Abstract
Activatable cell-penetrating peptide (ACPP) is a tumour-targeting cell-penetrating peptide. Here, we used ACPP to carry anti-p21Ras scFv for Ras-driven cancer therapy. The ACPP-p21Ras scFv fusion protein was prepared by a prokaryotic expression system and Ni-NTA column purification. The human tumour cell lines A549, SW480, U251 and Huh7 and the normal cell line BEAS 2B were used to study the tumor-targeting and membrane-penetrating ability of ACPP-p21Ras scFv. The antitumour activity of ACPP-p21Ras scFv on A549 cells and H1299 cells in vitro was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, scratch wound healing, plate cloning and apoptosis assays. The penetration pathway of ACPP was determined by enhanced green fluorescent protein. The ACPP-p21Ras scFv fusion protein was successfully obtained at a concentration of 1.8 mg/ml. We found that ACPP-p21Ras scFv could penetrate tumour cell membranes with high expression of matrix metalloproteinase-2 (MMP-2), effectively inhibit the migration and proliferation of A549 cells and H1299 cells, and promote the apoptosis of A549 cells and H1299 cells. The membrane penetration experiment demonstrated that ACPP could enter A549 cells by direct penetration. The ability of ACPP to penetrate the membrane was affected by the addition of a membrane affinity inhibitor and a change in the potential difference across the cell membrane but not by the addition of endocytosis inhibitors and a change in temperature. The ACPP-p21Ras scFv fusion protein can penetrate tumour cells with MMP-2 expression and has antitumour activity against A549 cells and H1299 cells in vitro. This molecule is expected to become a potential antitumour drug for Ras gene-driven lung cancer.
Keywords: Activatable cell-penetrating peptide-p21Ras scFv, antitumor, carrier, cell-penetrating peptide, lung cancer, matrix metalloproteinases-2, therapy
Introduction
Lung cancer is one of the most common cancers worldwide and is the main cause of cancer-related death [1]. In recent years, the mortality rate of lung cancer in China has been increasing [2]. The conventional therapy for lung cancer is surgery combined with radiotherapy and chemotherapy. However, many patients are in the middle and late stages of the disease when they are diagnosed and show local infiltration or distant metastasis [3,4], which makes surgery difficult. Unfortunately, nonspecific cell uptake [5] leads to serious side effects, which makes the curative effects of radiotherapy and chemotherapy unsatisfactory. In addition, insufficient accumulation of chemotherapeutic drugs [6] and drug resistance [7,8] limit the efficacy of chemotherapy. Targeted therapy is a method to treat tumours by acting on the corresponding targets at the cellular and molecular levels [5], which provides a new strategy to solve the above problems. Currently, the Food and Drug Administration has approved some molecular targeted drugs for the clinical treatment of lung cancer, such as gefitinib (an inhibitor of epidermal growth factor receptor) and crizotinib (an inhibitor of anaplastic lymphoma kinase) [9]. However, the secondary resistance of these drugs leads to a low response rate, which is a major disadvantage in treatment [10–14]. Therefore, more drugs with other molecular targets are urgently needed.
Ras is the most mutated oncogene in human cancer [15]. K-ras mutation is found in 20–25% of non-small cell lung cancer (NSCLC) cases and is the most common codriver gene [16]. Although many reagents targeting Ras, such as SCH-54292 (direct inhibition of K-ras), ISIS 2503 (inhibition of RAS protein expression) and farnesyltransferase inhibitors (inhibitors of the enzymes involved in the post-translational modifications of Ras), have been studied, they were abandoned due to poor efficacy [17,18]. Hence, the development of promising Ras-targeted drugs has received much attention. The anti-p21Ras scFv, which reacted with wild-type and mutant Ras proteins [19], was prepared in our laboratory. We used adenovirus to carry the anti-p21Ras scFv gene to enter tumour cells and to express scFv in cells. In both in-vitro and in-vivo experiments, the intracellularly expressed scFv could block the Ras-related signalling pathway, effectively inhibit tumour cell proliferation and promote apoptosis [20]. However, the safety and stability of adenovirus are uncertain in vivo, which limits its clinical application [21,22]. Therefore, identification of a well tolerated and stable intracellular delivery vector has become our next research direction.
Cell-penetrating peptides (CPPs) are peptides with effective intracellular delivery performance, low cytotoxicity and low immunogenicity [23,24]. These molecules can carry a variety of proteins into the cell, such as Rp3 (13.29 kDa) [25] and CARM1 (66.89 kDa) [26]. Although this strategy overcomes the defects of adenovirus, the lack of cell selectivity of CPPs limits its application as a drug delivery tool [27]. Activatable cell-penetrating peptide (ACPP) solves this problem [28]. ACPP is a modified CPPs that can target tumour cells and deliver anticancer drugs into cells. In this study, we designed and expressed the ACPP-p21Ras scFv fusion protein in Escherichia coli by genetic engineering and then investigated the immune activity, cell-penetrating effect and antitumour activity in vitro.
Materials and methods
Cell lines and cell culture
E. coli strain DH5α and E. coli strain BL21 (DE3) were purchased from Kunming Shuoqing Biotechnology Co., Ltd. (Kunming, China). Human tumour cell lines with matrix metalloproteinase-2 (MMP-2) overexpression, including the human NSCLC cell line A549 (KRASG12S) [29] and H1299 (NRASQ61K) [29], human colorectal cancer cell line SW480, human glioma cell line U251, human hepatoma cell line Huh7 and human bronchial epithelial cell line BEAS 2B with low MMP-2 expression, were purchased from Kunming Cell Bank of Chinese Academy of Sciences (Kunming, China). All the above cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (HyClone, USA) containing 100 U/ml penicillin, 100 mg/ml streptomycin and 10% foetal bovine serum (FBS; Biological Industries, Israel) at 37 °C with 5% CO2.
Construction of recombinant expression plasmids
The ACPP gene sequence was reported by Li et al., [28] (Fig. 1a). The anti-p21Ras scFv gene sequence was constructed previously in our laboratory [19]. The enhanced green fluorescent protein (EGFP) gene sequence is listed in National Center for Biotechnology Information GenBank (ID: 20473140). The ACPP gene sequence was linked to the 5′ end of the target gene to synthesise three kinds of inserts, ACPP-p21Ras scFv, ACPP-L-p21Ras scFv and ACPP-L-EGFP. Then, the insert fragments were cloned between the NcoI and HindIII sites of the pET28a (+) vector to construct three prokaryotic expression plasmids (Kunming Shuoqing Biotechnology Co., Ltd., China). The recombinant plasmids were identified by PCR to determine whether the inserted fragments were correct.
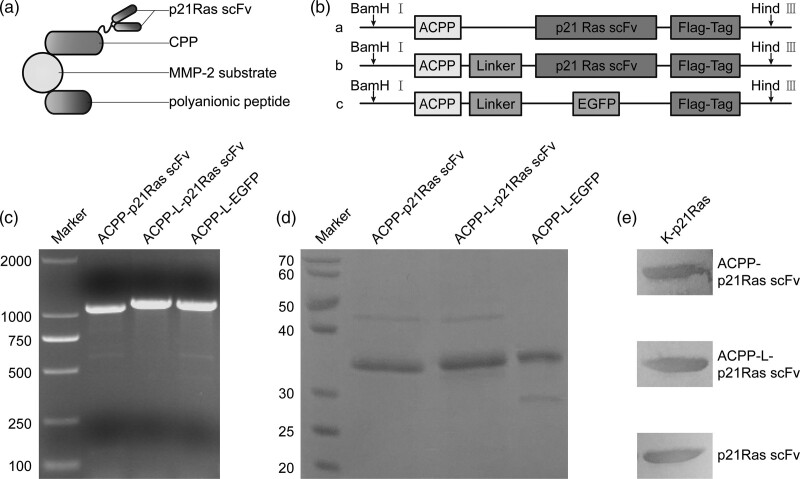
Fig. 1.
Construction and expression of the recombinant activatable cell-penetrating peptide (ACPP)-p21Ras scFv fusion protein. (a) Schematic structure of the ACPP-p21Ras scFv fusion protein. Cell-penetrating peptide (CPP): polycationic peptide with the cell-penetrating property. MMP-2 substrate: oligopeptide that binds to MMP-2. Polyanionic peptide: anionic peptide that blocks the membrane-penetrating function of CPP. Once the MMP-2-sensitive oligopeptide is cleaved, the polyanionic peptide drifts away, and the polycationic CPP is activated to carry p21Ras scFv into cells. (b) Schematic diagram of the recombinant plasmids design. A Flag tag was attached to the 3 ‘end of the p21Ras scFv, while the ACPP peptide was designed at the 5’ end of the p21Ras scFv. The sequence was inserted between BamH I and Hind III sites of plasmid. a, ACPP-p21Ras scFv; b, ACPP-L-p21Ras scFv; c, ACPP-L-EGFP. (c) PCR analysis showed that the sizes of ACPP-p21Ras scFv, ACPP-L-p21Ras scFv and ACPP-L-EGFP were 1185, 1230 and 1197 bp, respectively, which were consistent with the expected values. (d) The molecular weights of ACPP-p21Ras scFv, ACPP-L-p21Ras scFv and ACPP-L-EGFP was identified by SDS-PAGE, that is, 36, 37 and 37 KD, respectively. (e) The binding ability of the recombinant antibodies to K-p21Ras was identified by WB. After electrophoresis and membrane transfer, K-p21Ras was incubated with ACPP-p21Ras scFv, ACPP-L-p21Ras scFv or p21Ras scFv. All the antibodies could combine with K-p21Ras. EGFP, enhanced green fluorescent protein; MMP-2, matrix metalloproteinase-2; WB, western blot.
Expression and purification of fusion proteins
Three recombinant plasmids were transformed into E. coli BL21 (DE3). Transformed bacteria were cultured in LB liquid medium containing 10 μg/ml kanamycin and shaken at 200 rpm at 37 °C. When the optical density value reached 0.6, 1 mM isopropyl-β-D-thiogalactoside (IPTG) was added and shaken at 22 °C for 5 h to induce the expression of the target proteins. The bacteria were collected by centrifuging at 4 °C and 5000 g for 15 min and then resuspended in 50 mM pH 8.0 Tris HCl and sonicated at 4 °C for 130 W for 20 min (JY92-IIN, Scientz, China). The supernatant was collected by centrifugation at 10000 rpm for 15 min and loaded on a prebalanced Ni-NTA column (HisPurTM Ni-NTA purification kit; Thermo Scientific, USA) for affinity chromatography to obtain target proteins. The bicinchoninic acid method was used to determine the protein concentration, and SDS-PAGE was used to analyse the molecular weight and purity of the proteins.
Membrane penetration experiment of ACPP
Effect of different inhibitors on the penetration of ACPP
A total of 5 × 104 A549 cells per well were seeded in a 12-well plate and cultured at 37 °C for 24 h. When the cell confluence reached 80%, the cells were washed twice with PBS and incubated with a membrane affinity inhibitor (10 mM heparin) or endocytosis inhibitors [29 µM chlorpromazine, 50 µM amiloride (EIPA), and 5 mM methyl-β-cyclodextrin (MβCD)] in RPMI 1640 medium containing 10% FBS (300 µl) [30]. After incubation at 37 °C for 0.5 h, the supernatant was discarded, and the cells were subsequently incubated at 37 °C for 1 h in 1640 medium containing 20 µM CPP-L-EGFP (ACPP-L-EGFP digested by MMP-2) (300 µl) [31]. The cells were washed twice with PBS, and the number of green fluorescent cells was determined under a fluorescence microscope.
Effect of temperature on the penetration of ACPP
A total of 5 × 104 A549 cells per well were seeded in a 12-well plate and cultured at 37 °C for 24 h. When the cell confluence reached 80%, the cells were cultured at 4 °C and 37 °C for 0.5 h and washed twice with PBS after discarding the medium. The cells were subsequently incubated at 4 °C or 37 °C for 1 h in 1640 medium containing 20 µM CPP-L-EGFP (300 µl) [31]. The cells were washed twice with PBS, and the number of green fluorescent cells was determined under a fluorescence microscope.
Effect of weakening the potential difference across the cell membrane on the penetration of ACPP
A549 cells were seeded in a 12-well plate as described above. When the cell confluence reached 80%, the culture medium was discarded, and the cells were washed twice with PBS (K+) (Na+ in PBS buffer solution was replaced by equimolar amounts of K+, pH 7.4) or PBS. The cells were subsequently incubated at 37 °C for 1 h in PBS (K+) or PBS solution containing 20 µM ACPP-L-EGFP (300 µl) [31]. The cells were washed twice with PBS, and the number of green fluorescent cells was determined under a fluorescence microscope.
ACPP targeting test
A total of 5 × 104 tumour cells (A549, SW480, Huh7 and U251) and normal bronchial epithelial cells (BEAS-2B) per well were seeded in a 12-well plate and cultured at 37 °C for 24 h. When the cell confluence reached 80%, the culture medium was discarded, and the cells were washed twice with PBS. The cells were subsequently incubated at 37 °C for 2 h in 1640 medium containing 100 µM ACPP-L-EGFP (300 µl). The cells were washed twice with PBS, and the number of green fluorescent cells was determined under a fluorescence microscope.
Detection of ACPP-p21Ras scFv immune activity
The K-p21Ras proteins [19] were subjected to SDS-PAGE on a 12% gel and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% skim milk powder in tris buffered saline with tween 20 (TBST) at room temperature for 1 h with shaking (70 rpm). Each membrane was incubated with scFv (laboratory preparation), ACPP-p21Ras scFv and ACPP-L-p21Ras scFv fusion proteins (dilution 1:100) at 4 °C for 12 h. Next, PVDF membranes were incubated with anti-FLAG tag mAb (dilution 1:1000; MAB9744, Abnova, USA) at 37 °C for 1 h, washed with TBST and incubated with horseradish peroxidase (HRP)-conjugated goat antimouse/rabbit IgG antibody (dilution 1:4000; 2050D0708, Zhongshanjinqiao Co., Ltd., China) at 37 °C for 1 h. Finally, diaminobenzidine was used to develop the colour.
Detection of the ACPP-p21Ras scFv titre
Each well of a 96-well ELISA plate was coated with 5 µg/ml pH 9.6 K-p21Ras protein in 100 µl of carbonate buffer, and the plate was incubated overnight at 4 °C. Following three washes with phosphate buffered solution (PBST) (pH 7.4), the wells were blocked with 100 µl of 1% BSA in PBST at 37 °C for 1 h. Then, 150 µl of the fusion proteins of scFv, ACPP-p21Ras scFv and ACPP-L-p21Ras scFv at varying concentrations (12, 6, 3, 1.5, 0.75, 0.38, 0.19, 0.9 μg/ml) were added to separate wells, and the plate was incubated at 37 °C for 1 h. After three washes with PBST, anti-FLAG tag mAb (dilution 1:1000) and HRP-conjugated goat antimouse/rabbit IgG antibody (dilution 1:2000) were used as primary and secondary antibodies, respectively. Tetramethylbenzidine was added to separate wells, and the plate was incubated for 15 min in the dark. Then, the reaction was terminated with 98% H2SO4. The OD450 was determined by an ELISA reader.
Detection of the membrane-penetrating activity of ACPP-p21Ras scFv by immunohistochemistry
A549 cells were incubated with 20 µM p21Ras scFv, CPP-p21Ras scFv (ACPP-p21Ras scFv digested by MMP-2) and CPP-L-p21Ras scFv (ACPP- L-p21Ras scFv digested by MMP-2) in 1640 medium (4 ml) at 37 °C for 1 h. The culture medium was discarded, and the cells were treated with trypsin, after which the cells were collected by centrifugation (800 rpm, 5 min). The cells were fixed in formalin and embedded in paraffin to prepare paraffin sections. The sections were routinely dewaxed, hydrated, antigen repaired, blocked with H2O2 and washed with PBS three times. Then, the sections were incubated overnight with anti-FLAG tag mAb (dilution 1:100) at 4 °C and HRP-conjugated goat antimouse/rabbit IgG secondary antibody at room temperature for 30 min, and DAB staining was performed.
Detection of the membrane penetrating activity of ACPP-p21ras scFv by immunofluorescence
A549 cell climbing slides were prepared by adding the cells to slides and culturing at 37 °C for 10 h. The slides were washed twice with PBS. Fifty microlitres of 1640 medium containing 20 µM p21Ras scFv, CPP-p21Ras scFv and CPP-L-p21Ras scFv was dripped onto the slides and incubated at 37 °C for 1 h. The slide was fixed with 4% paraformaldehyde, treated with Triton 100 for permeation, and then incubated with anti-FLAG tag mAb (dilution 1:400) at 37 °C for 40 min, followed by rhodamine-conjugated goat antirabbit IgG (dilution 1:150; Zhongshanjinqiao Co., Ltd., China) at 37 °C for 40 min in the dark. Finally, the cells were incubated with 4',6-diamidino-2-phenylindole (Promega, USA) for 5 min in the dark and then observed under a fluorescence microscope.
Detection of ACPP-p21Ras scFv targeting of tumour cells
A549, SW480, Huh7, U251 and BEAS-2B cells were incubated with 100 µM ACPP-p21Ras scFv and 100 µM ACPP-L-p21Ras scFv fusion proteins at 37 °C for 2 h. The cells were washed twice with PBS, treated with trypsin and collected by centrifugation at 800 rpm for 5 min. The cells were suspended in RIPA cell lysate (R0010, Solarbio, China) for 0.5 h on ice. Total cell protein was obtained by centrifugation at 12,000 rpm at 4 °C for 5 min. The western blot (WB) procedures are described above.
Cell proliferation assay
A total of 1 × 104 A549 cells or H1299 cells per well were seeded in a 96-well plate and cultured at 37 °C. When the cell confluence reached 70%, the cells were washed twice with PBS and incubated with 20 µM p21Ras scFv, CPP-p21Ras scFv or CPP-L-p21Ras scFv in 1640 medium (150 µl) at 37 °C. On the 1st, 2nd and 3rd days of incubation with the fusion protein, 20 µl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well, and the supernatant was discarded after 5 h of incubation at 37 °C. Then, 150 µl dimethyl sulfoxide was added and shaken for 10 min. The absorbance was measured at 490 nm with an enzyme-labelled instrument.
Scratch wound healing assay
A549 cells or H1299 cells (5 × 105 cells per well) were seeded in a 6-well plate and cultured at 37 °C until the cells formed a monolayer and covered the bottom of the plate. A scratch was made in the centre of the monolayer using a 200 µl pipette tip. The cells were washed twice with PBS and incubated with 20 µM p21Ras scFv, CPP-p21Ras scFv or CPP-L-p21Ras scFv in 1640 medium (2 ml) at 37 °C. The images were taken under a microscope at 0, 24 and 48 h. Cell migration was determined according to the wound healing area.
Clone formation assay
A colony formation assay was used to detect the effect of ACPP-p21Ras scFv on the proliferation of A549 cells and H1299 cells. A total of 1 × 102 A549 cells or H1299 cells per well were seeded in a 6-well plate and cultured overnight at 37 °C. The cells were washed twice with PBS and incubated with 20 µM p21Ras scFv, CPP-p21Ras scFv or CPP-L-p21Ras scFv in 1640 medium (2 ml) at 37 °C. The medium was changed every 3 days until visible cell colonies formed, and then, the cells were fixed with 4% paraformaldehyde for 15 min and stained with Giemsa for 30 min. PS software was used to count the clones and calculate the clone formation rate. The calculation formula was as follows: clone formation rate (%) = clone number/inoculation number × 100%.
TUNEL staining for apoptosis detection
The paraffin sections were prepared by paraffin blocking of A549 cells or H1299 cells. The slices were dewaxed with xylene, hydrated, digested by protease K and washed with PBS. Terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick end labeling (TUNEL) reaction solution (TUNEL kit; Roche, Switzerland) was added, and the cells were incubated in the dark for 1 h. Then, the slices were rinsed three times with PBS and incubated 30 min with Converter-POD (TUNEL kit; Roche, Switzerland). The nuclei of apoptotic cells were stained with DAB. All cells and apoptotic cells were observed by microscopy.
Apoptosis measurement by flow cytometry
To confirm the apoptosis-inducing effect of ACPP-p21Ras scFv, the A549 cells or H1299 cells were treated with 20 µM p21Ras scFv, CPP-p21Ras scFv or CPP-L-p21Ras scFv in 1640 medium (12 ml) at 37 °C for 48 h. Next, the cells were collected and washed with PBS, and then collected again and resuspended in 195 μl annexin V-FITC binding buffer. Finally, the cells were incubated with 5 μl annexin V-FITC and 10 μl propidium iodide (annexin V-FITC/propidium iodide Apoptosis Detection Kit; Beyotime Biotechnology, China) for 20 min in the dark and then detected by flow cytometry.
Statistical analysis
The data of each experimental group and control group were analysed using SPSS version 24.0. All the data are expressed as the mean ± SD. Statistical analysis using multiple comparisons was performed with one-way ANOVA and least significant difference tests. Statistical significance was indicated by a value of P < 0.05.
Ethics committee approval
This article does not contain any studies with human participants or animals performed by any of the authors. All experimental protocols used cell culture in vitro.
Results
Expression and purification of fusion proteins
Based on the pET28a (+) plasmid, we constructed three prokaryotic expression plasmids, pET28a(+)-ACPP-p21Ras scFv, pET28a(+)-ACPP-L-p21Ras scFv and pET28a(+)-ACPP-L-EGFP (Fig. 1b). PCR analysis demonstrated that the target gene fragments were successfully inserted into the pET28a (+) plasmid vector, and the gene sizes of ACPP-p21Ras scFv, ACPP-L-p21Ras scFv and ACPP-L-EGFP were 1185, 1230 and 1197 bp, respectively (Fig. 1c). After these plasmids were transformed into E. coli BL21, the soluble recombinant proteins were expressed in E. coli BL21 by induction of IPTG and purified by a Ni-NTA column. Finally, we obtained mg level fusion proteins. SDS-PAGE analysis showed that the molecular weights of scFv, ACPP-p21Ras scFv, ACPP-L-p21Ras scFv and ACPP-L-EGFP were 35, 36, 37 and 37 KD, respectively (Fig. 1d). ELISA and WB analysis indicated that scFv, ACPP-p21Ras scFv and ACPP-L-p21Ras scFv could respond specifically to K-p21Ras expressed in prokaryotic cells (Fig. 1e). The effective titres of the three antibodies were 1:800. These results suggested that the ACPP does not affect the immunoreactivity of scFv to p21Ras.
Factors affecting the penetration of the ACPP
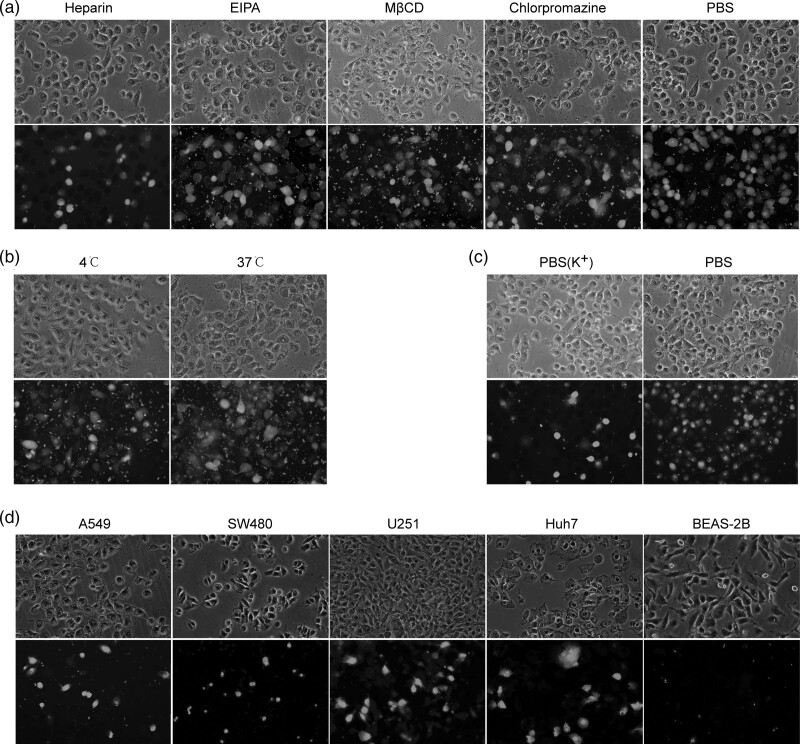
We found that when A549 cells were cocultured with CPP-L-EGFP, green fluorescence was observed in the cells, indicating that ACPP-L-EGFP penetrated the cell membrane and entered the cells. When heparin, a membrane affinity inhibitor, was added to the culture medium, the percentage of cells with green fluorescence decreased to 30.33 ± 5.51%, which was significantly different compared to that with PBS (94.67 ± 1.53%) (P < 0.05). When EIPA, mβCD or chlorpromazine was added, the green fluorescent cells comprised 88.33 ± 3.51, 89.00 ± 0.26 and 89.67 ± 3.51%, respectively (P > 0.05). The results indicated that the membrane affinity inhibitor could significantly inhibit the penetration of the ACPP-L-EGFP fusion protein, but the inhibitory effect of the endocytosis inhibitor was not obvious. These results indicated that ACPP enters the cells by direct penetration rather than endocytosis (Fig. 2a). When A549 cells were cocultured with CPP-L-EGFP at 4 °C and 37 °C, the percentages of green fluorescent cells were 89.67 ± 2.08 and 94.00 ± 3.60% (P > 0.05), demonstrating that the transmembrane transport of ACPP does not require energy, that is, nonendocytosis (Fig. 2b). When A549 cells were cocultured with CPP-L-EGFP in PBS (K+) and PBS solution, the green fluorescent cells comprised 21.67 ± 3.51 and 94.33 ± 3.21% (P < 0.05), respectively, indicating that the penetration of the ACPP-L-EGFP fusion protein could be significantly inhibited by weakening the potential difference across the cell membrane. This finding revealed that the driving force of ACPP penetrating the membrane comes from the potential difference between inside and outside the cell (Fig. 2c).
Fig. 2.
Mechanism and targeting of activatable cell-penetrating peptide (ACPP) uptake. (a) The uptake of ACPP-L-EGFP was inhibited in A549 cells treated with heparin, but not by endocytosis inhibitors [50 µM amiloride (EIPA), 5 mM methyl-β-cyclodextrin (MβCD) and chlorpromazine]. (b) The uptake of ACPP-L-EGFP by A549 cells was not inhibited at 4 °C. (c) The uptake of ACPP-L-EGFP in A549 cells was significantly inhibited by weaken the potential difference across the cell membrane [PBS (K+)]. (d) The uptake of ACPP-L-EGFP was higher by tumour cells with high expression of MMP-2 (A549, SW480, Huh7 and U251) than by normal cells without MMP-2 expression (BEAS-2B). EGFP, enhanced green fluorescent protein; MMP-2, matrix metalloproteinase-2.
Penetrating ability of ACPP-p21Ras scFv
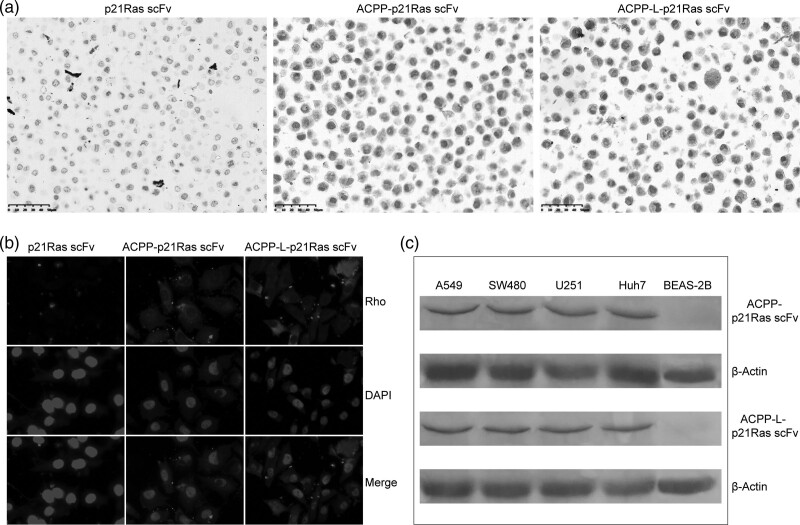
The ability of ACPP-p21Ras scFv to penetrate A549 cells was analysed by immunohistochemistry and immunofluorescence. We found that scFv alone could not enter A549 cells, whereas scFv carried by ACPP could enter A549 cells (Fig. 3a and b). The membrane penetrating efficiencies of ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were similar.
Fig. 3.
The cell-penetrating ability of activatable cell-penetrating peptide (ACPP)-p21Ras scFv was detected. (a) ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were found in the A549 cells (brown), while p21Ras scFv was not found in the A549 cells. Immunohistochemical staining, ×40. (b) ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were observed by immunofluorescence assay in A549 cells, while p21Ras scFv was not observed in the cells. Red: Rho-stained of p21Ras scFv, ACPP-p21Ras scFv and ACPP-L-p21Ras scFv, blue: DAPI-stained nuclei (×1000). (c) ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were detected by WB in A549, SW480, Huh7 and U251 cells, but not in Beas-2B cell. DAPI, 4’,6-diamidino-2-phenylindole; WB, western blot.
The ability of ACPP-p21Ras scFv to target tumour cells in vitro
To test the ability of the ACPP to target tumour cells, we incubated ACPP-L-EGFP with cell lines with high expression of MMP-2 (A549, SW480, Huh7 and U251) and normal bronchial BEAS-2B cells with low expression of MMP-2. ACPP-L-EGFP entered tumour cells with high expression of MMP-2 but not BEAS-2B cells with low expression of MMP-2 (Fig. 2d). In the WB assay, the ACPP-p21Ras scFv and ACPP-L-p21Ras scFv fusion proteins were detected in A549, SW480, Huh7 and U251 cells but not in BEAS-2B cells (Fig. 3c). This phenomenon occurs because ACPP can carry scFv into tumour cells with high expression of MMP-2 but not into normal cells with low expression of MMP-2.
Antitumour effect of the ACPP-p21Ras scFv fusion protein in vitro
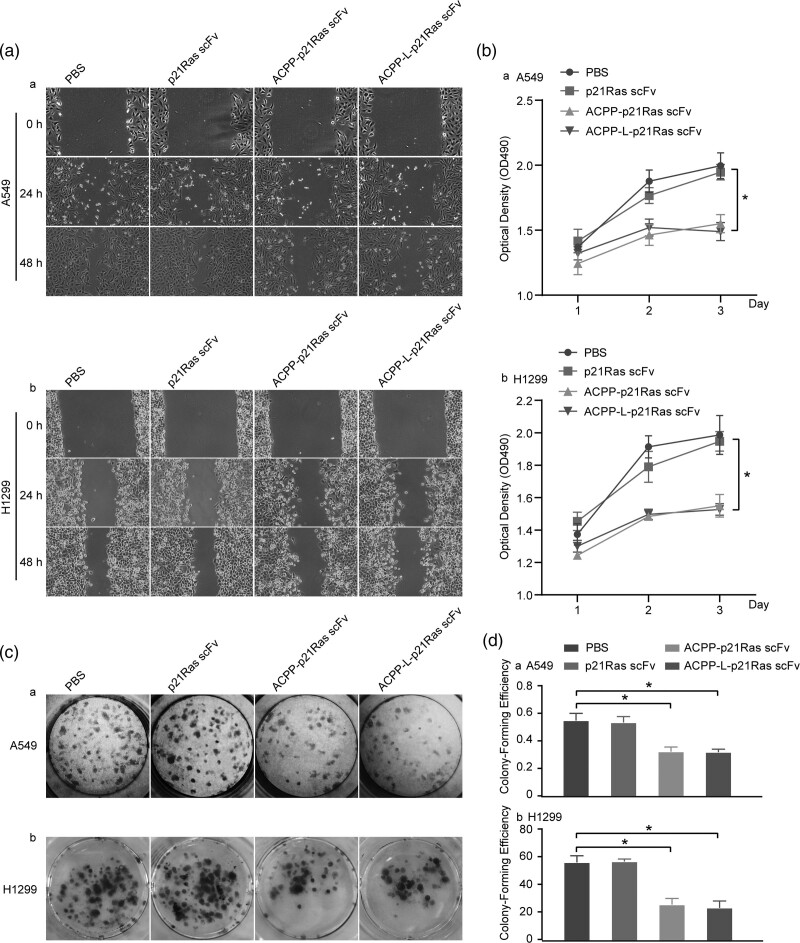
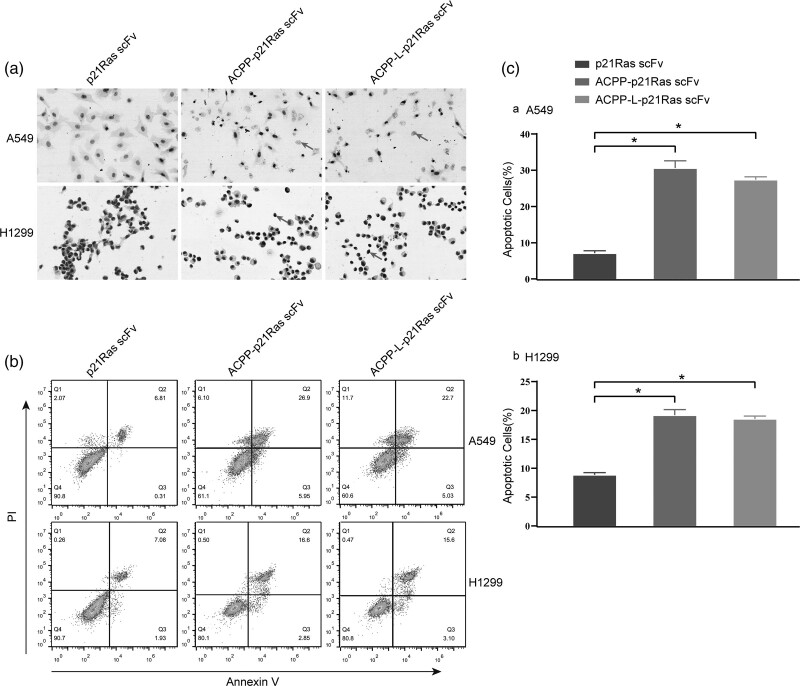
We detected the inhibitory effect of the ACPP-p21Ras scFv fusion protein on A549 cells and H1299 cells in vitro by the cell scratch test, MTT assay and plate cloning test, and the apoptotic effect of the ACPP-p21Ras scFv fusion protein was detected by TUNEL staining and annexin V and propidium iodide staining. The scratch test indicated that the area of cell migration in the PBS and scFv groups was larger than that in the ACPP-p21Ras scFv and ACPP-L-p21Ras scFv groups (Fig. 4a), suggesting that A549 cells and H1299 cells were significantly inhibited in the ACPP-p21Ras scFv and ACPP-L-p21Ras scFv groups. MTT assays showed that the number of living cells continued to increase in the PBS and scFv groups from day 2 after treatment, but the number of living cells decreased gradually in the ACPP-p21Ras scFv and ACPP-L-p21Ras scFv groups after 24 h. These results showed that ACPP-p21Ras scFv and ACPP-L-p21Ras scFv can significantly inhibit the growth of A549 and H1299 tumour cells and the inhibitory effect was similar (Fig. 4b). The plate clone experiment showed that ACPP-p21Ras scFv and ACPP-L-p21Ras scFv significantly inhibited the proliferation of A549 cells and H1299 cells, and the inhibitory effects were similar (Fig. 4c and d). In addition, the TUNEL and annexin V/propidium iodide assay results suggested that the apoptosis percentage of A549 cells and H1299 cells in the ACPP-p21Ras scFv and ACPP-L-p21Ras scFv groups was significantly higher than that of the control group (Fig. 5), indicating that ACPP-p21Ras scFv and ACPP-L-p21Ras scFv could promote the apoptosis of A549 cells and H1299 cells.
Fig. 4.
Anti tumour effects of activatable cell-penetrating peptide (ACPP)-p21Ras scFv on A549 cells and H1299 cells in vitro. (a) The scratch experiment revealed that the healing of A549 cells and H1299 cells treated with ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were slower than those treated with p21Ras scFv and PBS at 24 and 48 h, indicating that ACPP-p21Ras scFv can inhibit the migration of A549 cells and H1299 cells. (b) The MTT assay was used to investigate the killing effect of ACPP-p21Ras scFv to A549 cells and H1299 cells. The OD490 (living cell) of A549 cells and H1299 cells treated with ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were lower than those treated with p21Ras scFv and PBS (P < 0.05) on 2 d and 3 d (P < 0.05). (c and d) The clone formation test demonstrated that the proliferation ability of A549 cells and H1299 cells treated with ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were lower than that treated with p21Ras scFv and PBS (P < 0.05). MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Fig. 5.
Activatable cell-penetrating peptide (ACPP)-p21Ras scFv induce apoptosis on A549 cells and H1299 cells in vitro. (a) The cells treated with ACPP-p21Ras scFv, ACPP-L-p21Ras scFv and p21Ras scFv, then were stained with TUNEL kit and then detected by microscope, and the apoptotic cells are indicated with arrows. (b and c) Apoptotic cells are stained with annexin V/propidium iodide, then detected by flow cytometry. Annexin V/propidium iodide assay showed that the apoptotic percentage of A549 cells and H1299 cells treated with ACPP-p21Ras scFv and ACPP-L-p21Ras scFv were higher than that with p21Ras scFv (P < 0.05). TUNEL, terminal deoxynucleotidyl transferase-mediated fluorescein-dUTP nick end labeling.
Discussion
ACPP is a kind of CPPs that consists of a polycationic CPP, an MMP-2-sensitive linker and a polyanionic peptide [28]. In short, in normal tissue without MMP-2 expression, the cell-penetrating function of the CPP is blocked by the polyanionic peptide through intramolecular electrostatic interactions [32–34]. However, in tumour tissue, the linker is recognised and cleaved by MMP-2 [35–37] to activate CPP. Activated ACPP can penetrate the cell membrane to produce a substantial targeted delivery capacity. In this study, we successfully developed an ACPP-p21Ras scFv fusion protein by prokaryotic expression. Tumour-targeting and membrane-penetrating experiments showed that the ACPP-p21Ras scFv fusion protein could enter human lung cancer A549 cells rather than normal cells. In-vitro experiments showed that the ACPP-p21Ras scFv fusion protein can effectively inhibit the migration and proliferation of A549 and H1299 cells and promote the apoptosis of A549 and H1299 cells. These results are similar to our previous results using a specific adenovirus carrying the scFv gene to inhibit tumours [20]. These results revealed that the ACPP-p21Ras scFv fusion protein has the ability to target tumour cells effectively and enter the cytoplasm of tumour cells and may be used as an effective intracellular antitumour drug. MMP-2 is an extracellular protease that exists in the tumour microenvironment, can degrade the extracellular matrix [38] and plays an important role in tumour invasion and metastasis [39]. MMP-2 is overexpressed in many tumours [40], such as lung cancer [41], colorectal carcinoma [42,43], breast carcinoma [40] and prostatic neoplasia [41]. Because ACPP can target MMP-2, the ACPP-p21Ras scFv fusion protein may also be used to treat other MMP-2-overexpressing tumours.
It has been reported that CPP-protein covalent conjugation may lead to adverse changes in target protein structure and function [44]. In other words, ACPP and antitumour protein fusion may affect the biological activity of antitumour proteins, further affecting the antitumour efficacy of drugs. Nevertheless, Robinson et al., [45] used different linkers to prove the role of linkers in protein stability and folding, indicating that a linker can maintain the structure and function of each fusion partner in the fusion protein [46]. To determine whether the binding of the ACPP will affect the function of the p21Ras scFv protein, we also designed the ACPP-L-p21Ras scFv fusion protein. These results showed that p21Ras scFv, ACPP-p21Ras scFv and ACPP-L-p21Ras scFv could specifically bind to the K-p21Ras protein with the same reaction intensity. It was proven that the ACPP did not affect the immunoreactivity of the p21Ras scFv protein.
In general, the transmembrane pathways of CPPs include direct penetration and endocytosis [23,24]; they depend on the type of target cells [47], the cargo carried and the physicochemical properties of the penetrating peptide sequence [48]. Although Bouquier et al., [49] proved that ACPP-TAMRA enters hippocampal neurons through endocytosis, the ACPP used in our experiment [28] is different from ACPP-TAMRA [49] in the peptide sequence composition of the linker, so the membrane penetration mechanism of the ACPP used in our experiment is not clear. Therefore, we studied the mechanism by which ACPP enters A549 cells by EGFP tracing. The results showed that the number of cells with green fluorescence decreased significantly under the action of membrane affinity inhibitors, whereas the number of cells with green fluorescence changed little under the action of endocytosis inhibitors. When cells were cultured at different temperatures, the number of cells with green fluorescence changed little, but the number of cells with green fluorescence decreased significantly when the potential difference across the cell membrane was reduced. These phenomena revealed that ACPP entered A549 cells through direct penetration.
Conclusion
In this study, we successfully expressed the ACPP-p21Ras scFv fusion protein, which has the ability to penetrate the MMP-2-overexpressing A549 cell membrane and has antitumour activity against A549 cells and H1299 cells in vitro. Therefore, the ACPP-p21Ras scFv fusion protein may be a potential drug against Ras-mutated lung cancer and other MMP-2-overexpressing tumours. However, the antitumour effect of the fusion protein needs to be further verified by in-vivo experiments because only an in-vitro antitumour study of the fusion protein has been performed.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81460464) and Major special projects of science and technology plan of Yunnan Province (2018ZF009).
I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Jl.Y. designed the project. Xr.L., Q.F., Xy.P. and Sl.S. performed the experiments. Y.D. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dr. Yu Du and Dr. Xinrui Lin contributed equally to the writing of this article.
References
- 1.Trujillo-Reyes JC, Seijo L, Martínez-Tellez E, Couñago F. Lung cancer screening, what has changed after the latest evidence? World J Radiol 2020; 12:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng R, Zeng H, Zhang S, Fan Y, Qiao Y, Zhou Q, Chen W. Lung cancer incidence and mortality in China, 2010. Thorac Cancer 2014; 5:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA 2019; 322:764–774. [DOI] [PubMed] [Google Scholar]
- 4.Fu J, Wang X, Yue Q. Functional loss of TAGLN inhibits tumor growth and increases chemosensitivity of non-small cell lung cancer. Biochem Biophys Res Commun 2020; 529:1086–1093. [DOI] [PubMed] [Google Scholar]
- 5.Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol 2018; 834:188–196. [DOI] [PubMed] [Google Scholar]
- 6.Valiahdi SM, Egger AE, Miklos W, Jungwirth U, Meelich K, Nock P, et al. Influence of extracellular pH on the cytotoxicity, cellular accumulation, and DNA interaction of novel pH-sensitive 2-aminoalcoholatoplatinum(II) complexes. J Biol Inorg Chem 2013; 18:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Zhang Y, Deng H, Tang Z, Mao J, Wang L. Pursuing for the better lung cancer therapy effect: comparison of two different kinds of hyaluronic acid and nitroimidazole co-decorated nanomedicines. Biomed Pharmacother 2020; 125:109988. [DOI] [PubMed] [Google Scholar]
- 8.Van Den Borg R, Leonetti A, Tiseo M, Giovannetti E, Peters GJ. Novel targeted strategies to overcome resistance in small-cell lung cancer: focus on PARP inhibitors and rovalpituzumab tesirine. Expert Rev Anticancer Ther 2019; 19:461–471. [DOI] [PubMed] [Google Scholar]
- 9.Roskoski R., Jr. Anaplastic lymphoma kinase (ALK) inhibitors in the treatment of ALK-driven lung cancers. Pharmacol Res 2017; 117:343–356. [DOI] [PubMed] [Google Scholar]
- 10.Curran MP. Crizotinib: in locally advanced or metastatic non-small cell lung cancer. Drugs 2012; 72:99–107. [DOI] [PubMed] [Google Scholar]
- 11.Frampton JE. Crizotinib: a review of its use in the treatment of anaplastic lymphoma kinase-positive, advanced non-small cell lung cancer. Drugs 2013; 73:2031–2051. [DOI] [PubMed] [Google Scholar]
- 12.Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, Yun T, et al. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res 2011; 17:1553–1560. [DOI] [PubMed] [Google Scholar]
- 13.Johnson ML, Riely GJ, Rizvi NA, Azzoli CG, Kris MG, Sima CS, et al. Phase II trial of dasatinib for patients with acquired resistance to treatment with the epidermal growth factor receptor tyrosine kinase inhibitors erlotinib or gefitinib. J Thorac Oncol 2011; 6:1128–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Sun JM, Lim SH, Kim HS, Yoo KH, Jung KS, et al. A Phase Ib/II Study of Afatinib in Combination with Nimotuzumab in Non-Small Cell Lung Cancer Patients with Acquired Resistance to Gefitinib or Erlotinib. Clin Cancer Res 2016; 22:2139–2145. [DOI] [PubMed] [Google Scholar]
- 15.Murugan AK, Grieco M, Tsuchida N. RAS mutations in human cancers: roles in precision medicine. Semin Cancer Biol 2019; 59:23–35. [DOI] [PubMed] [Google Scholar]
- 16.Friedlaender A, Drilon A, Weiss GJ, Banna GL, Addeo A. KRAS as a druggable target in NSCLC: rising like a phoenix after decades of development failures. Cancer Treat Rev 2020; 85:101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marín-Ramos NI, Ortega-Gutiérrez S, López-Rodríguez ML. Blocking Ras inhibition as an antitumor strategy. Semin Cancer Biol 2019; 54:91–100. [DOI] [PubMed] [Google Scholar]
- 18.Uprety D, Adjei AA. KRAS: from undruggable to a druggable Cancer Target. Cancer Treat Rev 2020; 89:102070. [DOI] [PubMed] [Google Scholar]
- 19.Yang JL, Liu DX, Zhen SJ, Zhou YG, Zhang DJ, Yang LY, et al. A novel anti-p21Ras scFv antibody reacting specifically with human tumour cell lines and primary tumour tissues. BMC Cancer 2016; 16:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin XR, Zhou XL, Feng Q, Pan XY, Song SL, Fang H, et al. CIK cell-based delivery of recombinant adenovirus KGHV500 carrying the anti-p21Ras scFv gene enhances the anti-tumor effect and safety in lung cancer. J Cancer Res Clin Oncol 2019; 145:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervera-Carrascon V, Havunen R, Hemminki A. Oncolytic adenoviruses: a game changer approach in the battle between cancer and the immune system. Expert Opin Biol Ther 2019; 19:443–455. [DOI] [PubMed] [Google Scholar]
- 22.Nemerow G, Flint J. Lessons learned from adenovirus (1970-2019). FEBS Lett 2019; 593:3395–3418. [DOI] [PubMed] [Google Scholar]
- 23.Di Pisa M, Chassaing G, Swiecicki JM. Translocation mechanism(s) of cell-penetrating peptides: biophysical studies using artificial membrane bilayers. Biochemistry 2015; 54:194–207. [DOI] [PubMed] [Google Scholar]
- 24.Falanga A, Lombardi L, Galdiero E, Genio VD, Galdiero S. The world of cell penetrating: the future of medical applications. Future Med Chem 2020; 12:1431–1446. [DOI] [PubMed] [Google Scholar]
- 25.Favaro MT, de Toledo MA, Alves RF, Santos CA, Beloti LL, Janissen R, et al. Development of a non-viral gene delivery vector based on the dynein light chain Rp3 and the TAT peptide. J Biotechnol 2014; 173:10–18. [DOI] [PubMed] [Google Scholar]
- 26.Choi S, Jo J, Seol DW, Cha SK, Lee JE, Lee DR. Regulation of pluripotency-related genes and differentiation in mouse embryonic stem cells by direct delivery of cell-penetrating peptide-conjugated CARM1 recombinant protein. Dev Reprod 2013; 17:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habault J, Poyet JL. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules 2019; 24:E927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li SY, Cheng H, Qiu WX, Liu LH, Chen S, Hu Y, et al. Protease-activable cell-penetrating peptide-protoporphyrin conjugate for targeted photodynamic therapy in vivo. ACS Appl Mater Interfaces 2015; 7:28319–28329. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Baek DS, Lee S, Park D, Kang HN, Cho BC, Kim YS. Dual-targeting of EGFR and Neuropilin-1 attenuates resistance to EGFR-targeted antibody therapy in KRAS-mutant non-small cell lung cancer. Cancer Lett 2019; 466:23–34. [DOI] [PubMed] [Google Scholar]
- 30.Lim KJ, Sung BH, Shin JR, Lee YW, Kim DJ, Yang KS, Kim SC. A cancer specific cell-penetrating peptide, BR2, for the efficient delivery of an scFv into cancer cells. PLoS One 2013; 8:e66084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Duijnhoven SM, Robillard MS, Nicolay K, Grüll H. Development of radiolabeled membrane type-1 matrix metalloproteinase activatable cell penetrating peptide imaging probes. Molecules 2015; 20:12076–12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A 2004; 101:17867–17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstain R, Savariar EN, Felsen CN, Tsien RY. In vivo targeting of hydrogen peroxide by activatable cell-penetrating peptides. J Am Chem Soc 2014; 136:874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitney M, Savariar EN, Friedman B, Levin RA, Crisp JL, Glasgow HL, et al. Ratiometric activatable cell-penetrating peptides provide rapid in vivo readout of thrombin activation. Angew Chem Int Ed Engl 2013; 52:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao W, Xiang B, Meng TT, Liu F, Qi XR. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 2013; 34:4137–4149. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Wang X, Zhong W, Ren X, Sha X, Fang X. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: preparation and in vitro and in vivo evaluation. Int J Nanomedicine 2016; 11:1643–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou Z, He X, He D, Wang K, Qing Z, Yang X, et al. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials 2015; 58:35–45. [DOI] [PubMed] [Google Scholar]
- 38.Samuelson LE, Scherer RL, Matrisian LM, McIntyre JO, Bornhop DJ. Synthesis and in vitro efficacy of MMP9-activated NanoDendrons. Mol Pharm 2013; 10:3164–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo HY, Huang YS, Tseng CH, Chen YC, Chang YW, Shih HM, Wu CW. PML represses lung cancer metastasis by suppressing the nuclear EGFR-mediated transcriptional activation of MMP2. Cell Cycle 2014; 13:3132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2:161–174. [DOI] [PubMed] [Google Scholar]
- 41.Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 2005; 87:287–297. [DOI] [PubMed] [Google Scholar]
- 42.Levy AT, Cioce V, Sobel ME, Garbisa S, Grigioni WF, Liotta LA, Stetler-Stevenson WG. Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma. Cancer Res 1991; 51:439–444. [PubMed] [Google Scholar]
- 43.Ring P, Johansson K, Höyhtyä M, Rubin K, Lindmark G. Expression of tissue inhibitor of metalloproteinases TIMP-2 in human colorectal cancer–a predictor of tumour stage. Br J Cancer 1997; 76:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behzadipour Y, Hemmati S. Considerations on the rational design of covalently conjugated cell-penetrating peptides (CPPs) for intracellular delivery of proteins: a guide to CPP selection using glucarpidase as the model cargo molecule. Molecules 2019; 24:E4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson CR, Sauer RT. Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc Natl Acad Sci U S A 1998; 95:5929–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janczak M, Bukowski M, Górecki A, Dubin G, Dubin A, Wladyka B. A systematic investigation of the stability of green fluorescent protein fusion proteins. Acta Biochim Pol 2015; 62:407–411. [DOI] [PubMed] [Google Scholar]
- 47.He Y, Li F, Huang Y. Smart cell-penetrating peptide-based techniques for intracellular delivery of therapeutic macromolecules. Adv Protein Chem Struct Biol 2018; 112:183–220. [DOI] [PubMed] [Google Scholar]
- 48.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med 2012; 18:385–393. [DOI] [PubMed] [Google Scholar]
- 49.Bouquier N, Girard B, Aparicio Arias J, Fagni L, Bertaso F, Perroy J. Gelatinase biosensor reports cellular remodeling during epileptogenesis. Front Synaptic Neurosci 2020; 12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]