Curbing climate change goes hand-in-hand with decarbonizing energy production. But how can communities continue to meet the global demand for electricity without releasing more CO2? A cadre of chemists says one solution may be hiding in an unlikely source: ammonia—the pungent, clear, nitrogen-rich gas, or liquid, that’s most often used as an agricultural fertilizer. Most of the ammonia produced in the world goes to crops, with the rest ending up in plastics, explosives, fabrics, and other materials.
Ammonia is appealing as a means of storing and transporting energy, and it could offer a way to package and store hydrogen. But the conventional process for making ammonia at plants like this one involves liberating hydrogen from the hydrocarbons in fossil fuels and hence results in CO2 emissions. Image credit: Shutterstock/saoirse2013.
But to researchers looking for carbon-neutral energy, ammonia makes an appealing fuel. Made of nitrogen and hydrogen, it burns without releasing carbon. [In 2014, Japanese researchers unveiled the first turbine powered by ammonia combustion (1).] Ammonia is also appealing as a way to store energy that’s transported from where it’s made to where it’s needed. And some researchers say that ammonia could be used to package and store hydrogen, which could be readily cracked out of the liquid or gas and used in fuel cells.
And yet, several challenges remain if ammonia is to help with the globe’s massive carbon emissions conundrum. The conventional process for making ammonia involves liberating hydrogen from the hydrocarbons in fossil fuels, often methane, which adds CO2 to the atmosphere. By some estimates, ammonia production is responsible for roughly 2% of fossil fuel use, worldwide, and releases more than 400 millions of tons of CO2, representing more than 1% of total annual global emissions of the greenhouse gas. Those numbers aren’t likely to fall: Agricultural demand for ammonia is predicted to double in the next few decades. In addition, most ammonia applications can trigger side reactions that produce nitrogen oxide compounds, which are potent greenhouse gases that trap more heat than CO2. Ammonia-burning engines would require catalytic systems like those long used in other power plants to capture and reuse those gases, notes Douglas Macfarlane at Monash University in Melbourne, Australia.
Even if it’s made without carbon, ammonia has to be carefully managed to avoid worsening climate change in other ways. Much of the nitrogen in today’s ammonia-based fertilizers ends up in runoff; if too much ends up in a stream, for example, it can cause a widespread fish kill. Denitrificiation—part of the nitrogen cycle in which soil microbes convert nitrites and nitrates into gas—can produce nitrogen oxides.
There are also safety concerns to address. Ammonia is dangerous when inhaled. If ammonia is to play an outsized role in the future energy economy, then researchers need to ensure that it can be handled, transported, and used safely, says Macfarlane. Plus, a major scaling effort will be necessary, because ammonia-producing technologies that work in the laboratory will have to be adapted to real-world settings.
But in principle, these challenges have solutions, and ammonia could serve as both fuel and energy carrier. Chemists and engineers have started to develop ways to make ammonia without using fossil fuels, instead relying on electrochemical reactions or electrolysis. Promising results from pilot projects, such as a wind-to-ammonia plant in Minnesota, or models of a wind-and-solar-to-ammonia plant by researchers in Finland, suggest that ammonia can be made cleanly on large scales. “There’s a whole kind of space race happening at the moment in the area of green ammonia,” says chemical engineer Patrick “PJ” Cullen at the University of Sydney, Australia.
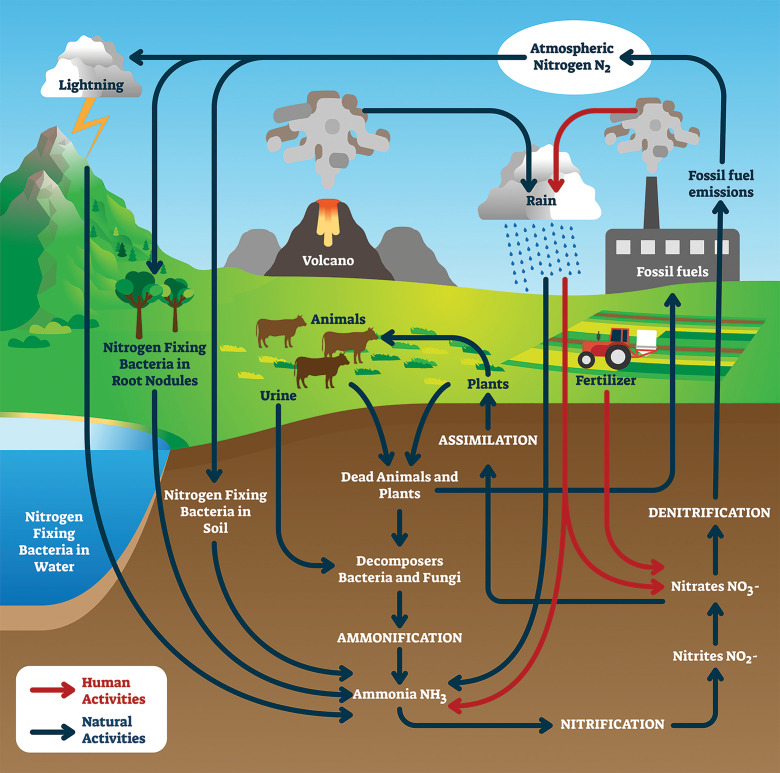
The nitrogen cycle entails the fixation of atmospheric nitrogen, typically via lightning, microbes, or the addition of synthetic fertilizers. This can produce nitrates, nitrites, or ammonia that aids in crop production. Green ammonia adds fertilizer to the cycle without releasing nearly as much greenhouse gas. Image credit: Shutterstock/VectorMine.
The Farm and Beyond
Already, ammonia is a mainstay of farms. That’s where Cullen, who is developing new technologies for producing ammonia by using nonthermal, atmospheric plasmas, would like to see future ammonia plants. Instead of buying and storing fertilizer, Cullen says, farmers could make their own, onsite, when they need it (ideally using wind or some other renewable energy). What they don’t use for fertilizer they could use as fuel—for corn dryers or to power tractors, for example.
“This is the concept of sector coupling—of bringing agriculture and power generation together,” says Mike Reese, who directs the renewable energy program at the University of Minnesota West Central Research and Outreach Center, in Morris. The program has been running a US Department of Energy-funded pilot project for converting wind power to ammonia. “When you need power and the wind isn’t blowing in July or August, you’d have ammonia.”
Ammonia-based fertilizer didn’t become a major player in agriculture until just after the turn of the 20th century, when a pair of German chemists found a new way to make lots of it. This chemical reaction, named the Haber-Bosch process after its inventors, forever changed the world. Now, ammonia is used globally as a fertilizer because it provides an efficient way to deliver nitrogen—critical for making chlorophyll, among other roles—to plants. The Haber-Bosch process allowed ammonia to be produced on large scales and shipped anywhere; as a result, crop yields soared.
In the air, nitrogen atoms pair up. The Haber-Bosch process removes those molecules of nitrogen and breaks the bond between them. Though a series of steps, it then couples those nitrogen atoms to hydrogen extracted from oil, coal, or natural gas in an industrial chemical reaction that uses metal—usually iron—as a catalyst. The process requires a temperature of more than 400 °C and pressures at or exceeding 200 atmospheres. That means it’s carbon expensive, releasing 1–3 tons of CO2 into the atmosphere for every ton of usable ammonia (2). Although improvements in the process have led to more energy-efficient production, researchers predict that at current rates the global production of ammonia will likely double in the next 30 y, from about 150 million metric tons in 2019–350 million by 2050 (2).
The solution isn’t to abandon ammonia, argues Macfarlane, but rather to make it more environmentally friendly. Some researchers tout carbon sequestration, meaning capturing and storing CO2 produced by the Haber-Bosch process. Carbon capture technology for power plants has been around since the 1970s, but large-scale attempts to implement these systems often run into financial or logistical challenges. Worldwide, two-thirds of the 149 carbon capture systems that had been proposed to go online by 2020 have been abandoned or indefinitely delayed. Progress has been slow, despite tens of billions of dollars invested into sequestration projects over the last 20 y, which means it may not be feasible for ammonia plants, either (3). (Within the ammonia industry, conventional ammonia is called “gray,” and ammonia with carbon capture is classified as “blue.”)
A second option, Macfarlane says, is to extract hydrogen from something other than a greenhouse gas like methane. Water might be that source, using electrolysis to split water molecules. But electrolysis is very expensive. Even so, plants have announced plans to install electrolyzers in some ammonia plants.
Seeking Alternatives
A third option, phased in gradually over time, could be more appealing in the long run: Abandon the Haber-Bosch process entirely and find other ways to fix nitrogen with renewable energy. This is the dream of “green” ammonia. “That’s the option that’s a bit further out but would ultimately be the most flexible,” says Macfarlane. “Ammonia could be produced in much smaller units, even located on individual farms.”
That suggests, for example, that fertilizer could be produced on-site and on-demand, which Macfarlane says would lessen the costs, both financial and environmental, associated with transporting and storing ammonia. At the same time, he acknowledges a paucity of research on how distributed green ammonia production (and use) will affect emissions of NH3 and nitrogen oxides, as well as on how these chemicals are processed in the planetary nitrogen cycle. Losses are inevitable, he says, and a better understanding of those processes and cycles is necessary to find and fix inefficiencies that could produce additional greenhouse gases.
One way to sidestep Haber-Bosch is through the electrochemical nitrogen reduction reaction, or ENRR, which fuses hydrogen from water and nitrogen gas from the air to produce ammonia. The ammonia yield from ENRR is lower than that of Haber-Bosch, but several studies published in the last two years suggest that new metal catalysts and approaches, based on renewable energy sources, could improve on current performance (4, 5).
“This process hasn’t been working efficiently in existing approaches,” Macfarlane says. “There are a lot of side reactions that tend to happen at the same time.” In an article published in June, however, his group reported on an efficient, productive approach to ENRR using a new kind of proton shuttle—the molecule that conveys the hydrogen ion from the source to the ammonia. Previous approaches, he says, required “sacrificial” molecules that, after releasing the proton, would go on to trigger those unwanted side effects. His group found a new carrier that could reverse course and be recycled in the cell, to carry more protons (6).
ENRR is promising in general, says Cullen, because it can be carried out at room temperature and ordinary pressures, it’s readily adaptable to renewable energy sources, and it can be used on small scales. But the process is still hindered by low rates, partly because atmospheric nitrogen molecules become unstable when they’re broken apart. In an article published in January, Cullen proposes a different way to source the nitrogen: using cold plasma (7).
Plasma can be generated by applying an electrical current through a gas, including the air in the atmosphere. The high voltage liberates electrons from their atoms, creating a mix of ions, charges, and neutral atoms. Nitrogen ions are highly reactive—they’re called “reactive species”—and can readily combine with hydrogen to form ammonia. The power required to make the plasma, Cullen says, could come from a wind turbine. He envisions a future farm with its own wind-powered, plasma-based ammonia plant, capable of producing fertilizer and liquid energy—to be used on site or shipped elsewhere.
“The whole goal is to make it locally, on a farm,” says Cullen. “But then you also use it directly as a fuel. In your tractors.”
An Ammonia Pipeline
In 2010, the University of Minnesota launched a state-funded pilot project, in Morris, that used a turbine to convert wind power into hydrogen; in 2013 they added a process that could capture and split nitrogen molecules to start making ammonia. “We believe it was the first wind-to-ammonia plant in the world at the time,” says Reese, who has been involved since the beginning. Now, there are at least three similar plants around the world, all experimental, and several planned commercial projects. The DOE is also funding a larger, next-generation pilot project by the university that converts wind and solar energy into ammonia.
The Great Plains offers a natural testing ground for the technology, says Reese. An area of favorable winds overlaps with the part of the country where most corn is grown. In the spring and summer wind-generated ammonia could be used as fertilizer, and during the remainder of the year it could be generated andstored, or used as a hydrogen carrier in power generation or for producing thermal energy.
Areas like western Minnesota and the Dakotas have huge wind resources but only limited power lines to shuttle that power to urban areas, Reese notes. “We can pretty dramatically reduce the carbon intensity of farming by using ammonia as a fuel,” he says.
Building an ammonia infrastructure won’t mean starting from scratch, however. Today’s resources could be repurposed, Reese points out. More than 100 ports around the world already have facilities for transporting and loading ammonia, and some natural gas terminals could be of some use, according to the Ammonia Energy Association and researchers from the firm Black & Vietch.
A major test of the ammonia potential will play out in the northeast kingdom of Saudi Arabia. Last year, an international group of energy companies announced they would build a $5 billion green ammonia plant in NEOM, a planned zero-carbon city that began construction in early 2021. The plant will use electrolysis, powered by solar and wind sources, to produce 1.2 megatons of ammonia annually, in addition to 650 daily tons of hydrogen.
Last year, Macfarlane and his colleagues published a “roadmap” describing how renewable, green ammonia might be produced at efficient, economic scales that could significantly decrease our dependence on carbon-based fuels, both as fertilizer and as fuel source (8). “Scaling up is definitely the next step,” he says, whether it’s in testing new technologies or adapting existing plants to go green.
References
- 1.Iki N., et al. , “Micro gas turbine firing kerosene and ammonia” in Proceedings of the ASME Turbo Expo 2015: Turbine Technical Conference and Exposition. Volume 8: Microturbines, Turbochargers and Small Turbomachines; Steam Turbines (American Society of Mechanical Engineers, 2015), p. V008T23A023.
- 2.Industrial Efficiency Technology Database, Ammonia benchmarks. http://www.iipinetwork.org/wp-content/Ietd/content/ammonia.html#benchmarks. Accessed 15 July 2021.
- 3.Abdulla A., Hanna R., Schell K. R., Babacan O., Victor D. G., Core Concept: Explaining successful and failed investments in U.S. carbon capture and storage using empirical and expert assessments. Environ. Res. Lett. 16, 014036 (2020). [Google Scholar]
- 4.Guo X., Du H., Qu F., Li J., Core Concept: Recent progress in electrocatalytic nitrogen reduction. J. Mater. Chem. A Mater. Energy Sustain. 7, 3531–3543 (2019). [Google Scholar]
- 5.Zou H., Rong W., Wei S., Ji Y., Duan L., Core Concept: Regulating kinetics and thermodynamics of electrochemical nitrogen reduction with metal single-atom catalysts in a pressurized electrolyser. Proc. Natl. Acad. Sci. U.S.A. 117, 29462–29468 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suryanto B. H. R., et al. , Core Concept: Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle. Science 372, 1187–1191 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Sun J., et al. , Core Concept: A hybrid plasma electrocatalytic process for sustainable ammonia production. Energy Environ. Sci. 14, 865–872 (2021). [Google Scholar]
- 8.MacFarlane D. R., et al. , Core Concept: A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020). [Google Scholar]