Abstract
Objective
To evaluate changes over 3 years in the thickness of inner retinal layers including the peripapillary retinal nerve fiber layer (pRNFL), and combined macular ganglion cell and inner plexiform layers (mGCIPL), in individuals with relapsing‐remitting multiple sclerosis (RRMS) versus healthy controls; to determine whether optical coherence tomography (OCT) is sufficiently sensitive and reproducible to detect small degrees of neuroaxonal loss over time that correlate with changes in brain volume and disability progression as measured by the Expanded Disability Status Scale (EDSS).
Methods
Individuals with RRMS from 28 centers (n = 333) were matched with 64 healthy participants. OCT scans were performed on Heidelberg Spectralis machines (at baseline; 1 month; 6 months; 6‐monthly thereafter).
Results
OCT measurements were highly reproducible between baseline and 1 month (intraclass correlation coefficient >0.98). Significant inner retinal layer thinning was observed in individuals with multiple sclerosis (MS) compared with controls regardless of previous MS‐associated optic neuritis––group differences (95% CI) over 3 years: pRNFL: −1.86 (−2.54, −1.17) µm; mGCIPL: −2.03 (−2.78, −1.28) µm (both p < 0.0001; effect sizes 0.39 and 0.34). Greater inner retinal layer atrophy was observed in individuals diagnosed with RRMS <3 years versus >5 years (pRNFL: p < 0.05; mGCIPL: p < 0.01). Brain volume decreased by 1.3% in individuals with MS over 3 years compared to 0.5% in control subjects (effect size 0.76). mGCIPL atrophy correlated with brain atrophy (p < 0.0001). There was no correlation of OCT data with disability progression.
Interpretation
OCT has potential to estimate rates of neurodegeneration in the retina and brain. The effect size for OCT, smaller than for magnetic resonance imaging based on Heidelberg Spectralis data acquired in this study, was increased in early disease.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system, characterized by inflammation, demyelination, gliosis, and axonal loss. 1 While the inflammatory aspect is routinely monitored in clinical practice using conventional magnetic resonance imaging (MRI), easily accessible biomarkers of neurodegeneration are needed to monitor disease progression and assess the efficacy of putative neuroprotective drugs. 2 , 3 One such potential candidate is an imaging biomarker that is found in the retina, namely, peripapillary retinal nerve fiber layer (pRNFL). 4 , 5 , 6 , 7 Because the retina is unmyelinated, measurements of retinal layer thinning are not confounded by myelin and are thereby ideal for assessing neuroaxonal degeneration. Both the pRNFL, containing unmyelinated axons emerging from retinal ganglion cell neurons, and the combined macular ganglion cell and inner plexiform layers (mGCIPL), containing retinal ganglion cell bodies (i.e., from which the axons forming pRNFL originate) and dendrites may show abnormal thinning over time and their atrophy is a sequelae of optic neuritis and optic neuropathy. 8 Several MS‐related studies have shown that thinning of pRNFL and mGCIPL is associated with increased clinical disability and brain volume loss. 5 , 9 , 10 , 11 , 12 , 13 , 14 , 15
Optical coherence tomography (OCT) noninvasively captures cross‐sectional images of the retina to generate three‐dimensional high‐resolution maps of the retinal architecture. One significant advantage of OCT compared with MRI is that it can be more sensitive in detecting small increments of neurodegeneration over time owing to its spatial resolution on the order of 5 microns, approximately 2–3 orders of magnitude better than MRI.
OCT may detect, and follow longitudinally, the degeneration of retinal axons and neurons by measuring change in pRNFL and mGCIPL thickness in patients with MS. 16 , 17 OCT technology therefore has the potential to provide a reliable and convenient tool to monitor disease progression, quantify the rate of neurodegeneration, and assess the potential neuroprotective treatment effect of MS therapies.
Although it is acknowledged as an easily applicable technique, OCT repeat measurement variability must be considered when assessing within‐subject longitudinal changes or when applied in multicenter settings. 18 Factors that influence the quality of scans and affect measurements, including signal strength, location of blood vessels in the inner retinal layers, acquisition procedures, etc., have been well described. 19 , 20 Post‐acquisition segmentation of healthy and thinned retinal layers also influences reproducibility, and can be affected by the robustness of the software algorithm. It is therefore essential to assess the test‐retest variability, that is, the intersession measurement difference in pRNFL that reflects the technical and/or operator‐based limitations of the method but is not attributable to the underlying disease pathology.
The OCTiMS (Optical Coherence Tomography in Multiple Sclerosis) study is the first large prospective multicenter study designed to determine whether OCT imaging is sufficiently sensitive to detect progression in retinal neuroaxonal loss over 3 years in patients with relapsing‐remitting MS (RRMS) compared with healthy participants in a multicenter trial‐like setting. Changes in average pRNFL and mGCIPL thickness were assessed, along with their potential association with brain volume loss, Expanded Disability Status Scale (EDSS) score, and other clinical and paraclinical measures.
Methods
Participants
The OCTiMS study (NCT02907281) was a 36‐month, prospective, multicenter, noninterventional study, which enrolled 397 participants (333 RRMS and 64 reference participants) across 28 sites in Australia, Europe and North America. The study was approved by respective institutional review boards and all participants provided written informed consent before enrollment. All data were captured and monitored by an independent clinical research organization (CRO) using electronic case record forms (eCRF). As pRNFL thinning may also occur as a result of MS‐associated optic neuritis (MS‐ON), patients were categorized into those with prior history of MS‐ON and those without MS‐ON (non‐MS‐ON) and assessed accordingly. For patients with a history of MS‐ON in one eye, the eye with prior MS‐ON was studied. For patients with a history of MS‐ON in both eyes, or no optic neuritis (ON) at all, the eye with the lowest respective pRNFL thickness at baseline was included. Healthy, age‐matched, control participants were included as the reference group in all models. Participants who experienced an adverse event of MS‐ON during the study were re‐categorized to the MS‐ON subgroup.
Objectives
The primary study objective was to evaluate change in average pRNFL thickness using OCT in patients with RRMS over a 36‐month follow‐up period and compare it with the findings from a group of healthy participants to determine whether the technology is sufficiently sensitive to detect disease‐related pRNFL changes over time. Secondary objectives were as follows: to evaluate short‐term reproducibility of pRNFL thickness measurements over a 4‐week interval by test re‐test estimation; to evaluate the correlation between change in pRNFL thickness with change in brain volume and disability progression in patients with RRMS. In addition, we evaluated change in mGCIPL thickness over 36 months in patients with RRMS and reference participants, as well as the relationship between change in mGCIPL thickness and change in brain volume and disability progression in patients with RRMS. pRNFL and mGCIPL thickness changes were also compared between subgroups according to duration of MS diagnosis determined at the time of enrollment. Finally, we explored the correlation of change in pRNFL thickness with change in other clinical and paraclinical measures and patient‐reported outcomes in patients with RRMS including visual acuity, MRI measures, cognitive performance, and quality of life.
Key inclusion/exclusion criteria
All participants (aged 18–65 years inclusive) were enrolled and followed up between 29 May 2012 and 24 July 2017. Key inclusion criteria for the MS group were as follows: a diagnosis of RRMS as defined by the 2005 revision to the McDonald criteria 21 ; MS disease duration of more than 1 year (from diagnosis of MS) before study entry (screening). The list of exclusion criteria is included in Table S1.
Optical coherence tomography
OCT scans in all participants were performed on Spectralis OCT1 machines (Heidelberg Engineering, Heidelberg, Germany), software version 1.6.2.0 or higher, with the eye tracking function enabled for best accuracy and the use of the baseline scan for alignment of subsequent scans on the same area of the retina. Data were collected from a peripapillary ring, a macular volume scan, the optic nerve head volume scan, and the papillomacular bundle volume scan (see supportive information Data S1 for more details on techniques used). All OCT scans underwent quality control (QC) assessment by the central reading center (Vienna Reading Center [VRC]). Figure S1 gives an overview of the scan patterns and the evaluation fields for each pattern used in the analysis.
OCT scans were performed at baseline and at every visit (1, 6 months, and every 6 months thereafter) for both groups. All operators at participating sites were certified by the VRC for the use of the correct scanning protocol, correct pseudonymization of study‐specific image labelling, and image transfer before patient inclusion according to standardized certification processes to obtain images for the above‐described acquisitions.
Automated segmentation was performed with the manufacturer's software (HEYEX version 1.9.10.0, Viewing Module version 6.0.9.0) and subsequently corrected by certified and trained graders of the VRC, supervised by a retina specialist, according to a predefined reading protocol. All graders were masked to the clinical status of the study participants (MS or healthy control group) and any other diagnostic information. Studies were performed and described in accordance to the QC criteria at the VRC, OSCAR‐IB, and the Advised Protocol for OCT Study Terminology and Elements (APOSTEL) criteria. 20 , 22 The detailed OCT scanning protocol is provided as supporting information.
Ophthalmology examination
An ophthalmologic examination was performed at screening and every 12 months thereafter. The examination included ophthalmic medical history, slit lamp examination, dilated ophthalmoscopy, best corrected visual acuity testing at low 2.5% contrast and high contrast (number of letters read correctly on an Early Treatment Diabetic Retinopathy Study scale), and perimetry testing (optional at sites where required technical equipment was available). If, during the study, worsening of visual acuity or any clinically acute ON were suspected, an unscheduled ophthalmology examination was performed.
Magnetic resonance imaging
All study participants underwent MRI scanning (1.5 or 3T) of the brain at screening and at yearly intervals. In the RRMS patient group, pulse‐sequences included 3DT1 for brain volume measurement, proton density (PD)/T2 for new lesion count and volume, T1 for new hypointense lesion count and volume, and gadolinium‐enhanced T1 sequences for lesion count and volume. Lesions were identified by expert radiology readers and quantified by trained technicians using MIPAV software with local thresholding. Brain volume measurements were performed using SIENA[X] (http://fsl.fmrib.ox.ac.uk ). In the reference participant group, 3DT1 sequences for brain volume measurements (all MRI scans) and T2 lesions (screening scan only) were performed. All MRIs were performed in a standardized manner according to the MRI manual upon performance of a successful dummy‐scan. Each MRI scan performed was previewed by the local neuroradiologist for incidental findings before the scans were sent to and further processed by the MRI central reading center at VU University Medical Center in Amsterdam.
Clinical assessments
EDSS
Disability was assessed every 6 months by certified EDSS raters in clinical MS studies. 23 Disability progression was defined as a one‐point EDSS increase for those with a baseline score of ≤5.0, or a 0.5‐point increase for those with a baseline score >5.0, with the EDSS progression confirmed after 6 months.
Symbol digit modalities test
The symbol digit modalities test (SDMT) was performed every 12 months to assess attention and information processing speed. During the test, subjects substituted symbols in a row by the corresponding number and responded verbally. 24 , 25
Patient‐reported indices for multiple sclerosis
The patient‐Reported Indices for Multiple Sclerosis (PRIMuS) instrument was used to assess patients’ quality of life (QoL) and included activities specifically designed for MS. The test was performed every 12 months. 26
Statistical methodology
Statistical analyses were performed using SAS (version 9.4). All hypothesis tests were evaluated at a 0.05 level of significance. All confidence intervals (CIs) were two sided at a 95% confidence level.
Average pRNFL thickness was summarized by group and ON history at each visit, including change from baseline. A mixed‐model repeated measures (MMRM) approach was applied to compare the change from baseline in average pRNFL thickness between groups, considering results from all available time points, and adjusting for age, gender, and baseline pRNFL thickness. The reference subjects were included as the reference group for both the MS‐ON and non‐MS‐ON subgroups. The group difference in least squares (LS) mean of change from baseline in average pRNFL thickness (patients with MS reference subjects), which reflects the change from baseline for the MS group, after adjusting for the change in the reference group (e.g., to account for age‐related change in healthy subjects), and the associated 95% CI and p‐value are presented for each time point. The LS mean of pRNFL change from baseline within each group is presented for Month 36. The analysis of the primary variable was repeated for the subfield measures of pRNFL thickness: papillomacular bundle, overall temporal quadrant, and temporal fields 6.
An additional analysis was performed to evaluate the change in mGCIPL thickness at Month 36 based on a mixed effect model. Change from baseline in both pRNFL and mGCIPL thickness at Month 36 was stratified by disease duration [defined as time from MS diagnosis to study screening (≤3 years, >3 to ≤5 years, >5 years)] for patients with MS and subgroups of ON history (MS‐ON, non‐MS‐ON) and groups of different disease duration were compared using an analysis of variance model (ANOVA).
Regression analyses were applied to assess the relationship between the change from baseline in EDSS/percentage change in brain volume and the change from baseline in average pRNFL/mGCIPL thickness at Month 36. All results are presented with nominal p‐value with no adjustment for multiplicity due to the observational character of the study.
Results
Baseline characteristics
A total of 333 patients with MS and 64 reference participants without neurologic disease provided a baseline and at least one post baseline pRNFL measurement in at least one eye. Thirty‐nine (11.7%) patients with MS and eight (12.5%) reference participants discontinued the study (Fig. 1). Demographics were well balanced between patients with MS and reference participants, with a small difference found for age (38.9 vs. 39.4, p = 0.003), which was not deemed clinically relevant. Normalized brain volume was lower in the MS groups (p < 0.0001) (Table 1). Differences between MS‐ON and non‐MS‐ON patients in terms of cognitive performance, normalized brain volume and disease characteristics including MS duration, EDSS, and T2 lesion volume were all statistically non‐significant (Table 2). Ocular characteristics, including measures of visual acuity and pRNFL/mGCIPL thickness, were highest in eyes of heathy participants, lowest in eyes with past MS‐ON, and intermediate in eyes of patients without MS‐ON (Table 3). All differences between groups (reference vs. MS and MS‐ON vs. non‐MS‐ON) were significant. Six patients developed MS‐ON during the study period. Prior to the episode, these patients had been included in the non‐MS‐ON group, but they were re‐categorized to the MS‐ON group at follow‐up (after the episode). While non‐severe glaucoma was not part of the exclusion criteria, it should be noted that only two patients (0.6%) had glaucoma in the MS group and 1 (1.4%) in the reference group.
Figure 1.
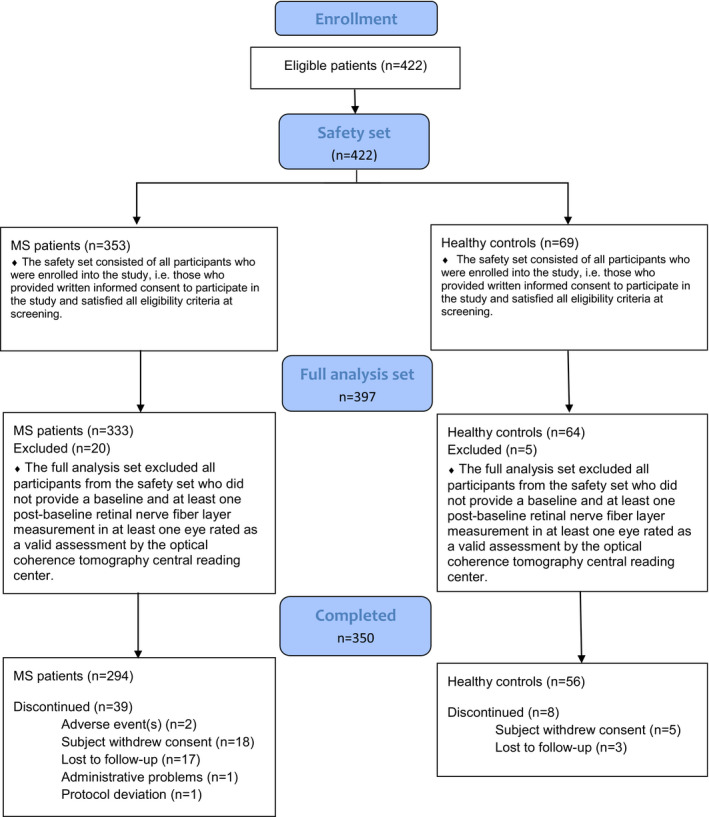
CONSORT diagram.
Table 1.
Baseline demographics and clinical characteristics.
| Characteristics | MS group | Reference group | Group difference | ||
|---|---|---|---|---|---|
|
All patients with MS (N = 333) |
Patients without MS‐ON 1 (N = 235) |
Patients with MS‐ON (N = 98) |
Healthy participants (N = 64) |
p‐value* | |
| Age (years) | |||||
| Mean ± SD | 38.9 ± 8.56 | 38.7 ± 8.56 | 39.4 ± 8.60 | 35.4 ± 9.73 | 0.003 |
| Median (min, max) | 39.0 (19.0, 55.0) | 39.0 (19.0, 55.0) | 39.0 (21.0, 55.0) | 35.0 (21.0, 53.0) | |
| Sex, n (%) | |||||
| Female | 233 (70.0) | 166 (70.6) | 67 (68.4) | 45 (70.3) | 0.956 |
| Male | 100 (30.0) | 69 (29.4) | 31 (31.6) | 19 (29.7) | |
| Race, n (%) | |||||
| Caucasian | 318 (95.5) | 223 (94.9) | 95 (96.9) | 61 (95.3) | 0.752 |
| Black | 4 (1.2) | 3 (1.3) | 1 (1.0) | 0 | |
| Asian | 5 (1.5) | 4 (1.7) | 1 (1.0) | 2 (3.1) | |
| Other | 6 (1.8) | 5 (2.1) | 1 (1.0) | 1 (1.6) | |
| SDMT | |||||
| Mean ± SD | 55.6 ± 13.37 | 55.2 ± 13.10 | 56.4 ± 14.02 | n/a | n/a |
| Median (min, max) | 58.0 (0.0, 90.0) | 56.0 (16.0, 90.0) | 59.0 (0.0, 81.0) | n/a | |
| MRI normalized brain volume (cm3) | |||||
| Mean ± SD | 1511 ± 103 | 1515 ± 99 | 1501 ± 112 | 1589 ± 101 | <0.0001 |
| Median (min, max) | 1514.0 (1203.0, 1774.0) | 1519.0 (1203.0, 1774.0) | 1503.0 (1270.0, 1748.0) | 1603.0 (1333.0, 1895.0) | |
MRI, magnetic resonance imaging; MS, multiple sclerosis; MS‐ON, multiple sclerosis‐associated optic neuritis; n/a, not available; SD, standard deviation; SDMT, symbol digit modalities test. N is the total number of subjects in the analysis population (different from the number of subjects with data available).
Without previous MS‐ON.
All p‐values are two‐side. For continuous variables, p‐value is from two sample t‐test comparing the MS and reference groups. For categorical variables, if any of the entries is <10, then p‐value is from Fisher's exact test; if all entries are >=10, then p‐value is from Chi‐square test.
Table 2.
Baseline disease characteristics of the MS group.
| Characteristics |
All patients with MS (N = 333) |
Patients without MS‐ON 1 (N = 235) |
Patients with MS‐ON (N = 98) |
|---|---|---|---|
| Time since MS diagnosis | |||
| Years, mean (SD) | 7.28 ± 5.75 | n/a | n/a |
| 1–≤3 years, n (%) | 100 (30.0) | 69 (29.4) | 31 (31.6) |
| >3–≤5 years, n (%) | 44 (13.2) | 34 (14.5) | 10 (10.2) |
| >5 years, n (%) | 189 (56.8) | 132 (56.2) | 57 (58.2) |
| Previous DMT, n (%) | |||
| Fingolimod | 45 (13.5) | n/a | n/a |
| Natalizumab | 60 (18.0) | n/a | n/a |
| iDMT (interferons or glatiramer acetate) | 162 (48.6) | n/a | n/a |
| DMF or teriflunomide | 14 (4.2) | n/a | n/a |
| Other | 5 (1.5) | n/a | n/a |
| No treatment | 61 (18.3) | n/a | n/a |
| EDSS score | |||
| Mean ± SD | 1.9 ± 1.43 | 1.9 ± 1.42 | 2.1 ± 1.44 |
| Median (min, max) | 2.0 (0.0, 7.0) | 1.5 (0.0, 7.0) | 2.0 (0.0, 6.0) |
| MRI PD/T2 lesion volume (mm3) | |||
| Mean ± SD | 3663 ± 5095 | 3523 ± 4659 | 4037 ± 6036 |
| Median (min, max) | 1922.5 (0.0, 48514.0) | 1880.0 (0.0, 34627.0) | 2206.0 (66.0, 48514.0) |
DMF, dimethyl fumarate; iDMT, injectable disease modifying therapy; EDSS, expanded disability status scale; max, maximum; min, minimum; MRI, magnetic resonance imaging; MS, multiple sclerosis; MS‐ON, multiple sclerosis‐associated optic neuritis; n/a, not available; PD, proton density; SD, standard deviation. N is the total number of subjects in the analysis population (different from the number of subjects with data available).
Without previous MS‐ON.
Table 3.
Ocular baseline characteristics.
| Number of patients | MS group | Reference group | Group difference | ||
|---|---|---|---|---|---|
| All patients with MS | Patients without MS‐ON1 | Patients with MS‐ON | Healthy participants | p‐value* | |
| N = 333 | N = 235 | N = 98 | N = 64 | ||
| Retinal thickness (μm) | |||||
| pRNFL | 89.0 ± 15.30 | 91.7 ± 14.21 | 82.5 ± 15.85 | 100.2 ± 9.23 | <0.0001 |
| mGCIPL | 79.3 ± 14.22 | 82.2 ± 12.91 | 72.5 ± 14.94 | 91.9 ± 8.42 | <0.0001 |
| Visual acuity (ETDRS letters) | |||||
| High contrast | 56.1 ± 9.49 | 57.0 ± 8.25 | 53.8 ± 11.69 | 59.6 ± 5.42 | <0.0001 |
| Low contrast | 27.8 ± 11.65 | 29.8 ± 10.44 | 22.9 ± 12.95 | 34.3 ± 7.28 | <0.0001 |
Values are mean ± SD. N is the total number of subjects in the analysis population.
ETDRS, early treatment diabetic retinopathy study; mGCIPL, combined macular ganglion cell‐inner plexiform layer; MS, multiple sclerosis; MS‐ON; multiple sclerosis‐associated optic neuritis; ON, optic neuritis; pRNFL, peripapillary retinal nerve fiber layer; SD, standard deviation.
1Without previous MS‐ON
All p‐values are two‐sided from two sample t‐test comparing the MS and reference groups. p‐values for the subgroup comparison MS‐ON versus non‐MS‐ON were also calculated and were all <0.0001.
Changes in inner retinal layers thicknesses
The decrease in pRNFL thickness from baseline (unadjusted for differences in baseline thickness at the time of enrollment) over time was greater for patients with RRMS compared with the reference group (Fig. 2). The mean (standard deviation [SD]) average pRNFL thickness decrease from baseline at Month 36 was 1.61 (4.60) µm in patients with RRMS compared with 0.09 (2.98) µm in reference participants. Decreases in pRNFL thickness over time were similar in the non‐MS‐ON and MS‐ON subgroups.
Figure 2.
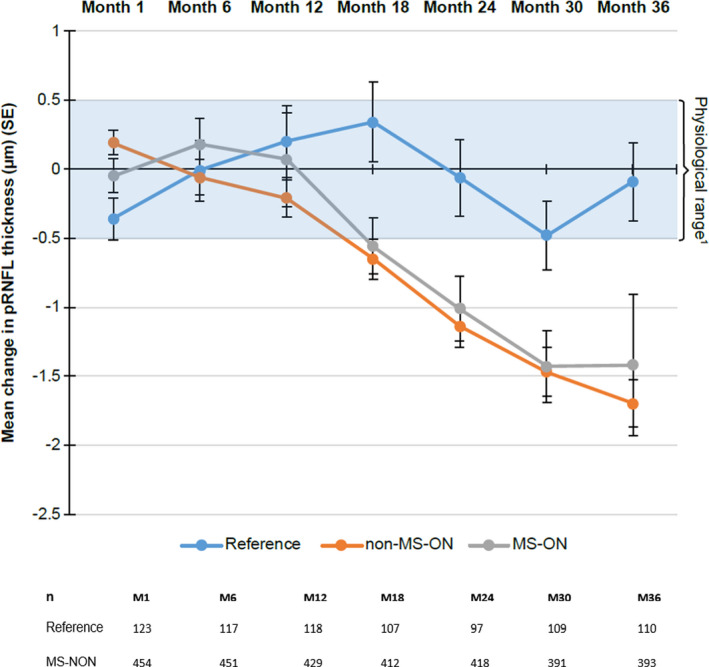
Mean change from baseline in pRNFL thickness over time by ON status. The change from baseline has been overlaid with the known physiological variation between measurements and is shown for months 1 to 36 1(Balk et al. Invest Ophthalmol Vis Sci 2012;53:1251–1257). SE, standard error; M, month; MS, multiple sclerosis; MS‐ON; multiple sclerosis‐associated optic neuritis; ON, optic neuritis; pRNFL, peripapillary retinal nerve fiber layer; n, number of eyes observed per time point.
After adjusting for potential imbalance between groups (i.e., MS vs. reference subjects) in baseline covariates including age, gender, and baseline RNFL score, the least square mean of change from baseline in RNFL at Month 36 was −1.74 with 95% CI (−2.04, −1.45) in the MS group versus 0.12 with 95% CI (−0.51, 0.74) in the reference group (Table 4). The LS mean difference (95% CI) between patients with RRMS and reference participants for change in average pRNFL thickness at Month 36 was −1.86 (−2.54, −1.17) μm (p < 0.0001; Table 4); this thus represents the change from baseline for the MS group after adjusting for changes (including age‐related change) in healthy subjects. The difference between the two groups for change in average pRNFL thickness were statistically significant from Month 18 onward: Month 18: −1.13 (−1.75, −0.52) μm (p = 0.0003); Month 24: −1.11 (−1.76, −0.46) μm (p = 0.0008); and Month 30: −1.16 (−1.82, −0.49) μm (p = 0.0007). At Month 6 and Month 12, the group differences were −0.10 (−0.65, 0.46) μm (p = 0.7289), and −0.55 (−1.13, 0.03) μm (p = 0.0621), respectively. The LS mean group differences in change in pRNFL thickness were similar regardless of MS‐ON history (Table 4).
Table 4.
Change from baseline in pRNFL and mGCIPL thickness and comparison between groups at Month 36.
|
ON history Visit group |
n |
LS mean of change from baseline (95% CI) |
Group difference (95% CI) in change from baseline (Patients with MS – Reference participants, adjusted for baseline) | p‐value |
|---|---|---|---|---|
| pRNFL thickness (µm) 1 | ||||
| All | ||||
| Patients with MS (N = 333) | 284 | −1.74 (−2.04, −1.45) | −1.86 (−2.54, −1.17) | <0.0001 |
| Reference participants (N = 64) | 56 | 0.12 (−0.51, 0.74) | ||
| Non‐MS‐ON | ||||
| Patients with MS (N = 235) | 200 | −1.79 (−2.12, −1.45) | −1.88 (−2.59, −1.16) | <0.0001 |
| Reference participants (N = 64) | 56 | 0.09 (−0.55, 0.72) | ||
| MS‐ON | ||||
| Patients with MS (N = 98) | 84 | −1.61 (−2.25, −0.96) | −1.80 (−2.71, −0.88) | 0.0001 |
| Reference participants (N = 64) | 56 | 0.19 (−0.41, 0.79) | ||
| mGCIPL thickness (µm) 2 | ||||
| All | ||||
| Patients with MS (N = 333) | 285 | −0.54 (−0.85, −0.24) | −2.03 (−2.78, −1.28) | <0.0001 |
| Reference participants (N = 64) | 55 | 1.49 (0.80, 2.18) | ||
| Non‐MS‐ON | ||||
| Patients with MS (N = 235) | 201 | −0.47 (−0.83, −0.12) | −1.77 (−2.51, −1.03) | <0.0001 |
| Reference participants (N = 64) | 55 | 1.30 (0.63, 1.97) | ||
| MS‐ON | ||||
| Patients with MS (N = 98) | 84 | −1.58 (−2.32, −0.85) | −3.89 (−5.17, −2.61) | <0.0001 |
| Reference participants (N = 64) | 55 | 2.31 (1.37, 3.25) | ||
N is the total number of participants in the analysis population, n is the number of participants with data available.
The average pRNFL thickness was the mean of the circular scan subfields 1 to 8.
Eye was nested within subject, and both were included as random effects. For participants with a history of ON, the unaffected eye was excluded. The reference participants were included as the reference group in all models. Participants who experienced an adverse event of ON during the study were re‐categorized and included in the ON history subgroup.
CI, confidence interval; mGCIPL, combined macular ganglion cell and inner plexiform layers; LS, least squares; MS, multiple sclerosis; MS‐ON, MS‐ associated optic neuritis; pRNFL, peripapillary retinal nerve fiber layer.
Mixed model for repeated measures (MMRM) included terms for group, visit, age, gender, and the interaction of group and visit with baseline average pRNFL thickness as a covariate.
Mixed model includes terms for group, age, gender, and baseline mGCIPL thickness as a covariate.
The mixed effect model analysis conducted on mGCIPL measurements yielded similar results at Month 36, demonstrating greater thinning of mGCIPL in the MS group compared with control participants over the 3 years. The mean (SD) average mGCIPL thickness at Month 36 decreased from baseline by 0.39 (3.096) μm in patients with MS and increased by 1.02 (5.063) μm in reference participants. The LS mean difference (95% CI) between patients with RRMS and reference participants for change in mGCIPL thickness at Month 36 (i.e., rate of thinning corrected with healthy age‐related attrition) was −2.03 (−2.78, −1.28) (p < 0.0001; Table 4). Results were similar regardless of MS‐ON history.
Comparison of change in papillomacular bundle, temporal quadrant pRNFL thickness, and temporal field 6 pRNFL thickness between groups at Month 36 are outlined in Table 5 (see Fig. S1 for the description of the fields). While there were no significant differences between groups for temporal field 6 RNFL thickness, there was a significant decrease in the papillomacular bundle and temporal quadrant RNFL thickness in patients with MS compared with reference participants.
Table 5.
Change from baseline in retinal parameters thickness, and comparison between groups at Month 36.
|
Patients with MS N = 333 |
Reference participants N = 64 |
|
|---|---|---|
| Papillomacular bundle thickness (µm) | ||
| LS mean of change from baseline | −0.55 (−0.85, −0.24) | 0.28 (−0.36, 0.92) |
| Group difference in change from baseline 1 (95% CI) | −0.82 (−1.53, −0.12), p = 0.0215 | |
| Temporal quadrant pRNFL thickness (µm) | ||
| LS mean of change from baseline | −0.72 (−1.0, −0.42) | 0.13 (−0.49, 0.75) |
| Group difference in change from baseline 1 (95% CI) | −0.85 (−1.53, −0.16), p = 0.0156 | |
| Temporal field 6 pRNFL thickness (µm) | ||
| LS mean of change from baseline | −0.75 (−1.11 −0.40) | −0.14 (−0.88, 0.59) |
| Group difference in change from baseline 1 (95% CI) | −0.61 (−1.42, 0.20), p = 0.1373 | |
CI, confidence interval; LS, least squares; MS, multiple sclerosis; pRNFL, peripapillary retinal nerve fiber layer; SD, standard deviation.
Group difference at Month 36 was analyzed using mixed model for repeated measures (MMRM) which included terms for group, visit, age, gender, and the interaction of group and visit with baseline value as a covariate. N is the total number of subjects in the analysis population (different from the number of subjects with data available).
Effect of time from diagnosis on OCT parameters
A greater decrease in both pRNFL and mGCIPL thickness was observed in patients diagnosed more recently (≤3 years) than in patients with a longer time from diagnosis (>5 years) (p < 0.05 for pRNFL and p < 0.01 for mGCIPL, based on an ANOVA model) (Fig. 3). This observation is in line with the primary analysis which demonstrated a positive correlation between baseline pRNFL thickness and the rate of pRNFL thinning (p < 0.0001). Patients with thicker pRNFL at baseline (as observed in early disease) are therefore more likely to show faster pRNFL loss.
Figure 3.
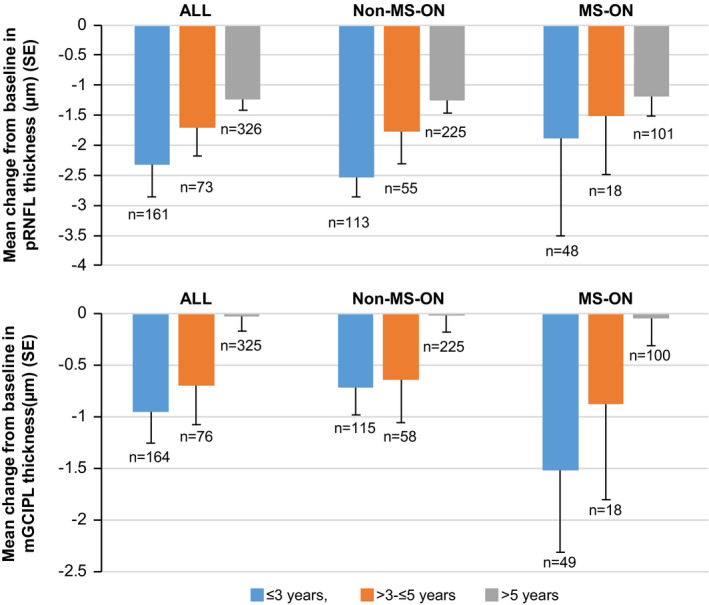
Change from baseline in pRNFL and mGCIPL thickness at Month 36, stratified by time from MS diagnosis. Pairwise comparisons between the three MS duration subgroups were conducted based on an analysis of variance (ANOVA) using Tukey's HSD test (unadjusted for differences in baseline thickness). Only the differences between <=3 and >5 groups were found significant (p < 0.05 and p < 0.01 for pRNFL and mGCIPL, respectively). Note that no patient with disease <1 year was included, as per the inclusion criteria. n is the number of eyes with data available. mGCIPL, combined macular ganglion cell and inner plexiform layers; MS, multiple sclerosis; MS‐ON; multiple sclerosis‐associated optic neuritis; pRNFL, peripapillary retinal nerve fiber layer; SE, standard error.
Test re‐test reproducibility of pRNFL OCT measurements
The test re‐test reproducibility analysis of all pRNFL measurements at baseline and 1 month by OCT reported intraclass correlation coefficient (ICC) values of ≥0.98, regardless of MS status or ON status (Table 6).
Table 6.
Test re‐test reproducibility of pRNFL OCT measurements.
| MS group | Reference group | |||
|---|---|---|---|---|
|
All patients with MS N = 656 |
Patients without MS‐ON1 N = 537 | Patients with MS‐ON N = 119 |
Healthy participants N = 122 |
|
| n | 642 | 524 | 118 | 121 |
| Baseline, (µm) | 88.84 ± 15.35 | 91.52 ± 13.89 | 76.86 ± 15.87 | 100.45 ± 9.15 |
| Month 1, (µm) | 88.96 ± 15.41 | 91.64 ± 14.01 | 77.03 ± 15.78 | 100.13 ± 9.34 |
| Difference, (µm) | 0.12 ± 1.82 | 0.14 ± 1.86 | 0.01 ± 1.60 | −0.35 ± 1.73 |
| 95% CI for mean | (−0.02, 0.26) | (−0.02, 0.30) | (−0.28, 0.30) | (−0.66, −0.04) |
| p‐value | 0.1036 | 0.0840 | 0.9429 | 0.0271 |
| ICC (95% CI) | 0.993 (0.992, 0.994) | 0.991 (0.989, 0.992) | 0.995 (0.993, 0.996) | 0.982 (0.974, 0.987) |
Values are mean ± SD unless otherwise indicated
CI, confidence interval; ICC, intraclass correlation coefficient; MS, multiple sclerosis; MS‐ON; multiple sclerosis‐associated optic neuritis; N is the total number of eyes in the analysis population, n is the number of eyes with data available.
1Without previous MS‐ON
Brain volume
The mean percentage (SD) decrease in brain volume from baseline to Month 36 was 1.3% (1.32) for patients with MS and 0.5% (0.67) for reference participants. While the percentage change from baseline in brain volume was not significantly associated with the change in average pRNFL thickness, it was significantly associated with change in mGCIPL thickness (p < 0.0001, Table 7). Additionally, baseline brain volume was significantly associated with baseline pRNFL thickness (p = 0.0047) but not with baseline mGCIPL (p = 0.0518) Table 8.
Table 7.
Regression analysis: relationship between percentage brain volume change from baseline to Month 36 and change in overall average mGCIPL thickness (µm) from baseline to Month 36.
| Regression parameter (A) | Parameter estimate (SE) | p‐value |
|---|---|---|
| mGCIPL thickness (µm) | 0.022 (0.005) | <0.0001 |
| Age (years) | 0.002 (0.008) | 0.7986 |
| Duration of disease (years) | 0.014 (0.013) | 0.2734 |
| MS‐ON history (yes/no) | −0.081 (0.176) | 0.6468 |
| Goodness of fit: adjusted R‐squared = 0.061 | ||
The most affected eye was used for analyses. Full analysis set (MS and reference individuals, N = 397).
mGCIPL, macular ganglion cell and inner plexiform layers; MS‐ON, multiple sclerosis‐associated optic neuritis; SE, standard error.
Table 8.
Regression analysis: relationship between normalized brain volume at baseline and (A) pRNFL thickness at baseline; (B) mGCIPL thickness at baseline.
| Regression parameter (A) | Parameter estimate (SE) | p‐value |
|---|---|---|
| pRNFL thickness (µm) | 1.0 (0.35) | 0.0047 |
| Age (years) | −3.3 (0.60) | <0.0001 |
| Duration of disease (years) | −2.8 (0.96) | 0.0043 |
| MS‐ON history (yes/no) | −3.9 (12.57) | 0.7552 |
| Goodness of fit: adjusted R‐square = 0.166 | ||
| Regression parameter (B) | Parameter estimate (SE) | p‐value |
|---|---|---|
| mGCIPL thickness (µm) | 0.8 (0.39) | 0.0518 |
| Age (years) | −3.2 (0.63) | <0.0001 |
| Duration of disease (years) | −2.5 (1.01) | 0.0124 |
| MS‐ON history (yes/no) | −3.2 (13.60) | 0.8168 |
| Goodness of fit: adjusted R‐squared = 0.138 | ||
The most affected eye was used for analyses. Full analysis set (MS and reference individuals, N = 397).
mGCIPL, macular ganglion cell and inner plexiform layers; MS‐ON, multiple sclerosis‐associated optic neuritis; ON, optic neuritis; pRNFL, peripapillary retinal nerve fiber layer; SE, standard error.
Expanded disability status scale
The mean (SD) EDSS increased from baseline by 0.15 (0.86) (median = 0.0 [0.00–0.50]) at Month 36 in the MS patient group, with a mean (SD) time to onset of EDSS progression of 18.6 (8.5) months. Twenty‐three (7%) patients had a 6 month confirmed disability progression by end of study.
No linear relationship was observed between the change from baseline in EDSS score and change from baseline in average pRNFL thickness, regardless of disease progression. The change from baseline in mGCIPL thickness was not correlated with the change in EDSS score either.
MRI lesions
Changes from baseline in T1 gadolinium enhancing and PD/T2 lesion count and volume as well as in the percentage of patients with T1 gadolinium enhancing lesions and new PD/T2 lesions are presented in (Table 9). There was no linear relationship (as assessed by a regression analysis) observed between the change from baseline in T1 gadolinium enhancing lesion count or volume and the change from baseline in average pRNFL thickness at different time points. However, there was a statistically significant inverse relationship between the number of new PD/T2 lesions from baseline and change from baseline in pRNFL thickness at Month 36, with a parameter estimate (SD) for pRNFL thickness of −0.69 (0.24) (p = 0.0048), suggesting that patients with a greater increase in the number of T2 lesions had more pRNFL thinning (Table 10).
Table 9.
Change in MRI lesions in patients with MS over the study period.
| Patients with MS, N = 333 | |
|---|---|
| Presence of T1 Gd‐enhancing lesions, n (%) | |
| Baseline | 50 (15.2) |
| Month 12 | 67 (22.1) |
| Month 24 | 75 (26.8) |
| Month 36 | 77 (27.5) |
| T1 Gd‐enhancing lesion count , mean ± SD | |
| Baseline | 0.3 ± 1.07 |
| Month 12 | 0.5 ± 1.48 |
| Month 24 | 0.8 ± 2.00 |
| Month 36 | 0.9 ± 2.77 |
| T1 Gd‐enhancing lesion volume (mm3), mean ± SD | |
| Baseline | 30.2 ± 119.19 |
| Month 12 | 54.9 ± 167.89 |
| Month 24 | 83.8 ± 267.85 |
| Month 36 | 100.3 ± 323.88 |
| Presence of new PD/T2 lesion, n (%) | |
| Change from baseline at Month 12 | 132 (43.1) |
| Change from baseline at Month 24 | 171 (59.8) |
| Change from baseline at Month 36 | 180 (64.3) |
| PD/T2 lesion count , mean ± SD | |
| Change from baseline at Month 12 | +2.4 ± 6.34 |
| Change from baseline at Month 24 | +4.7 ± 10.09 |
| Change from baseline at Month 36 | +5.9 ± 12.81 |
| PD/T2 lesion volume (mm3) , mean ± SD | |
| Change from baseline at Month 12 | + 92.9 ± 1045.11 |
| Change from baseline at Month 24 | +153.2 ± 1156.19 |
| Change from baseline at Month 36 | +247.5 ± 1311.52 |
Gd‐enhancing, gadolinium‐enhancing; MRI, magnetic resonance imaging; MS, multiple sclerosis; PD, proton density. N is the total number of subjects in the analysis population (different from the number of subjects with data available).
Table 10.
Regression analysis: relationship between the number of new PD/T2 lesions from baseline and the change in pRNFL thickness from baseline.
| Regression parameter | Parameter estimate (SE) | p‐value |
|---|---|---|
| pRNFL thickness (µm) | −0.69 (0.24) | 0.0048 |
| Baseline PD/T2 lesion volume (mm3) | 0.00 (0.00) | 0.0699 |
| Age (years) | −0.43 (0.09) | <0.0001 |
| Duration of disease (years) | −0.05 (0.14) | 0.7117 |
| MS‐ON history (yes/no) | 37.19 (8.42) | <0.0001 |
| Goodness of fit: adjusted R‐squared = 0.161 | ||
The most affected eye was used for analyses.
pRNFL, peripapillary retinal nerve fiber layer; MS‐ON, multiple sclerosis‐associated optic neuritis; SE, standard error.
There was no linear relationship between the change from baseline in PD/T2 lesion volume and change from baseline in pRNFL thickness observed at any time point.
Visual function
Changes from baseline in mean visual acuity were observed in both groups (MS and reference), and for high contrast and low contrast visual acuity at all time‐points. These changes were small, ranging from −1.6 to 1.1 letters in the RRMS group and from −1.5 to 0.0 letters in the reference group.
A significant linear correlation (Pearson's correlation) was observed between change from baseline in low contrast visual acuity (total number of letters recognized at low contrast level) and change from baseline in GCIPL thickness at month 36 (p < 0.01). No such correlation was observed for high contrast visual acuity (total number of letters recognized at high contrast level). There was no linear relationship observed between the change from baseline in average pRNFL thickness and the change from baseline in high or low contrast visual acuity.
Symbol digit modalities test
The mean (SD) SDMT total score increased in patients with MS from 55.7 (13.2) at baseline to 58.2 (14.22) at Month 36. There appears to be no visible linear correlation as observed graphically between the change from baseline in SDMT total score and average pRNFL thickness (Pearson correlation coefficient = 0.097634).
Patient‐reported indices for multiple sclerosis
There was no change from baseline in PRIMuS QoL total score observed in patients with MS at Month 24 and 36. There was no visible linear correlation as observed graphically between the change from baseline in PRIMuS QoL total score or PRIMuS activities total score and change in average pRNFL thickness (Pearson correlation coefficients −0.01668 and −0.0074, respectively).
Safety
None of the serious adverse events reported were related to study procedures. There were no new adverse safety signals identified from vital sign measurements during the study.
Discussion
The OCTiMS study was designed to evaluate change in pRNFL thickness in patients with RRMS followed for up to 3 years, compared with a group of reference participants (without neurologic or ophthalmic disease), to determine whether retinal OCT measurements are sufficiently accurate, reproducible, and sensitive to measure disease progression over time. In this study, the biggest multicenter OCT MS study performed to date, we detected a greater decrease in pRNFL thickness in patients with MS compared with reference participants over a 36‐month period. The accuracy of repeated measurements was excellent, indicated by an ICC greater than 0.98.
The mean (SD) average pRNFL thickness decreased from baseline by 1.61 (4.600) µm at Month 36 in patients with MS compared with only 0.09 (2.982) µm in reference control participants, representing an effect size of 0.39 (Cohen's d). The mean change from baseline for the MS group after adjusting for age‐related changes in healthy subjects were −1.86 (95% CI −2.54, −1.17) μm over the 3‐year period. The difference in change from baseline between the two groups for average pRNFL thickness was statistically significant from Month 18 onwards. The changes observed in pRNFL thickness between patients with MS and healthy controls are consistent with numerous clinical studies and meta‐analyses. 16 , 27 , 28 , 29 Separate analyses performed on the temporal quadrant and the papillomacular bundle also demonstrated significant differences between groups at Month 36, consistent with reports that the temporal quadrant is the most involved pRNFL region in MS. 30 , 31 However, the statistical significance of the differences were less marked than for the pRNFL.
To accurately assess the change in pRNFL thickness during the OCTiMS study, it was important to control for the presence of MS‐ON, a key confounding factor in absolute pRNFL thickness values. 28 , 29 Indeed, the average degree of pRNFL atrophy following MS‐ON is −20.38 µm, representing about one fifth of normal pRNFL thickness. In eyes without clinically manifested MS‐ON, the level of atrophy is about one third of this (~7 µm). 29 In our study, pRNFL thickness decreased similarly in patients with MS regardless of MS‐ON history, indicating that the rate of further pRNFL thinning after the initial optic neuritis‐related thinning has occurred is no different than eyes of patients with RRMS without a history of optic neuritis. However, we cannot exclude a history of subclinical MS‐ON in the absence of confounding ophthalmological pathology in apparent non‐MS‐ON patients. Moreover, in light of recent work on inter‐eye difference thresholds for detecting unilateral MS‐ON, a different assignment strategy of patients to MS‐ON and non‐MS‐ON subgroups might have changed diagnostic groupings. 32 , 33 In this study we adhered to the 2005 revision of the MS diagnostic criteria. 21 Interestingly, the pRNFL changes we observed are similar to those measured by the older time domain OCT technology used in a longitudinal multicenter US study. 34 As in OCTiMS, this work showed similar patterns of longitudinal pRNFL thinning in non‐MS‐ON and MS‐ON eyes. However, it should be noted that in OCTiMS, due to differences in standard deviations the effect size in the subset of patients without MS‐ON (0.5 [Cohen's d]) is numerically higher than that of MS‐ON patients (0.26 [Cohen's d]).
Abnormal thinning of the mGCIPL layer has also been associated with MS. 28 The mGCIPL analysis presented here supports the primary endpoint. Results show a decrease in mean (SD) mGCIPL thickness of 0.39 (3.096) µm in patients with MS over 36 months compared with an increase of 1.02 (5.063) µm in reference participants, with a rate of thinning corrected with healthy age‐related attrition of −2.03 (95% CI −2.78, −1.28) µm over the 3‐year period. The increase in mGCIPL observed in the reference group reflects the degree of physiological variation also observed with pRNFL (Fig. 2) and consistent with earlier observations. 35 , 36 An additional factor may be that the dendritic layer of GCIPL may be dynamic and changing more over time in normal eyes compared to eyes where damage is present.
Interestingly, we observed greater thinning of both pRNFL and mGCIPL in patients with a shorter disease duration, which aligns with previous smaller studies. 37 , 38 , 39 This observation is in line with the positive correlation between baseline pRNFL thickness and the rate of pRNFL thinning. It may be due to a larger dynamic range from which the retinal layer can thin, or to faster pRNFL loss in earlier disease, perhaps as a consequence of axonal loss driven by more active inflammation. In the subgroup of patients with earlier disease, the effect size for pRNFL is numerically higher compared with the overall MS population (0.43 vs. 0.39). The largest effect size is observed in non‐MS‐ON patients with ≤3 years of MS duration (0.75). This finding suggests that OCT measures may be more useful for documenting neuroaxonal loss in early disease, especially as MRI measures of brain atrophy are less sensitive at this stage.
In the present study, we confirm that neurodegeneration occurring in early stage of disease onset may not only reflect axonal degeneration, but also neuronal degeneration (as measured by mGCIPL change), and both appear to decrease over time. Similarly, another recent study reported mGCIPL thinning at a very early stage, followed by a plateau. 40 The data suggest that the mGCIPL complex may be capable of showing thickness changes at earlier time points than the pRNFL layer containing primarily axons.
A secondary endpoint of OCTiMS was to evaluate the short‐term reproducibility of the pRNFL thickness measure at study start by test/re‐test estimation after a 4‐week interval in patients with MS and control participants. The purpose of this analysis was to determine if adjustments in the assumptions and sample size were required if the measurement variability was larger than expected. Reproducibility for the average pRNFL thickness was excellent at a group level, with ICC values greater than 0.98. The minute average change in pRNFL from baseline to Month 1 was 0.12 µm for MS patient eyes and −0.35 µm for the reference eyes. Overall, the difference was not statistically significant and remains well within the recognized degree of physiological variation. 36 Our results are in line with prior evidence that demonstrate the high reliability of repeated scans utilizing eye tracking, Automatic Real‐Time (ART) averaging, auto‐registration to a baseline scan during subsequent scan acquisitions, and automated segmentation with manual correction of macular OCT scans, thereby supporting their use in multicenter settings. 41
Other secondary and exploratory objectives were to identify a potential relationship between the extent of pRNFL thinning and different measures of neuronal damage and disease severity. No clear associations were observed between change in pRNFL thickness and change in visual function, EDSS, brain volume, cognitive performance, or measures of QoL. By contrast, change in mGCIPL did significantly correlate (p < 0.01) with low contrast visual acuity, a sensitive measure of macular function. In addition, patients with more new T2/PD lesions showed greater RNFL thinning at Month 36 (p < 0.01), aligning with previous work that suggest an interrelation of inflammatory activity and retinal tissue loss. 42 There was no observed correlation between EDSS worsening and change in average RNFL thickness, contrary to the findings of previous studies. 13 , 39 Although recent evidence suggests that EDSS may correlate better with mGCIPL thickness than with pRNFL thickness, 40 , 43 this was not the case in our study either. However, it is important to note that the majority of the RRMS population in this study had well‐controlled disease, with only 7% of patients progressing during the 3 years, and a mean (SD) EDSS worsening at Month 36 of 0.15 (0.86), which may account for the failure to detect any correlation between EDSS progression and pRNFL/mGCIPL thinning.
A decrease of 1.3% and 0.5% from baseline in brain volume was observed at Month 36 in patients with MS and control subjects, respectively, representing an effect size of 0.76 (Cohen's d). While baseline brain volume was associated with baseline pRNFL thickness, there was no clear association between the extent of brain volume loss and the change in pRNFL thickness from baseline. However, we did find that decreases in average mGCIPL thickness were correlated with more brain atrophy over the 3‐year study. This finding is in line with results from a 4‐year study by Saidha et al., 12 suggesting that mGCIPL atrophy may mirror the severity of disease activity and whole‐brain atrophy. In their study, the correlation between mGCIPL and brain atrophy in patients with RRMS was stronger when MS‐ON effects were excluded.
It should be noted that Caucasians made up 94% of the study population, which is a limitation given that RNFL thickness and rates of thinning are known to differ between ethnic groups. 44 , 45 Another limitation is that only the affected eye of patients with unilateral MS‐ON was studied, which, in this subgroup, may increase the risk of a floor‐effect which could lead to underestimating the level of neurodegeneration. Furthermore, in spite of considerable collaborative network efforts of the neuro‐ophthalmological community, a multi‐rater, validated quality control metric for OCT has not yet been established and implemented in multicenter studies. 19 , 22 Likewise, reproducibility of inner nuclear layer thickness of bipolar cells, a promising biomarker for inflammation in MS, has not yet been optimized and applied. 46 , 47
A strength of this study is that there were substantial efforts made in to ensure that identical machines and software are used across sites for optimal comparability of OCT measures, which may not be possible in all multicenter studies. However, mGCIPL change measured in OCTiMS may not be very accurate, as shown in patients with MS > 5 years who had no mGCIPL change (Fig. 3), likely due to the limitations of the OCT imaging technique used and less frequent sampling. It should be noted that since the completion of this work the performance and sensitivity of OCT imaging have increased substantially. At the time of the protocol initiation, the standard recommended macular acquisition on the Heidelberg Spectralis was 25 B scans. The current Heidelberg segmentation software requires at least 61 B scan density, highlighting the technical advances that are rapidly occurring in OCT acquisition and post‐acquisition analyses. This will likely improve the accuracy of these measurements, as has happened in the MRI arena over the last three decades, and further supports the potential benefits of incorporating OCT measures in RRMS clinical trials in the future.
Conclusion
The OCTiMS study prospectively assessed pRNFL thickness change using OCT in patients with MS in a multicenter setting. The OCT measurements were shown to be highly reproducible, and a significant difference in pRNFL thinning was observed between patients with MS from 18 months onwards compared with healthy controls. This was supported by similar results obtained for mGCIPL thickness, of which changes were found to correlate with brain volume loss. Based on Heidelberg Spectralis data acquired in this study, the effect size for the OCT measures (RNFL, GCIPL) was between small to medium in this overall stable RRMS population, and smaller than that found for MRI brain volume. Of note, the effect size for OCT measures was larger in patients in the early stages of the disease course and without any history of MS‐ON. Considering the rapid advances in OCT imaging toward higher resolution, OCT measures are getting more and more reliable, increasing their potential to become a convenient tool to monitor disease progression, and quantify the level of neurodegeneration in MS populations, particularly in the early disease course.
Conflicts of Interest
Friedemann Paul is a member of the Novartis OCTIMS study steering committee and the MedImmune/Viela Bio steering committee; has received research support and personal compensation for activities with Alexion, Chugai, Biogen, Bayer, Merck Serono, Teva, Genzyme, Novartis, Shire, Roche, Actelion, Celgene, and MedImmune.
Peter A. Calabresi has received research funding from Biogen, Genentech and Annexon; consulting honoraria from Biogen, Disarm Therapeutics, and Nervgen.
Frederik Barkhof has received grants from National Institute for Health, Research University College London Hospitals, Biomedical Research Centre. He reports other from Neurology, other from Brain, other from Radiology, other from MSJ, other from Neuroradiology, personal fees from Springer, personal fees and other from Bayer, personal fees from Biogen, personal fees from Roche, grants from TEVA, grants from Merck, grants from Biogen, personal fees from IXICO Ltd, personal fees from Novartis, personal fees and other from GeNeuro, grants from IMI‐EU, grants from GE Healthcare, grants from UK MS Society, grants from Dutch Foundation MS Research, grants from NWO, grants from NIHR, outside the submitted work.
Ari J. Green reports grants and other support from Inception Biosciences; grants from the National Multiple Sclerosis Society and from the US National Institutes of Health; other support from MedImmune, Mylan, Sandoz, Dr Reddy, Amneal, Momenta, Synthon, and JAMA Neurology, outside the submitted work; and has received grant support from Novartis for participating in the OCTIMS study.
Randy Kardon served on the OCTIMS Steering Committee and receives honoraria from Novartis for this activity. RK reports other support from MedFace LLC and FaceX LLC and has a patent to use pupil and eye movement recordings to diagnose eye and CNS disorders, such as traumatic brain injury. RK has received an unrestricted grant from Heidelberg Engineering in support of development of a Neuro‐Toolbox analysis suite for optic nerve disorders using OCT.
Jaume Sastre‐Garriga has received speaking or consulting honoraria from Merck, Biogen, Genzyme, Celgene, and Novartis.
Sven Schippling received fees for speaking or consulting from Bayer Healthcare, Biogen, Genzyme/Sanofi, Merck, Novartis, Roche and Teva; research grants from Genzyme/Sanofi, Novartis, and Swiss Multiple Sclerosis Society; and he is now an employee of Roche, which was not involved in the study.
Patrick Vermersch received honoraria and consulting fees from Biogen, Sanofi‐Genzyme, Novartis, Teva, Merck, Roche, Imcyse, AB Science, and Celgene. He also received research supports from Novartis, Sanofi‐Genzyme, and Roche.
Shiv Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Genzyme, Genentech Corporation, EMD Serono & Celgene. He is the PI of investigator‐initiated studies funded by Genentech Corporation and Biogen Idec, and received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer.
Bianca Gerendas reports consulting fees from Roche and Novartis Ophthalmology (not relevant to the work presented herein) and receives funding from DXS.
Ursula Schmidt‐Erfurth has received personal fees from Bayer, Novartis, Genentech, and Roche.
Axel Petzold is part of the steering committee of the ANGI network which is sponsored by ZEISS, steering committee of the OCTiMS study which is sponsored by Novartis and reports speaker fees from Heidelberg‐Engineering.
Catherine Agoropoulou, Ying Zhang, and Gustavo Seifer are employees of Novartis.
Supporting information
Table S1. List of exclusion criteria for participants in the MS group and reference group (when applicable).
Data S1. Optical coherence tomography (OCT).
Figure S1. Different scan patterns used in the OCTiMS study for OCT imaging and respective evaluation areas for each scan.
Table S2. OCTiMS Principal Investigators (ordered alphabetically by countries).
Acknowledgments
The authors wish to thank Carolyn M. Ervin for her substantial contribution in the data analyses, as well as Mark Kirby, Aisling Towell, and Marie‐Catherine Mousseau (Novartis Ireland Ltd.) for their writing support, funded by Novartis Pharma AG, Basel, Switzerland. Frederik Barkhof is supported by the NIHR biomedical research center at UCLH. Sincere gratitude also to Alina Czechner, the senior reader at the Vienna Reading Center, who has supervised most of the manual OCT analysis, to Gordon Francis and Göril Karlsson (Novartis Pharma AG) who contributed to the design and initiation of the study and to Annik Laflamme (Novartis Pharma AG, Basel at time of study) who assisted in the initial development of this publication. The authors also thank patients, investigators, and site staff who participated in these studies, including all OCTiMS Principal Investigators (Table S2). In particular, the authors would like to extend their special thanks to Dr Pablo Villoslada for his ongoing commitment and fundamental contribution towards the completion of this work.
Funding Information
The authors wish to thank Carolyn M. Ervin for her substantial contribution in the data analyses, as well as Mark Kirby, Aisling Towell, and Marie‐Catherine Mousseau (Novartis Ireland Ltd.) for their writing support, funded by Novartis Pharma AG, Basel, Switzerland. FB is supported by the NIHR biomedical research center at UCLH.
Funding Statement
This work was funded by Novartis Pharma .
References
- 1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tur C, Moccia M, Barkhof F, et al. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat Rev Neurol. 2018;14(2):75‐93. [DOI] [PubMed] [Google Scholar]
- 3. Sastre‐Garriga J, Pareto D, Battaglini M, et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol. 2020;16(3):171‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40(11):2520‐2527. [PubMed] [Google Scholar]
- 5. Gordon‐Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69(16):1603‐1609. [DOI] [PubMed] [Google Scholar]
- 6. Frau J, Fenu G, Signori A, et al. A cross‐sectional and longitudinal study evaluating brain volumes, RNFL, and cognitive functions in MS patients and healthy controls. BMC Neurol. 2018;18(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuchling J, Paul F. Visualizing the central nervous system: imaging tools for multiple sclerosis and neuromyelitis optica spectrum disorders. Frontiers in Neurology. 2020;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frohman EM, Balcer LJ, Calabresi PA. Multiple sclerosis: can retinal imaging accurately detect optic neuritis? Nat Rev Neurol. 2010;6(3):125‐126. [DOI] [PubMed] [Google Scholar]
- 9. Siger M, Dzięgielewski K, Jasek L, et al. Optical coherence tomography in multiple sclerosis: thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol. 2008;255(10):1555‐1560. [DOI] [PubMed] [Google Scholar]
- 10. Dörr J, Wernecke KD, Bock M, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6(4):e18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saidha S, Sotirchos ES, Oh J, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70(1):34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saidha S, Al‐Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four‐year study. Ann Neurol. 2015;78(5):801‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez‐Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574‐584. [DOI] [PubMed] [Google Scholar]
- 14. Shi CE, Jiang H, Gameiro GR, et al. Visual function and disability are associated with focal thickness reduction of the ganglion cell‐inner plexiform layer in patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2019;60(4):1213‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vidal‐Jordana A, Pareto D, Cabello S, et al. Optical coherence tomography measures correlate with brain and spinal cord atrophy and multiple sclerosis disease‐related disability. Eur J Neurol. 2020;27(11):2225‐2232. [DOI] [PubMed] [Google Scholar]
- 16. Oertel FC, Zimmermann HG, Brandt AU, Paul F. Novel uses of retinal imaging with optical coherence tomography in multiple sclerosis. Expert Rev Neurother. 2019;19(1):31‐43. [DOI] [PubMed] [Google Scholar]
- 17. You Y, Graham EC, Shen T, et al. Progressive inner nuclear layer dysfunction in non‐optic neuritis eyes in MS. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lange AP, Sadjadi R, Costello F, Guber I, Traboulsee AL. Reproducibility of retinal nerve fiber layer measurements with manual and automated centration in healthy subjects using spectralis spectral‐domain optical coherence tomography. ISRN Ophthalmol. 2012;2012:860819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tewarie P, Balk L, Costello F, et al. The OSCAR‐IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schippling S, Balk LJ, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR‐IB criteria. Mult Scler. 2015;21(2):163‐170. [DOI] [PubMed] [Google Scholar]
- 21. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005;58(6):840‐846. [DOI] [PubMed] [Google Scholar]
- 22. Cruz‐Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303‐2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444‐1452. [DOI] [PubMed] [Google Scholar]
- 24. Langdon DW, Amato MP, Boringa J, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler. 2012;18(6):891‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):721‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doward LC, McKenna SP, Meads DM, Twiss J, Eckert BJ. The development of patient‐reported outcome indices for multiple sclerosis (PRIMUS). Mult Scler. 2009;15(9):1092‐1102. [DOI] [PubMed] [Google Scholar]
- 27. Khanifar AA, Parlitsis GJ, Ehrlich JR, et al. Retinal nerve fiber layer evaluation in multiple sclerosis with spectral domain optical coherence tomography. Clin Ophthalmol. 2010;20(4):1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta‐analysis. Lancet Neurol. 2010;9(9):921‐932. [DOI] [PubMed] [Google Scholar]
- 29. Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta‐analysis. Lancet Neurol. 2017;16(10):797‐812. [DOI] [PubMed] [Google Scholar]
- 30. Bock M, Brandt AU, Dörr J, et al. Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg. 2010;112(8):647‐652. [DOI] [PubMed] [Google Scholar]
- 31. Lamirel C, Newman NJ, Biousse V. Optical coherence tomography (OCT) in optic neuritis and multiple sclerosis. Rev Neurol. 2010;166(12):978‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nolan‐Kenney RC, Liu M, Akhand O, et al. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: an international study. Ann Neurol. 2019;85(5):618‐629. [DOI] [PubMed] [Google Scholar]
- 33. Xu SC, Kardon RH, Leavitt JA, Flanagan EP, Pittock SJ, Chen JJ. Optical coherence tomography is highly sensitive in detecting prior optic neuritis. Neurology. 2019;92(6):e527‐e535. [DOI] [PubMed] [Google Scholar]
- 34. Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balk LJ, Oberwahrenbrock T, Uitdehaag BM, Petzold A. Physiological variation of retinal layer thickness is not caused by hydration: a randomised trial. J Neurol Sci. 2014;344(1‐2):88‐93. [DOI] [PubMed] [Google Scholar]
- 36. Balk LJ, Sonder JM, Strijbis EM, et al. The physiological variation of the retinal nerve fiber layer thickn1ess and macular volume in humans as assessed by spectral domain‐optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(3):1251‐1257. [DOI] [PubMed] [Google Scholar]
- 37. Balk LJ, Cruz‐Herranz A, Albrecht P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263(7):1323‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Brück W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125(Pt 10):2202‐2212. [DOI] [PubMed] [Google Scholar]
- 39. Cilingir V, Batur M. Axonal degeneration independent of inflammatory activity: is it more intense in the early stages of relapsing‐remitting multiple sclerosis disease? Eur Neurol. 2020;83(5):508‐516. [DOI] [PubMed] [Google Scholar]
- 40. Pietroboni AM, Carandini T, Dell’Arti L, et al. Evidence of retinal anterograde neurodegeneration in the very early stages of multiple sclerosis: a longitudinal OCT study. Neurol Sci. 2020;41(11):3175‐3183. [DOI] [PubMed] [Google Scholar]
- 41. Oberwahrenbrock T, Traber GL, Lukas S, et al. Multicenter reliability of semiautomatic retinal layer segmentation using OCT. Neurol Neuroimmunol Neuroinflamm. 2018;5(3):e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80(1):47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17(12):1449‐1463. [DOI] [PubMed] [Google Scholar]
- 44. Samarawickrama C, Wang JJ, Huynh SC, et al. Ethnic differences in optic nerve head and retinal nerve fibre layer thickness parameters in children. Br J Ophthalmol. 2010;94(7):871‐876. [DOI] [PubMed] [Google Scholar]
- 45. Kimbrough DJ, Sotirchos ES, Wilson JA, et al. Retinal damage and vision loss in African American multiple sclerosis patients. Ann Neurol. 2015;77(2):228‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11(11):963‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gelfand JM, Cree BA, Nolan R, Arnow S, Green AJ. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol. 2013;70(5):629‐633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of exclusion criteria for participants in the MS group and reference group (when applicable).
Data S1. Optical coherence tomography (OCT).
Figure S1. Different scan patterns used in the OCTiMS study for OCT imaging and respective evaluation areas for each scan.
Table S2. OCTiMS Principal Investigators (ordered alphabetically by countries).


