Abstract
Objectives
The purpose of this study was to examine critical care continuous electroencephalography (cEEG) utilization and downstream anti‐seizure treatment patterns, their association with outcomes, and generate hypotheses for larger comparative effectiveness studies of cEEG‐guided interventions.
Methods
Single‐center retrospective study of critically ill patients (n = 14,523, age ≥18 years). Exposure defined as ≥24 h of cEEG and subsequent anti‐seizure medication (ASM) escalation, with or without concomitant anesthetic. Exposure window was the first 7 days of admission. Primary outcome was in‐hospital mortality. Multivariable analysis was performed using penalized logistic regression.
Results
One thousand and seventy‐three patients underwent ≥24 h of cEEG within 7 days of admission. After adjusting for disease severity, ≥24 h of cEEG followed by ASM escalation in patients not on anesthetics (n = 239) was associated with lower in‐hospital mortality (OR 0.76 [0.53–1.07]), though the finding did not reach significance. ASM escalation with concomitant anesthetic use (n = 484) showed higher odds for mortality (OR 1.41 [1.03–1.94]). In the seizures/status epilepticus subgroup, post cEEG ASM escalation without anesthetics showed lower odds for mortality (OR 0.43 [0.23–0.74]). Within the same subgroup, ASM escalation with concomitant anesthetic use showed higher odds for mortality (OR 1.34 [0.92–1.91]) though not significant.
Interpretation
Based on our findings we propose the following hypotheses for larger comparative effectiveness studies investigating the direct causal effect of cEEG‐guided treatment on outcomes: (1) cEEG‐guided ASM escalation may improve outcomes in critically ill patients with seizures; (2) cEEG‐guided treatment with combination of ASMs and anesthetics may not improve outcomes in all critically ill patients.
Introduction
Continuous Electroencephalography (cEEG) is frequently used in critical care to detect and guide treatment of seizures, monitor depth of sedation, and inform prognosis. 1 In recent years there has been an increase in critical care cEEG utilization, with reports of 33%/year increase in cEEG use nationally, and doubling of the number of hospitals utilizing cEEG. 2 Despite this increased utilization, there is limited and conflicting data on whether cEEG use improves outcomes. 2 , 3 , 4 , 5 , 6 , 7 , 8 Critically ill patients, including patients with acute brain dysfunction, receive complex care with numerous clinical confounding factors that present challenges in designing studies that assess diagnostic interventions, treatments, and outcomes. Randomized clinical trials in critical and neurocritical care are often infeasible, difficult to design due to the challenges of creating stringent inclusion and exclusion criteria, and do not reflect real‐world clinical practice. Not surprisingly, there is limited success of traditional randomized controlled trials in neurocritical care. 9 Therefore, an emphasis has been placed on comparative effectiveness research using high‐quality data from large collaborative administrative, healthcare utilization, and electronic health record (EHR) based datasets. 9 As a result, effectiveness of cEEG monitoring has been investigated through real‐world data from administrative and claims based databases and in pragmatic clinical trials. 2 , 3 , 4 Limitations of existing studies assessing cEEG effectiveness in critically ill patients include lack of inclusion of anti‐seizure treatment/downstream interventions, variable cEEG start time, variable duration of cEEG, and inclusion of cardiac arrest patients in whom cEEG is used primarily for prognostication. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9
In this study we used our local EHRs to examine cEEG utilization patterns and downstream treatment interventions in patients admitted across neurological, medical, and surgical intensive care units (ICUs). The primary objectives were to leverage real‐world observational data to (1) explore the association of post cEEG anti‐seizure medication (ASM) escalation with outcomes, (2) generate hypotheses on effectiveness of cEEG‐guided ASM treatment that can be tested in future studies, and (3) assesse the feasibility of using EHR and similar observational datasets for comparative effectiveness studies of cEEG‐guided ASM treatment.
Methods
This is a retrospective cohort study of patients admitted to six critical care units at our center between January 2016 and December 2019. The study was approved by the Institutional Review Board of Mass General Brigham (protocol number 2013P001024). Informed consent was not required for this retrospective study. We included patients with, (1) age ≥18 years; (2) admission for >7 days. We excluded patients with a diagnosis of cardiac arrest, and patients that were discharged or died prior to 7 days. Patients admitted to the epilepsy monitoring unit were not included in this study. Figure 1 displays the inclusion and exclusion flowchart.
Figure 1.
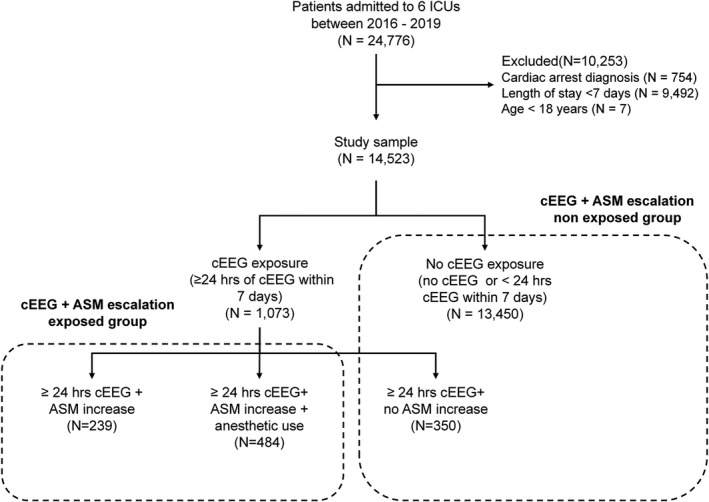
Inclusion, exclusion, and exposure flowchart. Inclusion and exclusion criteria along with exposed and unexposed groups are shown. ASM, anti‐seizure medication; cEEG, continuous electroencephalography; ICU, intensive care unit.
Clinical covariates
Demographic and clinical data were collected from EHRs. Admission data included ICU type (neurological, medical, or surgical), admitting service (neurology, neurosurgery, medical, and surgical specialties), admission pathway (emergent vs. elective), duration of hospital stay, and discharge disposition. Primary and secondary ICD 10 codes were recorded. The Elixhauser Comorbidity Index was calculated to measure baseline chronic health conditions. 10 Disease severity was measured by the admission Sequential Organ Failure Assessment (SOFA) score. 11
We recorded whether patients underwent cEEG monitoring, hospitalization day of cEEG initiation, and cEEG duration. ASM administration data including doses, dates, and times of administration were obtained. ASMs recorded included: phenytoin, fosphenytoin, phenobarbital, valproic acid, divalproex, levetiracetam, lacosamide, lamotrigine, carbamezapine, oxcarbezapine, clobazam, lorazepam, gabapentin, topirmate, diazepam, zonisamide, and clonazepam. Anesthetic medication infusion data including dose, date, and time of administration were recorded. Anesthetic infusions included propofol, midazolam, ketamine, and pentobarbital.
Exposure definitions
Continuous EEG (cEEG) exposure: We defined cEEG exposure as ≥24 h of continuous EEG monitoring initiated within 7 days of admission. We used a 24‐h cut‐off based on consensus statements that recommend at least 24 h of recording for diagnosis of nonconvulsive seizures, as the majority of nonconvulsive seizures can be identified in this time frame. 1 The likelihood of detecting epileptiform abnormalities and electrographic seizures, if not detected in the first 30 min to 2 h of recording, significantly decreases with increased monitoring duration, and drops to <5% after 16 h of monitoring. 1 , 12 We restricted the exposure window to the first 7 days of admission as this time window includes the at‐risk period for development of acute symptomatic seizures in patients with systemic illness or acute brain injuries. 13 Using strict criteria for cEEG duration and the exposure window also increase homogeneity of the exposure group. The unexposed group included patients who underwent no cEEG monitoring or <24 h of cEEG (Fig. 1).
Post cEEG ASM escalation: Among patients with ≥24 h of cEEG, ASM escalation was defined as initiation or increase in dose or number of ASMs between the cEEG start and stop times. Our objective was to capture ASM escalation that is, likely to be in response to cEEG findings. Therefore the escalation had to occur during the cEEG monitoring window and sustained for ≥48 h. As detailed above, we also used a cEEG threshold of 24 h to leverage the diagnostic yield of longer monitoring 1 , 12 and maximize the number of potential patients in the exposure group with the indication of ASM escalation likely to be in response to cEEG findings. These criteria resulted in a standardized and homogenous treatment exposure definition that can be refined in future comparative effectiveness studies. We divided post cEEG ASM escalation into two levels: (1) Initiation or increase in dose/number of ASMs for ≥48 h without concomitant use of anesthetics, (2) Initiation or increase in dose/number of ASMs for ≥48 h with concomitant use of any anesthetics. This categorization was used to capture the higher underlying illness severity in patients on anesthetics. Indications for anesthetics in these patients included sedation for intubation and procedures, mechanical ventilation management, and pharmacological sedation for treatment of seizures and refractory intracranial pressure. Exposed and unexposed groups are shown in Figure 1.
Outcomes
Our primary outcome measure was in‐hospital mortality. Our secondary outcome measure was prescription of ASMs at discharge. We selected our secondary outcome measure to determine the potential impact of cEEG use on long‐term continuation of ASMs.
Statistical analysis
Mean, standard deviation, median, and lower and upper quartiles were calculated for descriptive statistics. Univariate analyses were performed using Chi‐squared tests for dichotomized and categorical variables, and the Mann‐Whitney U‐test for continuous variables. Significance was set at 0.05, and 2‐sided p values are reported.
Multivariable analysis was performed using elastic‐net logistic regression to predict the likelihood of outcomes among patients receiving ≥24 h of cEEG and subsequent ASM escalation, with or without concomitant anesthetic use, compared to patients receiving no cEEG monitoring or <24 h of cEEG monitoring. The elastic‐net model combines L1 and L2 penalties and was chosen for the analysis as it performs automatic covariate selection and can reduce both multi‐collinearity and model over‐fitting. Candidate covariates for the regression analysis are shown in Tables S1 and S2. We included baseline clinical variables, comorbid conditions (Elixhauser score), measures of disease severity (e.g., SOFA score and admission pathway), and underlying diagnostic category. To account for differences in length of hospital stay, we also included length of hospitalization from day 7 (post exposure window) until discharge.
Nested 10‐fold cross‐validation (CV) was performed for model validation and tuning hyper‐parameters. First, the data were split into 10 groups and for each unique fold of CV, one group was selected as a testing set and the rest were used as training sets. Grid search‐based hyper‐parameter tuning was performed with the help of 10‐fold inner CV on the outer CV training data. In the inner CV training data, the majority class was down‐sampled to the size of the minority class to balance the data. Optimal hyper‐parameters for the elastic‐net model were selected using the maximum mean area under the receiver operating curve (AUROC). The elastic‐net model was trained on the optimal hyper‐parameters from the grid search on the balanced outer CV training data and the model was evaluated on the outer CV test set using AUROC. The 10‐fold outer CV cycle yielded 10 different elastic‐net models. The best hyper‐parameters were selected by averaging the mean AUROC over the 10‐fold outer CV. 1000 rounds of bootstrapping were performed to estimate confidence intervals. The optimal parameters from the nested 10‐fold CV were used to train the final elastic‐net model on the complete balanced bootstrapped data. For each iteration of bootstrapping, the coefficients of the model were recorded. Once bootstrapping was completed, the mean odds ratio and 95% confidence interval were calculated.
Subgroup analysis
We developed regression models to quantify the association between cEEG and ASM exposure with respect to the outcomes of interest within each of three diagnostic categories based on primary and secondary diagnosis ICD 10 codes: (1) Seizures/status epilepticus (ICD codes: G40.x, R56.x, I69.398) (2) Primary neurologic diagnosis excluding seizures (specific codes within: G00–G99 excluding G40.x, I60–I69, C70–72, D32–33, D35.2–4, Q85.x, F01–3, S00–S09, R25–29, R40–46, R47–49, C79.3–C79.4, R40.x, R51.x, R.20x; specific codes: I77.71, I77.74, E51.2, Q28.2) (3) Altered mental status (specific codes within: G93.4x, G92.x, I67.4, R41.x, F05.x, R40.x, F10–F16, F18–F19; code E51.2). Diagnostic categories 1 and 2 were mutually exclusive. The diagnostic category of altered mental status was not mutually exclusive and included patients with simultaneous diagnosis of seizures or other primary neurologic diagnosis.
Results
Overall, 14,523 patients met inclusion criteria: 1073 (7.39%) patients underwent ≥24 h of cEEG within the first 7 days of admission, and 13,450 underwent no EEG or <24 h of EEG within the first 7 days of admission. Table 1 summarizes the clinical and demographic variables. Patients who underwent ≥24 h of cEEG monitoring had higher illness severity as measured by SOFA scores, and more comorbid illnesses as measured by the Elixhauser index. Patients with seizure or primary neurological diagnosis were more likely to undergo ≥24 h of cEEG monitoring. Patients admitted to neurology and neurosurgical specialties were also more likely to undergo ≥24 h of cEEG monitoring. Patients who underwent ≥24 h of cEEG monitoring had longer lengths of hospital stay.
Table 1.
Clinical and demographic variables.
| Variable |
Total N = 14,523 |
≥24 h of cEEG N = 1073 |
No or <24 h of cEEG N = 13,450 |
p‐value |
|---|---|---|---|---|
| Age (median, Q1–Q3) | 65 [55–75] | 64 [51–73] | 66 [55–75] | <0.0001 |
| Gender, female, n (%) | 5755 (39.63%) | 528 (49.21%) | 5227 (38.88%) | <0.0001 |
| Race, n (%) | ||||
| African American | 823 (5.67%) | 77 (7.18%) | 746 (5.55%) | 0.0005 a |
| American Indian/Alaska Native | 13 (0.09%) | 0 (0.00%) | 13 (0.1%) | |
| Asian | 476 (3.28%) | 41 (3.82%) | 435 (3.23%) | |
| Native Hawaiian/Other pacific Islander | 2 (0.01%) | 0 (0.00%) | 2 (0.01%) | |
| Other | 756 (5.21%) | 52 (4.85%) | 704 (5.23%) | |
| Two or more | 26 (0.18%) | 1 (0.09%) | 25 (0.19%) | |
| Unknown | 813 (5.60%) | 99 (9.23%) | 714 (5.31%) | |
| White | 11,614 (79.97%) | 803 (74.84%) | 10,811 (80.38%) | |
| Ethnicity | ||||
| Hispanic | 860 (5.92%) | 62 (5.78%) | 798 (5.93%) | <0.0001 |
| Non‐Hispanic | 12,184 (83.89%) | 852 (79.40%) | 11,332 (84.25%) | |
| Unknown | 1479 (10.18%) | 159 (14.82%) | 1320 (9.81%) | |
| Elixhauser score (median, IQR) | 3 [0–7] | 5 [5–6] | 0 [0–7] | <0.0001 |
| SOFA score (median, IQR) | 3 [1–6] | 6 [2–8] | 3 [1–6] | <0.0001 |
| Diagnostic category b | ||||
| Seizure/status epilepticus | 1560 (10.74%) | 844 (78.66%) | 716 (5.32%) | <0.0001 |
| Neurologic non‐seizure | 2603 (17.92%) | 182 (16.96%) | 2421 (18.00%) | |
| Non‐neurologic diagnosis | 10,360 (71.34%) | 47 (4.38%) | 10,313 (76.68%) | |
| Altered mental status | 1034 (7.12%) | 184 (17.15%) | 850 (6.32%) | <0.0001 |
| Admission unit, n (%) | ||||
| Neuro ICU | 2332 (16.06%) | 761 (70.92%) | 1571 (11.68%) | <0.0001 |
| Medical ICUs | 3812 (26.25%) | 115 (10.72%) | 3697 (27.49%) | |
| Surgical ICUs | 7105 (48.92%) | 109 (10.16%) | 6996 (52.01%) | |
| Multi Departments c | 1274 (8.77%) | 88 (8.20%) | 1186 (8.82%) | |
| Admission service, n (%) | ||||
| Neurology | 1293 (8.90%) | 410 (38.21%) | 883 (6.57%) | <0.0001 |
| Neurosurgery | 1170 (8.06%) | 362 (33.74%) | 808 (6.01%) | |
| Medical specialties | 5753 (39.61%) | 215 (20.04%) | 5538 (41.17%) | |
| Surgical specialties | 6307 (43.43%) | 86 (8.01%) | 6221 (46.25%) | |
| Admission triage, n (%) | ||||
| Elective | 2425 (16.70%) | 79 (7.36%) | 2346 (17.44%) | <0.0001 |
| Urgent | 2905 (20.00%) | 225 (20.97%) | 2680 (19.93%) | |
| Emergency | 9193 (63.30%) | 769 (71.67%) | 8424 (62.63%) | |
| ASM use during the first 7 days of admission (exposure window) | ||||
| Any ASM use during first 7 days | 7126 (49.07%) | 979 (91.24%) | 6147 (45.70%) | |
| ASM treatment for <48 h | 1851 (12.75%) | 119 (11.09%) | 1732 (12.88%) | <0.0001 |
| ASM treatment for ≥48 h | 5275 (36.32%) | 860 (80.15%) | 4415 (32.83%) | |
| ASM treatment duration in the first 7 days (median [Q1–Q3]) | 6 [2–7] | 7 [5–8] | 5 [2–7] | |
| Preadmission ASM (%) | 1005 (6.92%) | 68 (6.34%) | 937 (6.97%) | 0.47 |
| Anesthetic use during the first 7 days of admission (exposure window) | ||||
| Any anesthetic use during first 7 days | 8242 (56.75%) | 775 (72.23%) | 7467 (55.52%) | <0.0001 |
| Anesthetic treatment for <48 h | 4972 (34.24%) | 279 (26.00%) | 4693 (34.89%) | |
| Anesthetic treatment for ≥48 h | 3270 (22.52%) | 496 (46.23%) | 2774 (20.62%) | |
| Anesthetic treatment duration in the first 7 days (median [Q1–Q3]) | 2 [1–4] | 4 [2–6] | 2 [1–3] | |
| Discharge disposition | ||||
| Home | 6218 (42.81%) | 205 (19.11%) | 6013 (44.71%) | <0.0001 |
| Rehab | 2059 (14.18%) | 298 (27.77%) | 1761 (13.09%) | |
| Long‐term acute care | 1457 (10.03%) | 150 (13.98%) | 1307 (9.72%) | |
| Skilled nursing facility | 2525 (17.39%) | 130 (12.12%) | 2395 (17.81%) | |
| Hospice | 374 (2.58%) | 47 (4.38%) | 327 (2.43%) | |
| Other hospital acute care | 274 (1.89%) | 30 (2.80%) | 244 (1.81%) | |
| Discharge mortality | 1616 (11.13%) | 213 (19.85%) | 1403 (10.43%) | |
| Length of hospital stay (median days, IQR) | 13 [10–21] | 16 [11–25] | 13 [9–21] | <0.0001 |
Abbreviations: ASM, anti‐seizure medications; cEEG, continuous electroencephalography; ICU, intensive care unit; IQR, inter‐quartile range; SOFA, Sequential Organ Failure Assessment score on admission.
Fisher’s exact test.
Diagnostic category defined by primary and secondary diagnosis.
Patients transferred across departments during hospitalization.
Table S3 summarizes clinical and demographic variables across patients with exposure to post cEEG ASM escalation. Patients that received ASM escalation with concomitant anesthetic use had higher illness severity compared to those that received ASM escalation without anesthetic use. Length of stay was not significantly different comparing patients with no ASM escalation to those with ASM escalation without anesthetics. However, patients that received ASM escalation with concomitant anesthetic use had significantly longer lengths of stay.
Among patients undergoing ≥24 h of cEEG, 212 (19.76%) had electrographic seizures and 735 (68.50%) had periodic or rhythmic patterns on EEG (including generalized and lateralized period discharges, and generalized and lateralized rhythmic delta activity). Table 2 shows the frequency of electrographic seizures and periodic and rhythmic patterns in patients that underwent ASM escalation. Across all diagnostic categories, periodic and rhythmic patterns were more frequent than electrographic seizures. Interestingly there was no significant difference in the frequency of electrographic seizures and periodic or rhythmic patterns when comparing patients that received ASM escalation only versus ASM escalation with concomitant anesthetic use (Table S3).
Table 2.
Frequency of electrographic seizures and periodic and rhythmic patterns in patients exposed to ASM escalation.
| N (%) | |
|---|---|
| All patients exposed to post cEEG ASM escalation | N (% of 1073) |
| Electrographic seizures | 212 (19.76%) |
| Periodic and rhythmic patterns | 735 (68.50%) |
| Seizures/status epilepticus exposed to post cEEG ASM escalation | N (% of 601) |
| Electrographic seizures | 174 (28.95%) |
| Periodic and rhythmic patterns | 458 (76.21%) |
| Neurologic non‐seizure patients exposed to post cEEG ASM escalation | N (% of 105) |
| Electrographic seizures | 19 (18.10%) |
| Periodic and rhythmic patterns | 66 (62.86%) |
| Altered mental status patients exposed to post cEEG ASM escalation | N (% of 123) |
| Electrographic seizures | 35 (28.46%) |
| Periodic and rhythmic patterns | 94 (76.42%) |
Frequency of electrographic seizures and periodic and rhythmic patterns in patients exposed to post cEEG ASM escalation with and without concomitant anesthetics. ASM, anti‐seizure medication; cEEG, continuous electroencephalography.
In patients receiving ASM escalation, the median time to treatment escalation was 5.23 h [Q1–Q3=2.29–9.04]. Among patients with seizures/status the median time to ASM escalation was 4.97 h [Q1–Q3=2.17–8.93]. Among patients with neurological non‐seizure diagnosis, the median time to ASM escalation was 6.11 h [Q1–Q3=3.27–9.92].
Primary outcomes
Figure 2 shows the odds for in‐hospital mortality with cEEG use and ASM escalation after multivariable analysis. Across the cohort, after adjusting for clinical covariates, ≥24 h of cEEG followed by ASM escalation without concomitant anesthetic use was associated with lower risk of in‐hospital mortality compared to patients receiving no cEEG monitoring or <24 h of cEEG monitoring (OR 0.76 [0.53–1.07]), though this finding did not achieve significance. Patients who underwent ASM escalation with concomitant anesthetic had higher odds for in‐hospital mortality, compared to unexposed patients (OR 1.41 [1.03–1.94]).
Figure 2.
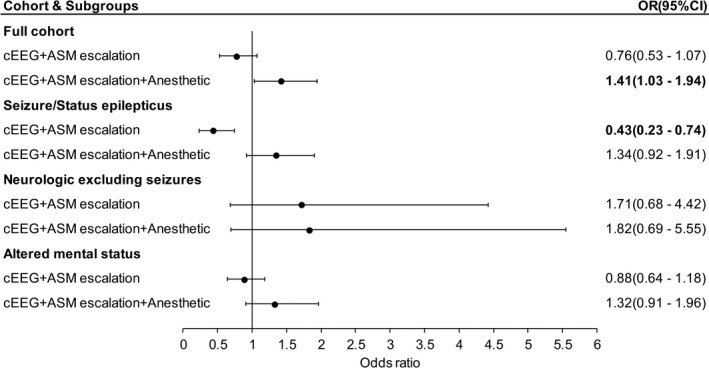
In‐hospital mortality with cEEG use and ASM escalation. Forest plot showing odds ratios for in‐hospital mortality with ≥24 h of cEEG and downstream ASM escalation +/− anesthetics, after multivariable analysis. Outcomes in the entire cohort, as well as subgroups are shown. ASM, anti‐seizure medication; cEEG, continuous electroencephalography; OR (95% CI), odds ratio (95% confidence interval).
When we restricted the analysis to patients with a primary diagnosis of seizures/status epilepticus, post cEEG ASM escalation without anesthetics was associated with lower odds for in‐hospital mortality (OR 0.43 [0.23–0.74]). Within the same subgroup, post cEEG ASM escalation with concomitant anesthetic use was associated with higher odds for in‐hospital mortality (OR 1.34 [0.92–1.91]), though not significant. Among patients with a primary neurological diagnosis excluding seizures, and in patients with altered mental status ASM escalation was not significantly associated with outcomes.
Among patients with ASM escalation and concomitant anesthetic use, we also compared differences in outcomes based on the timing of anesthetic administration. Three hundred and seventy‐nine (78.31%) received anesthetics prior to cEEG initiation. Patients who received anesthetics after cEEG initiation had increased mortality compared to those receiving anesthetics prior to cEEG initiation, after adjusting for baseline clinical covariates and disease severity (OR 1.88 [1.07–3.78]). Finally, among patients with ASM escalation and anesthetic use, we found no difference in outcomes between patients receiving <48 h of anesthetics versus those who receiving >48 h of anesthetics (OR 1.08 [0.96–1.27]).
We performed several additional sensitivity analysis detailed in the Supplemental material. In all the analysis we found similar trends where cEEG+ASM escalation showed lower odds for in‐hospital mortality, whereas cEEG+ASM escalation with concomitant anesthetics showed higher odds for in‐hospital mortality.
Secondary outcomes
After adjusting for clinical covariates, patients who underwent ≥24 h of cEEG within 7 days of admission were more likely to be discharged on ASMs when compared with patients who did not undergo cEEG or underwent <24 h of cEEG (Fig. 3). This association held in the subgroup analysis across all disease diagnostic categories.
Figure 3.
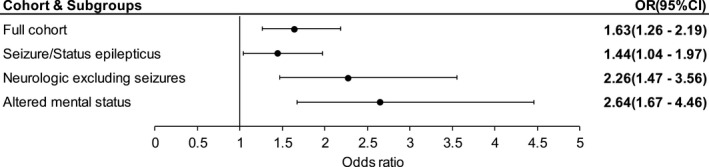
Discharge ASM prescription with cEEG use. Forest plot showing odds ratios for ASM prescription at discharge with ≥24 h of cEEG within 7 days, after multivariable analysis. Discharge ASM prescription in the entire cohort, as well as subgroups are shown. ASM, anti‐seizure medication; cEEG, continuous electroencephalography; OR (95% CI), odds ratio (95% confidence interval).
Discussion
In this hospital data driven study, we found that in patients with seizures/status epilepticus, exposure to ≥24 h of cEEG monitoring and downstream ASM escalation in the absence of anesthetics were associated with lower in‐hospital mortality. Exposure to ≥24 h of cEEG monitoring and downstream ASM escalation, was associated with a similar trend toward lower mortality across the larger cohort of neurological, medical, and surgical patients, although did not reach significance. In patients receiving anesthetics (indicative of more severe illness), exposure to ≥24 h of cEEG, and downstream ASM escalation was associated with higher in‐hospital mortality across the entire cohort. Furthermore, among these patients, those who had anesthetic initiation downstream from cEEG had higher mortality. Although these findings based on observational data explore associations and not causation and may still be biased by unmeasured confounding, they suggest the hypothesis that in certain ICU patients’ treatment of cEEG abnormalities with ASMs in combination with anesthetics may not improve outcomes. However, there may not be the same concerns for cEEG‐guided lower intensity/less aggressive ASM treatment, particularly in patients with recent or acute seizures.
In patients with seizures/status, ASM escalation with concomitant anesthetics was associated with increased risk of in‐hospital mortality, though the findings did not reach statistical significance. While our analysis adjusted for illness severity, this relation with outcomes may still be explained by higher illness severity, and higher likelihood of refractory and super‐refractory status epilepticus. At the same time, the adverse effects of anesthetics, risk‐benefit ratio, and their direct impact on outcomes in patients with seizures/status need to be explored further. Currently there is not enough evidence to guide management of refractory and super‐refractory status epilepticus. 14 , 15 While guidelines suggest use of anesthetics, this is based on low quality evidence (Level U). 14 Multiple series and cohort studies have found that aggressive treatment with anesthetics do not appear to improve outcomes, and may also worsen outcomes. 16 , 17 Our study did not differentiate between indications of anesthetics, and used anesthetics as another marker for illness severity. Larger comparative effectiveness studies are indicated to delineate the causal effect of cEEG‐guided anesthetic treatment on outcomes in patients with seizures and status epilepticus.
In the subgroup of neurological patients that did not have a seizure diagnosis ASM escalation both with and without anesthetics was associated with increased odds for in‐hospital mortality, though the finding did not reach significance. Reasons for ASM escalation in these patients likely include electrographic periodic and rhythmic patterns. In our entire cohort, and across all diagnostic categories, periodic, and rhythmic patterns were more frequent than electrographic seizures. Physicians frequently increase ASMs in response to periodic and rhythmic patterns, with considerable practice variation. 18 , 19 , 20 Based on our findings, we hypothesize that not all patients with periodic and rhythmic patterns may benefit from ASM escalation. Periodic and rhythmic patterns with higher frequency and prevalence are associated with a higher risk for seizures and secondary brain injury. 21 , 22 , 23 Therefore, ASM escalation may only be associated with improved outcomes in patients with high frequency periodic and rhythmic patterns, and in patients presenting with acute seizures. Future studies are indicated to test this hypothesis.
Finally among patients with altered mental status, we did not find any significant association between exposures to cEEG followed by ASM escalation, with in‐hospital mortality. While this diagnostic category also included patients with seizures, patients with delirium and encephalopathy secondary to metabolic derangements and primary medical/systemic illnesses were included in this subgroup. In altered mental status patients with metabolic derangements and cEEG abnormalities, particularly those not presenting with acute clinical seizures, studies are indicated to determine whether correcting the underlying metabolic derangement alone versus also treating with low‐dose ASMs result in improved outcomes.
Prior studies have shown that patients undergoing continuous EEG monitoring have longer lengths of stay. 2 In our cohort we found that patients with ≥24 h of cEEG within the first week of admission had greater lengths of stay compared with patients with no cEEG or <24 h of cEEG. However when examining post cEEG interventions, there was no significant difference in length of stay between patients with no treatment escalation and those who received ASM escalation in the absence of anesthetic use. Patients who received both ASM and anesthetics had longer lengths of stay. This may be a result of both higher illness severity and combined anesthetic/ASM‐related adverse effects.
Patients undergoing ≥24 h of cEEG monitoring were more likely to be discharged on ASMs. This association was significant across all diagnostic categories. This finding is similar to prior studies that show patients receiving cEEG are more likely to receive downstream ASMs and be discharged on ASMs. 4 , 24 Long‐term ASM use is associated with adverse effects including cognitive slowing, impaired balance and gait instability, and sedation. 25 , 26 , 27 , 28 , 29 , 30 This increases the impetus to not only determine whether acute treatment of EEG findings with ASMs improves outcomes, but also to determine whether post discharge continuation of ASMs impacts long‐term cognitive and functional outcomes. Across all our analysis, we found that patients who do not have a diagnosis of seizures showed increased odds for in‐hospital mortality with ASM escalation. Often patients are empirically started on ASM prophylaxis based on a clinical concern for seizures. In such patients, cEEG may not only guide ASM escalation, but also ASM weaning or discontinuation if seizures are ruled out and treatment is no longer indicated.
Limitations of our study include its retrospective nature. As this is a single center study, our findings may not generalize. We used hospitalization ICD 10 codes for determining hospitalization diagnosis, and categorization of subgroups which may result in information bias, and may capture suspected or clinically unconfirmed diagnosis. With regards to our secondary outcomes, we did not differentiate between indications for discharge ASM. Finally, we did not differentiate between indications for anesthetic. Although we adjusted for illness severity and underlying diagnosis, residual unmeasured confounding limits the ability to draw causal conclusions. However, the primary purpose of the study was to use real‐world data to generate causal hypotheses on effectiveness of post cEEG treatment interventions that can be investigated in larger comparative studies.
In this study of real‐world data, we found that in critically ill patients with seizures who are not on anesthetics, exposure to ASM escalation after cEEG monitoring showed lower odds for in‐hospital mortality. In patients with higher illness severity, and those requiring anesthetics, post cEEG ASM escalation may not be associated with improved outcomes. Based on our findings we propose larger comparative effectiveness studies to test the following hypotheses: (1) in critically ill patients presenting with non‐refractory seizures, cEEG‐guided ASM treatment may result in improved outcomes compared with non cEEG‐guided ASM treatment, and (2) cEEG‐guided ASM escalation or high intensity treatment in all patients with periodic and rhythmic patterns may not improve outcomes. Further studies are also needed to disentangle the independent effect of post cEEG ASM escalation, underlying illness severity and anesthetic use on outcomes in patients with higher illness severity, and refractory seizures. Randomized clinical trials to address these questions may be infeasible, challenging to recruit in and would not capture real‐world clinical care. Our study demonstrates that EHR and observational datasets can provide sufficient sample sizes to address varying exposures and interventions of interest. Application of robust statistical analysis and advanced causal inference methods in comparative studies using large observational real‐world datasets, can pave the way to identify which patients benefit the most from cEEG and cEEG‐guided ASM treatment. 31
Author Contributions
Rajesh Amerineni: designed the study, performed data collection and management, performed analysis and drafted the original, revised and final manuscript. Haoqi Sun: designed the analysis reviewed and revised, and the final manuscript. Hang Lee: performed data analysis and critically reviewed, and revised the manuscript. John Hsu: designed the study and critically reviewed, and revised the manuscript. Elisabettal Patorno: designed the study and critically reviewed, and revised the manuscript. M. Brandon Westover: designed the study and analysis, critically reviewed, and revised the manuscript. Sahar F. Zafar: conceptualized and designed the study, performed analysis drafted, reviewed, and revised the manuscript.
Conflict of Interest
MBW is a cofounder of Beacon Biosignals unrelated to this work. All other authors report no conflict of interest.
Supporting information
Table S1. Candidate covariates for multivariable regression analysis for primary outcome (in‐hospital mortality).
Table S2. Candidate covariates for multivariable regression analysis for discharge ASM.
Table S3. Clinical and demographic variables across patients with exposure to post cEEG ASM escalation +/− anesthetics.
Acknowledgment
This study received research support from NIH K23NS114201 (SFZ).
Funding Information
This study received research support from NIH K23NS114201.
Funding Statement
This work was funded by NIH grant K23NS114201.
References
- 1. Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ney JP, van der Goes DN, Nuwer MR, et al. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005–2009. Neurology. 2013;81(23):2002–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hill CE, Blank LJ, Thibault D, et al. Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology. 2019;92(1):e9–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossetti AO, Schindler K, Sutter R, et al. Continuous vs routine electroencephalogram in critically ill adults with altered consciousness and no recent seizure: a multicenter randomized clinical trial. JAMA Neurol. 2020;77(10):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khawaja AM, Wang G, Cutter GR, Szaflarski JP. Continuous electroencephalography (cEEG) monitoring and outcomes of critically ill patients. Med Sci Monit. 2017;23:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eskioglou E, Stähli C, Rossetti AO, Novy J. Extended EEG and non‐convulsive status epilepticus: benefit over routine EEG? Acta Neurol Scand. 2017;136(3):272–276. [DOI] [PubMed] [Google Scholar]
- 7. Alvarez V, Sierra‐Marcos A, Oddo M, Rossetti AO. Yield of intermittent versus continuous EEG in comatose survivors of cardiac arrest treated with hypothermia. Crit Care. 2013;17(5):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fatuzzo D, Beuchat I, Alvarez V, et al. Does continuous EEG influence prognosis in patients after cardiac arrest? Resuscitation. 2018;132:29–32. [DOI] [PubMed] [Google Scholar]
- 9. Lazaridis C, Maas AI, Souter MJ, et al. Alternative clinical trial design in neurocritical care. Neurocrit Care. 2015;22(3):378–384. [DOI] [PubMed] [Google Scholar]
- 10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 11. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 2015;126(3):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671–675. [DOI] [PubMed] [Google Scholar]
- 14. Glauser T, Shinnar S, Gloss D, et al. Evidence‐based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. [DOI] [PubMed] [Google Scholar]
- 16. Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol. 2005;62(11):1698–1702. [DOI] [PubMed] [Google Scholar]
- 17. Lin J‐J, Chou C‐C, Lan S‐Y, et al. Therapeutic burst‐suppression coma in pediatric febrile refractory status epilepticus. Brain Dev. 2017;39(8):693–702. [DOI] [PubMed] [Google Scholar]
- 18. Zafar SF, Postma EN, Biswal S, et al. Effect of epileptiform abnormality burden on neurologic outcome and antiepileptic drug management after subarachnoid hemorrhage. Clin Neurophysiol. 2018;129(11):2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alvarez V, Ruiz AAR, LaRoche S, et al. The use and yield of continuous EEG in critically ill patients: a comparative study of three centers. Clin Neurophysiol. 2017;128(4):570–578. [DOI] [PubMed] [Google Scholar]
- 20. Sivaraju A, Gilmore EJ. Understanding and managing the ictal‐interictal continuum in neurocritical care. Curr Treat Options Neurol. 2016;18(2):8. [DOI] [PubMed] [Google Scholar]
- 21. Ruiz AR, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74(2):181–188. [DOI] [PubMed] [Google Scholar]
- 22. Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016;79(4):579–590. [DOI] [PubMed] [Google Scholar]
- 23. Witsch J, Frey H‐P, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency‐dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol. 2009;66(6):723–728. [DOI] [PubMed] [Google Scholar]
- 25. Baker GA, Jacoby A, Buck D, et al. Quality of life of people with epilepsy: a European study. Epilepsia. 1997;38(3):353–362. [DOI] [PubMed] [Google Scholar]
- 26. Brodie MJ, Richens A, Yuen AWC; Group ULMT . Double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet. 1995;345(8948):476–479. [DOI] [PubMed] [Google Scholar]
- 27. Perucca P, Carter J, Vahle V, Gilliam FG. Adverse antiepileptic drug effects: toward a clinically and neurobiologically relevant taxonomy. Neurology. 2009;72(14):1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon SJ, Joo J‐Y, Kim YB, et al. Effects of prophylactic antiepileptic drugs on clinical outcomes in patients with a good clinical grade suffering from aneurysmal subarachnoid hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2015;17(3):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005;36(3):583–587. [DOI] [PubMed] [Google Scholar]
- 30. Naidech AM, Beaumont J, Muldoon K, et al. Prophylactic seizure medication and health‐related quality of life after intracerebral hemorrhage. Crit Care Med. 2018;46(9):1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Candidate covariates for multivariable regression analysis for primary outcome (in‐hospital mortality).
Table S2. Candidate covariates for multivariable regression analysis for discharge ASM.
Table S3. Clinical and demographic variables across patients with exposure to post cEEG ASM escalation +/− anesthetics.


