Supplemental Digital Content is available in the text.
Keywords: children, mortality, organ dysfunction, sepsis, septic shock, severe sepsis
Abstract
Objective:
To determine the associations of demographic, clinical, laboratory, organ dysfunction, and illness severity variable values with: 1) sepsis, severe sepsis, or septic shock in children with infection and 2) multiple organ dysfunction or death in children with sepsis, severe sepsis, or septic shock.
Data Sources:
MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from January 1, 2004, and November 16, 2020.
Study Selection:
Case-control studies, cohort studies, and randomized controlled trials in children greater than or equal to 37-week-old postconception to 18 years with suspected or confirmed infection, which included the terms “sepsis,” “septicemia,” or “septic shock” in the title or abstract.
Data Extraction:
Study characteristics, patient demographics, clinical signs or interventions, laboratory values, organ dysfunction measures, and illness severity scores were extracted from eligible articles. Random-effects meta-analysis was performed.
Data Synthesis:
One hundred and six studies met eligibility criteria of which 81 were included in the meta-analysis. Sixteen studies (9,629 patients) provided data for the sepsis, severe sepsis, or septic shock outcome and 71 studies (154,674 patients) for the mortality outcome. In children with infection, decreased level of consciousness and higher Pediatric Risk of Mortality scores were associated with sepsis/severe sepsis. In children with sepsis/severe sepsis/septic shock, chronic conditions, oncologic diagnosis, use of vasoactive/inotropic agents, mechanical ventilation, serum lactate, platelet count, fibrinogen, procalcitonin, multi-organ dysfunction syndrome, Pediatric Logistic Organ Dysfunction score, Pediatric Index of Mortality-3, and Pediatric Risk of Mortality score each demonstrated significant and consistent associations with mortality. Pooled mortality rates varied among high-, upper middle-, and lower middle-income countries for patients with sepsis, severe sepsis, and septic shock (p < 0.0001).
Conclusions:
Strong associations of several markers of organ dysfunction with the outcomes of interest among infected and septic children support their inclusion in the data validation phase of the Pediatric Sepsis Definition Taskforce.
Infections account for 26.5% of the global burden of disease (1) and 25% of deaths in children worldwide (2). However, the clinical manifestations of these infections vary from minimal symptoms to multiple organ failure and death. The currently accepted definitions of sepsis, severe sepsis, and septic shock were developed and refined using different criteria to help identify, treat, and study patients with infections who are at higher risk of significant morbidity and mortality (3, 4). However, specific variables identifying children with sepsis and their resulting outcomes have never been rigorously evaluated in a systematic review.
The 2016 sepsis definition update in adult patients (Sepsis-3) included a systematic review of reported criteria used to identify adults with septic shock (5). This review focused on hemodynamic criteria, was primarily limited to studies from upper middle-income countries (UMICs) and high-income countries (HICs), and specifically excluded pediatric studies. Furthermore, results of adult trials cannot be extrapolated to children because of differences in epidemiology (6), mortality rates (7), underlying diseases (8), disease-specific outcomes (9, 10), and differing responses to therapy (11, 12).
Therefore, the Society of Critical Care Medicine (SCCM) convened the Pediatric Sepsis Definition Taskforce to evaluate, develop, and validate criteria for the identification of sepsis in children. As part of this process, the Taskforce conducted a systematic review with the explicit goal of determining the ability of demographic, clinical, laboratory, organ dysfunction, and illness severity variables to capture children with more severe infections. For this purpose, we assessed association of these variables with: 1) sepsis, severe sepsis, or septic shock in children with suspected or confirmed infection and 2) with new or progressive multiple organ dysfunction (NPMODS) or mortality in children with sepsis, severe sepsis, or septic shock.
MATERIALS AND METHODS
The protocol including search strategy has been previously published (13) and is summarized below.
Eligibility Criteria
Inclusion criteria for studies were: 1) the words “sepsis,” “septic shock,” or “septicemia” present in the title or abstract; 2) publication between January 1, 2004, and November 16, 2020; 3) sepsis, septic shock, septicemia, NPMODS, or mortality reported as an outcome; 4) case-control study, cohort study, or randomized trial; and 5) study population of children greater than or equal to 37-week-old postconception to less than 18 years. Studies meeting the following criteria were excluded: 1) no reported data on children with sepsis, severe sepsis, or septic shock; 2) less than 50 children with sepsis, septicemia, severe sepsis, or septic shock; 3) abstracts, case studies, narrative reviews, or surveys; 4) variable values within 24 hours of admission not provided; 5) no comparator group for variable in question; 6) sepsis criteria not specified; 7) article not available; or 8) focused on criteria only available for research (e.g., gene-expression data). Only 27 non-English language articles (0.4%, 27/7502) were identified by the search (17 at abstract screening and 10 following full-text review). Therefore, non-English language studies were excluded.
Data Sources
We identified eligible studies by searching MEDLINE (including Epub Ahead of Print), Embase, and the Cochrane Central Register of Controlled Trials databases.
Study Selection
The titles, abstracts, and full texts were screened using a previously validated platform Insight Scope (14). Each title and full-text article were screened by two reviewers, and at each screening level and for data extraction, conflicts were resolved by a third reviewer.
Data Extraction and Management
Data were extracted from full-text articles using a Research Electronic Data Capture (REDCap) platform (15) hosted at the Children’s Hospital of Eastern Ontario Clinical Research Unit. Corresponding authors were contacted twice for missing data. The quality of selected articles was determined using the first four domains of the Quality in Prognostics Studies tool for assessment of risk of bias in observational studies (16). The last two domains pertain to confounding and statistical analysis that were not applicable to the unadjusted data used in our meta-analysis. The overall risk of bias was determined as the highest risk of bias attributed to any criterion. Unadjusted data were extracted since many studies did not report adjusted data and others did not specify the variables they adjusted for or adjusted for different variables (17). Studies were categorized as being conducted in low-income countries (LICs), low-middle-income countries (LMICs), UMICs, and HICs according to the World Bank classification of 2019–2020 (18).
Outcomes
The primary outcome for the meta-analysis of articles describing children with infection was the presence of sepsis, severe sepsis, or septic shock as defined in each individual study. The primary outcome for the meta-analysis of articles describing children with sepsis, severe sepsis, or septic shock was the development of NPMODS and/or death. Mortality was defined as at or prior to hospital discharge.
Data Synthesis and Analysis
Frequencies and descriptive statistics are reported for study demographics and patient characteristics from included studies. We pooled outcomes reported by two or more studies. We calculated unadjusted prognostic odds ratios (ORs) and 95% CIs for dichotomous variables and calculated the mean difference with 95% CIs for continuous variables. We imputed the mean and sd when median, interquartile range, or range and sample size were reported (19, 20). Statistical heterogeneity was assessed using I2 statistic and visual inspection of the forest plots, and DerSimonian-Laird random-effects model was employed for all comparisons. All analyses were conducted using Stata (StataCorp, Release 16.1. College Station, TX) (21). Baseline sepsis, severe sepsis, and septic shock rates among HIC, UMIC, and LMIC were compared using Kruskal-Wallis tests weighted for study sample sizes.
RESULTS
Overview of Included Studies
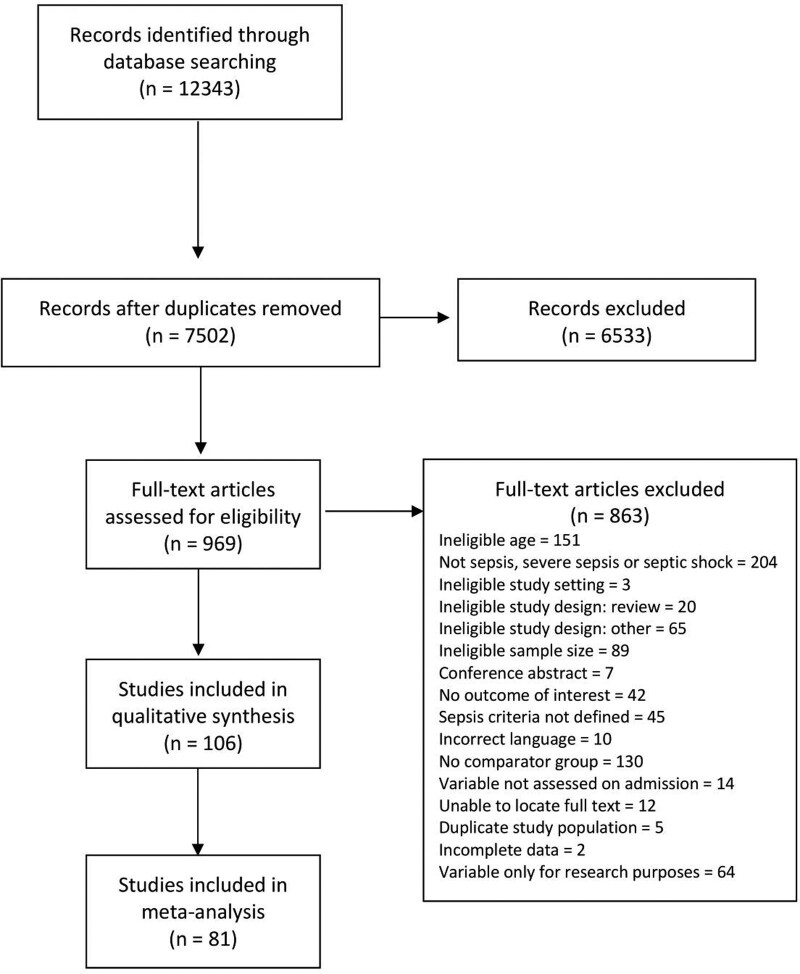
The search yielded 12,343 citations of which 969 underwent full-text review for eligibility. Of these, 863 were excluded (Fig. 1); 106 citations, representing 35 countries, were retained for the systematic review and 81 articles (154,674 patients) provided sufficient data for the meta-analysis. The remaining 25 articles met the inclusion criteria but studied individual variables that were unable to be combined in the meta-analysis and were, therefore, described in the narrative review. Characteristics of all included studies are summarized in Table 1. Studies represented all regions from the World Bank economies (18) with 46.2% (47/106) being conducted in HICs, 30.2% (35/106) in UMICs, 22.6% (23/106) in LMICs, and one in a LIC. All multicenter studies except one (10) included sites from the same income level. The remaining study (10) was conducted in 23/26 HIC and 3/26 UMIC settings and was, therefore, classified as an HIC study. The patient characteristics for included studies are shown in Table 2. More than half the patients were male (pooled estimate 55.7%; 95% CI, 54.8–56.6). The majority of studies were of PICU patients (70.8%, 75/106) followed by those from the emergency department (ED) (10.4%, 11/106). The most commonly used definition of sepsis was the 2005 International Pediatric Sepsis Consensus Conference (2005 IPSCC) criteria (69.8%, 74/106; Supplementary Table 1, http://links.lww.com/CCM/G817) (3).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for included studies.
TABLE 1.
Characteristics of All Included Studies
| Characteristic | Studies in Meta-Analysis (n = 81), n (%) | Patients From Meta-Analysis (n = 154,674), n (%) | Narrative Studies (n = 25), n (%) | Patients From Narrative Studies (n = 5,812) |
|---|---|---|---|---|
| Publication year | ||||
| 2004–2008 | 8 (9.9) | 7,861 (5.1) | 6 (24.0) | 374 (6.4) |
| 2009–2012 | 5 (6.2) | 1,499 (0.1) | 0 (0) | 0 (0) |
| 2013–2016 | 13 (16.0) | 53,340 (34.4) | 7 (28.0) | 1,046 (18.0) |
| 2017–2020 | 55 (67.9) | 91,974 (59.4) | 12 (48.0) | 4,392 (75.6) |
| Participating sites | ||||
| 1 | 58 (71.6) | 17,937 (11.6) | 22 (88.0) | 4,185 (72.0) |
| 2–5 | 3 (3.7) | 1,281 (0.8) | 0 (0) | 0 (0) |
| 6–10 | 6 (7.4) | 2,035 (1.3) | 0 (0) | 0 (0) |
| > 10 | 14 (17.3) | 133,421 (86.3) | 3 (12.0) | 1,627 (28.8) |
| Region of primary sitea | ||||
| North America | 15 (18.5) | 136,120 (88.0) | 8 (32.0) | 2,312 (39.8) |
| Latin America and Caribbean | 13 (16.0) | 3,387 (2.2) | 2 (8.0) | 57 (1.0) |
| Europe and Central Asia | 13 (16.0) | 3,807 (2.5) | 7 (28.0) | 694 (11.9) |
| East Asia and Pacific | 19 (23.5) | 8,053 (5.2) | 1 (4.0) | 1,510 (26.0) |
| South Asia | 14 (17.3) | 1,585 (1.0) | 3 (12.0) | 249 (4.3) |
| Middle East and North Africa | 5 (6.2) | 541 (0.3) | 3 (12.0) | 585 (10.1) |
| Sub-Saharan Africa | 2 (2.5) | 1,181 (0.8) | 1 (4.0) | 405 (7.0) |
| World Bank Income classification | ||||
| High income country | 33 (40.7) | 142,364 (92.0) | 14 (56.0) | 4,351 (7.5) |
| Upper middle-income country | 30 (37.0) | 9,377 (6.1) | 5 (20.0) | 528 (9.1) |
| Lower middle-income country | 17 (21.0) | 1,812 (1.2) | 6 (24.0) | 923 (15.9) |
| Lower income country | 1 (1.2) | 1,121 (0.7) | 0 (0) | 0 (0) |
| Study design | ||||
| Randomized controlled trialb | 1 (1.2)a | 50 (0.03) | 0 (0) | 0 (0) |
| Prospective cohort | 38 (46.9) | 9,634 (6.2) | 14 (56.0) | 1,160 (20.0) |
| Retrospective cohort | 37 (45.7) | 145,291 (94.1) | 10 (40.0) | 4,130 (71.1) |
| Case-control | 5 (6.2) | 499 (0.3) | 1 (4.0) | 72 (1.2) |
| Primary study settingc | ||||
| PICU | 68 (84.0) | 136,599 (88.3) | 21 (84.0) | 5,072 (87.3) |
| Emergency department | 8 (9.9) | 2,078 (1.3) | 4 (16.0) | 932 (16.0) |
| Ward | 7 (8.6) | 12,423 (8.0) | 0 (0) | 0 (0) |
| Other | 3 (3.7) | 3,574 (2.3) | 0 (0) | 0 (0) |
aSite of the corresponding author and or location of research ethics approval using the World Bank Classification of 2019–2020.
bSecondary analysis of a randomized controlled trial.
cTwo settings were unspecified and one included all hospital locations. In addition, some studies included more than one specified study location resulting in a total of more than 81 study locations.
TABLE 2.
Patient Characteristics for All Included Studies
| Characteristic | Studies in Meta-Analysis (n = 81), n (%) | Patients in Meta-Analysis (n = 154,674), n (%) | Narrative Studies (n = 25), n (%) | Patients in Narrative Studies (n = 5,812), n (%) |
|---|---|---|---|---|
| Age groups includeda | ||||
| Neonates (0–30 d) | 41 (50.6) | 127,574 (82.5) | 11 (44.0) | 3,657 (62.9) |
| Babies (31–90 d) | 70 (86.4) | 151,730 (98.1) | 22 (88.0) | 1,510 (26.0) |
| Infants (91 d to 1 yr) | 80 (98.8) | 154,452 (99.9) | 24 (96.0) | 5,719 (98.4) |
| Toddlers (2–5 yr) | 79 (97.5) | 154,380 (99.8) | 24 (96.0) | 5,812 (100) |
| School age (6–12 yr) | 73 (90.1) | 152,810 (98.8) | 22 (88.0) | 5,652 (97.2) |
| Adolescents (13–16 yr) | 62 (76.5) | 151,283 (97.8) | 19 (76.0) | 5,248 (90.3) |
| Young adults (17–18 yr) | 44 (54.3) | 144,616 (93.5) | 13 (52.0) | 3,757 (64.6) |
| Population studied | ||||
| Bronchiolitis | 1 (1.2) | 72 (0.0) | 0 (0) | |
| Meningococcal infections | 2 (2.5) | 1,151 (0.7) | 1 (4.0) | 151 (2.6) |
| Pneumonia | 1 (1.2) | 222 (0.1) | 1 (4.0) | 160 (2.8) |
| Diarrheal illness | 2 (2.5) | 270 (0.2) | 0 (0) | 0 (0) |
| Severe acute malnutrition | 1 (1.2) | 50 (0.0) | 0 (0) | 0 (0) |
| Bone marrow transplant | 0 (0) | 0 (0) | 1 (4.0) | 567 (9.8) |
| Oncology—general | 4 (4.9) | 768 (0.5) | 1 (4.0) | 99 (1.7) |
| Oncology—febrile neutropenia | 1 (1.2) | 151 (0.1) | 0 (0) | 0 (0) |
| Emergency department patients | 5 (6.2) | 1,664 (1.1) | 4 (16.0) | 740 (12.7) |
| Hospital ward patients | 6 (7.4) | 24,778 (16.0) | 0 (0) | 0 (0) |
| Any PICU admission | 58 (71.6) | 125,539 (81.2) | 17 (68.0) | 4,095 (70.5) |
| Sepsis definition usedb | ||||
| 2001 SCCM/ACCP criteria | 7 (8.6) | 2,317 (1.5) | 2 (8.0) | 93 (1.6) |
| 2005 International Pediatric Sepsis Consensus Conference | 57 (70.4) | 56,377 (36.4) | 17 (68.0) | 5,274 (90.7) |
| ACCM 2002 | 2 (2.5) | 126 (0.1) | 1 (4.0) | 57 (0.1) |
| ACCM 2007 | 1 (1.2) | 1,299 (0.8) | 1 (4.0) | 71 (1.2) |
| International Classification of Diseases, 9th Edition codes | 6 (7.4) | 86,594 (56.0) | 0 (0) | 0 (0) |
| Bone criteria | 2 (2.5) | 431 (0.3) | 2 (8.0) | 166 (2.9) |
| Sepsis-3 | 2 (2.5) | 7,091 (4.6) | 0 (0) | 0 (0) |
| Other | 4 (4.9)c | 439 (0.3) | 2 (8.0)d | 151 (2.6) |
ACCM = American College of Critical Care Medicine, ACCP = American College of Chest Physicians, SCCM = Society of Critical Care Medicine.
aValues for age groups from eligible articles were included in category that provided the closest approximation to the classification used in the article. Articles could have patients from more than one age group resulting in totals being > 100%.
b1. Bone RC, et al: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. 2. Levy et al (22). 3. Carcillo JA, et al: Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med 2002; 30:1365–1378. 4. Goldstein et al (3). 5. Brierley J, et al: Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009; 37:666–688. 6. Singer M, et al: The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–810.
cThree papers referred to hospital guidelines and one defined sepsis as tachycardia plus hypothermia (35.0°C) or hyperthermia (38.5°C), or abnormal WBC count plus poor peripheral perfusion (mean arterial pressure 50 mm Hg and/or absent peripheral pulses or capillary refilling time 3 s) in the absence of clinical dehydration.
dOne paper used Carrol ED, et al: The role of RANTES in meningococcal disease. J Infect Dis 2000; 182:363–366 and one referenced Abraham E, et al: Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: Time for a re-evaluation. Crit Care Med 2000; 28:232–235.
Included studies along with the variables assessed in the meta-analysis and narrative review are detailed in Supplementary Table 2 (http://links.lww.com/CCM/G818) and Supplementary Table 3 (http://links.lww.com/CCM/G819), respectively. Forest plots for variables with significant findings are shown in Supplementary Figure 1 (http://links.lww.com/CCM/G820), Supplementary Figure 2 (http://links.lww.com/CCM/G821), Supplementary Figure 3 (http://links.lww.com/CCM/G822), Supplementary Figure 4 (http://links.lww.com/CCM/G823), Supplementary Figure 5 (http://links.lww.com/CCM/G824), Supplementary Figure 6 (http://links.lww.com/CCM/G825), Supplementary Figure 7 (http://links.lww.com/CCM/G826), and Supplementary Figure 8 (http://links.lww.com/CCM/G827), and associations of these variables with the outcomes of sepsis and mortality are summarized in Table 3.
TABLE 3.
Summary of Variables With Significant Associations With Outcomes of Interest
| Variable | No. of Studies | No. of Participants With Outcomea | No. of Participants Without Outcomea | Pooled Estimateb (95% CI) | Mean Value in Two Groupsc | p for Heterogeneity | I2 Value (%) |
|---|---|---|---|---|---|---|---|
| Variables significantly associated with outcome of sepsis, severe sepsis, or septic shock | |||||||
| Decreased LOC | 4 | 172/369 | 354/2,565 | 9.8 (5.8–16.7) | 0.080 | 55.7 | |
| PRISM score | 2 | 1,695 | 3,612 | 6.0 (4.0–8.0) | 9.5 vs 3.5 | < 0.0001 | 93.3 |
| Variables significantly associated with outcome of mortality | |||||||
| Demographic variables | |||||||
| Severe acute malnutrition | 3 | 30/135 | 57/450 | 4.7 (1.4–16.3) | 0.094 | 57.8 | |
| Chronic conditions | 11 | 859/1,464 | 13,013/25,664 | 2.4 (1.4–4.1) | < 0.0001 | 85.7 | |
| Oncologic conditions | 8d | 104/402 | 616/2,422 | 2.3 (1.7–3.1) | 0.63 | 0.0 | |
| Clinical variables | |||||||
| Hypotension | 4 | 1,013/1,910 | 10,828/41,283 | 2.3 (1.8–2.9) | 0.052 | 61.1 | |
| Vasoactive agents | 20 | 623/739 | 1,831/3,475 | 6.5 (4.2–10.0) | < 0.0001 | 59.7 | |
| Vasoactive-inotropic score | 6 | 175 | 468 | 23.5 (3.4–43.6) | 49.3 vs 20.4 | < 0.0001 | 87.5 |
| Stroke index | 3 | 165 | 295 | 0.2 (0.1–0.4) | 1.8 vs 1.7 | 0.42 | 0.0 |
| Mechanical ventilation | 30 | 2,778/3,350 | 22,874/51,151 | 11.0 (7.4–16.3) | < 0.0001 | 84.2 | |
| Decreased LOC | 3 | 1,147/1,813 | 10,975/38,744 | 4.1 (2.9–5.9) | 0.22 | 33.6 | |
| Glasgow Coma Scale | 3 | 134 | 176 | –4.0 (–6.2 to –1.8) | 6.6 vs 11.0 | 0.10 | 56.5 |
| Laboratory variables | |||||||
| pH | 4 | 203 | 334 | –0.10 (–0.14 to –0.05) | 7.21 vs 7.31 | 0.077 | 56.1 |
| Lactate (mmol/L) | 17 | 900 | 3,867 | 1.9 (1.2–2.6) | 4.6 vs 2.7 | < 0.0001 | 94.4 |
| Base deficit | 6 | 570 | 2,377 | –3.2 (–5.8 to –0.6) | –9.1 vs –5.9 | < 0.0001 | 98.1 |
| Urea (mg/dL) | 4 | 326 | 1,750 | 1.5 (0.7–2.3) | 9.4 vs 6.2 | 0.075 | 56.6 |
| Creatinine (µmol/L) | 8 | 471 | 2,148 | 13.0 (4.6–21.5) | 62.4 vs 42.8 | < 0.0001 | 89.0 |
| Potassium (meq/L) | 3 | 268 | 1,447 | 0.2 (0.02–0.44) | 4.5 vs 4.3 | 0.92 | 0.0 |
| Platelet count (109/L) | 14 | 585 | 3,196 | –87 (–107 to –67) | 90 vs 178 | < 0.0001 | 90.9 |
| Fibrinogen (g/L) | 5 | 324 | 2,503 | –1.5 (–2.5 to –0.6) | 2.0 vs 3.6 | < 0.0001 | 97.9 |
| Albumin (g/L) | 3 | 237 | 563 | –4.3 (–8.4 to –0.2) | 31.0 vs 35.4 | < 0.0001 | 92.5 |
| Procalcitonin (ng/ml) | 9 | 463 | 1,266 | 4.0 (2.0–6.0) | 7.8 vs 4.8 | < 0.0001 | 92.2 |
| Alanine aminotransferase (units/L) | 3 | 298 | 1,262 | 10.1 (4.0–16.2) | 97.3 vs 65.8 | 0.46 | 0.0 |
| Organ dysfunction and illness severity variables | |||||||
| No. of organ dysfunctions | 4 | 1,065 | 4,683 | 0.9 (0.3–1.5) | 3.4 vs 2.5 | < 0.0001 | 92.6 |
| Renal dysfunction | 4 | 77/160 | 84/323 | 4.0 (1.0–18.4) | < 0.0001 | 86.2 | |
| Multiple organ dysfunction syndrome | 9c | 388/467 | 816/1,986 | 7.8 (3.9–15.6) | < 0.0001 | 75.2 | |
| PELOD | 12c | 442 | 1,748 | 6.1 (2.5–9.8) | 16.7 vs 8.7 | < 0.0001 | 97.7 |
| PELOD-2 | 3 | 110 | 1,320 | 8.7 (5.7–11.6) | 10.4 vs 1.2 | < 0.0001 | 91.2 |
| Sequential Organ Failure Assessment | 2 | 95 | 647 | 3.8 (2.7–4.9) | 9.9 vs 5.9 | 0.16 | 50.4 |
| Pediatric Sequential (Sepsis-related) Organ Failure Assessment | 4 | 595 | 756 | 4.8 (3.7–5.8) | 10.0 vs 4.2 | 0.52 | 0.0 |
| PRISM | 19 | 821 | 3,871 | 11.0 (5.6–16.5) | 22.5 vs 11.5 | < 0.0001 | 99.6 |
| PIM-2 | 2 | 63 | 397 | 12.1 (9.3–14.9) | 35.6 vs 18.0 | 0.33 | 0.0 |
| PIM-3 | 5c | 245 | 1,455 | 7.8 (2.5–13.1) | < 0.0001 | 89.1 | |
LOC = level of consciousness, PELOD = pediatric logistic organ dysfunction, PIM = Pediatric Index of Mortality, PRISM = Pediatric Risk of Mortality.
aNumbers reported are totals for those with and without the listed outcome for each parameter for continuous variables. For categorical variables, the numbers shown are the number with the parameter over the total with or without the outcome of interest.
bPooled estimate is for the odds ratio for categorical variables and the mean difference for continuous variables.
cThe mean values of nonsurvivors versus survivors are provided for all continuous variables.
dThe study by Thakkar et al (23) reported on two nonoverlapping cohorts of medical and surgical patients that were, therefore, analyzed separately and counted as two studies.
Variables Associated With Sepsis, Severe Sepsis, Septic Shock in Children With Suspected Infection
Sixteen studies on 9,629 patients provided data for the meta-analysis assessing the association of 16 variables among children with suspected infection with the outcome of sepsis, severe sepsis, or septic shock (for study and patient characteristics, see Supplementary Table 4, http://links.lww.com/CCM/G828; and Supplementary Table 1, http://links.lww.com/CCM/G817). Sepsis and severe sepsis among infected children were associated with decreased level of consciousness (24–27) and higher Pediatric Risk of Mortality (PRISM) scores (28, 29), respectively (Supplementary Fig. 2, http://links.lww.com/CCM/G821; and Supplementary Fig. 8, http://links.lww.com/CCM/G827). Our meta-analysis did not demonstrate an association among age, age groups, gender or malnutrition (30–34), and sepsis, severe sepsis, or septic shock (Supplementary Table 5, http://links.lww.com/CCM/G829). Sepsis among infected children was not associated with pooled estimates of hemoglobin (35–37), C-reactive protein (CRP) (25, 27), or procalcitonin (38, 39).
Variables Associated With NPMODS and Mortality in Children With Sepsis, Severe Sepsis, or Septic Shock
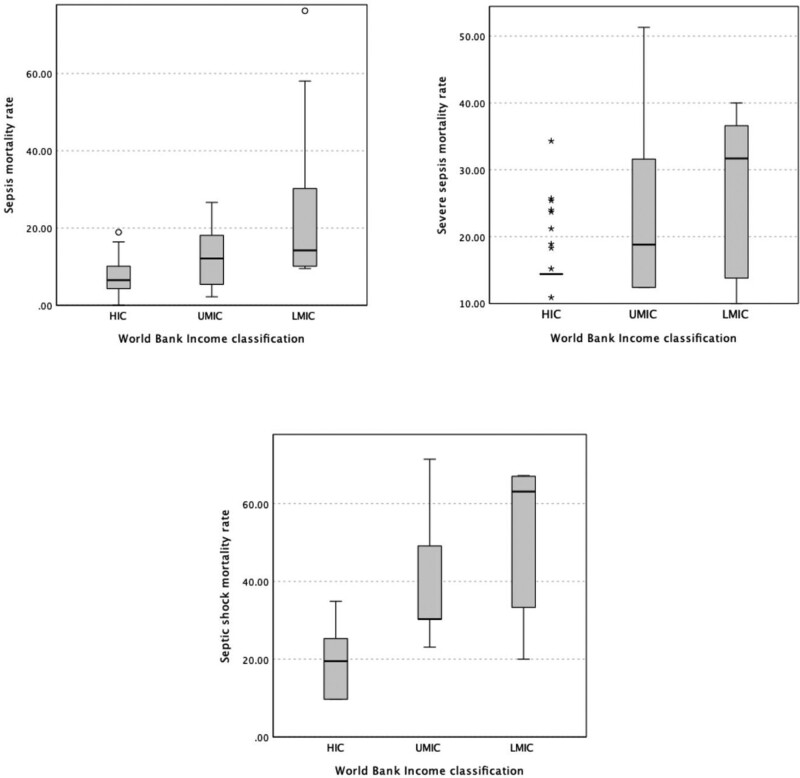
Mortality rates for sepsis, severe sepsis, and/or septic shock were provided in 86 of 106 included studies. The pooled mortality rate using a random-effects model for patients with sepsis was 10.9% (n = 47 studies; 95% CI, 8.9–13.2), for severe sepsis was 23.0% (n = 26 studies; 95% CI, 19.6–26.9), and for septic shock was 36.8% (n = 28 studies; 95% CI, 29.4–44.9). The pooled mortality rates varied among HIC, UMIC, and LMIC locations for each of sepsis, severe sepsis, and septic shock patient groups (p < 0.0001) (Fig. 2). The mortality analysis did not include LICs as there was only one study with eligible data.
Figure 2.
Pooled mortality rates for sepsis, severe sepsis, and septic shock in included studies across World Bank Income classifications. HIC = high-income country, LMIC = low-middle-income country, UMIC = upper-middle-income country.
Sixty-nine studies on 145,461 patients provided data for the meta-analysis of the association of 54 variables with the primary outcome of mortality (for patient and study characteristics, see Supplementary Table 1, http://links.lww.com/CCM/G817; and Supplementary Table 4, http://links.lww.com/CCM/G828, respectively). One study reported separately on two populations that were, therefore, reported as two studies in the meta-analysis (23). Only one study reported NPMODS as an outcome, and two reported a composite outcome of NPMODS and death. Meta-analysis with NPMODS as the outcome was not possible as none of these studies assessed the same variables.
Pooled estimates supported an increased odds of mortality in patients with severe acute malnutrition (31, 40, 41), chronic conditions (31, 33, 42–50), and oncologic conditions (23, 31, 47, 51–54) (Supplementary Fig. 1, http://links.lww.com/CCM/G820). The evidence did not support an association between age, age groups, or gender with mortality. In addition, no association was noted between, obesity (55–57) or malnutrition (30–34) and mortality, but only a small number of studies assessed these variables.
Clinical Variables
Among children with sepsis, severe sepsis, and septic shock, pooled estimates provide strong support for increased mortality with hypotension (46, 47, 58, 59), use of vasoactive agents/inotropes (26, 31–33, 40, 41, 44, 49, 50, 58, 60–69), increased vasoactive-inotropic score (VIS) (51, 53, 68, 70–72), increased shock index (58, 73, 74), decreased level of consciousness (58, 59, 67), decreased Glasgow Coma Scale (GCS) (53, 70, 75), and mechanical ventilation (26, 28, 31, 32, 40–44, 46, 49–51, 53, 58–62, 65–69, 71, 72, 75–80) (Supplementary Fig. 2, http://links.lww.com/CCM/G821). There were no mortality differences significantly associated with heart rate (47, 53, 58, 71, 74, 76), mean blood pressure (53, 71), systolic blood pressure (47, 58, 67, 74, 76), central venous pressures (51, 53, 71), and arterial oxygen saturations (47, 58).
Laboratory Variables
Pooled estimates provide strong support for a difference in the following laboratory measures between nonsurvivors and survivors: lower serum pH (53, 58, 72, 75), higher lactate (43, 50, 51, 53, 60–62, 65, 68, 71, 72, 74–76, 81–83), higher serum base deficit (62, 71, 75, 76, 84, 85), higher urea (58, 76, 81, 86), higher creatinine (53, 58, 71, 76, 81, 83, 85, 86), lower platelet count (41, 43, 50, 53, 58, 62, 71, 77, 81, 83–85, 87, 88), lower fibrinogen (62, 81, 84, 85, 87), higher potassium (62, 71, 76), lower albumin (53, 76, 83), higher procalcitonin (26, 35, 43, 76, 83, 85, 89–91), and higher alanine aminotransferase (ALT) (58, 76, 81) (Supplementary Fig. 3, http://links.lww.com/CCM/G822; Supplementary Fig. 4, http://links.lww.com/CCM/G823; Supplementary Fig. 5, http://links.lww.com/CCM/G824; and Supplementary Fig. 6, http://links.lww.com/CCM/G825). Pooled estimates did not support a difference between nonsurvivors and survivors in mean glucose (50, 68, 71, 72, 76), total bilirubin (53, 58, 76, 81, 85, 86), WBC (26, 43, 50, 53, 58, 62, 71, 75–77, 81, 83, 85, 87, 89), hemoglobin (26, 43, 50, 71, 83, 85), international normalized ratio (62, 81), prothrombin time (53, 71, 76, 81, 92), activated partial thromboplastin time (62, 71, 81, 92), and brain natriuretic peptide (51, 66, 76).
Organ Dysfunction Measures and Illness Severity Scores
Our meta-analysis provides strong support for greater organ dysfunction in nonsurvivors compared with survivors as shown by the pooled estimates for renal dysfunction (50, 64, 67, 70), multi-organ dysfunction (MODS) (23, 33, 41, 50, 69, 70, 93, 94), number of organ dysfunctions (28, 40, 64, 83), PEdiatric Logistic Organ Dysfunction (PELOD) score (23, 28, 40, 44, 50, 53, 55, 60, 65, 72, 85), and PELOD-2 (85, 86, 95). Our meta-analysis also provides strong support for greater illness severity in nonsurvivors compared with survivors as shown by the pooled estimates pediatric Sequential (Sepsis-related) Organ Failure Assessment (pSOFA) (50, 58, 70, 95) and Sequential (Sepsis-related) Organ Failure Assessment (SOFA) (85, 86, 95), PRISM (32, 43, 51, 53, 55, 60–62, 64, 68, 70–72, 75, 77, 81, 84, 85, 88), Pediatric Index of Mortality (PIM)-2 (79, 85), and PIM-3 (23, 61, 65, 85) (Supplementary Fig. 7, http://links.lww.com/CCM/G826; and Supplementary Fig. 8, http://links.lww.com/CCM/G827).
Narrative Review of n = 25 Studies
Three studies reported risk factors for developing sepsis or septic shock among children with infection. Two studies reported differing thresholds of CRP (81.9 and 154.3 nmol/dL) and procalcitonin levels (43 and 19.1 ng/mL) for association with septic shock in patients with meningococcemia (96) and sepsis (97), respectively. One study of children presenting to the ED with suspected infection found that a lactate level of greater than 3 mmol/L was associated with higher risk of sepsis (98).
In one study of patients with septic shock, those with hematopoietic cell transplants had increased odds of mortality (OR 4.74; 95% CI, 2.56–8.77) (99), and those with progressively higher Logistic Organ Dysfunction Score and alert, verbal, pain, unresponsive score demonstrated increasing positive predictive values for early mortality from 40% to 60% and 39.3% to 50%, respectively (100). Several studies assessed cardiovascular variables. In one study, the Tp-e interval/QT on an electrocardiogram was an independent predictor of mortality in patients with septic shock (101). A VIS of greater than 20 was associated with increased mortality (102). Another study suggested time-dependant cutoffs for shock index values from 0- to 6-hour postadmission (103), and two studies each found an association of a decreased left ventricular ejection fraction (45% and 55%) with mortality (104, 105).
Laboratory values that showed an association with mortality in single studies included red cell distribution width elevation (106); antithrombin III levels below 41.5% (< 1 yr old) and 67.5% (≥ 1 yr old) (92); 25-hydroxy vitamin D less than 50 nmol/L (107); baseline cortisol cutoff of 20 µg/dL and postadrenocorticotropic hormone stimulation level of less than or equal to 9 µg/dL (108); lower serum zinc levels (109); lower high-density lipoprotein, low-density lipoprotein, and cholesterol levels (110); and lower total T3 and T4, and free T3 and T4 hormone levels (111). Two studies assessed serum troponin in sepsis with one reporting an association of serum troponin greater than 1 ng/dL with mortality (112), whereas the other found higher levels of troponin in nonsurvivors compared with survivors (71). Serum lactate levels were studied using three criteria. Serum-lactate-to-albumin ratio greater than 1.17 was associated with increased mortality (113), and lack of lactate clearance (decrease of ≤ 10%) or normalization (< 2 mmol/L) was associated with persistent MODS (114).
DISCUSSION
Our systematic review evaluated over 50 variables and derived scores in studies on over 150,000 patients from diverse global settings. To our knowledge, this is the largest systematic review assessing a broad range of variables associated with severity of infection in children (115). The majority of included studies in this review described features among septic children associated with higher mortality. We found evidence of increased odds of mortality for septic patients with severe acute malnutrition, chronic conditions, oncologic disorders, hypotension, use of inotropes, mechanical ventilation, decreased level of consciousness, and lower GCS. In addition, we found significant differences in VIS, base deficit, pH, lactate, platelets, fibrinogen, urea, creatinine, albumin, potassium, ALT, and procalcitonin between nonsurvivors and survivors. These findings provide support for using the above measures of organ dysfunction as hallmarks of sepsis and serve to inform data-driven development of revised pediatric sepsis criteria.
Our study evaluated data from 35 countries in diverse geographic regions and income levels of the World Bank Income classification (18). This is important given that up to 85% of all sepsis cases and related deaths occur in lower income and middle-income countries (2). However, although 18 included studies were conducted in LIC and LMIC countries, these represented only 1.8% (2,784/154,674) of the patients analyzed. The lower representation of LIC/LMIC patients may have resulted in our findings being more applicable to HIC/UMIC settings as a result of distinct causes of sepsis (116), limited access to and availability of treatments (117), and higher mortality rates (2) in patients with sepsis from LMIC/LIC settings. Large studies in LMIC/LIC settings remain challenging to perform due to the lack of comprehensive registries, electronic health records, and limited laboratory resources, which has important implications for the derivation, dissemination, and uptake of a revised definition of pediatric sepsis.
Only a small proportion of eligible studies (8/81, 9.9%), contributing 1.3% of included patients (2078/154,674), was from pre-ICU settings. This may have resulted in underrepresentation of early clinical variables used to differentiate self-limited febrile illness from critical illness and that may be important in sepsis definitions designed for the pre-ICU phase of illness (118). Considering that most children with sepsis initially present to non-ICU settings, it is imperative that the future work of the Pediatric Sepsis Definition Taskforce also develops and validates tools for the recognition of sepsis outside of the ICU.
Previous sepsis definitions, such as the 2001 Consensus Conference (22) and 2005 IPSCC (3) definitions, included markers of organ dysfunction such as lactate, but their inclusion was the result of a consensus process and was never formally validated. The present systematic review allows prioritization of markers showing robust association with mortality for future revisions of sepsis criteria. Interestingly, bilirubin, used as the sole marker of liver dysfunction in the adult-adapted pSOFA score, was not associated with mortality, whereas another marker of liver dysfunction, ALT, performed well. In addition, measures of metabolic failure (increased serum lactate, acidosis, and base deficit) were confirmed as relevant markers despite not being part of the SOFA score. This review assessed individual variables as well as illness severity and organ dysfunction scores that incorporate combinations of the studied variables. This is an important contribution as many of the studied scores were derived and validated in critically ill children but not specifically studied in those with sepsis.
This review has several limitations. The first is that several variables included in the meta-analysis demonstrated significant heterogeneity. However, since the purpose of this review was to identify potential variables for use in an updated definition of pediatric sepsis rather than draw conclusions regarding a treatment effect, the actual effect size and its associated I2 value may be less relevant. Second, our pragmatic approach resulted in the inclusion of studies with different definitions of sepsis. Although this may have limited our ability to find associations of some variables with our outcomes of interest, it may also have contributed to the robustness of the associations for other variables. Finally, for continuous variables, we were not able to determine thresholds for the development of sepsis or for mortality due to lack of data. However, we determined overall mean values for survivors and nonsurvivors for variables with a significant mean difference that could provide initial thresholds in the data validation phase of the Pediatric Sepsis Definition Taskforce project.
CONCLUSIONS
This systematic review rigorously assessed the association of individual variables with development of sepsis in children with infections and the odds of mortality in children with sepsis, severe sepsis, and septic shock. The included studies were from economically diverse regions of the world, populations with diverse underlying conditions, and varying definitions of sepsis. Despite the clinical heterogeneity and limited number of studies for some variables, strong associations with the outcomes of interest were seen for many of the variables assessed, predominantly reflecting measures of organ dysfunction and supporting the inclusion of these variables in the data validation phase of the Pediatric Sepsis Definition Taskforce.
ACKNOWLEDGMENTS
We thank Kathy Vermoch and Lori Harmon from the SCCM for all their help with this project and article, Margaret Sampson, librarian at the Children’s Hospital of Eastern Ontario (CHEO) for her invaluable help in creating and executing the search for this systematic review, and Katie O’Hearn, research coordinator at CHEO for her help with screening and data extraction to update the review.
Members of the Pediatric Sepsis Definition Taskforce of the Society of Critical Care Medicine are: Luregn J. Schlapbach (Cochair), Pediatric and Neonatal ICU, University Children`s Hospital Zurich, Switzerland, and Child Health Research Centre, The University of Queensland, Brisbane, Australia; R. Scott Watson (Cochair), Center for Child Health, Behavior and Development, Seattle Children’s Research Institute, Seattle Children’s Hospital, Seattle, WA; Andrew Argent (Vice Chair), Department of Paediatrics and Child Adolescent Health, Red Cross War Memorial Children’s Hospital and University of Cape Town, Cape Town, South Africa; Lauren R. Sorce (Vice Chair), Ann & Robert H. Lurie Children’s Hospital AND Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL; Elizabeth R. Alpern, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Fran Balamuth, Children’s Hospital of Philadelphia, Philadelphia, PA; Tellen D. Bennett, University of Colorado, Denver, CO; Paolo Biban, Verona University Hospital, Verona, Italy; Joe Carcillo, Department of Critical Care Medicine, UPMC Children’s Hospital, Pittsburg, Pennsylvania, PA; Enitan Carrol, University of Liverpool, Liverpool, United Kingdom; Kathleen Chiotos, Children’s Hospital of Philadelphia, Philadelphia, PA; Mohammod Jobayer Chisti, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh; Idris Evans, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA; Lu Guoping, Children’s Hospital of Fudan University, Shanghai, China; Mark W. Hall, Nationwide Children’s Hospital, Columbus, OH; David Inwald, Addenbrooke’s Hospital, Cambridge University Hospital NHS Trust, Cambridge, United Kingdom; Paul Ishimine, University of California San Diego, San Diego, CA; Michael Levin, Imperial College London, London, United Kingdom; Niranjan Kissoon, British Columbia Women and Children’s Hospital, Vancouver, Canada; Rakesh Lodha, All India Institute of Medical Sciences, New Delhi, India; Kathryn Maitland, Imperial College, London, United Kingdom; Simon Nadel, St. Mary’s Hospital, London, United Kingdom; Satoshi Nakagawa, National Center for Child Health & Development, Tokyo, Japan; Claudio Flauzino Oliveira, Associação de Medicina Intensiva Brasileira, São Paulo, Brazil; Mark Peters, University College London Great Ormond Street Institute of Child Health, London, United Kingdom; Adrienne G. Randolph, Boston Children’s Hospital, Boston, MA; Suchitra Ranjit, Apollo Hospitals, Chennai, India; L. Nelson Sanchez-Pinto, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Halden F. Scott, Children’s Hospital of Colorado, Denver, CO; Daniela Carla Souza, University Hospital of The University of São Paulo, Sao Paulo, Brazil; Pierre Tissieres, Pierre Tissieres, Pediatric Intensive Care, AP-HP Paris Saclay University, Bicêtre Hospital, Le Kremlin-Bicêtre, France; Juliane Bubeck Wardenburg, Department of Pediatrics Washington School of Medicine St. Louis, MN; Scott L. Weiss, Children’s Hospital of Philadelphia, Philadelphia, PA; Wilson Milton Were, Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization, Geneva, Switzerland; Matt Wiens, University of British Columbia, Canada/Africa; James L. Wynn, University of Florida, Gainsville, FL; Jerry J. Zimmerman, Seattle Children’s Hospital, Seattle, WA.
Supplementary Material
Footnotes
*See also p. 148.
Members of the Pediatric Sepsis Definition Taskforce of the Society of Critical Care Medicine are listed in the Acknowledgments.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the Society of Critical Care Medicine and a Tier 2 Clinical Research Chair of the University of Ottawa, Faculty of Medicine, Ottawa, Canada.
Dr. Menon has a Canadian Institutes of Health Research grant for the study of corticosteroids in pediatric septic shock. Dr. Menon’s institution received funding from Crowdsourcing; he disclosed that he is a principal investigator on an international trial on Steroids in Pediatric Sepsis Shock. Drs. Menon and Watson received funding from Society of Critical Care Medicine. Dr. Argent received funding from the World Federation of Pediatric Intensive and Critical Care Societies as a part of the process of working with the Pediatric Sepsis Definitions working group. Dr. Biban received funding from Chiesi and Getinge. Dr. Nadel received funding from Abbvie. Dr. Sadeghirad received funding from the Pre-Interventional Preventative Risk Assessment AG, Mitacs Canada, and Accelerate internship in partnership with Nestlé Canada. Dr. Scott’s institution received funding from the Agency for Healthcare Research and Quality (AHRQ K08HS025696); he received support for article research from the AHRQ. Dr. Tissieres received funding from Sedana, Inotrem, and Baxter. Dr. Wynn received funding from the National Institutes of Health (NIH) (R01GM128452; R01HD089939, R01HD097081, R43EB029863). Dr. Zimmerman’s institution received funding from the NIH, the National Institute of Child Health and Human Development, and Immunexpress; he received funding from Elsevier Publishing. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The work was coordinated at the Children’s Hospital of Eastern Ontario, Ottawa, ON K1H 8L1.
REFERENCES
- 1.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020; 396:1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 2020; 395:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 4.Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020; 21:e52–e106 [DOI] [PubMed] [Google Scholar]
- 5.Shankar-Hari M, Phillips GS, Levy ML, et al. ; Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016; 315:775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agyeman PKA, Schlapbach LJ, Giannoni E, et al. ; Swiss Pediatric Sepsis Study. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: A population-based cohort study. Lancet Child Adolesc Health. 2017; 1:124–133 [DOI] [PubMed] [Google Scholar]
- 7.Volakli EA, Sdougka M, Drossou-Agakidou V, et al. Short-term and long-term mortality following pediatric intensive care. Pediatr Int. 2012; 54:248–255 [DOI] [PubMed] [Google Scholar]
- 8.Pollack MM, Holubkov R, Funai T, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Simultaneous prediction of new morbidity, mortality, and survival without new morbidity from pediatric intensive care: A new paradigm for outcomes assessment. Crit Care Med. 2015; 43:1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberico AM, Ward JD, Choi SC, et al. Outcome after severe head injury. Relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. 1987; 67:648–656 [DOI] [PubMed] [Google Scholar]
- 10.Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choong K, Bohn D, Fraser DD, et al. ; Canadian Critical Care Trials Group. Vasopressin in pediatric vasodilatory shock: A multicenter randomized controlled trial. Am J Respir Crit Care Med. 2009; 180:632–639 [DOI] [PubMed] [Google Scholar]
- 12.Lauzier F, Lévy B, Lamarre P, et al. Vasopressin or norepinephrine in early hyperdynamic septic shock: A randomized clinical trial. Intensive Care Med. 2006; 32:1782–1789 [DOI] [PubMed] [Google Scholar]
- 13.Menon K, Schlapbach LJ, Akech S, et al. Pediatric sepsis definition-a systematic review protocol by the pediatric sepsis definition taskforce. Crit Care Explor. 2020; 2:e0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nama N, Sampson M, Barrowman N, et al. Crowdsourcing the citation screening process for systematic reviews: Validation study. J Med Internet Res. 2019; 21:e12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013; 158:280–286 [DOI] [PubMed] [Google Scholar]
- 17.Voils CI, Crandell JL, Chang Y, et al. Combining adjusted and unadjusted findings in mixed research synthesis. J Eval Clin Pract. 2011; 17:429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The World Bank. World Bank Country and Lending Groups. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed April 12, 2021
- 19.Higgins JPT, Thomas J, Chandler J, et al. (Eds). Cochrane Handbook for Systematic Reviews of Interventions. Second Edition. Chichester, United Kingdom, John Wiley & Sons, 2019 [Google Scholar]
- 20.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, et al. ; International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003; 29:530–538 [DOI] [PubMed] [Google Scholar]
- 23.Thakkar RK, Weiss SL, Fitzgerald JC, et al. ; SPROUT Investigators; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Risk factors for mortality in pediatric postsurgical versus medical severe sepsis. J Surg Res. 2019; 242:100–110 [DOI] [PubMed] [Google Scholar]
- 24.Chisti MJ, Salam MA, Bardhan PK, et al. Severe sepsis in severely malnourished young Bangladeshi children with pneumonia: A retrospective case control study. PLoS One. 2015; 10:e0139966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santolaya ME, Alvarez AM, Aviles CL, et al. Predictors of severe sepsis not clinically apparent during the first twenty-four hours of hospitalization in children with cancer, neutropenia, and fever: A prospective, multicenter trial. Pediatr Infect Dis J. 2008; 27:538–543 [DOI] [PubMed] [Google Scholar]
- 26.Shah S, Kaul A, Jadhav Y, et al. Clinical outcome of severe sepsis and septic shock in critically ill children. Trop Doct. 2020; 50:186–190 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Lin X, Yue H, et al. ; Collaborative Study Group for Pediatric Sepsis in Huai’an. Evaluation of systemic inflammatory response syndrome-negative sepsis from a Chinese regional pediatric network. BMC Pediatr. 2019; 19:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza DC, Shieh HH, Barreira ER, et al. ; LAPSES Group. Epidemiology of sepsis in children admitted to PICUs in South America. Pediatr Crit Care Med. 2016; 17:727–734 [DOI] [PubMed] [Google Scholar]
- 29.Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017; 171:e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branco RG, Garcia PC, Piva JP, et al. Glucose level and risk of mortality in pediatric septic shock. Pediatr Crit Care Med. 2005; 6:470–472 [DOI] [PubMed] [Google Scholar]
- 31.Jabornisky R, Sáenz SS, Capocasa P, et al. ; Miembros del Grupo de Investigación Clínica y Epidemiológica en Terapia Intensiva Pediátrica de la Sociedad Argentina de Pediatría (SAP). Epidemiological study of pediatric severe sepsis in Argentina. Arch Argent Pediatr. 2019; 117:S135–S156 [DOI] [PubMed] [Google Scholar]
- 32.Kaur G, Vinayak N, Mittal K, et al. Clinical outcome and predictors of mortality in children with sepsis, severe sepsis, and septic shock from Rohtak, Haryana: A prospective observational study. Indian J Crit Care Med. 2014; 18:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan MR, Maheshwari PK, Masood K, et al. Epidemiology and outcome of sepsis in a tertiary care PICU of Pakistan. Indian J Pediatr. 2012; 79:1454–1458 [DOI] [PubMed] [Google Scholar]
- 34.Villegas D, Echandia CA. Factors associated with mortality through sepsis syndrome in children 31 days to 14 years of age. Colombia Medica. 2010; 41:349–357 [Google Scholar]
- 35.Sakyi SA, Enimil A, Adu DK, et al. Individual and combined bioscore model of presepsin, procalcitonin, and high sensitive C-reactive protein as biomarkers for early diagnosis of paediatric sepsis. Heliyon. 2020; 6:e04841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiens MO, Larson CP, Kumbakumba E, et al. Application of sepsis definitions to pediatric patients admitted with suspected infections in Uganda. Pediatr Crit Care Med. 2016; 17:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Ma Y, Mao M, et al. Application of regression model combined with computer technology in the construction of early warning model of sepsis infection in children. J Infect Public Health. 2020; 13:253–259 [DOI] [PubMed] [Google Scholar]
- 38.Alejandre C, Guitart C, Balaguer M, et al. Use of procalcitonin and C-reactive protein in the diagnosis of bacterial infection in infants with severe bronchiolitis. Eur J Pediatr. 2021; 180:833–842 [DOI] [PubMed] [Google Scholar]
- 39.Smok B, Domagalski K, Pawłowska M. Diagnostic and prognostic value of IL-6 and sTREM-1 in SIRS and sepsis in children. Mediators Inflamm. 2020; 2020:8201585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baranwal AK, Deepthi G, Rohit MK, et al. Longitudinal study of CPK-MB and echocardiographic measures of myocardial dysfunction in pediatric sepsis: Are patients with shock different from those without? Indian J Crit Care Med. 2020; 24:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah S, Deshmukh CT, Tullu MS. The predictors of outcome and progression of pediatric sepsis and septic shock: A prospective observational study from western India. J Postgrad Med. 2020; 66:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ames SG, Davis BS, Angus DC, et al. Hospital variation in risk-adjusted pediatric sepsis mortality. Pediatr Crit Care Med. 2018; 19:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.İşgüder R, Ceylan G, Ağin H, et al. Increased mean platelet volume in children with sepsis as a predictor of mortality. Turk J Pediatr. 2016; 58:503–511 [DOI] [PubMed] [Google Scholar]
- 44.Lanziotti VS, Póvoa P, Prata-Barbosa A, et al. Patterns of C-reactive protein ratio response to antibiotics in pediatric sepsis: A prospective cohort study. J Crit Care. 2018; 44:217–222 [DOI] [PubMed] [Google Scholar]
- 45.Prout AJ, Talisa VB, Carcillo JA, et al. Children with chronic disease bear the highest burden of pediatric sepsis. J Pediatr. 2018; 199:194–199.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlapbach LJ, MacLaren G, Festa M, et al. ; Australian & New Zealand Intensive Care Society (ANZICS) Centre for Outcomes & Resource Evaluation (CORE) and Australian & New Zealand Intensive Care Society (ANZICS) Paediatric Study Group. Prediction of pediatric sepsis mortality within 1 h of intensive care admission. Intensive Care Med. 2017; 43:1085–1096 [DOI] [PubMed] [Google Scholar]
- 47.Scott HF, Brou L, Deakyne SJ, et al. Association between early lactate levels and 30-day mortality in clinically suspected sepsis in children. JAMA Pediatr. 2017; 171:249–255 [DOI] [PubMed] [Google Scholar]
- 48.Shime N, Kawasaki T, Saito O, et al. Incidence and risk factors for mortality in paediatric severe sepsis: Results from the national paediatric intensive care registry in Japan. Intensive Care Med. 2012; 38:1191–1197 [DOI] [PubMed] [Google Scholar]
- 49.Tonial CT, Costa CAD, Andrades GRH, et al. Performance of prognostic markers in pediatric sepsis. J Pediatr (Rio J). 2021; 97:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vila Pérez D, Jordan I, Esteban E, et al. Prognostic factors in pediatric sepsis study, from the Spanish Society of Pediatric Intensive Care. Pediatr Infect Dis J. 2014; 33:152–157 [DOI] [PubMed] [Google Scholar]
- 51.Choi SJ, Ha EJ, Jhang WK, et al. Elevated central venous pressure is associated with increased mortality in pediatric septic shock patients. BMC Pediatr. 2018; 18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dagher GA, Safa R, Hajjar K, et al. Characteristics and outcomes of pediatric septic patients with cancer: A retrospective cohort study. J Emerg Med. 2019; 57:216–226 [DOI] [PubMed] [Google Scholar]
- 53.Nazir M, Wani W, Dar SA, et al. Lactate clearance prognosticates outcome in pediatric septic shock during first 24 h of intensive care unit admission. J Intensive Care Soc. 2019; 20:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pound CM, Johnston DL, Armstrong R, et al. The morbidity and mortality of pediatric oncology patients presenting to the intensive care unit with septic shock. Pediatr Blood Cancer. 2008; 51:584–588 [DOI] [PubMed] [Google Scholar]
- 55.Ibrahiem SK, Galal YS, Youssef MR, et al. Prognostic markers among Egyptian children with sepsis in the intensive care units, Cairo University Hospitals. Allergol Immunopathol (Madr). 2016; 44:46–53 [DOI] [PubMed] [Google Scholar]
- 56.Peterson LS, Gállego Suárez C, Segaloff HE, et al. Outcomes and resource use among overweight and obese children with sepsis in the pediatric intensive care unit. J Intensive Care Med. 2020; 35:472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross PA, Klein MJ, Nguyen T, et al. Body habitus and risk of mortality in pediatric sepsis and septic shock: A retrospective cohort study. J Pediatr. 2019; 210:178–183.e2 [DOI] [PubMed] [Google Scholar]
- 58.Jaiswal P, Dewan P, Gomber S, et al. Early lactate measurements for predicting in-hospital mortality in paediatric sepsis. J Paediatr Child Health. 2020; 56:1570–1576 [DOI] [PubMed] [Google Scholar]
- 59.Peters C, Murthy S, Brant R, et al. Mortality risk using a pediatric quick sequential (sepsis-related) organ failure assessment varies with vital sign thresholds. Pediatr Crit Care Med. 2018; 19:e394–e402 [DOI] [PubMed] [Google Scholar]
- 60.Alam A, Gupta S. Lactate measurements and their association with mortality in pediatric severe sepsis in India: Evidence that 6-hour level performs best. J Intensive Care Med. 2020; 36:443–450 [DOI] [PubMed] [Google Scholar]
- 61.Boeddha NP, Schlapbach LJ, Driessen GJ, et al. ; EUCLIDS consortium. Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: A prospective cohort study from the European Childhood Life-threatening Infectious Disease Study (EUCLIDS). Crit Care. 2018; 22:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couto-Alves A, Wright VJ, Perumal K, et al. A new scoring system derived from base excess and platelet count at presentation predicts mortality in paediatric meningococcal sepsis. Crit Care. 2013; 17:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carvalho MV, Maluf MA, Catani R, et al. Cytokines and pediatric open heart surgery with cardiopulmonary bypass. Cardiol Young. 2001; 11:36–43 [DOI] [PubMed] [Google Scholar]
- 64.Fiser RT, West NK, Bush AJ, et al. Outcome of severe sepsis in pediatric oncology patients. Pediatr Crit Care Med. 2005; 6:531–536 [DOI] [PubMed] [Google Scholar]
- 65.Gorgis N, Asselin JM, Fontana C, et al. Evaluation of the association of early elevated lactate with outcomes in children with severe sepsis or septic shock. Pediatr Emerg Care. 2019; 35:661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Ning B, Wang Y, et al. The prognostic value of left ventricular systolic function and cardiac biomarkers in pediatric severe sepsis. Medicine (Baltimore). 2019; 98:e15070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarmin M, Afroze F, Sharifuzzaman , et al. Predictor of death in diarrheal children under 5 years of age having severe sepsis in an urban critical care ward in Bangladesh. Glob Pediatr Health. 2019; 6:2333794X19862716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verhoeven JJ, den Brinker M, Hokken-Koelega AC, et al. Pathophysiological aspects of hyperglycemia in children with meningococcal sepsis and septic shock: A prospective, observational cohort study. Crit Care. 2011; 15:R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Cui Y, Miao H, et al. Circulating vitronectin predicts liver injury and mortality in children with sepsis: A prospective observational study. Clin Appl Thromb Hemost. 2020; 26:1076029620935201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angurana SK, Bansal A, Muralidharan J, et al. Cytokine levels in critically ill children with severe sepsis and their relation with the severity of illness and mortality. J Intensive Care Med. 2020; 36:576–583 [DOI] [PubMed] [Google Scholar]
- 71.El-Zayat RS, Shalaby AG. Mitral annular plane systolic excursion as a predictor of mortality in children with septic shock. Pediatr Crit Care Med. 2018; 19:e486–e494 [DOI] [PubMed] [Google Scholar]
- 72.Sachdev A, Raheja K, Gupta N, et al. Association of urinary albumin:creatinine ratio with outcome of children with sepsis. Indian J Crit Care Med. 2020; 24:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez-Reyes CS, Baca-Velazquez LN, Villasis-Keever MA, et al. Shock index utility to predict mortality in pediatric patients with septic shock or severe sepsis. Boletin Medico del Hospital Infantil de Mexico. 2018; 75:192–198 [DOI] [PubMed] [Google Scholar]
- 74.Rousseaux J, Grandbastien B, Dorkenoo A, et al. Prognostic value of shock index in children with septic shock. Pediatr Emerg Care. 2013; 29:1055–1059 [DOI] [PubMed] [Google Scholar]
- 75.Choudhary R, Sitaraman S, Choudhary A. Lactate clearance as the predictor of outcome in pediatric septic shock. J Emerg Trauma Shock. 2017; 10:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen M, Lu X, Hu L, et al. Development and validation of a mortality risk model for pediatric sepsis. Medicine (Baltimore). 2017; 96:e6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi SJ, Ha E, Jhang WK, et al. Platelet indices as predictive markers of prognosis in pediatric septic shock patients. Iran J Pediatr. 2017; 27:e2712 [Google Scholar]
- 78.da Silva ED, Koch Nogueira PC, Russo Zamataro TM, et al. Risk factors for death in children and adolescents with cancer and sepsis/septic shock. J Pediatr Hematol Oncol. 2008; 30:513–518 [DOI] [PubMed] [Google Scholar]
- 79.Goonasekera CDA, Carcillo JA, Deep A. Oxygen delivery and oxygen consumption in pediatric fluid refractory septic shock during the first 42 h of therapy and their relationship to 28-day outcome. Front Pediatr. 2018; 6:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ostrowski JA, MacLaren G, Alexander J, et al. The burden of invasive infections in critically ill indigenous children in Australia. Med J Aust. 2017; 206:78–84 [DOI] [PubMed] [Google Scholar]
- 81.Tang X, Shao L, Dou J, et al. Fibrinogen as a prognostic predictor in pediatric patients with sepsis: A database study. Mediators Inflamm. 2020; 2020:9153620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tonial CT, Costa CAD, Andrades GRH, et al. Prediction of poor outcomes for septic children according to ferritin levels in a middle-income setting. Pediatr Crit Care Med. 2020; 21:e259–e266 [DOI] [PubMed] [Google Scholar]
- 83.Xie X, Li M, Xiong TT, et al. Nested case-control study of multiple serological indexes and Brighton pediatric early warming score in predicting death of children with sepsis. World J Clin Cases. 2019; 7:431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maat M, Buysse CM, Emonts M, et al. Improved survival of children with sepsis and purpura: Effects of age, gender, and era. Crit Care. 2007; 11:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niederwanger C, Varga T, Hell T, et al. Comparison of pediatric scoring systems for mortality in septic patients and the impact of missing information on their predictive power: A retrospective analysis. PeerJ. 2020; 8:e9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhong M, Huang Y, Li T, et al. Day-1 PELOD-2 and day-1 “quick” PELOD-2 scores in children with sepsis in the PICU. J Pediatr (Rio J). 2020; 96:660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niederwanger C, Bachler M, Hell T, et al. Inflammatory and coagulatory parameters linked to survival in critically ill children with sepsis. Ann Intensive Care. 2018; 8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sayed SZ, Mahmoud MM, Moness HM, et al. Admission platelet count and indices as predictors of outcome in children with severe sepsis: A prospective hospital-based study. BMC Pediatr. 2020; 20:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lawang SA, Jayaganda DD. White blood cell, procalcitonin, C-reactive protein and TNF-alpha as prognostic factors in pediatric sepsis. Indian J Public Health Res Dev. 2019; 10:708– 713 [Google Scholar]
- 90.Liu GB, Cui XQ, Wang ZB, et al. Detection of serum procalcitonin and hypersensitive C-reactive protein in patients with pneumonia and sepsis. J Biol Regul Homeost Agents. 2018; 32:1165–1169 [PubMed] [Google Scholar]
- 91.Wu Q, Nie J, Wu FX, et al. Prognostic value of high-sensitivity C-reactive protein, procalcitonin and pancreatic stone protein in pediatric sepsis. Med Sci Monit. 2017; 23:1533–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niederwanger C, Hell T, Hofer S, et al. Antithrombin deficiency is associated with mortality and impaired organ function in septic pediatric patients: A retrospective study. PeerJ. 2018; 6:e5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaramillo-Bustamante JC, Marín-Agudelo A, Fernández-Laverde M, et al. Epidemiology of sepsis in pediatric intensive care units: First Colombian multicenter study. Pediatr Crit Care Med. 2012; 13:501–508 [DOI] [PubMed] [Google Scholar]
- 94.Xiao C, Wang S, Fang F, et al. Epidemiology of pediatric severe sepsis in main PICU centers in Southwest China. Pediatr Crit Care Med. 2019; 20:1118–1125 [DOI] [PubMed] [Google Scholar]
- 95.Mianling Z, Yuge H, Tufeng L, et al. Performance of the pediatric sequential organ failure assessment score in assessing the prognosis of children with sepsis in a PICU of a developing country: A single-center retrospective observational study. Iran J Pediatr. 2019; 29:e89024 [Google Scholar]
- 96.Carrol ED, Newland P, Thomson AP, et al. Prognostic value of procalcitonin in children with meningococcal sepsis. Crit Care Med. 2005; 33:224–225 [DOI] [PubMed] [Google Scholar]
- 97.Rey C, Los Arcos M, Concha A, et al. Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med. 2007; 33:477–484 [DOI] [PubMed] [Google Scholar]
- 98.Reed L, Carroll J, Cummings A, et al. Serum lactate as a screening tool and predictor of outcome in pediatric patients presenting to the emergency department with suspected infection. Pediatr Emerg Care. 2013; 29:787–791 [DOI] [PubMed] [Google Scholar]
- 99.Lindell RB, Gertz SJ, Rowan CM, et al. ; Sepsis PRevalence, OUtcomes, and Therapies Study Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. High levels of morbidity and mortality among pediatric hematopoietic cell transplant recipients with severe sepsis: Insights from the sepsis PRevalence, OUtcomes, and Therapies International Point Prevalence Study. Pediatr Crit Care Med. 2017; 18:1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kortz TB, Sawe HR, Murray B, et al. Clinical presentation and outcomes among children with sepsis presenting to a public tertiary hospital in Tanzania. Front Pediatr. 2017; 5:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozdemir R, Isguder R, Kucuk M, et al. A valuable tool in predicting poor outcome due to sepsis in pediatric intensive care unit: Tp-e/QT ratio. J Trop Pediatr. 2016; 62:377–384 [DOI] [PubMed] [Google Scholar]
- 102.Haque A, Siddiqui NR, Munir O, et al. Association between vasoactive-inotropic score and mortality in pediatric septic shock. Indian Pediatr. 2015; 52:311–313 [DOI] [PubMed] [Google Scholar]
- 103.Gupta S, Alam A. Shock index-a useful noninvasive marker associated with age-specific early mortality in children with severe sepsis and septic shock: Age-specific shock index cut-offs. J Intensive Care Med. 2020; 35:984–991 [DOI] [PubMed] [Google Scholar]
- 104.Lautz AJ, Wong HR, Ryan TD, et al. Myocardial dysfunction is independently associated with mortality in pediatric septic shock. Crit Care Explor. 2020; 2:e0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sankar J, Das RR, Jain A, et al. Prevalence and outcome of diastolic dysfunction in children with fluid refractory septic shock–a prospective observational study. Pediatr Crit Care Med. 2014; 15:e370–e378 [DOI] [PubMed] [Google Scholar]
- 106.Khanbabaee G, Hashemi SM, Salarian S, et al. Red cell distribution width elevation and sepsis in pediatric critically ill patients. Arch Pediatr Infect Dis. 2010; 6:e12210 [Google Scholar]
- 107.Onwuneme C, Carroll A, Doherty D, et al. Inadequate vitamin D levels are associated with culture positive sepsis and poor outcomes in paediatric intensive care. Acta Paediatr. 2015; 104:e433–e438 [DOI] [PubMed] [Google Scholar]
- 108.Pizarro CF, Troster EJ, Damiani D, et al. Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med. 2005; 33:855–859 [DOI] [PubMed] [Google Scholar]
- 109.Saleh NY, Abo El Fotoh WMM. Low serum zinc level: The relationship with severe pneumonia and survival in critically ill children. Int J Clin Pract. 2018; 72:e13211. [DOI] [PubMed] [Google Scholar]
- 110.Vermont CL, den Brinker M, Kâkeci N, et al. Serum lipids and disease severity in children with severe meningococcal sepsis. Crit Care Med. 2005; 33:1610–1615 [DOI] [PubMed] [Google Scholar]
- 111.Yildizdas D, Yapicioglu H, Celik U, et al. Terlipressin as a rescue therapy for catecholamine-resistant septic shock in children. Intensive Care Med. 2008; 34:511–517 [DOI] [PubMed] [Google Scholar]
- 112.Oliveira NS, Silva VR, Castelo JS, et al. Serum level of cardiac troponin I in pediatric patients with sepsis or septic shock. Pediatr Crit Care Med. 2008; 9:414–417 [DOI] [PubMed] [Google Scholar]
- 113.Moustafa AA, Antonios MA, Abdellatif EM, et al. Association of lactate/albumin ratio level to organ failure and mortality in severe sepsis in a pediatric intensive care unit in Egypt. Turk J Pediatr. 2018; 60:691–701 [DOI] [PubMed] [Google Scholar]
- 114.Scott HF, Brou L, Deakyne SJ, et al. Lactate clearance and normalization and prolonged organ dysfunction in pediatric sepsis. J Pediatr. 2016; 170:149–155.e1 [DOI] [PubMed] [Google Scholar]
- 115.Rambaud-Althaus C, Althaus F, Genton B, et al. Clinical features for diagnosis of pneumonia in children younger than 5 years: A systematic review and meta-analysis. Lancet Infect Dis. 2015; 15:439–450 [DOI] [PubMed] [Google Scholar]
- 116.Maitland K, Kiguli S, Opoka RO, et al. ; FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011; 364:2483–2495 [DOI] [PubMed] [Google Scholar]
- 117.Wiens MO, Kumbakumba E, Kissoon N, et al. Pediatric sepsis in the developing world: Challenges in defining sepsis and issues in post-discharge mortality. Clin Epidemiol. 2012; 4:319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Balamuth F, Schlapbach LJ. Paediatric patient stratification in the emergency department. Lancet Child Adolesc Health. 2020; 4:557–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.