rabitsch KP, Galova M, Schleiffer A, Buonomo SB, Nasmyth K. Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis i. Cell. 2000;103(7):1155–68. [DOI] [PubMed] [Google Scholar]
- 103.Rabitsch KP, Petronczki M, Javerzat JP, Genier S, Chwalla B, Schleiffer A, et al. Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I. Dev Cell. 2003;4(4):535–48. [DOI] [PubMed] [Google Scholar]
- 104.Corbett KD, Yip CK, Ee LS, Walz T, Amon A, Harrison SC. The monopolin complex crosslinks kinetochore components to regulate chromosome-microtubule attachments. Cell. 2010;142(4):556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corbett KD, Harrison SC. Molecular architecture of the yeast monopolin complex. Cell Rep. 2012;1(6):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clyne RK, Katis VL, Jessop L, Benjamin KR, Herskowitz I, Lichten M, et al. Polo-like kinase Cdc5 promotes chiasmata formation and cosegregation of sister centromeres at meiosis I. Nat Cell Biol. 2003;5(5):480–5. [DOI] [PubMed] [Google Scholar]
- 107.Lee BH, Kiburz BM, Amon A. Spo13 maintains centromeric cohesion and kinetochore coorientation during meiosis I. Curr Biol. 2004;14(24):2168–82. [DOI] [PubMed] [Google Scholar]
- 108.Katis VL, Matos J, Mori S, Shirahige K, Zachariae W, Nasmyth K. Spo13 facilitates monopolin recruitment to kinetochores and regulates maintenance of centromeric cohesion during yeast meiosis. Curr Biol. 2004;14(24):2183–96. [DOI] [PubMed] [Google Scholar]
- 109.Monje-Casas F, Prabhu VR, Lee BH, Boselli M, Amon A. Kinetochore orientation during meiosis is controlled by Aurora B and the monopolin complex. Cell. 2007;128(3):477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Llano E, Gomez R, Gutierrez-Caballero C, Herran Y, Sanchez-Martin M, Vazquez-Quinones L, et al. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22(17):2400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, et al. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol. 2008;10(1):42–52. [DOI] [PubMed] [Google Scholar]
- 112.Rattani A, Wolna M, Ploquin M, Helmhart W, Morrone S, Mayer B, et al. Sgol2 provides a regulatory platform that coordinates essential cell cycle processes during meiosis I in oocytes. Elife. 2013;2:e01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Katis VL, Galova M, Rabitsch KP, Gregan J, Nasmyth K. Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr Biol. 2004;14(7):560–72. [DOI] [PubMed] [Google Scholar]
- 114.Tseng TC, Chen SH, Hsu YP, Tang TK. Protein kinase profile of sperm and eggs: cloning and characterization of two novel testis-specific protein kinases (AIE1, AIE2) related to yeast and fly chromosome segregation regulators. DNA Cell Biol. 1998;17(10):823–33. [DOI] [PubMed] [Google Scholar]
- 115.Gopalan G, Chan CS, Donovan PJ. A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators. J Cell Biol. 1997;138(3):643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, et al. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59(4):249–63. [DOI] [PubMed] [Google Scholar]
- 117.Slattery SD, Moore RV, Brinkley BR, Hall RM. Aurora-C and Aurora-B share phosphorylation and regulation of CENP-A and Borealin during mitosis. Cell Cycle. 2008;7(6):787–95. [DOI] [PubMed] [Google Scholar]
- 118.Slattery SD, Mancini MA, Brinkley BR, Hall RM. Aurora-C kinase supports mitotic progression in the absence of Aurora-B. Cell Cycle. 2009;8(18):2984–94. [PubMed] [Google Scholar]
- 119.Schindler K, Davydenko O, Fram B, Lampson MA, Schultz RM. Maternally recruited Aurora C kinase is more stable than Aurora B to support mouse oocyte maturation and early development. Proc Natl Acad Sci U S A. 2012;109(33):E2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yanai A, Arama E, Kilfin G, Motro B. ayk1, a novel mammalian gene related to Drosophila aurora centrosome separation kinase, is specifically expressed during meiosis. Oncogene. 1997;14(24):2943–50. [DOI] [PubMed] [Google Scholar]
- 121.Balboula AZ, Schindler K. Selective disruption of aurora C kinase reveals distinct functions from aurora B kinase during meiosis in mouse oocytes. PLoS Genet. 2014;10(2):e1004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang KT, Li SK, Chang CC, Tang CJ, Lin YN, Lee SC, et al. Aurora-C kinase deficiency causes cytokinesis failure in meiosis I and production of large polyploid oocytes in mice. Mol Biol Cell. 2010;21(14):2371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li X, Sakashita G, Matsuzaki H, Sugimoto K, Kimura K, Hanaoka F, et al. Direct association with inner centromere protein (INCENP) activates the novel chromosomal passenger protein, Aurora-C. J Biol Chem. 2004;279(45):47201–11. [DOI] [PubMed] [Google Scholar]
- 124.Yan X, Cao L, Li Q, Wu Y, Zhang H, Saiyin H, et al. Aurora C is directly associated with Survivin and required for cytokinesis. Genes Cells. 2005;10(6):617–26. [DOI] [PubMed] [Google Scholar]
- 125.Sharif B, Na J, Lykke-Hartmann K, McLaughlin SH, Laue E, Glover DM, et al. The chromosome passenger complex is required for fidelity of chromosome transmission and cytokinesis in meiosis of mouse oocytes. J Cell Sci. 2010;123(Pt 24):4292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Uzbekova S, Arlot-Bonnemains Y, Dupont J, Dalbies-Tran R, Papillier P, Pennetier S, et al. Spatio-temporal expression patterns of aurora kinases a, B, and C and cytoplasmic polyadenylation-element-binding protein in bovine oocytes during meiotic maturation. Biol Reprod. 2008;78(2):218–33. [DOI] [PubMed] [Google Scholar]
- 127.Avo Santos M, van de Werken C, de Vries M, Jahr H, Vromans MJ, Laven JS, et al. A role for Aurora C in the chromosomal passenger complex during human preimplantation embryo development. Hum Reprod. 2011;26(7):1868–81. [DOI] [PubMed] [Google Scholar]
- 128.Tang CJ, Lin CY, Tang TK. Dynamic localization and functional implications of Aurora-C kinase during male mouse meiosis. Dev Biol. 2006;290(2):398–410. [DOI] [PubMed] [Google Scholar]
- 129.Balboula AZ, Nguyen AL, Gentilello AS, Quartuccio SM, Drutovic D, Solc P, et al. Haspin kinase regulates microtubule-organizing center clustering and stability through Aurora kinase C in mouse oocytes. J Cell Sci. 2016;129(19):3648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shuda K, Schindler K, Ma J, Schultz RM, Donovan PJ. Aurora kinase B modulates chromosome alignment in mouse oocytes. Mol Reprod Dev. 2009;76(11):1094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kimmins S, Crosio C, Kotaja N, Hirayama J, Monaco L, Hoog C, et al. Differential functions of the Aurora-B and Aurora-C kinases in mammalian spermatogenesis. Mol Endocrinol. 2007;21(3):726–39. [DOI] [PubMed] [Google Scholar]
- 132.Fernandez-Miranda G, Trakala M, Martin J, Escobar B, Gonzalez A, Ghyselinck NB, et al. Genetic disruption of aurora B uncovers an essential role for aurora C during early mammalian development. Development. 2011;138(13):2661–72. [DOI] [PubMed] [Google Scholar]
- 133.Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, et al. Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal. 2011;4(179):ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nguyen AL, Gentilello AS, Balboula AZ, Shrivastava V, Ohring J, Schindler K. Phosphorylation of threonine 3 on histone H3 by haspin kinase is required for meiosis I in mouse oocytes. J Cell Sci. 2014;127(Pt 23):5066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Quartuccio SM, Dipali SS, Schindler K. Haspin inhibition reveals functional differences of interchromatid axis-localized AURKB and AURKC. Mol Biol Cell. 2017;28(17):2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Q, Wei H, Du J, Cao Y, Zhang N, Liu X, et al. H3 Thr3 phosphorylation is crucial for meiotic resumption and anaphase onset in oocyte meiosis. Cell Cycle. 2016;15(2):213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.El Yakoubi W, Buffin E, Cladiere D, Gryaznova Y, Berenguer I, Touati SA, et al. Mps1 kinase-dependent Sgo2 centromere localisation mediates cohesin protection in mouse oocyte meiosis I. Nat Commun. 2017;8(1):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hached K, Xie SZ, Buffin E, Cladiere D, Rachez C, Sacras M, et al. Mps1 at kinetochores is essential for female mouse meiosis I. Development. 2011;138(11):2261–71. [DOI] [PubMed] [Google Scholar]
- 139.Meyer RE, Kim S, Obeso D, Straight PD, Winey M, Dawson DS. Mps1 and Ipl1/Aurora B act sequentially to correctly orient chromosomes on the meiotic spindle of budding yeast. Science. 2013;339(6123):1071–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cairo G, MacKenzie AM, Lacefield S. Differential requirement for Bub1 and Bub3 in regulation of meiotic versus mitotic chromosome segregation. J Cell Biol. 2020;219(4). [DOI] [PMC free article] [PubMed] [Google Scholar]