Abstract
People with low incomes have a disproportionate prevalence of diabetes and its complications and experience many barriers to self-management, which community health workers (CHWs) may help address. We sought to examine the effects of an in-home CHW-led intervention for adults with diabetes and incomes <250% of the federal poverty line on self-management behaviors and test mediators and moderators. From 2010 to 2013, we randomized participants from three Washington State health systems with type 2 diabetes and hemoglobin A1c (HbA1c) ≥ 8% to the CHW intervention (N = 145) or usual care control (N = 142) arms. We examined effects on 12-month self-management: physical activity, dietary behaviors, medication taking, blood glucose monitoring, foot care, and tobacco use. For behaviors with significant intervention-control group differences, we tested mediation by self-efficacy and social support. We also investigated whether intervention-associated changes in behaviors varied by race/ethnicity, gender, and baseline values of HbA1c, diabetes distress, depression, and food insecurity (moderators). Compared to controls, intervention participants engaged in more physical activity and reported better dietary behaviors for some measures (general diet, frequency of skipping meals, and frequency of eating out) at 12-months, but there was no evidence of mediation by self-efficacy or social support. Evidence of moderation was limited: improvements in the frequency of skipping meals were restricted to participants with baseline HbA1c < 10%. Study findings suggest CHWs could be integrated into diabetes care to effectively support lifestyle changes around physical activity and some eating behaviors among adults with low incomes. More research is needed to understand mechanisms of change.
Keywords: Diabetes mellitus, type 2, Community health workers, Self-management, Diet, Exercise, Randomized controlled trial
Implications.
Practice: In-home community health worker-led programs can promote physical activity and some healthy eating behaviors among adults with diabetes and low incomes.
Policy: Policymakers should consider how to effectively integrate community health workers into health care systems to support diabetes self-management among adults with low incomes.
Research: Future research is needed to understand the mechanisms by which community health worker-led programs lead to positive changes in health behaviors among adults with diabetes.
Introduction
Diabetes remains a major public health problem, affecting more than 30 million American adults [1]. Socioeconomic disparities exist in the prevalence of diabetes and its complications, with populations with low incomes bearing a disproportionate burden [2, 3]. Individuals with low incomes are less likely to receive self-management education and support [4] and experience greater barriers to self-management related to their physical and social environments, [5] which may contribute to higher hemoglobin A1c (HbA1c) levels and diabetes complications [6]. Therefore, there is a need to develop effective diabetes self-management interventions that address challenges adults with low incomes face that can be implemented broadly across health care systems to improve access.
One potential strategy is the use of community health workers (CHWs) to provide self-management education and support. CHWs are trusted members of and/or have intimate knowledge of the community served whose goal is to promote health through education, assistance, client advocacy, social support, informal counseling, and linkages to community resources [7, 8]. CHW-led interventions have shown modest improvements in blood glucose levels in randomized controlled trials among individuals with diabetes [9, 10]; however, the mechanisms by which these changes occur are not well understood [10]. CHWs may be particularly well-suited to provide self-management support among ethnically diverse households with low incomes because they are able to address underlying socioeconomic contributors to health and act as bridges between the community and health care systems [8]. Furthermore, CHWs are less costly than other types of health professionals, which improves the feasibility and scalability of these interventions.
We completed a randomized controlled trial of Peer Support for Achieving Independence in Diabetes (PeerAID): a home-based, low-intensity CHW intervention among adults with diabetes and incomes <250% of the federal poverty line (FPL), which was intended to improve HbA1c [11]. PeerAID focused on promoting the adoption of self-management behaviors that improve HbA1c, such as blood glucose monitoring, healthy eating, physical activity, medication taking, and smoking cessation. PeerAID was based on Social Cognitive Theory [12] and Self-Regulation Theory [13], leading to an individualized intervention approach. CHWs met with participants in their homes and worked collaboratively to develop and revise diabetes self-management plans, including setting behavioral goals, identifying actions to achieve those goals, evaluating progress, addressing challenges and concerns, and providing education and referrals to group activities and community resources. Because of links to improved self-management and HbA1c [14, 15], self-efficacy and social support were important targets through which the intervention was intended to improve outcomes.
In PeerAID primary analyses, there were no statistically significant improvements in HbA1c in the intervention arm relative to usual care in the full sample, but improvements were observed among the prespecified subgroup of participants with HbA1c ≥ 10% at baseline [16]. Although reductions in HbA1c are commonly used to measure whether diabetes interventions are successful, improvements in self-management behaviors that can lead to changes in HbA1c are also meaningful given their overlap with health-promoting behaviors and independent associations with quality of life and mortality [17, 18]. Examining effects on behavioral outcomes may illuminate which behaviors are most susceptible to the influence of CHW interventions. Understanding mechanisms (i.e., mediators) by which any behavioral changes occur provides valuable information about how CHWs should address them in future interventions, which has not been a focus of most previous intervention evaluations. Identifying whether the intervention was more or less effective in subgroups of participants (i.e., moderators) allows focusing on participants most likely to benefit and identifying intervention modifications to improve outcomes in those without benefit, leading to a more effective and efficient intervention [19].
Therefore, the aims of this study were to (a) examine effects of PeerAID on diabetes self-management behaviors; (b) evaluate whether effects on behaviors were mediated by self-efficacy or social support; and (c) determine whether intervention effects on behaviors were moderated by gender, race/ethnicity, and baseline levels of HbA1c, diabetes distress, depression, or food insecurity.
METHODS
Study setting
Details of PeerAID have been previously published [11]. Briefly, the study was a randomized controlled trial in which the local health department (Public Health-Seattle King County [PHSKC]) provided CHW services to several health systems. Participants were recruited from September 2010 to May 2013 from three health systems: Harborview Medical Center (a local public hospital); VA Puget Sound Health Care System (a tertiary hospital serving Veterans); and Sea Mar Community Health Centers (a community health center network specializing in service to Latinxs). Follow-up concluded in November 2014. University of Washington and VA Puget Sound Institutional Review Boards approved the study, and all participants provided written informed consent.
Participants
We recruited individuals with type 2 diabetes and HbA1c ≥ 8% who were 30–70 years of age, English or Spanish-speaking, and had a household income <250% of the FPL. We excluded individuals currently participating in other diabetes studies, who had received diabetes education in the past 3 years, or who had a serious illness (e.g., cancer, end-stage renal disease, and dementia).
Intervention and controls
The PeerAID intervention focused on promoting the adoption of self-management behaviors that improve HbA1c, such as blood glucose monitoring, healthy eating, physical activity, medication taking, and smoking cessation. CHWs met with participants in their homes at baseline, with follow-up visits at 0.5, 1.5, 3.5, and 7 months and an optional 10-month visit. Behavior change techniques employed in the intervention, according to Michie et al.’s 2013 taxonomy, included shaping knowledge (e.g., instruction on how to perform behaviors), feedback and monitoring (e.g., feedback on behavior, self-monitoring of behavior), goals and planning (e.g., goal setting, problem-solving, and action planning), and social support (e.g., emotional and practical) [20]. At the baseline visit, the CHW developed an individualized diabetes self-management plan with the participant. At follow-up visits, the CHW assessed diabetes and blood pressure control, learned more about participant challenges and concerns, reviewed progress on implementing the self-management plan, provided targeted education, revised the self-management plan, and made referrals to group activities and community resources.
Self-efficacy and social support were key mechanisms by which the intervention was intended to influence self-management behaviors. CHWs addressed self-efficacy, an individual’s confidence in their ability to perform the desired behavior, using motivational interviewing to help participants consider and resolve their ambivalence toward making behavioral changes, set achievable health goals, and identify barriers to goals and strategies to overcome them [21]. CHWs directly provided social support to participants and encouraged family members and other social network members to help participants by supporting lifestyle changes and medication taking, attending clinic visits, and providing emotional support. CHWs also linked interested participants to group support, such as group-based diabetes education classes.
Two full-time CHWs who were employed by PHSKC delivered the intervention to all intervention arm participants. Intervention arm participants were allocated equally between the two CHWs. The CHWs had high school or equivalent degrees and 5–8 years of experience as CHWs on asthma projects. CHWs were Latina and bilingual in Spanish and English and were from the communities the project served. The CHWs received 40 hr of comprehensive training, including didactic sessions, in-class exercises, and field practice. CHWs received training in health coaching and motivational interviewing delivered by a professional health coach and Certified Diabetes Educator (CDE). They received education on diabetes self-management from the CDE. CHWs passed a competency test prior to working with clients. Throughout the intervention, CHWs had biweekly training sessions with the health coach. Participant educational materials and assessments were provided in Spanish and English; CHWs conducted home visits in the patient’s primary language.
Participants in the control group received usual care and were offered one optional diabetes self-management educational visit after completing the 12-month outcome assessment. We randomized individual participants to the intervention or usual care control with equal probability using a stratified (by health system), permuted block design with varying block size. Study coordinators enrolled participants and assigned them to the intervention or control arm, based on the allocation sequence generated by the study biostatistician. The average time from randomization to the first CHW visit among intervention arm participants was 20.9 days. The nature of the intervention made it impossible to blind participants and staff to group assignments.
Outcomes
Outcomes included self-management, including eating behaviors, physical activity, medication taking, blood glucose monitoring, foot care, and tobacco use. Outcomes were assessed in-home by the CHWs at baseline and 12-months for both intervention and control participants. For intervention participants, the CHW who did not deliver the intervention collected these data to minimize bias.
We used the Summary of Diabetes Self-Care Activities to assess self-management engagement in days per week (range: 0–7) for specific diet (fruit/vegetable consumption, full-fat dairy/red meat consumption), general diet (following a healthful eating plan), carbohydrate spacing, foot care, and blood glucose monitoring [22]. We measured additional diabetes-specific diet variables with the Diabetes Self-Management Assessment Report Tool, including the frequency of eating more than one should, the frequency of skipping a meal or scheduled snack, and the location where meals are prepared [23]. We dichotomized frequency variables as at least several times a week versus a few times a month or less and location of meal preparation as at least regularly eat out or take out versus mostly at home.
We assessed self-reported physical activity using the short form of the International Physical Activity Questionnaire, from which we calculated total weekly minutes of physical activity from vigorous, moderate, and walking activity [24]. Walking was included because it is encouraged as a way to meet national physical activity recommendations [25] and is the most common modality to achieve recommended physical activity [26]. We measured medication taking with the Morisky Medication Adherence Scale (range: 0–11) [27]. We classified respondents’ smoking status as current smoker or nonsmoker based on self-report.
Mediators
We measured self-efficacy with the Australian/English version of the Diabetes Management Self-Efficacy Scale, which measures respondent’s confidence in managing their blood glucose level, foot care, medication, diet, and physical activity [28]. Item scores were summed for a composite score (range: 0–200) where higher scores reflect greater self-efficacy. We measured diabetes-related social support from significant others, family, friends, and health professionals with the Multidimensional Diabetes Questionnaire [29]. We calculated an average score across items (range: 0–6), with higher scores indicating greater support. We used change score values (12-month to baseline) for self-efficacy and social support in mediation analyses.
Moderators
Potential moderators included those we believed could influence the effectiveness of the intervention, including demographic characteristics (race/ethnicity, gender), baseline values of clinical (HbA1c), and baseline psychosocial variables (diabetes distress, depression, and food insecurity) [30–34]. Participants self-reported demographic characteristics at baseline. We categorized race/ethnicity as White, Black, Latinx, or other. We obtained HbA1c values at the baseline home visit using a collection kit (Home Access Health Corporation) that allowed individuals to self-test with a finger stick blood sample, assisted by the CHW. All testing is performed in a laboratory regulated by Clinical Laboratory Improvement Amendments and certified by the College of American Pathologists [35]. We collected baseline home testing data for 90% of participants, which was supplemented with clinic values for missing data (n = 28). Due to logistical challenges, most samples were non-fasting. Consistent with the parent study, we dichotomized baseline HbA1c as <10% or ≥10% [16].
We measured diabetes-related distress with the Diabetes Distress Scale [36]. Scores for all items were summed and divided by the total number of questions answered to obtain an average item score; average scores ≥ 2.0 were categorized as moderate/high distress [37]. We measured depression with the Patient Health Questionnaire depression scale (PHQ-8) and categorized values of ≥10 as clinically significant symptoms [38]. We ascertained food insecurity using the United States Department of Agriculture’s validated Six-Item Short Form Food Security Survey Module [39]. Items were totaled; total scores ≥ 2 were categorized as food insecure, a standard cutoff.
Statistical analyses
Demographic and clinical characteristics
We first examined the distribution of demographic and clinical characteristics of participants in each study arm. We also examined whether characteristics of participants included and excluded from analyses differed overall and within each arm using chi-square or Fisher’s exact tests for categorical variables and t-tests for continuous variables.
Baseline to 12-month changes in self-management behaviors (within-group changes)
We estimated unadjusted baseline to 12-month changes in self-management behaviors in each of the intervention and control arms using t-tests for continuous and McNemar’s test for categorical outcomes.
Intervention effects on self-management behaviors (between-group changes)
To compare intervention and control arms, we estimated associations between study assignment and 12-month values of self-management behaviors with linear regression for continuous outcomes and modified Poisson regression for dichotomous outcomes [40], along with 95% confidence intervals (CIs). Modified Poisson regression directly estimates the relative risk (RR) for common outcomes where the odds ratio from logistic regression no longer approximates the RR. We adjusted for baseline values of outcomes so that estimates reflected changes from baseline to 12 months, as well as clinic site because randomization was stratified by site, and body mass index calculated from CHW-measured height and body weight because it was unbalanced at baseline [16].
Mediation of intervention effects on self-management behaviors
We examined mediation only among self-management outcomes for which there were statistically significant 12-month differences between control and intervention arms. We used a common approach to mediation analysis that defines causal mediation effects within a potential outcomes framework [41] and is implemented in Stata (Version 15; medeff command; [42]). Within this framework, we estimated the average causal mediation effect, which is the indirect effect of the treatment through the mediator. This is defined as the average difference between two potential outcomes: the self-management outcome experienced under a given treatment status and its associated mediator value, and the self-management outcome experienced under the same treatment status, but this time under a mediator value associated with the other treatment. Only one of these potential outcomes is observable since each participant was assigned to only one treatment group. All models adjusted for clinic site, baseline BMI, and baseline self-management behaviors.
Moderation of intervention effects on self-management behaviors
To examine moderators of the intervention on diabetes self-management, we fit separate linear regression models for each moderator with an interaction term between study assignment and moderator. We restricted analyses to self-management outcomes with significant 12-month differences between intervention and control arms. We used Wald tests to evaluate the significance of moderation at the p = 0.05 level. We adjusted for clinic site, baseline BMI, and baseline self-management behaviors.
Sensitivity analyses
To account for missing data, we used multiple imputations by chained equations and repeated analyses testing intervention effects on 12-month self-management outcomes and testing for moderation [43]. We created N = 20 imputed data sets using all baseline demographic and clinical characteristics (see Table 1), baseline and 12-month self-management behaviors, and baseline and 12-month mediators and pooled results across data sets.
Table 1.
Distribution of baseline participant characteristics by PeerAID study assignment, King County, Washington, 2010–2014
| Controla N = 133 |
Interventiona N = 130 |
|||
|---|---|---|---|---|
| Site (n, %) | ||||
| Local public hospital | 71 | 53.4 | 64 | 49.2 |
| Community health center network | 50 | 37.6 | 51 | 39.2 |
| Department of Veterans Affairs Medical Center | 12 | 9.0 | 15 | 11.5 |
| Age (mean, SD) | 51.8 | 9.4 | 53.5 | 8.9 |
| Female (n, %) | 71 | 53.4 | 58 | 44.6 |
| Race/ethnicity (n, %) | ||||
| Black | 38 | 28.6 | 31 | 23.8 |
| Latinx | 54 | 40.6 | 55 | 42.3 |
| White | 28 | 21.1 | 28 | 21.5 |
| Other | 13 | 9.8 | 16 | 12.3 |
| Less than high school education (n, %) | 44 | 33.1 | 47 | 36.2 |
| Unemployed (n, %) | 100 | 75.2 | 90 | 69.2 |
| Uninsured (n, %) | 57 | 42.9 | 54 | 41.5 |
| Food insecure (n, %) | 66 | 49.6 | 61 | 46.9 |
| Obesity (n, %) | 86 | 64.7 | 71 | 54.6 |
| Depression (n, %) | 35 | 26.3 | 35 | 26.9 |
| High diabetes distress (n, %) | 86 | 64.7 | 66 | 50.8 |
| Self-efficacy (mean, SD) | 144.9 | 31.1 | 150.6 | 39.1 |
| Social support (mean, SD) | 5.1 | 1.7 | 5.1 | 1.8 |
| HbA1c ≥ 10% (n, %) | 34 | 25.6 | 36 | 27.7 |
HbA1c hemoglobin A1c; SD standard deviation.
aNumbers may not add to totals and percents to 100 due to missing data; 9 participants randomized to the control arm and 15 to the intervention arm were excluded from all analyses due to missing data (all 12-month self-management behaviors or body mass index).
RESULTS
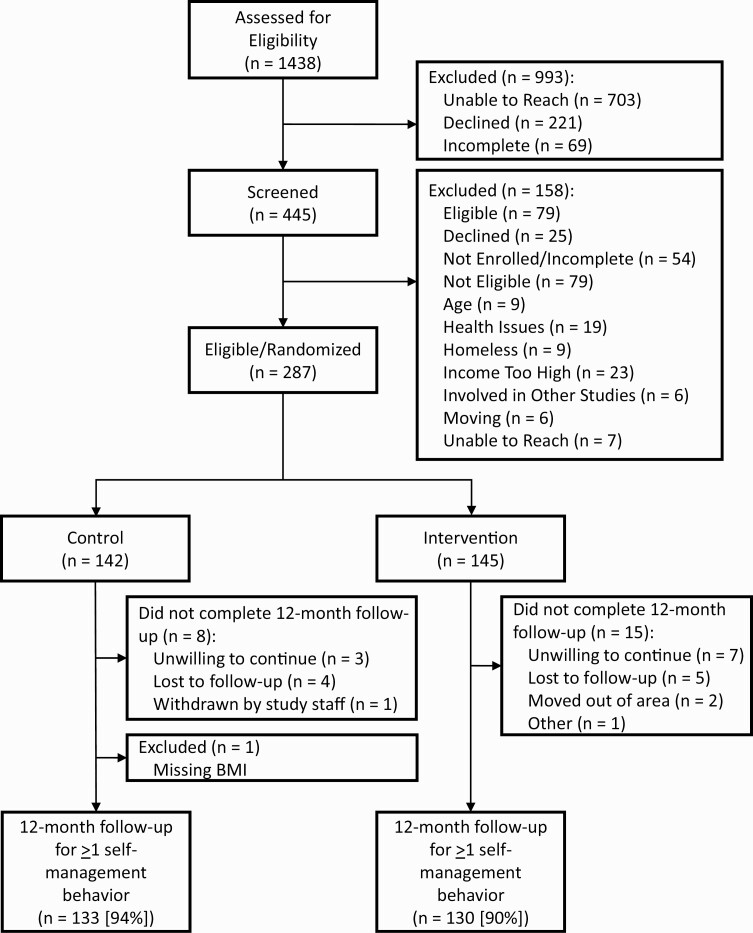
We randomized 287 participants (5% of those identified as potentially eligible from medical records, Fig. 1), and 264 had data on ≥1 12-month self-management outcome (92%). Twenty-three participants (control: N = 8 [6%], intervention: N = 15 [10%]) did not complete the 12-month exit visit. Among intervention arm participants with baseline and 12-month self-management outcomes, all but one received at least four CHW visits. One control arm participant was excluded from analyses because they were missing data on BMI. Therefore, analyses include N = 130 control and N = 133 intervention arm participants. Participants across arms were similar for most baseline characteristics (Table 1), except controls were more likely to be female, to have obesity, and have high diabetes distress. There were few significant differences between participants included and excluded from analyses due to missing data, with excluded participants having: higher baseline physical activity in the full sample and intervention arm, a greater proportion with high diabetes distress in the intervention arm, and a smaller proportion with food insecurity in the control arm (Supplemental Table 1).
Fig 1.
Flow diagram for PeerAID inclusion, exclusion, randomization, and follow-up. The figure displays the recruitment and retention of the study sample of participants with hemoglobin A1c ≥ 8% and incomes < 250% of the Federal Poverty Line in King County, Washington from 2010 to 2014.
Baseline to 12-month changes in self-management behaviors
Both intervention and control arms demonstrated within group improvements from baseline to 12-months in general diet, specific diet, carbohydrate spacing, foot care, and medication taking (Table 2). From baseline to 12-months, a larger proportion of intervention arm participants reported skipping meals a few times a month or less, and a smaller proportion of control arm participants reported preparing meals mostly at home.
Table 2.
Effect of PeerAID intervention on 12-month diabetes self-management behaviors, King County, Washington, 2010–2014
| Unadjusted | Adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Intervention effect | |||||||
| Continuous self-management behaviors | Baseline | 12-months | Within group change | Baseline | 12-months | Within group change | p-values | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Diffa | 95% CI | ||||
| Eating behaviors (days/week) | |||||||||
| General diet | 3.8 (2.5) | 4.7 (2.2) | 0.84b | 4.1 (2.2) | 5.4 (1.7) | 1.29b | 0.54 | 0.07, 1.01 | 0.03 |
| Specific diet | 3.6 (1.9) | 4.2 (1.9) | 0.57b | 3.8 (1.8) | 4.6 (1.8) | 0.78b | 0.32 | −0.11, 0.75 | 0.14 |
| Carbohydrate spacing | 4.7 (2.8) | 5.5 (2.5) | 0.74b | 4.8 (2.7) | 5.8 (2.3) | 0.99b | 0.21 | −0.36, 0.78 | 0.46 |
| Physical activity (minutes/week) | 240.1 (364.1) | 199.9 (350.0) | −40.2 | 271.3 (421.6) | 344.2 (455.6) | 72.8 | 141.4 | 46.4, 236.4 | 0.004 |
| Blood glucose monitoring (days/ week) | 3.7 (2.9) | 3.9 (2.9) | 0.15 | 3.1 (2.9) | 3.8 (2.9) | 0.71b | 0.37 | −0.25, 0.98 | 0.24 |
| Foot care (days/week) | 4.2 (2.3) | 5.4 (2.2) | 1.17b | 4.0 (2.3) | 5.2 (2.1) | 1.26b | −0.09 | −0.58, 0.41 | 0.74 |
| Medication taking | 5.8 (1.8) | 6.1 (1.8) | 0.30 | 5.8 (1.9) | 6.1 (1.9) | 0.39b | 0.04 | −0.37, 0.45 | 0.84 |
| Control | Intervention | Intervention effect | |||||||
| Dichotomous self-management behaviors | Baseline | 12-months | Baseline | 12-months | p-values | ||||
| N (%) | N (%) | N (%) | N (%) | RRa | 95% CI | ||||
| Eating behaviors | |||||||||
| Skipping meals a few times a month or less | 70 (52.6) | 76 (57.1) | 49 (37.7) | 84 (64.6)b | 1.24 | 1.02, 1.50 | 0.03 | ||
| Preparing meals mostly at home | 107 (82.9) | 96 (74.4)b | 109 (85.8) | 113 (89.0) | 1.19 | 1.07, 1.34 | 0.002 | ||
| Overeating a few times a month or less | 97 (74.0) | 97 (74.0) | 90 (69.2) | 101 (77.7) | 1.04 | 0.91, 1.18 | 0.59 | ||
| Current nonsmoker | 103 (77.4) | 105 (78.9) | 98 (75.4) | 101 (77.7) | 1.01 | 0.93, 1.08 | 0.88 |
CI confidence interval; Diff difference from linear regression model; RR relative risk from modified Poisson regression model (control is reference); SD standard deviation.
aAdjusted for baseline value of the self-management behavior, baseline body mass index, and study site.
bWithin-group baseline to 12-month changes in self-management behaviors significant at the p < 0.05 level.
Intervention effects on self-management behaviors
At 12 months, participants in the intervention arm followed general diet recommendations more days per week (difference [diff] = 0.54, 95% CI 0.07, 1.01), were more likely to skip meals a few times a month or less (RR = 1.24, 95% CI 1.02, 1.50), and were more likely to prepare meals mostly at home (RR = 1.19, 95% CI 1.07, 1.34) compared to controls (Table 2). Intervention arm participants also had 141 min of additional weekly physical activity compared to controls at 12 months (95% CI 46.4, 236.4).
Mediators of intervention effects on self-management behaviors
We found no evidence that intervention-control differences in eating behaviors and physical activity were mediated by changes in self-efficacy or social support (Table 3).
Table 3.
Average causal mediation effect (indirect effect) of changes in self-efficacy and social support on 12-month diabetes self-management behaviors associated with the PeerAID intervention, King County, Washington, 2010–2014
| Self-efficacy | Social support | |||
|---|---|---|---|---|
| Self-management behavior | ACME estimatea | 95% CI | ACME estimatea | 95% CI |
| General diet (days/week) | −0.001 | −0.04, 0.04 | −0.01 | −0.08, 0.06 |
| Skipping meals a few times a month or less | −0.001 | −0.01, 0.01 | −0.001 | −0.01, 0.01 |
| Eating out a few times a month or less | −0.0004 | −0.01, 0.01 | −0.001 | −0.02, 0.01 |
| Physical activity (minutes/week) | −1.57 | −18.78, 15.57 | −1.60 | −14.26, 8.62 |
ACME average causal mediation effect; CI confidence interval.
aAdjusted for study site and baseline values of the self-management behavior, mediator, and body mass index.
Moderation of intervention effects on self-management behaviors
The only statistically significant moderator of the intervention was baseline HbA1c on the frequency of skipping meals (p-value = 0.03; Supplemental Table 2). Among participants with HbA1c < 10% at baseline, the intervention group was more likely to skip meals several times a month or less compared to controls (RR = 1.41, 95% CI = 1.13, 1.74), whereas there was no difference between intervention and control group participants with HbA1c ≥ 10% (RR = 0.81, 95% CI 0.53, 1.26).
Sensitivity analyses
Findings accounting for missing data using multiple imputations were similar to complete case analyses except that differences between arms in 12-month general diet were slightly smaller and no longer statistically significant (diff = 0.43, 95% CI −0.07, 0.94, p = 0.09; data not shown).
Discussion
We found that participants in a CHW-delivered self-management intervention for individuals with diabetes and low incomes engaged in more weekly physical activity and had more favorable dietary behaviors for some measures after the intervention compared to usual care controls. Intervention participants followed a healthful eating plan more days per week (general diet measure), skipped meals less frequently, and prepared meals at home more regularly at 12-month follow-up compared to controls. Intervention participants also reported an additional 2 hr and 21 min of physical activity per week compared to controls. Differences in preparing meals at home and physical activity may, in part, be influenced by declines in physical activity and in frequency of preparing meals at home among the control group. We found no evidence that changes in self-efficacy and social support mediated improvements in self-management behaviors. Furthermore, we found minimal evidence that the intervention’s effects on the subset of dietary behaviors and physical activity varied by demographic, clinical, and psychosocial variables.
Observations of increases in physical activity and improvements in a subset of dietary behaviors associated with the CHW intervention are important from a public health perspective. Our results demonstrate that CHWs can improve or mitigate typical declines in health behaviors in adults with diabetes who have low incomes. Many adults with diabetes do not meet physical activity or dietary recommendations [44, 45], yet meeting these targets is estimated to prevent 14% of deaths among older adults in the general population [46]. Therefore, interventions that successfully improve dietary behaviors and physical activity could have substantial impacts among individuals with diabetes. Because these behaviors are important for self-management of a variety of other common chronic conditions, such as cardiovascular disease and obesity, and with disease prevention, CHWs may be effective in changing health behaviors more broadly. Among populations with low incomes, in particular, CHWs may be one solution to help improve access to diabetes self-management education, address social and environmental barriers to self-management, and mitigate adverse health outcomes disproportionately experienced by this population.
Although the intervention targeted a wide range of self-management behaviors, intervention-control differences were only found for a subset of dietary behaviors and physical activity. Consistent with our findings, these behaviors were the most commonly improved outcomes among other CHW-led diabetes self-management interventions, despite differences in intervention design, delivery, and target populations [47]. These behaviors may be more susceptible to change than other skilled medical tasks (e.g., blood glucose monitoring, medication taking) [48] because they are socially influenced or occur in social settings. Additionally, action plans and goals developed by participants and CHWs in the study may have focused on these behaviors more frequently and thus were more likely to improve. Future CHW-led interventions could consider more explicitly incorporating education and goal setting related to medication taking and blood glucose monitoring by requiring these during CHW visits. Alternatively, CHW interventions could incorporate additional evidence-based strategies to improve medication taking or blood glucose monitoring, including provision of electronic medicine boxes for real-time medication monitoring, text message reminders, teach back or pictorial images for education purposes, pharmacist counseling, or remote patient monitoring of blood glucose [49, 50].
We found limited evidence of mediation and moderation, for which there are several plausible explanations. We may not have had an adequate sample size for detecting mediation or moderation, as the study was powered to test for clinically significant changes in HbA1c [11]. Regarding mediation, the intervention may have not sufficiently targeted social support and self-efficacy. Other unmeasured mediators, such as diabetes knowledge or perceived control, may account for improvements in self-management behaviors [51], which should be measured in future studies. Furthermore, null findings for social support may reflect that the measure assessed support mainly from family and friends, which may not have been substantially improved by the intervention. Future studies should consider directly including supportive others in the intervention to better enhance social support [52]. The lack of significant moderation may reflect that the intervention was equally effective across subgroups examined. There was evidence of moderation by baseline HbA1c for skipping meals, with intervention-associated decreases in skipping meals only among those with HbA1c < 10% at baseline. This contrasts primary study findings in which improvements in HbA1c were restricted to participants with HbA1c ≥ 10% at baseline [16]. Findings may reflect participants’ disease burden interfering with the ability to follow complex dietary recommendations or may be due to chance.
Study limitations and strengths
Our findings should be interpreted considering several important limitations. The trial was powered to detect changes in HbA1c and therefore we may have had insufficient power to detect changes in self-management behaviors or mediation or moderation of these changes. Furthermore, most self-management behaviors, mediators, and moderators were subject to social desirability bias, as they were self-reported. There is a possibility that social desirability bias was more pronounced among intervention arm participants, as blinding of participants and CHWs was not possible. To reduce possible bias, the CHW who did not deliver the intervention to a particular participant collected the participant’s 12-month outcome data. If social desirability bias was more extensive in the intervention arm, this would result in an overestimation of differences between intervention and control arm participants. Another limitation is that the potential outcomes framework employed in our analyses relies on strong assumptions, including that the mediators are independent of the outcome given treatment assignment and baseline confounders. Lastly, because we tested many hypotheses, at least some of the statistically significant findings may be due to chance. Rather than formally control for multiple comparisons by adjusting our significance threshold (e.g., via a Bonferroni correction), we followed the methods suggested by Althouse to present effect sizes, confidence intervals, and p-values to facilitate individual judgments about the relative weight of the conclusions [53]. Strengths of the study include a randomized controlled trial design; good participant retention; the racially/ethnically diverse study population recruited from multiple health care systems, which enhances generalizability; use of validated measures of many important health behaviors; and application of the intervention in a community health setting.
Conclusions
CHW-led interventions have been proposed as an effective strategy for improving self-management across a variety of chronic conditions. Particularly for underserved populations, such as individuals with low incomes, CHWs can fill a gap in self-management support [8]. However, efforts are needed to bolster the sustainability of CHWs, including predictable and sustained funding for CHWs; standardization of CHW trainings, roles, and responsibilities; greater integration of CHWs into health care systems and health care teams; and development of established mechanisms for reimbursement commensurate with CHW skills [54–56].
Our findings support that CHWs are effective for changing some diabetes self-management behaviors among adults with low incomes, particularly some eating behaviors and physical activity. For physical activity, these improvements were especially notable as intervention arm participants reported almost 2.5 additional hours per week of activity than controls. However, further research is needed to understand the mechanisms of these changes, as there was no evidence of changes being mediated by social support or self-efficacy, through which the intervention was designed and hypothesized to act. Given that eating behaviors and physical activity are critical components of disease prevention and self-management of other chronic conditions, and that only a small proportion of U.S. adults meet basic recommendations for both [57], CHWs may be effective in addressing health promotion broadly among individuals with low incomes.
Supplementary Material
Acknowledgments
We thank the study participants, health care systems, community health workers, and study staff for their assistance. This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases [grant number 5R18DK088072] and the United States Department of Veterans Affairs, Health Services Research & Development Program [grant numbers IK2HX002189 and TPP 61-029]. Supplemental funding was provided by the Veterans Health Administration, Diabetes Quality Enhancement Research Initiative to support recruitment efforts.
Compliance with Ethical Standards
Conflicts of Interest: All authors declare that they have no conflicts of interest.
Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the University of Washington and VA Puget Sound Institutional Review Boards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
Transparency Statements
The study was pre-registered at ClinicalTrials.gov (NCT02152852). The analysis plan was not formally pre-registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author. Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author. Materials used to conduct the study are not publicly available.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2017. [Google Scholar]
- 2. Beckles GL, Chou CF. Disparities in the prevalence of diagnosed diabetes – United States, 1999–2002 and 2011–2014. MMWR Morb Mortal Wkly Rep. 2016;65(45):1265–1269. [DOI] [PubMed] [Google Scholar]
- 3. Tatulashvili S, Fagherazzi G, Dow C, Cohen R, Fosse S, Bihan H. Socioeconomic inequalities and type 2 diabetes complications: A systematic review. Diabetes Metab. 2020;46(2):89–99. [DOI] [PubMed] [Google Scholar]
- 4. Hardman R, Begg S, Spelten E. What impact do chronic disease self-management support interventions have on health inequity gaps related to socioeconomic status: A systematic review. BMC Health Serv Res. 2020;20(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bachmann MO, Eachus J, Hopper CD, et al. . Socio-economic inequalities in diabetes complications, control, attitudes and health service use: A cross-sectional study. Diabet Med. 2003;20(11):921–929. [DOI] [PubMed] [Google Scholar]
- 7. American Public Health Association. Community health workers; 2019. Available at https://www.apha.org/apha-communities/member-sections/community-health-workers. Accessed April 30, 2018. [DOI] [PubMed]
- 8. American Public Health Association. Support for Community Health Workers to Increase Health Access and to Reduce Health Inequities. Policy No.: 20091. Washington, DC: American Public Health Association; 2009. [Google Scholar]
- 9. Palmas W, March D, Darakjy S, et al. . Community health worker interventions to improve glycemic control in people with diabetes: a systematic review and meta-analysis. J Gen Intern Med. 2015;30(7):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trump LJ, Mendenhall TJ. Community health workers in diabetes care: A systematic review of randomized controlled trials. Fam Syst Health. 2017;35(3):320–340. [DOI] [PubMed] [Google Scholar]
- 11. Nelson K, Drain N, Robinson J, et al. . Peer Support for Achieving Independence in Diabetes (Peer-AID): design, methods and baseline characteristics of a randomized controlled trial of community health worker assisted diabetes self-management support. Contemp Clin Trials. 2014;38(2):361–369. doi: 10.1016/j.cct.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. [DOI] [PubMed] [Google Scholar]
- 13. Clark NM, Gong M. Management of chronic disease by practitioners and patients: Are we teaching the wrong things? BMJ. 2000;320(7234):572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stopford R, Winkley K, Ismail K. Social support and glycemic control in type 2 diabetes: A systematic review of observational studies. Patient Educ Couns. 2013;93(3):549–558. [DOI] [PubMed] [Google Scholar]
- 15. Krichbaum K, Aarestad V, Buethe M. Exploring the connection between self-efficacy and effective diabetes self-management. Diabetes Educ. 2003;29(4):653–662. [DOI] [PubMed] [Google Scholar]
- 16. Nelson K, Taylor L, Silverman J, et al. . Randomized controlled trial of a community health worker self-management support intervention among low-income adults with diabetes, Seattle, Washington, 2010-2014. Prev Chronic Dis. 2017;14:E15. doi: 10.5888/pcd14.160344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laxy M, Mielck A, Hunger M, et al. . The association between patient-reported self-management behavior, intermediate clinical outcomes, and mortality in patients with type 2 diabetes: Results from the KORA-A study. Diabetes Care. 2014;37(6):1604–1612. [DOI] [PubMed] [Google Scholar]
- 18. Ausili D, Bulgheroni M, Ballatore P, et al. . Self-care, quality of life and clinical outcomes of type 2 diabetes patients: an observational cross-sectional study. Acta Diabetol. 2017;54(11):1001–1008. [DOI] [PubMed] [Google Scholar]
- 19. Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. [DOI] [PubMed] [Google Scholar]
- 20. Michie S, Richardson M, Johnston M, et al. . The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]
- 21. Smith DE, Heckemeyer CM, Kratt PP, Mason DA. Motivational interviewing to improve adherence to a behavioral weight-control program for older obese women with NIDDM. A pilot study. Diabetes Care. 1997;20(1):52–54. [DOI] [PubMed] [Google Scholar]
- 22. Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. [DOI] [PubMed] [Google Scholar]
- 23. Peyrot M, Peeples M, Tomky D, Charron-Prochownik D, Weaver T; AADE Outcomes Project and AADE/UPMC Diabetes Education Outcomes Project . Development of the American Association of Diabetes Educators’ Diabetes Self-management Assessment Report Tool. Diabetes Educ. 2007;33(5):818–826. [DOI] [PubMed] [Google Scholar]
- 24. Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–762. [DOI] [PubMed] [Google Scholar]
- 25. U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 26. Arem H, Moore SC, Patel A, et al. . Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. McDowell J, Courtney M, Edwards H, Shortridge-Baggett L. Validation of the Australian/English version of the Diabetes Management Self-Efficacy Scale. Int J Nurs Pract. 2005;11(4):177–184. [DOI] [PubMed] [Google Scholar]
- 29. Talbot F, Nouwen A, Gingras J, Gosselin M, Audet J. The assessment of diabetes-related cognitive and social factors: The Multidimensional Diabetes Questionnaire. J Behav Med. 1997;20(3):291–312. [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez JS, Peyrot M, McCarl LA, et al. . Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care. 2008;31(12):2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burke V, Beilin LJ, Cutt HE, Mansour J, Mori TA. Moderators and mediators of behaviour change in a lifestyle program for treated hypertensives: A randomized controlled trial (ADAPT). Health Educ Res. 2008;23(4):583–591. [DOI] [PubMed] [Google Scholar]
- 32. Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campbell JA, Walker RJ, Smalls BL, Egede LE. Glucose control in diabetes: The impact of racial differences on monitoring and outcomes. Endocrine. 2012;42(3):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heerman WJ, Wallston KA, Osborn CY, et al. . Food insecurity is associated with diabetes self-care behaviours and glycaemic control. Diabet Med. 2016;33(6):844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. U.S. Department of Health and Human Services. K141944. Silver Spring, MD: Food and Drug Administration, U.S. Department of Health and Human Services; 2015. [Google Scholar]
- 36. Polonsky WH, Fisher L, Earles J, et al. . Assessing psychosocial distress in diabetes: Development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. [DOI] [PubMed] [Google Scholar]
- 37. Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 39. Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89(8):1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 41. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15(4):309–334. [DOI] [PubMed] [Google Scholar]
- 42. Hicks R, Tingley D. Causal mediation analysis. Stata J. 2011;11(4):1–15. [Google Scholar]
- 43. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 44. Johnson PJ, Ghildayal N, Rockwood T, Everson-Rose SA. Differences in diabetes self-care activities by race/ethnicity and insulin use. Diabetes Educ. 2014;40(6):767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pilkerton CS, Singh SS, Bias TK, Frisbee SJ. Changes in cardiovascular health in the United States, 2003–2011. J Am Heart Assoc. 2015;4(9):e001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Behrens G, Fischer B, Kohler S, Park Y, Hollenbeck AR, Leitzmann MF. Healthy lifestyle behaviors and decreased risk of mortality in a large prospective study of U.S. women and men. Eur J Epidemiol. 2013;28(5):361–372. [DOI] [PubMed] [Google Scholar]
- 47. Norris SL, Chowdhury FM, Van Le K, et al. . Effectiveness of community health workers in the care of persons with diabetes. Diabet Med. 2006;23(5):544–556. [DOI] [PubMed] [Google Scholar]
- 48. Rosland AM, Piette JD, Lyles CR, et al. . Social support and lifestyle vs. medical diabetes self-management in the Diabetes Study of Northern California (DISTANCE). Ann Behav Med. 2014;48(3):438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zullig LL, Gellad WF, Moaddeb J, et al. . Improving diabetes medication adherence: Successful, scalable interventions. Patient Prefer Adherence. 2015;9:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Greenwood DA, Young HM, Quinn CC. Telehealth remote monitoring systematic review: Structured self-monitoring of blood glucose and impact on A1C. J Diabetes Sci Technol. 2014;8(2):378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Steed L, Barnard M, Hurel S, Jenkins C, Newman S. How does change occur following a theoretically based self-management intervention for type 2 diabetes. Psychol Health Med. 2014;19(5):536–546. [DOI] [PubMed] [Google Scholar]
- 52. Sorkin DH, Mavandadi S, Rook KS, et al. . Dyadic collaboration in shared health behavior change: The effects of a randomized trial to test a lifestyle intervention for high-risk Latinas. Health Psychol. 2014;33(6):566–575. [DOI] [PubMed] [Google Scholar]
- 53. Althouse AD. Adjust for multiple comparisons? It’s not that simple. Ann Thorac Surg. 2016;101(5):1644–1645. [DOI] [PubMed] [Google Scholar]
- 54. Humphry J, Kiernan J. Insights in Public health: Community health workers are the future of health care: how can we fund these positions? Hawaii J Health Soc Welf. 2019;78(12):371–374. [PMC free article] [PubMed] [Google Scholar]
- 55. Hynes DM, Buscemi J, Quintiliani LM; Society of Behavioral Medicine Health Policy Committee . Society of Behavioral Medicine (SBM) position statement: SBM supports increased efforts to integrate community health workers into the patient-centered medical home. Transl Behav Med. 2015;5(4):483–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stupplebeen DA, Sentell TL, Pirkle CM, et al. . Community health workers in action: Community-clinical linkages for diabetes prevention and hypertension management at 3 community health centers. Hawaii J Med Public Health. 2019;78(6 suppl 1):15–22. [PMC free article] [PubMed] [Google Scholar]
- 57. Kruger J, Yore MM, Solera M, Moeti R. Prevalence of fruit and vegetable consumption and physical activity by race/ethnicity—United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(13):301–304. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.