Abstract
Since the treatment of lung squamous cell carcinoma (SCC) was limited due to a lack of appropriate biomarkers and novel target agents. Immune checkpoint inhibitors can offer an effective treatment for patients with advanced non-small cell lung cancer. Here, we described the cases of two patients with SCC who showed a good response following treatment with tislelizumab. We encountered two patients with unresectable lung SCC who were treated with immunotherapy and chemotherapy. One patient had negatively programmed death-ligand 1 expression, and the primary lesion becomes a thick wall cavity after the tislelizumab combined with chemotherapy. Another patient was diagnosed with advanced lung SCC with negative programmed death-ligand 1 expression. After the treatment, the fluorine-18-fluorodeoxyglucose PET/computed tomography indicated that no abnormal increase in radioactivity uptake and tend to complete remission. We found a significant response or even complete response in unresectable SCC treated with tislelizumab combined with chemotherapy. Our cases added evidence of the feasibility and efficacy of tislelizumab combined with chemotherapy in unresectable lung SCC.
Keywords: case report, immune checkpoint inhibitor, lung squamous cell carcinoma, lymphocyte-to-monocyte ratio
Introduction
According to the 2020 Global Cancer Statistics, the incidence and mortality rate of lung cancer, respectively, ranked second and first in all cancers, and nearly 30–40% of patients had locally advanced disease or distant metastases at the time of diagnosis [1,2]. The treatment of lung squamous cell carcinoma (SCC) was limited due to a lack of appropriate biomarkers and novel target agents. Recent research has found that immune checkpoint inhibitor (ICI) therapy may improve lung SCC survival benefit [3].
Tislelizumab, a mAb with high binding affinity to the programmed death 1 (PD-1) receptor, was specifically engineered to minimize Fcγ receptor binding on macrophages to abrogating antibody-dependent phagocytosis and potential resistance to anti-PD-1 therapy [4]. Pharmacological analysis showed that the dissociation rate of tislelizumab was about 30-fold slower than that of nivolumab and 80-fold less than of pembrolizumab, respectively, resulting in a 35-fold to 60-fold higher target affinity of tislelizumab compared with the other two antibodies [5]. Initially, for most lung cancers, tislelizumab and chemotherapy in combination demonstrated encouraging antitumor activity [6]. Subsequently, Wang et al. showed that adding tislelizumab to chemotherapy significantly prolonged progress-free survival regardless of programmed death-ligand 1 (PD-L1) expression [7]. At the same time, the adverse effects (AEs) of tislelizumab were tolerable, and most of them were grade 1 or 2 AEs [8,9]. However, grades 3–5 adverse events occurred in 74.1% of patients receiving pembrolizumab plus chemotherapy [10]. This year, the National Medical Products Administration of China approved tislelizumab combined with two chemotherapy regimens as the first-line drugs for advanced lung SCC.
Here, we described the cases of two patients with SCC who showed a good response following treatment with tislelizumab.
Case reports
This study was approved by the ethics committee of the Affiliated Hospital of Zunyi Medical University. Informed consent was obtained from the two patients.
Case 1
A 69-year-old man was sent to the pneumology department due to hemoptysis for 5 months. At that time, a computed tomography (CT) revealed a 6.68 cm × 6.16 cm × 6.9 cm mass in the upper lobe of the left lung (Fig. 1), a 3.4 cm × 2.5 cm × 4.5 cm mass in the lower lobe of the right lung and multiple metastases in lung (Fig. 1). He had been smoking one packet of cigarettes per day for 40 years. Lung SCC was confirmed (Fig. 2) using fiberoptic bronchoscopy biopsy. Immunohistochemistry showed CK5/6 (+), P40 (+), Ki-67 (60%), TTF-1 (–), CK7(–) (Fig. 2). The membranous PD-1 and PD-L1 of tumor cells were both negative by immune staining with MX003 and MXR033, respectively (Fig. 2). The cystic lesion of the liver disappeared after treatment, so we regarded the lesion as distant liver metastasis. A cT4N2M1c stage IVB disease of the left lung and a cT2N2M1c stage IVB disease of the right lung were conclusive (according to the eighth edition of American Joint Committee on Cancer).
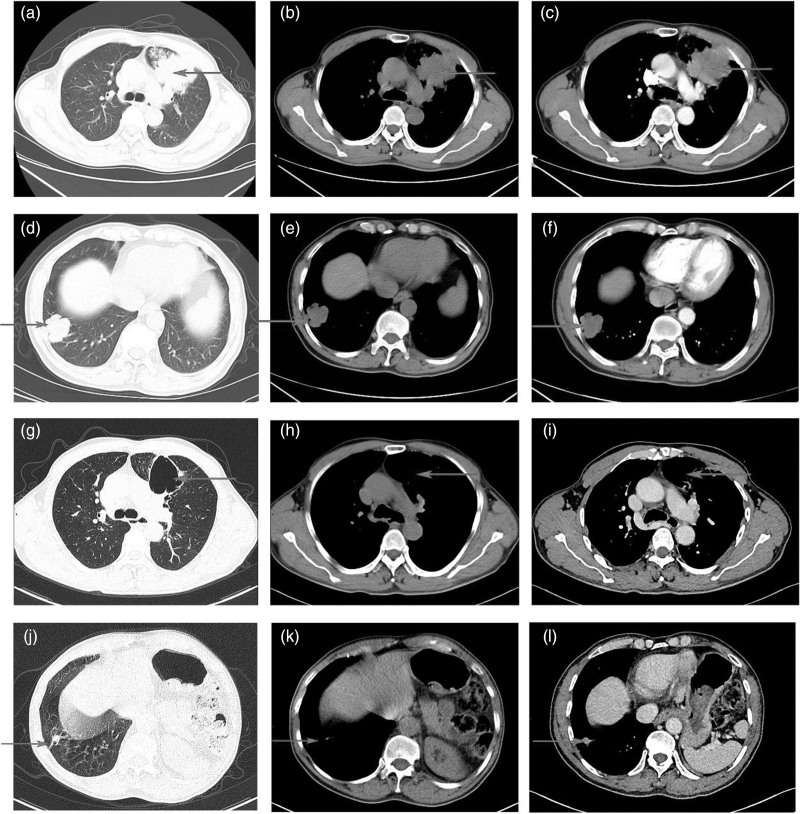
Fig. 1.
CT of the primary lung lesion for case 1. (a, b, c) The primary lesion in the lung at diagnosis (arrow). (d, e, f) The second primary lesion in the lung at diagnosis (arrow). (g, h, i, j, k, l) After four cycles therapy showed the tumor response with CT scan (arrow). CT, computed tomography.
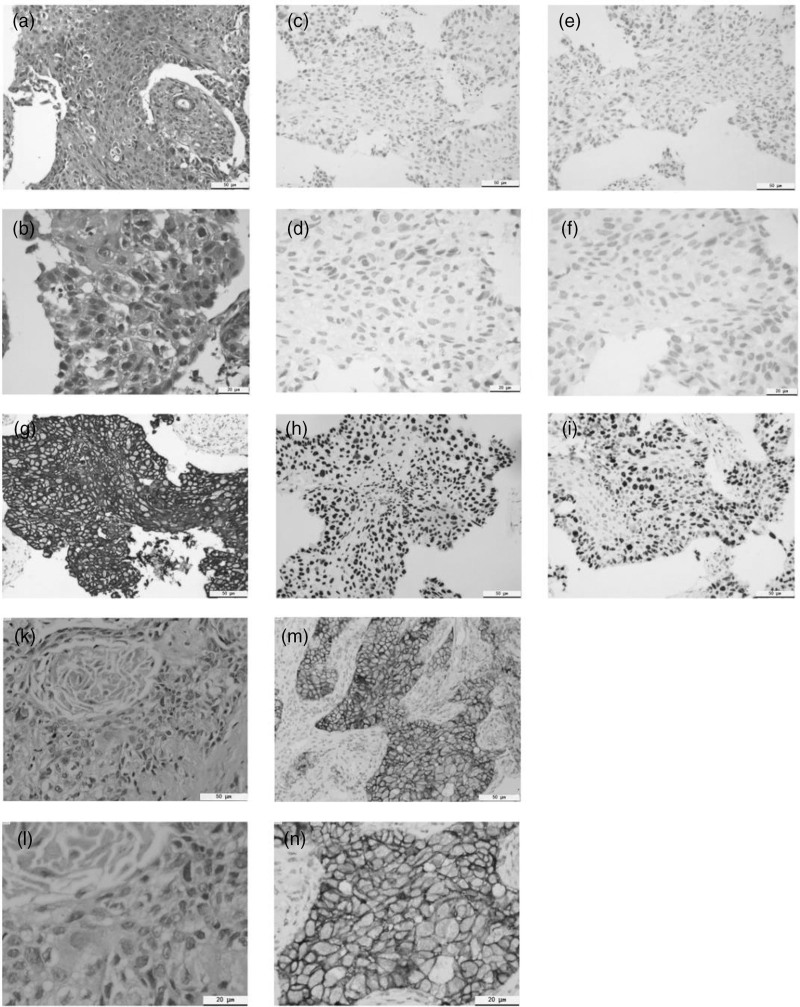
Fig. 2.
Results of pathological section and immunohistochemistry. case 1: (a, b) hematoxylin-eosin staining and images were acquired (magnification ×200, 400); (c, d) the result of PD-1 testing was negative (magnification ×200, 400); (e, f) The result of PD-L1 testing was negative (magnification ×200, 400); (g) by immunostaining, the tumor cells were diffusely positive for CK5/6 (magnification ×200); (h) the diffusely positive for p40 (magnification ×200); (i) by immunostaining, the tumor cells were 67% for ki-67 (magnification ×200). Case 2: (k, m) hematoxylin-eosin staining and images were acquired (magnification ×200, 400); (h, i) positive for PD-L1 (≥70%, magnification ×200, 400). PD-L1, programmed death-ligand 1.
Survival benefits can still be improved by ICI therapy, although PD-L1 and PD-1 were both negative [11]. We treated the patient with cisplatin 75 mg/m2, paclitaxel for injection (albumin-bound) 260 mg/m2 and tislelizumab 200 mg, every 3 weeks for three cycles. Because of side effects in the digestive system, we changed the scheme to carboplatin (area under the cure = 5) for three cycles.
According to response evaluation criteria in solid tumors RECIST version 1.1, the patient showed a good response and reached partial remission after four cycles. The CT scan results showed that the main primary lesion disappeared and formed a thick wall cavity in the upper lobe of the left lung (Fig. 1) and the right lung lesion was shrunk to 1.3 cm × 1.3 cm × 1.0 cm (Fig. 1). Subsequently, the patient has been receiving tislelizumab as the maintenance therapy. During the treatment, the patient appeared ICI-related hypothyroidism (G2) after three cycles of ICI, and performed the symptomatic treatment with levothyroxine sodium tablets.
Case 2
A 54-year-old man, with a 30-year history of smoking and no family history of cancer, was diagnosed with lung SCC of the inferior lobe of the left lung. The CT scan revealed that the mass measured about 6.3 cm × 4.0 cm × 6.8 cm accompanied by a small amount of pleural effusion on the left side (Fig. 3). Since the pleural effusion cannot be definitively diagnosed as malignant, the patient was staged as T3N2Mx. In other hospitals, fiberoptic bronchoscopy biopsy showed SCC. Immunohistochemistry showed: CK5/6 (+), P40 (+), P63 (+), Ki-67 (30%), TTF-1 (–), PSA (–), Syn (–) and Napsin A (–) (Fig. 2).
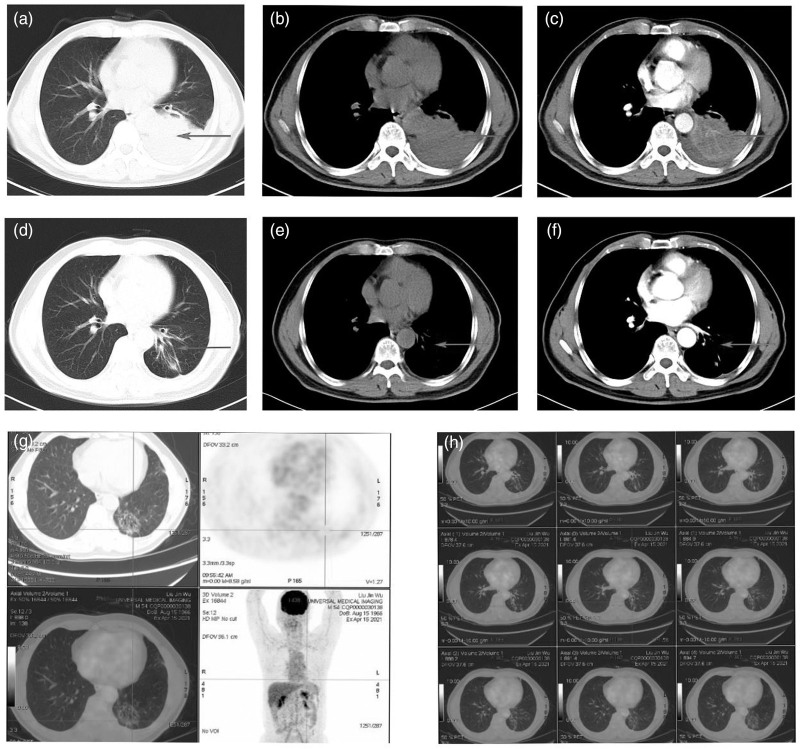
Fig. 3.
CT and PET/CT of the primary lung lesion for case 2. (a, b, c) The primary lesion and partial atelectasis in the lung at diagnosis (arrow); (d, e, f) after four cycles therapy showed the tumor response and remission of the pulmonary atelectasis with CT scan (arrow); (g, h) after six cycles therapy showed the tumor response and remission of the pulmonary atelectasis with PET/CT scan (PET/CT indicated no abnormal increase in radioactivity uptake and maximum SUV was 2.45 and suggested suppressed tumor activity). CT, computed tomography.
PD-L1 expression of tumor cells was 70% positive using the E1L3N in our hospital (Fig. 2). This patient was difficult to operate and pleural metastasis cannot be ruled out, so we opted for systemic therapy. Tislelizumab (200 mg) combined with cisplatin (75 mg/m2) and paclitaxel for injection (albumin bound) (260 mg/m2) were carried out.
The patient showed a good tumor response and reached remission of the pulmonary atelectasis after four cycles of therapy, the CT showed that the pleural effusion was significantly reduced (Fig. 3). After six cycles of therapy, fluorine-18-fluorodeoxyglucose PET/CT indicated no abnormal increase in radioactivity uptake and maximum standard uptake value was 2.45, and revealed that tumor activity was suppressed (Fig. 3). The patient was then treated with radical radiotherapy (PTV 6,000 cGy/ 200 cGy/ 30 F). At present, the patient has been receiving tislelizumab as maintenance therapy. There were no side effects during the therapeutic course.
Hematological results
We analyzed the changes in the patient’s white blood cells before each immunochemotherapy session. The lymphocyte percentage gradually increased and the lymphocyte-to-monocyte ratio (LMR) rose to about 3.0 and tended to stabilize. In addition, the neutrophil–lymphocyte ratio (NLR) which is recognized as a predictive biomarker of ICI therapy decreased below 4.0 after one cycle of ICI for case 1 and below 2.0 for case 2 (Table 1), which indicated that immunotherapy was effective.
Table 1.
Serial changes in white cell counts
| Cycle 0 | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 | |
|---|---|---|---|---|---|---|---|
| Case 1 | |||||||
| WBCs (×109/L) | 6.89 | 6.64 | 5.62 | 5.07 | 2.92 | 4.33 | 3.07 |
| Neutrophil (%) | 0.74 | 0.68 | 0.6 | 0.71 | 0.48 | 0.64 | 0.64 |
| Lymphocyte (%) | 0.14 | 0.18 | 0.21 | 0.22 | 0.4 | 0.24 | 0.26 |
| Monocyte (%) | 0.08 | 0.11 | 0.16 | 0.05 | 0.08 | 0.1 | 0.06 |
| Platelet | 350 | 258 | 260 | 160 | 171 | 127 | 116 |
| Lymphocyte count | 0.96 | 1.2 | 1.18 | 1.12 | 1.17 | 1.04 | 0.8 |
| LMR | 1.75 | 1.63 | 1.31 | 4.4 | 5 | 2.4 | 4.33 |
| NLR | 5.28 | 3.78 | 2.85 | 3.22 | 1.2 | 2.67 | 2.46 |
| Case 2 | |||||||
| WBCs (×109/L) | 10.73 | 5.8 | 5.69 | 5.07 | 4.25 | 3.94 | 4.6 |
| Neutrophil (%) | 0.86 | 0.52 | 0.45 | 0.58 | 0.46 | 0.55 | 0.55 |
| Lymphocyte (%) | 0.08 | 0.38 | 0.38 | 0.3 | 0.42 | 0.33 | 0.33 |
| Monocyte (%) | 0.06 | 0.08 | 0.14 | 0.11 | 0.11 | 0.11 | 0.1 |
| Platelet | 395 | 197 | 176 | 175 | 185 | 149 | 126 |
| Lymphocyte count | 0.86 | 2.2 | 2.16 | 1.52 | 1.78 | 1.3 | 1.52 |
| LMR | 1.33 | 4.75 | 2.71 | 2.72 | 3.82 | 3 | 3.3 |
| NLR | 10.75 | 1.36 | 1.18 | 1.93 | 1.09 | 1.67 | 1.67 |
The lymphocyte percentages increased after immunotherapy and the NLR was decreased to the stationary state.
LMR, lymphocyte monocyte ratio; NLR, neutrophil–lymphocyte ratio; WBC, white blood cells.
Discussion
We presented the two cases of the patients with unresectable lung SCC and received tislelizumab and chemotherapy as the first-line treatment. The two patients had a significant remission of their lung lesions. According to the present reported cases, patients with lung cancer for tislelizumab were extremely rare [6,12], and we did not find any case report with a significant response or even complete response in SCC treated with tislelizumab. Similarly, a few cases show the effectiveness of pembrolizumab for lung SCC [13–15].
NLR and LMR are two easily available clinical indicators that can predict the response of ICI. Some reports revealed that patients with on-treatment NLR<5 had significantly longer overall survival, and the changes in NLR were associated with gastric cancer survival during nivolumab monotherapy [16–18]. In both two patients, NLR was gradually reduced and maintained at a level, which remained a marker for treatment efficacy.
The patient in case 1 achieved a significant remission, although both PD-L1 and PD-1 were negative, which is consistent with the results of some studies [19]. The reason may include conflicting results between different detection antibodies, heterogeneity in PD-L1 expression within and across tumor sites, and temporally dynamic PD-L1 expression [20]. Hence, looking for a maker that can reflect the expression level of PD-L1 is promising.
ICIs were better tolerated than chemotherapy overall [21]. The patient in case 1 developed ICI-related hypothyroidism (G2) after three cycles of ICI. Thyroid function fluctuated at normal levels after symptomatic treatment.
In conclusion, in our study, we reported two patients with lung SCC who were successfully treated with tislelizumab as the first-line therapy. Our cases added pieces of evidence of the feasibility and efficacy of tislelizumab combined with chemotherapy in patients with lung SCC which is unresectable or not radiotherapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81860469), China, and by the Science and Technology Foundation of Basic Research Program of Guizhou Province ([2020]1Z063), Guizhou, P. R. China.
The data are not available for public access because of patient privacy concerns but are available from the corresponding author on reasonable request.
Signed informed consents were obtained from the two patients.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Qiaoyuan Wu and Yunliang Cao are the co-first authors.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018; 553:446–454. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al.; KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67:1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong Y, Feng Y, Sun H, Zhang B, Wu H, Zhu Q, et al. Tislelizumab uniquely binds to the CC’ loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio 2021; 11:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Zhao J, Ma Z, Cui J, Shu Y, Liu Z, et al. A phase 2 study of tislelizumab in combination with platinum-based chemotherapy as first-line treatment for advanced lung cancer in Chinese patients. Lung Cancer 2020; 147:259–268. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021; 7:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai J, Deva S, Lee JS, Lin CC, Yen CJ, Chao Y, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer 2020; 8:e000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai Y, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020; 8:e000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 2020; 15:1657–1669. [DOI] [PubMed] [Google Scholar]
- 11.Borghaei H, Langer CJ, Paz-Ares L, Rodríguez-Abreu D, Halmos B, Garassino MC, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: a pooled analysis of 3 randomized controlled trials. Cancer 2020; 126:4867–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous non-small cell lung cancer (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol 2021; 16:1512–1522. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi E, Okamoto Y, Takahashi N, Morizumi S, Toyoda Y, Kuroda N, Yorita K. Complete response of squamous cell carcinoma of the lung following treatment with pembrolizumab in an elderly patient: a case report. Thorac Cancer 2021; 12:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CP, Sung YC, Lo CY, Yen MH. Pathological complete response of initially inoperable lung squamous cell carcinoma treated by immunochemotherapy: a case report. Asian J Surg 2020; 43:393–395. [DOI] [PubMed] [Google Scholar]
- 15.Maller B, Kaszuba F, Tanvetyanon T. Complete tumor response of tracheal squamous cell carcinoma after treatment with pembrolizumab. Ann Thorac Surg 2019; 107:e273–e274. [DOI] [PubMed] [Google Scholar]
- 16.Ayers KL, Ma M, Debussche G, Corrigan D, McCafferty J, Lee K, et al. A composite biomarker of neutrophil-lymphocyte ratio and hemoglobin level correlates with clinical response to PD-1 and PD-L1 inhibitors in advanced non-small cell lung cancers. BMC Cancer 2021; 21:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol 2020; 85:265–272. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Spakowicz D, Burkart J, Patel S, Husain M, He K, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol 2019; 145:2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol 2021; 32:1137–1147. [DOI] [PubMed] [Google Scholar]
- 20.Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev 2015; 41:868–876. [DOI] [PubMed] [Google Scholar]
- 21.Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist 2017; 22:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]