SUMMARY
Virtually all land mammals and birds have two sleep states: slow-wave sleep (SWS) and rapid eye movement (REM) sleep [1, 2]. After deprivation of REM sleep by repeated awakenings, mammals increase REM sleep time [3], supporting the idea that REM sleep is homeostatically regulated. Some evidence suggests that periods of REM sleep deprivation for a week or more cause physiological dysfunction and eventual death [4, 5]. However, separating the effects of REM sleep loss from the stress of repeated awakening is difficult [2, 6]. The northern fur seal (Callorhinus ursinus) is a semiaquatic mammal [7]. It can sleep on land and in seawater. The fur seal is unique in showing both the bilateral SWS seen in most mammals and the asymmetric sleep previously reported in cetaceans [8]. Here we show that when the fur seal stays in seawater, where it spends most of its life [7], it goes without or greatly reduces REM sleep for days or weeks. After this nearly complete elimination of REM, it displays minimal or no REM rebound upon returning to baseline conditions. Our data are consistent with the hypothesis that REM sleep may serve to reverse the reduced brain temperature and metabolism effects of bilateral nonREM sleep, a state that is greatly reduced when the fur seal is in the seawater, rather than REM sleep being directly homeostatically regulated. This can explain the absence of REM sleep in the dolphin and other cetaceans and its increasing proportion as the end of the sleep period approaches in humans and other mammals.
In Brief
Lyamin et al. show that although fur seals have 80 min of REM sleep a day on land, they have little or no REM sleep when in water for as long as 2 weeks. When they return to land they show little or no REM rebound. Their switch to from bilateral to unihemispheric nonREM sleep in water may make REM sleep unnecessary.
RESULTS
We examined the sleep of fur seals when they were in seawater with and without access to a dry platform. These two experimental conditions approximate that of fur seals during the breeding and migratory seasons [7] (see Table S1). We continuously recorded electroencephalogram (EEG), electromyogram, electrooculogram, and electrocardiogram using a data logger. Slow-wave sleep (SWS) and REM sleep on land occurred when fur seals were lying or sitting (Figures 1 and 2). In agreement with our prior studies [9–11], fur seals on land displayed REM and nonREM sleep. SWS includes both bilateral SWS (BSWS) and unihemispheric SWS (USWS; see Figure 1E and Table S2), the latter resembling the unihemispheric slow-wave pattern seen in dolphins, with unilateral eye opening. After a baseline period of 2 days with access to a dry platform, the platform was removed for 10–14 days. In seawater, the seals slept at the surface predominantly in the lateral position (88% ± 7% of total sleep time, n = 4) with the remaining time in the prone position (Figures 1C, 1D, 1F, and 1G). SWS in seawater was predominantly USWS (94% ± 1% of all SWS compared to 61% ± 4% of SWS when on land; see Figures 1E–1G and Table S2). There was a clear association between the lateralization of EEG slow waves in fur seals sleeping in seawater and the asymmetric lateral posture and eye opening [8] (Figure 1F).
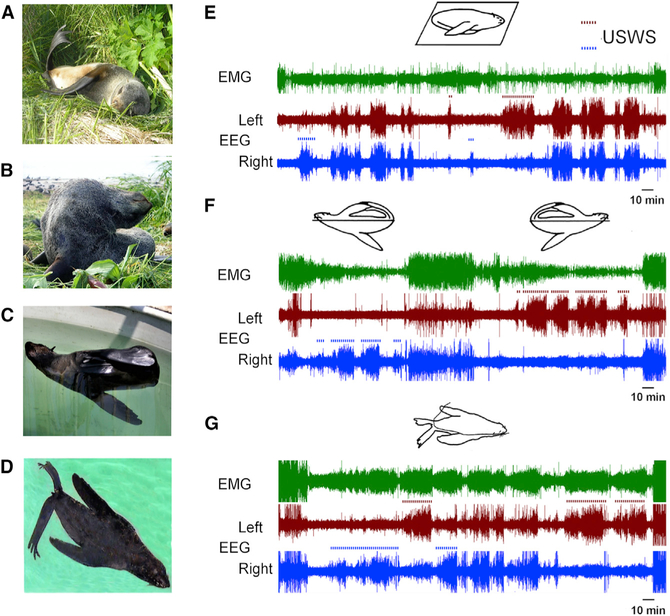
Figure 1. Sleep in Fur Seals.
(A and B) Sleep on land while lying (A) and sitting (B).
(C and D) Sleep in seawater at the surface in the lateral (C) and in a prone (D) position.
(E–G) Periods of sleep and wakefulness on land and in the lateral and prone positions as shown by EEG of the two cerebral hemispheres and neck electromyogram (EMG). Brown dotted lines indicate left unihemispheric slow-wave sleep (USWS), and blue dotted lines indicate right USWS. The drawings above each panel indicate the predominant fur seal posture during the recording. (E) shows the predominant posture while sleeping on land with relatively little EEG asymmetry. (F) illustrates the most common posture of sleep in the fur seal in water. The initial posture, with the right front flipper moving in the water, is asssoicated with right USWS, and the second posture, with the left front flipper moving in the water, is associated with left USWS. (G) illustrates a prone posture in water, which is less common, as the fur seals begins sleeping in right USWS, then left, then right then left again without changing the position. The photo in (D) and polygrams in (E)–(G) are from [8].
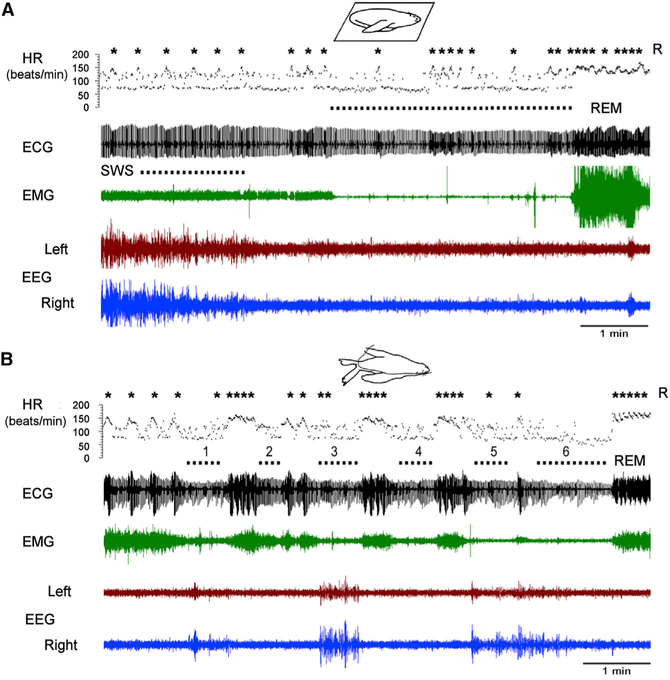
Figure 2. SWS and REM Sleep in the Fur Seal under Dry Baseline Conditions and in Seawater.
(A) Episodes of REM sleep in fur seals under baseline conditions were characterized by maximal muscle tone reduction and lasted up to 16 min (on average, 5 min). A representative episode is displayed.
(B) Episodes of REM sleep in seawater were much shorter (on average 23 s, no longer than 2 min) than under baseline conditions on land. The cluster of REM episodes that is presented is among the longest that we observed in water. The short episodes occurred while the head was held above seawater (episodes 1–4) and when the head was submerged (episodes 5 and 6). As described above and in Figure 3 and Tables S2 and S3, when fur seals were in the water, on many days, there was no REM sleep at all. REM sleep in fur seals was characterized by pronounced heart rate arrhythmia and apneas. High-voltage deflection in the EEG during episodes 1, 3, and 5 are movement artifacts caused by repeated immersion of head. ECG, electrocardiogram; HR, instantaneous heart rate; R, respiratory acts (inhale and exhale).
Throughout the period in seawater, the average daily amount of REM sleep in seals was reduced to 3 min a day versus 80 min under baseline conditions (a 96.4% ± 1.0% reduction; one-way ANOVA, F13,33 = 27.506, p = 3.21E–13; see Table S2). This is a greater amount and percentage reduction than the average reduction seen even with automated REM-sleep-deprivation techniques in the rat [4]. In seawater, the number of REM sleep episodes per day decreased to 20% ± 3% of the baseline values (F13,33 = 10.754, p = 9.98E–08), and the average duration of REM sleep episodes decreased to 13% ± 1% of baseline (F13,33 = 6.508, p = 2.68E–05; see also Figures 2A, 2B, and 3A–3C and Table S2). No REM sleep was recorded in the seals during the first 3–7 days in seawater. In one of the four seals, REM sleep was recorded on only one of 11 days (Figure 3A).
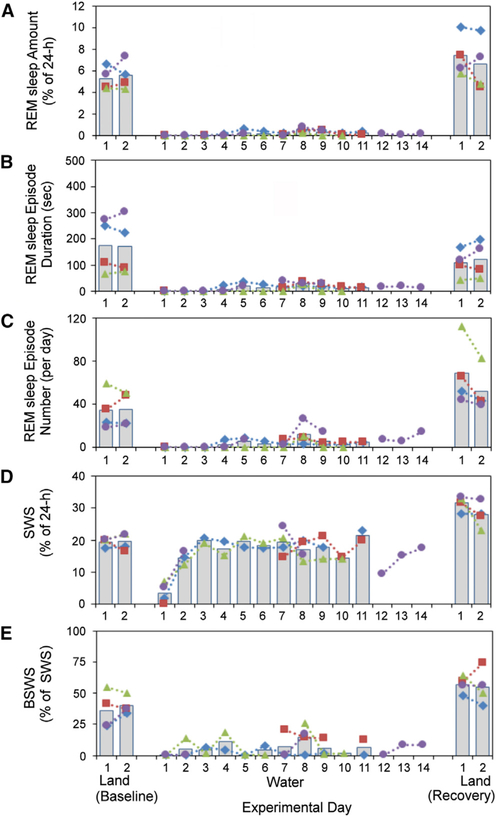
Figure 3. REM Sleep Is Suppressed When Fur Seals Are in Seawater with Little or No Rebound When Returned to Baseline Conditions.
The amount (A), duration (B), and number (C) of REM sleep episodes were substantially decreased in fur seals during the entire period in seawater. (D) shows the percentage of slow-wave sleep (BSWS plus USWS), and (E) shows the percentage of bilateral SWS (BSWS). No significant REM rebound was recorded when the fur seal returned to land. The total amounts of SWS decreased only on the first day in seawater and increased above baseline values when the seals were returned to baseline conditions (D). BSWS was almost completely absent in seawater (E; see also Table S2). The colored lines and symbols mark individual seals, and the gray bars indicate the average values. See also Table S4.
By the end of the 10th day, an accumulated “loss” of the expected amounts of REM sleep under baseline conditions averaged 765 ± 72 min (12.8 hr) or 974% ± 8% of projected daily baseline amounts (Figure 4A and Table S3). The episodes of REM sleep in seawater were characterized by head jerks, submergence of the head with apneas lasting up to 75 s, heart rate deceleration, and arrhythmia (Figure 2B; see also STAR Methods). Apneas (respiratory pauses longer than 30 s) were not recorded during waking or SWS on land.
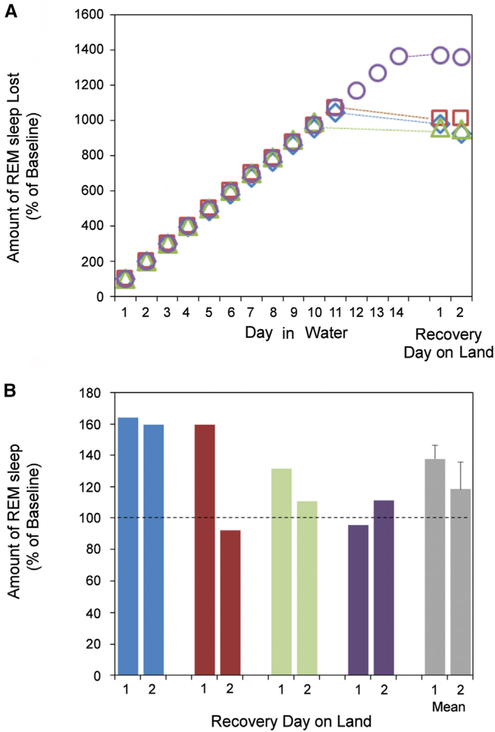
Figure 4. Cumulative Loss of Projected REM Sleep Time in Seawater without Significant Recovery with Return to Baseline Conditions.
(A) During 10–14 days in seawater, the estimated deficit of REM sleep in fur seals reached 995%–1,370% of the daily baseline amount. Different seals are coded by color, and average values are shown as gray bars. The dotted lines connect the last day in seawater (the 10th, 11th, or 14th) and the first recovery day in each seal.
(B) Despite their REM sleep loss, only two out of four seals (marked blue and brown) displayed a substantial increase in REM sleep beyond baseline values after return to baseline conditions. In two other seals (labeled light green and dark blue), REM sleep amounts were similar to the daily variation seen under baseline conditions. Average values are shown as mean ± SEM (see also Table S3). See also Table S4.
On the first day the seals were returned to baseline conditions (“recovery” day 1, R1), the amounts of REM sleep were not significantly greater than during baseline (Tukey’s post hoc test, df = 6, p > 0.05; see Table S2) (Figure 4B). On average, REM percent of 24 hr was 38% ± 16% above baseline level on R1 and 18% ± 14% above baseline on R2, with two of the four seals accounting for most of this increase. The amounts of REM sleep in the other two seals on R1 and R2 were within the range of day-to-day variation under baseline conditions.
For all seals combined, the average amount above baseline values on R1 was just 28 ± 17 min of the average 895 ± 147 min lost, or 3% ± 2% of REM sleep lost in seawater (Table S3). The estimated loss of REM sleep in the fourth seal, which stayed in seawater for 14 days, the longest period studied, was 21.5 hr or 1,369% of the daily baseline. However, the amount of REM sleep on R1 and R2 was comparable to its baseline prior to the period in seawater (Figure 4B, seal 4). Across seals, the number of REM sleep episodes did not significantly differ from baseline on R1, as was the case with REM sleep amounts. The amount of REM sleep in the seals on R1 did not correlate with the amount of REM sleep lost. On R2, the amounts of REM sleep remained substantially greater than baseline values in only one of the four seals (Tables S2 and S3).
In contrast to the near elimination of REM sleep, the total amounts of SWS (USWS plus BSWS) in fur seals in seawater ranged from 45% to 129% of baseline values except for the first day in water (W1) when it was reduced on average to 20% of baseline. The drop in SWS in the seawater was significant only on W1 (p < 0.01 compared to the baseline and other days in seawater, ANOVA, F13,28 = 18.961, p = 7.63E–10; see Table S2). The time spent in SWS as depicted in Figure 3D overestimates the amount of SWS that each hemisphere acquired, as SWS was largely unihemispheric in the water (Figure 3E). This hemispheric reduction of SWS may explain the increased time spent in SWS on R1. During all days in seawater, the amount of BSWS was significantly smaller than that under baseline conditions, with a decrease from 7.5% of the 24 hr to 0.5% of the 24 hr (p = 1.18E–09, F12,25 = 22.868) and the proportion of USWS was significantly greater with an increase from 62.0% to 93.8% of SWS time (p = 2.03E–08, F12,25 = 17.540; see Table S2 and Figure 3E). After a return to baseline conditions on both recovery days, the amount of BSWS more than doubled with respect to the average baseline amounts (p < 0.001; Table S2). This represented a reversal of the decreased BSWS in the water. The increase of total SWS over baseline values on R1 (62%; p < 0.001, df = 6) and R2 (43%; p = 0.05) may also have been a partial consequence of the thermoregulatory and activity adjustments produced by the animals’ return to a dry platform after the 10–14 day in-water period. The proportion of USWS dropped back to 69% of baseline values (Table S2).
DISCUSSION
When returned from their 10–14 days in seawater to baseline conditions (sleeping on a dry platform), the seals were in good condition, as judged by their appearance, activity, interaction with the experimenters, weight, and appetite. We did not undertake any further examination of changes in their health. The condition of fur seals after the extended REM sleep suppression in water is in dramatic contrast to that of rats deprived of REM sleep by the disk-over-water method for a similar period. The REM-sleep-deprived rats show weight loss, skin lesions, hypothermia, and a generally debilitated appearance, leading to death [4, 12].
In further contrast, REM sleep deprivation in the rat produced a significant increase of 560% of baseline REM amounts on recovery day 1 and 180% of baseline amounts on days 2–15 [4, 12, 13]. This difference in recovery amounts between fur seals and rats occurred despite the much greater loss of REM sleep in the fur seal, since the automated deprivation apparatus used in the rat had to first detect sleep states before each disruption, i.e., a considerable amount of REM sleep occurred during each of the hundreds of detections each day. In these rat studies, as in all conventional REM-sleep-deprivation studies, a considerable disruption of nonREM sleep also occurred [4, 13].
Prompted by the current findings, we re-analyzed the data we previously reviewed [14] for spontaneous REM sleep time versus nonREM sleep time across mammalian species. All land mammals so far examined have bilateral nonREM sleep exclusively. We find that REM sleep time is correlated with nonREM sleep time (r = +0.488, p = 0.000279; see Table S4). Thus, REM sleep amounts are a function of bilateral nonREM amounts, even as these nonREM amounts vary across terrestrial mammalian species.
The assumption has been that animals have a daily need for REM sleep and display a rebound of REM sleep amount and intensity after it has been selectively eliminated or reduced, even if the loss is not fully recovered [3, 15–17]. The northern fur seal is a semiaquatic mammal. During the summer breeding season, fur seals alternate between staying on land for a period of several days and foraging in the ocean for periods of up to 2 weeks. During their winter migration, fur seals remain continuously pelagic for up to 10 months [7]. We have found that REM sleep is eliminated or greatly reduced when fur seals enter seawater. This lasts for a period of at least 2 weeks. We also found that after a return to baseline conditions, REM sleep was substantially increased in only two out of the four fur seals. In addition, the increase of REM sleep was not proportional to REM sleep lost. REM sleep and BSWS amounts are reduced in fur seals in seawater, at a time when the animal requires high levels of alertness, cognitive performance, learning, and motor activity to navigate, locate prey, and avoid predators compared to when the animals are resting in large, safe groups on land and have daily REM sleep.
One can appreciate why suppression of REM sleep and high-voltage bilateral SWS would be adaptive in fur seals when they stay in seawater. It is known that animals sleeping under the risk of predation have smaller amounts of REM sleep [1]. Muscle tone reduction and elevated arousal thresholds are features of REM sleep and bilateral SWS [2]. Thus, long episodes of bilateral sleep at the surface of seawater with bilateral eye closure would be potentially dangerous for fur seals. Aspiration of seawater during sleep is also a considerable risk since the nostrils must be held above the waves. Thermoregulation is impaired in REM sleep [18], and long episodes in cold water might cause hypothermia [8, 11]. Phocidae seals (the other group of pinnipeds) deal with some of these issues by holding their breath and hiding in the seawater’s depths during which REM sleep of normal duration may occur [19–21]. Phocids, unlike otariids, do not have USWS in seawater or on land.
An absence or nearly complete absence or REM sleep may be a characteristic of unihemispherically sleeping animals. Repeated studies of cetaceans (dolphins and whales) have failed to find evidence for REM sleep in any amount [11]. Cetaceans solve the problem of breathing and maintaining vigilance in the ocean environment by never showing bilateral high-voltage EEG activity. Rather, they have periods of high-amplitude unihemispheric slow waves, which can occur during swimming, while the other hemisphere shows a waking EEG. We find that fur seals convert their bilateral nonREM sleep on land to a largely unihemispheric pattern in seawater, in parallel with a profound reduction or elimination of REM sleep.
It has been hypothesized that the function of REM sleep is linked to nonREM sleep [22–24]. In land mammals, REM sleep normally follows nonREM periods, and the duration and intensity of phasic events in REM increases throughout the sleep period, being maximal near the time of spontaneous awakening [22]. We hypothesize that a major function of REM sleep may be the reversal of the metabolic depression and cooling of the brainstem that result from prolonged bilateral nonREM sleep. Novel evidence for this is the correlation of REM sleep amount with nonREM sleep across mammalian species (see Table S4). Further work is necessary to determine the strength of this relation across species, the timing and duration of individual REM periods in relation to prior nonREM periods, and the role of circadian and environmental variables in the occurrence of REM sleep.
The brainstem is necessary and sufficient for REM sleep generation [2]. In a decerebrate cat, in which hypothalamic thermoregulatory systems are removed or disconnected from the brainstem, thermoregulation is abolished and brain temperature drifts toward the controlled environmental temperature. Under these conditions, low brain temperature is associated with greatly increased REM sleep, with REM comprising up to 70% of recording time at a brain temperature of 25°C, an amount of REM never seen in intact cats (or any other mammal). Average baseline REM sleep amounts in intact cats at a brain temperature of 39°C is 13% [2, 25]. Thus, the brainstem generators of REM sleep are triggered by brain cooling, consistent with a role of the smaller reduction in temperature which occurs in nonREM sleep in triggering the smaller REM durations seen in intact mammals [26].
Total sleep deprivation by the disk over water technique in the rat elicits a surprising, and very large, increase in REM sleep, rather than total sleep or nonREM sleep when the deprivation ends [13]. But this method of deprivation not only decreases sleep, it also decreases body and brain temperature. This is consistent with a triggering of REM sleep to counter the brain hypothermia that occurs with the disk-over-water deprivation technique.
Further studies might be profitably aimed at recording temperature from both cerebral hemispheres and bilaterally in the brainstem of the fur seal in seawater and on land and determining the temperature dependence of REM triggering. Alertness and brain temperature are considerably greater when animals awaken at the end of periods of REM than after nonREM sleep [27–29] as is brain metabolic rate [30]. The alternation of nonREM and REM sleep allows animals to achieve the energetic savings of nonREM sleep [31, 32], without the relatively impaired awakening that occurs in nonREM sleep arousals.
The loss of REM sleep for long periods of time in fur seals, or its complete absence in cetaceans is not incompatible with long lifespans and large brain size. Cetaceans have the largest brains and among the longest lifespans of any mammal [33] and are considered by some to rival primates in their complex cognition [34, 35]. Pathologies resulting from sleep deprivation in land mammals may be the result of stress induced by the sleep deprivation procedure, particularly the repeated awakenings, which may reach 1,000 a day, linked to the cortisol awakening response [6, 36, 37] and phasic changes in brain neuronal activity and neurochemistry, including monoamine release [2].
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jerome Siegel (jsiegel@ucla.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Data were collected from 4 juvenile northern fur seals (Callorthinus ursinus L). The animals (3 males and 1 female, 18–25 kg, were 2–5 years old; See also Table S1) were captured on the Commander Islands (the Western Pacific, Russia) 1 or 2 years before the study and were well adapted to captivity. The capture permits were issued by the Russian Federation Federal Agency for Fishery. Experiments were conducted on land and in an indoor pool measuring 4 ×4 ×1.8 m.
All procedures were approved by the Institutional Animal Care and Use Committees of the University of California Los Angeles, the Veterans Affairs Greater Los Angeles Healthcare System Committees and the A.E. Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences. Studies were conducted in accordance with the National Institute of Health Guide for the Care and Use of Experimental Animals. The experiments were performed at the Utrish Marine Station of the Severtsov Institute (the Black Sea, Russia).
METHOD DETAILS
Surgical Procedures
Fur seals were implanted under general anesthesia (isoflurane, 1–3%) with two pairs of stainless steel electrodes for electroencephalogram (EEG) recording from symmetrical fronto-occipital or fronto-parietal derivations of the right and left hemispheres. A pair of electrodes was also implanted into the supraorbital bone above the eyes to record the electrooculogram (EOG). Four Teflon-coated multistranded stainless steel wires (0.3 mm in diameter) were inserted into the neck muscles to record the electromyogram (EMG). Two longer wires were implanted subcutaneously to record electrocardiogram (EKG). Surgical procedures were described in detail in our prior publications (e.g., [9, 10, 38]). After implantation, the seals were returned to the pool and allowed at least 5 days to recover before the experiments started. As in our prior studies, by the second day after surgery the implanted seals resumed eating fish and appeared to be in good condition.
Experimental design
Recordings (EEG, EMG, ECG, EOG and ECG) were conducted using a portable 8-channel data logger (Neurologger, bandwidth 1–115 Hz, acquisition rate 200 Hz) previously used to study sleep in birds [16, 17, 39], land mammals [40] and marine mammals [17, 41]. The datalogger and a battery were placed in a custom-made waterproof box measuring 4 × 4 × 2.5 cm and weighing 120 g. The box was attached to a platform on the seal’s back using cable ties. The platform (6 × 10 × 0.5 cm) was made from a neoprene resin and glued to the seal’s fur with a neoprene adhesive (McNett, WA) during the surgery. The electrodes were connected to the data logger via a flexible 45 cm long, low noise coaxial cable.
We monitored seals over 2 “baseline” (B1,B2) days when they had access to seawater and a dry platform, followed by 10 – 14 “in seawater” days with seals restricted to the seawater, followed by an additional 2 “recovery” (R1,R2) days under baseline conditions. The seals were instrumented with the recording equipment 24 hr before the first baseline day started, to allow adaptation to the recording equipment (the datalogger and cable). A wooden island (0.6 × 1 m) was positioned in the center of the pool under baseline and recovery conditions, so the seals could sleep on a dry surface as they do in the wild during the summer season. Under these conditions, the seals rested and slept while lying on the wooden platform. They also slept while sitting on the platform or in the shallow seawater at the bottom of the pool. Following 2 baseline days the platform was removed and the pool was filled with seawater to a level of 1.2 m starting the “in seawater” period. The seals were restricted to the seawater for 10–14 days. They then were returned to the baseline conditions and recording continued for 2 continuous days (“recovery”; See also Table S1).
The data were downloaded from the datalogger every 5 days. In order to do this, the seal was restrained for about 5 min between 07:30 and 8:00 when the pool was completely drained and refilled with fresh seawater pumped in from the Black Sea. The seals were accustomed to this short procedure which did not appear to cause any stress. The seals approached the personnel and picked up fish soon after they had been released.
All recording days started at 08:00 and included simultaneous recording of EEG from two symmetrical cortical derivations (fronto-occipital and fronto-parietal), EMG of neck muscles, EOG and ECG. The behavior of all seals was continuously video recorded with one panorama camera and 3 high resolution remote control cameras (520 lines, 0.01 Lux, 35 optical zoom, 360 degree view) fitted with infrared light sources. The data were downloaded from the datalogger and converted into Spike 2 software format (Cambridge Electronic Design, UK) for further analysis.
During the entire period, the seals were fed fish twice a day (at 08:00 and 18:00) and kept on a 12 hr light (300 lux at floor level or 400 lux at seawater level when afloat; onset at 08:00) and 12 hr dim light (30 lux; onset at 20:00) cycle. During recording, the average air temperature above the pool varied between 18–25°C. The temperature of the seawater corresponded to that in the Black Sea, with which it was exchanged daily. The average temperature varied between 10 and 27°C (Table S5). However, for each individual seal the daily variation of the air temperature did not exceed 5°C and the daily variation of the seawater temperature did not exceed 3°C.
Scoring behavioral states and polygrams
SWS and REM sleep were quantified for two baseline and two recovery days. Out of a total of the 46 days fur seals stayed in seawater, SWS and REM sleep were scored and quantified for 33 and 38 days, respectively. On the remaining days reliable scoring was not possible due to artifacts in the EEG of either one or both hemispheres (Table S1). These data losses were caused by the damage to the recording wires or failure of the data logger.
The behavior of seals was scored in 20 s epochs. Behavioral features of active waking (AW), quiet waking (QW), slow wave sleep (SWS) and REM sleep in fur seals while on land have been described in our prior publications [8–10, 38].
AW in fur seals in seawater included swimming in the pool, grooming and feeding. QW was scored when the animal rested at the surface, was looking around, slowly grooming or being motionless, as long as low voltage desynchronized EEG was recorded in both cortical hemispheres. SWS occurred in seawater at the surface in two characteristic postures: the lateral and prone positions. Most of the time the head was above the surface and the nostrils were in the air.
On the infrequent occasions when REM sleep occurred in seawater, the animal could be in either the lateral or prone positions. Isolated REM episodes or a series of short episodes were characterized by sinking of the head toward the seawater, eye and muscle jerks, body twitches, breathing, heart rate irregularity and release of air bubbles. These short REM episodes were terminated by brief awakening during which the seals opened both eyes and paddled more intensively to resume the sleep posture.
SWS was subdivided into unihemispheric SWS (USWS), asymmetrical SWS and bilateral SWS (BSWS). Waking was scored when low voltage EEG activity was recorded in both cerebral hemispheres. USWS was scored when EEG slow waves at least 2-fold above the baseline desynchronized EEG activity occurred in one hemisphere for at least of 50% of the epoch time simultaneously with the waking EEG pattern in the other hemisphere. Asymmetrical SWS was scored when low voltage EEG slow waves (amplitude at least 2 times the waking desynchronized activity but smaller than maximal, occupying at least 50% of the epoch time) were recorded in one hemisphere in parallel with EEG slow waves of maximal amplitude (occupying at least 50% of the epoch). BSWS was scored when either low or high voltage EEG slow wave stage was recorded in both hemispheres simultaneously. The majority of asymmetrical SWS in fur seals was characterized by highly pronounced interhemispheric EEG asymmetry resembling USWS in cetaceans. Those asymmetrical SWS episodes are herein referred to as USWS. More information on scoring can be found in our prior publications [8, 9, 38].
In addition to visual scoring, for each hemisphere, EEG spectral power from 1.2–4 Hz (SWA) was computed in consecutive 5 s epochs by fast Fourier transformation using Spike 2 software. Epochs containing artifacts were excluded. The average SWA in each hemisphere was then calculated for 20 s epochs. To quantify the expression of interhemispheric EEG asymmetry during BSWS, left and right USWS, we used the asymmetry index [AI = (L − R) ⁄ (L + R)], where L and R are the spectral powers in the left and right hemispheres, respectively (36). REM sleep was scored by the criteria described in [8, 9, 38].
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were performed using Sigma Plot 11 and Excel. Data were assessed for significance using one-way ANOVA followed by Tukey’s post hoc multiple-comparison tests, t test or Pearson Product Moment correlation test. Values are given as mean ± SEM.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Northern fur seal Callorhinus ursinus | N/A | N/A |
Highlights.
Fur seals have no or little REM sleep when they stay in water
Moving from land to water, fur seals switch from bilateral to unihemispheric sleep
Unihemispheric sleep may explain the absence of REM sleep in dolphins
REM may serve to reverse the brain cooling that occurs in bilateral nonREM sleep
ACKNOWLEDGMENTS
This study was supported by the NIH (DA034748), VA (2I01-BX001753), NSF (0919929), Russian Fund for Basic Research (13-04-01704 and 16-04-01306), and Utrish Dolphinarium. The authors thank Dr. J. Lapierre for assistance with the surgeries and animal care.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes five tables and can be found with this article online at https://doi.org/10.1016/j.cub.2018.05.022.
REFERENCES
- 1.Lesku JA, and Rattenborg NC (2014). Avian sleep. Curr. Biol. 24, R12–R14. [DOI] [PubMed] [Google Scholar]
- 2.Siegel JM (2017). Rapid eye movement sleep. In Principles and Practice of Sleep Medicine, Kryger MK, Roth T, and Dement WC, eds. (Elsevier; ), pp. 78–95. [Google Scholar]
- 3.Dement W (1960). The effect of dream deprivation. Science 131, 1705–1707. [DOI] [PubMed] [Google Scholar]
- 4.Kushida CA, Bergmann BM, and Rechtschaffen A (1989). Sleep deprivation in the rat: IV. Paradoxical sleep deprivation. Sleep 12, 22–30. [DOI] [PubMed] [Google Scholar]
- 5.Venancio DP, and Suchecki D (2015). Prolonged REM sleep restriction induces metabolic syndrome-related changes: mediation by pro-inflammatory cytokines. Brain Behav. Immun. 47, 109–117. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JM (2009). Sleep viewed as a state of adaptive inactivity. Nat. Rev. Neurosci. 10, 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentry RL (1997). Behavioral Ecology of the Northern Fur Seal (Princeton University Press; ). [Google Scholar]
- 8.Lyamin OI, Mukhametov LM, and Siegel JM (2017). Sleep in the northern fur seal. Curr. Opin. Neurobiol. 44, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyamin OI, Kosenko PO, Lapierre JL, Mukhametov LM, and Siegel JM (2008). Fur seals display a strong drive for bilateral slow-wave sleep while on land. J. Neurosci. 28, 12614–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapierre JL, Kosenko PO, Kodama T, Peever JH, Mukhametov LM, Lyamin OI, and Siegel JM (2013). Symmetrical serotonin release during asymmetrical slow-wave sleep: implications for the neurochemistry of sleep-waking states. J. Neurosci. 33, 2555–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, and Siegel JM (2008). Cetacean sleep: an unusual form of mammalian sleep. Neurosci. Biobehav. Rev. 32, 1451–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, and Gilliland MA (1989). Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep 12, 68–87. [PubMed] [Google Scholar]
- 13.Everson CA, Gilliland MA, Kushida CA, Pilcher JJ, Fang VS, Refetoff S, Bergmann BM, and Rechtschaffen A (1989). Sleep deprivation in the rat: IX. Recovery. Sleep 12, 60–67. [PubMed] [Google Scholar]
- 14.Siegel JM (2005). Clues to the functions of mammalian sleep. Nature 437, 1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiromani PJ, Lu J, Wagner D, Thakkar J, Greco MA, Basheer R, and Thakkar M (2000). Compensatory sleep response to 12 h wakefulness in young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 278, R125–R133. [DOI] [PubMed] [Google Scholar]
- 16.Lesku JA, Rattenborg NC, Valcu M, Vyssotski AL, Kuhn S, Kuemmeth F, Heidrich W, and Kempenaers B (2012). Adaptive sleep loss in polygynous pectoral sandpipers. Science 337, 1654–1658. [DOI] [PubMed] [Google Scholar]
- 17.Rattenborg NC, Voirin B, Cruz SM, Tisdale R, Dell’Omo G, Lipp HP, Wikelski M, and Vyssotski AL (2016). Evidence that birds sleep in mid-flight. Nat. Commun. 7, 12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmeggiani PL, Zamboni G, Cianci T, and Calasso M (1977). Absence of thermoregulatory vasomotor responses during fast wave sleep in cats. Electroencephalogr. Clin. Neurophysiol. 42, 372–380. [DOI] [PubMed] [Google Scholar]
- 19.Lyamin OI (1993). Sleep in the harp seal (Pagophilus groenlandica). Comparison of sleep on land and in water. J. Sleep Res. 2, 170–174. [DOI] [PubMed] [Google Scholar]
- 20.Castellini MA, Milsom WK, Berger RJ, Costa DP, Jones DR, Castellini JM, Rea LD, Bharma S, and Harris M (1994). Patterns of respiration and heart rate during wakefulness and sleep in elephant seal pups. Am. J. Physiol. 266, R863–R869. [DOI] [PubMed] [Google Scholar]
- 21.Mitani Y, Andrews RD, Sato K, Kato A, Naito Y, and Costa DP (2010). Three-dimensional resting behaviour of northern elephant seals: drifting like a falling leaf. Biol. Lett. 6, 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carskadon MA, and Dement WC (2005). Normal human sleep. In Principles and Practice of Sleep Medicine, Kryger MH, Roth T, and Dement WC, eds. (W.B. Saunders; ), pp. 13–23. [Google Scholar]
- 23.Ursin R (1968). The two stages of slow wave sleep in the cat and their relation to REM sleep. Brain Res. 11, 347–356. [DOI] [PubMed] [Google Scholar]
- 24.Benington JH, and Heller HC (1994). Does the function of REM sleep concern non-REM sleep or waking? Prog. Neurobiol. 44, 433–449. [DOI] [PubMed] [Google Scholar]
- 25.Jouvet M, Buda C, and Sastre JP (1990). Progressive hypothermia is accompanied by an almost permanent paradoxical sleep in pontine cats. Sleep Res. 19, 38. [Google Scholar]
- 26.Szymusiak R, and McGinty D (2008). Hypothalamic regulation of sleep and arousal. Ann. N Y Acad. Sci. 1129, 275–286. [DOI] [PubMed] [Google Scholar]
- 27.Wehr TA (1992). A brain-warming function for REM sleep. Neurosci. Biobehav. Rev. 16, 379–397. [DOI] [PubMed] [Google Scholar]
- 28.Horner RL, Sanford LD, Pack AI, and Morrison AR (1997). Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 778, 127–134. [DOI] [PubMed] [Google Scholar]
- 29.Parmeggiani PL (2007). REM sleep related increase in brain temperature: a physiologic problem. Arch. Ital. Biol. 145, 13–21. [PubMed] [Google Scholar]
- 30.Buchsbaum MS, Hazlett EA, Wu J, and Bunney WE Jr. (2001). Positron emission tomography with deoxyglucose-F18 imaging of sleep. Neuropsychopharmacology 25 (5, Suppl), S50–S56. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt MH (2014). The energy allocation function of sleep: a unifying theory of sleep, torpor, and continuous wakefulness. Neurosci. Biobehav. Rev. 47, 122–153. [DOI] [PubMed] [Google Scholar]
- 32.Siegel JM (2011). Sleep in animals: a state of adaptive inactivity. In Principles and Practice of Sleep Medicine, Kryger MH, Roth T, and Dement WC, eds. (Elsevier; ), pp. 126–138. [Google Scholar]
- 33.Keane M, Semeiks J, Webb AE, Li YI, Quesada V, Craig T, Madsen L-B, van Dam S, Brawand D, Marques PI, et al. (2015). Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 10, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manger PR (2013). Questioning the interpretations of behavioral observations of cetaceans: is there really support for a special intellectual status for this mammalian order? Neuroscience 250, 664–696. [DOI] [PubMed] [Google Scholar]
- 35.Marino L, Connor RC, Fordyce RE, Herman LM, Hof PR, Lefebvre L, Lusseau D, McCowan B, Nimchinsky EA, Pack AA, et al. (2007). Cetaceans have complex brains for complex cognition. PLoS Biol. 5, e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clow A, Law R, Evans P, Vallence AM, Hodyl NA, Goldsworthy MR, Rothwell JR, and Ridding MC (2014). Day differences in the cortisol awakening response predict day differences in synaptic plasticity in the brain. Stress 17, 219–223. [DOI] [PubMed] [Google Scholar]
- 37.Shibuya I, Nagamitsu S, Okamura H, Ozono S, Chiba H, Ohya T, Yamashita Y, and Matsuishi T (2014). High correlation between salivary cortisol awakening response and the psychometric profiles of healthy children. Biopsychosoc. Med. 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyamin OI, Lapierre JL, Kosenko PO, Mukhametov LM, and Siegel JM (2008). Electroencephalogram asymmetry and spectral power during sleep in the northern fur seal. J. Sleep Res. 17, 154–165. [DOI] [PubMed] [Google Scholar]
- 39.Vyssotski AL, Serkov AN, Itskov PM, Dell’Omo G, Latanov AV, Wolfer DP, and Lipp HP (2006). Miniature neurologgers for flying pigeons: multichannel EEG and action and field potentials in combination with GPS recording. J. Neurophysiol. 95, 1263–1273. [DOI] [PubMed] [Google Scholar]
- 40.Voirin B, Scriba MF, Martinez-Gonzalez D, Vyssotski AL, Wikelski M, and Rattenborg NC (2014). Ecology and neurophysiology of sleep in two wild sloth species. Sleep (Basel) 37, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyamin OI, Kosenko PO, Vyssotski AL, Lapierre JL, Siegel JM, and Mukhametov LM (2012). Study of sleep in a walrus. Dokl. Biol. Sci. 444, 188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.