Abstract
Background
About 50% of all primary breast cancers show a low-level expression of HER2 (HER2-low), defined as immunohistochemically 1+ or 2+ and lack of HER2 gene amplification measured by in situ hybridization. This low HER2 expression is a promising new target for antibody–drug conjugates (ADCs) currently under investigation. Until now, little is known about the frequency and the prognostic value of low HER2-expression in metastatic breast cancer (MBC).
Patients and methods
The MBC-Registry of the Austrian Study Group of Medical Tumor Therapy (AGMT) is a multicenter nationwide ongoing registry for MBC patients in Austria. Unadjusted, univariate survival probabilities of progression-free survival (PFS) and overall survival (OS) were calculated by the Kaplan–Meier method and compared by the log-rank test. Multivariable adjusted hazard ratios were estimated by Cox regression models. In this analysis, only patients with known HER2 status and available survival data were included.
Results
As of 11/15/2020, 1,973 patients were included in the AGMT-MBC-Registry. Out of 1,729 evaluable patients, 351 (20.3%) were HER2-positive, 608 (35.2%) were HER2-low and 770 (44.5%) were completely HER2-negative (HER2-0). Low HER2-expression was markedly more frequent in the hormone-receptor(HR)+ subgroup compared to the triple-negative subgroup (40% vs. 23%). In multivariable analysis, low HER2 expression did not significantly influence OS neither in the HR+ (HR 0.89; 95% CI 0.74–1.05; P = 0.171) nor in the triple-negative subgroup (HR 0.92; 95% CI 0.68–1.25; P = 0.585), when compared to completely HER2-negative disease. Similar results were observed when HER2 IHC 2+ patients were compared to IHC 1+ or 0 patients.
Conclusion
Low-HER2 expression did not have any impact on prognosis of metastatic breast cancer in this real-world population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-021-01492-x.
Keywords: Metastatic breast cancer, HER2-low, HER2-positive, HER2-negative, OS, PFS, Registry, Real-world data
Introduction
Amplification of human epidermal growth factor receptor 2 (HER2) is a well-established negative prognostic factor both in early and metastatic breast cancer (MBC). HER2-directed therapies, however, have changed the natural course of this disease. Nowadays, adequately treated HER2+ /hormone-receptor(HR)+ breast cancer belongs to the subtypes with the most favorable prognosis both in the early and the advanced stage [1, 2].
In contrast to HER2 positivity, defined as immunohistochemically (IHC) 3+ or IHC 2+ and HER2 gene amplification measured by in situ hybridization (ISH) [3], the significance of a low-level expression of HER2 (HER2-low) is less clear. HER2-low is defined as IHC 1+ or IHC 2+ without HER2 gene amplification and compromises about 50 to 55% of all primary breast cancers [3, 4]. In general, these tumors do not respond to trastuzumab [5] or T-DM1 [6], even if there seems to be a subgroup of patients—selected by a novel poly-ligand profiling technique—who might benefit from trastuzumab [7]. HER2-low, however, is a potential target of new antibody–drug conjugates (ADCs). In contrast to T-DM1, these new ADCs show a higher bystander killer effect, by using cleavable linkers and a higher drug-to-antibody ratio [8, 9] and are therefore not only active in HER2-overexpressing tumors [10] but also in tumors with low HER2 expression. Two of these ADCs have already shown promising activity in phase I trials including HER2-low MBC [11, 12]: trastuzumab deruxtecan and trastuzumab duocarmazine. The former ADC is already approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of HER2+ MBC pretreated with two or more anti-HER2-based regimens. For the treatment of HER2-low MBC, however, none of the ADCs is approved today. Currently, two phase III trials are investigating trastuzumab deruxtecan in patients with HER2-low MBC (DESTINY-Breast04; ClinicalTrials.gov identifier: NCT03734029 and DB-06; NCT04494425). Furthermore, another novel HER2-targeting ADC (RC-48) is tested in HER2-low MBC in a phase I/II trial (NCT03052634). Besides several ADCs, bispecific antibodies as well as HER2 vaccines are under investigation in HER2-low breast cancer as reviewed by Tarantino et al. [13].
Several retrospective studies investigated the prognostic value of low HER2 expression in early breast cancer (EBC) [14–20]. Most of these studies showed a negative impact of a IHC 2+ HER2-expression on the risk of recurrence or survival but no or little influence on outcome by HER2 IHC 1+. In contrast to EBC, little is known about the real frequency and the prognostic significance of this new breast cancer subtypes in MBC. Only a few retrospective studies have currently been published, which investigated the prognostic value of low HER2 expression in MBC [20–22]. One trial showed a negative prognostic value of HER2 IHC 2+ only in patients older than 55 years, while there was no OS difference between IHC 2+ and 0 or 1+ tumors in the overall population [20]. The other two publications did not find any difference in OS between patients with HER2-low tumors compared to patients with completely HER2-negative tumors irrespective of the hormone receptor status [21, 22], however the patient numbers in these studies were rather low.
Here, we present data from a large nation-wide registry for MBC in Austria. Both the incidence and the prognostic value of low HER2 expression were investigated in dependence of the HR status.
Methods
The MBC-registry of the Austrian Study Group of Medical Tumor Therapy (AGMT) is a multicenter nationwide ongoing retrospective and prospective registry for MBC patients in Austria.
HER2+ was defined as IHC 3+ or IHC 2+ and ISH+ according to the American Society of Clinical Oncology/College of American Pathologists Clinical Practice (ASCO/CAP) guidelines [3]. HER2-low was defined as IHC 1+ or IHC 2+ and ISH-. Completely, HER2-negative (HER2-0) was defined as IHC 0 and ISH- (if available). The classification was based on local pathology reports. No central pathology review was performed.
In this analysis, only patients with known HER2 status and available survival data were included. For progression-free survival (PFS) analyses, only patients with at least one line of therapy for metastatic disease and sufficiently documented medical records allowing calculation of PFS were included.
In patients with more than one available tumor sample, the following hierarchy was applied: if a tumor sample from a metastatic site was accessible, which was taken within 3 months after diagnosis of metastatic disease and included at least ER- and HER2-status, the receptor status (as well as grade, Ki-67 and histologic subtype) of this biopsy was used. Otherwise, the receptor status of the latest primary tumor (or local recurrence) diagnosed before (or within 3 months of) the diagnosis of metastatic disease was used.
The primary goal of this analysis was to determine the frequency of HER2+, HER2-low and HER2-0 in this MBC population in dependency of the HR status. Furthermore, the impact of low HER2-expression on overall survival (OS) in the HR+ and triple negative population was investigated, respectively. Primarily, HER2-low was compared with HER2-0 regarding OS and first-line PFS, both in univariate and in multivariable analysis in the HR+ and triple-negative population, respectively. Secondarily, IHC 2+ was compared to IHC 1+ or IHC 0 in the two mentioned populations.
Overall survival was calculated from diagnosis of metastatic disease until death from any cause. First-line PFS was defined as time from start of first-line therapy until progression or death from any cause. In order to prevent falsely long PFS times in this retrospective analysis, patients who died more than two month after the end of therapy were censored with date of last dose.
Unadjusted and univariate PFS and OS probabilities between subgroups and were compared by the log-rank test. Multivariable adjusted hazard ratios (HR) were estimated by Cox proportional hazards models. Multivariable analysis was performed separately for HR+ and HR- subgroups with HER2-status (low vs. 0), disease-free survival (DFS; ≥ 24 months or de novo metastatic vs. < 24 months) and visceral disease (yes vs. no) as minimum model. For inclusion of age (continuous), menopausal status (premenopausal vs. postmenopausal) and number of metastatic sites (2–3 vs. 1 and ≥ 4 vs. 1) stepwise backward selection according to Akaike’s information criterion (AIC) were performed. As a result of the algorithm (Additional file 1: Table S5–S8), all variables were included in the final models. Subsequently model stability investigations were performed according to Heinze G et al. [14]. Due to nonlinear influence of age on survival, age was finally included according to menopausal status (interaction).
All tests were carried out at the 5% significance level, no p-value correction was applied. All statistical analyses were performed using R (version 4.0.2). Important packages: survminer (for survival analysis), bootStepAIC (for variable selection).
Results
Frequency of low HER2 expression
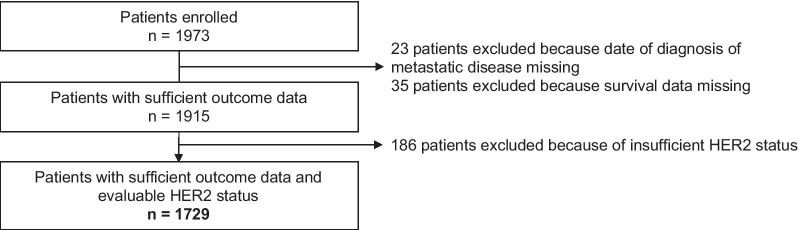
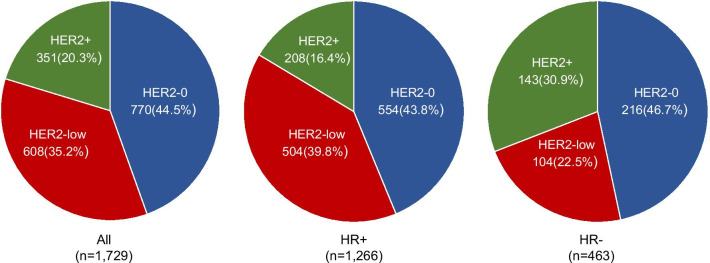
As of 11/15/2020, 1,973 patients were included in the AGMT-MBC-Registry (Fig. 1). Out of 1,729 evaluable patients, who were diagnosed with MBC between November 2000 and August 2020, 351 (20.3%) were HER2-positive, 608 (35.2%) were HER2-low and 770 (44.5%) were completely HER2-negative (HER2-0) (Fig. 2). In 459 patients (26.5%), the receptor status was determined in metastatic tissue and in 1270 patients (73.5%) in the primary tumor. Low HER2-expression was markedly more frequent in the HR+ subgroup compared to the HR- (triple-negative) subgroup (40% vs. 23%). The frequencies of all three HER2 subgroups in dependency of the HR status are shown in Fig. 2. When HER2patients were excluded, 44% of all HER2-negative patients, 48% of patients with HR+ /HER2- tumors and 33% of patients with triple-negative tumors showed a low-level expression of HER2, respectively.
Fig. 1.
Consort diagram
Fig. 2.
Frequencies of the different expression levels of HER2 in dependency of the hormone receptor (HR) status. HER2+ = HER2-positive (immunohistochemically [IHC] 3+ or IHC 2+ and ISH+); HER2-low = low HER2 expression (IHC 1 or IHC 2+ and ISH−); HER2-0 = completely HER2-negative (IHC 0)
Compared to HER2-0 patients, patients with HER2-low tumors were significantly older, were significantly more frequent de novo metastatic, HR+ and of no special type (NST) histology, respectively. Detailed patient characteristics for the overall population as well as for the three different expression levels of HER2 are provided in Table 1.
Table 1.
Patient characteristics
| All (n = 1729) N (%) |
HER2-0 (n = 770) N (%) |
HER2-low (n = 608) N (%) |
HER2+ (n = 351) N (%) |
P HER2-low versus HER2-0 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median age (range)* | 63 (24–97) | 62 (27–97) | 64 (26–95) | 61 (24–94) | 0.017‡ | ||||
| Stage at initial diagnosis | |||||||||
| Stage I–III | 1119 | (64.7) | 536 | (69.6) | 388 | (63.8) | 195 | (55.6) | 0.066 |
| DFS < 24 months | 366 | (32.7) | 179 | (33.4) | 119 | (30.7) | 68 | (34.9) | |
| DFS ≥ 24 months | 696 | (62.2) | 328 | (61.2) | 253 | (65.2) | 115 | (59) | |
| DFS NA** | 57 | (5.1) | 29 | (5.4) | 16 | (4.1) | 12 | (6.2) | |
| De novo metastatic | 602 | (34.8) | 231 | (30.0) | 217 | (35.7) | 154 | (43.9) | |
| Unknown | 8 | (0.5) | 3 | (0.4) | 3 | (0.5) | 2 | (0.6) | |
| Menopausal status* | |||||||||
| Postmenopausal | 1148 | (66.4) | 501 | (65.1) | 429 | (70.6) | 218 | (62.1) | 0.029 |
| Premenopausal | 226 | (13.1) | 108 | (14.0) | 60 | (9.9) | 58 | (16.5) | |
| Unknown | 336 | (19.4) | 154 | (20.0) | 109 | (17.9) | 73 | (20.8) | |
| Male*** | 19 | (1.1) | 7 | (0.9) | 10 | (1.6) | 2 | (0.6) | |
| Metastatic sites* | |||||||||
| Visceral disease | 836 | (48.4) | 337 | (43.8) | 279 | (45.9) | 220 | (62.7) | 0.464 |
| Non-visceral disease only | 893 | (51.6) | 433 | (56.2) | 329 | (54.1) | 131 | (37.3) | |
| Number of metastatic sites* | |||||||||
| Median (range) | 1 | (1–9) | 1 | (1–6) | 1 | (1–8) | 1 | (1–9) | 0.793 |
| 1 | 951 | (55) | 432 | (56.1) | 332 | (54.6) | 187 | (53.3) | |
| 2–3 | 661 | (38.2) | 288 | (37.4) | 232 | (38.2) | 141 | (40.2) | |
| ≥ 4 | 117 | (6.8) | 50 | (6.5) | 44 | (7.2) | 23 | (6.6) | |
| Hormone-receptor (HR) status | |||||||||
| Positive | 1266 | (73.2) | 554 | (71.9) | 504 | (82.9) | 208 | (59.3) | < 0.001 |
| Negative | 463 | (26.8) | 216 | (28.1) | 104 | (17.1) | 143 | (40.7) | |
| Histologic subtype | |||||||||
| No special type (NST) | 1205 | (69.7) | 503 | (65.3) | 433 | (71.2) | 269 | (76.6) | 0.010 |
| Invasive lobular | 228 | (13.2) | 129 | (16.8) | 80 | (13.2) | 19 | (5.4) | |
| Other | 224 | (13.0) | 101 | (13.1) | 82 | (13.5) | 41 | (11.7) | |
| Unknown | 72 | (4.2) | 37 | (4.8) | 13 | (2.1) | 22 | (6.3) | |
| Grade | |||||||||
| 1 | 70 | (4.0) | 36 | (4.7) | 29 | (4.8) | 5 | (1.4) | 0.032 |
| 2 | 751 | (43.4) | 314 | (40.8) | 295 | (48.5) | 142 | (40.5) | |
| 3 | 645 | (37.3) | 293 | (38.1) | 202 | (33.2) | 150 | (42.7) | |
| Unknown | 263 | (15.2) | 127 | (16.5) | 82 | (13.5) | 54 | (15.4) | |
| Treatment for early stage | |||||||||
| (Neo)adj. chemotherapy | 705 | (63.0) | 337 | (62.9) | 226 | (58.2) | 142 | (72.8) | 0.176 |
| (Neo)adj. trastuzumab ± pertuzumab | 133 | (11.9) | 13 | (2.4) | 17 | (4.4) | 103† | (52.8)† | 0.142 |
| Adj. endocrine therapy (HR+ only) | 623 | (78.9) | 299 | (79.7) | 245 | (79.8) | 79 | (73.1) | 1.000 |
| Treatment for metastatic disease | |||||||||
| Chemotherapy | 1161 | (67.1) | 499 | (64.8) | 378 | (62.2) | 284 | (80.9) | 0.341 |
| Trastuzumab | 315 | (18.2) | 12 | (1.6) | 29 | (4.8) | 274 | (78.1) | 0.001 |
| Pertuzumab | 170 | (9.8) | 5 | (0.6) | 20 | (3.3) | 145 | (41.3) | 0.001 |
| T-DM1 | 105 | (6.1) | 5 | (0.6) | 8 | (1.3) | 92 | (26.2) | 0.322 |
| Lapatinib | 119 | (6.9) | 3 | (0.4) | 9 | (1.5) | 107 | (30.5) | 0.061 |
| Endocrine therapy (HR+ only) | 1033 | (81.6) | 465 | (83.9) | 429 | (85.1) | 139 | (66.8 | 0.655 |
| CDK4/6 inhibitor (HR+ only) | 443 | (35.0) | 213 | (38.4) | 215 | (42.7) | 15 | (7.2) | 0.183 |
Statistically significant p-values are highlighted in bold
*At diagnosis of metastatic disease
**No surgery or incomplete surgery date
***Not included in subgroup comparison with chi-square tests
†17 patients did not receive any (neo-)adjuvant therapy, 11 patients were diagnosed before trastuzumab was available, 20 patients had HER2- primary tumors but HER2+ metastatic disease
‡Calculated with Wilcoxon test. All other p-values result from Chi-square tests
Impact of low HER2 expression on OS
Patients with HER2+ MBC had a significantly better prognosis compared to both patients with HER2-low and HER2-0 tumors (median OS 38.3 vs. 34.2 vs. 26.8 months; HR 0.69; 95% CI 0.59–0.81; P < 0.001 and HR 0.84; 95% CI 0.73–0.95; P = 0.006) months respectively; Additional file 1: Fig. S1).
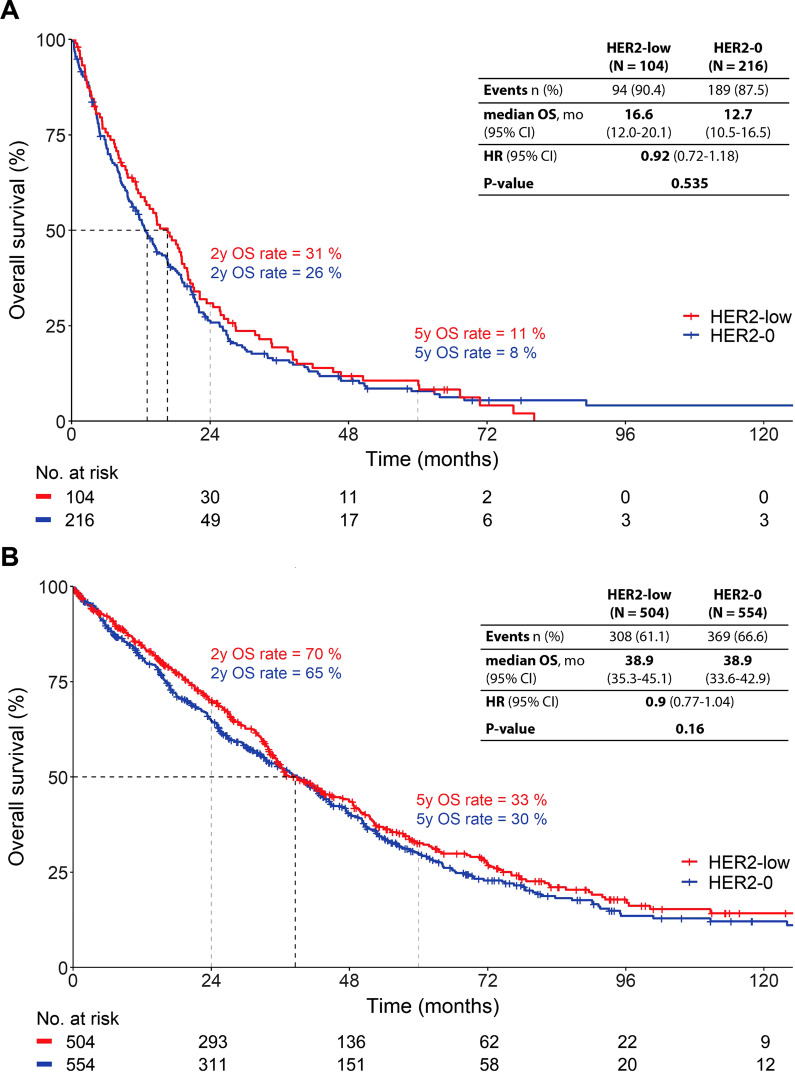
In this analysis, we focused on the prognostic differences between the HER2-low and HER2-0 subgroup. The median follow-up in this population was 68.2 months (95% CI 61.6–72.3 months). In univariate analysis, HER2-low was significantly associated with a longer OS compared to completely HER2-negative disease (HR 0.84; 95% CI 0.73–0.95; P = 0.006; Additional file 1: Fig. S2). Given the unalterable influence of the HR-status on prognosis and the uneven distribution of HR-positivity between the two HER2-subgroups, all further analyses were performed in HR+ and HR- patients separately. In the triple-negative subgroup, median OS was 16.6 months in HER2-low patients and 12.7 months in HER2-0 patients (HR 0.92; 95% CI 0.72–1.18; P = 0.535; Fig. 3A). In the HR+ subgroup, the median OS was 38.9 months both in the HER2-low and in the HER2-0 subgroup (HR 0.90; 95% CI 0.77–1.04, P = 0.160; Fig. 3B).
Fig. 3.
OS of patients with HER2-low tumors and patients with completely HER2-negative tumors (HER2-0) in (A) the HR-negative [n = 320] and (B) the HR-positive subgroup [n = 1058], respectively
Similarly, in multivariable analysis including the known prognostic factors age (according to menopausal status), duration of disease-free survival and presence of visceral disease and metastatic sites at diagnosis of metastatic disease, we did not observe a statistically significant difference between patients with HER2-low and HER2-0 tumors both the HR+ (HR 0.89; 95% CI 0.74–1.05; P = 0.171; Table 2) and in the triple-negative subgroup (HR 0.92; 95% CI 0.68–1.25; P = 0.585; Table 3).
Table 2.
Multivariable analysis (Cox proportional hazard model) of OS for HR+ MBC
| N = 832 (events 525) | HR | 95% CI | P |
|---|---|---|---|
| Age (continuous) according to menopausal status | |||
| Premenopausal | 1.04 | 1.03–1.06 | < 0.001 |
| Postmenopausal | 1.03 | 1.02–1.04 | < 0.001 |
| DFS | |||
| ≥ 24 months or de novo versus < 24 months | 0.75 | 0.59–0.95 | 0.017 |
| Visceral versus no visceral disease* | 1.26 | 1.02–1.55 | 0.030 |
| Number of metastatic sites* | |||
| 2–3 versus 1 | 1.25 | 1.02–1.53 | 0.029 |
| ≥ 4 versus 1 | 1.73 | 1.16–2.59 | 0.007 |
| HER2-low versus HER2-0 | 0.89 | 0.74–1.05 | 0.171 |
Statistically significant p-values are highlighted in bold
*At diagnosis of metastatic disease
Table 3.
Multivariable analysis (Cox proportional hazard model) of OS for HR-negative MBC
| N = 227 (events 199) | HR | 95% CI | P |
|---|---|---|---|
| Age (continuous) according to menopausal status | |||
| Premenopausal | 1.04 | 1.01–1.06 | 0.004 |
| Postmenopausal | 1.02 | 1.01–1.04 | 0.002 |
| DFS | |||
| ≥ 24 months or de novo versus < 24 months | 0.59 | 0.44–0.80 | 0.001 |
| Visceral versus no visceral disease* | 1.59 | 1.15–2.20 | 0.005 |
| Number of metastatic sites* | |||
| 2–3 versus 1 | 1.23 | 0.89–1.68 | 0.210 |
| ≥ 4 versus 1 | 2.01 | 1.17–3.48 | 0.012 |
| HER2-low versus HER2-0 | 0.92 | 0.68–1.25 | 0.585 |
Statistically significant p-values are highlighted in bold
*At diagnosis of metastatic disease
Impact of low HER2 expression on first-line PFS
In univariate analysis, HER2-low did not show a significant influence on PFS when compared to HER2-0: in the HR+ subgroup, median PFS was 15.9 months in the HER2-low subgroup and 13.6 months in HER2-0 subgroup (HR 0.91; 95% CI 0.79−1.05; P = 0.189; Additional file 1: Fig. S3). In the triple-negative subgroup, the median PFS was 5.9 months in the HER2-low and 5.5 months in the HER2-0 subgroup (HR 0.93; 95% CI 0.71−1.21; P = 0.590; Additional file 1: Fig. S4).
Similar to the OS analysis, in multivariable analysis, we did not find a statistically significant difference between the first-line PFS of patients with HER2-low and HER2-0 tumors both the HR+ (HR 0.92; 95% CI 0.78–1.08; P = 0.308; Additional file 1: Table S1) and in the triple-negative subgroup (HR 0.98; 95% CI 0.70–1.37; P = 0.908; Additional file 1: Table S2).
Comparison of HER2 2+ and HER2 1+ /0
As next step, we compared HER2 2+ tumors with HER2 0 or 1+ tumors in the HR+ and triple negative cohort, respectively. Similar to the previous OS analysis, we did not find any prognostic differences between these two HER2-expression groups in the univariate analysis. This was true for the whole HER2-negative cohort (HR 0.99; 95% CI 0.8−1.23; P = 0.945; Additional file 1: Fig. S5), the HR+ /HER2- cohort (HR 1.01; 95% CI 0.78−1.31; P = 0.957; Additional file 1: Fig. S6) and the triple-negative cohort (HR 0.93; 95% CI 0.63−1.39; P = 0.732; Additional file 1: Fig. S7).
Discussion
Previous studies have shown a negative prognostic impact of HER2 IHC 2+ expression in EBC even in the absence of HER2 amplification [14–20]. Tumor biologic findings in early breast cancer cannot simply be transferred to the metastatic stage, since the genetic background is different between the early and the advanced disease [23] and prognostic factors can behave differently depending on the context. For example, androgen receptor (AR) expression predicted a better prognosis in HR+ EBC [24], while there was no influence on time-to-progression (TTP) on first-line endocrine therapy in MBC [25].
Here we provide, complementary evidence for the incidence of HER2-low in MBC an its influence on prognosis in the HR+ and triple-negative subgroup. Out of the whole MBC cohort (n = 1,729), 35% of patients had HER2-low tumors defined as HER2 IHC 1+ or IHC 2+ and ISH-. This corresponds to 44% of all HER2-negative patients, 48% of patients with HR+/HER2- tumors and 33% of patients with TNBC.
Patients with low HER2 expression did not have a significantly different OS compared to patients without any HER2 expression both in the triple-negative cohort (HR 0.92; 95% CI 0.72–1.18; P = 0.535; Fig. 3A) and in the HR+ cohort (HR 0.90; 95% CI 0.77–1.04, P = 0.160; Fig. 3B). These results did not chance when HER2 IHC 2+ patients were compared to IHC 1+ or 0 patients.
The large and very detailed database of the AGMT MBC-registry allowed adjusting for important risk factors like age, disease-free survival and location of metastases. Similar to the univariate analyses, in multivariable analysis, the differences in OS between patients with HER2-low and HER2-0 were not statistically significant both in the HR+ (HR 0.89; 95% CI 0.74–1.05; P = 0.171) and in the triple-negative subgroup (HR 0.92; 95% CI 0.68–1.25; P = 0.585).
The major limitation of our analysis is that the receptor status was extracted from the pathology report and no central HER2 (and ER) testing was performed. The known inter-pathologist variability [26] could have potential impact on our results. Furthermore, the technique of staining and the details of interpretation have slightly changed over time [3]. Since the diagnosis of MBC ranged in a timeframe of 20 years, this is another potential confounder.
Our data are of importance, because new therapeutic options are on the horizon for this new breast cancer subtype. Several antibody–drug conjugates (ADCs), vaccines and bispecific antibodies are currently under development in HER2-low MBC [13]. In a phase Ib study, including 54 extensively pretreated patients with HER2-low MBC (median 7.5 prior therapies), the ADC trastuzumab deruxtecan showed a confirmed objective response rate (ORR) of 37.0% (95% CI 24.3–51.3%) and a median PFS of 11.1 months (95% CI 7.6 months-not evaluable). No difference in activity was seen between tumors with 1+ and 2+ HER2 expression, respectively (ORR 35.7% and 38.5%) [11]. Similarly, trastuzumab duocarmazine showed an ORR of 28% (95% CI 13.8–46.8) in patients with HER2-low/HR+ MBC (n = 32) and of 40% (95% CI 16.3–67.6) in patients with HER2-low/HR- breast cancer in phase I [12].
Currently, two phase III trials are randomizing between trastuzumab deruxtecan and investigator's choice chemotherapy in patients with HER2-low MBC. DESTINY-Breast04 (ClinicalTrials.gov identifier: NCT03734029) includes patients with HR+ and HR- disease pretreated with one or two lines of chemotherapy. DB-06 (NCT04494425), instead, recruits only patients with HR+/HER2-low MBC who have had disease progression on at least two previous lines of endocrine therapies but are naïve for chemotherapy.
Our data are well in line with those from other retrospective analyses, showing no difference in OS between patients with low HER2 expression compared to patients with HER2 0 (or 1+) [20–22]. In one analysis, patients older than 55 years had a statistically significant worse prognosis in case of 2+ HER2 expression compared to HER2 0/1+ patients (HR 1.45; 95% CI 1.01–2.07; P = 0.044) [20]. This observation was not confirmed in our study. In an exploratory multivariable analysis, we did not find a statistically significant influence of HER2-low on OS neither in premenopausal (HR 1.10; 95% CI 0.67–1.82; P = 0.705; Additional file 1: Table S3) nor in postmenopausal women (HR 0.84; 95% CI 0.70–1.01; P = 0.069; Additional file 1: Table S4) with HR+ MBC.
Conclusion
In our analysis, about 44% of all patients with MBC, defined as HER2-negative according to the ASCO/CAP guidelines [3], showed a low expression of HER2. HER2-low was more frequently in patients with HR+ tumors compared to patients with TNBC (48% vs. 33%). This potentially new target for anti-HER2 ADCs, however, did not show any impact on OS or first-line PFS in this real-world population when HR-expression and other prognostic factors were considered.
Supplementary Information
Additional file 1: Figure S1. OS of patients with HER2-low tumors, patients with completely HER2-negative tumors (HER2-0) and patients with HER2-positive tumors (HER2-pos) in the overall population (n = 1,729). Figure S2. OS of patients with HER2-low tumors and patients with completely HER2-negative tumors (HER2-0) in the overall population (n = 1,378). Figure S3. PFS of patients with HER2-low tumors and patients with completely HER2-negative tumors (HER2-0) in the HR+ population (n = 961). Figure S4. PFS of patients with HER2-low tumors and patients with completely HER2-negative tumors (HER2-0) in the HR-negative population (n = 272). Figure S5. OS of patients with HER2 2+ tumors and patients with HER2 0 or 1+ tumors in the overall population (n = 1,378). Figure S6. OS of patients with HER2 2+ tumors and patients with HER2 0 or 1+ tumors in the HR+ population (n = 1,058). Figure S7. OS of patients with HER2 2+ tumors and patients with HER2 0 or 1+ tumors in the HR-negative population (n = 320). Table S1. Multivariate analysis (Cox proportional hazard model) of PFS for HR+ MBC. Table S2. Multivariate analysis (Cox proportional hazard model) of PFS for HR-negative MBC. Table S3. Multivariate analysis (Cox proportional hazard model) of OS for premenopausal patients with HR+ MBC. Table S4. Multivariate analysis (Cox proportional hazard model) of OS for postmenopausal patients with HR+ MBC. Table S5. HR+ model stability investigations according Heinze G. et al. [17]. Table S6. HR+ model selection frequencies according Heinze G. et al. [17]. Table S7. HR- Model stability investigations according Heinze G. et al. [17]. Table S8. HR- Model selection frequencies according Heinze G. et al. [17].
Acknowledgements
The authors gratefully acknowledge the support from the AGMT office (particularly Mag. Katrin Dorfinger, Sebastian Schütz and Dr. Daniela Wolkersdorfer) and all trial coordinators at the contributing centers.
Abbreviations
- ADC
Antibody–drug conjugate
- AGMT
Austrian Study Group of Medical Tumor Therapy
- AIC
Akaike’s information criterion
- ASCO
American Society of Clinical Oncology
- CAP
College of American Pathologists
- CI
Confidence interval
- DFS
Disease-free survival
- EBC
Early breast cancer
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- HER2
Human epidermal growth factor receptor 2
- HER3
Human epidermal growth factor receptor 3
- HR
Hazard ratio
- HR
Hormone receptor
- IHC
Immunohistochemically
- ISH
In-situ hybridization
- MBC
Metastatic breast cancer
- NST
No special type
- OS
Overall survival
- PFS
Progression-free survival
- TNBC
Triple negative breast cancer
Authors' contributions
SPG was involved in the conceptualization of the registry, in methodology, investigation, data curation, formal analysis, visualization, project administration and original draft writing; GR was involved in the conceptualization of the registry, in methodology, investigation, data curation, formal and statistical analysis, visualization, project administration and review and editing of the manuscript; SPG, GR, MH, MK, CFS and RG are part of the steering committee of the registry; CT, AP, MB, SH, CS, AFZ, DE, MS, CFS, FR, CH and JA provided resources and were involved in project administration, review and editing of the manuscript. RG was involved in the conceptualization of the registry, in methodology, formal analysis, supervision and review and editing of the manuscript and provided resources. All authors read and approved the final manuscript.
Funding
The AGMT MBC-registry is supported by grants from Roche, Daiichi Sankyo, Pfizer and AstraZeneca. The supporters did not have any involvement in study design, selection or enrollment of patients, data collection, storage, analysis, interpretation of the data, preparation of the manuscript or the decision to submit the manuscript for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The registry was approved by the Ethics Committee of the province Salzburg (IRB number: 415-E/1836). All patients provided written informed consent or died before data entry.
Consent for publication
Not applicable.
Competing interests
SPG received honoraria from Roche, Daiichi Sankyo, Seagen, Novartis, BMS, AstraZeneca, Eli Lilly and MSD, travel support from Roche, Amgen, Novartis, Pfizer, Daiichi Sankyo, and a research grant from Roche. GR received honoraria from Roche, Novartis, Amgen, Pfizer, Daiichi Sankyo, MSD, Eli Lilly, and Bristol-Myers Squibb, declared speaker bureau/expert testimony for Amgen, Astra Zeneca, Novartis, Bristol-Myers Squibb, Roche, Pfizer, and Eli Lilly, and a research grant from Roche. AP received honoraria from Pfizer, Roche, Novartis, Celgene-BMS, Celgene, Eli Lilly, AstraZeneca, Saegen, Daiichi Sankyo, and Gilead, and travel support from Roche, and Pfizer. MB received honoraria from Amgen, AstraZeneca, Bayer, Celgene, Pfizer, Eli Lilly, Novartis, Roche, MSD, Samsung and Pierre Fabre, declared speaker bureau/expert testimony for Amgen, AstraZeneca, Bayer, Celgene, Pfizer, Eli Lilly, Novartis, Roche, MSD, Samsung and Pierre Fabre, received research grants (institution) from Amgen, Celgene, Eli Lilly, Novartis and Pfizer, and travel support from Amgen, AstraZeneca, Bayer, Celgene, Pfizer, Eli Lilly, Novartis, Roche, MSD, Samsung and Pierre Fabre. SH received honoraria from Celgene, Novartis, AOP Orphan, Takeda, and Roche, travel support from Roche, Sanofi, AbbVie, and Pfizer, and research grants (institution) from Celgene, and AOP Orphan. AFZ received honoraria from Roche, BMS, Lilly, Astra Zeneca, Takeda, and MSD, and travel support from Roche, Takeda, BMS, and Lilly. DE received honoraria from Roche, Pfizer, Novartis, AstraZeneca, Pierre Fabre, Lilly, and MSD, and travel support from Roche, Pfizer, Novartis, AstraZeneca, Pierre Fabre, Lilly, and MSD. MS received honoraria from Novartis, Lilly, Roche, AstraZeneca, Pfizer, Myriad, Pierre Fabre, and Amgen, and travel support from Pfizer, Roche, Celgene, Lilly, Novartis, and Merck. CFS received honoraria from Amgen, Roche, Novartis, and AstraZeneca, travel support from Pfizer, AstraZeneca, and Tesaro, and research grants from Amgen, Roche, and AstraZeneca. FR received honoraria from Pfizer, Roche, Daiichi Sankyo, and Pierre Fabre (institution), and travel support from Pfizer, Eli Lilly, Roche, Pierre Fabre. MH received honoraria from Amgen, Roche, Lilly, and Pfizer, and travel support from Roche, Pfizer. MK received honoraria travel support from Roche, and a research grant (institution) from Agendia. RG received honoraria from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, and Daiichi Sankyo, travel support from Roche, Amgen, Janssen, Astra Zeneca, Novartis, MSD, Celgene, Gilead, BMS, AbbVie, and Daiichi Sankyo, and research grants from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, Abbvie, Gilead, and Daiichi Sankyo. CT, CS, CH, and JA declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hwang KT, et al. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: a population-based study using SEER database. Clin Cancer Res. 2019;25(6):1970–1979. doi: 10.1158/1078-0432.CCR-18-2782. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi K, et al. Impact of immunohistological subtypes on the long-term prognosis of patients with metastatic breast cancer. Surg Today. 2016;46(7):821–826. doi: 10.1007/s00595-015-1252-x. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 4.Schalper KA, et al. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch Pathol Lab Med. 2014;138(2):213–219. doi: 10.5858/arpa.2012-0617-OA. [DOI] [PubMed] [Google Scholar]
- 5.Fehrenbacher L, et al. NSABP B-47/NRG Oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38(5):444–453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogitani Y, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 7.Domenyuk V, et al. Poly-ligand profiling differentiates trastuzumab-treated breast cancer patients according to their outcomes. Nat Commun. 2018;9(1):1219. doi: 10.1038/s41467-018-03631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–1742. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 10.Cortés J, et al. Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32(suppl_5):S1283–S1346. doi: 10.1016/annonc/annonc741. [DOI] [Google Scholar]
- 11.Modi S, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerji U, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–1135. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 13.Tarantino P, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 14.Birner P, et al. Evaluation of the United States Food and Drug Administration-approved scoring and test system of HER-2 protein expression in breast cancer. Clin Cancer Res. 2001;7(6):1669–1675. [PubMed] [Google Scholar]
- 15.Camp RL, et al. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res. 2003;63(7):1445–1448. [PubMed] [Google Scholar]
- 16.Eggemann H, et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer. 2015;22(5):725–733. doi: 10.1530/ERC-15-0335. [DOI] [PubMed] [Google Scholar]
- 17.Gilcrease MZ, et al. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol. 2009;33(5):759–767. doi: 10.1097/PAS.0b013e31819437f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ménard S, et al. Biology, prognosis and response to therapy of breast carcinomas according to HER2 score. Ann Oncol. 2008;19(10):1706–1712. doi: 10.1093/annonc/mdn369. [DOI] [PubMed] [Google Scholar]
- 19.Rossi V, et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist. 2012;17(11):1418–1425. doi: 10.1634/theoncologist.2012-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MH, et al. Intermediate HER2 expression is associated with poor prognosis in estrogen receptor-positive breast cancer patients aged 55 years and older. Breast Cancer Res Treat. 2020;179(3):687–697. doi: 10.1007/s10549-019-05505-4. [DOI] [PubMed] [Google Scholar]
- 21.Hein A, et al. Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. Eur J Cancer. 2021;155:1–12. doi: 10.1016/j.ejca.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Schettini F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul MR, et al. Genomic landscape of metastatic breast cancer identifies preferentially dysregulated pathways and targets. J Clin Invest. 2020;130(8):4252–4265. doi: 10.1172/JCI129941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozovic-Spasojevic I, et al. The prognostic role of androgen receptor in patients with early-stage breast cancer: a meta-analysis of clinical and gene expression data. Clin Cancer Res. 2017;23(11):2702–2712. doi: 10.1158/1078-0432.CCR-16-0979. [DOI] [PubMed] [Google Scholar]
- 25.Bronte G, et al. Androgen receptor in advanced breast cancer: is it useful to predict the efficacy of anti-estrogen therapy? BMC Cancer. 2018;18(1):348. doi: 10.1186/s12885-018-4239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson TA, et al. HER-2/neu in breast cancer: interobserver variability and performance of immunohistochemistry with 4 antibodies compared with fluorescent in situ hybridization. Mod Pathol. 2001;14(11):1079–1086. doi: 10.1038/modpathol.3880440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. OS of patients with HER2-low tumors, patients with completely HER2-negative tumors (HER2-0) and patients with HER2-positive tumors (HER2-pos) in the overall population (n = 1,729). Figure S2. OS of patients with HER2-low tumors and patients with completely HER2-negative tumors (HER2-0) in the overall population (n = 1,378). Figure S3. PFS of patients with HER2-low tumors and patients with completely HER2-negative tumors (HER2-0) in the HR+ population (n = 961). Figure S4. PFS of patients with HER2-low tumors and patients with completely HER2-negative tumors (HER2-0) in the HR-negative population (n = 272). Figure S5. OS of patients with HER2 2+ tumors and patients with HER2 0 or 1+ tumors in the overall population (n = 1,378). Figure S6. OS of patients with HER2 2+ tumors and patients with HER2 0 or 1+ tumors in the HR+ population (n = 1,058). Figure S7. OS of patients with HER2 2+ tumors and patients with HER2 0 or 1+ tumors in the HR-negative population (n = 320). Table S1. Multivariate analysis (Cox proportional hazard model) of PFS for HR+ MBC. Table S2. Multivariate analysis (Cox proportional hazard model) of PFS for HR-negative MBC. Table S3. Multivariate analysis (Cox proportional hazard model) of OS for premenopausal patients with HR+ MBC. Table S4. Multivariate analysis (Cox proportional hazard model) of OS for postmenopausal patients with HR+ MBC. Table S5. HR+ model stability investigations according Heinze G. et al. [17]. Table S6. HR+ model selection frequencies according Heinze G. et al. [17]. Table S7. HR- Model stability investigations according Heinze G. et al. [17]. Table S8. HR- Model selection frequencies according Heinze G. et al. [17].
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.