Abstract
Oncolytic viruses have been combined with standard cancer therapies to increase therapeutic efficacy. Given the sequential activation of herpes viral genes (HSV-1) and the temporal cellular changes induced by ionizing radiation, we hypothesized an optimal temporal sequence existed in combining oncolytic HSV-1 with ionizing radiation. Murine U-87 glioma xenografts were injected with luciferase encoding HSV-1 and ionizing radiation was given at times prior or after viral injection. HSV-1 replication and tumor volume response were followed. Radiation given 6–9 hours after HSV-1 injection resulted in maximal viral luciferase expression and infectious viral production in tumor xenografts. The greatest xenograft regression was also seen with radiation given 6 hr after viral injection. We then tested if HSV-1 replication had a dose response to ionizing radiation. HSV-1 luciferase expression exhibited a dose response as xenografts were irradiated from 0 to 5 Gy. There was no difference in viral luciferase expression as ionizing radiation dose increase from 5 Gy up to 20 Gy. These results suggest that the interaction of ionizing radiation with the HSV-1 lytic cycle can be manipulated for therapeutic gain by delivering ionizing radiation at a specific time within viral replicative cycle.
Keywords: Oncolysis, Radiation, Glioma, HSV-1
Introduction
High grade gliomas remain one of the most therapy-resistant tumors. Current therapy consists of maximal surgical resection followed by radiotherapy and chemotherapy1. However, median survival for the most aggressive tumors, glioblastoma multiforme, is 12–14 months. The predominant site of tumor recurrence following both surgery and radiation is local2. Because most tumor recurrences are within the initial tumor bed, direct injection of attenuated oncolytic viruses such as herpes simplex virus-1 (HSV-1) has been proposed as an adjuvant treatment and utilized in Phase I clinical trials for high grade gliomas3–7.
HSV-1 is a double stranded DNA virus expressing its genes in a sequential cascade of immediate-early (α), early (β), and late (γ) genes8. Immediate-early genes encode viral transcriptional regulators. Early viral genes predominantly encode for proteins involved in viral DNA synthesis. Late genes largely encode structural proteins used in progeny virions. Oncolytic viruses have been genetically engineered by deleting specific viral genes to restrict viral replication to cancer cells and also attenuate virulence to increase patient safety7,9. A major strategy has focused on deleting the γ134.5 late gene of HSV-1, which in part mediates neurovirulence10. Phase I clinical trials in patients with glioma have been completed with neuro-attenuated HSV-1 deleted of γ134.5 that have demonstrated the safety and feasibility of this approach4–6. However in creating clinically safe, replication-competent viruses, the therapeutic tradeoff is decreased viral replication to maintain safety which hampers anti-tumor efficacy.
We and others have shown that mutant HSV-1 and other classes of oncolytic viruses (adenovirus, measles virus, reovirus, VSV) can be combined with ionizing radiation (IR) to improve tumor xenograft control in experimental animal tumor model systems11–23. Specifically, γ134.5 deleted HSV-1 combined with IR resulted in increased viral replication and glioma xenograft regression in hindlimb and orthotopic preclinical murine glioma models11,17. Mechanistic studies have elucidated multiple pathways through which IR enhances viral replication. The HSV-1 immediate-early gene product ICP0 has been reported to interfere with DNA repair following irradiation of glioma cells resulting in increased tumor cell death18. IR also increases transcription of cellular genes which trans-complement the functions of deleted viral genes. IR enhanced expression of cellular ribonucleotide reductase assists the replication of viruses deleted of viral ribonucleotide reductase19. Another example of trans-complementation involves HSV-1 viruses deleted in γ134.5. Growth arrest and DNA damage 34 (GADD34) is a cellular homologue to the HSV-1 gene γ134.524. GADD34 is upregulated following IR and has been shown to increase the replication of HSV-1 viruses deleted in γ134.520,21,25. A third mechanism by which IR can enhance viral replication is by increased expression of viral proteins through upregulation of viral promoters. It has been shown that IR can enhance transcription of late HSV-1 viral promoters by activating the p38 MAPK pathway22. The elucidation of mechanisms through which IR enhances viral replication predicts that sequencing of IR and oncolytic viruses may be clinically relevant.
The current series of studies aimed to define optimal parameters for combining oncolytic HSV-1 and ionizing radiation for high grade glioma therapy. Our previous studies have shown that flank and orthotopic glioma models for studying the combination of IR and oncolytic HSV-1 produce similar results11,17. We therefore utilized the flank glioma model to gain in understanding in how to best combine oncolytic HSV with IR. Given that the effects of radiation are specific to different phases of the cell cycle and viral replication proceeds in a sequential cascade, we hypothesized an optimal time to deliver IR during the replicative cycle of oncolytic HSV-1 may exist to maximize oncolytic HSV-1 replication. Such a strategy could provide a rational basis for sequencing radiotherapy and oncolytic viral therapy that will facilitate developing future clinical trials.
Results
Delivering radiation during onset of late viral gene expression results in increased oncolytic viral gene expression
The cellular effects of radiation are specific to different phases of the cell cycle. Viral replication requires a temporal expression of viral genes and IR has been previously demonstrated to enhance late viral promoter genes. We hypothesized an optimal time to deliver IR during the replicative cycle of oncolytic HSV-1 to maximize HSV-1 replication existed. R2636 is a neuro-attenuated HSV-1 deleted in both copies of γ134.5 and encodes the luciferase gene22. R2636 was injected into glioma xenografts in the hindlimb of athymic nude mice on day 0 at time 0 hr. In irradiated glioma xenografts, 20 Gy was given either before (6 hr) or after (0.5, 6, 12, or 24 hr) viral injection. Glioma xenografts were serially imaged on days 1, 2, 3, 4, 5, and 7 days post infection to detect viral luciferase expression (Figure 1A). The photon flux from non-irradiated R2636 injected glioma xenografts on day 1 was arbitrarily set to 1, and photon flux for all other measurements were expressed relative to this value. In non-irradiated R2636 injected glioma xenografts, luciferase expression increased up to day 4 (2.7 fold) post injection after which it declined. In general, all irradiated glioma xenografts expressed higher levels of viral luciferase compared to non-irradiated glioma xenografts. Notably when IR was given at 6 hours following R2636 injection, a 3.5 fold increase in luciferase expression was observed compared to non-irradiated R2636 luciferase expression at day 4 post injection (p = 0.047) (Figure 1A).
Figure 1.
Optimal Timing of Radiation in Glioma Xenografts Injected with Oncolytic HSV-1 for Viral Gene Expression and Tumor Regression. HSV-1 R2636 was injected at time 0, and 20 Gy of IR was delivered before or after viral injection. 1A: Luciferase expression was quantified on days 1, 2, 3, 4, 5, and 7 after viral injection in non-irradiated and irradiated mice by measuring photon flux. The mean bioluminescence of 5 xenografts per group are shown, and normalized to values obtained in non-irradiated R2636 injected xenografts on day 1. 1B: Fractional tumor volumes of glioma xenografts imaged in Figure 1A Tumors volume measurements were normalized to tumor volumes on day 0.
Enhanced oncolytic viral gene expression following ionizing radiation results in increased infectious viral particle production
We next determined if IR enhanced viral gene expression resulted in increased infectious viral particle formation within glioma xenografts. R3616, the prototypical neuro-attenuated HSV-1 deleted for both copies of γ134.5, was injected into glioma xenografts and 20 Gy was given either before or after viral injection13. Glioma xenografts were harvested 3 days post viral injection and infectious viral particles were quantified (Figure 2). Infectious viral particles were normalized to non-irradiated R3616 injected glioma xenografts at day 3. Similar to the in-situ imaging results, IR delivered 6–9 hrs post viral injection resulted in the highest recovery of infectious viral particles, 2.3–2.9 fold (p =0.056). Interestingly, IR delivered 12 hrs post-infection did not result in as much infectious viral particle production, but IR given 24 hrs resulted in levels of infectious particles similar to when IR was delivered 6–9 hrs post infection. These results may be explained by the fact that it takes approximately 16–18 hrs for HSV-1 to replicate, therefore giving IR 24 hrs after infection may represent 6 – 8 hrs into the course of the replicative cycle of progeny virus from the initial viral injection.
Figure 2.
Influence of Timing Sequence of Oncolytic HSV-1 and Radiation on HSV-1 Progeny Production. Infectious viral particles were quantified from xenografts harvested 3 days after injection with R3616 and irradiated with 20 Gy before or after viral injection. Viral particle production was normalized to non-irradiated R3616 infected glioma xenografts.
Optimal timing of oncolytic HSV-1 and ionizing radiation results in increased tumor xenograft regression
Since oncolytic HSV-1 and IR sequencing resulted in differential levels of viral gene expression and infectious viral particle production we next determined if IR and viral sequencing translated to increased glioma xenograft regression. Glioma xenografts treated as above in Figure 1A and serially imaged were also measured to assess tumor cell destruction. The greatest glioma xenograft regression was observed with IR delivered 6 hr after R2636 injection, correlating directly with maximal luciferase expression (Figure 1A and 1B). Glioma xenograft regression for this treatment schedule was significantly increased by day 8 compared to non-irradiated glioma xenografts (p = 0.00018) and importantly also increased compared to the groups treated with R2636 and IR at times other than 6 hr post infection (p = 0.036). This is consistent with our previous studies where we showed that combining oncolytic HSV-1 with IR resulted in significantly greater U87 xenograft regression starting at day 10 post initiation of therapy through day 6011. We also tested if combining IR with oncolytic HSV-1 resulted in increased tumor xenograft regression in another glioma cell line, D54 (Supplementary Figure 1). IR was given 24 hours post intratumoral viral injection. The combination of HSV and IR resulted in significantly greater tumor regression than IR alone or virus alone (p<0.04).
HSV-1 late gene promoter activation exhibits a dose response to ionizing radiation
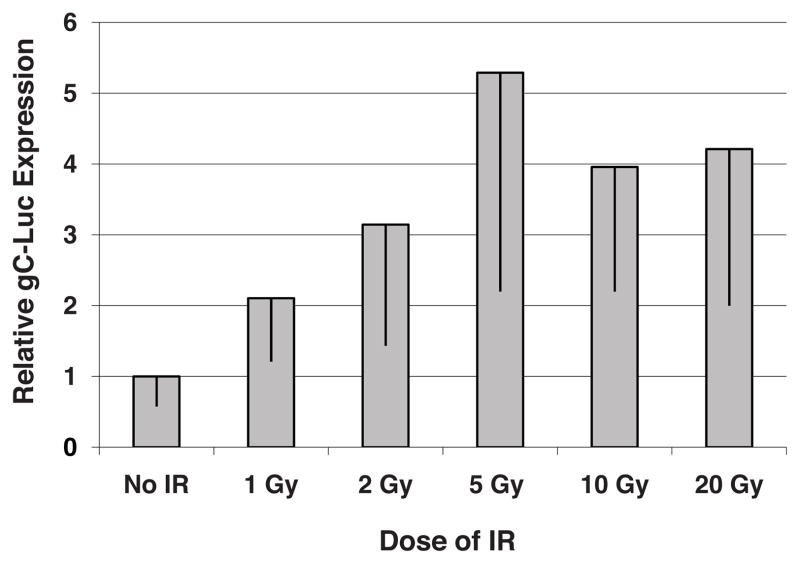
We then determined if an IR dose response to enhance oncolytic HSV-1 gene expression existed. Glioma xenografts were injected with R2636 at day 0, time 0 hr and then 0, 1, 2, 5, 10, or 20 Gy of IR was delivered 6 hrs post viral injection, the optimal temporal sequence from the above series of experiments (Figure 1 and 2). Viral luciferase expression was measured 4 days following injection (Figure 3). Viral luciferase expression increased with increasing doses of IR from 0 to 5 Gy after which the detection of luciferase leveled off up to 20 Gy. The combined groups of 5, 10, and 20 Gy had significantly higher luciferase expression than non-irradiated tumors (p=0.012). While increasing doses of IR are result in greater cell kill, these data suggest that at a minimum 5 Gy should be delivered to take advantage of the effect of IR on viral replication.
Figure 3.
Irradiation Dose Response of Viral gC-luciferase Expression. U87 xenografts were injected with R2636 and irradiated 6 hrs later, bioluminescence imaging was done 4 days after viral injection. Photon fluxes were normalized to non-irradiated R2636 injected xenografts.
Discussion
In this series of studies, we have shown that the interaction of IR with the HSV-1 lytic cycle can be manipulated for a therapeutic gain. Viral replication occurs in a temporal sequential cascade of gene expression of immediate early, early, and late gene expression8. Ionizing radiation’s effects on cellular gene expression is also temporal, suggesting a favorable sequence of oncolytic virus and IR delivery. In our preclinical animal tumor model system, the replicative and oncolytic potential of attenuated HSV-1 is optimized by delivering IR at a specific period within sequential cascade of the viral replicative cycle (Figure 4A). While delivering radiation at other times in the viral lytic cycle enhanced viral replication and increased tumor regression as compared to non-irradiated tumors, delivering IR 6–9 hrs into the course of viral infection resulted in the largest increase in viral transcription as measured by viral encoded luciferase expression. Enhanced viral gene expression correlated with increased production of infective viral particles and maximal glioma xenograft regression (Figure 1, 2). While the current studies measured immediate effects of IR on viral replication and tumor regression, our previous studies have shown longer term tumor xenograft regression in glioma flank models and survival in glioma intracranial modsl when IR is combined with oncolytic HSV-111,17. To our knowledge this is the first report showing how optimally delivering IR with oncolytic virus results in increased viral gene expression, infectious viral particle production and tumor xenograft regression. Differential glioma xenograft responses as a consequence of only altering the temporal sequence of IR and HSV-1 directly implicate an interaction of IR and HSV-1 in mediating enhanced glioma xenograft regression.
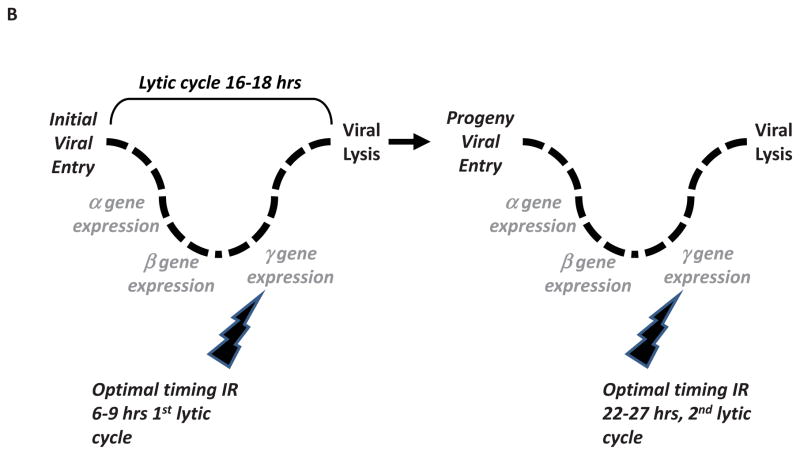
Figure 4.
Mechanistic Model of the Interaction of Radiation and Oncolytic HSV-1. 4A: The interaction of IR during a single HSV-1 replicative cycle. Following receptor mediated internalization of HSV-1, a temporal cascade of HSV-1 gene expression occurs. Initial α gene expression is followed by β genes (function to replicate viral DNA), and finally γ genes (involved in assembly of progeny virus). Ionizing radiation has been shown to enhance replication of HSV-1 by multiple mechanisms. As depicted, IR delivered at different times in relation to the viral replicative cycle can enhance the oncolytic ability of viruses. 4B: Clinically incorporating radiotherapy with oncolytic HSV-1. As oncolytic viruses replicate and spread within tumor, radiotherapy could be optimally delivered so as to coincide with late viral gene expression (i.e. 6–9 hrs after viral entry) in later viral replicative life cycles. This interaction may be clinically incorporated into hypofractionated radiotherapy.
IR enhanced oncolysis has been shown to occur through transcriptional and post-translational changes of cellular and viral proteins (Figure 4A)23. In regards to HSV-1, viral ICP0 has been shown to interfere with IR mediated DNA damage repair through its actions on DNA-PK18. IR has been shown to increase transcription of cellular genes that trans-complement viral gene deletions (i.e. ribonucleotide reductase and GADD34)19–21. Also, IR enhances transcription of viral late promoters by phosphorylating and activating p38 MAPK22. These multiple mechanisms of IR mediated enhancement of HSV-1 viral replication are supported by our data. IR delivered at various time points in the HSV-1 replicative cycle increased viral replication, suggesting the pleiotropic role of IR in enhancing viral replication. However, IR mediated enhancement of viral replication was greatest when IR was delivered 6–9 hours following viral infection. The increased tumor xenograft regression observed when IR is delivered at this time point post viral injection compared to other time points provides direct evidence for the interactive nature of IR and oncolytic HSV-1. Interestingly, the second best time to deliver IR was 24 hrs post viral injection. One explanation for this result may be the fact that it takes approximately 16–18 hrs for HSV-1 to replicate, therefore giving IR 24 hrs after infection may represent 6 hrs into the course of the replicative cycle of progeny virus (i.e. 2nd life cycle) from the initial viral injection (Figure 4B).
The other major radiotherapeutic parameter is the dose of IR to deliver following oncolytic HSV-1 injection. Our data indicates a radiation dose response from 0 to 5 Gy after which a plateau is reached to 20 Gy. This has several important clinical implications. Radiotherapy has traditionally been delivered in 2 Gy fractions to a total dose of 60 Gy for high grade gliomas1. Technological advancements in the delivery of radiotherapy have allowed for altered fractionation schemes to be delivered, such as hypofractionated (5 Gy fractions) and higher radiosurgical doses (20 Gy)26,27. Also, critical structure tolerances (such as the brainstem or optic chiasm) to IR guide the choice of radiation dose that can be safely delivered. Our data suggest that a single radiation dose of 5 Gy may be sufficient to enhance viral replication and serve as the basis for future clinical trials with oncolytic HSV-1 and radiotherapy to enhance viral gene expression and infectious viral production. A recent study has shown that the dose rate of IR is also an important consideration when combining IR with oncolytic viruses28.
Phase I clinical trials have already begun to explore the feasibility of combining oncolytic viruses with radiotherapy29–31. A clinical trial has also been undertaken to demonstrate the safety and feasibility of combining IR (single 5 Gy fraction) and oncolytic HSV-1 (JM Markert, personal communication). The data from the current study in part provided the basis for this trial in which radiotherapy is rationally integrated into the viral replicative cycle. These data suggest a new therapeutic role for IR in cancer therapy, that in addition to its inherent tumoricidal activity, IR can enhance viral gene expression and replication which results in increased tumor regression. In such a treatment paradigm, the spatial and temporal regulation of IR delivery is used to increase the therapeutic ratio of attenuated oncolytic viruses.
Materials and Methods
Cells and viruses
Vero and U-87MG (glioma) cell lines were obtained from American Type Culture Collection. R3616 was derived from HSV-1(F) and lacks both copies of γ134.5 as previously described10. R2636 is also a HSV-1 mutant deleted for both copies of γ134.5, but encodes the luciferase gene under the control of the HSV-1 gC promoter as previously described22.
Glioma xenograft bioluminescence imaging
U-87MG xenografts were grown in the right hindlimb of athymic nude mice by subcutaneous injection of 1×107 cells in 100 μl of PBS. For timing experiments, tumor xenografts were injected simultaneously with 2×107 pfu R2636 in 10 ul of buffer at time defined as day 0, time 0 hr from the same aliquot of stock of virus. 20 Gy of IR was given at time points prior or after viral injection as indicated in the Results as previously described using a superficial X-ray generator11. Luciferase expression was imaged serially in mice from days 1 through 7 post injection using an IVIS 200 Imaging System and Living Image software. Prior to imaging, mice were i.p. injected with D-luciferin (15 mg/kg) and anesthetized with xylazine (5 mg/kg) and ketamine (75 mg/kg). Glioma xenografts were imaged 15 minutes after luciferin injection. For radiation dose response experiments, tumor xenografts were injected with 2×107 pfu of R2636 on day 0 at time 0 hr from the same aliquot of stock virus. Six hours after viral injection 0, 1, 2, 5, 10, or 20 Gy was delivered. Tumor xenografts were imaged for luciferase expression 4 days post viral injection. All animal studies were done in accordance with the University of Chicago Animal Care and Use Committee standards.
Glioma xenograft viral titers
U-87MG xenografts were injected with 2×107 pfu of R3616 on day 0, time 0 hr from the same aliquot of virus. 20 Gy was given at time points prior to or after viral injection as indicated in the Results. Three days post viral injection, tumor xenografts were harvested and infectious viral particles were quantified on Vero cells as previously described11.
Glioma xenograft regression studies
Mice imaged for luciferase expression also had their tumor xenografts measured biweekly as previously described11. Fractional tumor volume (FTV) is defined as the tumor volume at the specified day divided by the initial tumor volume (V/V0).
Statistical analysis
One-tailed t-tests were performed using Microsoft Excel to compare experimental groups to untreated controls. We and others have previously shown that IR increased oncolytic HSV-1 replication11–23. The hypothesis tested in this series of experiments was that if varying the timing of IR in relation to the timing of viral injection would result in a further enhancement of viral replication.
Supplementary Material
Acknowledgments
This research was supported from grants from the US National Institutes of Health, CA 071933 and CA 097247. We thank Jennifer Coleman for assistance with manuscript preparation.
Footnotes
Conflict of Interest:
The authors declare no conflict of interest
Supplementary Information is available at Gene Therapy website
References
- 1.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 3.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 4.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 5.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34. 5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 6.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker JN, Bauer DF, Cody JJ, Markert JM. Oncolytic viral therapy for malignant glioma. Neurotherapeutics. 2009;6:558–569. doi: 10.1016/j.nurt.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 10.Chou J, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134. 5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 11.Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- 12.Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Sarkaria JN, Petell CA, Paraskevakou G, Zollman PJ, Schroeder M, et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res. 2007;12:7155–7165. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- 14.Twigger K, Vidal L, White CL, De Bono JS, Bhide S, Coffey M, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008;14:912–923. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- 15.Alajez NM, Mocanu JD, Shi W, Chia MC, Breitbach CJ, Hui AB, et al. Efficacy of systemically administered mutant vesicular stomatitis virus (VSVDelta51) combined with radiation for nasopharyngeal carcinoma. Clin Cancer Res. 2008;14:4891–4897. doi: 10.1158/1078-0432.CCR-07-4134. [DOI] [PubMed] [Google Scholar]
- 16.Qian J, Yang J, Dragovic AF, Abu-Isa E, Lawrence TS, Zhang M. Ionizing radiation-induced adenovirus infection is mediated by dynamin 2. Cancer Res. 2005;65:5493–5497. doi: 10.1158/0008-5472.CAN-04-4526. [DOI] [PubMed] [Google Scholar]
- 17.Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, et al. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. 1999;5:1517–1522. [PubMed] [Google Scholar]
- 18.Hadjipanayis CG, DeLuca NA. Inhibition of DNA repair by a herpes simplex virus vector enhances the radiosensitivity of human glioblastoma cells. Cancer Res. 2005;65:5310–5316. doi: 10.1158/0008-5472.CAN-04-3793. [DOI] [PubMed] [Google Scholar]
- 19.Stanziale SF, Petrowsky H, Joe JK, Roberts GD, Zager JS, Gusani NJ, et al. Ionizing radiation potentiates the antitumor efficacy of oncolytic herpes simplex virus G207 by upregulating ribonucleotide reductase. Surgery. 2002;132:353–359. doi: 10.1067/msy.2002.125715. [DOI] [PubMed] [Google Scholar]
- 20.Adusumilli PS, Chan MK, Hezel M, Yu Z, Stiles BM, Chou TC, et al. Radiation-induced cellular DNA damage repair response enhances viral gene therapy efficacy in the treatment of malignant pleural mesothelioma. Ann Surg Oncol. 2007;14:258–269. doi: 10.1245/s10434-006-9127-4. [DOI] [PubMed] [Google Scholar]
- 21.Adusumilli PS, Stiles BM, Chan MK, Chou TC, Wong RJ, Rusch VW, et al. Radiation therapy potentiates effective oncolytic viral therapy in the treatment of lung cancer. Ann Thorac Surg. 2005;80:409–416. doi: 10.1016/j.athoracsur.2005.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezhir JJ, Advani SJ, Smith KD, Darga TE, Poon AP, Schmidt H, et al. Ionizing radiation activates late herpes simplex virus 1 promoters via the p38 pathway in tumors treated with oncolytic viruses. Cancer Res. 2005;65:9479–9484. doi: 10.1158/0008-5472.CAN-05-1927. [DOI] [PubMed] [Google Scholar]
- 23.Advani SJ, Mezhir JJ, Roizman B, Weichselbaum RR. ReVOLT: radiation-enhanced viral oncolytic therapy. Int J Radiat Oncol Biol Phys. 2006;66:637–646. doi: 10.1016/j.ijrobp.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 24.He B, Chou J, Liebermann DA, Hoffman B, Roizman B. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the gamma(1)34. 5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J Virol. 1996;70:84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollander MC, Zhan Q, Bae I, Fornace AJ., Jr Mammalian GADD34, an apoptosis- and DNA damage-inducible gene. J Biol Chem. 1997;272:13731–13737. doi: 10.1074/jbc.272.21.13731. [DOI] [PubMed] [Google Scholar]
- 26.Hudes RS, Corn BW, Werner-Wasik M, Andrews D, Rosenstock J, Thoron L, et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999;43:293–298. doi: 10.1016/s0360-3016(98)00416-7. [DOI] [PubMed] [Google Scholar]
- 27.Torcuator RG, Thind R, Patel M, Mohan YS, Anderson J, Doyle T, et al. The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol. 2010;97:401–407. doi: 10.1007/s11060-009-0034-y. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Zhang Y, Liu MM, Zhou H, Chowdhury W, Lupold SE, et al. Evaluation of low dose rate versus acute single high dose rate radiation combined with oncolytic viral therapy in prostate cancer. Int J Radiat Biol. 2010;86:220–229. doi: 10.3109/09553000903419338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 30.Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. 2010;16:3067–3077. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.