Abstract
PURPOSE:
Clinical models to identify patients at high risk of primary graft dysfunction (PGD) after heart transplantation (HT) are limited, and the underlying pathophysiology of this common post-transplant complication remains poorly understood. We sought to identify whether pre-transplant levels of circulating proteins reporting on immune activation and inflammation are associated with incident PGD.
METHODS:
The study population consisted of 219 adult heart transplant recipients identified between 2016 and 2020 at Duke University Medical Center, randomly divided into derivation (n = 131) and validation (n = 88) sets. PGD was defined using modified ISHLT criteria. Proteomic profiling was performed using Olink panels (n = 354 proteins) with serum samples collected immediately prior to transplantation. Association between normalized relative protein expression and PGD was tested using univariate and multivariable (recipient age, creatinine, mechanical circulatory support, and sex; donor age; ischemic time) models. Significant proteins identified in the derivation set (p < 0.05 in univariate models), were then tested in the validation set. Pathway enrichment analysis was used to test candidate biological processes. The predictive performance of proteins was compared to that of the RADIAL score.
RESULTS:
Nine proteins were associated with PGD in univariate models in the derivation set. Of these, only CLEC4C remained associated with PGD in the validation set after Bonferroni correction (OR [95% CI] = 3.04 [1.74,5.82], p = 2.8×10−4). Patterns of association were consistent for CLEC4C in analyses stratified by biventricular/left ventricular and isolated right ventricular PGD. Pathway analysis identified interferon-alpha response and C-type lectin signaling as significantly enriched biologic processes. The RADIAL score was a poor predictor of PGD (AUC = 0.55). CLEC4C alone (AUC = 0.66, p = 0.048) and in combination with the clinical covariates from the multivariable model (AUC = 0.69, p = 0.018) improved discrimination for the primary outcome.
CONCLUSIONS:
Pre-transplantation circulating levels of CLEC4C, a protein marker of plasmacytoid dendritic cells (pDCs), may identify HT recipients at risk for PGD. Further studies are needed to better understand the potential role pDCs and the innate immune response in PGD.
Keywords: primary graft dysfunction, heart transplant, proteomics, innate immunity, plasmacytoid dendritic cell
Recent innovations in heart transplantation (HT), such as recipient optimization, changes in organ allocation, and diversified mechanical circulatory support (MCS) strategies, have led to improved outcomes for HT patients. Nonetheless, HT patients remain at risk for early post-transplant morbidity and mortality, including rejection, infection, and primary graft dysfunction (PGD).1 PGD in particular is a poorly understood entity that is responsible for over 60% of deaths occurring in the first 30 days after HT.2 Defined as failure of the new allograft to provide sufficient support to the recipient circulation within the first 24h following transplantation, in the absence of hyperacute rejection or surgical complication, PGD leads to multisystem organ failure, prolonged intubation, and increased risk of infection. Despite this, the underlying pathophysiology of PGD remains ill-defined and there exist no disease-specific treatments.
Risk stratification of HT recipients for the development of PGD is a clinical challenge. To date, the RADIAL score, derived in Europe using data collected between 2006 and 2014, is the only clinical risk score validated to predict PGD.3 However, the major components of the RADIAL score (recipient right atrial pressure ≥ 10 mmHg, recipient age ≥ 60, diabetes mellitus, inotrope dependence, donor age ≥ 30, and donor heart ischemic time ≥ 240 minutes) fail to capture contemporary challenges in HT, including bridge to transplant with left ventricular assist devices (LVAD) and other short term MCS, which have been previously associated with increased risk of severe PGD.4 A more precise understanding of recipient-level biologic risks (e.g., circulating biomarkers) could enhance mechanistic knowledge, improve risk stratification for PGD, and ultimately improve donor-recipient matching, organ procurement and preservation strategies, and peri-transplant management algorithms.
Based upon previously published small, single-center studies, we hypothesized that pre-transplant innate immune activation and inflammation in HT recipients may contribute to post-HT PGD risk, and that relevant biomarkers in these pathways may be used to identify patients at high risk for this devastating complication.5,6 The objective of this study was to use high-throughput proteomic profiling of pre-transplant serum from HT recipients to identify relevant biomarkers and pathways involved the development of PGD.
Materials and methods
Study population
Of the 324 HT performed at Duke University Medical Center between March 2016 and April 2020, we identified 219 adult, first time, single-organ recipients who had available pre-transplant serum available for retrospective analysis. Those patients whose organ was procured using investigational warm ex vivo perfusion and/or received organs following donor after circulatory death (DCD) were excluded from the current study, as these procurement and preservation strategies were considered investigational during this time period. There were no exclusion criteria based on pre-transplant pulmonary pressures or perioperative crossmatch results. Eligible patients were randomly assigned to either derivation (60%) or validation (40%) sets, with equal proportions of patients transplanted after October 18, 2018 and patients with PGD. HT recipient serum samples used for this study were residual material from samples collected for routine clinical care within 24 hours prior to HT. Blood samples were collected via venous phlebotomy into EDTA tubes, processed to serum, and stored at −80°C.
Definition of PGD
PGD was defined and adjudicated for this study using modified criteria from the ISHLT 2014 consensus conference.2 PGD cases for this study were identified when intraoperative transesophageal echocardiogram after HT demonstrated a left ventricular ejection fraction (LVEF) < 40% and/or moderate to severe right ventricular dysfunction. These cases were further categorized as moderate PGD when requiring intra-aortic balloon pump (IABP) placement or high dose inotropes (defined as vasoactive inotrope score >10) 7; or severe PGD when requiring cannulation for veno-arterial extracorporeal membranous oxygenation (VA-ECMO) or placement of a surgical ventricular assist device. Controls were defined as patients who did not meet these criteria for either moderate or severe PGD. All adjudications for PGD were performed within the first 24 hours following HT. No patients who developed secondary graft dysfunction in the setting of surgical complications (e.g. hemorrhage) were included in the current study. All recipients underwent endomyocardial biopsy approximately one week after transplantation.
Proteomic profiling
Serum samples were stored frozen until proteomic profiling was performed using the Olink platform (Uppsala, Sweden).8 Olink is based on a Proximity Extension Assay (PEA) that uses a dual recognition, DNA-coupled immunoassay that rapidly allows for protein identification and relative quantification with high sensitivity and specificity. After normalization and adjustment for technical variation between plates, data from each assay are rendered as normalized log2 protein expression (NPX) values, a measure of relative quantification between samples for a given analyte. For the current study, we utilized four Olink panels (Cardiovascular II, Cardiovascular III, Inflammation, and Immune Activation), which together quantify 354 distinct protein biomarkers.
Samples were run in two batches, with quality control (QC) performed separately in each batch on a per-panel basis. Seven samples were flagged for having control probe values > 0.3 NPX from the plate median. All problematic control probe values were close to the cutoff of 0.3, so the data were retained in the primary analysis but removed in a subsequent sensitivity analysis. Assay CVs for each panel ranged from 4% to 6%. Samples were examined for high proportions of proteins below the limit of detection (LOD) and for outlying NPX values (median and IQR). Two samples were removed for having extremely low NPX values across all 4 panels. The mean per-sample percentage of assays below LOD was 3.0% in the first batch and 4.7% in the second. Twelve representative samples that performed well in the first batch were re-run in the second batch and used for reference sample normalization, which allowed the 2 batches to be analyzed jointly. After normalization, NPX values for proteins with < 25% of measurements below the LOD for both the discovery and validation sets were converted to a Z-score (number of standard deviations from the mean) for analysis, while proteins with 25% to 75% of NPX values below the LOD in either set were instead treated as a binary variable (detected vs undetected). Proteins with > 75% values below the LOD in either set were removed from analysis.
Statistical methods
Clinical variables were summarized and compared between PGD cases and controls. Variables are presented as mean (standard deviation) for normally distributed continuous variables, median [IQR] for non-normally distributed continuous variables, and as counts and percentages for categorical data. Groups were compared using a t-test, Wilcoxon rank-sum test, or chi-assessed using (1) a univariate logistic regression model adjusted for assay batch only, and (2) a full multivariable logistic regression model also adjusted for recipient age, recipient sex, recipient creatinine, pre-transplant MCS, donor age, and ischemic time. Protein biomarkers found to be significantly associated with PGD in univariate models in the derivation set (p < 0.05) were then tested in the validation set (with Bonferroni adjustment for multiple tests). Pathway analysis was performed on pre-specified candidate gene sets: 4 hallmark gene sets (TNF-α signaling via NFκβ, inflammatory response, interferon-alpha response, and interferon-gamma response) and 8 KEGG pathways (cytokine-cytokine receptor interaction, viral protein interaction with cytokine and cytokine receptor, TNF signaling pathway, NFκβ signaling pathway, Toll-like receptor signaling pathway, natural killer cell mediated cytotoxicity, C-type lectin receptor signaling pathway, and IL-17 signaling pathway).9,10 Pathway over-representation analysis (ORA) was conducted via a hypergeometric test of proteins that were nominally significant in univariate modeling of the combined set (derivation and validation) on a background of all tested proteins. Gene set enrichment analysis (GSEA) was performed using the p-values from the univariate models for all tested proteins in the combined derivation and validation set.11 Receiver operating characteristic (ROC) curve analysis was also performed to assess the discrimination of protein biomarkers and associated models for the primary outcome and to compare their performance with that of the RADIAL clinical score. The RADIAL score was calculated by adding one point for each of the six risk factors present in a given HT: right atrial pressure ≥ 10 mmHg, recipient age ≥ 60, recipient diabetes mellitus, recipient inotrope dependence, donor age ≥ 30, and ischemic time ≥ 240 minutes. All statistical analyses were conducted using R v4.0.5.12
The study, including retrospective collection of clinical data and use of associated residual serum samples, was approved by the Duke University Institutional Review Board. This study adheres to and is compliant with the ISHLT Ethics statement on transplantation.
Results
Study population
A total of 131 patients were included in the derivation set, of which 39 (29.8%) were PGD cases (24 moderate and 15 severe PGD), and a total of 88 patients were included in the validation set, of which 26 (29.5%) were PGD cases (18 moderate and 8 severe PGD). Baseline clinical characteristics, hemodynamics, and laboratory values of cases and controls in the derivation and validation sets are displayed in Table 1. In the derivation and validation sets, there were no significant differences in demographics, past medical history, or wait list time between cases and controls (Supplemental Table 1). Pre-transplant hemodynamics, laboratory values, pre-transplant IABP use, and ischemic times were also similar. When the derivation and validation sets were compared, only recipient age, race, and total bilirubin remained unbalanced in the groups.
Table 1.
Clinical Characteristics of the Recipients and Donors in the Derivation Set
| Derivation Set | Validation Set | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Overall(n = 131) | Controls(n = 92) | PGD Cases(n = 39) | p-value | Overall(n = 88) | Controls(n = 62) | PGD Cases(n = 26) | p-value |
| Recipient Clinical Characteristics | ||||||||
| Recipient Age (years), mean(SD) | 52.03 (12.37) | 52.02 (12.69) | 52.05 (11.74) | 1 | 55.43 (10.92) | 56.48 (11.35) | 52.92 (9.56) | 0.2 |
| Male Sex, n(%) | 90 (68.7) | 65 (70.7) | 25 (64.1) | 0.6 | 63 (71.6) | 45 (72.6) | 18 (69.2) | 1.0 |
| Diabetes Mellitus, n(%) | 30 (23.1) | 24 (26.1) | 6 (15.8) | 0.3 | 22 (25.0) | 17 (27.4) | 5 (19.2) | 0.6 |
| Ethnicity, n(%) | 0.9 | 0.8 | ||||||
| White | 91 (69.5) | 64 (69.6) | 27 (69.2) | 47 (53.4) | 34 (54.8) | 13 (50.0) | ||
| Black | 34 (26.0) | 24 (26.1) | 10 (25.6) | 39 (44.3) | 27 (43.5) | 12 (46.2) | ||
| Other | 6 (4.6) | 4 (4.3) | 2 (5.1) | 2 (2.3) | 1 (1.6) | 1 (3.8) | ||
| BMI (kg/m2), mean(SD) | 27.51 (4.66) | 27.40 (4.91) | 27.76 (4.06) | 0.7 | 27.41 (4.45) | 27.30 (4.41) | 27.68 (4.61) | 0.7 |
| Diagnosis, n(%) | 0.4 | 0.6 | ||||||
| Non-Ischemic CM | 90 (68.7) | 66 (71.7) | 24 (61.5) | 54 (61.4) | 40 (64.5) | 14 (53.8) | ||
| Ischemic CM | 27 (20.6) | 18 (19.6) | 9 (23.1) | 24 (27.3) | 16 (25.8) | 8 (30.8) | ||
| HCM/RCM/Congenital | 14 (10.7) | 8 (8.7) | 6 (15.4) | 10 (11.4) | 6 (9.7) | 4 (15.4) | ||
| Prior Sternotomy, n(%) | 50 (39.4) | 35 (39.3) | 15 (39.5) | 1 | 30 (34.5) | 23 (37.7) | 7 (26.9) | 0.5 |
| Total Days on Wait List, median[IQR] | 37.00 [9.50, 170.00] |
34.00 [10.75, 212.75] |
37.00 [8.00, 100.00] |
0.5 | 39.00 [12.00, 141.25] |
51.50 [14.00, 157.00] |
23.50 [6.50, 87.25] |
0.1 |
| MCS as BTT, n(%) | 38 (29.0) | 27 (29.3) | 11 (28.2) | 1 | 30 (34.1) | 24 (38.7) | 6 (23.1) | 0.2 |
| IABP, n(%) | 57 (43.5) | 36 (39.1) | 21 (53.8) | 0.2 | 36 (40.9) | 24 (38.7) | 12 (46.2) | 0.7 |
| Inotrope Dependence, n(%) | 73 (55.7) | 49 (53.3) | 24 (61.5) | 0.5 | 48 (54.5) | 34 (54.8) | 14 (53.8) | 1.0 |
| Recipient Hemodynamics | ||||||||
| CVP (mmHg), median[IQR] | 11.00 [7.00, 14.00] |
11.00 [7.00, 13.00] |
12.00 [7.00, 16.00] |
0.7 | 9.00 [6.00, 13.00] |
9.00 [6.00, 13.00] |
9.00 [6.00, 15.00] |
0.8 |
| Mean PA Pressure (mmHg), mean(SD) | 29.34 (9.04) | 28.98 (9.08) | 30.18 (9.00) | 0.5 | 29.38 (9.49) | 30.07 (8.22) | 27.77 (11.99) | 0.3 |
| Pulmonary Capillary Wedge Pressure (mmHg), mean(SD) | 19.42 (8.45) | 18.92 (8.42) | 20.59 (8.49) | 0.3 | 19.46 (8.05) | 19.66 (7.70) | 19.04 (8.92) | 0.7 |
| Cardiac Index (L/min/m2), mean(SD) | 2.15 (0.53) | 2.16 (0.57) | 2.12 (0.45) | 0.7 | 2.11 (0.51) | 2.10 (0.52) | 2.13 (0.49) | 0.8 |
| Pulmonary Vascular Resistance (Woods units), mean(SD) | 2.51 (1.31) | 2.50 (1.39) | 2.54 (1.13) | 0.9 | 2.70 (1.59) | 2.72 (1.29) | 2.66 (2.15) | 0.9 |
| Recipient Laboratory Values | ||||||||
| Sodium (mEq), mean(SD) | 135.16 (11.41) | 135.00 (13.42) | 135.54 (3.91) | 0.8 | 135.69 (3.92) | 135.70 (4.28) | 135.65 (3.01) | 1.0 |
| Blood Urea Nitrogen (mg/dL), mean(SD) | 18.84 (7.64) | 19.46 (7.84) | 17.38 (7.04) | 0.2 | 19.01 (9.28) | 19.58 (10.18) | 17.65 (6.61) | 0.4 |
| Creatinine (mg/dL), mean(SD) | 1.21 (0.34) | 1.24 (0.35) | 1.14 (0.33) | 0.1 | 1.18 (0.31) | 1.17 (0.32) | 1.18 (0.30) | 0.9 |
| Total Bilirubin (g/dL), median [IQR] | 1.00 [0.80, 1.50] | 1.10 [0.80, 1.50] | 1.00 [0.80, 1.35] | 0.3 | 0.90 [0.60, 1.30] | 0.90 [0.70, 1.30] | 0.80 [0.60, 1.30] | 0.4 |
| Albumin (g/dL), mean(SD) | 3.61 (0.54) | 3.65 (0.50) | 3.54 (0.63) | 0.3 | 3.53 (0.56) | 3.54 (0.54) | 3.52 (0.61) | 0.9 |
| Donor Characteristics | ||||||||
| Donor Age (years), mean ± SD | 32.31 (10.90) | 33.14 (11.04) | 30.36 (10.43) | 0.2 | 33.44 (9.97) | 33.13 (9.25) | 34.19 (11.68) | 0.7 |
| Ischemic Time (hours) mean ± SD | 2.95 (0.76) | 2.87 (0.75) | 3.12 (0.76) | 0.08 | 2.95 (0.87) | 2.83 (0.88) | 3.23 (0.80) | 0.1 |
| Gender Mismatch, n(%) | 14 (10.7) | 11 (12.0) | 3 (7.7) | 0.7 | 5 (5.7) | 5 (8.1) | 0 (0.0) | 0.3 |
BMI, body mass index; CM, cardiomyopathy; HCM, hypertrophic cardiomyopathy; RCM, restrictive cardiomyopathy; MCS, mechanical circulatory support – temporary or durable; IABP, intra-aortic balloon pump; CVP, central venous pressure; PA, pulmonary artery.
Gender mismatch defined by male recipient of female donor.
Proteomic profiling identifies CLEC4C as significantly associated with PGD
Proteomic profiling in the derivation set revealed a total of 342 proteins that passed quality control and had ≤ 75% of NPX values below LOD; of these, nine proteins were nominally significant in univariate analysis with six proteins remaining significant in the multivariable model (Table 2). These nine proteins were carried forward for testing in the validation set, where significance was determined by a Bonferroni adjustment for multiple tests (p < 0.0056). In the validation set, only CLEC4C (C-Type Lectin Domain Family 4 Member C) was significantly associated with PGD (OR [95% CI] = 3.04 [1.74,5.82], p = 2.8×10−4); it remained significant in the full model after adjustment for clinical covariates (OR [95% CI] = 3.13 [1.76,6.11], p = 2.8×10−4) and in a sensitivity analysis that included race (OR [95% CI] = 3.44 [1.86,7.14], p = 2.6×10−4), a variable whose distribution was significantly different in the derivation and validation sets. CLEC4C expression was higher in cases as compared to controls in both the derivation and validation set (Figure 1). When the derivation and validation sets were combined in a sensitivity analysis, CLEC4C remained the protein that was most significantly associated with PGD (OR [95% CI] = 1.89 [1.38,2.64], p = 1.3×10−4) (Figure 2).
Table 2.
Proteins Associated with PGD
| Derivation set | Validation set | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay | Univariate OR (95%CI) | Univariate p-value | Full model OR (95% CI) | Full model p-value | Assay | Univariate OR (95%CI) | Univariate p-valuea | Full model OR (95% CI) | Full model p-valuea |
| CLEC4C | 1.51 (1.02,2.30) | 0.045 | 1.52 (1.02,2.35) | 0.048 | CLEC4C | 3.04 (1.74,5.82) | 0.00028 | 3.13 (1.76,6.11) | 0.00028 |
| PSGL-1 | 0.65 (0.43,0.95) | 0.029 | 0.57 (0.35,0.87) | 0.012 | PSGL-1 | 1.96 (1.17,3.68) | 0.020 | 1.97 (1.10,4.06) | 0.041 |
| LIF | 1.48 (1.04,2.15) | 0.031 | 1.53 (1.04,2.32) | 0.035 | LIF | 1.6 (0.988,2.73) | 0.063 | 1.75 (1.04,3.17) | 0.042 |
| CXCL11 | 1.61 (1.04,2.57) | 0.038 | 1.72 (1.07,2.85) | 0.029 | CXCL11 | 1.4 (0.844,2.44) | 0.22 | 1.49 (0.83,2.83) | 0.20 |
| ADAM-TS13 | 0.63 (0.40,0.94) | 0.029 | 0.69 (0.44,1.06) | 0.097 | ADAM-TS13 | 1.38 (0.836,2.46) | 0.24 | 1.7 (0.97,3.25) | 0.08 |
| FS | 0.69 (0.47,0.98) | 0.044 | 0.69 (0.47,1) | 0.056 | FS | 1.31 (0.789,2.22) | 0.30 | 1.41 (0.82,2.52) | 0.22 |
| Ep-CAM | 0.66 (0.44,0.99) | 0.048 | 0.66 (0.42,1) | 0.059 | Ep-CAM | 1.22 (0.789,1.89) | 0.37 | 1.44 (0.89,2.41) | 0.15 |
| CCL24 | 1.54 (1.04,2.34) | 0.035 | 1.69 (1.1,2.69) | 0.020 | CCL24 | 1.15 (0.707,1.86) | 0.58 | 1.34 (0.77,2.38) | 0.31 |
| TR-AP | 1.67 (1.07,2.75) | 0.032 | 1.67 (1.03,2.83) | 0.046 | TR-AP | 1.11 (0.691,1.8) | 0.67 | 1.1 (0.67,1.83) | 0.70 |
In the validation set, p-values < 0.0056 are significant after Bonferroni correction
Figure 1.
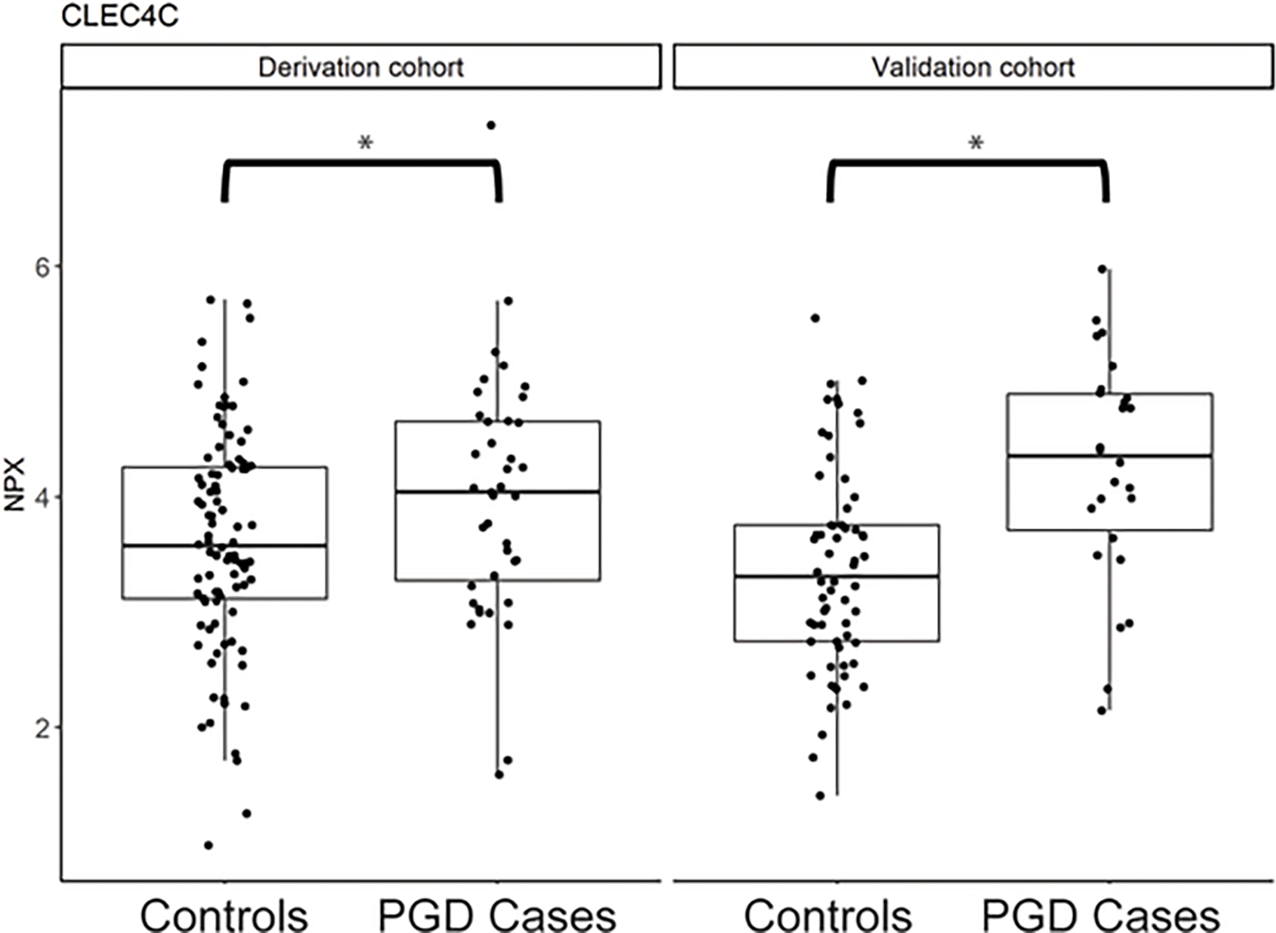
Relative protein expression (NPX) of CLEC4C between PGD cases and controls in the derivation and validation set.
Figure 2.
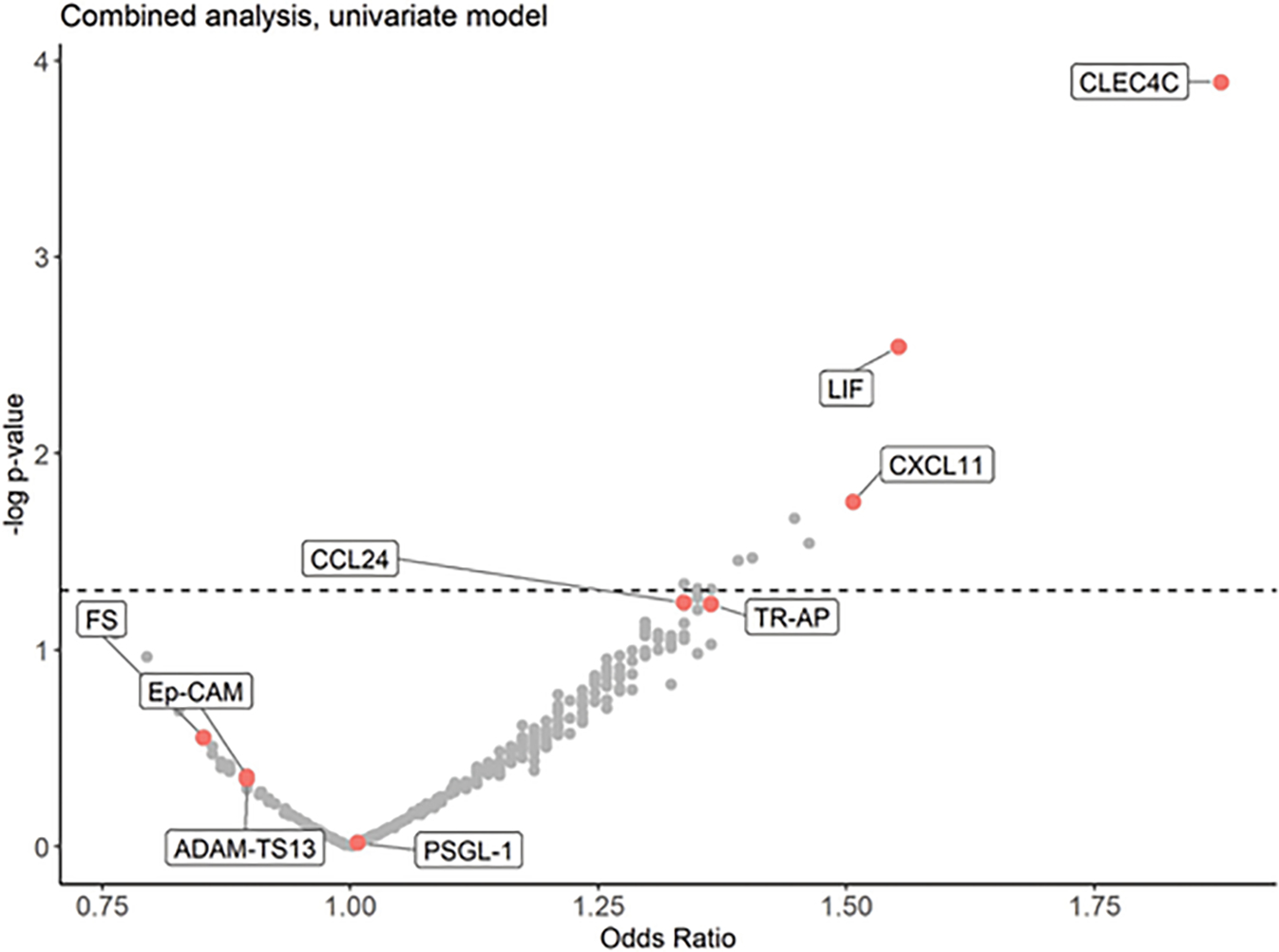
Volcano plot of proteins associated with PGD in a sensitivity analysis combining the derivation and validation sets (x-axis: Odds Ratio, y-axis: −log 10[p-value]). CLEC4C was the protein most significantly associated with PGD. All labeled proteins were nominally significant in the derivation set.
Right ventricular v. biventricular PGD
We performed stratified analysis in the combined set to test whether the association of CLEC4C with PGD was primarily driven by a subset of either right ventricular (RV) PGD cases (n = 22) or LV/biventricular (BiV) PGD cases (n = 43). CLEC4C remained significantly associated with the outcome in each model (RV: OR [95% CI] = 2.20 [1.37,3.76], p = 2.0×10−3; LV/BiV: OR [95%CI] = 1.84 [1.26,2.77], p = 2.3×10−3), suggesting its association with PGD is not specific to a particular phenotype.
CLEC4C can discriminate moderate and severe PGD risk
In a sensitivity analysis in the combined set, we also looked at relative expression of CLEC4C across PGD strata (no-mild PGD (controls), moderate PGD, and severe PGD). One-way ANOVA was significant after adjustment for all clinical covariates included in the multivariable model. Using Tukey’s method for p-value adjustments, there were significant pairwise differences between no and/or mild and moderate (p = 0.006), and no/mild and severe (p = 0.003). There was not a significant difference between moderate and severe (p = 0.73) (Supplemental Figure 1).
Pathway analyses
Twelve candidate KEGG pathways and hallmark gene sets associated with inflammation and immune function were selected for pathway analysis using ORA and GSEA. In ORA, we tested these gene sets for over-representation of proteins that were nominally significant in the univariate analysis performed on the combined derivation and validation sets. Of the 12 candidate gene sets, 6 had at least 3 hit proteins and were tested; hallmark interferon pathways and the KEGG C-type lectin receptor signaling pathway all showed over-representation of hit proteins (FDR-adjusted p-value < 0.05). The GSEA analysis was performed using p-values from all 342 proteins that passed QC in the univariate analysis of the combined derivation and validation sets. The hallmark interferon-alpha response, hallmark inflammatory response, and KEGG C-type lectin receptor signaling gene sets remained significant after FDR adjustment (FDR-adjusted p-value < 0.05), while other hallmark gene sets reporting on interferon signaling and the inflammatory response were nominally significant (Table 3).
Table 3.
Gene Set Enrichment Analysis and Over-Representation Analysis in the Combined Set
| Pathway name | Pathway size | Tested genes | Hit genes | ORA p-value | ORA FDR p-value | GSEA p-value | GSEA FDR p-value |
|---|---|---|---|---|---|---|---|
| Interferon Alpha Response | 97 | 10 | 3 | 0.0026 | 0.016 | 0.00062 | 0.0074 |
| C-type Lectin Receptor Signaling Pathway | 104 | 15 | 3 | 0.0091 | 0.036 | 0.012 | 0.046 |
| Inflammatory Response | 200 | 42 | 4 | 0.034 | 0.10 | 0.011 | 0.046 |
| TNF-a Signaling via NFKB | 200 | 31 | 2 | 0.024 | 0.072 | ||
| Interferon Gamma Response | 200 | 24 | 4 | 0.0044 | 0.022 | 0.040 | 0.094 |
| IL-17 Signaling Pathway | 94 | 27 | 0 | 0.047 | 0.094 | ||
| TNF Signaling Pathway | 112 | 26 | 2 | 0.068 | 0.12 | ||
| Natural Killer Cell Mediated Cytotoxicity | 131 | 13 | 1 | 0.13 | 0.19 | ||
| Cytokine-Cytokine Receptor Interaction | 295 | 93 | 5 | 0.15 | 0.30 | 0.14 | 0.19 |
| Viral Protein Interaction with Cytokine Receptor | 100 | 48 | 3 | 0.19 | 0.30 | 0.32 | 0.38 |
| Toll-Like Receptor Signaling Pathway | 104 | 16 | 1 | 0.35 | 0.38 | ||
| NFKB Signaling Pathway | 104 | 27 | 1 | 0.46 | 0.46 |
Discrimination of risk models with CLEC4C versus the RADIAL score
The distributions of the RADIAL score between PGD cases and controls are shown in Supplemental Figure 2. In the combined dataset, the RADIAL score was not associated with PGD (p-value = 0.29) and demonstrated poor discriminative capability in ROC analysis (AUC 0.55) (Figure 3). CLEC4C levels alone (AUC 0.66) demonstrated improved discrimination as compared to RADIAL score (p = 0.048). The combination of CLEC4C and the clinical covariates from the multivariable model displayed the best performance for identification of PGD cases (AUC 0.69, p = 0.018).
Figure 3.

Receiver operator curve analysis showing the sensitivity and specificity of the RADIAL score, the clinical model, CLEC4C alone, RADIAL + CLEC4C, and clinical + CLEC4C for the discrimination of PGD. p-values shown are for the comparison to the RADIAL score alone.
Discussion
Using high-throughput proteomic profiling in a unique cohort of samples collected from a large number of HT patients, we have identified CLEC4C as a circulating protein biomarker whose pre-transplant levels are elevated in recipients who develop PGD. The important findings of the study include: (1) relative expression of CLEC4C, a surface marker of pDCs, is higher in the pre-transplant serum of PGD cases as compared to controls, (2) proteins associated with PGD are over-represented in C-type lectin and interferon signaling pathways, (3) CLEC4C is significantly associated with both the RV and LV/BiV PGD phenotype, and (4) CLEC4C improves discrimination of PGD risk as compared to the RADIAL score. Taken together, our findings suggest that CLEC4C expression may serve as a biomarker of PGD risk and could also inform how we understand the underlying pathogenesis of PGD.
PGD risk profiling
A variety of clinical and perioperative risk factors have been previously associated with PGD. High-risk recipient-related factors that have been identified include increasing age, diabetes mellitus, elevated central venous pressures, amiodarone use, and bridge to transplant with durable LVADs.3,4,13 High risk donor and perioperative features include advanced donor age, prolonged ischemic time, and length of cardiopulmonary bypass run.14 To date, only the RADIAL score has been validated for the prediction of PGD based upon recipient and donor clinical risk factors.3 Unfortunately, its utility is limited by poor calibration and the fact that contemporary recipient management strategies, particularly temporary and durable MCS, are not reflected in its patient population. More recently the PREDICTA score was derived in a more contemporary cohort of HT recipients from the UK and demonstrated improved discrimination when compared to the RADIAL score.14 However, this model has yet to be internally or externally validated and includes cardiopulmonary bypass time which may be a result of–and not a predictor for–moderate to severe PGD. Thus, the ability to use clinical risk factors alone to predict incident PGD remains elusive. In the current study, even a more comprehensive clinical model including recipient age, recipient sex, recipient creatinine, pre-transplant mechanical circulatory support, donor age, and ischemic time performed relatively poorly (AUC 0.62), highlighting the need for a more precisionmedicine and biomarker driven approach to assessing and understanding PGD risk at the recipient level.
More broadly, there exists a paucity of contemporary studies investigating potential biomarkers of PGD in HT recipients. In one published abstract, pre-transplant exosomes were analyzed using mass spectroscopy from 8 patients with severe PGD and 8 controls.5 Using unsupervised multidimensional analysis, the authors reported differential expression of a total of 176 proteins with pathway analysis suggesting that immune and acute-phase responses, in particular IL-6 signaling, were upregulated in HT recipients who developed PGD. This study, however, was limited by a very small sample size and the complete data set has yet to be published. Our current study generally confirms the findings of this previous small study in identifying upregulation of pre-transplant immune activation and inflammation, and does so in the largest study of HT recipient biomarkers to date. We specifically identified CLEC4C, a surface marker of pDCs, as a biomarker that can be used independent of clinical covariates to risk stratify patients for PGD. Adjustment for a number of variables previously associated with PGD did not significantly improve its risk discrimination, further emphasizing the great potential of CLEC4C as a biomarker for PGD with practical clinical applications.
Potential role of pDCs in PGD
pDCs are a unique subset of dendritic cells whose primary role is the production of type I interferon and pro-inflammatory cytokines in response to viral or “self” nucleic acid detection by Toll-like receptor (TLR)-7 and TLR-9.15 In particular, unmethylated CpG sequences such as those found on bacterial or viral DNA and oxidized mitochondrial DNA (mtDNA) are known to elicit the immunostimulatory potential of pDCs. Activation of TLR-7 or TLR-9 by these damage-associated molecular patterns (DAMPs) results in increased pDC-mediated production of type I interferon (~1000x fold higher than by any other single cell type), release of proinflammatory cytokines (including IL-6 and TNF-α), increased expression of co-stimulatory molecules (including CD-86 to activate the adaptive immune response), and expression of TRAIL and granzyme B to mediate cytotoxicity.16
In the context of HT, we speculate that ischemia and/or reperfusion injury of the donor heart may result in accumulation of pro-inflammatory stimuli, such as oxidized mtDNA, that could activate the innate immune response via recipient pDCs. Interestingly, it has been shown that the circulating pDC population is rapidly depleted in the first week following HT and subsequently returns to normal cell counts.17 While previously attributed to the effects of immunosuppression, this population-specific depletion may also be the result of the pDC activation by TLR ligand binding, resulting in interferon-alpha-dependent apoptosis.17,18 Taken together with what is known about the negative inotropic effects of pro-inflammatory cytokines such as TNF and IL-6, our findings suggest a biologically plausible hypothesis for the development of PGD, wherein those patients whose peripheral blood mononuclear cell populations are enriched for pDCs (reflected in our study by higher protein expression of CLEC4C) may be at higher risk of developing transient interferon- and TNF-mediated cardiotoxicity due to the activation of pDCs by oxidized mtDNA that is released during ischemia and/or reperfusion injury of the cardiac allograft (Figure 4).19 If an underlying role of pDCs in PGD is confirmed, this could facilitate a more targeted and personalized approach to induction immunosuppression as a means to mitigate PGD risk. Future studies are warranted to test this hypothesis.
Figure 4.
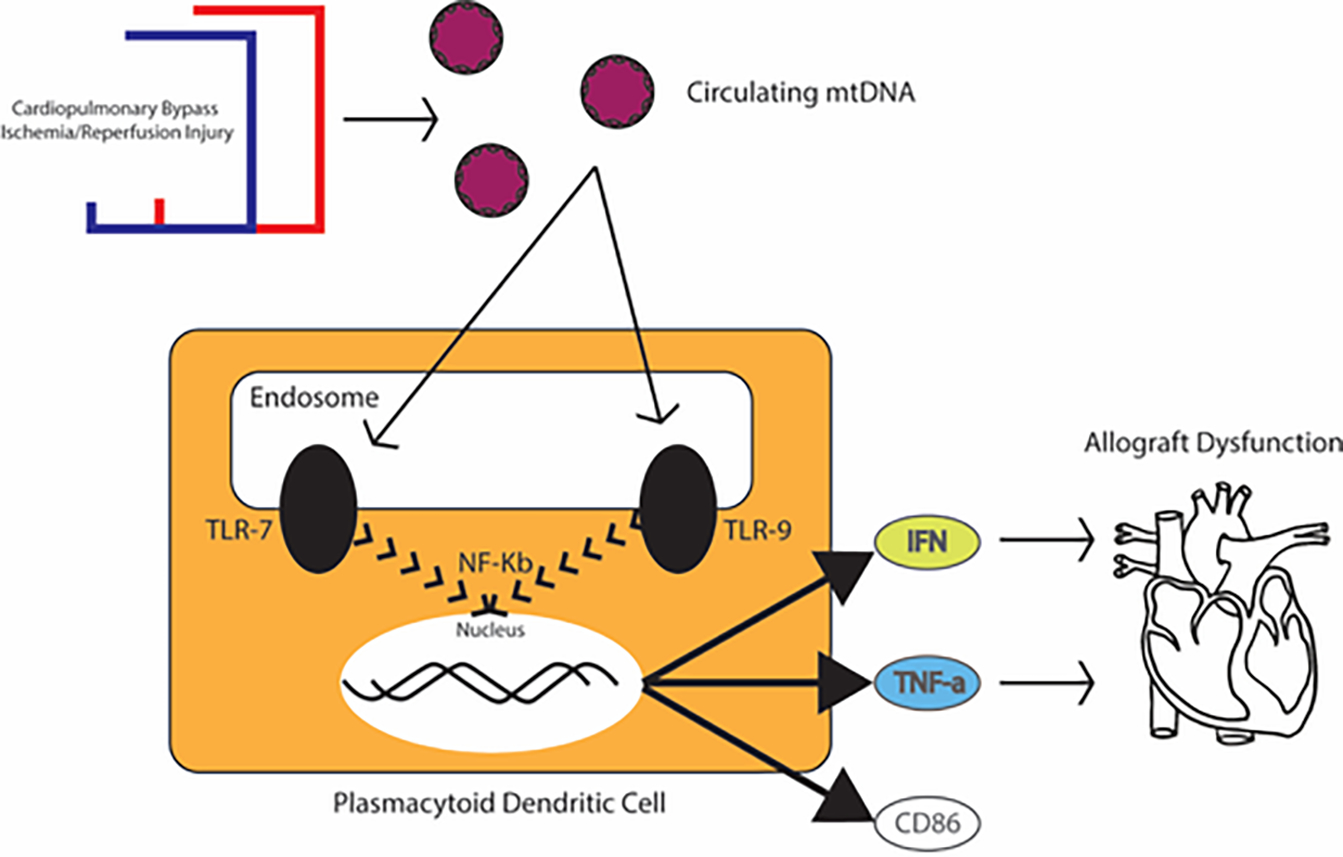
Proposed biologic model for the role of the innate immune system and pDCs in the pathogenesis of PGD. After release of the aortic cross clamp and reperfusion of the donor allograft, ischemic reperfusion injury results in a release of pro-inflammatory stimuli including oxidized mitochondrial DNA, which has the potential to activate Toll-like receptor (TLR) signaling pathways in pDCs. This may result in the rapid release of large amount of type I interferon and other pro-inflammatory cytokines which may produce the transient allograft dysfunction that characterizes PGD.
Study strength and limitations
The present study’s strength is the inclusion of a large number of HT recipients in the contemporary landscape of cardiac transplantation. Furthermore, the use of samples that were collected immediately prior to HT eliminates confounders that result from use of samples collected more distant to the operation, and is a timepoint with higher clinical utility to identify those highest risk patients. While we did assay a relatively large number of targeted immune and inflammatory proteins using the Olink platform, the 354 proteins assayed do not provide a comprehensive view of the pre-transplant proteome associated with PGD. Additionally, because of the retrospective nature of the study, certain previously described risk factors for PGD (e.g. pre-transplant amiodarone use) were not available for analysis. Future studies to identify biomarkers of PGD could benefit from a multi-institutional approach to study even larger numbers of patients using a non-targeted approach to proteomic profiling and may include measurement of CLEC4C levels at serial timepoints. Finally, because of the retrospective nature of this study, we were unable to perform flow-cytometric profiling of these patients to directly validate whether the elevated levels of CLEC4C were associated with elevated numbers and/or differences in functionality of pDCs in the blood (although CLEC4C is thought to be one of the best protein markers to quantify pDC abundance20).
Conclusions
In conclusion, relative expression of CLEC4C may represent a novel biomarker of PGD risk in the pre-transplant serum of HT recipients and appears to add significant risk discrimination to clinical covariates alone. In addition, interferon and C-type lectin receptor signaling pathways related appear to be upregulated in cases as compared to controls. Taken together, our findings suggest a novel role for the innate immune response–specifically via pDCs–in the pathogenesis of PGD. Additional mechanistic studies are warranted to test this hypothesis.
Supplementary Material
Acknowledgments
LKT has received research support from the National Institute of Health (T32HL007101 and T32HL069749.) and the Mandel Family Foundation. CLH has received funding from the Translating Duke Health Initiative. SHS has received funding from the American Heart Association 16SFRN31800010. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Disclosure statement
The authors report no disclosures relevant to the current study.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2021.07.024.
References
- 1.Khush KK, Potena L, Cherikh WS, et al. , The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: 37th adult heart transplantation report-2020; focus on deceased donor characteristics, J Heart Lung Transplant, 39, 2020, 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobashigawa J, Zuckermann A, Macdonald P, et al. , Report from a consensus conference on primary graft dysfunction after cardiac transplantation, J Heart Lung Transplant, 33, 2014, 327–340. [DOI] [PubMed] [Google Scholar]
- 3.Segovia J, Cosio MD, Barcelo JM, et al. , RADIAL: a novel primary graft failure risk score in heart transplantation, J Heart Lung Transplant, 30, 2011, 644–651. [DOI] [PubMed] [Google Scholar]
- 4.Truby LK, Takeda K, Topkara VK, et al. , Risk of severe primary graft dysfunction in patients bridged to heart transplantation with continuous-flow left ventricular assist devices, J Heart Lung Transplant, 37 (12), 2018, 1433–1442. [DOI] [PubMed] [Google Scholar]
- 5.Giangreco N, Lebreton G, Restaino S, et al. , Plasma kallikrein predicts primary graft dysfunction after heart transplant, J Heart Lung Transplant, 2021, doi: 10.1016/j.healun.2021.07.001, Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee D, Primary graft failure following cardiac transplantation is associated with elevated pre-transplant levels of pro-inflammatory factors, J Heart Lung Transplant, 35, 2016, S297–S298. [Google Scholar]
- 7.Belletti A, Lerose CC, Zangrillo A and Landoni G, Vasoactive-inotropic score: volution, clinical utility, and pitfalls, J Cardiothorac Vasc Anesth, (Epub Ahead of Print), 2020, doi: 10.1053/j.jvca.2020.09.117. [DOI] [PubMed] [Google Scholar]
- 8.Assarsson E, Lundberg M, Holmquist G, et al. , Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability, PLoS One, 9, 2014, e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP and Tamayo P, The molecular signatures database (MSigDB) hallmark gene set collection, Cell Syst, 1, 2015, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanehisa M and Goto S, KEGG: kyoto encyclopedia of genes and genomes, Nucleic Acids Res, 28, 2000, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mootha VK, Lindgren CM, Eriksson KF, et al. , PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes, Nat Genet, 34, 2003, 267–273. [DOI] [PubMed] [Google Scholar]
- 12.A language and environment for statistical computing. [computer program]. R foundation for statistical computing. [Google Scholar]
- 13.Wright M, Takeda K, Mauro C, et al. , Dose-dependent association between amiodarone and severe primary graft dysfunction in orthotopic heart transplantation, J Heart Lung Transplant, 36, 2017, 1226–1233. [DOI] [PubMed] [Google Scholar]
- 14.Avtaar Singh SS, Das De S, Rushton S, Berry C and Al Attar N, PREDICTA: a model to predict primary graft dysfunction after adult heart transplantation in the United Kingdom, J Card Fail, 25, 2019, 971–977. [DOI] [PubMed] [Google Scholar]
- 15.Swiecki M and Colonna M, The multifaceted biology of plasmacytoid dendritic cells, Nat Rev Immunol, 15, 2015, 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karrich JJ, Jachimowski LC, Uittenbogaart CH and Blom B, The plasmacytoid dendritic cell as the Swiss army knife of the immune system: molecular regulation of its multifaceted functions, J Immunol, 193, 2014, 5772–5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athanassopoulos P, Vaessen LM, Maat AP, Balk AH, Weimar W and Bogers AJ, Peripheral blood dendritic cells in human end-stage heart failure and the early post-transplant period: evidence for systemic Th1 immune responses, Eur J Cardiothorac Surg, 25, 2004, 619–626. [DOI] [PubMed] [Google Scholar]
- 18.Zhan Y, Chow KV, Soo P, et al. , Plasmacytoid dendritic cells are short-lived: reappraising the influence of migration, genetic factors and activation on estimation of lifespan, Sci Rep, 6, 2016, 25060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann DL, Stress-activated cytokines and the heart: from adaptation to maladaptation, Annu Rev Physiol, 65, 2003, 81–101. [DOI] [PubMed] [Google Scholar]
- 20.Murray L, Xi Y and Upham JW, CLEC4C gene expression can be used to quantify circulating plasmacytoid dendritic cells, J Immunol Methods, 464, 2019, 126–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


