Abstract
Previous studies show that intermittent social defeat (ISD) stress increases self-administration of psychostimulants, which suggests that ISD promotes reward-seeking behavior and, ultimately, increases vulnerability to develop drug abuse. The present study investigates whether ISD alters cost/benefit evaluations to promote reward-seeking behavior and whether these alterations are time-dependent. Male rats performed two different tasks that assessed their motivation to seek and consume food rewards. An effort-discounting task in which rats chose between less and more effortful options (i.e., 1 lever-press versus 2, 5, 10 or 20 lever-presses) associated with low- and high-reward (i.e., 1 sugar pellet versus 3 sugar pellets), respectively; and a progressive ratio task in which rats had to increase their effort (more lever presses) to obtain a sugar pellet. ISD consisted of exposing animals to social defeat once every three days for ten days (4 stress episodes). Rats were tested 24-48 h after stress episodes, and 1 week and 6 weeks after the last stress episode. In the effort-discounting task, stressed animals showed a decrease in their preference for high rewards associated with more effort (i.e., 10 and 20 lever-presses). These effects were transient and not maintained one week after stress. In the progressive ratio task, stressed animals showed an increase in the number of lever presses to obtain rewards that emerged six weeks after the last stress episode. These results suggest different short- and long-term effects on the motivation for rewards after ISD and indicate temporal dynamic adaptations in the function of the brain reward system.
Keywords: social stress, motivation, decision-making, effort, reward-seeking
INTRODUCTION
The repeated exposure to social adversity increases vulnerability to develop substance use disorders [1]. Studies in humans and animals show that stressful experiences increase drug-seeking behavior and relapse [1-4]. In particular, intermittent (episodic) social defeat (ISD) stress in rats has been consistently shown to increase drug self-administration (i.e., psychostimulants) [5-7] as well as the activity of the mesolimbic dopamine system [5,8], which suggests that ISD enhances the motivation to seek rewards. However, how the intermittent exposure to social stress changes reward processing is unclear [9] and therefore, how ISD increases reward-seeking behavior is poorly understood. Pharmacological studies show that the activation of the mesolimbic dopamine system alters cost/benefit evaluations and increases the motivation to pursue food as well as drug rewards [10-15]. Based on these studies, the present study investigates whether ISD alters cost/benefit evaluations in order to promote reward-seeking behavior. Clarifying this will help understand the transition from social adversity to drug abuse.
Previous studies assessing the effects of stress on cost/benefit decision-making focused on acute and chronic stress [16]. Thus, by using an effort-discounting task, it has been shown that acute restraint stress decreases the preference for high magnitude rewards when animals are required to put more effort (i.e. more lever presses) to earn them [17,18]. Similarly, by using a progressive ratio schedule, studies have shown that acute restraint and chronic social stress [6,19,20] as well as chronic corticosterone administration [21,22], decrease the motivation to work for rewards, as they reduce the number of lever presses that animals are willing to make to obtain rewards. The effects of chronic social stress have been associated with depression-like behaviors and anhedonia [23-26]. Overall, these studies suggest that stress alters effort-based reward seeking and decreases the motivation to work for rewards. It is important to emphasize, however, that the effects of repeated social stress on motivation depend critically on the stress protocol utilized –continuous (i.e., every day) versus intermittent exposure to social defeat [6]–, and therefore, these studies do not provide evidence of whether ISD changes effort-based reward-seeking behavior.
Importantly, the effects of repeated social stress on motivation and reward seeking seem to depend on the time when these effects are evaluated [25-29]. In fact, it has been shown that the alterations in reward processing (i.e., anhedonia) produced by chronic social defeat are transient in some animals (i.e., resilient to social defeat) and not maintained after the last stress episode [25,26]. Moreover, one study shows that chronic social defeat increased the motivation for rewards ten weeks after stress [29], while the opposite was found when the effects of social defeat were evaluated immediately or up to three weeks after stress [6,26]. These studies point to long-term changes in the function of the brain reward system during the post-stress period and open the possibility that ISD produces time-dependent effects on effort-based reward-seeking behavior [30].
The present study investigates whether ISD alters cost/benefit decision-making and the motivation to pursue rewards (i.e. sugar pellets) and whether these alterations are time dependent. In the current study we focus on the intermittent exposure to social defeat (i.e., ISD) versus other social defeat protocols (i.e., chronic social defeat) because ISD consistently increases reward seeking (i.e., drug-self administration) [5-7], while the opposite is found (i.e., depressive-like anhedonia) by most studies utilizing chronic social defeat [6,26,31,32]. ISD is also an optimal protocol for investigating time-dependent and cumulative effects of stress since animals can be tested on the days in between stress episodes as well as weeks after stress. We trained animals in an effort-discounting task [18] and then exposed them to ISD as in our previous work [30]. In this task, animals choose between high- and low-reward choices based on the effort (i.e., number of lever presses) to obtain the high-reward option. The terms low-reward and high-reward refer to the magnitude of rewards (i.e., one sugar pellet versus three sugar pellets). Food deprived rats value higher magnitude rewards more than lower magnitude. Additionally, we tested animals in a progressive ratio task [18] to evaluate whether they were more motivated to work (i.e. press lever more times) for rewards. To assess the temporal profile of the effects of ISD on behavior, we tested animals’ performance in both tasks in between social stress episodes and one and six weeks after the last stress episode. This timeline is based on previous studies showing that ISD increases drug self-administration five weeks after the last stress episode [5,33].
2. METHODS
2.1. Animals
Sixteen adult male Long Evans rats (Envigo, Indianapolis, IN) (375–425 g) were pair-housed in standard polycarbonate cages (45 x 24 x 20 cm) on a 12 h light/dark cycle (lights on at 9:00 P.M.). All experiments were performed during the dark phase when the animals are most active. The rats were placed on a mild food-restricted diet (15 g of chow per rat and day) two days before starting behavioral experiments [30]. This feeding regime –extended throughout all phases of the experiment–, resulted in a 7-10% weight loss for both groups of rats (basal: Control= 528 ±11, Stress= 540 ±14; pre-ISD: Control= 483 ±11, Stress= 503 ±14; post-ISD: Control= 477 ±11, Stress= 500 ±10). All procedures were approved by the University of Mississippi Institutional Animal Review Board and were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Experimental design
Two-three weeks after their arrival to the animal facility, all rats were handled for at least three days and then habituated to the operant chambers and trained in the effort-discounting task. Once animals reached stable performance (see below), they were divided in two groups: control (n= 8) and stress (n= 8). The stress group was exposed to ISD following the protocol used in our previous study [30] and depicted in Figure 1A. The control group was taken to a different room and handled for 5 min. Rats were tested in the effort-discounting task 24-48 h after stress episodes and one week after the end of the stress protocol. Rats were tested in the progressive ratio task 48 h after the second and fourth stress episodes, and one and six weeks after the end of the stress protocol.
Figure 1:
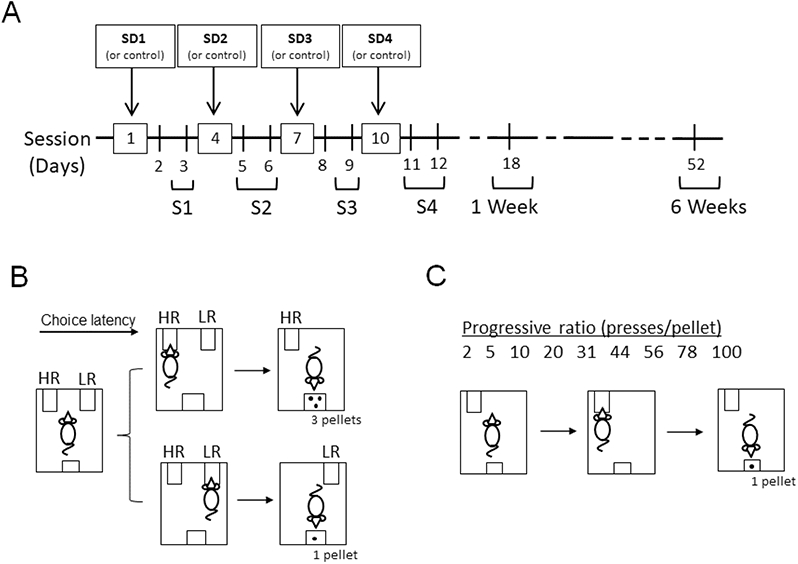
(A) Timeline for the intermittent social stress and the effort-based reward-seeking behavior testing sessions. Rats were exposed to social defeat stress once every three days (SD1-4) for ten days. Control animals were handled. (B) Effort-discounting task protocol. Rats chose between a High-Reward/High-Effort lever (HR) and a Low-Reward/Low-Effort lever (LR). Choosing the HR delivered three sugar pellets and choosing the LR delivered one sugar pellet. The effort-discounting task was performed 24-48 h after stress episodes and 1 week after the last stress episode (days 3, 5, 9, 11 and 18). (C) Progressive ratio task protocol. Rats were required to increase their effort (presses per pellet) to obtain rewards (one sugar pellet). The progressive ratio task was performed 48 h after the second and the fourth stress episodes, and 1 and 6 weeks after the last stress episode (days 6, 12, 18 and 52).
2.3. Effort-discounting task
We utilized an effort-discounting task modified from Bryce and Floresco [18] (Figure 1B). The apparatus consisted of an operant chamber with two retractable levers (right and left) and a food trough in between them in the same wall. The chamber contained a house light that was on during the entire session. First, animals were trained to establish the lever–response contingency through a reward-shaping procedure using both levers (3-4 days) (dustless sugar pellets, 45mg; Bio-Serv). Second, they were trained to discriminate between a high-reward lever (HR) and a low-reward lever (LR) (FR1, 30 min sessions). Pressing the HR delivered 3 sugar pellets while pressing the LR delivered 1 sugar pellet. This training phase lasted until they pressed the LR <25% of the trials (5-6 days). Then, the effort-discounting training started. The task consisted of 4 blocks of 12 trials each (48 trials total, 32 min sessions). In each block, the first 2 trials were force choice trials in which only one of the two levers was randomly extended. The right lever was associated with a low reward (1 sugar pellet) and the left lever was associated with a high reward (3 sugar pellets). The value of the levers varied for each animal. The remaining 10 trials were free choice trials in which both levers were extended, and animals were required to make a choice. For all sessions and blocks, one lever was designated as the LR, and the other lever was designated as the HR. This designation was counterbalanced among animals. Every trial had a duration of 40 s and started with a variable intertrial interval (25-35 s) after which the levers were extended for a maximum of 10 s. If the animals press the LR, both levers were retracted, and a single food pellet was delivered to the food trough after 1 s. The light of the food trough was turned on then. If the animals pressed the HR only the LR retracted, and the HR remained extended until the animals made the required number of presses to obtained 3 food pellets. If animals did not press any lever within the 10 s both levers were retracted, and the trial was considered an omission. Similarly, if the animals did not perform the required number of presses, the lever retracted, and no pellet was delivered. The number of presses required to earn rewards (ratio) increased over blocks, initially set at 2 presses, and increasing to 5, 10, and 20 presses for subsequent blocks. Animals were trained until stable performance (3 consecutive sessions choosing the HR lever at least 80% of trials during the first block), which typically took 5-7 days. Choice latency (the time between lever extension and choice) and rates of pressing on the HR lever were also evaluated.
2.4. Progressive ratio schedule
Control and stressed rats were tested in a progressive ratio schedule to assess changes in the motivation to work for rewards (Figure 1C). In the progressive ratio the number of presses required to obtain a pellet increased exponentially. The ratio was modified from previous work [18] and increased in the following manner: 2, 5, 10, 20, 31, 44, 56, 78 and 100 presses. Rats had a maximum of 10 min to complete each ratio and obtain rewards. The sessions ended when rats failed to complete a ratio. The total number of lever presses and pellets obtained over the course of a session and the last ratio obtained before a session terminated (breakpoint) were measured.
2.5. Intermittent social defeat stress (ISD)
After stable performance in the effort-discounting task, rats were exposed to repeated social defeat stress [30,34] (Resident-Intruder paradigm, modified from Miczek et al., [6]). Rats were housed individually one week before starting the stress protocol. Resident males (Long Evans retired breeders; 500-600 g) were housed in transparent PVC cages (H x L x W: 45 x 61 x 61 cm) with females for at least 10 days before starting the procedure. Female rats were previously sterilized by ligation of the oviducts. Animals of the stress group were exposed to social defeat once every three days for ten days. Control animals were moved to a new room and handled for 5 min. Every social stress session started by removing the female rat from the resident cage at least 30 min before. Then, first, the intruder rat was placed in the cage with the resident male separated by a divider wall that contained wire mesh (allowing sensory exposure) –both rats could see and smell each other–, for 10 minutes. Second, the divider wall was removed, allowing the rats to interact. The interaction was stopped when either 6 attacks were witnessed, the intruder was in supine position for 5 seconds, or 5 minutes had elapsed. To avoid injuries, the interaction was also stopped if an aggressive bite occurred. The latency to the first attack was also recorded. Third, the divider wall was reinserted, and the intruder remained in the cage for 10 more minutes. After this time, intruders returned to their home cages. Intruders were not exposed to the same resident more than twice.
2.6. Data analysis
A three-way ANOVA with repeated measures was utilized to analyze performance in the effort-discounting and progressive ratio tasks. This analysis considered group (Control and Stress) as between subjects and dependent variables measured in the effort-discounting task (i.e., number of choices for high reward lever, choice latency, rate of lever presses) and the progressive ratio task (i.e., lever presses, pellets consumed, break point) as within subjects; and focused on the cumulative (i.e., after the second stress episode) and time-dependent (i.e., across sessions) effects of ISD. One- and Two- way ANOVAs with repeated measures as well as Student t tests were also utilized for further analysis. The statistical computations were performed with SPSS software and the statistical significance was set at p= 0.05.
RESULTS
Effects of ISD on the effort-discounting task
The rats were tested in the effort-discounting task during the days in between stress episodes and one week after the last stress episode (see Figure 1A). A three-way ANOVA with repeated measures with group (Control, Stress) as between subjects and ratio (2, 5, 10 and 20) and session (S2-Post 1Week) as within subjects was performed to compare the choice of the more effortful option (choice HR lever) as well as choice latency and rate of lever presses. As shown in Figure 2A, animals decreased their preference for the HR lever when they were required to put more effort (i.e., press the lever more times) to receive a food pellet (ratio effect: F(3,42)= 21.45, p< 0.001, ηp2= 0.61). The three-way ANOVA did not detect overall significant effects across sessions (F(3,42)= 2.34, p= 0.082, ηp2= 0.14). However, it did reveal a significant session x group interaction (F(3,42)= 3.44, p= 0.025, ηp2= 0.20) indicating that the effects of stress on the choice HR lever were different across sessions.
Figure 2:
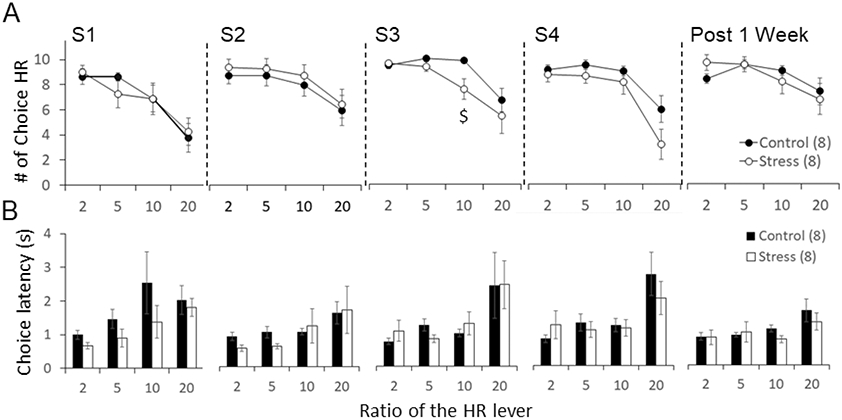
Effort-discounting task performance for control and stressed animals. (A) Preference for the more effortful option (HR lever) at different ratios (2-20) and across sessions (S1-Post 1 Week). Data are the mean ± SEM of the number of HR choices. (B) Choice latency at different ratios (2-20) and across sessions (S1-Post 1 Week). Data are the mean ± SEM of the choice latency in seconds. Number of animals per group in parenthesis. $ p< 0.05 compared to control.
We performed two-way ANOVAs to further assess how stress changed the preference for the HR lever across sessions for every ratio (Figure 3). We found that stress decreased the preference for HR compared to controls at the 10 presses ratio (session x group interaction: F(3,42)= 3.40, p= 0.026, ηp2= 0.20). This effect was significant after the third stress episode (S3) (t14= 2.75, p= 0.016, ηp2= 0.35; independent t test) (Figures 2A and 3). We also found that the preference for HR changed across sessions at the 20 presses ratio (session effect: F(3,42)= 4.79, p= 0.006, ηp2= 0.25). This effect was significant for stressed animals (F(3,21)= 6.47, p= 0.003, ηp2= 0.48; one-way ANOVA with repeated measures), but not controls (F(3,21)= 0.94, p= 0.44, ηp2= 0.12; one-way ANOVA with repeated measures), which indicates a decrease in the preference for HR across sessions in the Stress group. As shown in Figure 3, this decrease was lowest after the last stress episode (S4) compared to the previous sessions S3 (t7= 2.34, p= 0.050) and S2 (t7= 3.26, p= 0.014; paired t test). This effect was not maintained 1 week after the last stress episode since HR choices increased significantly at 1 week compared to S4 (t7= 2.55, p= 0.038; paired t test). The two-way ANOVA did not find group differences for HR preference across sessions at the 20 presses ratio (session x group interaction: F(3,42)= 2.13, p= 0.110, ηp2= 0.13).
Figure 3:
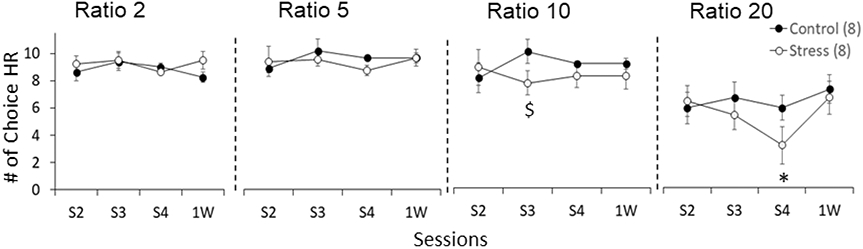
Effort-discounting task performance across sessions (S2-Post 1 week) for each ratio (2-20) for control and stressed animals. Data are the mean ± SEM of the number of HR choices. $ p< 0.05 compared to control. Number of animals per group in parenthesis. $ p< 0.05 compared to control; * p< 0.05 compared to S3 and 1W sessions.
Figure 2B shows the choice latency to the HR lever for every ratio across sessions. The three-way ANOVA analysis did not detect significant differences for group (F(1,11)= 0.28, p= 0.60, ηp2= 0.02) or session (F(3,33)= 0.85, p= 0.47, ηp2= 0.07). However, there was a ratio effect (F(3,33)= 11.92, p< 0.001, ηp2= 0.52) indicating that the choice latency increased with higher ratios.
We also show the rate of lever presses in both groups for every ratio across sessions (Table 1). The three-way ANOVA analysis did not detect significant differences for group (F(1,11)= 0.65, p= 0.43, ηp2= 0.05) or session (F(3,33)= 0.53, p= 0.66, ηp2= 0.04). However, there was a ratio effect (F(3,33)= 11.61, p< 0.001, ηp2= 0.51) indicating that animals in both groups reduce the number of presses per second at higher ratios. In addition, we also evaluated the number of omissions during the task after the third and fourth stress episodes. There were few omitted trials after the third (control= 0.25 ±0.25; stress= 0.75 ±0.75) and fourth (control= 0.62 ±0.37, stress= 1.62 ±1.16) stress episodes in both groups. The number of omitted trials was not different between groups (group effect: F(1,14)= 0.58, p= 0.45, ηp2= 0.04; session x group interaction: F(1,14)= 0.67, p= 0.42, ηp2= 0.04) or across sessions (F(1,14)= 4.21, p= 0.060, ηp2= 0.23).
Table 1.
Rate of lever presses (presses/s) during the effort-discounting task for every ratio (2-20) scheduled across sessions (S1-Post 1 Week). Animals decreased their rate of lever presses at higher ratios (F(3,33)= 11.61, p< 0.001, ηp2= 0.51). Data are the mean ±SEM (n= 8 per group).
| S1 | S2 | S3 | S4 | Post 1 Week | ||
|---|---|---|---|---|---|---|
| Ratio 2 | Control | 3.40 ±0.58 | 4.22 ±0.76 | 3.44 ±0.55 | 3.67 ±0.46 | 3.60 ±0.57 |
| Stress | 3.58 ±0.56 | 3.97 ±0.53 | 3.82 ±0.63 | 3.60 ±0.73 | 3.07 ±0.40 | |
| Ratio 5 | Control | 2.37 ±0.28 | 2.25 ±0.29 | 2.51 ±0.21 | 2.48 ±0.27 | 2.64 ±0.20 |
| Stress | 3.16 ±0.40 | 3.35 ±0.41 | 3.06 ±0.29 | 2.95 ±0.38 | 2.77 ±0.37 | |
| Ratio 10 | Control | 2.22 ±0.21 | 2.39 ±0.26 | 2.54 ±0.22 | 2.70 ±0.29 | 2.55 ±0.21 |
| Stress | 3.21 ±0.48 | 3.08 ±0.45 | 2.87 ±0.29 | 3.16 ±0.58 | 3.07 ±0.47 | |
| Ratio 20 | Control | 1.95 ±0.24 | 1.96 ±0.16 | 2.16 ±0.21 | 1.99 ±0.18 | 2.10 ±0.13 |
| Stress | 2.67 ±0.46 | 2.67 ±0.39 | 2.93 ±0.73 | 2.62 ±0.44 | 2.85 ±0.39 |
Effects of ISD on the progressive ratio task
Rats were tested in a progressive ratio schedule after the second (S2) and fourth (S4) stress episodes, and 1 and 6 weeks after the last stress episode. A three-way ANOVA with repeated measures with group (Control, Stress) as between subjects and ratio (2, 5, 10, 20, 31) and session (S2-Post 6Weeks) as within subjects, was performed to compare the number of lever presses during the progressive ratio task. As shown in Figure 4A, in every session, both control and stressed animals pressed the lever more times whenever the ratio to receive a reward (i.e., press/g) was lower (ratio effect: F(4,168)= 24.59, p< 0.001, ηp2= 0.63). Also, there was an increase in the number of lever presses across sessions (session effect: F(3,168)= 3.88, p= 0.015, ηp2= 0.22). The three-way ANOVA did not detect overall group effects (F(1,14)= 0.64, p= 0.43, ηp2= 0.04), or significant group x ratio (F(4,56)= 0.46, p= 0.76, ηp2= 0.03) and group x session (F(3,42)= 1.84, p= 0.15, ηp2= 0.11) interactions. However, this analysis did reveal a significant session x ratio interaction (F(3,12)= 3.41, p= 0.001, ηp2= 0.19), and therefore we performed two-way ANOVAs with repeated measures to further assess how the number of lever presses changed across sessions for every ratio (2, 5, 10, 20 and 31) in both groups. The two- way ANOVAs demonstrated increases in lever presses across sessions for ratio 2 (F(3,42)=6.76, p= 0.001, ηp2= 0.32), ratio 5 (F(3,42)= 4.89, p= 0.005, ηp2= 0.60) and ratio 10 (F(3,42)= 6.76, p= 0.001, ηp2= 0.32). They also revealed a session x group interaction for ratio 5 (price of 100 press/g) (F(3,42)= 3.39, p= 0.027, ηp2= 0.19) indicating that stressed animals pressed the lever more times than controls at this ratio. Specifically, stressed animals pressed the lever more times compared to controls 6 weeks after the last stress episode (t14= 2.86, p= 0.012, ηp2= 0.37; independent t test), when the price to receive rewards was low (Figure 4A).
Figure 4:
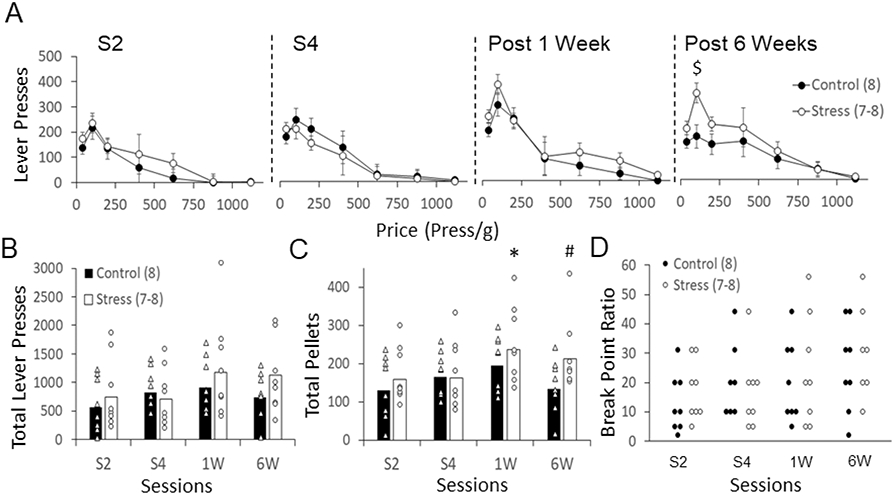
Progressive ratio task performance for control and stressed animals. (A) Lever presses at different prices (presses per grams of sugar) and across sessions (S2-Post 6 Weeks). Data are the mean ± SEM of the number of lever presses. (B) Total number of lever presses across sessions. Data are the mean ± SEM of the total number of lever presses. (C) Total number of pellets consumed across sessions. Data are the mean ± SEM of the total number of pellets consumed. (D) Break point ratio across sessions. Each dot represents one rat. Number of animals per group in parenthesis. $ p< 0.05 compared to control; * p< 0.05 compared to S4; # p= 0.056 compared to control.
We also analyzed whether social stress changed the total number of lever presses and the total number of pellets consumed across sessions by performing the corresponding two-way ANOVAs with group and sessions as between and within subjects, respectively. Figure 4B shows the total number of lever presses across sessions. There was an increase in the total number of lever presses across sessions (session effect: F(3,42)= 4.56, p= 0.007, ηp2= 0.24). However, this analysis did not detect overall group effects (F(1,14)= 0.66, p= 0.42, ηp2= 0.045) or significant session x group interaction (F(3,42)= 1.80, p= 0.161, ηp2= 0.11).
Figure 4C shows the total number of pellets consumed across sessions. Similar to total lever presses, there were not overall group effects (F(3,42)= 1.33, p= 0.26, ηp2= 0.09) or significant session x group interaction (F(3,42)= 2.27, p= 0.094, ηp2= 0.14), but there was a significant change in the number of pellets consumed across sessions (session effect: F(3,42)= 6.95, p= 0.001, ηp2= 0.33). This effect was significant for stressed (F(3,21)= 4.54, p= 0.013, ηp2= 0.39) and control (F(3,21)= 4.72, p= 0.011, ηp2= 0.40; one-way ANOVA with repeated measures) animals. However, a further analysis revealed that stressed animals, but not controls, consumed significantly more pellets at 6 weeks (stressed: t7= 2.64, p= 0.033; control: t7= 0.17, p= 0.860; paired t test) and 1 week (stressed: t7= 3.50, p= 0.010; control: t7= 1.59, p= 0.160; paired t test) compared to the previous sessions S2 and S4, respectively. Furthermore, there was a strong tendency for stressed animals to consume significantly more pellets than controls 6 weeks after the last stress episode (t13= 2.07, p= 0.056, independent t test).
Figure 4D shows the break point for stressed and control animals, which is the ratio (price) at which animals stop pressing the lever. We performed a two-way ANOVA with group and sessions as between and within subjects, respectively, and found that there were not significant differences for group (F(1,13)= 0.10, p= 0.75, ηp2= 0.008) or session x group interaction (F(3,39)= 0.39, p= 0.76, ηp2= 0.02), but there was a significant effect across sessions (F(3,39)= 3.64, p= 0.021, ηp2= 0.22). According to these results animals stop pressing the lever later at later sessions but both groups of animals stop pressing the lever at similar ratios (i.e., price).
DISCUSION
We assessed whether the exposure to intermittent episodes of social stress changes the effort that animals are willing to make to obtain rewards (i.e., sugar pellets). In the effort-discounting task, we found that ISD decreases the preference for larger rewards associated with a larger effort (i.e., more lever presses), which suggests that ISD biases animal’s choices to move away from costlier rewards. Yet these effects were observed 24-48 h after stress but not one week after the last stress episode. In addition, we found that ISD increases the number of lever presses and the total number of food pellets consumed during the progressive ratio task six weeks after stress, but not before, which suggests that ISD increases reward seeking in the long term. These results suggest that ISD produces different short- and long-term effects on the motivation for rewards and point to temporal dynamic adaptations in the function of the brain reward system that are relevant to better understand the transition from social adversity to drug abuse.
During the effort-discounting task animals are required to choose between low effort/low reward and high effort/high reward [10,18]; the high reward choice requires animals to press the lever more times in order to earn a larger reward (i.e. three sugar pellets instead of one). Food deprived rats value higher magnitude rewards more than lower magnitude. As expected, both control and stressed animals decreased their preference for the high reward option as the effort requirements increased from two to twenty lever presses. This effect paralleled an increase in the choice latency to the high reward lever. These results agree with previous studies using the same task [17,18], and confirm that animals are making their choices according to cost/benefit subjective evaluations.
Exposing animals to ISD decreased the preference for high rewards at higher ratios (i.e. higher prices) 24-48 h after the third and fourth stress episodes, which indicates that the effects of ISD on task performance are cumulative and depend on the effort involved in the choice. Similar effects have been found after acute restraint and chronic social stress [17,18,20]. Furthermore, these effects of ISD were time-dependent since they were not observed one week after stress. In addition, we also found that ISD did not change performance in the progressive ratio task 48 h after single stress episodes. The progressive ratio task evaluates whether animals are willing to work harder to earn rewards [12,14]. As shown, stressed and control animals made similar number of lever presses and put the same effort to obtain sugar pellets (i.e. lever presses). Taken together, our results indicate that ISD alters effort-based decision-making in the short term and biases animal’s behavior to avoid higher effort options when a lower effort (lower reward) option is concurrently available but does not change the motivation for rewards. These results resemble previous studies which suggest that stress reduces effort-related motivated behaviors [18,20,22].
In contrast to the short-term effects, the animals exposed to ISD were more motivated to seek rewards in the long term. Thus, stressed animals pressed the lever more times (at low ratios) to obtain rewards and consumed more sugar pellets than control animals, during the progressive ratio task. Importantly, these effects were observed six weeks after the termination of the stress protocol. In line with these results, it has been shown that chronic social defeat can increase the motivation for rewards ten weeks after stress [29]. The increased consumption of sugar pellets long after the termination of stress could be related to metabolic changes after ISD. Thus, it has been shown that chronic social stress alters metabolism and increases food consumption, and that this effect can be facilitated by using highly palatable foods like sugar [35,36]. However, it is currently unknown whether ISD and chronic social stress produce similar effects on metabolism.
The increased reward-seeking behavior after ISD shown here can be interpreted in the context of previous studies showing that ISD increases drug-self administration. Thus, by using the same ISD protocol as the one utilized here, studies have shown that ISD increases cocaine self-administration during a 24-h continuous access session (i.e. binge) several weeks after the last stress episode. Interestingly, the same studies show that ISD does not change the breakpoint for cocaine infusions in a progressive ratio schedule [5-7,37,38], which is in agreement with our results using food. In fact, as shown here, stressed animals did not “pay” higher prices for sugar pellets and stopped pressing the lever at similar ratios (i.e. breakpoint) compared to controls. These results suggest that stressed animals have more motivation to seek out food and drug rewards but not to work harder to obtain them. These results also point to long-term adaptations in the function of the brain reward system leading to an enhanced reward-seeking behavior. In this context, the enhanced seeking and consumption of rewards (i.e., food, drugs) after ISD might function as a stress-reliever, which ultimately could increase the risk for developing drug abuse [2,9,39].
At the mechanistic level, and based on previous work, ISD could alter effort-based reward seeking behavior by changing the activity of the hypothalamus-pituitary-adrenal (HPA) axis, and the release of the corticotropic-releasing-factor (CRF) and glucocorticoids [5,16,18,40]. Thus, previous studies suggest that increases of CRF during stress alter cost/benefit decision-making and decreases the preference for high magnitude rewards [18,41]. Similarly, it has been shown that the long-term administration of corticosterone biases animal’s behavior to low-cost rewards [22]. In addition, studies have also demonstrated that the activation of CRF receptors in the ventral tegmental area can increase drug-seeking behavior [5,8] and have suggested a link between ISD effects and long-term changes in the function of the mesolimbic dopamine system. Overall, these studies support the idea that both short- and long-term effects of ISD on effort-based reward-seeking behavior are produced by dynamic adaptations in the function of the HPA axis and the mesolimbic dopamine system. Critically, both the HPA axis (i.e., CRF, glucocorticoids) and the mesocorticolimbic dopamine system are involved in drug abuse vulnerability and substance use disorders [1,42-44].
Several methodological limitations should be considered for the present study. First, our experiments were performed in male rats and therefore the reported effects on effort-based reward-seeking behavior cannot be extended to both sexes. In fact, sex differences have been reported on reward seeking after social stress [20,45]. Second, some studies suggest that the effort-discounting task can be influenced by the order of experience on effort choice [46]. In the current study, both stressed and control animals experienced the task in the same order every session and therefore, it is difficult to know the actual impact of this factor on our results. Third, previous studies have shown that food-deprived animals can develop sucrose craving [36,47,48]. It is possible that food deprivation modulates performance in the progressive ration task by having a stronger impact on stressed compared to control animals. Yet whether food deprivation interacts with the effects of other treatments, such as stress, is uncertain and requires further investigation. Finally, our results show an important individual variability, especially for stressed animals, during task performance. Individual differences most likely reflect the complexity of the neurobiological and physiological mechanisms involved in the effects of stress [49], and ultimately suggest that our results should be interpreted cautiously since all animals are not equally affected by ISD [50,51].
In summary, our study shows that ISD produces time-dependent changes in effort-based reward-seeking behavior by decreasing the motivation for high magnitude rewards–associated with high effort, in the short term, and increasing the motivation to obtain and consume rewards in the long term. These results suggest dynamic changes in the function of the reward system (i.e., CRF, dopamine) during and after the repeated exposure to social stress. It can be hypothesized that the short-term effects on effort- based decision-making after ISD can predict the long-term effects on the motivation for rewards. For instance, a decrease in the preference for high rewards shortly after stress might be associated with lower risk to increasing reward-seeking behavior and, ultimately lower vulnerability to drug abuse, in the long term. Further studies will be needed to substantiate this hypothesis. Our findings are relevant to better understand the link between social adversity and vulnerability to drug abuse, which is essential to help predict and prevent the development of substance use disorders.
ACKNOWLEDGEMENTS
This study has been supported by NIH/NIGMS P30GM122733-01A1 (AD) and the Neuroscience Summer Research Education Program (CL) from the University of Mississippi.
Footnotes
DECLARATION OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sinha R, How does stress increase risk of drug abuse and relapse?, Psychopharmacology (Berl). 158 (2001) 343–359.. [DOI] [PubMed] [Google Scholar]
- [2].Koob GF, Schulkin J, Addiction and stress: An allostatic view, Neurosci. Biobehav. Rev 106 (2019) 245–262. [DOI] [PubMed] [Google Scholar]
- [3].Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y, Stress-induced reinstatement of drug seeking: 20 years of progress, Neuropsychopharmacology. 41 (2016) 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Logrip ML, Zorrilla EP, Koob GF, Stress modulation of drug self-administration: Implications for addiction comorbidity with post-traumatic stress disorder, Neuropharmacology. 62 (2012) 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, Miczek KA, Social Stress and CRF-Dopamine Interactions in the VTA: Role in Long-Term Escalation of Cocaine Self-Administration, J. Neurosci 34 (2014) 6659–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miczek KA, Nikulina EM, Shimamoto A, Covington HE, Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats., J. Neurosci 31 (2011) 9848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Covington HE, Miczek KA, Repeated social-defeat stress, cocaine or morphine: Effects on behavioral sensitization and intravenous cocaine self-administration “binges,” Psychopharmacology (Berl). 158 (2001) 388–398. [DOI] [PubMed] [Google Scholar]
- [8].Holly EN, Debold JF, Miczek KA, Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: Modulation by corticotropin releasing factor receptors in the ventral tegmental area, Psychopharmacology (Berl). 232 (2015) 4469–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tunstall BJ, Carmack SA, Social Stress-Induced Alterations in CRF Signaling in the VTA Facilitate the Emergence of Addiction-like Behavior, J. Neurosci 36 (2016) 8780–8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Salamone JD, Correa M, Ferrigno S, Yang J-H, Rotolo RA, Presby RE, The Psychopharmacology of Effort-Related Decision Making: Dopamine, Adenosine, and Insights into the Neurochemistry of Motivation, Pharmacol. Rev 70 (2018) 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boekhoudt L, Wijbrans EC, Man JHK, Luijendijk MCM, de Jong JW, van der Plasse G, Vanderschuren LJMJ, Adan RAH, Enhancing excitability of dopamine neurons promotes motivational behaviour through increased action initiation, Eur. Neuropsychopharmacol 28 (2018) 171–184. [DOI] [PubMed] [Google Scholar]
- [12].Lorrain DS, Arnold GM, Vezina P, Previous exposure to amphetamine increases incentive to obtain the drug: Long-lasting effects revealed by the progressive ratio schedule, Behav. Brain Res 107 (2000) 9–19. [DOI] [PubMed] [Google Scholar]
- [13].Wyvell CL, Berridge KC, Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward., J. Neurosci 21 (2001) 7831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang M, Balmadrid C, Kelley AE, Nucleus accumbens opioid, GABAergic, and dopaminergic modulation of palatable food motivation: Contrasting effects revealed by a progressive ratio study in the rat, Behav. Neurosci 117 (2003) 202–211. [DOI] [PubMed] [Google Scholar]
- [15].Hosking JG, Floresco SB, Winstanley CA, Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: A comparison of two rodent cost/benefit decision-making tasks, Neuropsychopharmacology. 40 (2015) 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hollon NG, Burgeno LM, Phillips PEM, Stress effects on the neural substrates of motivated behavior, Nat. Neurosci 18 (2015) 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shafiei N, Gray M, Viau V, Floresco SB, Acute stress induces selective alterations in cost/benefit decision-making., Neuropsychopharmacology. 37 (2012) 2194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bryce CA, Floresco SB, Perturbations in Effort-Related Decision-Making Driven by Acute Stress and Corticotropin-Releasing Factor, Neuropsychopharmacology. 101 (2016) 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wanat MJ, Bonci A, Phillips PEM, CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors, Nat. Neurosci 16 (2013) 383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dieterich A, Liu T, Samuels BA, Chronic non-discriminatory social defeat stress reduces effort-related motivated behaviors in male and female mice, Transl. Psychiatry 11 (2021) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Olausson P, Kiraly DD, Gourley SL, Taylor JR, Persistent effects of prior chronic exposure to corticosterone on reward-related learning and motivation in rodents, Psychopharmacology (Berl). 225 (2013) 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dieterich A, Stech K, Srivastava P, Lee J, Sharif A, Samuels BA, Chronic corticosterone shifts effort-related choice behavior in male mice, Psychopharmacology (Berl). 237 (2020) 2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spierling SR, Mattock M, Zorrilla EP, Modeling hypohedonia following repeated social defeat: Individual vulnerability and dopaminergic involvement, Physiol. Behav 177 (2017) 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U, Anhedonia and motivational deficits in rats: Impact of chronic social stress, Behav. Brain Res 162 (2005) 127–134. [DOI] [PubMed] [Google Scholar]
- [25].Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A, Enduring deficits in brain reward function after chronic social defeat in rats: Susceptibility, resilience, and antidepressant response, Biol. Psychiatry 76 (2014) 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barthas F, Hu MY, Siniscalchi MJ, Ali F, Mineur YS, Picciotto MR, Kwan AC, Cumulative Effects of Social Stress on Reward-Guided Actions and Prefrontal Cortical Activity, Biol. Psychiatry 88 (2020) 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Holmes A, Wellman CL, Stress-induced prefrontal reorganization and executive dysfunction in rodents, Neurosci. Biobehav. Rev 33 (2009) 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ortiz JB, Conrad CD, The impact from the aftermath of chronic stress on hippocampal structure and function: Is there a recovery?, Front. Neuroendocrinol 49 (2018) 114–123. [DOI] [PubMed] [Google Scholar]
- [29].Riga D, Theijs JT, De Vries TJ, Smit AB, Spijker S, Social defeat-induced anhedonia: effects on operant sucrose-seeking behavior, Front. Behav. Neurosci 9 (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sullivan L, Shaffer H, Hill C, Del Arco A, Time-dependent changes in cognitive flexibility performance during intermittent social stress: Relevance for motivation and reward-seeking behavior, Behav. Brain Res 370 (2019) 111972. [DOI] [PubMed] [Google Scholar]
- [31].Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A, Enduring deficits in brain reward function after chronic social defeat in rats: Susceptibility, resilience, and antidepressant response, Biol. Psychiatry 76 (2014) 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vasconcelos M, Stein DJ, de Almeida RMM, Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: a systematic review of the last decade, Trends Psychiatry Psychother. 37 (2015) 51–66. [DOI] [PubMed] [Google Scholar]
- [33].Cruz FC, Quadros IM, Hogenelst K, Planeta CS, Miczek KA, Social defeat stress in rats: Escalation of cocaine and “speedball” binge self-administration, but not heroin, Psychopharmacology (Berl). 215 (2011) 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans P.J. a, The resident-intruder paradigm: a standardized test for aggression, violence and social stress., J. Vis. Exp (2013) e4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell’Omo G, Parmigiani S, Palanza P, Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress, PLoS One. 4 (2009) e4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ulrich-Lai YM, Self-medication with sucrose, Curr. Opin. Behav. Sci 9 (2016) 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yap JJ, Chartoff EH, Holly EN, Potter DN, Carlezon WA, Miczek KA, Social defeat stress-induced sensitization and escalated cocaine self-administration: The role of ERK signaling in the rat ventral tegmental area, Psychopharmacology (Berl). 232 (2015) 1555–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boyson CO, Holly EN, Burke AR, Montagud-Romero S, DeBold JF, Miczek KA, Maladaptive choices by defeated rats: link between rapid approach to social threat and escalated cocaine self-administration, Psychopharmacology (Berl). 233 (2016) 3173–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, De Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP, Pleasurable behaviors reduce stress via brain reward pathways, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 20529–20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Covington HE, Miczek KA, Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: Dissociation from corticosterone activation, Psychopharmacology (Berl). 183 (2005) 331–340. [DOI] [PubMed] [Google Scholar]
- [41].Peciña S, Schulkin J, Berridge KC, Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: Paradoxical positive incentive effects in stress?, BMC Biol. 4 (2006) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tunstall BJ, Carmack SA, Koob GF, Vendruscolo LF, Dysregulation of brain stress systems mediates compulsive alcohol drinking, Curr. Opin. Behav. Sci 13 (2017) 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Volkow ND, Wise RA, Baler R, The dopamine motive system: Implications for drug and food addiction, Nat. Rev. Neurosci 18 (2017) 741–752. [DOI] [PubMed] [Google Scholar]
- [44].Kalivas PW, Ph D, Volkow ND, The Neural Basis of Addiction : A Pathology of Motivation and Choice, Am J Psychiatry. (2005) 1403–1413. [DOI] [PubMed] [Google Scholar]
- [45].Holly EN, Shimamoto A, Debold JF, Miczek KA, Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats, Psychopharmacology (Berl). 224 (2012) 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Knauss ZT, Filipovic M, Smith KA, Queener MM, Lubera JA, Bolden-Hall NM, Smith JP, Goldsmith RS, Bischoff JE, Miller MK, Cromwell HC, Effort-reward balance and work motivation in rats: Effects of context and order of experience, Behav. Processes 181 (2020) 104239. [DOI] [PubMed] [Google Scholar]
- [47].Grimm J, Barnes J, North K, Collins S, Weber R, A general method for evaluating incubation of sucrose craving in rats, J. Vis. Exp e3335 (2011) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Counotte D, Schiefer C, Shaham Y, O’Donnell P, Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats, Psychopharmacology (Berl). 231 (2014) 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sapolsky RM, Stress and the brain: individual variability and the inverted-U, Nat. Neurosci 18 (2015) 1344–1346. [DOI] [PubMed] [Google Scholar]
- [50].Shimamoto A, Holly EN, Boyson CO, Debold JF, Miczek KA, Individual differences in anhedonic and accumbal dopamine responses to chronic social stress and their link to cocaine self-administration in female rats, Psychopharmacology (Berl). 232 (2015) 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions, Cell. 131 (2007) 391–404. [DOI] [PubMed] [Google Scholar]


