Significance
Both plant biodiversity and soil fertility are in decline. We find that restoration of plant biodiversity on a nutrient-poor, unfertilized soil led to greater increases in soil fertility than occurred when these same plant species grew in monocultures. The plant species in this biodiversity experiment fell along a trade-off surface in their nutrient content traits, precluding any one species, or any one type of species, from markedly increasing soil fertility. Our results have implications for degraded agroecosystems, suggesting that increasing plant functional biodiversity may help restore their soil fertility. Creative applications of our findings to pastures, cover crops, and intercropping systems may provide greenhouse gas benefits from soil carbon storage and reduce the amounts of fertilizers needed for optimal yields.
Keywords: biodiversity, soil fertility, carbon storage, trait trade-offs, soil restoration
Abstract
Fertile soils have been an essential resource for humanity for 10,000 y, but the ecological mechanisms involved in the creation and restoration of fertile soils, and especially the role of plant diversity, are poorly understood. Here we use results of a long-term, unfertilized plant biodiversity experiment to determine whether biodiversity, especially plant functional biodiversity, impacted the regeneration of fertility on a degraded sandy soil. After 23 y, plots containing 16 perennial grassland plant species had, relative to monocultures of these same species, ∼30 to 90% greater increases in soil nitrogen, potassium, calcium, magnesium, cation exchange capacity, and carbon and had ∼150 to 370% greater amounts of N, K, Ca, and Mg in plant biomass. Our results suggest that biodiversity, likely in combination with the increased plant productivity caused by higher biodiversity, led to greater soil fertility. Moreover, plots with high plant functional diversity, those containing grasses, legumes, and forbs, accumulated significantly greater N, K, Ca, and Mg in the total nutrient pool (plant biomass and soil) than did plots containing just one of these three functional groups. Plant species in these functional groups had trade-offs between their tissue N content, tissue K content, and root mass, suggesting why species from all three functional groups were essential for regenerating soil fertility. Our findings suggest that efforts to regenerate soil C stores and soil fertility may be aided by creative uses of plant diversity.
For a soil to be fertile, it must supply sufficient amounts of the multiple nutrients that may limit plant growth, such as nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg), and have sufficient organic matter to retain water and nutrients (1, 2). Low levels of one or more of these factors would reduce plant productivity. In natural ecosystems, plants contribute to the creation of fertile soils through the fixation of carbon (C) and N, through root chemical liberation of unavailable forms of soil minerals and their movement from deep to surface soils, and through the uptake and retention of nutrients (3–12). However, if plant species differ in their capacity to liberate, capture, or retain particular limiting soil nutrients (5), then any one species growing alone might lead to the creation of a soil relatively deficient in those nutrients that it has difficulty obtaining. If plants species have trade-offs between their abilities to acquire different nutrients, with each species being better at acquiring some nutrients but poorer for others (13), then a diversity of plant species may be essential for the long-term accrual of the multiple elements that are required for a soil to be fertile.
Here we use a long-term grassland biodiversity field experiment to explore the potential role that different perennial grassland plant species, plant traits, and plant biodiversity may play in generating and restoring soil fertility. While greater plant biodiversity is associated with greater primary productivity and soil C accumulation (14–17), increased soil C alone does not make a soil more fertile. Greater fertility also requires increases in all potentially limiting nutrients such as N, P, K, Ca, and Mg, as well as optimal soil pH and adequate soil cation exchange capacity (CEC) (1, 2). Here we test the hypothesis “that the sustainability of soil nutrient cycles and thus of soil fertility depends on biodiversity” (18).
Because greater plant species richness has been associated with greater uptake of available soil nutrients and greater plant biomass production (18), higher plant biodiversity might increase soil fertility if the increased nutrients in plant biomass are returned to the soil as plant tissue decomposes (3, 4, 6, 7, 12, 19–21) and if greater plant diversity leads to lower leaching losses of these nutrients (22). This increase in soil fertility could then increase biomass production, creating a positive feedback as even more nutrients were added to the soil from greater biomass inputs (7, 19, 23). In particular, as roots, leaves, and other plant parts are shed, soil bacteria, fungi, and invertebrates modify and stabilize these organic matter inputs and release nutrients as they decompose plant tissue (9, 10, 21, 24–29). Nutrients released by decomposition can increase plant growth and thus the amount of plant biomass that subsequently gets returned to the soil (7). On a nutrient-poor soil, greater plant diversity may lead to greater accumulation of soil nutrients and organic matter and therefore may cause plant productivity to increase more through time than in low-diversity ecosystems (21, 30, 31).
Our experiment, planted in the spring of 1994, manipulated the composition and diversity of perennial grassland plant species growing on a sandy, degraded soil. In August 1993, the upper 6 cm to 8 cm of topsoil was removed from an abandoned agricultural field to eliminate a weedy soil seed bank. The field was then plowed and disked multiple times, and had bare soil from August 1993 until planted in spring 1994. The one hundred fifty-four 9 × 9 m plots established for this experiment were seeded to have 1, 2, 4, 8, or 16 perennial grassland species randomly chosen from a pool of 18 species. Here, “plant diversity” refers to the number of species planted in a plot. We additionally calculated plant “functional group diversity” based on a functional grouping commonly applied to grasslands, classifying plant species as grasses, legumes, or forbs (32). Plots were never fertilized, were annually burned in early spring before green-up, and were fenced to exclude large vertebrate herbivores. The glacial outwash sandplain soils of our site in east-central Minnesota are agronomically classified as “very low” in organic matter, N, and K, but “very high” in P (SI Appendix, Table S1). Using archived soil samples collected from each plot before planting in 1994 and samples collected after 23 y of growth in 2017, we measured soil total N and C, exchangeable K, Ca, and Mg, soil CEC, soil pH, and extractable Bray P in the upper 0 cm to 20 cm of the soil profile. In August 2017, both aboveground and belowground (root; 0- to 30-cm depth) plant biomass were measured, as were N, P, K, Ca, and Mg in both aboveground and root biomass. We additionally measured aboveground plant tissue chemistry for each individual plant species.
Results
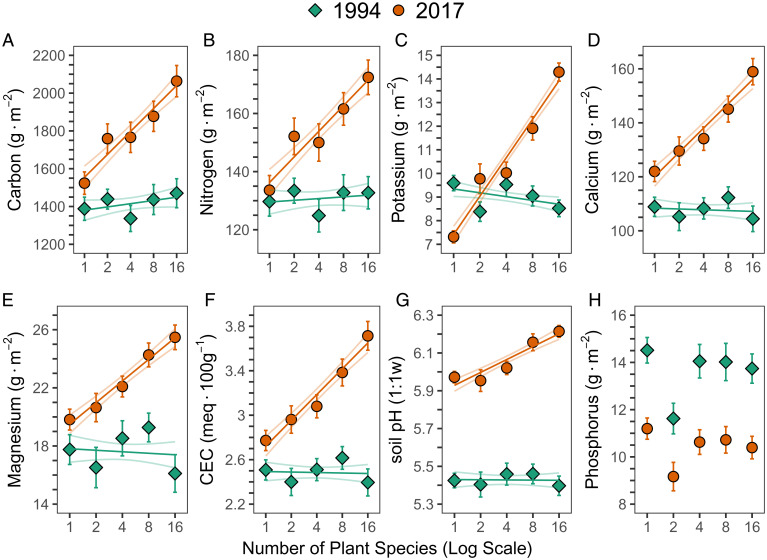
Higher levels of plant diversity led to increases in numerous factors that contribute to soil fertility. Comparison of pretreatment 1994 soils to 2017 soils shows that plots with higher plant diversity had significantly greater increases in soil N, K, Ca, Mg, and C, in CEC and in soil pH (Fig. 1 and SI Appendix, Figs. S1 and S2 and Table S2). Soil P levels, which were very high before planting, remained very high in 2017, with no detectable effect of plant diversity (Fig. 1H and SI Appendix, Table S2). Although no plots were ever fertilized, by 2017, the soils of the 16-species treatment had 29% more total soil N (0- to 20-cm depth) than the monoculture mean of these same species, 95% more soil K, 30% more soil Ca, 29% more soil Mg, 35% more total soil C, 34% greater CEC, and a less acidic soil (0.2 pH increase from monocultures) (Fig. 1). Although soil bulk density declined with plant diversity from a mean ± SE of 1.46 g⋅cm−3 ± 0.015 in the monocultures to 1.37 g⋅cm−3 ± 0.018 in 16 species plots (F1,85 = 23, R2 = 0.21, P < 0.001), expressing soil elemental levels on a concentration basis and on an area density basis were qualitatively similar (SI Appendix, Figs. S3–S5 and Table S3).
Fig. 1.
Soil chemistry vs. plant diversity. Mean ±1 SE of soil chemistry (0- to 20-cm depth) before planting in 1994 in green (diamond) and in 2017 in orange (circle) of (A) total carbon, (B) total nitrogen, (C) exchangeable potassium, (D) exchangeable calcium, (E) exchangeable magnesium, (F) CEC, (G) soil pH, and (H) extractable Bray phosphorus versus number of planted species (1, 2, 4, 8, or 16; log scale). Lines are linear regressions ± 1 SE (n = 154 plots). The quantity (grams per square meter) for C, N, P, K, Ca, and Mg were calculated using soil bulk density. Sample sizes for each diversity level (1 to 16 species) are 1 species = 32 plots; 2 = 28; 4 = 29; 8 = 30; and 16 = 35.
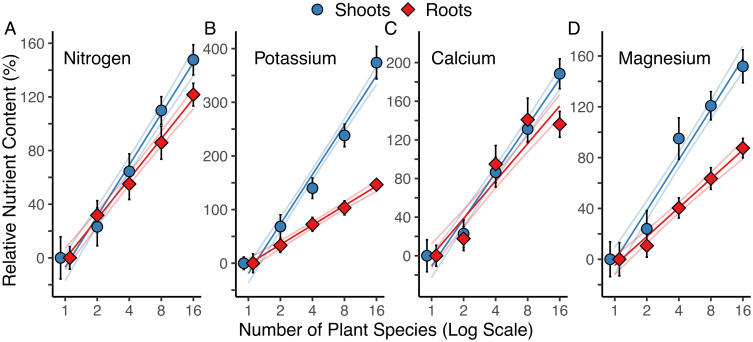
The greater accumulation of N, K, Ca, and Mg in surface soils (0- to 20-cm depth) at higher plant diversity was accompanied by even greater percent increases relative to monocultures in the pool size of these nutrients in both aboveground and belowground plant biomass in 2017 (Fig. 2). Linear regressions show that tissue pools of N, K, Ca, and Mg in aboveground and in belowground biomass were, relative to average levels across all monocultures, positively dependent on the log of plant diversity (all P < 0.001; Fig. 2 and SI Appendix, Figs. S6 and S7 and Table S4).
Fig. 2.
Nutrient contents relative to monoculture levels. The 2017 relative shoot (blue; circle) and root (red; diamond) nutrient content of biomass for each diversity treatment is expressed as the percent of the 2017 mean nutrient content of all monocultures combined (mean ± 1 SE). Percent change relative to the mean of all monocultures for (A) nitrogen, (B) potassium, (C) calcium, and (D) magnesium contained in aboveground shoot biomass and belowground (0 cm to 30 cm) root biomass. Lines are linear regressions ± 1 SE (n = 154 plots). Shoots are dried aboveground biomass, and roots are dried belowground biomass (0 cm to 30 cm). Biomass was multiplied by the concentration of each element.
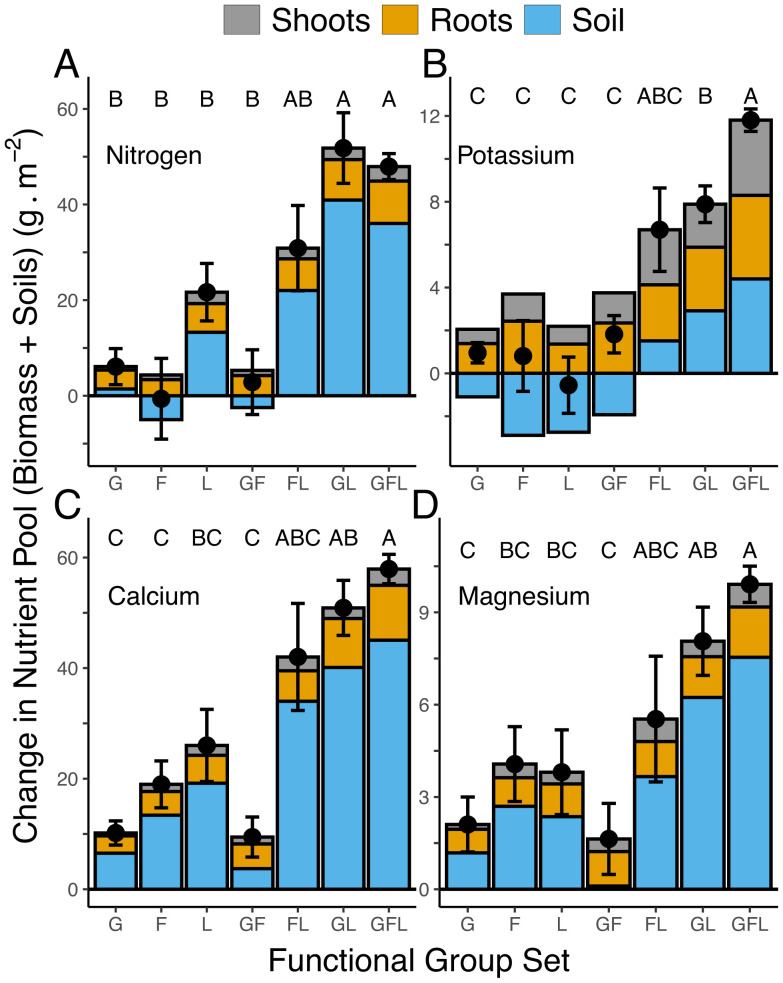
Why, though, might the production of greater plant biomass and the accumulation of soil nutrients depend on plant diversity? Diversity is thought to impact ecosystem processes because of functional differences between species (32). Because the herbaceous perennial species of tallgrass prairie are often functionally classified as grasses (Poaceae), legumes (Fabaceae), and forbs (not including legumes; Asteraceae, Lamiaceae, and Apocynaceae), we tested whether the rate of ecosystem accumulation of particular nutrients was related to the presence of these plant functional groups. To do this, we classified each plot by its presence of grass (G), legume (L), or forb (F) species. This gave seven functional group compositions: G, L, F, G+F, L+F, G+L, and G+L+F. Ecosystem pools of accumulated N, K, Ca, and Mg (Fig. 3) were calculated as the change in each plot of each element in the soil from 1994 to 2017 (as grams per square meter of each element in the 0- to 20-cm soil depth increment) plus the total amount of each element accumulated in shoots and roots by 2017 (as grams per square meter), since all plant biomass had been removed the year before planting.
Fig. 3.
Change in ecosystem total nutrient pools (black points) for each functional group composition for (A) nitrogen, (B) potassium, (C) calcium, and (D) magnesium. Each black point shows the mean of the total ecosystem pool ± 1 SE. Pools were defined as the change from 1994 to 2017 in soil levels of a nutrient (0- to 20-cm-depth increment) plus amounts of that nutrient in aboveground biomass and in roots (0 cm to 30 cm) in 2017; sum expressed as grams of nutrient per square meter. Bars show the value for each nutrient in aboveground biomass (gray), in belowground biomass (yellow), and in soil (blue). Bars with negative values, shown below the zero line, indicate a reduction from 1994 to 2017 for an element. Functional group compositions: G = grasses only, n = 22; F = nonlegume forb only, n = 10; L = legumes only, n = 11; FL = at least one forb and one legume, n = 5; GL = at least one grass and one legume, n = 23; GF = at least one grass and one forb, n = 14; GFL = at least one grass, one legume, and one forb, n = 69. Letters indicate whether means for a particular nutrient differ (P < 0.05) following a Tukey correction.
The presence of all three functional groups, the G+F+L plots, was associated with the largest increases in ecosystem pools of N, K, Ca, and Mg compared to when just a single functional group was present (Fig. 3). In particular, plots containing all three functional groups (G+L+F) had significantly greater accumulation in soils plus plant biomass of each of the four nutrients, N, K, Ca, and Mg, than did the plots with just a single functional group (the F, G, or L plots; Fig. 3 and SI Appendix, Table S5). This was not the case for any combination of just two functional groups (Fig. 3). Neither the G+F nor the F+L plots accumulated significantly greater ecosystem pools of N, K, Ca, or Mg than did the G, F, or L plots (Fig. 3). Results were intermediate for the G+L plots, which were not significantly different from F or L in Mg accumulation or from L in Ca accumulation, but had greater N accumulation than plots planted with a single functional group or with G+F. Finally, although G+F+L and G+L did not differ in ecosystem pools of N, Ca, or Mg, G+F+L had significantly greater K pools than all other functional compositions except F+L, suggesting that the presence of forbs was an important cause of the observed large increases in K at high plant biodiversity.
In total, plots planted with a single functional group accumulated significantly lower ecosystem pools of most nutrients than did G+F+L plots, and those planted with two functional groups only significantly exceeded single functional groups in one-fourth of the pairwise comparisons (Fig. 3). Separate analyses for each of the plant, root, and soil nutrient pools demonstrates that, when compared to plots planted with a single functional group, G+F+L produced more aboveground and belowground biomass and accumulated more C, N, K, Ca, and Mg in 87% of the comparisons (39 out of 45 comparisons) (SI Appendix, Fig. S8).
On an even finer scale, for amounts of each of the four nutrients in aboveground biomass, G+F+L was significantly greater than G+L, but was never significantly greater than F+L (SI Appendix, Fig. S8). For root nutrients, G+F+L was significantly higher in root K than both F+L and G+L. The only functional group with root K levels as high as those of G+F+L was F, the forb-only plots. For root Mg, F+L did not differ from G+F+L, but G+F+L had significantly more Mg than G+L. For root Ca, the opposite occurred: G+F+L did not differ from G+L but had significantly more Ca than F+L. In total, these results suggest that forbs, and the joint presence of forbs and legumes, are important contributors of K and Mg to ecosystem pools and that legumes and the joint presence of legumes and grasses are more important contributors of N and Ca.
Since not all of these nutrients may be limiting to the production of plant biomass, we determined which soil variables were more strongly correlated with observed diversity-dependent changes in productivity while accounting for the effect of plant diversity. We used linear multiple regressions and multimodel inference, finding that total plant biomass depended positively on the loge of the number of species (156 ± 25.6 g⋅m−2 biomass per 1 loge(number of plant species), P < 0.001), soil exchangeable K (51.3 ± 8.8 g⋅m−2 biomass per g⋅m−2 of K, P < 0.001), total soil N (2.88 ± 0.67 g⋅m−2 biomass per g⋅m−2 of N, P < 0.001), and total soil C (0.23 ± 0.05 g⋅m−2 biomass per g⋅m−2 of C, P < 0.001) (SI Appendix, Table S6). This analysis suggests that total soil C, total soil N, and exchangeable soil K are the soil variables most strongly associated with the amount of plant biomass produced in this field experiment, which is consistent with our soil analyses that indicated agronomically low soil levels of N, K, and organic matter at the start of our experiment (SI Appendix, Table S1).
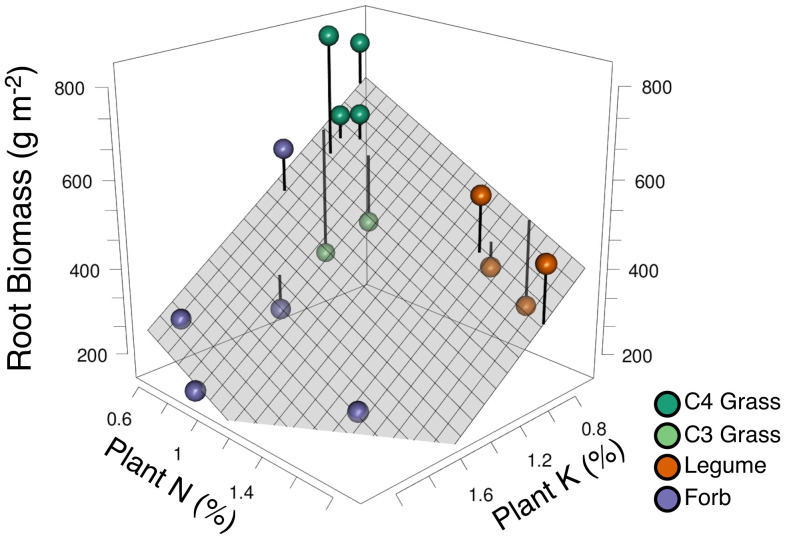
Finally, we determined how the plant species in the three functional groups might differ in traits relevant to the accumulation of soil K, N, and C. For K and N, we used average measured aboveground tissue concentrations of K and N for each species in monoculture and 16-species plots. For soil C accumulation, we used average monoculture root mass for each species because prior results of this experiment showed that greater root biomass (as gram per square meter) was the variable most strongly associated with greater increases in soil C (14, 17).
These three measured traits of the species (root mass, tissue %N, tissue %K) defined a regression plane (Fig. 4 and SI Appendix) (F2,12 = 6.3, R2 = 0.51, P = 0.014). The species within each functional group tended to be similar to each other, as evident by their tendency to cluster (Fig. 4). On this trade-off surface, perennial C4 grasses were low in both tissue %N and %K but had the highest root biomass. Forbs and legumes, which had less root biomass than C4 grasses, were further differentiated: Legumes had higher %N but markedly lower %K. Forbs, in contrast, had higher %K but lower %N (Fig. 4). Forbs and legumes had similar %Ca and %Mg levels, and their levels were greater than for grasses (SI Appendix, Fig. S10).
Fig. 4.
Empirical trade-off surface among plant traits for the 15 herbaceous perennial plant species that persisted in the experiment. A regression plane (F2,12 = 6.3, R2 = 0.51, P = 0.014) is fitted to species-specific measured values of percent aboveground tissue potassium (K) (x axis), percent aboveground tissue nitrogen (N) (y axis), and mean monoculture root biomass (grams per square meter; 0- to 30-cm depth; z axis). Each point represents the three measured traits of each of 15 species (SI Appendix, Table S7) classified as grasses (C4 grasses in dark green and C3 grasses in light green), forbs (purple), and legumes (orange). The %N and %K represent the mean across each species' monocultures and the biomass of each species in five 16-species plots. Root biomass represents the mean root mass (0- to 30-cm depth) of each species' monocultures. Removing the two C3 grasses (lighter green; below the plane), which are subdominant species in this ecosystem and grew poorly in monoculture, increased the fit of the plane to F2,10 = 15, R2 = 0.75, P = 0.001 (not shown). The point for Andropogon gerardii (C4 grass) was slightly jittered in the x and y axis to avoid overplotting with Sorghastrum nutans (C4 grass).
Discussion
Early in this experiment, greater plant diversity was associated with greater capture of soil nitrate (18) and with 16 species plots being ∼100% more productive than the average of these species in monocultures. After 23 y, we find that greater plant diversity was associated with higher levels of soil C, N, K, Ca, and Mg and with 16 species plots being ∼200% more productive than monocultures. The progressively greater primary productivity observed through time at higher diversity in this and other long-term biodiversity experiments (refs. 30 and 31, but see ref. 33) and the greater accumulation of multiple nutrients and C in soil and in plant biomass (Figs. 1 and 2) suggest the existence of a positive feedback effect (7, 23) of plant diversity on soil fertility that increased primary productivity through time.
We hypothesize that high plant diversity, and especially the joint presence of grass, legume, and forb species, leads to greater liberation and capture of limiting soil nutrients (18). This, in turn, allows greater production of plant biomass. We suggest that the nutrient and C contents of the greater biomass (roots and shoots) produced by diverse mixtures of grass, legume, and forb species is then recycled when it senesces, helping create a more fertile soil. This more fertile soil would then further increase plant biomass production and biomass nutrient pools, in a positive feedback loop that would persist until an equilibrium is reached (7, 19, 20).
We note, however, that the annual early spring burning in our experiment likely volatilizes some of the C and N that had been in litter from senesced aboveground biomass, but also likely deposits biologically available forms of other elements in ash (e.g., Ca, Mg, and K). Because root mass in our prairie-like high-diversity plots is about 4 times the aboveground biomass, senesced biomass from root turnover may add C, N, and other nutrients to soil (6–8, 10, 11, 19, 34).
The three-way trade-off shown in Fig. 4 suggests why plant functional diversity may have been essential for increasing soil fertility in our experiment, which was unfertilized. It suggests that no single species and no functional group could, by itself, span the full space of the root biomass–N–K trade-off surface and thus cause soil C, N, and K, which seem to limit productivity, to all increase. Increases in all three of these were associated with greater productivity in our experiment, and thus with the hypothesized feedback effect of greater productivity and its nutrient contents on soil fertility. In contrast, increased N, P, and K fertilization is often required to increase the productivity of an agricultural monoculture crop, and such fertilization can also lead to increased soil C (35). Our monocultures, however, were never as productive as our high-diversity plots (17). At our site, soil P was at agronomically very high levels both prior to planting and 23 y later. While root biomass, N, and K differed among functional groups, and while soil C, soil N, and soil K increased with diversity, we found that soil P was not significantly dependent on plant diversity or functional groups, and tissue P levels did not differ among functional groups when growing on this P-rich soil (Fig. 1H and SI Appendix, Figs. S9 and S10).
The trait differences (Fig. 4) between grasses, legumes, and forbs suggest a mechanistic link from plant traits to the effect of functional diversity on primary productivity and the accumulation of soil nutrients. The trade-off surface shows that each functional group should contribute to the soil more of one of root biomass, N, or K, but less of the other two. Because legumes were high in %N and %Ca while forbs were high in %K, %Ca, and %Mg, the presence of each of these functional groups should have particularly enriched soil for those elements that were in higher relative concentration in its biomass (Fig. 3 and SI Appendix, Figs. S8 and S10). The high root biomass of C4 grasses may have decreased nutrient losses via leaching and helped increase soil organic C (14, 17, 18, 22). Moreover, no functional group growing alone increased soil C and nutrients as much as occurred when all three groups were present (Fig. 3 and SI Appendix, Fig. S8).
The increases in surface soil exchangeable K, Ca, and Mg may have come from root uptake of these elements in deeper soils that was concentrated at the surface as aboveground tissues and shallow roots died and decomposed or as elements were deposited as ash from litter during early spring burns (6). Ca, for example, tends to move unidirectionally from roots to shoots, with limited resorption when tissues senesce (34, 36). If greater root uptake and recycling of nutrients from ash or root turnover is the mechanism for the accumulation of soil fertility in high-diversity plots, then one would expect a coupling of the accumulation of plant and soil pools (37). For example, soil K was highly dependent on plant diversity (R2 = 0.47), and K was the nutrient with the largest % increase (370%) in its aboveground plant pool when comparing 16-species plots to the mean of all monocultures (Fig. 2). For K, Ca, and Mg, 16 species plots had ∼150 to 370% greater aboveground pools and ∼90 to 150% greater root pools than monocultures, indicating that higher plant diversity led to greater ecosystem capture and retention in biomass of these cations (Fig. 2). Moreover, in these sandy soils, increases in soil organic C were correlated with increases in CEC (1994: R2 = 0.20, P < 0.001; 2017: R2 = 0.52, P < 0.001; SI Appendix, Fig. S11), which should increase K, Ca, and Mg retention in these soils.
Our long-term experiment revealed surprisingly large diversity-dependent increases in soil fertility. This magnitude, however, might depend on our initial soil characteristics. The soils of our site, which formed on a glacially deposited sand plain, are classified taxonomically as entisols, which have limited horizon development (38). At the beginning of this experiment, some of the topsoil and its organic matter were removed, and soils were plowed and disked, which tended to homogenize the remaining topsoil with deeper soil layers. The low initial levels of soil C and N and high levels of P in our starting soils are characteristic of geologically young soils undergoing progressive development (39). Thus, if degraded and abandoned agricultural soils at our site accumulate C and nutrients in a logistic manner (40), the rates of increase in soil C, N, K, Ca, and Mg that we observed at high plant diversity may be greater than if our soil had initially been higher in these elements.
Our results, and their likely mechanistic basis, may provide insights into methods to restore soil C and increase limiting soil macronutrients in agroecosystems and managed forests. For instance, incorporating greater plant functional diversity via appropriate choice of the plant species used in crop rotations, intercropping, or cover crops may lead to long-term increases in soil fertility and subsequent reductions in the amount of fertilizer needed (41–44). Because our results suggest it is not simply the number of plant species that matters, but rather the appropriate suite of complementary plant traits, it would be interesting to determine whether as few as perhaps three such plant species might offer notable soil benefits relative to monocultures.
In our study, the increased inputs of senesced plant biomass that occurred at higher diversity had to be transformed and mineralized by the soil microbial and invertebrate communities, suggesting that soil microbial biodiversity may also help explain the results in Fig. 1 (45, 46), which is an intriguing possibility (9, 16, 25, 27, 28). Greater accumulation of plant or soil pathogens in monocultures, or increases in soil mutualists, or decreases in soil pathogens at high plant diversity are other possible ways that microbial biodiversity might impact ecosystem functioning through time (25, 47).
In total, our results show that plant diversity, including plant functional diversity, can play a significant role in the generation of soil fertility, likely via positive feedback effects of diversity-dependent increases in nutrient capture and productivity on soil fertility. Our results raise the interesting possibility that the high plant diversity of most natural ecosystems may have been an important factor leading to the creation of fertile soils around the world. Efforts to increase soil C stores and fertility of degraded soils may be aided by creative uses of plant diversity.
Methods
Experimental Design.
The experimental field had been abandoned from agriculture for more than 15 y when, in August of 1993, the herbicide glyphosate was applied and surface vegetation, once dead and dried, was burned. The top 6 cm to 8 cm of soil was scraped off to reduce the presence of weedy annual plant seeds in the soil seed bank. This also reduced soil carbon and soil nutrient levels. The site was then plowed twice and harrowed multiple times that year, and again in May 1994 before planting. The 168 plots, initially 13 m by 13 m but subsequently reduced to the central 9 m by 9 m portion, were planted with 1, 2, 4, 8, or 16 perennial plant species randomly chosen from a species pool of 18. The species pool consisted of common perennial grassland species of regional tallgrass prairie and two oaks common in nearby oak savannas. Herbaceous species were functionally categorized as C4 grasses, C3 grasses, legumes, and nonleguminous forbs, with four species in each functional group.
Because of poor establishment, 14 plots were dropped from the experiment, leaving 154 plots. In particular, the two oak species failed to survive because of annual burning. Two of the four C3 grasses, Agropyron smithii and Elymus canadensis, initially germinated but failed to survive long term. The final experimental design thus consisted of 154 plots seeded with 1, 2, 4, 8, or 16 randomly selected perennial grassland species, and with 32, 28, 29, 30, and 35 replicates of each diversity level, respectively. Monocultures were not, by design, replicated; rather, the monoculture treatment was based on random draws of single species from the species pool. Most species were randomly assigned to two monocultures. However, Poa pratensis and Panicum virgatum have one monoculture; Liatris aspera, Lespedeza capitata, Dalea purpureum, and Schizachyrium scoparium have three monocultures; and Sorghastrum nutans has four. In addition, one forb species, Solidago rigida, failed to germinate during the first year and was planted with another forb, Monarda fistulosa, in spring 1995. In the third year, S. rigida germinated and eventually became well established and dominated its monocultures.
Plots were annually burned early each spring before green-up but received no fertilizer. Each plot was annually weeded by hand to remove nonplanted species. The experiment was fenced to exclude white-tailed deer. Additional details can be found on the Long-Term Ecological Research (LTER) program website for the Cedar Creek Ecosystem Science Reserve under experiment name “e120: Biodiversity II: Effects of Plant Biodiversity on Population and Ecosystem Processes” (https://www.cedarcreek.umn.edu/research/experiments/e120).
Calculation of Plant Functional Groups.
For each plot in the experiment, we determined if it had been planted with grasses (“G,” Poaceae), legumes (“L,” Fabaceae) or forbs (“F,” Asteraceae, Lamiaceae, and Apocynaceae). We then categorized each plot by the functional groups planted in it. This grouping gave seven functional group compositions: G, L, F, G+F, L+F, G+L, and G+L+F. Sample sizes were as follows: G = grasses only, n = 22 plots; F = forb only, n = 10; L = legumes only, n = 11; FL = at least one forb and one legume, n = 5; GL = at least one grass and one legume, n = 23; GF = at least one grass and one forb, n = 14; GFL = at least one grass, one legume, and one forb, n = 69.
Field Collection of Soil Samples.
Nine soil cores per plot were collected in an evenly spaced 3 × 3 sampling grid pattern in September of 2017 to a depth of 60 cm in 20-cm increments using a 1.9-cm-diameter soil corer. The nine cores from a plot were then combined, dried at 60 °C, sieved to a 2-mm fraction, and then well mixed. Soil samples were similarly taken and processed in 1994 prior to planting. Those samples remained in glass archived vials until analysis. The 2017 soil samples were similarly archived.
Plant Biomass Sampling.
In August, near the time of peak aboveground biomass, two parallel 0.10-m by 6-m strips were clipped at the soil surface in each plot each year to sample plant biomass. Strips were located in the middle half of each plot, separated by about 1 m to 2 m, and located so as to not clip an area that had previously been clipped within the past decade. One strip per plot was sorted to species; the other was unsorted. Root biomass was subsequently sampled in the clipped area, with a 5.1-cm-diameter soil probe used to collect three cores per strip (six per plot) to a depth of 30 cm. Roots were washed over a mesh screen to remove soil. Roots and aboveground biomass were dried in a dehumidified drying room at 60 °C until achieving constant mass. Aboveground biomass was sampled annually starting in 1996. Belowground biomass has been sampled in years 1997, 1998, 1999, 2000, 2001, 2002, 2003, 2004, 2006, 2010, 2015, and 2017.
Laboratory Analysis of Soil Samples.
The University of Minnesota Research Analytical Laboratory analyzed soil samples that we collected from depth increments of 0 cm to 20 cm, 20 cm to 40 cm, and 40 cm to 60 cm in 2017 and 0 cm to 20 cm in 1994 for exchangeable cations (calcium, magnesium, potassium, sodium) using a pH 7 ammonium acetate extraction and for aluminum using a 1 M KCl extraction followed by analysis using an Inductively Coupled Argon Plasma Optical Emission Spectrometer (iCap 7600 Duo ICP-OES Analyzer, Thermo Fisher Scientific) (48). Effective CEC was measured by the summation method of exchangeable Ca, Mg, K, Na, and Al (48). Extractable P was measured using a standard Bray-1 extract (49) (0.025 M HCl and 0.03 M NH4F) and analyzed colorimetrically on a Brinkmann PC 900 probe colorimeter (Thermo Fisher Scientific). A commercial laboratory, Waypoint Analytical, analyzed soil samples at depths 0 cm to 20 cm, 20 cm to 40 cm, and 40 cm to 60 cm in 2017 and 0 cm to 20 cm, 20 cm to 40 cm, and 40 cm to 60 cm in 1994 for soil pH in a 1:1 soil:deionized water slurry. Soil total carbon and nitrogen were analyzed in soil samples from depth increments of 0 cm to 20 cm, 20 cm to 40 cm, and 40 cm to 60 cm in 1994, 2015, and 2017. The average of 2015 and 2017 values was used to reduce sampling noise. Ground soil was analyzed using dry combustion gas chromatography on an Elemental Analyzer (Costech ECS 4010 CHNSO Analyzer). Because of the lack of carbonate minerals in these soils (38), total C represents total organic C. In order to evaluate the agronomic status of our starting soil (SI Appendix, Table S1), Waypoint Analytical measured total soil organic matter using loss on ignition for 1994 soil.
Laboratory Analysis of Plant Samples.
The dried aboveground and belowground biomasses sampled in August of 2017 were analyzed for their chemical composition. The homogenate unsorted clipped strip of dried biomass for each of 154 plots was ground completely and then subsampled. Additionally, for all monoculture plots and five 16-species plots, the total sorted quantity, including leaves, stems, and inflorescences if present, of each of the 15 plant species was additionally ground to provide estimates of their individual traits. Because the legume, Lupinus perennis, has a spring growth and seed shedding pattern that required a separate spring biomass sampling to determine its tissue nutrient contents, samples of L. perennis from June in 2019 were also analyzed and used instead of the 2017 sample. Plant samples were ground using a Model 4 Wiley Mill (Thomas Scientific) and analyzed at a commercial laboratory, Waypoint Analytical, for tissue chemistry using method 3050B of the Environmental Protection Agency Manual SW-846. Specifically, each plant sample was digested in concentrated nitric acid followed by heating for 15 min at 95 °C. Then 30% hydrogen peroxide was added until effervescence was no longer observed, followed by 5 mL of concentrated HCl and continued heating for 30 min. Five to ten milliliters of water was finally added to the sample following filtration using Whatman #2 and analyses using Inductively Coupled Plasma Optical Emission Spectrometry (Perkin-Elmer Optima 8300).
Estimation of Ecosystem Nutrient Pools.
The concentration of each measured soil variable was adjusted to an area density quantity (grams per square meter) with soil bulk density. Bulk density was measured in 2018 to a depth of 60 cm in 20-cm increments using an AMS Inc. split soil core sampler with a removal jack (part numbers 400.99, 403.41, 403.73, 211.05, and 211.06) in a subset of plots (87) with a sample size of 26, 16, 15, 15, and 15 at 1, 2, 4, 8, and 16 number of species, respectively, randomly chosen while including two replicates for each species in monoculture where available. For unmeasured plots, we estimated bulk density using a linear regression of the dependency of bulk density (0 cm to 20 cm) on % soil C (0 cm to 20 cm) measured in 2017. Bulk density was not measured in 1994. We estimated bulk density (0 cm to 20 cm) values in each plot in 1994 using the measured % soil C values in 1994 (0 cm to 20 cm) and the regression fit with % soil C in 2017 (0 cm to 20 cm). The predicted mean bulk density of 1.45 g⋅cm−3 (0 cm to 20 cm) in 1994 approximates measured soil bulk density at the site's soil survey of the Nymore series of 1.4 g⋅cm−3 (0 cm to 23 cm) (ref. 38, table 5, p. 22). Estimated bulk density in 1994 at 0 cm to 20 cm had treatment means ± 1 SE of 1.45 g⋅cm−3 ± 0.01, 1.44 g⋅cm−3 ± 0.00, 1.45 g⋅cm−3 ± 0.01, 1.44 g⋅cm−3 ± 0.01, and 1.44 g⋅cm−3 ± 0.01 at 1, 2, 4, 8, and 16 number of species, respectively. Bulk density measured in 2018 at 0 cm to 20 cm had treatment means ± 1 SE of 1.46 g⋅cm−3 ± 0.015, 1.43 g⋅cm−3 ± 0.015, 1.42 g⋅cm−3 ± 0.019, 1.36 g⋅cm−3 ± 0.019, and 1.37 g⋅cm−3 ± 0.018 at 1, 2, 4, 8, and 16 number of species, respectively. We then used the equivalent soil mass approach to adjust for sampling 20 cm deep across all plots, despite an assumed change in density, by adding mass from the 20- to 40-cm-depth increment or subtracting mass at 0 cm to 20 cm relative to the change in bulk density from the reference value in 1994 (SI Appendix) (50). The pool of nutrients in aboveground biomass was calculated using the percent of each element in the biomass from the unsorted clipped strip multiplied by the dry biomass in each plot. Aboveground biomass was calculated as the dry-weight (grams per square meter) average of both sorted and unsorted strips as has been done historically in this experiment. Plant litter, dead biomass on the soil surface, was not included in this measurement, but there were negligible quantities of litter given that the field is annually burned. Belowground biomass was calculated as the dry weight (grams per square meter) (0 cm to 30 cm) for each plot. To reduce sampling noise from interannual variability, the average of aboveground and belowground biomass measured in 2015 and 2017 was used for all statistical analysis and calculations. To improve readability, we refer to these as 2017 within the text as the year when the plant tissue was chemically analyzed. The change in ecosystem nutrient pools for N, K, Ca, and Mg was estimated as the change in each plot of each element in the soil from 1994 to 2017 (as grams per square meter of each element in the 0–20 cm soil depth increment) plus the total amount of each element measured in aboveground and belowground biomass (0–30 cm) in 2017 (as grams per square meter).
Statistical Analysis.
Analyses were performed using R version 4.1.1 and JMP 14 Pro. Linear regressions were used to test the dependence of soil and plant variables on experimental plant biodiversity using the natural log of plant species number (1, 2, 4, 8, 16). For analysis of the plant biomass pools, the percent increase from the monoculture mean was used as the response variable. For each set of analysis, a false discovery rate (51) correction was applied to the P value for each regression. The regression results were robust to a variety of transformations of the y variable and are presented on the untransformed y scale. The dependance of the sum of aboveground and belowground biomass (total biomass) on the log of plant biodiversity and soil variables (C, N, Ca, Mg, K, P, pH) was tested using a generalized least-squares model with a power variance structure (varPower) on the fitted values (R package nlme). Multimodel inference (R package MuMIn) was used as a model selection approach with each soil variable and the natural log of plant biodiversity with the conditional average using the Bayesian Information Criterion. The dependance of ecosystem nutrient pools (N, K, Ca, and Mg) on the presence of different plant functional group sets was tested using a generalized least-squares model with a variance structure for the factor to account for unequal variance (varIdent, nlme). Differences among means were compared using least-squares means (R package emmeans) followed by a Tukey correction using the Satterthwaite estimation of the degrees of freedom. As supplemental analyses, we ran these same tests of the effects of functional group presence on the change in soil C, N, K, Ca, and Mg on aboveground and belowground biomass (belowground log transformed) and on the pools of N, K, Ca, and Mg in aboveground and belowground biomass.
A trade-off surface among plant species in their traits was tested by analyzing the dependence of belowground biomass (0 cm to 30 cm) in monoculture on percent aboveground N and percent aboveground K using a linear regression. The time series mean of belowground biomass for each species' in monoculture was the response variable, and the average tissue chemistry (%N and %K) for each species measured in its monocultures and five 16-species plots were the explanatory variables. R graphical package rgl was used to generate a regression plane in Fig. 4 with an aspect ratio of 1:1:1 (x:y:z).
Supplementary Material
Acknowledgments
We thank Troy Mielke and the numerous interns and staff who, starting in 1994, managed experimental treatments and collected data. NSF LTER Grants DEB-9411972, DEB-0080382, DEB-0620652, DEB-1234162, and DEB-1831944 funded this work, as did a Balzan Foundation award to D.T.
Footnotes
Reviewers: A.H., University of Oxford; and G.P.R., Michigan State University.
The authors declare no competing interest.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2111321118/-/DCSupplemental.
Data Availability
All data used in this paper have been deposited and are available from the Environmental Data Initiative. These datasets contain the following: soil C (https://doi.org/10.6073/pasta/9ed3740e181a3c41ec2cb787ef3a615b), soil N (https://doi.org/10.6073/pasta/9b55ae5a418c59fb8e3d7088c3591fc5), soil Bray P (https://doi.org/10.6073/pasta/b37f4e38718784480259f3d92fb7a9d7), soil CEC, K, Ca, Mg (https://doi.org/10.6073/pasta/0fa7f5b395f104ed346602a66696fa53), soil pH (https://doi.org/10.6073/pasta/810ebc0a46361bd6a4d1693f17b440fb), soil OM (https://doi.org/10.6073/pasta/6521113c6115b8fbeae7ef0ab6ebca9e), aboveground biomass (https://doi.org/10.6073/pasta/7ef2de3865062d7352f7b20753ecd39b), belowground biomass (https://doi.org/10.6073/pasta/0479da667672693c3cf2a6b2c8d14002), whole plot plant tissue chemistry (% N, P, K, Ca, Mg), (https://doi.org/10.6073/pasta/f433650417c15afb33d1dd0ad602a2e6), soil bulk density (https://doi.org/10.6073/pasta/270fbd77c7bd6d1cbb59647a3eccb639), individual plant chemistry traits (% N, P, K, Ca, Mg) (https://doi.org/10.6073/pasta/cea0d715283e4f0c2d71d28c8d146558). All data were submitted by the Cedar Creek Ecosystem Science Reserve site of the LTER Network. All study data are additionally included in the supporting information. Data to reproduce figures and analyses in the main text are included in Dataset S1 (Figs. 1–3) and Dataset S2 (Fig. 4). SI Appendix, Figs. S1, S2, S6–S9, and S11 can be reproduced using Dataset S1. SI Appendix, Figs. S3–S5 can be reproduced using Dataset S3. Data to reproduce the conversion of soil concentrations to area density quantities are available in Dataset S4. Data to reproduce the conversion of tissue concentrations to area density quantities are available in Dataset S5. Data to reproduce the derived response variables in Fig. 2 are available in Dataset S6. Data to reproduce SI Appendix, Fig. S10 are in Dataset S7. Metadata for these datasets are provided in the SI Appendix. R code to reproduce analyses is archived on Zenodo and will be maintained by G.N.F. (https://doi.org/10.5281/zenodo.5565171).
References
- 1.Hartemink A., “Soil fertility decline: Definitions and assessment” in Encyclopedia of Soil Science, Rattan L., Ed. (CRC, ed. 2, 2005), vol. 2, pp. 1618–1621. [Google Scholar]
- 2.Karlen D. L., Andrews S. S., Doran J. W., “Soil quality: Current concepts and applications” in Advances in Agronomy, Sparks D. L., Ed. (Elsevier, 2001), vol. 74, pp. 1–40. [Google Scholar]
- 3.Jenny H., Role of the plant factor in the pedogenic functions. Ecology 39, 5 (1958). [Google Scholar]
- 4.Zinke P. J., The pattern of influence of individual forest trees on soil properties. Ecology 43, 130–133 (1962). [Google Scholar]
- 5.Aerts R., Chapin F. S. III, “The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns” in Advances in Ecological Research, Fitter A. H., Raffaelli D. G., Eds. (Elsevier, 2000), vol. 30, pp. 1–67. [Google Scholar]
- 6.Jobbágy E. G., Jackson R. B., The uplift of soil nutrients by plants: Biogeochemical consequences across scales. Ecology 85, 2380–2389 (2004). [Google Scholar]
- 7.Ehrenfeld J. G., Ravit B., Elgersma K., Feedback in the plant-soil system. Annu. Rev. Environ. Resour. 30, 75–115 (2005). [Google Scholar]
- 8.Reich P. B., et al. , Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 8, 811–818 (2005). [Google Scholar]
- 9.Bardgett R. D., Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change (Oxford University Press, 2010). [Google Scholar]
- 10.Hobbie S. E., Plant species effects on nutrient cycling: Revisiting litter feedbacks. Trends Ecol. Evol. 30, 357–363 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Waring B. G., et al. , Pervasive and strong effects of plants on soil chemistry: A meta-analysis of individual plant ‘Zinke’ effects. Proc. Biol. Sci. 282, 20151001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berendse F., Effects of dominant plant species on soils during succession in nutrient-poor ecosystems. Biogeochemistry 42, 73–88 (1998). [Google Scholar]
- 13.Tilman D., Resource Competition and Community Structure (Princeton University Press, 1982). [PubMed] [Google Scholar]
- 14.Fornara D. A., Tilman D., Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 96, 314–322 (2008). [Google Scholar]
- 15.Cong W. F., et al. , Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 102, 1163–1170 (2014). [Google Scholar]
- 16.Lange M., et al. , Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 6, 6707 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Yang Y., Tilman D., Furey G., Lehman C., Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 10, 718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilman D., Wedin D., Knops J., Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379, 718–720 (1996). [Google Scholar]
- 19.Walker L. R., Primary Succession and Ecosystem Rehabilitation (Cambridge University Press, 2003). [Google Scholar]
- 20.Vitousek P. M., Reiners W. A., Ecosystem succession and nutrient retention: A hypothesis. Bioscience 25, 376–381 (1975). [Google Scholar]
- 21.Dybzinski R., Fargione J. E., Zak D. R., Fornara D., Tilman D., Soil fertility increases with plant species diversity in a long-term biodiversity experiment. Oecologia 158, 85–93 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Dijkstra F. A., West J. B., Hobbie S. E., Reich P. B., Trost J., Plant diversity, CO2, and N influence inorganic and organic N leaching in grasslands. Ecology 88, 490–500 (2007). [DOI] [PubMed] [Google Scholar]
- 23.DeAngelis D., Post W. M., Travis C. C., Positive Feedback in Natural Systems (Springer-Verlag, 1986). [Google Scholar]
- 24.Zak D. R., Holmes W. E., White D. C., Peacock A. D., Tilman D., Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 84, 2042–2050 (2003). [Google Scholar]
- 25.Eisenhauer N., Reich P. B., Scheu S., Increasing plant diversity effects on productivity with time due to delayed soil biota effects on plants. Basic Appl. Ecol. 13, 571–578 (2012). [Google Scholar]
- 26.van der Putten W. H., et al. , Plant–soil feedbacks: The past, the present and future challenges. J. Ecol. 101, 265–276 (2013). [Google Scholar]
- 27.Lange M., et al. , “How plant diversity impacts the coupled water, nutrient and carbon cycles” in Advances in Ecological Research, Eisenhauer N., Bohan D. A., Dumbrell A. J., Eds. (Elsevier, 2019), vol. 61, pp. 185–219. [Google Scholar]
- 28.Bardgett R. D., van der Putten W. H., Belowground biodiversity and ecosystem functioning. Nature 515, 505–511 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Kravchenko A. N., et al. , Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 10, 3121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero-Ramírez N. R., et al. , Diversity-dependent temporal divergence of ecosystem functioning in experimental ecosystems. Nat. Ecol. Evol. 1, 1639–1642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reich P. B., et al. , Impacts of biodiversity loss escalate through time as redundancy fades. Science 336, 589–592 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Tilman D., “Functional diversity” in Encyclopedia of Biodiversity, Levin S. A., Ed. (Academic, ed. 2, 2001), pp. 587–596. [Google Scholar]
- 33.Kardol P., Fanin N., Wardle D. A., Long-term effects of species loss on community properties across contrasting ecosystems. Nature 557, 710–713 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Dijkstra F. A., Smits M. M., Tree species effects on calcium cycling: The role of calcium uptake in deep soils. Ecosystems (N. Y.) 5, 385–398 (2002). [Google Scholar]
- 35.Bundy L. G., Andraski T. W., Ruark M. D., Peterson A. E., Long-term continuous corn and nitrogen fertilizer effects on productivity and soil properties. Agron. J. 103, 1346–1351 (2011). [Google Scholar]
- 36.Hanger B. C., The movement of calcium in plants. Commun. Soil Sci. Plant Anal. 10, 171–193 (1979). [Google Scholar]
- 37.Amundson R., Richter D. D., Humphreys G. S., Jobbágy E. G., Gaillardet J., Coupling between biota and earth materials in the critical zone. Elements 3, 327–332 (2007). [Google Scholar]
- 38.Grigal D. F., “Soils of the Cedar Creek Natural History Area” (Agricultural Experiment Station Rep. 123-1974, University of Minnesota, 1974).
- 39.Peltzer D. A., et al. , Understanding ecosystem retrogression. Ecol. Monogr. 80, 509–529 (2010). [Google Scholar]
- 40.Knops J., Tilman D., Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology 81, 88–98 (2000). [Google Scholar]
- 41.McDaniel M. D., Tiemann L. K., Grandy A. S., Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 24, 560–570 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Yu Y., Stomph T.-J., Makowski D., van der Werf W., Temporal niche differentiation increases the land equivalent ratio of annual intercrops: A meta-analysis. Field Crops Res. 184, 133–144 (2015). [Google Scholar]
- 43.Li C., et al. , Syndromes of production in intercropping impact yield gains. Nat. Plants 6, 653–660 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Finney D. M., Kaye J. P., Functional diversity in cover crop polycultures increases multifunctionality of an agricultural system. J. Appl. Ecol. 54, 509–517 (2017). [Google Scholar]
- 45.van der Heijden M. G., Bardgett R. D., van Straalen N. M., The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Bennett J. A., et al. , Resistance of soil biota and plant growth to disturbance increases with plant diversity. Ecol. Lett. 23, 119–128 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Thakur M. P., et al. , Plant-soil feedbacks and temporal dynamics of plant diversity-productivity relationships. Trends Ecol. Evol. 36, 651–661 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Soil Survey Staff, “Kellogg Soil Survey Laboratory methods manual, version 5.0” (Soil Survey Laboratory Investigations Rep. 42, US Department of Agriculture, 2017).
- 49.Eliason R., Goos R. J., Hoskins B., Recommended Chemical Soil Test Procedures for the North Central Region (Missouri Agricultural Experiment Station, rev. ed., 2015). [Google Scholar]
- 50.Ellert B. H., Bettany J. R., Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 75, 529–538 (1995). [Google Scholar]
- 51.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this paper have been deposited and are available from the Environmental Data Initiative. These datasets contain the following: soil C (https://doi.org/10.6073/pasta/9ed3740e181a3c41ec2cb787ef3a615b), soil N (https://doi.org/10.6073/pasta/9b55ae5a418c59fb8e3d7088c3591fc5), soil Bray P (https://doi.org/10.6073/pasta/b37f4e38718784480259f3d92fb7a9d7), soil CEC, K, Ca, Mg (https://doi.org/10.6073/pasta/0fa7f5b395f104ed346602a66696fa53), soil pH (https://doi.org/10.6073/pasta/810ebc0a46361bd6a4d1693f17b440fb), soil OM (https://doi.org/10.6073/pasta/6521113c6115b8fbeae7ef0ab6ebca9e), aboveground biomass (https://doi.org/10.6073/pasta/7ef2de3865062d7352f7b20753ecd39b), belowground biomass (https://doi.org/10.6073/pasta/0479da667672693c3cf2a6b2c8d14002), whole plot plant tissue chemistry (% N, P, K, Ca, Mg), (https://doi.org/10.6073/pasta/f433650417c15afb33d1dd0ad602a2e6), soil bulk density (https://doi.org/10.6073/pasta/270fbd77c7bd6d1cbb59647a3eccb639), individual plant chemistry traits (% N, P, K, Ca, Mg) (https://doi.org/10.6073/pasta/cea0d715283e4f0c2d71d28c8d146558). All data were submitted by the Cedar Creek Ecosystem Science Reserve site of the LTER Network. All study data are additionally included in the supporting information. Data to reproduce figures and analyses in the main text are included in Dataset S1 (Figs. 1–3) and Dataset S2 (Fig. 4). SI Appendix, Figs. S1, S2, S6–S9, and S11 can be reproduced using Dataset S1. SI Appendix, Figs. S3–S5 can be reproduced using Dataset S3. Data to reproduce the conversion of soil concentrations to area density quantities are available in Dataset S4. Data to reproduce the conversion of tissue concentrations to area density quantities are available in Dataset S5. Data to reproduce the derived response variables in Fig. 2 are available in Dataset S6. Data to reproduce SI Appendix, Fig. S10 are in Dataset S7. Metadata for these datasets are provided in the SI Appendix. R code to reproduce analyses is archived on Zenodo and will be maintained by G.N.F. (https://doi.org/10.5281/zenodo.5565171).