Significance
Humans have the proportionately smallest male canines among all anthropoids and little canine sexual dimorphism. However, the evolutionary emergence of this defining condition remains unclear because until now we have lacked a reliable method of determining dimorphism in weakly dimorphic fossil species. Using a probability-based method we recently developed, we estimated canine size dimorphism in the ∼4.5 million-year-old Ardipithecus ramidus and found it to be weak and comparable to that of modern humans. Our analysis of >300 fossils spanning 6 million years shows that male canine size reduction occurred early in human evolution, broadly coincident with the adoption of bipedality. This suggests a profound and evolutionarily deep sociobehavioral shift that minimized male–male aggression, most likely mediated by female choice.
Keywords: canine dimorphism, Bayesian estimate, Ardipithecus ramidus, Australopithecus, Homo
Abstract
Body and canine size dimorphism in fossils inform sociobehavioral hypotheses on human evolution and have been of interest since Darwin’s famous reflections on the subject. Here, we assemble a large dataset of fossil canines of the human clade, including all available Ardipithecus ramidus fossils recovered from the Middle Awash and Gona research areas in Ethiopia, and systematically examine canine dimorphism through evolutionary time. In particular, we apply a Bayesian probabilistic method that reduces bias when estimating weak and moderate levels of dimorphism. Our results show that Ar. ramidus canine dimorphism was significantly weaker than in the bonobo, the least dimorphic and behaviorally least aggressive among extant great apes. Average male-to-female size ratios of the canine in Ar. ramidus are estimated as 1.06 and 1.13 in the upper and lower canines, respectively, within modern human population ranges of variation. The slightly greater magnitude of canine size dimorphism in the lower than in the upper canines of Ar. ramidus appears to be shared with early Australopithecus, suggesting that male canine reduction was initially more advanced in the behaviorally important upper canine. The available fossil evidence suggests a drastic size reduction of the male canine prior to Ar. ramidus and the earliest known members of the human clade, with little change in canine dimorphism levels thereafter. This evolutionary pattern indicates a profound behavioral shift associated with comparatively weak levels of male aggression early in human evolution, a pattern that was subsequently shared by Australopithecus and Homo.
A small canine tooth with little sexual dimorphism is a well-known hallmark of the human condition. The small and relatively nonprojecting deciduous canine of the first known fossil of Australopithecus, the Taung child skull, was a key feature used by Raymond Dart for his inference that the fossil represented an early stage of human evolution (1). However, recovery of additional Australopithecus fossils led to the canine of Australopithecus africanus to be characterized as large (compared to that of humans or “robust australopithecines”) and its morphology primitive, based on a projecting main cusp and crown structures lacking or hardly expressed in Homo (2). Later, the perception of a large and primitive canine was enhanced by the discovery and recognition of Australopithecus afarensis and Australopithecus anamensis (3–8), the latter species extending back in time to 4.2 million years ago (Ma). Although assessments of canine size variation and sexual dimorphism in Au. afarensis were hampered by limited sample sizes (9, 10), some suggested that the species had a more dimorphic canine than do humans, equivalent in degree to the bonobo (11) or to chimpanzees and orangutans (12). Initially, Au. anamensis was suggested to express greater canine dimorphism than did Au. afarensis (13, 14). However, based on a somewhat larger sample size, this is now considered to be the case with the tooth root but not necessarily its crown (15–17).
Throughout the 1990s and 2000s, a pre-Australopithecus record of fossils spanning >6.0 to 4.4 Ma revealed that the canines of these earlier forms did not necessarily exceed those of Au. afarensis or Au. anamensis in general size (18–28). However, all these taxa apparently possessed canine crowns on average about 30% larger than in modern humans, which makes moderately high levels of sexual dimorphism potentially possible. Canine sexual dimorphism, combined with features such as body size dimorphism, inform sociobehavioral and ecological adaptations of past and present primates, and therefore have been of considerable interest since Darwin’s 1871 considerations (29–57). In particular, the relationship of canine size dimorphism (and/or male and female relative canine sizes) with reproductive strategies and aggression/competition levels in primate species have been a continued focus of interest (14, 33, 35–45, 49–56). Conspecific-directed agonistic behavior in primates related to mate and/or resource competition can be particularly intense among males both within and between groups (14, 44, 57). It is widely recognized that a large canine functions as a weapon in intra- and intergroup incidences of occasional lethal aggression (45, 58–61), and a large, tall canine has been shown or inferred to significantly enhance male fitness (50, 56). Hence, canine size and dimorphism levels in fossil species provide otherwise unavailable insights into their adaptive strategies.
Here, we apply a recently developed method of estimating sexual size dimorphism from fossil assemblages of unknown sex compositions, the posterior density peak (pdPeak) method (62), and reexamine canine sexual dimorphism in Ardipithecus ramidus at ∼4.5 Ma. We include newly available fossils recovered from the Middle Awash and Gona paleoanthropological research areas in the Afar Rift, Ethiopia (26, 63, 64) in order to obtain the most reliable dimorphism estimates currently possible. We apply the same method to Australopithecus, Homo, and selected fossil apes, and evaluate canine sexual dimorphism through evolutionary time.
We operationally define canine sexual dimorphism as the ratio between male and female means of basal canine crown diameters (the m/f ratio). Because the canines of Ar. ramidus, Au. anamensis, and extant and fossil apes are variably asymmetric in crown shape, we examine the maximum basal dimension of the crown. This can be either the mesiodistal crown diameter or a maximum diameter taken from the distolingual to mesiobuccal crown base (7, 27, 65). In the chronologically later Au. afarensis and all other species of Australopithecus sensu lato and Homo, we examine the more widely available conventional metric of buccolingual breadth, which corresponds to or approximates the maximum basal crown diameter. In anthropoid primates, canine height is more informative than basal canine diameter as a functional indicator of aggression and/or related display (14, 41–44). We therefore also examine available unworn and minimally worn fossil canines with reliable crown heights.
Estimating Canine Sexual Dimorphism, the Problem
Because the sexual identity of fossil teeth can only rarely be established by associated skeletal remains with sufficient indicators of sex, there is a long history of research on inferring dental sexual dimorphism. In anthropoid primates with strong canine sexual dimorphism, the canine can often be sexed on the basis of size and shape. However, this is not the case when dimorphism is only moderate or slight. With extant and Miocene fossil apes, Kelley (66, 67) has demonstrated that a set of morphological criteria tends to distinguish sex regardless of taxa, although boundary conditions vary by species. By this method, a clearly bimodal canine (and molar) size dimorphism was established in Lufengpithecus, Griphopithecus, and Kenyapithecus (68–70). In these taxa, the m/f ratios of basal canine diameters are greater than 1.4 or 1.6, values comparable to, or more dimorphic than, the most dimorphic extant great ape (Gorilla, with m/f ratios of ∼1.4 to 1.5). A similarly large m/f ratio (>1.5) has been reported for Nacholapithecus, based on bimodal nonoverlapping canine size distributions (71, 72). Somewhat lower m/f ratios of 1.34 and 1.37 were reported in Late Miocene Ouranopithecus (73) and Pleistocene Gigantopithecus (74), respectively, whereas taxonomic complexities and/or smaller samples make assessments difficult in other cases (67, 72, 75–78). The m/f ratios of Pan and Pongo range from around 1.2 to 1.4, with the lowest degree of dimorphism seen in the lower canine of the bonobo (11, 15, 27, 42, 75, 79).
In anthropoid primates, including extant great apes, sexual dimorphism of the canine is generally more enhanced in the upper than in the lower canine (27, 35, 41, 42, 66). The upper canines exhibit thin enamel, especially in males (80, 81), and are sharpened distolingually by the occluding lower premolar to form a sharp distal cutting edge (80, 82–84). This morphological complex is known as the honing complex (or the C/P3 complex in catarrhines), comprising sharp upper and lower canines interlocking in occlusion along vertical wear facets/zones supported by distinct crown structures/shapes (ref. 82, see also ref. 25 pp. 212−216 and supplementary online material in ref. 27, pp. 14− 16 on hominoid canine structures and occlusal relationships). Whereas the canine is generally a conical puncturing and grasping organ in carnivores, in anthropoid primates, the upper canine functions as a sharp-edged weapon as amply inferred from wounds inflicted in physical fights (45, 58–61). In humans, the ancestral C/P3 complex is highly modified to remnant vestigial expression, and the m/f ratio of basal crown diameters varies among populations predominantly between 1.03 and 1.09 (85), although the ratio exceeds 1.1 in some modern or recent human populations (Dataset S1).)
When shape indicators of sex are unclear or not demonstrable, and when female and male canine sizes (and shape) overlap substantially, as is the case in modern humans and some nonhuman anthropoids, accurately sexing individual canines is difficult or impossible. That is, the male canine can be “feminized” as in humans, or the female canine can be large and have “male morphology” indistinguishable from those of males, as in gibbons (66, 86). However, with the expectancy that mean sizes were larger in males than in females, canine sexual dimorphism in primate fossil assemblages has been estimated by a variety of methods (9, 11, 43, 87). Here, we briefly summarize the necessary limitations inherent in these methods and then present our findings via the pdPeak method (62) that enables a more accurate and meaningful dimorphism estimate than previously possible.
Of particular interest is the timing(s) when male canine reduction occurred in the lineages leading to humans, and whether or not emergence of a human-like low level of canine size dimorphism was gradual or focused on some particular time period and circumstances.
The Mean and Binomial Dimorphism Index Methods
The most commonly applied method is known as the mean method (MM). In this method, the fossil sample is divided into two nonoverlapping subgroups by the mean, and the ratio between the larger and smaller subsample means is considered the m/f ratio (88). Although the MM has been shown to return reliable estimates of dimorphism when the m/f ratio is >1.2 or >1.3, by definition, the MM overestimates dimorphism when male and female size distributions overlap (62, 87–90). Because our goal is to ascertain the evolutionary trajectory that led to the human condition, i.e., a transition of an m/f ratio from >1.2 to < ∼1.1, the MM is incapable of providing sufficient resolution. The binomial dimorphism index (BDI) (46, 91) was devised to alleviate some of the overestimating effects of the MM and other ratios such as maximum to minimum observed individuals. This is done by inputting binomial probabilities into the calculations to account for uneven sex compositions in small fossil assemblages when estimating dimorphism.
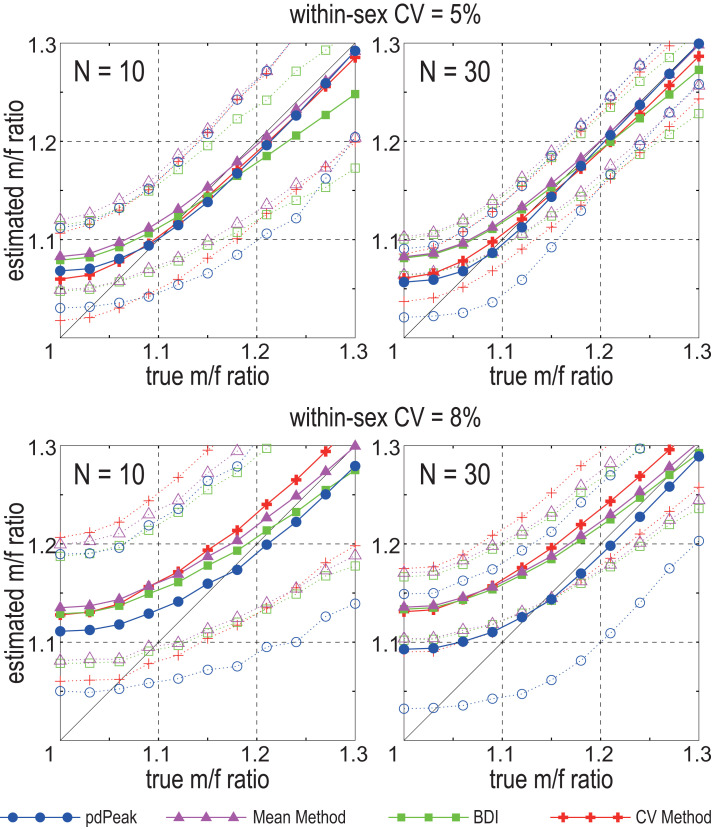
Since both MM and BDI assume that all males are larger in size than all females, these methods produce unbiased estimates of dimorphism only when the difference between sex means are greater than certain levels of within-sex variance (46, 88, and detailed in ref. 62). Strictly speaking, for an unbiased estimate to be obtained, bimodality needs to be well expressed in the combined-sex whole sample; a difference of sex means that is greater than four SD units of within-sex variance is required. However, this bias is relatively modest as long as bimodality is detectable, which corresponds to instances when sex means are separated by more than 2 SD units of within-sex variance (62). Fig. 1 depicts the degree of systematic bias inherent in the MM and BDI. If within-sex variation is low, for example when the within-sex coefficient of variation (wsxCV) is as low as 0.05, overestimation is slight for m/f ratios greater than 1.1. However, this is not the case when wsxCV is 0.08, a condition commonly seen in anthropoid canines including humans (Dataset S1, and figure 2 in ref. 62).
Fig. 1.
Simulation tests of the pdPeak, mean, BDI, and CV methods, adapted from figure 3 in ref. 62. The x axis is the m/f ratio (male mean divided by the female mean) of the hypothetical populations with either a within-sex CV of 0.05 (Upper plots) or 0.08 (Lower plots). The y axis is the estimated population m/f ratio. Solid lines are the means, and dotted lines are the 5th (Lower line) and 95th (Upper line) percentiles of the 2,000 samples (of n = 10 or n = 30) extracted from the simulated populations. This was done for simulated populations set at m/f ratios of 0.03 intervals. pdPeak, posterior density peak; BDI, binomial dimorphism index; CV, coefficient of variation. The CV method is that of Plavcan (87). Note that unbiased m/f ratio estimates of ∼1.10 and lower are possible only by the pdPeak method. The other methods systematically overestimate.
The CV Method
The widely used alternative method of assessing sexual size dimorphism in fossil assemblages is some form of the CV method (the coefficient of variation, CV, is the SD divided by the mean). It has been empirically demonstrated that the combined-sex CVs of a size dimorphic element, such as the canine of many anthropoid primates, are conspicuously elevated by the mixture of sex samples with large mean differences (9, 75, 92). Simply put, a large CV of a fossil assemblage likely indicates a large degree of sexual size dimorphism. The latter can then be estimated by regressing the m/f ratio on whole-sample (combined-sex) CV, using modern primate references of choice or comparably simulated hypothetical population sets. Such methods have been applied to the Australopithecus canine (11, 43), resulting in the inference that at least some Australopithecus species, such as Au. afarensis or Au. anamensis, had moderate dimorphism levels of canine basal crown size, as in bonobos or chimpanzees (11, 14, 43).
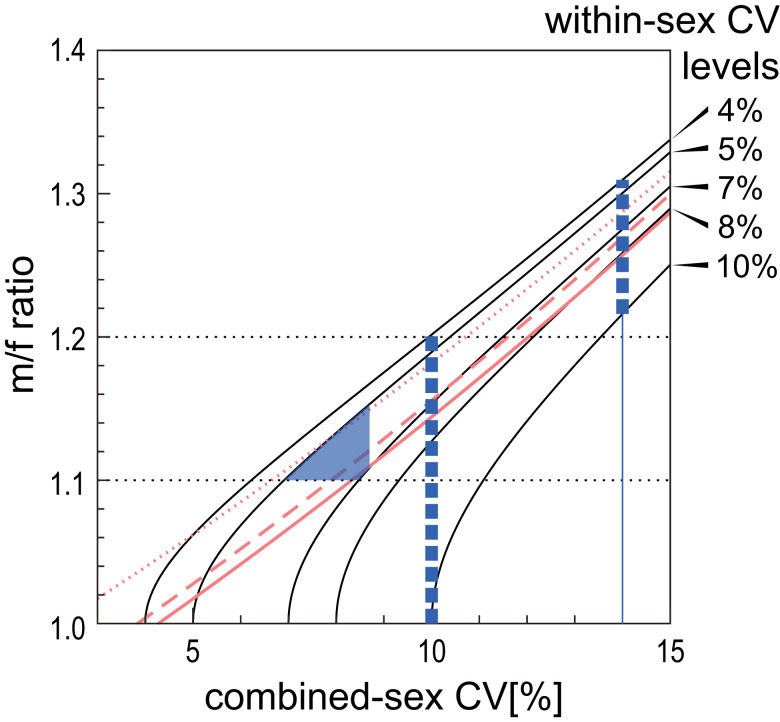
However, an elevated combined-sex CV can be caused either by divergent sex distributions or by high wsxCVs characteristic of the species or samples. Therefore, the validity of a CV-based estimate of sexual dimorphism is dependent entirely on the underlying within-sex variance of the sample (which is generally unknowable in fossils). Under some simplifying assumptions (equal male and female sample sizes, common within-sex CV, and a large sample size), a mathematical relationship between the m/f ratio (k), combined-sex CV (cmbCV), and wsxCV is given by the formula:
(supplementary online material and table S8 in ref. 27).
In Fig. 2, we illustrate this relationship, which shows that the CV method cannot reliably estimate population m/f ratios without a prior knowledge of wsxCV. In the case of canine dimensions, because the wsxCVs of modern human populations (Dataset S1) and geographically circumscribed populations and/or (sub)species of nonhuman anthropoids are empirically known to range from 0.04 to 0.10 (93–95), the CV method is thereby incapable of reliably estimating population m/f ratios in the <1.1 to 1.2 interval, our target of inquiry.
Fig. 2.
Computational interrelationship of the m/f ratio, combined-sex CV, and within-sex CV. Each curvilinear line represents the relationship between the combined-sex CV (x axis) and m/f ratio (male mean divided by the female mean) (y axis) of hypothetical populations with different levels of within-sex CV. Given that anthropoid within-sex CVs of canine diameters range from <0.04 to >0.10, the Left and Right vertical interrupted lines show the possible m/f ratio ranges of a population with a combined-sex CV of 0.10 and 0.14, respectively. Note that a population with a combined-sex CV of 0.10 can represent either no dimorphism (m/f ratio of 1.0) or the lower range of an extant great ape level of dimorphism (m/f ratio of 1.2). The red lines represent alternative CV-method regression estimates of the m/f ratio. The dotted red line is the Plavcan CV method equation: lnY = 2.14X − 0.047 (87). The continuous and interrupted lines are the empirical least squares regressions of ln(m/f ratio) on combined-sex CV of the upper and lower canine maximum diameters, respectively, using the screened Plavcan dataset of anthropoids (95) (SI Appendix, SI Text). The hatched area indicates the m/f ratio range delineated by the boundary conditions assumed in Suwa et al. (table S8 in ref. 27) wherein the Ar. ramidus canine m/f ratio was estimated as 1.10 to 1.15, with a “best estimate” of 1.12.
The Method of Moments
There have been few attempts to model sexual dimorphism by considering parameters (other than total variance) that vary according to separation of the sex means. Martin et al. (96) pointed out that the kurtosis of a mixed-sex sample changes in a predictable pattern by the magnitude of mean sex difference. The method of moments (MoM) takes a similar approach by using a formula that derives the m/f ratio from the first, second, and fourth moments (89). A combined-sex sample of a widely separated sex distribution should exhibit a “flatter” overall distribution, the opposite being the case with weak levels of dimorphism. However, when applying the MoM to actual samples, a problem occurs that the MoM cannot always calculate the m/f ratio estimate. This stems from its quartic equation that lacks real-number solutions depending on sample distribution (i.e., imaginary solutions may result). This can frequently occur when m/f ratios are low (e.g., <∼1.1). Focusing only on the real-number solutions, the MoM would tend to overestimate when wsxCV is >0.08 (albeit to a lesser extent than the MM or BDI) (SI Appendix, Fig. S1). Alternatively, if the imaginary solutions are considered to represent an m/f ratio of 1.0 (89), this results in an inability to distinguish between weak and no dimorphism (SI Appendix, Fig. S1). In a previous trial application to primate body mass data (97), the MoM was found to perform no better than the MM.
The pdPeak Method and Ar. ramidus Canine Sexual Dimorphism
The pdPeak method is a Bayesian-based method that in part solves the above problems (Materials and Methods) (62). The pdPeak method is similar to the MoM in modeling a mixture of two normal distributions of equal within-sex variance. However, instead of solving a formula from observed parameters, in the pdPeak method, population sex means and variance are estimated based on likelihoods of the observed fossil distributions. After extensive validation on both simulated and known-sex extant anthropoid datasets (62), we applied this method to the Ar. ramidus fossil assemblage of 13 upper canines and 11 lower canines (the latter also reported in ref. 62) and to a range of fossil apes, Australopithecus and Homo. Fig. 1 shows that the pdPeak method is capable of documenting m/f ratios of ∼1.1 if sample size is large (n ≥ 30) and/or the within-sex variance is low.
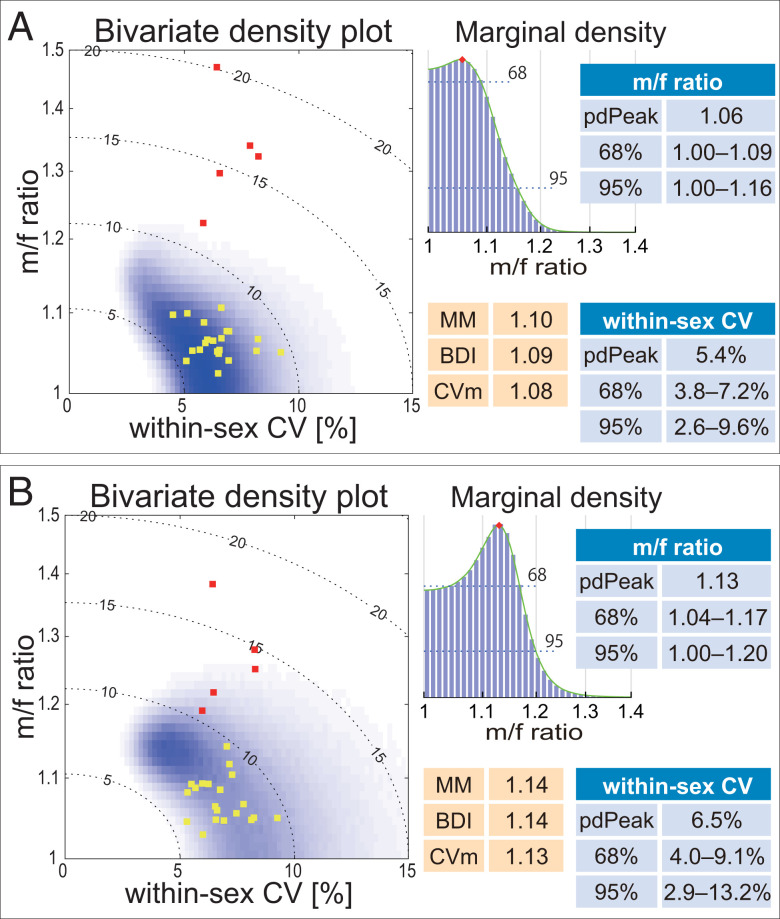
The results of the pdPeak method can be expressed in the form of an m/f ratio vs. wsxCV bivariate posterior distribution plot (Fig. 3) or as a male mean vs. female mean bivariate plot (SI Appendix, Fig. S2). We consider the pdPeak of the marginal distributions to be the best estimator of each parameter. The pdPeak estimates of the Ar. ramidus upper and lower canine m/f ratios were 1.06 and 1.13, respectively. This is within the modern human range of variation of population m/f ratios (Dataset S1). An important feature of the pdPeak method is that statistical probabilities of the estimates can be reliably evaluated by the Bayesian inference credible intervals. The Ar. ramidus upper canine m/f ratio is <1.09 at P = 0.68 and lies well within the modern human range of variation. The probability of the Ar. ramidus upper canine being at least as dimorphic as that of the least dimorphic extant great ape (the bonobo lower canine with a m/f ratio of 1.19) is P = 0.012. The same probability of the Ar. ramidus lower canine is P = 0.068. Thus, we can safely conclude that canine size dimorphism was weaker in Ar. ramidus than in any extant great ape and indistinguishable or close to the modern human condition.
Fig. 3.
Ar. ramidus canine sexual dimorphism. The bivariate probability distributions of within-sex CV (x axis) and the m/f ratio (male mean divided by the female mean) (y axis) are shown in gray scale. (A) Upper canine maximum diameter, (B) lower canine maximum diameter (modified from figure 6 in ref. 62). The probability densities were obtained in log scale (log-transformed data), and the axis labels were back transformed to original scale (see ref. 62 for details). Yellow and red squares, respectively, are the modern human (Dataset S1, n ≥ 20) and extant great ape (27) population or species/subspecies values. The dotted contour lines indicate combined-sex CV levels. The marginal probability densities (based on MCMC histograms) of the logarithm of m/f ratio are shown to the Right of the bivariate density plots. The pdPeak values and credible intervals are shown, as well as the m/f ratio estimates of the MM, BDI, and CV (87) methods. The pdPeak m/f ratio estimate of the n = 13 upper canine sample is 1.06, with a 95% highest density credible interval of 1.00 to 1.16. This indicates a 0.95 probability that the population m/f ratio is 1.16 or lower. In addition, it is possible to derive bootstrap confidence intervals (CIs) of the pdPeak estimate. Bootstrap CIs represent reproducibility of the estimate given the sample distribution in hand, but, in contradistinction to the credible interval, do not provide probabilities in relation to the population value. Based on 1,000 random iterations with replacement on the n = 13 upper canine sample, the bootstrap pdPeak m/f ratio average is 1.08 with a 95% CI of 1.02 to 1.16. Although this additionally confirms the reliability of the pdPeak estimate, the bootstrap CI represents the probable range of the estimate and not of the population value. With the lower canine sample (n = 11), the pdPeak m/f ratio estimate and its bootstrap average are both 1.13, with a bootstrap 95% CI of 1.05 to 1.19, subsumed within the 95% credible interval of 1.00 to 1.20. These examples show that the Bayesian credible interval of the pdPeak m/f ratio is (generally) more conservative than the bootstrap CI (see also ref. 62).
The pdPeak estimates of the Ar. ramidus m/f ratio are accompanied by wsxCV estimates of 0.054 and 0.065 for the upper and lower canines, respectively. Because of the suggested low wsxCV, the MM and BDI estimates are also low (1.10 and 1.09, respectively in the upper canine), a further indication of the low dimorphism level of the Ar. ramidus canine. Previously, assuming a low wsxCV of 0.05 to 0.07, we had estimated its canine m/f ratio as between 1.10 and 1.15 with a best estimate of 1.12 (27) (Fig. 2). These previous findings are in accord with our pdPeak estimates.
As briefly noted above, an important capacity of the pdPeak method is its ability to simultaneously estimate both m/f ratio and within-sex variance. Therefore, fossil assemblages with a high whole-sample CV can nevertheless return a low m/f ratio because of a high wsxCV, depending on the actual within-sample distribution. Furthermore, this is the case regardless of the source of the elevated sample variance; i.e., a high wsxCV can stem from factors other than inherent variance of the population. For example, the often-discussed effects of time averaging that may occur in fossil assemblages are at least in part considered by the pdPeak method. This is shown in SI Appendix, Fig. S3.
Canine Sexual Dimorphism in Human Evolution
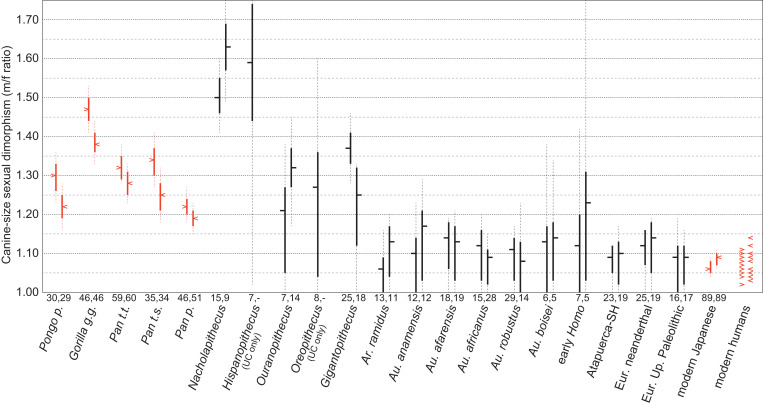
We applied the pdPeak method to selected fossil apes and to Pliocene and Pleistocene Australopithecus and Homo (Fig. 4, SI Appendix, Figs. S4–S11, Dataset S2, and see also SI Appendix, Figs. S12 and S13 for extant great ape sample tests). Nacholapithecus kerioi is characterized by a high level of canine size dimorphism with clear bimodal distributions (71, 72) (SI Appendix, Fig. S9B). Near identical m/f ratios of ∼1.5 were obtained either by the pdPeak or MM methods, and slight underestimations by the BDI method, all as expected (Fig. 1). Despite the small sample size, Hispanopithecus laietanus (SI Appendix, Fig. S10A) likewise suggests a comparably large degree of canine dimorphism. We also examined three fossil apes (SI Appendix, Figs. S10B and S11), Ouranopithecus macedoniensis, Oreopithecus bambolii, and Gigantopithecus blacki, considered by some to exhibit a reduced canine or C/P3 complex convergent on the human clade. In all three taxa, strong dimorphisms broadly equivalent to the chimpanzee or orangutan conditions are indicated. The 25 upper canines of G. blacki returned a pdPeak m/f ratio of 1.37 (>1.28 at P = 0.95), conclusively as dimorphic as in chimpanzees or orangutans. O. macedoniensis also shows a broadly comparable level of dimorphism with a lower canine pdPeak m/f ratio of 1.32, although uncertainty is greater because of smaller sample sizes.
Fig. 4.
Canine sexual dimorphism in fossil apes, Ar. ramidus, Australopithecus, and Homo. The m/f ratios (male mean divided by the female mean) of the fossil samples estimated by the pdPeak method are shown (in black) and compared to the actual population m/f ratios of the extant great apes and modern humans (in red). The Bayesian credible intervals of the pdPeak estimates provide reliable probable ranges of the actual population values (see ref. 62), and therefore the pdPeak mf/ratios and credible intervals are compared to the extant sample values and CIs. In black, the pdPeak m/f ratio estimate is shown by the horizontal tick, and the solid and dashed vertical lines indicate the 68% and 95% credible intervals, respectively. In red, the sample m/f ratio is shown by the horizontal arrowhead, and the solid and dashed vertical lines indicate the 68% and 95% CIs, respectively. For each taxon/population, upper canine on the Left, lower canine on the Right, sample sizes are indicated below the vertical lines. Note that the high m/f ratio obtained for the lower canine of early Homo is based on a small sample size of five. See SI Appendix, Figs. S4–S11 for a fuller presentation of the pdPeak results of each taxon/population. The extant great ape and modern Japanese samples are from Suwa et al. (27) and Sasaki et al. (62) and the other modern humans were taken from Dataset S1. The latter are based on population means available in the literature and shown as point values with the red arrowheads. P. t. t., P. t. s., and P. p. are Pan troglodytes troglodytes, P. t. schweinfurthi, and P. paniscus, respectively. See SI Appendix, Figs. S12 and S13 for test applications of the pdPeak method on the extant samples and how the pdPeak estimates relate to actual sample values and CIs and in relation to sample size. The latter results show that both uncertainty and overestimation bias at low levels of dimorphism become greater with smaller sample sizes and larger within-sex variance (as in the simulation results of Fig. 1), and that a modern human level of dimorphism of <1.10 would tend to be overestimated to an m/f ratio of generally 1.1 to 1.2 if within-sex variance was large (as estimated for some of the Australopithecus samples, see section Canine Sexual Dimorphism in Human Evolution above).
We next examined the post-Ar. ramidus taxa/populations of the human clade (Fig. 4 and SI Appendix, Figs. S5–S9A). It can be seen that canine dimorphism was weak and approximated the modern human condition not only in Ar. ramidus but also throughout human evolution in high probability. Au. anamensis and Au. afarensis (either whole species samples or their subsets, see Dataset S2) tend to have mandibular canines with high combined-sex CVs of >0.10, resulting in high m/f ratios of ∼1.2 by the MM, BDI, or CV methods, concordant with previous reports (11, 43). However, the pdPeak method shows that the large variation is explained by a high wsxCV of ∼0.09 to ∼0.10 and m/f ratios not significantly greater than 1.10. Thus, Au. anamensis and Au. afarensis canine dimorphism was probably weaker than in bonobos, and consistent with a low level of canine dimorphism inferred for the chronologically earlier sister taxon Ar. ramidus.
In Australopithecus and Homo, some of the species/populations are represented by relatively larger samples, enabling results that are statistically well constrained. For Australopithecus, the largest samples occur in Au. africanus (lower canine n = 28) and Australopithecus robustus (upper canine n = 29) (SI Appendix, Fig. S6). The pdPeak dimorphism of the Au. africanus lower canine is 1.09, with an m/f ratio <1.11 at P = 0.68 and <1.15 at P = 0.95. With the Au. robustus upper canine, the pdPeak m/f ratio is 1.11 (<1.14 at P = 0.68, and <1.17 at P = 0.95). This suggests that sexual dimorphism in Australopithecus was close to the modern human condition regardless of differences in absolute and relative crown sizes and crown morphologies.
The Early Pleistocene record of Homo is too scant to make detailed assessments, but the Middle Pleistocene Atapuerca Sima de los Huesos (SH) population sample is instructive. Previous evaluations suggested a canine size dimorphism slightly greater than in modern humans, via a whole-sample (combined-sex) CV of ∼0.08, perhaps with an m/f ratio as large as 1.15; the latter was inferred from associations with morphologically sexed mandibles (98). To the contrary, the currently available Atapuerca SH upper canine sample of n = 23 (99) returns a pdPeak m/f ratio of 1.09 (<1.12 at P = 0.95) (SI Appendix, Fig. S8A), well within the modern human range. This is concordant with a recent dimorphism assessment of the SH sample on canine tissue attributes (enamel and dentine volumes and surface areas) that concluded that sexual dimorphism did not exceed that of modern humans (100). The Homo neanderthalensis canine sample of the present study exhibits an upper canine (n = 25) pdPeak m/f ratio of 1.12 (<1.16 at P = 0.95), also only marginally greater than in modern humans (SI Appendix, Fig. S8B). Finally, the European Upper Paleolithic sample of sexed individuals (101) returns pdPeak m/f ratios of 1.09 in both upper and lower canines, corresponding to the modern human condition; the latter pdPeak estimates compare with m/f ratios of 1.08 and 1.05 for the upper and lower canines, respectively, based on sex assignments via their associated pelves.
Discussion
Taken together, our results suggest that a greatly reduced, weak canine dimorphism characterized the early members of the human clade. Our pdPeak estimates and probabilities indicate that canine size dimorphism was close to the modern human condition as early as in Ar. ramidus at ∼4.5 Ma. Despite the considerable size variation seen in early Australopithecus canines, by concurrently modeling within-sex variance, it now appears that dimorphism levels of early Australopithecus were likely as low as in Ar. ramidus. The slightly greater pdPeak estimates of the m/f ratio in Au. anamensis and Au. afarensis can be inferred to reflect a combination of sample bias, elevations of variance from mixing populations, and/or fluctuations within a common weak level of canine size dimorphism close to or only slightly greater than in modern humans. Because tooth crown size can covary with body size in mix-sexed samples (102), body size variation/dimorphism may also be a contributing factor to differences in canine size dimorphism levels. We await future fossil finds to test whether or not subtle differences in canine dimorphism occurred among Pliocene Australopithecus species and populations. We note that considerable population variation is seen in the m/f ratios of modern humans (Dataset S1).
In Ar. ramidus, and in early Australopithecus species, size dimorphism of the lower canine crown tends to be greater than that of the upper canine (but not in Au. africanus and Au. robustus). Although statistical uncertainties preclude a definitive conclusion, this may reflect how the C/P3 complex was modified early in human evolution, as inferred from Ar. ramidus (27). Assuming an ancestral C/P3 honing complex, the Ar. ramidus upper canine appears morphologically more derived than the lower canine. We previously interpreted this as selection having operated more strongly on the behaviorally important male upper canine, whereas morphological change in the lower canine could have lagged behind (25, 27, 65). That the m/f ratio pdPeak estimates are lower in the upper canines of Ar. ramidus, and perhaps also in early Australopithecus, supports this interpretation. This is contrary to the greater size dimorphism generally observed in the upper compared to lower canines in extant great apes and other anthropoids with significant degrees of canine sexual dimorphism (42, 43).
It is well known that canine crown height is more closely related to behavioral aggression and/or competition levels than is basal crown size (41, 43, 44, 103). This is clearly expressed metrically in the canines of anthropoids in which crown height dimorphism is considerably enhanced over basal crown diameter dimorphism (SI Appendix, Fig. S14 and Dataset S3). Some anthropoids (colobines and Ateles) exhibit only weak basal crown dimorphism (<1.2) but large degrees of height dimorphism (>1.5) (42, 43) (SI Appendix, Fig. S14), indicating that the latter is the selectively more crucial. In extant great apes, canine dimorphism in crown height is greater than in basal diameters, albeit to a lesser extent than in Old World monkeys. This is associated with a proportionately higher crowned canine in males, although overlap in relative heights occurs between sexes particularly in Pan (27, 66). The taller canine crown in males is attained predominantly by a prolonged growth period in apes (104) and in cercopithecids (105), and has been demonstrated to impact male reproductive success (50, 56).
From the foregoing, as previously noted (16, 27, 103), it is necessary to examine canine crown height and its dimorphism in Ar. ramidus and the other species. In the fossil record, however, reliable evaluation of unworn crown height is greatly constrained by the shortage of fully developed canine crowns lacking substantial wear and/or damage. We therefore followed Kelley (66) in compensating small amounts of cusp tip loss (<1∼2 mm), and assessed crown heights in six available Ar. ramidus upper canines (Dataset S4). Although small sample size precludes a statistically robust estimation, the pdPeak estimate of Ar. ramidus canine crown height m/f ratio is 1.10 (<1.14 at P = 0.95). The short crown heights of the probable males attest to the advanced “feminization” of the Ar. ramidus canine (Fig. 5), resulting in low sexual dimorphism in both basal crown diameter and crown height.
Fig. 5.

Ar. ramidus upper (maxillary) canine variation. Relatively well-preserved Ar. ramidus upper (maxillary) canines from the Middle Awash research area, Afar Rift, Ethiopia, demonstrate low crown height and weak sexual dimorphism. From Left to Right, Top row lingual and Bottom row occlusal views of ARA-VP-6/1 (Left reversed), ARA-VP-1/300 (Right), ARA-VP-1/3429 (Left reversed), SAG-VP-7/118 (Left reversed), ARA-VP-1/1818 (Right), and ARA-VP-6/500 Left. The five specimens on the Left are probable males, and the Far Right specimen, ARA-VP-6/500, a female. The ARA-VP-6/500 canine does not preserve the apical crown (translucent portion hypothetical), but its crown height was reasonably estimated ( SI Appendix, Fig. S15). The well-worn SAG-VP-7/118 canine is included as an example of apical wear ubiquitous in known Ar. ramidus upper canines and to show variation in shoulder heights and occlusal view crown shape. Mesial shoulder is high in ARA-VP-6/1, and low in SAG-VP-7/118 and ARA-VP-1/1818. In occlusal view, SAG-VP-7/118 and ARA-VP-1/300 are mesiodistally longer than buccolingually broad, whereas the other specimens are subequal in mesiodistal and buccolingual diameters (65). Note that all specimens exhibit a well-developed, voluminous mesiolingual ridge demarking a deep cleft-like mesiolingual groove. This structural pattern is best considered close to that of an ancestral honing C/P3 complex and is morphologically more primitive than in known examples of early Australopithecus (27).
Although weak or minimal canine dimorphisms occur in some nonhuman anthropoids (42, 43) (SI Appendix, Fig. S14), this is variably attained. Some taxa are characterized by females with large canines (hylobatids and some pitheciines), while others exhibit some combination of a moderately large female canine and a relatively small but still projecting male canine (callithrichids and Aotus) (Fig. 6 and Dataset S3). The canines of these taxa potentially function as weapons in territorial and/or mate competition in both sexes (see ref. 55 on competition/aggression in monogamous Aotus). The extreme degree of male canine reduction seen in the weakly dimorphic Ar. ramidus is unknown in nonhuman anthropoids with the exception of perhaps Callicebus and Brachyteles (Fig. 6) (33, 43, 44, 106–109).
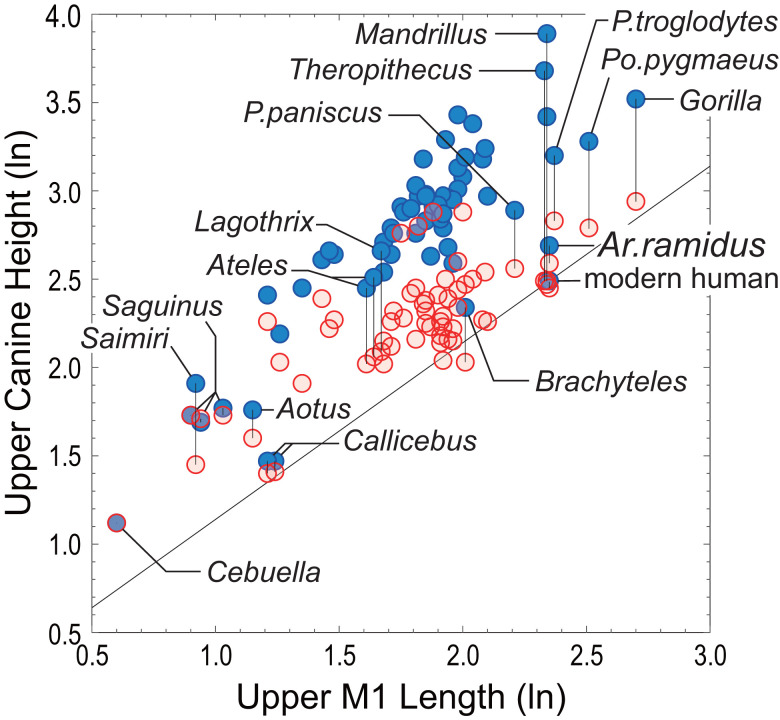
Fig. 6.
Upper canine crown height of Ar. ramidus compared with that of extant anthropoids. Mean labial crown height (y axis) plotted against mean upper first molar (M1) lengths (x axis), both in log scale. Each data point represents species and sex means: male (blue circle), female (red circle). Mean M1 lengths are combined sex so that both male and female canines can be compared in the same relative scale and because it is difficult to segregate sex in fossil taxa. The diagonal line indicates isometry passing through the mean of modern H. sapiens males (modern Japanese). The plotted data are shown in Dataset S3, and based on Suwa et al. (27) and a conservatively screened version of the Plavcan dataset (95) (SI Appendix, SI Text).
Relative to molar size, Ar. ramidus male upper canine height is decidedly shorter than in male Pan paniscus (bonobo), and broadly comparable to female extant great apes (and other female anthropoids) and male Brachyteles (Fig. 6). Brachyteles forms a male philopatric multimale/multifemale society with a polygynandrous reproductive strategy, and is known for their extreme intermale tolerance and weak aggression in competing for sexual access to receptive females (110, 111). Strier (110) considered male–female codominance as a key factor of this behavioral condition (see also ref. 112 regarding bonobos). This compares with Ateles characterized by male dominance over females and greater intra- and intergroup aggression than in Brachyteles, despite a generally low level of intragroup male–male aggression and a strong female choice component as in the other atelins (111, 113). Lagothrix is also known by male philopatry, polygynandry, and generally tolerant intermale relationships (111), but nevertheless exhibits large male canines (42, 106), moderate body size dimorphisms (114, 115), and male dominance over females (116). It is tempting to speculate that the Ar. ramidus condition was a part of a sociobehavioral complex that involved a strong inclination for male–female codominance. Under such sociobehavioral conditions, a less projecting and less dagger-like male canine could have evolved via selection mediated by female mate preference, as previously suggested (27, 51).
The available evidence of basal canine crown diameter and crown height is summarized in Fig. 7 spanning >6 million years. Although data are scarce before ∼4.5 Ma, there are now nine canines known from the 5- to 7-Ma time period attributed to Orrorin tugenensis (20, 21), Ardipithecus kadabba (19, 24, 25, 28), and Sahelanthropus tchadensis (22, 23). All are broadly equivalent to the canines of extant female chimpanzees in basal crown dimensions, but tend to be lower crowned. They are within the size range of Ar. ramidus and early Australopithecus canines, strongly suggesting that male canine reduction was already attained in these Late Miocene taxa.
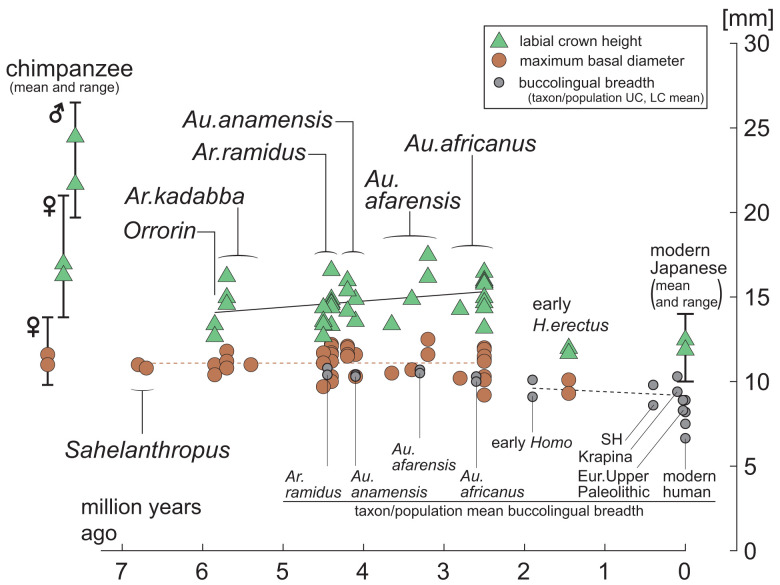
Fig. 7.
Canine crown height through time. Canine size of individual specimens with unworn crown heights are plotted: green triangles, labial height; orange circles, maximum basal diameter. Given the small sample sizes of unworn/minimally worn canines, the upper and lower canines are combined. Sample sizes are: O. tugenensis (n = 2), Ar. kadabba (n = 3, including two rough height estimates), Ar. ramidus (n = 11), Au. anamensis (n = 6), Au. afarensis (n = 4, including one rough height estimate), Au. africanus (n = 10), and Homo erectus (n = 2). The rough crown height estimates are included to indicate possible ranges. Two Sahelanthropus and one Ar. kadabba canine without crown heights are plotted. Specimen specifics are shown in Dataset S4. The gray circles are the upper and lower canine taxon/population means of buccolingual breadths (in each circle pair, the upper canine breadth is the larger). For scale, Pan troglodytes and modern human unworn canine crown heights (vertical line and green triangles), maximum diameters (vertical line and orange circles) and buccolingual breadths (gray circles) are shown at the Far Left and Right, respectively (symbol pairs are upper and lower canine means). Modern human mean buccolingual breadths are the maximum and minimum population means (of both upper and lower canines) of Dataset S1; the Upper two circles represent an Australian aborigine sample, and the Lower two circles are from a San sample. Solid and interrupted lines are the least squares best fit lines of individual crown height (black), individual maximum diameters (orange), and taxon/population mean buccolingual diameters (gray), respectively. With the current small samples, there are little apparent size changes between >6 Ma and ∼3 Ma. A slight reduction in canine size (basal diameter and height) occurred in Homo after ∼2.5 Ma.
At ∼4.5 Ma, canine sexual size dimorphism in Ar. ramidus was unambiguously weaker than in all extant great apes, including the least dimorphic bonobo. The Ar. ramidus dimorphism level was probably within or close to the modern human ranges of population variation. However, this seems to be the case more securely for the upper canines, whereas dimorphism in the lower canines may have remained somewhat greater. Canine sexual dimorphism in Australopithecus can be summarized as broadly equivalent to the Ar. ramidus condition, although it is possible that dimorphism levels in some species/populations were slightly higher. During the Pleistocene, a slight decrease of absolute canine size apparently occurred through time, together with crown structure simplification or modification, leading to the modern human condition. It is possible that this was accompanied by a slight decrease of size dimorphism, especially among lower canines. However, with the currently available fossil sample sizes, it is not possible to objectively assess the subtleties of such evolutionary trajectories. At the cruder scale of the available evidence, a drastic reduction of male canine size and projection can be hypothesized to have occurred early in human evolution, establishing minimal canine dimorphism deep in evolutionary time. Only minor perturbations in dimorphism levels occurred thereafter.
Our results considerably strengthen the idea that male canine reduction occurred very early in human evolution and broadly coincident with the adoption of bipedality (best exemplified by Ar. ramidus) (63, 65). Because Ar. ramidus and earlier fossils attributed to the human clade lack noticeable indications of postcanine expansion or enhanced mastication (18–28, 117), both classic and newer ideas (31, 34, 118) that attempted to explain initial canine reduction via tradeoffs with masticatory function are now rejected. Another long-standing explanation for canine reduction is tool use supplanting the canine as weapons in an increasingly open environment (29, 119). This notion, going back to Darwin (reviewed in ref. 120), is effectively negated by the observation that niche expansion into open habitats occurred largely after ∼4 Ma (63, 65, 121) and that complex tool technology beyond that known in nonhuman primates is routinely seen only after ∼2.5 Ma (122, 123), although the extent of organic tool use cannot generally be assessed in the fossil record.
Because relative canine size covaries with aggression and/or competition levels related with mate and/or resource acquisition among anthropoids, the canine evidence (feminized male canine shape and minimal size dimorphism) suggests a profound behavioral shift early in human evolution, and one precipitously characterized by comparatively weak levels of male–male aggression. A reduced general level of aggression can be considered an important evolutionary prerequisite for the later acquisition of enhanced interindividual cooperation and complex prosociality, attributes that are the hallmarks of our lineage (e.g., refs. 124, 125).
Materials and Methods
Datasets.
The fossil samples used in the present study are summarized in SI Appendix, Table S1. Many of the data are in common with previous work on Ar. ramidus fossils from the Middle Awash (27, 65) and Gona (26) study areas in Ethiopia. Twenty-four Ar. ramidus canines from 22 individuals are included in our metric analysis. Basal crown dimensions of eight Ar. ramidus canines are newly reported (five from Gona and three from the Middle Awash). All specimens and metrics are listed in Dataset S2 (basal crown diameters) and/or Dataset S4 (crown heights).
Metrics of a total of 170 Australopithecus and early Homo canines, attributed to Au. anamensis, Au. afarensis, Au. africanus, Au. robustus, Australopithecus boisei, and early Homo, were used in the present study. Most of these were taken on the original fossils by the two of us (T.D.W. and G.S.), and used in previous analyses of the Australopithecus garhi (126), Au. afarensis (127), Au. anamensis (7), Ar. ramidus (27), and Ardipithecus kadabba (25) dentitions. To this dataset, we added the canine metrics that subsequently became available (sources cited in Dataset S2) including some from the literature (n = 33), in which cases, methodological comparability between studies was cross-checked as necessary. For Middle Pleistocene and later Homo, in order to keep interobserver error minimal, we confined our analysis to few sources, our own metrics of H. neanderthalensis (n = 44 by T.D.W.), the Homo “heidelbergensis” Atapuerca SH sample (n = 42) in Martinón-Torres et al. (99), and the European Upper Paleolithic Homo sapiens metrics (n = 33) of Frayer (101). We also analyzed 111 fossil ape canines from five selected species, also by confining sources predominantly to our own metrics: N. kerioi (Y.K./M.N.), H. laietanus (T.D.W./G.S.), O. macedoniensis (G.S.), O. bambolii (G.S.), and G. blacki (Y.Z./R.T.K.). These were taken by the present authors adhering to the same measurement protocol, with the exception of six O. macedoniensis metrics taken from the literature (128). All specimens, metrics, and sources are listed in Dataset S2. Where necessary, notes on taxonomic treatment are spelled out in Dataset S2.
Summary statistics of modern anthropoid (including human) canine crown dimensions were used for comparisons. The modern human sample includes the upper and lower canine metrics of 23 populations, including the modern Japanese dataset (n = 89) measured by ourselves and the summary metrics of 22 populations taken from the literature. The samples, metrics, and sources are listed in Dataset S1. The modern Japanese data were also used in the canine crown height comparison across anthropoids (Dataset S3). The nonhuman anthropoid data were derived from the Plavcan dataset (95), except for the extant great ape metrics, which were taken from Suwa et al. (27).
Methods.
The maximum basal crown diameter and labial crown height of the canine were measured following established methods (7, 27) (SI Appendix, SI Text).
The pdPeak method is fully described in Sasaki et al. (62). Briefly, dimorphism is modeled by a mixture of two homoscedastic normal distributions in log scale. A balanced sex ratio in the source population (but not in the samples) is also assumed. Situations are specified by three parameters: male mean μm, female mean μf (μm≥μf), and SD σ common to the sexes (in log scale). Assuming independent uniform prior probability distributions and independent prior probabilities of 0.5 for sexes, the Bayes theorem yields the joint posterior probability distribution of P(μm,μf,σ,S∣d), where S is a vector of sex assignments and d is a vector of the log-transformed measurements. We followed the theories and procedures of MacLachlan and Peel (129), and based our Markov chain Monte Carlo (MCMC) sampling on the Gibbs sampler algorithm (130). To evaluate the posterior probabilities of the m/f ratio and within-sex variation simultaneously, a bivariate density plot of μm−μf (logarithm of m/f ratio) and σ was made from the MCMC samples. We obtained the marginal posterior probability distributions of μm−μf or σ by integrating out the other from the bivariate plot and determined its point of highest density using the expectation maximization algorithm (131). Then, we back transformed the values to normal scale: to the m/f ratio by exp [μm−μf] and to the within-sex CV (wsxCV) by exp [σ2]−1. We term these the posterior density peak (pdPeak) estimates and consider the pdPeak value to be the single best estimator of the population m/f ratio or wsxCV. The credible intervals of Bayesian inference were also determined from the marginal posterior distributions. The credible intervals shown in Fig. 3 (and in SI Appendix, Figs. S4–S11 and Dataset S2) are the 95% highest posterior density intervals (HDIs), the interval(s) enclosed by points whose probability density and above integrates to 0.95, or the likewise 68% HDIs (corresponding to ±1 SD range of a normal distribution).
We assessed the performance of the pdPeak method by using simulated (computationally generated) datasets that were sampled from hypothetical populations that conform to the model assumptions. The population m/f ratios were set to vary from 1.0 to 1.3, and wsxCVs were designated as either 5% or 8% (SI Appendix, SI Text). Results of simulation tests on deviations from assumptions are reported in ref. 62. Such effects were found to be generally slight, with the exception of a highly unbalanced sex ratio or a situation that we term a “head-to-head skew” (negative skew in females and positive skew in males). In these cases, the pdPeak m/f ratio estimates would tend to slightly underestimate (see ref. 62). Validation analyses using the known-sex samples of a wide range of extant anthropoids are detailed in ref. 62, and those on the extant great ape and modern Japanese samples are presented in SI Appendix, Figs. S12 and S13. The results of these extant sample tests were found to largely parallel the results of the computationally generated simulation tests.
Supplementary Material
Acknowledgments
We thank the Ministry of Culture and Tourism, the Authority for Research and Conservation of Cultural Heritage, the National Museum of Ethiopia, and the Afar Regional Government, Ethiopia, for permissions and facilitations; and the many individuals who have contributed to the recovery and study of the Ar. ramidus fossils. We thank the Authority for Research and Conservation of the Cultural Heritage of Ethiopia, National Museums of Kenya, Ditsong National Museum of Natural History, the Cleveland Museum of Natural History, the Royal Museum for Central Africa Tervuren, Naturalis Biodiversity Center, and B. Engesser, S. Moya-Sola, L. Rook, and G. Koufos for access and facilitation to the other materials. We thank C. Ward, J. M. Plavcan, F. Manthi, and Y. Haile-Selassie for information on Au. anamensis fossils, and J. M. Plavcan for the Plavcan anthropoid teeth dataset and related information (95). We thank the two reviewers for their constructive comments. This work was supported primarily by the Japan Society for the Promotion of Science (Kakenhi Grants 24000015 and 18H04007 to G.S.). Major grants for the Gona Project were made by the L. S. B. Leakey Foundation and European Union Marie Curie (FP7-PEOPLE-2011-CIG). The Late Miocene/Early Pliocene research was in part supported by the NSF (SBR-9910974 and RHOI BCS 0321893 to T.D.W. and F. C. Howell). Y.Z. has been supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB26000000).
Footnotes
Reviewers: R.F.K., Duke University; and P.R., Philadelphia College of Osteopathic Medicine.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2116630118/-/DCSupplemental.
Data Availability
All study data are presented in the article and/or supporting information.
References
- 1.Dart R. A., The dentition of Australopithecus africanus. Folia Anatomica Japonica 12, 207–221 (1934). [Google Scholar]
- 2.Robinson J. T., The Dentition of the Australopithecinae (Transvaal Museum Memoir No. 9, Transvaal Museum, Pretoria, South Africa, 1956). [Google Scholar]
- 3.Johanson D. C., White T. D., A systematic assessment of early African hominids. Science 203, 321–330 (1979). [DOI] [PubMed] [Google Scholar]
- 4.White T. D., Johanson D. C., Kimbel W. H., Australopithecus africanus: Its phyletic position reconsidered. S. Afr. J. Sci. 77, 445–470 (1981). [Google Scholar]
- 5.Leakey M. G., Feibel C. S., McDougall I., Walker A., New four-million-year-old hominid species from Kanapoi and Allia Bay, Kenya. Nature 376, 565–571 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Leakey M. G., Feibel C. S., McDougall I., Ward C., Walker A., New specimens and confirmation of an early age for Australopithecus anamensis. Nature 393, 62–66 (1998). [DOI] [PubMed] [Google Scholar]
- 7.White T. D., et al. , Asa Issie, Aramis and the origin of Australopithecus. Nature 440, 883–889 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Kimbel W. H., Delezene L. K., “Lucy” redux: A review of research on Australopithecus afarensis. Am. J. Phys. Anthropol. 140 (suppl. 49), 2–48 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Kay R. F., Sexual dimorphism in Ramapithecinae. Proc. Natl. Acad. Sci. U.S.A. 79, 209–212 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimbel W. H., White T. D., “Variation, sexual dimorphism and the taxonomy of Australopithecus” in Evolutionary History of the “Robust” Australopithecines, Grine F. E., Ed. (Aldine de Gruyter, New York, NY, 1988), pp. 175–192. [Google Scholar]
- 11.Leutenegger W., Shell B., Variability and sexual dimorphism in canine size of Australopithecus and extant hominoids. J. Hum. Evol. 16, 359–367 (1987). [Google Scholar]
- 12.Frayer D. W., Wolpoff M. H., Sexual dimorphism. Annu. Rev. Anthropol. 14, 429–473 (1985). [Google Scholar]
- 13.Ward C. V., Leakey M. G., Walker A., Morphology of Australopithecus anamensis from Kanapoi and Allia Bay, Kenya. J. Hum. Evol. 41, 255–368 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Plavcan J. M., Sexual dimorphism in primate evolution. Am. J. Phys. Anthropol. 44 (suppl. 33), 25–53 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Manthi F. K., Plavcan J. M., Ward C. V., New hominin fossils from Kanapoi, Kenya, and the mosaic evolution of canine teeth in early hominins. S. Afr. J. Sci. 108, 1–9 (2012). [Google Scholar]
- 16.Ward C. V., Manthi F. K., Plavcan J. M., New fossils of Australopithecus anamensis from Kanapoi, West Turkana, Kenya (2003-2008). J. Hum. Evol. 65, 501–524 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Ward C. V., Plavcan J. M., Manthi F. K., New fossils of Australopithecus anamensis from Kanapoi, West Turkana, Kenya (2012-2015). J. Hum. Evol. 140, 102368 (2020). [DOI] [PubMed] [Google Scholar]
- 18.White T. D., Suwa G., Asfaw B., Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature 371, 306–312 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Haile-Selassie Y., Late Miocene hominids from the Middle Awash, Ethiopia. Nature 412, 178–181 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Senut B., et al. , First hominid from the Miocene (Lukeino Formation, Kenya). C. R. Acad. Sci. 332, 137–144 (2001). [Google Scholar]
- 21.Senut B., Pickford M., Gommery D., Dental anatomy of the early hominid, Orrorin tugenensis, from the Lukeino Formation, Tugen Hills, Kenya. Rev. Paleobiol. 37, 577–591 (2018). [Google Scholar]
- 22.Brunet M., et al. , A new hominid from the Upper Miocene of Chad, Central Africa. Nature 418, 145–151 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Brunet M., et al. , New material of the earliest hominid from the Upper Miocene of Chad. Nature 434, 752–755 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Haile-Selassie Y., Suwa G., White T. D., Late Miocene teeth from Middle Awash, Ethiopia, and early hominid dental evolution. Science 303, 1503–1505 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Haile-Selassie Y., Suwa G., White T., “Hominidae” in Ardipithecus kadabba. Late Miocene Evidence from the Middle Awash, Ethiopia, Haile-Selassie Y., WoldeGabriel G., Eds. (University of California Press, Berkeley, CA, 2009), pp. 159–236. [Google Scholar]
- 26.Semaw S., et al. , Early Pliocene hominids from Gona, Ethiopia. Nature 433, 301–305 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Suwa G., et al. , Paleobiological implications of the Ardipithecus ramidus dentition. Science 326, 94–99 (2009). [PubMed] [Google Scholar]
- 28.Simpson S. W., et al. , Late Miocene hominin teeth from the Gona Paleoanthropological Research Project area, Afar, Ethiopia. J. Hum. Evol. 81, 68–82 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Washburn S. L., Tools and human evolution. Sci. Am. 203, 63–75 (1960). [PubMed] [Google Scholar]
- 30.Holloway R. L., Tools and teeth: Some speculations regarding canine reduction. Am. Anthropol. 69, 63–67 (1967). [Google Scholar]
- 31.Jolly C. J., The seed-eaters: A new model of hominid differentiation based on a baboon analogy. Man (Lond.) 5, 5–26 (1970). [Google Scholar]
- 32.Kinzey W. G., Evolution of the human canine tooth. Am. Anthropol. 73, 680–694 (1971). [Google Scholar]
- 33.Kinzey W. G., Canine teeth of the monkey, Callicebus moloch: Lack of sexual dimorphism. Primates 13, 365–369 (1972). [DOI] [PubMed] [Google Scholar]
- 34.Jungers W. L., On canine reduction in early hominids. Curr. Anthropol. 19, 155–156 (1978). [Google Scholar]
- 35.Leutenegger W., Kelly J. T., Relationship of sexual dimorphism in canine size and body size to social, behavioral, and ecological correlates in anthropoid primates. Primates 18, 117–136 (1977). [Google Scholar]
- 36.Harvey P. H., Kavanagh M., Clutton-Brock T. H., Sexual dimorphism in primate teeth. J. Zool. 186, 474–485 (1978). [Google Scholar]
- 37.Lovejoy C. O., The origin of man. Science 211, 341–350 (1981). [DOI] [PubMed] [Google Scholar]
- 38.Smith R. J., Interspecific scaling of maxillary canine size and shape in female primates: Relationships to social structure and diet. J. Hum. Evol. 10, 165–173 (1981). [Google Scholar]
- 39.Leutenegger W., Scaling of sexual dimorphism in body weight and canine size in primates. Folia Primatol. (Basel) 37, 163–176 (1982). [DOI] [PubMed] [Google Scholar]
- 40.Kay R. F., Plavcan J. M., Glander K. E., Wright P. C., Sexual selection and canine dimorphism in New World monkeys. Am. J. Phys. Anthropol. 77, 385–397 (1988). [DOI] [PubMed] [Google Scholar]
- 41.Greenfield L. O., Washburn A., Polymorphic aspects of male anthropoid canines. Am. J. Phys. Anthropol. 84, 17–34 (1991). [DOI] [PubMed] [Google Scholar]
- 42.Plavcan J. M., van Schaik C. P., Intrasexual competition and canine dimorphism in anthropoid primates. Am. J. Phys. Anthropol. 87, 461–477 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Plavcan J. M., van Schaik C. P., Interpreting hominid behavior on the basis of sexual dimorphism. J. Hum. Evol. 32, 345–374 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Plavcan J. M., van Schaik C. P., Kappeler P. M., Competition, coalitions and canine size in primates. J. Hum. Evol. 28, 245–276 (1995). [Google Scholar]
- 45.McGraw W. S., Plavcan J. M., Adachi-Kanazawa K., Adult female Cercopithecus diana employ canine teeth to kill another adult female C. diana. Int. J. Primatol. 23, 1301–1308 (2002). [Google Scholar]
- 46.Reno P. L., Meindl R. S., McCollum M. A., Lovejoy C. O., Sexual dimorphism in Australopithecus afarensis was similar to that of modern humans. Proc. Natl. Acad. Sci. U.S.A. 100, 9404–9409 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reno P. L., Lovejoy C. O., From Lucy to Kadanuumuu: Balanced analyses of Australopithecus afarensis assemblages confirm only moderate skeletal dimorphism. PeerJ 3, e925 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixson A., Dixson B., Anderson M., Sexual selection and the evolution of visually conspicuous sexually dimorphic traits in male monkeys, apes, and human beings. Annu. Rev. Sex Res. 16, 1–19 (2005). [PubMed] [Google Scholar]
- 49.Thorén S., Lindenfors P., Kappeler P. M., Phylogenetic analyses of dimorphism in primates: Evidence for stronger selection on canine size than on body size. Am. J. Phys. Anthropol. 130, 50–59 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Leigh S. R., Setchell J. M., Charpentier M., Knapp L. A., Wickings E. J., Canine tooth size and fitness in male mandrills (Mandrillus sphinx). J. Hum. Evol. 55, 75–85 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Lovejoy C. O., Reexamining human origins in light of Ardipithecus ramidus. Science 326, 74e1–74e8 (2009). [PubMed] [Google Scholar]
- 52.Grueter C. C., Van Schaik C. P., Sexual size dimorphism in Asian colobines revisited. Am. J. Primatol. 71, 609–616 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Plavcan J. M., Understanding dimorphism as a function of changes in male and female traits. Evol. Anthropol. 20, 143–155 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Plavcan J. M., Sexual size dimorphism, canine dimorphism, and male-male competition in primates: Where do humans fit in? Hum. Nat. 23, 45–67 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Duque E., Huck M., Till death (or an intruder) do us part: Intrasexual-competition in a monogamous primate. PLoS One 8, e53724 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galbany J., Tung J., Altmann J., Alberts S. C., Canine length in wild male baboons: Maturation, aging and social dominance rank. PLoS One 10, e0126415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clutton-Brock T., Social evolution in mammals. Science 373, eabc9699 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Palombit R. A., Lethal territorial aggression in a white-handed gibbon. Am. J. Primatol. 31, 311–318 (1993). [DOI] [PubMed] [Google Scholar]
- 59.Watts D. P., Muller M., Amsler S. J., Mbabazi G., Mitani J. C., Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am. J. Primatol. 68, 161–180 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Kaburu S. S. K., Inoue S., Newton-Fisher N. E., Death of the alpha: Within-community lethal violence among chimpanzees of the Mahale Mountains National Park. Am. J. Primatol. 75, 789–797 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Marzec A. M., et al. , The dark side of the red ape: Male-mediated lethal female competition in Bornean orangutans. Behav. Ecol. Sociobiol. 70, 459–466 (2016). [Google Scholar]
- 62.Sasaki T., et al. , Estimating sexual size dimorphism in fossil species from posterior probability densities. Proc. Natl. Acad. Sci. U.S.A. 118, e2113943118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White T. D., et al. , Ardipithecus ramidus and the paleobiology of early hominids. Science 326, 75–86 (2009). [PubMed] [Google Scholar]
- 64.Simpson S. W., Levin N. E., Quade J., Rogers M. J., Semaw S., Ardipithecus ramidus postcrania from the Gona Project area, Afar regional state, Ethiopia. J. Hum. Evol. 129, 1–45 (2019). [DOI] [PubMed] [Google Scholar]
- 65.White T. D., Lovejoy C. O., Asfaw B., Carlson J. P., Suwa G., Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proc. Natl. Acad. Sci. U.S.A. 112, 4877–4884 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelley J., Sexual dimorphism in canine shape among extant great apes. Am. J. Phys. Anthropol. 96, 365–389 (1995). [DOI] [PubMed] [Google Scholar]
- 67.Kelley J., Sex determination in miocene catarrhine primates. Am. J. Phys. Anthropol. 96, 391–417 (1995). [DOI] [PubMed] [Google Scholar]
- 68.Kelley J., Xu Q. H., Extreme sexual dimorphism in a Miocene hominoid. Nature 352, 151–153 (1991). [DOI] [PubMed] [Google Scholar]
- 69.Kelley J., Plavcan J. M., A simulation test of hominoid species number at Lufeng, China: Implications for the use of the coefficient of variation in paleotaxonomy. J. Hum. Evol. 35, 577–596 (1998). [DOI] [PubMed] [Google Scholar]
- 70.Kelley J., Andrews P., Alpagut B., A new hominoid species from the middle Miocene site of Paşalar, Turkey. J. Hum. Evol. 54, 455–479 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Ishida H., Mbua E., Nakano Y., Yasui K., “Sexual dimorphism in canine size of Kenyapithecus from Nachola” in Primatology Today, Ehara A., Kimura T., Takenaka O., Iwamoto M., Eds. (Elsevier, Amsterdam, 1991), pp. 517–520. [Google Scholar]
- 72.Kikuchi Y., et al. , Sexual dimorphism of body size in an African fossil ape, Nacholapithecus kerioi. J. Hum. Evol. 123, 129–140 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Schrein C. M., Metric variation and sexual dimorphism in the dentition of Ouranopithecus macedoniensis. J. Hum. Evol. 50, 460–468 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y., Harrison T., Gigantopithecus blacki: A giant ape from the Pleistocene of Asia revisited. Am. J. Phys. Anthropol. 162 (suppl. 63), 153–177 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Kay R. F., Sivapithecus simonsi, a new species of Miocene hominoid, with comments on the phylogenetic status of Ramapithecinae. Int. J. Primatol. 3, 113–173 (1982). [Google Scholar]
- 76.Kelley J., Misconceptions arising from the misassignment of non-hominoid teeth to the Miocene hominoid Sivapithecus. Palaeontol. Electronica 8, 16A (2005). [Google Scholar]
- 77.Pickford M., Senut B., Gommery D., Musiime E., Distinctiveness of Ugandapithecus from Proconsul. Estudios Geológicos 65, 183–241 (2009). [Google Scholar]
- 78.Bhandari A., et al. , First record of the Miocene hominoid Sivapithecus from Kutch, Gujarat state, western India. PLoS One 13, e0206314 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Almquist A. J., Sexual differences in the anterior dentition in African primates. Am. J. Phys. Anthropol. 40, 359–367 (1974). [DOI] [PubMed] [Google Scholar]
- 80.Walker A., Mechanisms of honing in the male baboon canine. Am. J. Phys. Anthropol. 65, 47–60 (1984). [DOI] [PubMed] [Google Scholar]
- 81.Schwartz G. T., Reid D. J., Dean C., Developmental aspects of sexual dimorphism in hominoid canines. Int. J. Primatol. 22, 837–860 (2001). [Google Scholar]
- 82.Zingeser M. R., Cercopithecoid canine tooth honing mechanisms. Am. J. Phys. Anthropol. 31, 205–213 (1969). [DOI] [PubMed] [Google Scholar]
- 83.Greenfield L. O., Washburn A., Polymorphic aspects of male anthropoid honing premolars. Am. J. Phys. Anthropol. 87, 173–186 (1992). [DOI] [PubMed] [Google Scholar]
- 84.Delezene L. K., Modularity of the anthropoid dentition: Implications for the evolution of the hominin canine honing complex. J. Hum. Evol. 86, 1–12 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Kieser J. A., Human Adult Odontometrics (Cambridge University Press, Cambridge, UK, 1990). [Google Scholar]
- 86.Frisch K., Sex-differences in the canines of the gibbon (Hylobates lar). Primates 4, 1–10 (1963). [Google Scholar]
- 87.Plavcan J. M., Comparison of four simple methods for estimating sexual dimorphism in fossils. Am. J. Phys. Anthropol. 94, 465–476 (1994). [DOI] [PubMed] [Google Scholar]
- 88.Godfrey L. R., Lyon S. K., Sutherland M. R., Sexual dimorphism in large-bodied primates: The case of the subfossil lemurs. Am. J. Phys. Anthropol. 90, 315–334 (1993). [DOI] [PubMed] [Google Scholar]
- 89.Josephson S. C., Juell K. E., Rogers A. R., Estimating sexual dimorphism by method-of-moments. Am. J. Phys. Anthropol. 100, 191–206 (1996). [DOI] [PubMed] [Google Scholar]
- 90.Kościński K., Pietraszewski S., Methods to estimate sexual dimorphism from unsexed samples: A test with computer generated samples. Anthropol. Rev. 67, 33–55 (2004). [Google Scholar]
- 91.Lovejoy C. O., Kern K. F., Simpson S. W., Meindl R. S., “A new method for estimation of skeletal dimorphism in fossil samples with an application to Australopithecus afarensis” in Hominidae, Giacobini G., Ed. (Jaca Book, Milano, Italy, 1989), pp. 103–108. [Google Scholar]
- 92.Gingerich P. D., Schoeninger M. J., Patterns of tooth size variability in the dentition of primates. Am. J. Phys. Anthropol. 51, 457–465 (1979). [DOI] [PubMed] [Google Scholar]
- 93.Leutenegger W., Metric variability in the anterior dentition of African colobines. Am. J. Phys. Anthropol. 45, 45–51 (1976). [DOI] [PubMed] [Google Scholar]
- 94.Cope D. A., “Systematic variation in Cercopithecus dental samples,” PhD dissertation, University of Texas at Austin, Austin, TX (1989).
- 95.Plavcan J. M., “Sexual dimorphism in the dentition of extant anthropoid primates,” PhD dissertation, Duke University, Durham, NC (1990).
- 96.Martin R. D., Willner L. A., Dettling A., “The evolution of sexual size dimorphism in primates” in The Differences Between the Sexes, Short R. V., Balaban E., Eds. (Cambridge University Press, Cambridge, UK, 1994), pp. 159–200. [Google Scholar]
- 97.Rehg J. A., Leigh S. R., Estimating sexual dimorphism and size differences in the fossil record: A test of methods. Am. J. Phys. Anthropol. 110, 95–104 (1999). [DOI] [PubMed] [Google Scholar]
- 98.Bermúdez de Castro J. M., Sarmiento S., Cunha E., Rosas A., Bastir M., Dental size variation in the Atapuerca-SH Middle Pleistocene hominids. J. Hum. Evol. 41, 195–209 (2001). [DOI] [PubMed] [Google Scholar]
- 99.Martinón-Torres M., Bermúdez de Castro J. M., Gómez-Robles A., Prado-Simón L., Arsuaga J. L., Morphological description and comparison of the dental remains from Atapuerca-Sima de los Huesos site (Spain). J. Hum. Evol. 62, 7–58 (2012). [DOI] [PubMed] [Google Scholar]
- 100.García-Campos C., et al. , Sexual dimorphism of the enamel and dentine dimensions of the permanent canines of the Middle Pleistocene hominins from Sima de los Huesos (Burgos, Spain). J. Hum. Evol. 144, 102793 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Frayer D. W., Evolution of the dentition in Upper Paleolithic and Mesolithic Europe. Univ. Kansas Publ. Anthropol. 10, 1–201 (1978). [Google Scholar]
- 102.Ramirez-Rozzi F. V., Romero A., Tooth dimensions and body size in a Pygmy population. Ann. Hum. Biol. 46, 467–474 (2019). [DOI] [PubMed] [Google Scholar]
- 103.Plavcan J. M., Ward C. V., Paulus F. L., Estimating canine tooth crown height in early Australopithecus. J. Hum. Evol. 57, 2–10 (2009). [DOI] [PubMed] [Google Scholar]
- 104.Schwartz G. T., Dean C., Ontogeny of canine dimorphism in extant hominoids. Am. J. Phys. Anthropol. 115, 269–283 (2001). [DOI] [PubMed] [Google Scholar]
- 105.Leigh S. R., Setchell J. M., Buchanan L. S., Ontogenetic bases of canine dimorphism in anthropoid primates. Am. J. Phys. Anthropol. 127, 296–311 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Zingeser M. R., Dentition of Brachyteles arachnoides with reference to Alouattine and Atelinine affinities. Folia Primatol. (Basel) 20, 351–390 (1973). [DOI] [PubMed] [Google Scholar]
- 107.Rosenberger A. L., Strier K. B., Adaptive radiation of the ateline primates. J. Hum. Evol. 18, 717–750 (1989). [Google Scholar]
- 108.Lemos de Sá R. M., Pope T. R., Struhsaker T. T., Glander K. E., Sexual dimorphism in canine length of woolly spider monkeys (Brachyteles arachnoides, E. Geoffroy 1806). Int. J. Primatol. 14, 755–763 (1993). [Google Scholar]
- 109.Leigh S. R., Jungers W. L., A re-evaluation of subspecific variation and canine dimorphism in woolly spider monkeys (Brachyteles arachnoides). Am. J. Phys. Anthropol. 95, 435–442 (1994). [DOI] [PubMed] [Google Scholar]
- 110.Strier K. B., “Causes and consequences of nonagression in the wooly spider monkey, or muriqui (Brachyteles arachnoides)” in Aggression and Peacefulness in Humans and other Primates, Silverberg J., Gray J. P., Eds. (Oxford University Press, New York, NY, 1992), pp. 100–116. [Google Scholar]
- 111.Di Fiore A., Campbell C. J., “The atelines: Variation in ecology, behavior, and social organization” in Primates in Perspective, Campbell C. J., Fuentes A., MacKinnon K. C., Panger M., Bearder S. K., Eds. (Oxford University Press, New York, NY, 2007), pp. 155–185. [Google Scholar]
- 112.Tokuyama N., Sakamaki T., Furuichi T., Inter-group aggressive interaction patterns indicate male mate defense and female cooperation across bonobo groups at Wamba, Democratic Republic of the Congo. Am. J. Phys. Anthropol. 170, 535–550 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Link A., Di Fiore A., Spehar S. N., “Female–directed aggression and social control in spider monkeys” in Sexual Coercion in Primates and Humans, Muller M. N., Wrangham R. W., Eds. (Harvard University Press, Cambridge, MA, 2009), pp. 157–183. [Google Scholar]
- 114.Napier P. H., Catalogue of Primates in the British Museum (Natural History) Part 1: Families Callithrichidae and Cebidae (British Museum Natural History, London, UK, 1981). [Google Scholar]
- 115.Ford S. M., Davis L. C., Systematics and body size: Implications for feeding adaptations in New World monkeys. Am. J. Phys. Anthropol. 88, 415–468 (1992). [DOI] [PubMed] [Google Scholar]
- 116.Di Fiore A., Fleischer R. C., Social behavior, reproductive strategies, and population genetic structure of Lagothrix poeppigii. Int. J. Primatol. 26, 1137–1173 (1995). [Google Scholar]
- 117.Suwa G., et al. , Ardipithecus ramidus skull and its implications for hominid origins. Science 326, 68e1–68e7. [PubMed] [Google Scholar]
- 118.Hylander W. L., Functional links between canine height and jaw gape in catarrhines with special reference to early hominins. Am. J. Phys. Anthropol. 150, 247–259 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Washburn S. L., Ciochon R. L., Canine teeth: Notes on controversies in the study of human evolution. Am. Anthropol. 76, 765–784 (1974). [Google Scholar]
- 120.White T. D., “Human evolution: How has Darwin done?” in Evolution Since Darwin: The First 150 Years, Bell M. A., Futuyma D. J., Eanes W. F., Levinton J. S., Eds. (Sinauer, Sunderland, MA, 2010), pp. 519–560. [Google Scholar]
- 121.Sponheimer M., et al. , Isotopic evidence of early hominin diets. Proc. Natl. Acad. Sci. U.S.A. 110, 10513–10518 (2013). [Google Scholar]
- 122.Semaw S., The world’s oldest stone artefacts from Gona, Ethiopia: Their implications for understanding stone technology and patterns of human evolution between 2.6–1.5 million years ago. J. Archaeol. Sci. 27, 1197–1214 (2000). [Google Scholar]
- 123.Stout D., Rogers M. J., Jaeggi A. V., Semaw S., Archaeology and the origins of human cumulative culture. Curr. Anthropol. 60, 309–340 (2019). [Google Scholar]
- 124.Layland K. N., Darwin’s Unfinished Symphony: How Culture Made the Human Mind (Princeton University Press, Princeton, NJ, 2017). [Google Scholar]
- 125.Ihara Y., A mathematical model of social selection favoring reduced aggression. Behav. Ecol. Sociobiol. 74, 91 (2020). [Google Scholar]
- 126.Asfaw B., et al. , Australopithecus garhi: A new species of early hominid from Ethiopia. Science 284, 629–635 (1999). [DOI] [PubMed] [Google Scholar]
- 127.White T. D., Suwa G., Simpson S., Asfaw B., Jaws and teeth of Australopithecus afarensis from Maka, Middle Awash, Ethiopia. Am. J. Phys. Anthropol. 111, 45–68 (2000). [DOI] [PubMed] [Google Scholar]
- 128.Koufos G. D., de Bonis L., Kugiumtzis D., New material of the hominoid Ouranopithecus macedoniensis from the Late Miocene of the Axios Valley (Macedonia, Greece) with some remarks on its sexual dimorphism. Folia Primatol. (Basel) 87, 94–122 (2016). [DOI] [PubMed] [Google Scholar]
- 129.MacLachlan G. J., Peel D., Finite Mixture Models (John Wiley & Sons, New York, NY, 2000). [Google Scholar]
- 130.Gilks W. R., Richardson S., Spiegelhalter D. J., Eds., Markov Chain Monte Carlo in Practice (Chapman & Hall, London, UK, 1996). [Google Scholar]
- 131.MacLachlan G. J., Krishnan T., The EM Algorithm and Extensions (John Wiley & Sons, New York, NY, ed. 2, 2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are presented in the article and/or supporting information.