Abstract
Aims:
Caveolae are specialized regions of the cell membrane that modulate signal transduction and alterations in these structures affect bladder smooth muscle (BSM) contraction. Since bladder dysfunctions are common in the elderly, we evaluated the effect of aging on the morphology of caveolae and caveolin protein expression in BSM.
Methods:
Caveolar morphology (number, size, and depth) in BSM was determined from electron microscopy images of young (10 weeks), adult (6-month old), and old (12-month old) rat urinary bladders. Changes in expression levels of caveolin proteins with age were investigated by Western blot and immunofluorescence microscopy. Caveolin-3 gene expression was determined by real-time RT-PCR in young and 19-month-old rat bladders.
Results:
Twelve-month-old animals exhibited 50% fewer BSM caveolae compared to young (P < 0.01). The area of caveolae was significantly decreased at 6 and 12 months. Despite a decrease in the number of BSM caveolae at 12 months, the expression of caveolin-1 and cavin-1 were unaltered with age. In contrast, caveolin-2 and caveolin-3 protein expression and immunoreactivity were reduced in BSM at 6 and 12 months of age. Caveolin-3 gene expression was also downregulated at 19 months compared to young animals.
Conclusion:
Biological aging significantly decreases BSM caveolae number and morphology with associated selective alteration in caveolin protein expression. Since caveolae are protected membrane regions that regulate signal transduction, age-related alterations in caveolae and caveolin protein expression could alter BSM contractility resulting in bladder dysfunctions of the elderly.
Keywords: aging, bladder smooth muscle, caveolae, caveolin
INTRODUCTION
Caveolae are flask-shaped invaginations of the plasma membrane that are particularly abundant in well-differentiated cells such as muscle. These structures provide a mechanism for compartmentalization and integration of signal transduction and play an important role in normal bladder smooth muscle (BSM) function.1,2 In aging human bladders, a distinct pattern of smooth muscle morphology has been described, which includes an apparent reduction in BSM membrane caveolae.3 Whether alterations in BSM caveolae are characteristic of the aging process has not been specifically addressed. However, both muscle and non-muscle cells in other organs exhibit age-associated changes in these membrane microdomains and expression of their structural proteins, caveolin-1, -2, and -3. For example, with aging, caveolin-1 expression increases in human prostatic4 but decreases in rat aortic,5 colonic,6 and penile7 smooth muscle resulting in functional alterations. Expression of caveolin proteins in rat heart is unchanged in adulthood but an age-dependent dissociation of caveolin-3 from muscle sarcolemma alters cardiomyocyte signaling.8 In rat skeletal muscle, expression of caveolin protein increases with age.9 Upregulation of caveolin proteins also occurs in non-muscle tissues such as brain, liver, and spleen of older rats,10 while caveolin-1 and -2 decrease in cell membranes of senescent human fibroblasts in association with loss of caveolae.11 Thus, aging significantly impacts caveolae and caveolin protein expression; moreover, these changes are cell type dependent and caveolin isoform specific.
Caveolin-1 is the principal caveolar protein required for caveolae biogenesis in most cell types. In lymphocytes, transient expression of caveolin-1 by gene transfection induces caveolar biogenesis.12 The development of caveolin gene knockout (KO) mice for caveolin-1, -2, and -3, have offered valuable insights regarding the effects of caveolin proteins on cell function. Caveolin-1 KO mice lack caveolae in cells that normally express caveolin-1 and exhibit age-dependent organ-specific abnormalities in non-muscle cells.13 Caveolin-2 KO mice exhibit normal caveolae formation14 suggesting that caveolin-2, normally co-expressed with caveolin-1, is not essential for caveolae biogenesis. Caveolin-3 KO mice show loss of caveolae from striated (skeletal/cardiac) muscles15–17; however, these muscles have normal caveolae in caveolin-1 KO mice, indicating that caveolin-3 is the principal caveolar protein in these muscle types. Thus, both caveolin-1 and -3 are capable of caveolar biogenesis independently.
All three caveolin proteins are expressed in BSM cells.1,14 Caveolae are almost absent in BSM of caveolin-1 KO mice but normal in caveolin-2 and -3 KO mice.14 Thus, caveolin-1 appears to be the principal structural protein for caveolar biogenesis in mouse BSM cells. Deletion of caveolin-1 causes marked structural changes in the bladder that are more apparent in older mice14 but the impact of aging on BSM caveolae is unclear. Since impaired bladder function is highly associated with aging and results from both genetic loss of caveolin-118 and biochemical depletion of caveolae,1 the relationship between caveolae and aging warrants investigation. This study was thus aimed to ascertain the effect of biological aging on caveolae morphology and caveolin protein expression in BSM.
MATERIALS AND METHODS
Urinary bladders were procured from male Sprague-Dawley rats that were categorized as young adult (controls; 10-week old), adult (6-month old), and old (1-year old).
Ultrastructure
Quantitative assessment of caveolae on BSM cells was made from young, 6- and 12-month-old animals by electron microscopy (EM). Bladder tissue was fixed in buffered glutaraldehyde, dehydrated in ascending concentrations of ethanol, and embedded in resin with aid of propylene oxide. Ultrathin sections cut on microtome and mounted on copper grids were examined. The length of plasmalemma (μm) visible in the photomicrographs was measured and number of caveolae along this length was counted using Metamorph software. The density of caveolae was calculated as number of caveolae per μm. The area (nm2), maximum depth from cell membrane (nm), and pore diameter (nm) of caveolae were determined. Values from all EM images (n = 10 images from three bladders in each group) were averaged for each age group.
Protein Extraction
The mucosa was carefully dissected from the detrusor using a stereomicroscope (Meiji Techno America, Santa Clara, CA). Visualization of the entire lamina propria vascular plexus in the detached continuous mucosal sheet confirmed that the mucosa was essentially removed from each bladder. Bladder tissue (n = 3 from each group) was then quickly frozen and stored at −80°C. Total protein was extracted from bladder tissue, maintaining 4°C temperature throughout the procedure. Bladder tissue was minced and transferred to a tube containing RIPA lysis buffer (3 μl/mg of tissue) supplemented with protease inhibitor cocktail (PIC), phenylmethanesulfonyl fluo-ride (PMSF), sodium orthovandate, octyl-β-d-glucopyranoside (60 mM), and incubated for 5 min on a shaker before sonication. Contents were transferred to a centrifuge tube and incubated for another 30 min on a rotating shaker followed by centrifugation at 20,000g repeated twice. The supernatant (total protein lysate) was collected after each centrifugation. Protein extraction kit (Calbiochem, San Diego, CA) was used to extract cytosolic and membrane fractions as per the instructions provided by manufacturer. Briefly, minced bladder tissue was sonicated and contents were transferred to a centrifuge tube and incubated for another 10 min before centrifugation at 1,000g. The supernatant (cytoplasmic fraction) was collected and the pellet incubated in given solution for 30 min on a rotating shaker. The supernatant (membrane fraction) was collected after centrifugation at 6,000g. Protein concentrations were determined using the bicinchoninic acid protein assay (BCA) by measuring the absorbance at 280 nm with a biophotometer (Eppendorf).
Western Blotting
Equal amounts of protein extracts (15 μg) from each group were loaded in duplicate onto a polyacrylamide 4–12% bis-tris gel and separated by electrophoresis. Proteins were transferred to a 0.2 μm nitrocellulose membrane over 90 min at 4°C. Membranes were rinsed in tris-buffered saline with 0.05% Tween-20 (TBST), blocked for 2 hr at room temperature (RT) with 5% non-fat dry milk in TBST and incubated overnight at 4°C with primary antibody (caveolin-2, 1:300, BD Transduction Laboratories, Sparks, MD; caveolin-1, 1:1,000; caveolin-3, 1:300; β-actin, 1:4,000; Santa-Cruz Biotechnology, Santa Cruz, CA, and Cavin-1:PTRF, 0.625 μg/ml, Abcam, Cambridge, MA) and then for 1 hr at RT with horse-radish peroxidase (HRP) secondary antibody (donkey anti-rabbit 1:15,000; donkey anti-goat 1:30,000; donkey anti-mouse 1:5,000; Santa-Cruz Biotech), diluted in blocking buffer. Molecular weight marker (MagicMark XP, Invitrogen, Carlsbad, CA) in 1-well identified relative position of proteins. Target proteins were detected with Western Lightning Plus ECL chemiluminescence agent (Perkin-Elmer, San Jose, CA) and exposure to film. Immunoreactive bands were quantified using image analysis software (Kodak Image Station 4000). Optical density for each protein band was normalized by that of β-actin.
Real-Time RT-PCR
RNA was isolated from 10 weeks (n = 3) and 79-week-old (n = 3) rat bladders after removal of the mucosa. Reverse transcribed total RNA (50 ng) was used for two-step quantitative real-time PCR. Taq-Man probe with forward and reverse primers were used in real-time PCR system (ABI Prism 7700, Applied Biosystems, Carlsbad, CA), with 18S serving as the internal control. All samples were amplified in duplicates. Caveolin-3 gene expression in BSM tissue was calculated as relative quantification of cycle threshold to the value in young animals and expressed as fold change in gene expression.
Confocal Microscopy
Bladder tissue obtained from each group was embedded in OCT and immediately frozen at −80°C for immunofluorescence confocal microscopy (n = 3 from each group). Sections of bladder tissue were cut (10 μm) with a cryostat and placed onto precleaned microscope slides. Three to four sections from each age group were placed on the same slide to ensure identical processing and imaging conditions. Sections were air dried after fixation in cold acetone and blocked in 5% chicken/donkey serum in phosphate-buffered saline (PBS) for 2 hr. Slides were incubated overnight in primary antibody (caveolin-2, 1:800, BD Transduction Lab; caveolin-1, 1:600, and caveolin-3, 1:200, Santa-Cruz Biotech) at 4°C and secondary antibody (Alexa Fluor 594 chicken anti-rabbit, Alexa Fluor 488 donkey anti-mouse or Alexa Fluor 568 chicken anti-goat, 1:1,000; Invitrogen) for 1 hr at RT and examined with confocal microscope (LSM 710, Carl Zeiss Microimaging, GmBH, Germany). Fluorescence intensity thresholds, laser power and gain were determined for images from the young group and the same settings were used for all age groups. From each bladder, 10 images were obtained from three separate tissue sections. One to three regions were selected from each image based on the number of smooth muscle bundles identified. The mean intensity of fluorescence from each region was determined using Zen imaging software. Areas with no staining were excluded as were non-BSM regions.
Data Analysis
After testing for normality, parametric data were assessed using one-way analysis of variance (ANOVA) followed by post hoc Holm-Sidak test. For comparing data with non-normal distribution, one-way ANOVA on ranks was used and significant differences between the groups were determined by post hoc Dunn’s method. P-value of 0.05 or less was considered significant. All data were expressed as mean ± standard error of mean unless otherwise specified.
RESULTS
Morphologic Characterization of BSM Caveolae
Changes in the morphology of BSM caveolae were quantified from images obtained from EM. In all the three age groups, caveolae were seen as distinct omega-shaped invaginations of the plasma membrane. A consistent age-dependent reduction in the number of membrane caveolae on BSM cells was evident, reaching significance at 12 months of age. Discernible caveolar structures were decreased by more than 50% at 12 months compared to young animals (Fig. 1). While in young, caveolae were predominantly spherical in shape with slight neck region, this particular contour was less apparent in 6 and 12 months animals, in which caveolae were more tubular with prominent neck region. The average area of BSM caveolae in 6- and 12-month age groups was significantly smaller than in the young group (Table I). The average diameter of the open caveolar neck at the plasma membrane was unaltered with age (Table I).
Fig. 1.
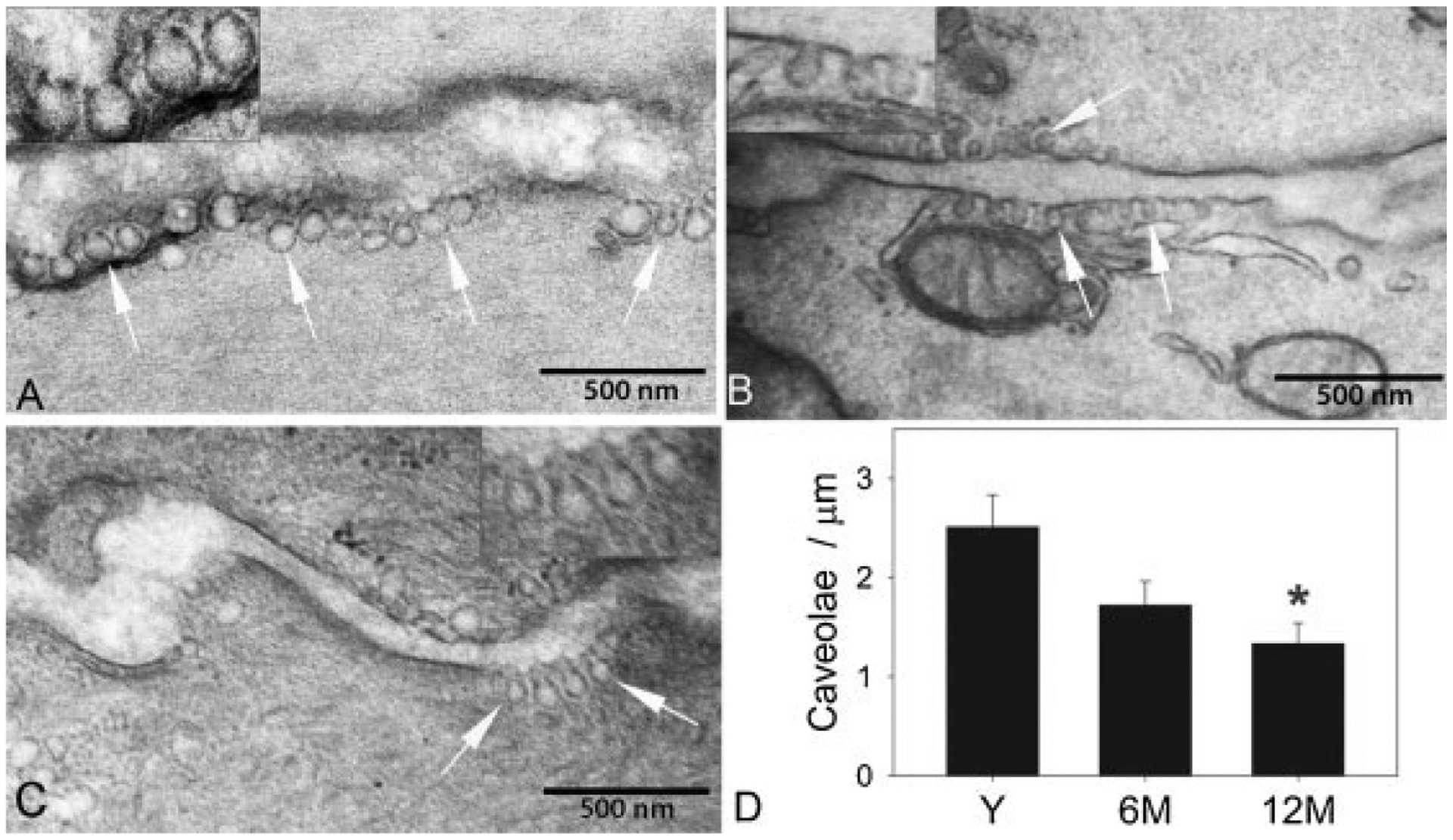
Alterations in density and dimensions of caveolae with age. Electron microscope images of bladder smooth muscle cells from young (A), 6-month-old (B), and 12-month-old (C) male Sprague-Dawley rats. Plasma membranes of two adjacent detrusor muscle cells are seen in each image with caveolae identified by white arrows. Mitochondria and sarcoplasmic reticulum are visible in proximity to caveolae in image B. Magnified regions of plasmalemma with caveolae are shown in insets. Note the apparent decrease in size of caveolae in 6- and 12-month-old animals compared to the young. D: Graph shows quantitation of caveolar density in each age group. Y, young; 6M, 6 months; 12M, 12 months. *Significant decrease compared to young (P < 0.01).
TABLE I.
Age-Related Alteration in Caveolar Dimensions
| Age group (months) | Caveolar area (nm2) | Caveolar depth (nm) | Caveolar pore diameter (nm) |
|---|---|---|---|
| Young adult (2.5) | 5456.2 ± 244.87 | 90.8 ± 2.04 | 31.3 ± 1.37 |
| Adult (6) | 2740.7 ± 122.24* | 63.9 ± 1.53* | 29.8 ± 1.24 |
| Old (12) | 4319.6 ± 153.22* | 83.9 ± 2.13† | 33.0 ± 2.31 |
Means ± SE.
Significant change from young (P < 0.05).
Significant change from 6 months (P < 0.05).
Effect of Age on Caveolin Expression
To determine whether an age-dependent decrease in caveolae is associated with altered caveolin protein expression in BSM, expression of caveolin proteins were determined by Western blotting in bladder tissue from which the mucosa had been removed. Expression of caveolin-1 in total bladder lysate was not altered with age (Fig. 2). To examine whether unchanged caveolin-1 expression was due to altered membrane targeting, caveolin-1 expression was determined in bladder lysate extracted from membrane and cytosolic fractions. Caveolin-1 expression was unaltered by age in both cytosolic and membrane fractions (Fig. 2). To exclude potential alterations in the relative expression of caveolin-1 isoforms, a different antibody that detects both α and β isoforms was used. There was no change in the expression of either caveolin-1 isoform in the studied age groups (Fig. 2). Finally, the expression of cavin-1 was determined in total bladder tissue lysate to examine whether an unchanged caveolin-1 expression with age was associated with alterations in other proteins regulating caveolin function. However, cavin-1 expression was also found to be unchanged with age (Fig. 2).
Fig. 2.
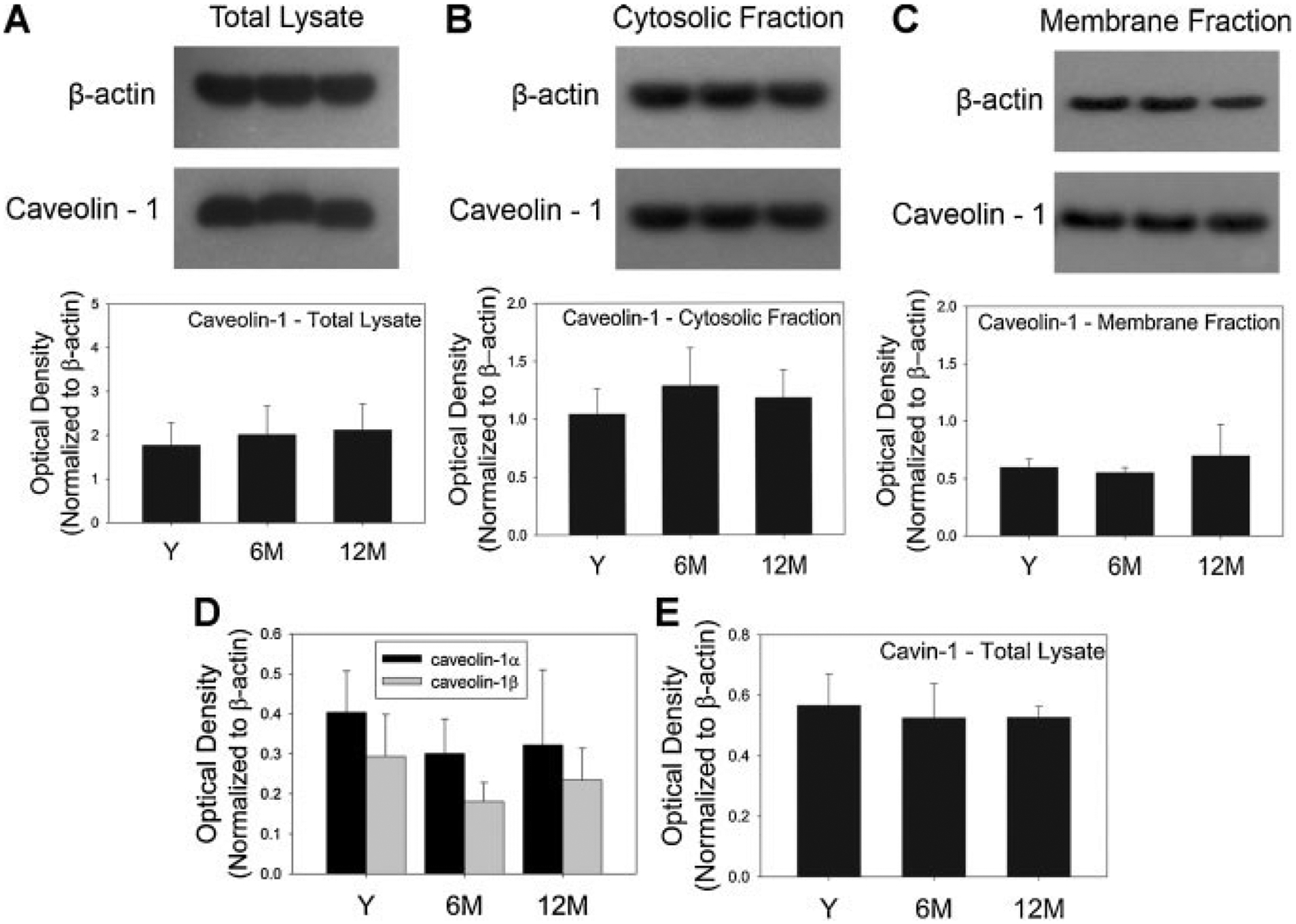
Effect of age on caveolin-1 expression. Expression of caveolin-1 in total (A), membrane (B), and cytosolic (C) fractions of lysate from rat bladder tissue in which the mucosa was removed. β-Actin expression (43 kDa) was used as control. Lane 1 = young; lane 2 = 6 months; lane 3 = 12 months. Corresponding graphs below show relative expression of caveolin-1 (normalized to β-actin) in total, membrane, and cytosolic fractions in the three age groups (n = 3). Relative expression of α and β isoforms (D) of caveolin-1 (normalized to β-actin) in the membrane lysate of bladder tissue was not different among the three age groups (n = 4 for young and 3 for the other age groups). E: Quantification of Western blot showing no change in the expression of cavin-1 in total lysate from each age group (n = 3). β-Actin was used as loading control. Bladders used for extraction of cytosolic and membrane fractions were procured from different animals than those used for extraction of total protein. Y, young; 6M, 6 months; 12M, 12 months.
For determining caveolin-2 protein expression, all three isoforms (α, β, γ) were quantitated collectively from total bladder lysate. Total caveolin-2 expression was significantly decreased at both 6 and 12 months (Fig. 3).
Fig. 3.
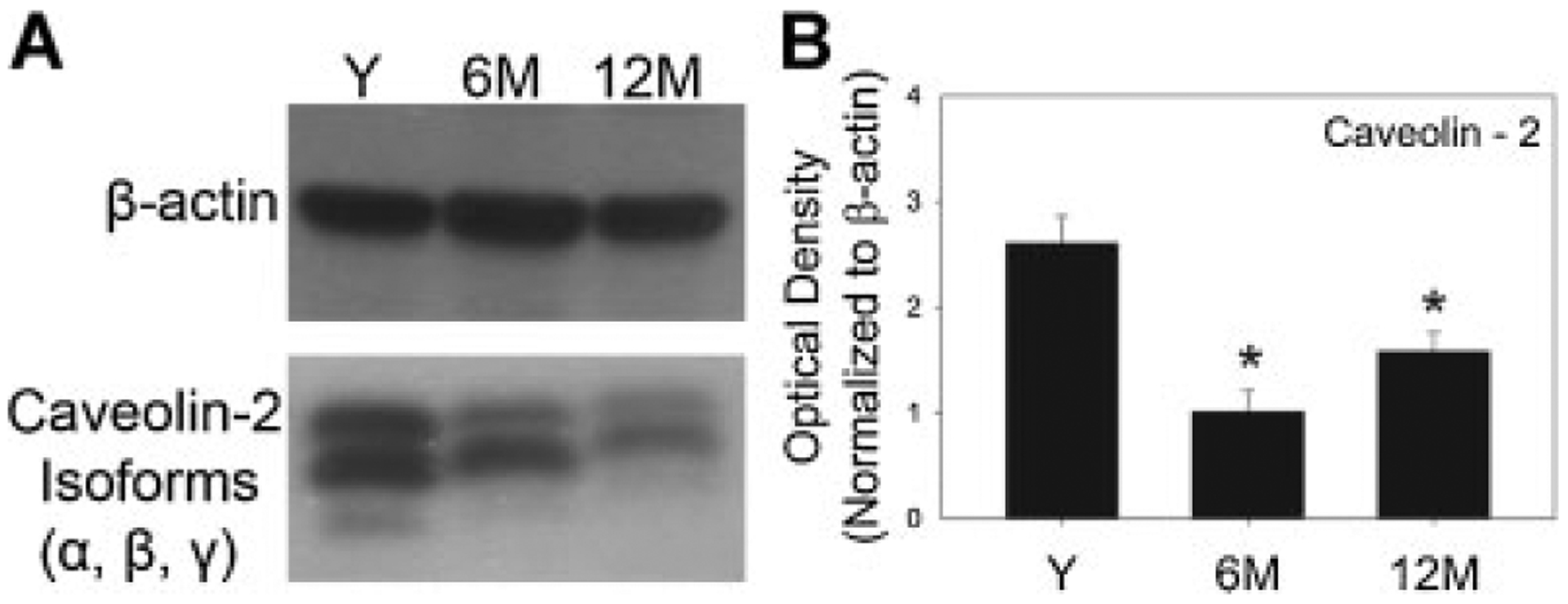
Effect of age on expression of caveolin-2 in rat bladder. A: Western blot for caveolin-2 (α, β, γ isoforms) in total protein lysate of bladders procured from young, 6 months, and 12-month-old animals (n = 3 for each group) from which the mucosa was removed. β-Actin (43 kDa) was used as control. B: Corresponding graph depicts the quantitative changes in caveolin-2 expression with age, normalized by β-actin. Y, young (n = 3); 6M, 6 months (n = 3); 12M, 12 months (n = 3). *Significant compared to young (P < 0.05).
Expression of caveolin-3 was significantly decreased at 6 and 12 months compared to that in young animals (Fig. 4A). Moreover, caveolin-3 gene expression was significantly reduced by more than threefold in 76-week-old rat bladders relative to young adult bladders (Fig. 4B).
Fig. 4.
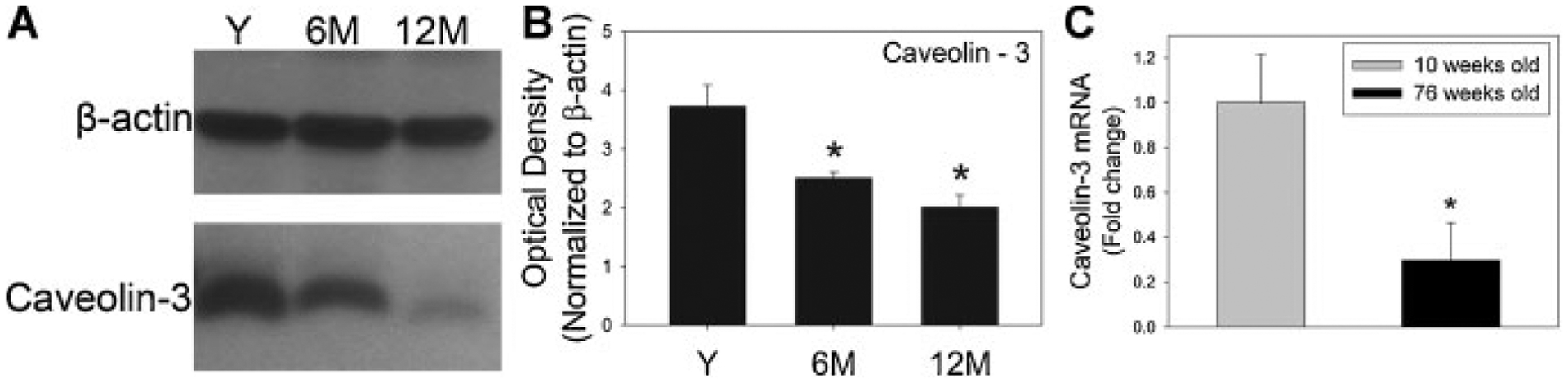
Effect of age on caveolin-3 expression in rat bladder smooth muscle. A: Representative example of Western blot of caveolin-3 expression in each age group. β-Actin was used as loading control. B: Densitometric analysis of caveolin-3 protein immunoreactivity expressed as optical density normalized by β-actin. Caveolin-3 expression decreased significantly with age. Y, young (n = 3); 6M, 6 months (n = 3); 12M, 12 months (n = 3). C: Expression of caveolin-3 mRNA, normalized to 18S and depicted relative to 10-week-old animals. Bars represent the mean ± SEM of the results from three bladders. *Significant compared to young group (P < 0.05).
Confocal microscopy was performed and immunofluorescence of caveolin-1, -2, and -3 in each age group was examined. Prominent staining of BSM cell membrane was apparent with all caveolin proteins. Of the three caveolin proteins, caveolin-1 staining was more homogenously distributed compared to caveolin-2 and -3, while the distribution of caveolin-3 was the least uniform in all age groups (Fig. 5). In fact caveolin-3 staining varied considerably within the same muscle bundle. The immunoreactivity for caveolin-1 was unchanged with age while that for caveolin-2 and -3 was significantly decreased at 6 and 12 months compared to young animals (Fig. 5).
Fig. 5.
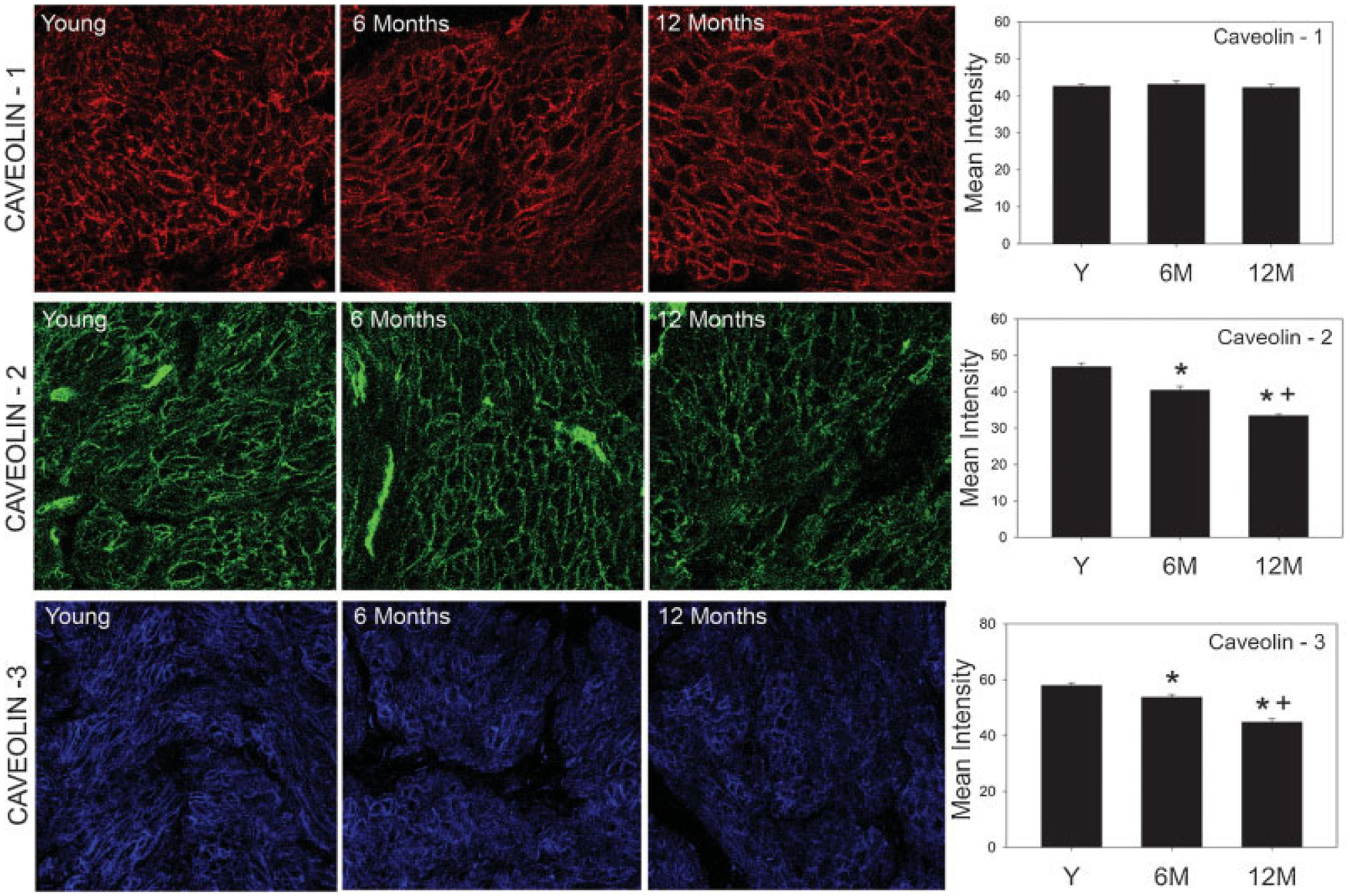
Effect of age on the distribution of caveolin proteins in bladder smooth muscle. Left: Image panels show immunofluorescence for caveolin-1 (top), caveolin-2 (middle), and caveolin-3 (bottom) in the young, 6 months, and 12 months age groups. Strong staining of blood vessels is apparent in caveolin-2 images. Right: Graphs provide a quantitative comparison of immunofluorescence in each group. Image analysis was performed in three bladders from each group. For each bladder, 10 images from three different tissue sections were used for quantitation. *Significantly lower than young group (P < 0.05); +Significantly lower than adult group (P < 0.05).
DISCUSSION
Aging is commonly associated with bothersome lower urinary tract symptoms affecting male and female populations alike. Despite its prevalence, the pathophysiology of the aging bladder is poorly understood. Several diseases that increase in incidence with age (Alzheimer’s disease, diabetes, atherosclerosis) have recently been linked with abnormalities in the structure and function of caveolae.19 In this study, we have quantitatively established an age-dependent reduction of caveolae and an alteration in caveolin protein expression in BSM cells. Previous studies have shown that caveolae play a crucial role in modulation of agonist-induced BSM contractions and depletion of BSM caveolae results in significant bladder dys-function.18 That alterations in these functionally specialized microdomains and their structural proteins are associated with bladder dysfunction is further exemplified by the fact that animal models of bladder outlet obstruction (BOO),20–21 a condition commonly associated with bladder dysfunction in men, also display alterations in BSM caveolae and caveolin protein expression. Together, these findings indicate that morphologic and functional defects of membrane caveolae could contribute to the aging bladder by altering BSM cell phenotype.
BSM caveolar density varies with age, sex, species, and strain of the animals. In contrast to the reported caveolae density in BSM of 1.3/μm in female Sprague-Dawley rats (roughly 8-week old),20 0.74/μm in female Wistar rats (8-week old),22 and 0.98/μm in female Fischer 344 rats (13-month old),23 we found a caveolae density of 2.5/μm in young 10-week old male Sprague-Dawley rats. In mice bladders, the BSM caveolae density is 1.1/μm in males (4-month old)14 and 2.1/μm in females (4- to 6-month old).2 Although differences in measurement techniques may account for some of the relatively small variation in published values, the density of caveolae in BSM measured in this study, as well as in previous reports, is similar in range to those described in other types of smooth muscle cells.24 In the present study, we demonstrated an age-dependent decrease in caveolae density amongst the studied age groups. The BSM cell caveolae density in the oldest age group was approximately half the density calculated in young animals. Thus, age-dependent depletion in BSM caveolae as found here could alter signal transduction in BSM resulting in bladder dysfunction in the elderly.
To focus on caveolin expression specifically in BSM, the mucosa was removed from all bladder samples. While other cell types are present in these samples, the contribution of BSM to protein expression undoubtedly predominates. Moreover, our protein expression data from Western blotting was corroborated by our quantification of caveolin immuoreactivity in bladder tissue sections, in which non-BSM structures were disregarded in the analysis.
In caveolin-1 KO mice, caveolae are nearly absent in BSM cells, an observation that has led to the notion that caveolin-1 is the major caveolae forming protein in these cells. Interestingly, our finding of an age-dependent reduction of BSM cell caveolae was not associated with a decrease in caveolin-1 expression in BSM tissue. It is possible that an age-dependent dissociation of caveolin-1 from the BSM cell membrane to the cytosol could account for caveolae depletion despite an unchanged total caveolin-1 expression, similar to the shift from membrane to cytosolic localization of caveolin-3 seen in cardiomyocytes with age.8 However, in the present study, while average caveolin-1 immunoreactivity in BSM was unaltered with age, caveolin-1 expression in the membrane and cytosolic fractions of bladder tissue extracts was also unchanged among the three age groups. Since caveolin-1 isoforms differ in their efficiency for caveolae formation25 age-dependent changes in the isoform ratio could potentially decrease BSM caveolae without a reduction in total caveolin-1 protein expression. We thus determined the expression of caveolin-1α and -1β in BSM cell membrane fractions, but found that both isoforms were also unaltered with age. Therefore, age-associated caveolae depletion in BSM does not appear to be associated with loss of caveolin-1 protein from the cell membrane.
Recently, a novel family of proteins termed “cavins” was shown to regulate caveolae formation. While cavins were not the primary focus of present study, we determined the cavin-1 protein expression in the bladder, since loss of cavin-1 is associated with depletion of caveolae, at least in PC3 cancer cell line.26 We found that, similar to caveolin-1, the expression of cavin-1 was also unaltered with age. These findings are consistent with the parallel regulation of caveolin-1 and cavin-1 expression in mice adipocytes and lung tissue.27 An unaltered expression of caveolin-1 and cavin-1 despite a significant change in BSM caveolae density has been reported in an animal model of BOO.20 Taken together, these findings suggest that the caveolar alterations in BSM of aging animals could result from alterations in expression of proteins other than caveolin-1 or cavin-1.
Mice with deletion of caveolin-1 have co-existing reduction of caveolin-2 expression suggesting that caveolin-1 regulates caveolin-2 expression.14 Interestingly, in the present study, caveolin-2 expression decreased with age despite an unaltered caveolin-1 expression, a finding inconsistent with caveolin-1-dependent regulation of caveolin-2 expression in physiologically normal BSM cells. In caveolin-2 KO mice, BSM cell caveolae density is not different from that in wild-type mice, thus supporting the view that caveolin-2 is not essential for caveolae formation in BSM cells. Yet in HepG2 cells, caveolin-2 enhances caveolin-1-dependent caveolae formation.25 Thus, an age-dependent effect of decreased caveolin-2 expression on the efficiency of caveolae formation in BSM cells cannot be ruled out. However, our results more likely point to a regulatory interaction between caveolin-2 and -3, consistent with the parallel changes in caveolin-2 and -3 expression in cardiomyocytes detected during cardiac development.28
Morphological variations in the depth of caveolae have been described, in which caveolin-2 was associated with the efficient formation of deep caveolae when expressed in HepG2 cells.25 Our finding of an age-dependent decrease in BSM caveolin-2 expression thus prompted us to investigate the possibility of alterations in size and depth of BSM caveolae in aged animals. The decrease in caveolin-2 expression in 6-month-old animals was paralleled by significant reductions in the depth and area of caveolae compared to young animals. However, the shape of caveolae appeared to partially recover in old animals, creating a biphasic trend that could suggest that with even older age, caveolar dimensions would return to those found in young adult animals. Thus, the age range selected for our study limits the interpretation of caveolar structural changes. Nevertheless, our data show that the potential role of caveolin-2 in affecting morphologic characteristics of BSM caveolae warrants further investigation. The size and depth of caveolae have also been shown to increase with cavin-2 expression, imparting caveolae with a more tubular profile than the typical spherical shape.27 Due to the lack of availability of a cavin-2 antibody, we were not able to determine its expression in the present study but differences in the relative cavin and caveolin expression could result in the altered caveolar dimensions we found among age groups. While the implications of variations in area and depth of caveolae with age are not clear, the physiological functioning of BSM caveolae may be affected by alterations in their morphology as well. These membrane microdomains provide a favorable microenvironment for conglomeration of receptors involved in bladder contraction that cross-talk upon activation. It has been proposed that cross-talk between GPCR receptors is a function of the distance between receptors.29 A reduction in the size of caveolae could potentially impact this receptor interaction within caveolae thus altering the dynamics of further downstream signaling.
Although caveolin-1 drives caveolae biogenesis in most cells, caveolin-3 is responsible for caveolae formation in striated muscle in which caveolin-1 is not expressed. In BSM cells, both caveolin-1 and -3 are expressed.1 Since caveolin-3 can perform functions that are independent from caveolin-130,31 and caveolin-1 KO mice do not exhibit a complete loss of BSM caveolae,14 caveolin-3 expression may generate caveolae exclusive of caveolin-1 and contribute to a heterogeneous population of BSM caveolae. In the present study, there was a significant age-dependent decrease in caveolin-3 expression. With unaltered caveolin-1 expression with age, the present finding of caveolae depletion by greater than 50% in 12-month-old bladders indicate that in physiologically normal BSM cells, caveolin-3 is essential for the formation and maintenance of a substantial number of caveolae. In fact, in an animal model of BOO, a reduction in BSM caveolae was associated with an approximately 70% decline in caveolin-3 expression.21 Although a parallel but smaller decrease in caveolin-1 expression was also detected, this finding is consistent with our assertion that loss of caveolin-3 could result in BSM caveolae depletion. Despite the age-dependent pattern of declining caveolae and caveolin-3 expression, the use of 12-month-old animals would not necessarily correspond with a geriatric age and thus a different pattern of caveolin protein expression may develop at later stages of life. However, the significant reduction in caveolin-3 gene expression detected in 19-month-old animals supports the premise that these changes are specific to the aging bladder. These findings, along with a previous study that showed a greater number of caveolae in adult compared to neonatal rat bladders,23 suggest that caveolae increase in number during maturation and decrease with aging. Latent changes in caveolin-1 expression that may appear much later in life cannot be ruled out.
Loss of caveolin-3 with age may have considerable functional implications as well. In murine and human bronchial smooth muscle cells, in which caveolin-1 and -3 are co-expressed and are closely associated, caveolin-3 rather than caveolin-1 regulates M2 muscarinic receptor-mediated signaling.28 Thus in BSM cells, caveolin-3 may be an important independent factor in caveolar modulation of signal transduction and the age-related decrease in caveolin-3 may alter bladder contractility by precipitating both structural and functional changes in BSM caveolae. Our future studies will identify age-dependent alterations in bladder contractility that may be mediated by loss of caveolae. Mice with caveolae deficiency, albeit induced by genetic ablation of caveolin-1, have been proposed as an animal model of the aging bladder.32 Given our findings regarding caveolin-3, an assessment of the functional consequences of caveolin-3 gene ablation may shed additional light on the pathophysiology of the aging bladder.
CONCLUSION
The present study established a significant age-dependent decline in BSM cell caveolae associated with isoform-specific alterations in caveolin protein expression. While the expression of caveolin-1 is unaltered, a significant decline in the expression of caveolin-2 and -3 proteins parallels caveolae depletion in older animals. Age-dependent variations in the expression of caveolin proteins in BSM cells not only affect caveolar biogenesis but also impinge upon caveolar morphology. Urodynamic features of the aging bladder including inadequate contractility and bladder overactivity, could be a function of both quantitative and morphological alterations in BSM caveolae that lead to perturbations in caveolae-mediated signaling.
Grant sponsor:
Medical Research Service, Department of Veteran’s Affairs, Washington, D.C.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Cristofaro V, Peters CA, Yalla SV, et al. Smooth muscle caveolae differentially regulate specific agonist induced bladder contractions. Neurourol Urodyn 2007;26:71–80. [DOI] [PubMed] [Google Scholar]
- 2.Sadegh MK, Ekman M, Rippe C, et al. Biomechanical properties and innervations of the female caveolin-1-deficient detrusor. Br J Pharm 2011;162:1156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dys-function. II. Aging detrusor: Normal versus impaired contractility. J Urol 1993;150:1657–67. [DOI] [PubMed] [Google Scholar]
- 4.Herbert Z, Botticher G, Aschoff A, et al. Changing caveolin-1 and oxytocin receptor distribution in the ageing human prostate. Anat Histol Embryol 2007;36:361–5. [DOI] [PubMed] [Google Scholar]
- 5.Schutzer WE, Reed JF, Mader SL. Decline in caveolin-1 expression and scaffolding of G protein receptor kinase-2 with age in Fischer 344 aortic smooth muscle. Am J Physiol Heart Circ Physiol 2005;288:H2457–64. [DOI] [PubMed] [Google Scholar]
- 6.Somara S, Gilmont RR, Martens JR, et al. Ectopic expression of caveolin-1 restores physiological contractile response of aged colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 2007;293:G240–9. [DOI] [PubMed] [Google Scholar]
- 7.Bakircioglu ME, Sievert K-D, Nunes L, et al. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol 2001;166:734–8. [PubMed] [Google Scholar]
- 8.Ratajczak P, Damy T, Heymes C, et al. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res 2003;57:358–69. [DOI] [PubMed] [Google Scholar]
- 9.Munoz P, Mora S, Sevilla L, et al. Expression and insulin-regulated distribution of caveolin in skeletal muscle. Caveolin does not colocalize with GLUT4 in intracellular membranes. J Biol Chem 1996;271:8133–9. [DOI] [PubMed] [Google Scholar]
- 10.Park WY, Park JS, Cho KA, et al. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem 2000;275: 20847–52. [DOI] [PubMed] [Google Scholar]
- 11.Wheaton K, Sampsel K, Boisvert F-M, et al. Loss of functional caveolae during senescence of human fibroblasts. J Cell Phys 2001;187:226–35. [DOI] [PubMed] [Google Scholar]
- 12.Fra AM, Williamson E, Simons K, et al. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA 1995;92:8655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Timme TL, Naruishi K, et al. Mice with cav-1 gene disruption have benign stromal lesions and compromised epithelial differentiation. Exp Mol Pathol 2008;84:131–40. [DOI] [PubMed] [Google Scholar]
- 14.Woodman SE, Cheung MW-C, Tarr M, et al. Urogenital alterations in aged male caveolin-1 knockout mice. J Urol 2004;171:950–7. [DOI] [PubMed] [Google Scholar]
- 15.Minetti C, Bado M, Broda P, et al. Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency. Am J Pathol 2002;160:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbiati F, Engelman JA, Volonte D, et al. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glyco-protein complex and T-tubule abnormalities. J Biol Chem 2001;276: 21425–33. [DOI] [PubMed] [Google Scholar]
- 17.Zhao YY, Liu Y, Stan R, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. PNAS 2002;99:11375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai HH, Boone TB, Yang G, et al. Loss of caveolin-1 expression is associated with disruption of muscarinic cholinergic activities in the UB. Neurochem Int 2004;45:1185–93. [DOI] [PubMed] [Google Scholar]
- 19.Ohno-Iwashita Y, Shimada Y, Hayashi M, et al. Plasma membrane microdomains in aging and disease. Geriatr Gerontol Int 2010;10:S41–52. [DOI] [PubMed] [Google Scholar]
- 20.Shakirova Y, Sward K, Uvelius B, et al. Biochemical and functional correlates of an increased membrane density of caveolae in hypertrophic rat urinary bladder. Eur J Pharm 2010;649:362–8. [DOI] [PubMed] [Google Scholar]
- 21.Polyak E, Boopathi E, Mohanan S, et al. Alterations in caveolin expression and ultrastructure after bladder smooth muscle hypertrophy. J Urol 2009; 182:2497–503. [DOI] [PubMed] [Google Scholar]
- 22.Popescu LM, Gherghiceanu M, Mandache E, et al. Caveolae in smooth muscles: Nanocontacts. J Cell Mol Med 2006;10:960–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Q, Resnick NM, Elbadawi A, et al. Estrogen and postnatal maturation increase caveolar number and caveolin-1 protein in bladder smooth muscle cells. J Urol 2004;171:467–71. [DOI] [PubMed] [Google Scholar]
- 24.Voldstedlund M, Thuneberg L, Tranum-Jensen J, et al. Caveolae, caveolin and cav-p60 in smooth muscle and rennin-producing cells in the rat kidney. Acta Physiol Scand 2003;179:179–88. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto T, Kogo H, Nomura R, et al. Isoforms of caveolin-1 and caveolar structure. J Cell Sci 2000;113:3509–17. [DOI] [PubMed] [Google Scholar]
- 26.Hill MM, Bastiani M, Luetterforst R, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 2008; 132:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briand N, Dugail I, Lay SL. Cavin proteins: New players in the caveolae field. Biochimie 2011;93:71–7. [DOI] [PubMed] [Google Scholar]
- 28.Rybin VO, Grabham PW, Elouardighi H, et al. Caveolae-associated proteins in cardiomyocytes: Caveolin-2 expression and interactions with caveolin-3. Am J Physiol Heart Circ Physiol 2003;285:H325–32. [DOI] [PubMed] [Google Scholar]
- 29.Woolf PJ, Linderman JJ. Self organization of membrane proteins via dimerization. Biophys Chem 2003;104:217–27. [DOI] [PubMed] [Google Scholar]
- 30.Schlenz H, Kummer W, Jositsch G, et al. Muscarinic receptor-mediated bronchoconstriction is coupled to caveolae in murine airways. Am J Physiol Lung Cell Mol Physiol 2010;298:L626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogo H, Ito S-y, Moritoki Y, et al. Differential expression of caveolin-3 in mouse smooth muscle cells in vivo. Cell Tissue Res 2006;324:291–300. [DOI] [PubMed] [Google Scholar]
- 32.Lai HH, Boone TB, Thompson TC, et al. Using caveolin-1 knockout mouse to study impaired detrusor contractility and disrupted muscarinic activity in the aging bladder. Urology 2007;69:407–11. [DOI] [PubMed] [Google Scholar]


