Abstract
Background.
Data about vaccine efficacy in solid organ transplant patients are limited. We previously reported our initial observation of a 6.2% immunogenicity rate in kidney transplant recipients (KTRs) after administration of 1 dose of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine. We sought to report our observations of anti-SARS-CoV-2 antibody in KTRs after 2 doses of the SARS-CoV-2 mRNA vaccine.
Methods.
We identified 105 KTRs who received 2 doses of the Pfizer-BioNTech or Moderna mRNA-1273 vaccine per availability and had anti-SARS-CoV-2 labs obtained at least 2 wk following administration of the second dose. Antibody testing was performed using 3 clinically validated qualitative and semiquantitative assays.
Results.
KTRs had a 36.2% antibody response rate, whereas an age ≥68 years and a longer time from transplant were factors associated with antibody response.
Conclusions.
The low antibody response in KTRs may be associated with the immunosuppressive state. More data are needed to evaluate if KTRs may require higher vaccine doses or an additional booster dose to increase their ability to mount an immune response to the SARS-CoV-2 vaccine.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) vaccine has provided an optimistic outlook to the otherwise devastating toll of the COVID-19 pandemic. With promising initial outcomes following vaccine administration in regards to safety and disease prevention in the general population,1-3 there has been a strong push to vaccinate vulnerable patient populations, such as solid organ transplant recipients (SOTRs).4 Although there are substantial efforts evaluating antibody response from the vaccine in the general population,5-8 only limited reports on vaccine efficacy in SOTRs exist. Kidney transplant recipients (KTRs) seem especially vulnerable, as researchers have observed a decline and loss of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies by 6 mo after SARS-CoV-2 infection.9 Additionally, KTRs exhibit a diminished antibody response to other vaccines, such as influenza A virus subtype H1N1 and influenza.10,11 It is unclear if the poor antibody response is due to the immunosuppressed state.
We previously published our initial experience of KTRs who received 1 dose of the mRNA vaccine.12 In that report, we showed that only 6.2% of our kidney transplant cohort demonstrated an antibody response compared with 87% of those on the kidney transplant waitlist. This is comparable to other reports evaluating antibody response in SOTRs following 1 vaccine dose.13,14 In an effort to further evaluate the immune response of the mRNA vaccines in transplant patients, we examined the overall antibody response rate in KTRs following 2 doses of the SARS-CoV-2 mRNA vaccine and sought to identify factors associated with anti-SARS-CoV-2 antibody response.
MATERIALS AND METHODS
This was an institutional review board approved (IRB0507-0053) retrospective review of KTRs who received 2 doses of either the Pfizer-BioNTech or Moderna mRNA-1273 vaccine at the Houston Methodist J.C. Walter Jr Transplant Center in Houston, TX, from January 2, 2021, to April 1, 2021. Patients received the specific vaccine brand based on availability, and the doses were administered per manufacturer guidelines. Anti-SARS-CoV-2 labs were obtained before each vaccine dose and at least 2 wk following administration of the second vaccine. Patient demographics (age, gender, and race), maintenance immunosuppression, induction agent, history of T-cell depleting therapy (ie, antithymocyte globulin) within 6 mo, history of rejection, and time between vaccine dose to transplant and labs were collected. Those with a positive COVID-19 polymerase chain reaction test, anti-SARS-CoV-2 antibodies at the time of their first vaccine dose‚ or evidence of anti-SARS-CoV-2 nucleocapsid antibodies were excluded from analysis. Per institutional protocols, patients who were within 1 mo of transplant were excluded from receiving the vaccine. Antibody response or reactivity was defined as the presence of either anti-SARS-CoV-2 immunoglobulin (Ig) IgG or total antibody or anti-SARS-CoV-2 Spike total Ig ≥1:50.
Clinical Assays
Anti-SARS-CoV-2 antibody testing used clinically validated assays and was performed in a Clinical Laboratory Improvement Amendments-certified laboratory at Houston Methodist Hospital. Qualitative anti-SARS-CoV-2 Spike total Ig and Anti-SARS-CoV-2 IgG-specific assays (Ortho Clinical Diagnostics, Markham, ON, Canada) were performed on the VITROS 3600 automated immunoassay analyzer according to the manufacturer’s protocol. Anti-SARS-CoV-2 Spike Ig titers were measured as <1:50, 1:50, 1:150, 1:450, and >1:1350, with reactivity defined as titers ≥1:50 as previously reported at our institution.15 A lab-developed semiquantitative test to detect anti-SARS-CoV-2 Spike protein IgG-specific ELISA test was performed on a Tecan Freedom EVO instrument as previously described.15 Anti-SARS-CoV-2 nucleocapsid IgG was tested using the Elecsys anti-SARS-CoV-2 serological assay (Roche Diagnostics, Indianapolis, IN) on a Cobas E602 instrument.
Institutional Immunosuppression Protocol
KTRs received an immunosuppression regimen per our institutional protocol.16 Patients considered at high risk of acute rejection (African Americans, retransplant, and highly sensitized recipients) received a 3-d course of rabbit antithymocyte globulin (Thymoglobulin; Genzyme, Cambridge, MA) at a dose of 1.5 mg/kg/d, beginning on the day of transplantation. Patients ≥70 years old were excluded from this group. All other subjects received 20 mg/kg of Basiliximab (Novartis, East Hanover, NJ) on the day of transplantation and on the third day posttransplant. Maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil, and prednisone. The dose of tacrolimus was adjusted to maintain a trough level of 8 to 10 ng/mL for the first 3-mo posttransplantation, tapered to 5 to 8 ng/mL thereafter. Mycophenolate mofetil was given at a dose of 1000 mg twice daily. Methylprednisolone (250 mg) was given on the day of transplantation, tapered to 25 mg by 5 d posttransplant, and then to 5 to 10 mg by 6 mo posttransplantation. Patients who had biopsy-proven acute cellular rejection as defined by the Banff criteria17 also received a 5-d course of rabbit antithymocyte globulin per institutional protocol.16
Statistical Analysis
Patient characteristics were reported as frequencies and proportions for categorical variables and as median and interquartile range (IQR) for continuous variables. Differences between groups were compared using the χ2 or Fisher exact tests for categorical variables and Wilcoxon rank-sum test for the continuous variables. The optimal thresholds of age (68 years of age) and time from transplant (6 mo) in discriminating the antibody response were determined by the Youden index.18
A generalized linear model (GLM) was used to determine factors associated with having a reactive antibody response to the COVID-19 vaccine. Variables for the multivariable models were selected based on the clinical importance and also by the least absolute shrinkage and selection operator (Lasso) method using the cross-validation selection option.19,20 Variables used in the univariable analysis, after being checked for biological plausibility and collinearity, were assessed by the LASSO program, which suggested good models that included the variables with the highest probability of being a risk factor. Potential risk factors were also discussed with senior clinicians to ensure the biological plausibility of the selected covariates. To avoid overfitting, variables which were significant in the univariate analysis but insignificant in the multivariable analysis were not selected in the final model if their exclusion did not affect the diagnostic performance of the final model (such as prednisone and mammalian target of rapamycin inhibitors). Induction type was included in the final model based on its clinical importance. Variables included in the final GLM model were age (< or ≥68 y), time from transplant to vaccination (in years), T-cell depleting therapy within 6 mo, and immunosuppression therapies (mycophenolate, prednisone, and mammalian target of rapamycin inhibitors). All analyses were performed on Stata version 17.0 (StataCorp LLC, College Station, TX). A P value of <0.05 was considered statistically significant.
RESULTS
Demographics
As of April 2021, 105 KTRs received 2 doses of either the Pfizer-BioNTech or Moderna mRNA-1273 vaccine and had antibody titers obtained at least 2 wk following the second vaccine dose at our institution. The median age of this cohort was 57 years (IQR, 46–65), with 61.9% (65 of 105) identified as male. The majority of these patients were Caucasian (62.9%, 66 of 105), followed by African American (17%, 18 of 105), Hispanic (10.5%, 11 of 105), and Asian (9.5%, 10 of 105). Only 13% (14 of 105) received T-cell depleting therapy within 6 mo before vaccine administration. Nineteen patients had rejection before vaccine, whereas 6 patients had rejection following vaccine administration. This data is summarized in Table 1.
TABLE 1.
Demographics of recipients with and without reactivity to the mRNA COVID-19 vaccine
| Total, N = 105 | Nonreactive (n = 67) | Reactive (n = 38) | P | |
|---|---|---|---|---|
| Age (y), median (IQR) | 57.0 (46.0–64.0) | 56.0 (46.0–64.0) | 57.5 (45.0–68.0) | 0.57 |
| Age (y) | 0.052 | |||
| <68 | 85 (81.0) | 58 (86.6) | 27 (71.1) | |
| ≥68 | 20 (19.0) | 9 (13.4) | 11 (28.9) | |
| Gender | 0.52 | |||
| Female | 40 (38.1) | 24 (35.8) | 16 (42.1) | |
| Male | 65 (61.9) | 43 (64.2) | 22 (57.9) | |
| Ethnicity | 0.10 | |||
| White | 66 (62.9) | 37 (55.2) | 29 (76.3) | |
| Black | 18 (17.1) | 15 (22.4) | 3 (7.9) | |
| Hispanic | 11 (10.5) | 9 (13.4) | 2 (5.3) | |
| Asian | 10 (9.5) | 6 (9.0) | 4 (10.5) | |
| Time from transplant to vaccination (y), median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 2.0 (1.0–7.0) | 0.002 |
| Time from transplant to vaccination (y) | 0.01 | |||
| <6 mo | 37 (35.2) | 30 (44.8) | 7 (18.4) | |
| ≥6 mo | 68 (64.8) | 37 (55.2) | 31 (81.6) | |
| Vaccine | 0.48 | |||
| Moderna mRNA-1273 | 45 (42.9) | 27 (40.3) | 18 (47.4) | |
| Pfizer-BioNTech | 60 (57.1) | 40 (59.7) | 20 (52.6) | |
| Days between vaccine 1 and vaccine 2, median (IQR) | 26.0 (21.0–28.0) | 26.0 (21.0–28.0) | 25.5 (21.0–28.0) | 0.97 |
| Days between vaccine 2 and last lab date, median (IQR) | 91.0 (45.0–110.0) | 89.0 (46.0–106.0) | 93.5 (42.0–122.0) | 0.38 |
| History of rejection | 0.48 | |||
| No | 79 (76.0) | 52 (78.8) | 27 (71.1) | |
| Yes | 25 (24.0) | 14 (21.2) | 11 (28.9) | |
| Rejection before or after vaccination (n = 25) | 0.55 | |||
| Before | 19 (76.0) | 10 (71.4) | 9 (81.8) | |
| After | 6 (24.0) | 4 (28.6) | 2 (18.2) | |
| Time from transplant to rejection (y), median (IQR) (n = 25) | 0.8 (0.4–1.5) | 0.8 (0.4–1.5) | 0.8 (0.1–2.1) | 0.70 |
| Immunosuppression therapy and induction | ||||
| T-cell depleting therapy, ≤6 mo | 0.02 | |||
| No | 91 (86.7) | 54 (80.6) | 37 (97.4) | |
| Yes | 14 (13.3) | 13 (19.4) | 1 (2.6) | |
| Tacrolimus | 1.00 | |||
| No | 11 (10.5) | 7 (10.4) | 4 (10.5) | |
| Yes | 94 (89.5) | 60 (89.6) | 34 (89.5) | |
| Mycophenolate | 0.003 | |||
| No | 20 (19.0) | 7 (10.4) | 13 (34.2) | |
| Yes | 85 (81.0) | 60 (89.6) | 25 (65.8) | |
| Prednisone | 0.02 | |||
| No | 6 (5.7) | 1 (1.5) | 5 (13.2) | |
| Yes | 99 (94.3) | 66 (98.5) | 33 (86.8) | |
| Azathioprine | 0.02 | |||
| No | 101 (96.2) | 67 (100.0) | 34 (89.5) | |
| Yes | 4 (3.8) | 0 (0.0) | 4 (10.5) | |
| Cyclosporine | 1.00 | |||
| No | 97 (92.4) | 62 (92.5) | 35 (92.1) | |
| Yes | 8 (7.6) | 5 (7.5) | 3 (7.9) | |
| Belatacept | 0.53 | |||
| No | 103 (98.1) | 65 (97.0) | 38 (100.0) | |
| Yes | 2 (1.9) | 2 (3.0) | 0 (0.0) | |
| mTOR inhibitors | 0.13 | |||
| No | 97 (92.4) | 64 (95.5) | 33 (86.8) | |
| Yes | 8 (7.6) | 3 (4.5) | 5 (13.2) | |
| Induction receipt | 1.00 | |||
| No | 9 (8.6) | 6 (9.0) | 3 (7.9) | |
| Yes | 96 (91.4) | 61 (91.0) | 35 (92.1) | |
| Induction type | 0.97 | |||
| None | 9 (8.6) | 6 (9.0) | 3 (7.9) | |
| Thymoglobulin | 70 (66.7) | 44 (65.7) | 26 (68.4) | |
| Simulect | 24 (22.9) | 16 (23.9) | 8 (21.1) | |
| Campath | 2 (1.9) | 1 (1.5) | 1 (2.6) | |
Values are in number (%) unless otherwise specified.
COVID-19. coronavirus disease 19; IQR, interquartile range; mTOR, mammalian target of rapamycin.
Vaccine Response and Associated Factors
The median time between kidney transplant and the first vaccine dose was 1 year (IQR, 0–3) and 57% (60 of 105) of patients received the Pfizer-BioNTech vaccine. The median time between vaccine doses was 26 days (IQR, 21–28), consistent with manufacturer recommendations, and the median follow-up after the second vaccine dose was 91 days (IQR, 45–110).
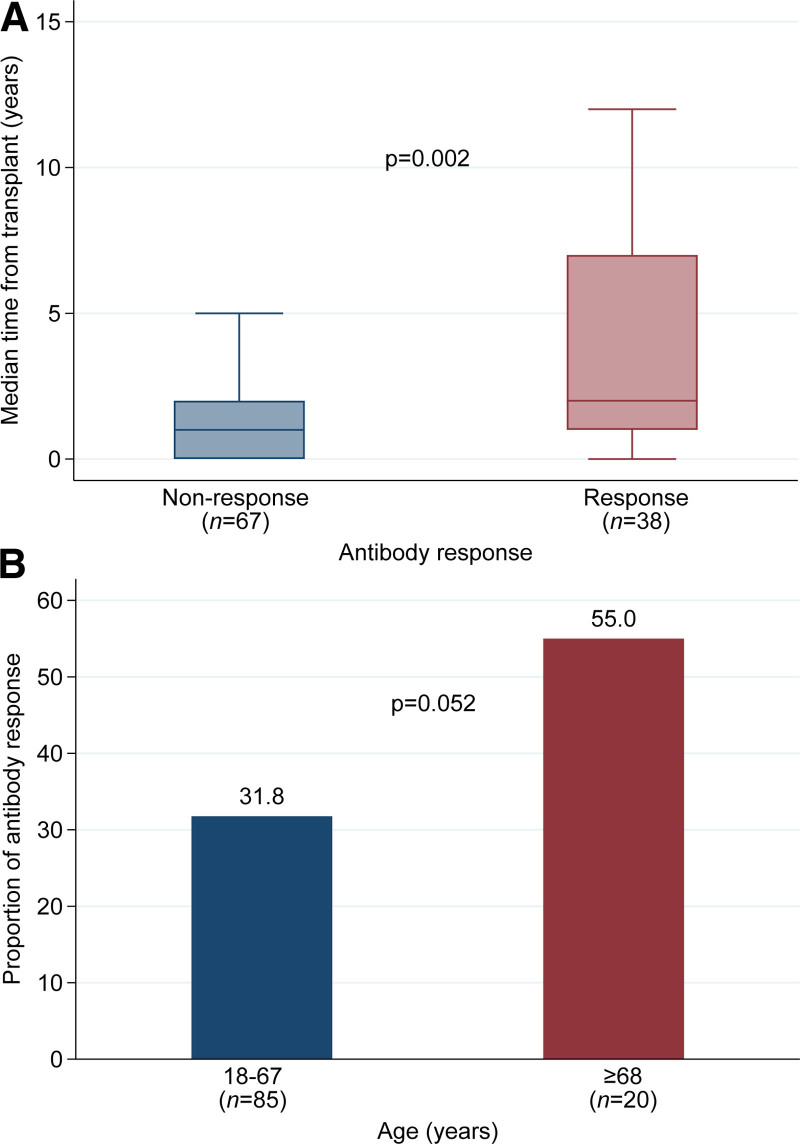
Only 36.2% (38 of 105) of KTRs exhibited an antibody response. Of these, 61% (22 of 38) had an anti-SARS-CoV-2 Spike Ig titer ≥1:50. Median time from transplant in the recipients with and without antibody response was 2.0 (IQR, 1.0–7.0) versus 1.0 (IQR, 0.0–2.0), respectively (P = 0.002) (Figure 1A). Those with a longer time from transplant were more likely to exhibit an antibody response (relative risk [RR], 1.07 [95% confidence interval (CI), 1.00-1.15]; P = 0.045) (Table 2). Increased age was likely to be associated with a likelihood to antibody response. Kidney transplant patients ≥68 years old had a higher proportion for antibody response (55.0% versus 31.8%; P = 0.052; Figure 1B) and a higher RR for antibody response than younger cohorts (RR, 3.14 [95% CI, 1.29-7.66]; P = 0.01) (Table 2). Immunosuppression regimen was also associated with antibody response. In the univariate analysis, maintenance therapy with mycophenolate (RR, 0.45 [95% CI, 0.29-0.72]; P = 0.001) or prednisone (RR, 0.40 [95% CI, 0.25-0.72]; P < 0.001), was associated with a lower likelihood for antibody response, whereas azathioprine was associated with a higher likelihood (RR, 1.84 [95%, 1.00-3.36]; P = 0.048). Only maintenance treatment with mycophenolate was significant in the GLM (RR, 0.42 [95%, 0.21-0.87]; P = 0.02). Additionally, patients who received T-cell depleting therapy within 6 mo of vaccine administration had a trend toward having a lower relative risk of reactive antibody response in the univariable analysis (P = 0.07); however, this finding was not significant in the GLM (RR, 0.27 [95%, 0.04-2.04]; P = 0.20) (Table 2). Of the 14 patients who received T-cell depleting therapy within 6 mo before vaccination, 9 were due to rejection‚ and 5 were due to induction. Rejection and induction type were not found to be statistically significant factors for vaccine-associated antibody response.
FIGURE 1.
Antibody response based on time from transplant or recipient age. A, Median time (y) from transplantation to vaccination by antibody response group. B, Proportion of antibody response by age group.
TABLE 2.
Characteristics associated with antibody response
| Univariable | Multivariable | |||
|---|---|---|---|---|
| RR (95% I) | P | RR (95% I) | P | |
| Age (y), median (IQR) | 1.01 (0.98–1.03) | 0.65 | – | – |
| Age (y) | ||||
| <68 | (Reference) | (Reference) | ||
| ≥68 | 1.73 (1.05-2.87) | 0.03 | 3.14 (1.29-7.66) | 0.01 |
| Gender | ||||
| Female | (Reference) | – | – | |
| Male | 0.85 (0.51-1.41) | 0.52 | – | – |
| Ethnicity | ||||
| White | (Reference) | – | – | |
| Black | 0.38 (0.13-1.10) | 0.08 | – | – |
| Hispanic | 0.41 (0.11-1.49) | 0.18 | – | – |
| Asian | 0.91 (0.41-2.04) | 0.82 | – | – |
| Time from transplant to vaccination (y), median (IQR) | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.00–1.15) | 0.045 |
| Time from transplant to vaccination (y) | ||||
| <6 mo | (Reference) | – | – | |
| ≥6 mo | 2.41 (1.18-4.93) | 0.02 | – | – |
| Vaccine | ||||
| Moderna mRNA-1273 | (Reference) | – | – | |
| Pfizer-BioNTech | 0.83 (0.50-1.38) | 0.48 | – | – |
| Days between vaccine 1 and vaccine 2, median (IQR) | 0.99 (0.93–1.05) | 0.66 | – | – |
| Days between vaccine 1 and last lab date, median (IQR) | 1.00 (1.00–1.01) | 0.46 | – | – |
| Days between vaccine 2 and last lab date, median (IQR) | 1.00 (1.00–1.01) | 0.42 | – | – |
| History of rejection | ||||
| No | (Reference) | – | – | |
| Yes | 1.29 (0.75-2.20) | 0.36 | – | – |
| Rejection before or after vaccination (n = 25) | ||||
| Before | (Reference) | – | – | |
| After | 0.70 (0.21-2.40) | 0.58 | – | – |
| Time from transplant to rejection (y), median (IQR) (n = 25) | 1.04 (0.71–1.52) | 0.85 | – | – |
| Immunosuppression therapy and induction | ||||
| T-cell depleting therapy, ≤6 mo | ||||
| No | (Reference) | (Reference) | ||
| Yes | 0.18 (0.03-1.18) | 0.07 | 0.27 (0.04–2.04) | 0.20 |
| Tacrolimus | ||||
| No | (Reference) | – | – | |
| Yes | 0.99 (0.44-2.27) | 0.99 | – | – |
| Mycophenolate | ||||
| No | (Reference) | (Reference) | ||
| Yes | 0.45 (0.29-0.72) | 0.001 | 0.42 (0.21–0.87) | 0.02 |
| Prednisone | ||||
| No | (Reference) | – | – | |
| Yes | 0.40 (0.25-0.63) | <0.001 | – | – |
| Cyclosporine | ||||
| No | (Reference) | – | – | |
| Yes | 1.04 (0.41-2.64) | 0.94 | – | – |
| mTOR inhibitors | ||||
| No | (Reference) | – | – | |
| Yes | 1.84 (1.00-3.36) | 0.048 | – | – |
| Induction | ||||
| No | (Reference) | – | – | |
| Yes | 1.09 (0.42-2.86) | 0.86 | – | – |
| Induction type | ||||
| None | (Reference) | (Reference) | ||
| Thymoglobulin | 1.11 (0.42-2.95) | 0.83 | 2.24 (0.59-8.52) | 0.24 |
| Simulect | 1.00 (0.34-2.95) | 1.00 | 1.65 (0.43-6.35) | 0.47 |
| Campath | 1.50 (0.28-7.93) | 0.63 | 4.91 (0.44-55.09) | 0.20 |
| C-statistic = 0.83 | ||||
Values are in number (%) unless otherwise specified.
IQR, interquartile range; mTOR, mammalian target of rapamycin; RR, relative risk.
DISCUSSION
Our findings showed that of the 105 KTRs who received 2 doses of the SARS-CoV-2 mRNA vaccine at our institution‚ only 36.2% (n = 38) had a reactive antibody response to the vaccine. Although this observation is higher than the 6.2% to 17% antibody response rate following 1 vaccine dose,12,13 our observation is significantly lower than the estimated 95% antibody response rate in the general population.21 An important difference between KTRs and the general population is that KTRs are immunosuppressed, and factors associated with antibody response in KTRs appear to be linked to the immunosuppressed state. In a multivariate analysis, recipients ≥68 years old and those with a longer time from transplant were more likely to elicit an antibody response than younger patients and those more recently transplanted. The older patients at our transplant center were also less likely to have received T-cell depleting therapy.
Our observation that older KTRs were more likely to exhibit an antibody response than younger KTRs differs from prior reports showing that the immunogenicity of the SARS-CoV-2 mRNA vaccine was lower in adults aged 65 to 85 years.8,13 In SOTRs, Boyarsky et al13 reported that older patients were less likely to exhibit an antibody response and identified those who were younger and not on antimetabolite immunosuppression to be more likely to have a response. When we looked specifically at KTRs, older patients were less likely to receive T-cell depleting therapy at the time of transplant and potentially have a lower level of maintenance immunosuppression. Per our institutional protocol, KTRs ≥70 years old do not receive T-cell depleting therapy for induction because of concerns for infection. Additionally, older KTRs are less likely to have allograft rejection,22 thus prompting a lower level of maintenance immunosuppression (ie, lower calcineurin inhibitor levels, half the antimetabolite dose ± prednisone) than the younger cohort.16 In our study, only 1 patient ≥68 years old received T-cell depleting therapy within 6 mo of receiving the vaccine. This finding may reflect the older KTR cohort’s ability to exhibit an antibody response to the vaccine because we also observed that KTRs receiving T-cell depleting therapy within 6 mo of vaccination were less likely to exhibit an antibody response.
Similar to the older KTRs, patients with a longer time from transplant were more likely to exhibit an antibody response as they were further from the time of their induction treatment and usually maintained on lower immunosuppression.16 All patients in the antibody-reactive group were beyond 2 years from transplant.
Recent studies examining antibody response to the COVID-19 vaccine in SOTRs have reported similar data to our own. In addition to providing one of the largest studies to date, we have also offered additional insight into associated factors related to antibody response in KTRs. Rusk et al14 presented 1 SORT who did not exhibit an antibody response following 2 doses of the COVID-19 vaccine. Boyarsky et al13 followed up with their initial series by evaluating their SOTRs after 2 vaccine doses and identified a similarly low antibody response rate to the vaccine.23 Specific to KTRs, Korth et al24 identified significantly lower immunogenicity with 2 doses of the Pfizer-BioNTech vaccine than with healthy controls. Likewise, our study did not suggest a difference in immunogenicity based on mRNA vaccine type. These early reports all identify low immunogenicity among SOTRs after 2 doses of the SARS-CoV-2 mRNA vaccine.
Our findings are similar to the immunogenic response rate for the influenza vaccine in SOTRs. When dosed for the general population, the influenza vaccine had a suboptimal response rate of about 15% to 70%.25,26 Studies utilizing higher-dose vaccines showed improved antibody response in these patients,27,28 and the current recommendations are for transplant recipients to receive the high-dose influenza vaccine. This experience can provide guidance for our evolving management of transplant patients receiving the COVID-19 vaccine.
Although we have identified several factors associated with antibody response in KTRs to the COVID-19 vaccine, there are a few limitations to our study. First, we had a relatively small sample size when variable groups were stratified. Second, our study was observational, as there was no randomization or control group. Third, we only studied the SARS-CoV-2 mRNA vaccines because of limited availability and restrictions of other COVID-19 vaccines. Last, the vaccine may induce important T-cell response in this population that we could not measure. Thus, despite a lack of antibody response to the SARS-CoV-2 mRNA vaccine, it remains possible that KTRs may convey some immunologic defense against SARS-CoV-2.
With increasing COVID-19 infections in the community, there is an opportunity to better understand the efficacy of the SARS-CoV-2 mRNA vaccine in KTRs in terms of infection rate and antibody response. There are new reports of break through infections following vaccination,29,30 with Wadei et al30 observing 7 COVID-19 positive SOTRs who received 1 or 2 doses of the mRNA vaccine. In this small cohort, none of the patients developed antibodies following vaccine administration. More data will be needed to guide our management in this vulnerable patient population.
In the growing field of research investigating SARS-CoV-2 vaccine efficacy in transplant patients, we have presented important data evaluating the antibody response in KTRs after 2 doses of the SARS-CoV-2 mRNA vaccine and suggest that the degree of immunosuppression likely contributes to the lack of antibody response. As the majority of COVID-19 positive cases in SOTRs at our institution are in KTRs, we chose to analyze this high-risk cohort given our routine use of induction agents (including T-cell depleting therapy) and relatively high level of maintenance immune suppression. Future studies will include evaluation of other COVID-19 vaccine types, outcomes of additional booster vaccines and vaccine dose adjustment, and identification of potential biomarkers of response.
ACKNOWLEDGMENTS
The authors acknowledge the Houston Methodist Hospital COVID-19 transplant task force and the J.C. Walter Jr Transplant Center nurses, coordinators‚ and staff who worked tirelessly to ensure the vaccination and safety of our transplant patients. Data availability: The data that support the findings of this study are available from the corresponding author, S.G. Yi, upon reasonable request.
Footnotes
The authors declare no funding or conflicts of interest.
Research design was done by S.G.Y. and R.J.K. Performance of research and data acquisition was done by S.G.Y. and R.J.K. Data analysis and interpretation were done by S.G.Y., E.A.G., D.T.N., R.J.K., and T.E. Writing of the paper was done by S.G.Y. and R.J.K. Critical review of the paper was done by S.G.Y., R.J.K., E.A.G., D.T.N., R.M.G., A.O.G., H.J.H., L.W.M., A.S., M.H., R.M., and H.I.
REFERENCES
- 1.Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb Mortal Wkly Rep. 2021;70:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslam S, Goldstein DR, Vos R, et al. COVID-19 vaccination in our transplant recipients: the time is now. J Heart Lung Transplant. 2021;40:169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones NK, Rivett L, Seaman S, et al. ; Cambridge COVID-19 Collaboration. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. Elife. 2021;10:e68808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. [DOI] [PubMed] [Google Scholar]
- 8.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavarot N, Leruez-Ville M, Scemla A, et al. Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int. 2021;99:486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birdwell KA, Ikizler MR, Sannella EC, et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulley WR, Visvanathan K, Hurt AC, et al. Mycophenolate and lower graft function reduce the seroresponse of kidney transplant recipients to pandemic H1N1 vaccination. Kidney Int. 2012;82:212–219. [DOI] [PubMed] [Google Scholar]
- 12.Yi SG, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105:e72–e73. [DOI] [PubMed] [Google Scholar]
- 13.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusk DS, Strachan CC, Hunter BR. Lack of immune response after mRNA vaccination to SARS-CoV-2 in a solid organ transplant patient. J Med Virol. 2021;93:5623–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar E, Perez KK, Ashraf M, et al. Treatment of Coronavirus Disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houston Methodist Hospital. Transplant center and procedures. Available at https://sharepoint.houstonmethodist.org/sites/hm/tmhs_transplant_center/MTCstaff/Protocol%20Books/Forms/AllItems.aspx. Accessed August 24, 2021.
- 17.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): ipdates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 19.Hastie T, Tibshirani R, Wainwright M. Statistical Learning With Sparsity: The Lasso and Generalizations. CRC Press; 2015. [Google Scholar]
- 20.StataCorp. Stata Reference Manual. 16th ed. College Station: Stata Press; 2019. [Google Scholar]
- 21.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabuig AS, Martínez EG, Berga JK, et al. Kidney transplantation in recipients older than 70 years old: a good option for our patients. Transplantation. 2018;102:S468. [Google Scholar]
- 23.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses. 2021;13:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar D, Blumberg EA, Danziger-Isakov L, et al. ; AST Infectious Diseases Community of Practice. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11:2020–2030. [DOI] [PubMed] [Google Scholar]
- 26.Baluch A, Humar A, Eurich D, et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 2013;13:1026–1033. [DOI] [PubMed] [Google Scholar]
- 27.Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66:1698–1704. [DOI] [PubMed] [Google Scholar]
- 28.Mombelli M, Rettby N, Perreau M, et al. Immunogenicity and safety of double versus standard dose of the seasonal influenza vaccine in solid-organ transplant recipients: a randomized controlled trial. Vaccine. 2018;36:6163–6169. [DOI] [PubMed] [Google Scholar]
- 29.Yamada M, Matsumoto E, Thomas CP, et al. Case report: severe COVID-19 in a kidney transplant recipient without humoral response to SARS-CoV-2 mRNA Vaccine Series. Transplant Direct. 2021;7:e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadei HM, Gonwa TA, Leoni JC, et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021;21:3496–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]