Abstract
Background
Noma (cancrum oris) is an ancient but neglected and poorly understood preventable disease, afflicting the most disenfranchised populations in the world. It is a devastating and often fatal condition that requires urgent and intensive clinical and surgical care, often difficult to access as most cases of noma occur in resource-limited settings. We conducted a scoping review of the literature published on noma to understand the size and scope of available research on the disease and identify research gaps that need to be addressed to evolve our understanding of how to address this disease.
Methods
We searched 11 databases and collected primary peer reviewed articles on noma in all languages, the final search was conducted on 24th August 2021. The oldest manuscript identified was from 28th March 1843 and the most recently published manuscript was from 3rd June 2021. Search terms included cancrum oris and noma. Data was extracted using a standardised data extraction tool and key areas of interest were identified. The Preferred Reporting Items for Systemic review and Meta-Analyses requirements were followed.
Results
The review included 147 articles, the majority of the studies (n = 94, 64%) were case reports. Most manuscripts (n = 81, 55%) were published in the 2000s, 49 (33%) were from the 1900s and 17 (12%) from the 1800s. The main areas of interest identified were the history and epidemiology of the disease, noma’s clinical progression and aetiology, treatment regimens, mortality rates and the risk factors for the development of noma.
Conclusions
Noma has been reported in the literature for hundreds of years; however important gaps in our understanding of the disease remain. Future research should focus on determining the burden and distribution of disease; the true mortality rate, pathogenic cause(s) and the factors that influence prognosis and outcomes after treatment.
Author summary
Noma is a devastating and often fatal condition that mainly affects children in severely disenfranchised communities. Noma is preventable and requires urgent basic medical care in the early stages of disease. Once the disease reaches the last stage, sequelae, survivors require expert surgical care, usually difficult to access as most cases of noma occur in resource-limited settings. We conducted a scoping review of the literature published on noma to understand the size and scope of available research on the disease and to identify research priorities that will evolve our understanding of how to eradicate this disease. Our review showed that noma has been reported in the literature for hundreds of years; however several major gaps in knowledge still exist. There is appreciation among the small community of clinicians and researchers involved in noma care and research that these gaps in knowledge impact on the ability to develop and implement sound evidence-based policies and activities aimed at eradicating noma from communities that continue to be afflicted by this ancient disease. The main focus of future research should be to study the burden and distribution of disease; the true mortality rate, and the pathogenic cause(s) and the factors that influence prognosis and outcomes after treatment.
Background
Noma is a rapidly progressing infection of the oral cavity, associated with a reported 90% mortality rate within weeks after onset, if left untreated [1]. Noma mostly affects disenfranchised children who lack access to basic nutrition, hygiene services, and health care, although cases are reported in immunocompromised adults [1]. The pathogenesis of noma is poorly understood [1]. Commonly available broad-spectrum antibiotics can be used to treat the early reversible stages of noma [1]. Once noma progresses past these stages, the sequelae of noma are numerous and include difficulty in eating, drinking, seeing and breathing[1,2]. For those who seek care for these sequelae, it can mean hospital stays of many months with multistage surgical treatments that can take years to complete. Therefore, noma is associated with a high degree of morbidity for survivors, and this often has a significant impact on family members given the long-lasting and often permanent sequelae. Noma is an important public health issue and its existence is a painful reminder of the existing global inequalities in food distribution, health care access and living conditions. We conducted a scoping review of the literature on noma to consolidate the information available and to understand the size and scope of available research on this disease.
Methods
This scoping review was conducted in line with Preferred Reporting Items for Systemic review and Meta-Analyses (PRISMA) requirements (S1 PRISMA checklist) [3].
Databases searched
The following databases were searched manually for articles to include in this literature review: PubMed; PsycINFO via Ebsco Host; Science Direct; Social Science Citation Index via Web of Science; MEDLINE via PubMed; Cumulative Index to Nursing and Allied Health Literature via Ebsco Host; Cochrane Library; Population Information Online; LILACS; SciELO and Scopus. The final search was conducted on 24 August 2021.
Searching methods utilized
An initial search of each database was done online. All articles identified were listed. Available full text articles were downloaded. Outstanding articles were sourced through the University of Cape Town library or via the corresponding authors.
Eligibility criteria
The databases and journals were searched using the following eligibility criteria: 1) noma, cancrum oris related; 2) peer reviewed; 3) primary study (a study including primary data collection, literature reviews and opinion pieces were not included); 4) addressed a main area of interest; 5) any publication date; 6) all study designs; and 7) all languages.
Search terms
Databases were searched with the following terms: "cancrum oris" OR “noma” OR "cancrum oris cases" OR "cancrum oris defects" OR "cancrum oris like lesions" OR "cancrum oris noma" OR "cancrum oris, noma".
Data extraction
Data from the eligible studies were extracted using a standardized data extraction tool. Data extracted included: title; author; journal; year of publication; geographic location of first author; geographic location of study; number of cases/individuals in study; research question/aim; methodology; analysis; results; area of interest; conclusions; implications for future research and practice; gaps in knowledge and any other noteworthy comments.
Our main areas of interest were the history and epidemiology of the disease, noma’s clinical progression and aetiology, treatment regimens, mortality rates and the risk factors for the development and progression of noma.
All non-English papers were translated into English using Google translate, anything unclear was checked with a native language speaker.
Analysis
A manual analysis was conducted by grouping individual factors within the areas of interest (the history and epidemiology of the disease, noma’s clinical progression and aetiology, treatment regimens, mortality rates and the risk factors for the development and progression of noma). These areas of interest were explored in-depth and findings on each area are concisely reported.
Results
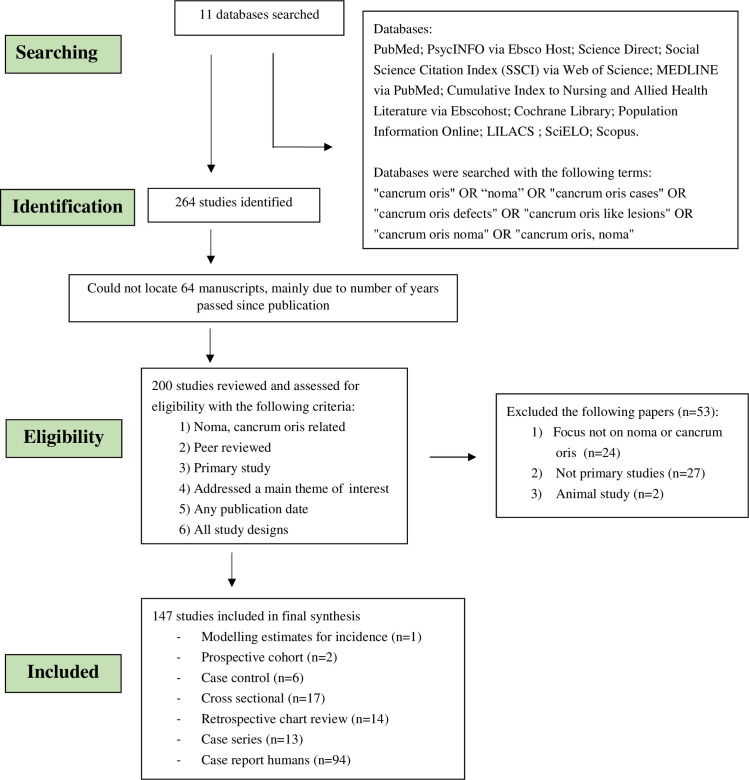
Our initial search identified 200 full text articles for review, of which 147 were included in the literature review (full list of included manuscripts attached in S1 Data). The oldest manuscript identified was from 28th March 1843, and the most recently published manuscript was from 3rd June 2021. Most manuscripts (n = 81, 55%) were published in the 2000s, 49 (33%) were from the 1900s and 17 (12%) were from the 1800s. Fifty three manuscripts were excluded as they either did not directly relate to noma, did not meet the inclusion criteria, or they were not considered to be primary research. The majority of the studies (n = 94, 64%) were case reports (Fig 1).
Fig 1. Flow diagram of databases searched and articles included in the noma scoping review.
History of noma and names for the disease
The word ‘noma’ is derived from a Greek word which, loosely translated, means ‘to devour’ [4]. It was first used by Dutch surgeon Cornelis van de Voorde in 1680, for a rapidly-spreading ulceration originating in wet soft tissues ‘typical of the mouth’ [5]. The term ‘noma’ and ‘cancrum oris’ are currently used interchangeably [6–9]. In the 1800’s, there were ongoing discussions about the usage of the terms with some viewing them as two separate diseases [10]. In a publication from 1862, ‘noma’ referred to ulcerative stomatitis (lesions on the skin or on the internal mucosal surface of the mouth) and ‘cancrum oris’ referred to gangrenous stomatitis (death of the tissue of the mouth) [10]. There are very early reports of clinical conditions similar to noma by physicians such as Hippocrates (460–370 BC) and Galen (129–200 AD) [11]. However; it was subsequently reported that this referred to general ulceration of the body and not noma as the disease is currently understood [12]. The first clinical description of the disease we now call noma, was written by Battus in 1620, who labelled it ‘water canker’ [5,12]. The 1848 definition of noma by Tourdes is similar to the modern medical understanding of the disease: “a gangrenous disease affecting the mouth and face of children living in bad hygiene conditions and suffering from debilitating diseases, especially eruptive fever, beginning with an ulcer on the oral mucosa rapidly spreading outside and destroying the soft and hard tissues of the face and almost always fatal” [13].
In Laos, the name commonly used for noma is ‘Pagnad Pak Poue’ meaning ‘disease of mouth rotting’ [14]. In Zambia, the disease has been labelled as ‘aka popo’, meaning the child has been fed a stillborn fetus, and the flesh is ‘coming out’ (describing the sloughing of the cheek) [15]. In Hausa, the most widely spoken language in northwest Nigeria, several names for noma have been documented including ‘ciwon iska’, ‘bakin kare’ [5], ‘danhurawa’, ‘tuareg’, ‘akin’ [16], ‘gaude’ and ‘sadde’ [17]. Several of these names are generalised terms and have reportedly caused confusion in patient recruitment drives for surgical interventions, as patients with ailments such as cleft lip and palate also identify with these names[5]. A further complicating etymological factor in the setting is that the word ‘noma’ means ‘farming’ in Hausa [16]. Names form a part of the understanding of the disease, and in this case, the beliefs about the causes of noma such as spirits, living creatures (insects and animals), and connections with previous illness [16]. These names and the beliefs about the disease have an impact on processes such as health-seeking behaviours and stigmatisation. If it is believed that the disease is caused by spirits or a bad omen, patients and their families are more likely to be ostracised [18].
Epidemiology
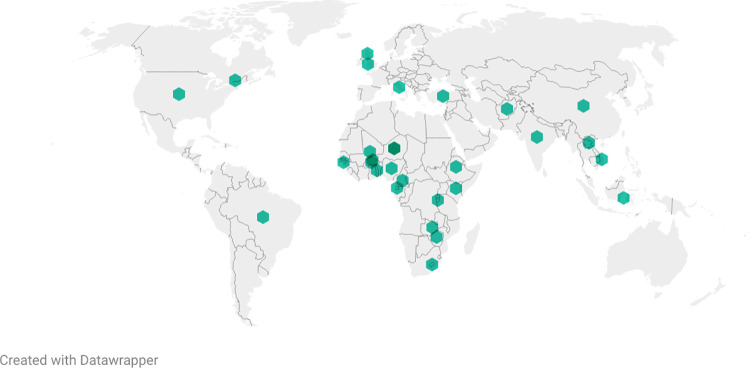
There is a shift in reporting of noma from primarily in Europe and India in the 1800’s [4,10,12,19–32], to parts of Africa and North America in the 1900’s [11,15,17,22,33–77], to Africa, South America and Asia in the 2000’s [6–9,14,16,78–150] (Fig 2). Noma cases were reported in Irish and British soldiers in India the 1880’s [26,28]; in Belsen and Auschwitz concentration camps during the Second World War [9,14,17,45,139,151,152] and in the general war-time population of the Netherlands following the famine in the winter of 1944/1945 [139]. Since the Second World War, as living conditions improved, the occurrence of noma in Europe dramatically decreased and is only sporadically reported in the region today [80,81,83,129]. In recent years, noma has been reported in many countries around the world, but primarily in low and middle income countries in Africa and Asia (Fig 2).
Fig 2. Map of location of noma studies published from 2000 to 2021 included in this review [6–9,14,16,78–150] (green dot represents at least one study in that country) (Created using Datawrapper, basemap: https://datawrapper.dwcdn.net/RE1zh/1/).
In 2007, the WHO carried out a survey in African member states, which found that 39 of the 46 countries surveyed had reported noma cases in the year prior to data collection [153]. Those with the highest number of reported cases were Burkina Faso, Ethiopia, Mali, Niger, Nigeria and Senegal which led to these countries being labelled the ‘noma-belt’ [153]. This term is commonly used when reporting the epidemiology of noma. However; the information gathered that led to this term was not standardized and not based on robust global prevalence or incidence estimates across countries [153]. Since 2000, cases have also been reported in a wide range of settings (Fig 2), indicating a much wider distribution than the usually reported ‘noma-belt’ [153].
The oldest estimate we found of the burden of disease, based on hospital admissions, indicated that, noma was diagnosed once out of every 5,000 cases of children admitted to hospital with an illness, between 1860 to 1871 in Edinburgh [4]. In 1997, Barnes et al. estimated that, based on records from three referral centres, the prevalence of noma was 1 case per 1,250 children aged two to six years per year in Nigeria [154]. In 1998, the World Health Organisation (WHO) estimated that 140,000 new cases of noma occur each year globally and that 770,000 patients were living with noma sequelae at that time [155], the origin of this estimate is unclear [156]. In 1999, it was estimated that there was an annual incidence of 4.2 acute noma cases per million Senegalese children aged 1–4 years [157]. This estimate was calculated using a WHO recommended formula (S1 Equation), based on a 5–20% presentation rate of patients with acute noma or sequelae, and an 80–90% mortality rate in the acute stages of the disease [157]. A Nigerian study (retrospective chart review from 2010 to 2018) estimated the incidence of noma in the north central zone was 8.3 per 100,000 population members at risk [135] and a further study from Nigeria (2018) estimated the community-based point prevalence in the northwest was 3,300 out of every 100,000 children aged 0–15 years [142]. The large variation in these results is due to the differing study designs and the different stages of noma (and case definitions for these stages) included in the estimates.
Through a retrospective chart review (n = 6,390) in 2003, Denloye et al. estimated seven cases per 1,000 children aged between one and 16 years had noma between 1986 and 2000 in Nigeria [118]. In that same year, a Fieger et al. modelled the incidence of noma in northwest Nigeria based on the number of clefts and concluded that the incidence of noma is estimated to be 6.4 per 1,000 children from 1996 to 2001 [139]. These estimates may not accurately reflect the present incidence of acute noma or the prevalence of patients with noma sequelae as they are based on expert opinion or historical data. It is also unclear which stages of noma are included in these estimates [158].
Risk factors
There is limited primary evidence on the risk factors for the development of noma. The table below explores the risk factors noted in the primary studies included in this review (Table 1). Reported risk factors for the development of noma in these primary studies include chronic malnutrition [11,15,17,75,111,114,118,147], comorbidities either at the time of noma diagnosis or in the three months leading up to diagnosis [11,15,17,41,51,75,104,107,111,114,118,122,147] and low vitamin A and vitamin C levels [7]. Social and environmental risk factors include being between two and five years of age [11,15,41,51,75,96,107,118], not being breastfed [114,143], lack of access to basic health care [41]- including a lack of childhood vaccinations [100,143], poor oral hygiene practices leading to gingivitis (Stage 0 noma) [100], low socioeconomic status [104], a lack of variety in the diet [143], the mother being unmarried, not the primary caretaker [143], and having a high number of previous pregnancies [111], and the absence of chickens at home [111].
Table 1. Risk factors for noma identified in primary research.
| Study | Study details | Risk factors identified |
|---|---|---|
| Osuji, 1990 [51] | Study type: Cross-sectional Location: Nigeria n: 58 cases of acute necrotizing gingivitis (Stage 1 noma as categorized under the WHO system [1]), 5 noma cases (diagnosed as advanced acute necrotizing gingivitis with sequestrum formation) |
• Respondents aged between 2–7 years (n = 49, 85% acute necrotizing gingivitis cases, n = 3, 60% noma cases) • Rainy season (n = 42, 67%) • History of recent febrile illness (n = 55, 87%) |
| Lazarus, 1997 [11] | Study type: Retrospective chart review, reviewing charts of cancrum oris patients from the previous 35 years Location: South Africa n: 26 respondents |
• Respondents mean age 4 years 4 months (range 1–15 years) • Malnutrition (n = 7/ 11 (whose records had comorbidity information), 64%) • Gastroenteritis (n = 4/11, 36%) • Measles (n = 3/11, 27%) |
| Nath, 1998 [15] | Study type: Retrospective chart review over 15 years n: 81 respondents |
• Respondents aged between 1 and 4 years (n = 67, 83%), • Diarrhoea (n = 13, 28.9%) • HIV (n = 26/45 (children admitted between 1989–93), 60.5%), • Malnutrition (n = 15/ 45 (no. children assessed for malnutrition), 33.3%) • Rainy season (n = 60, 74.1%), |
| Ndiaye, 1999 [41] | Study type: Prospective cohort Location: Senegal n: 25 later stage noma cases, 1058 acute necrotizing gingivitis cases |
• Noma respondents mostly aged >15 years (n = 13, 52%), acute necrotizing gingivitis respondents mostly aged between 1–4 years (n = 465, 44%) • No access to basic care (n = 20/25 (noma only), 80%) |
| Enwonwu, 1999 [17] | Study type: Case control study Location: Nigeria n: 86 noma cases |
• Respondents mean age 5.9 years (Standard Deviation) (SD) 2.6 years • Malnutrition (Weight-for-height Z score (WHZ) ≤ -2.0 SD) (n = 9, 10.2% controls, n = 17, 19.4% cases) |
| Oginni, 1999 [75] | Study type: Retrospective chart review of noma patients from 1982 to 1996 Location: Nigeria n: 146 noma patients, 133 acute, 13 sequelae (which was 1.7% of all patients admitted to the hospital during this time). |
• Respondents mean age 4.7 years (SD 2.6 years) • Malnutrition (n = 146, 100%) • Poor oral hygiene (n = 122, 83.6%) |
| Denloye, 2003 [118] | Study type: Retrospective chart review 1986 to 2000 Location: Nigeria n: 45 noma cases |
• Respondents mean age 4.2 years (SD 2.7 years) • Malnutrition (n = 45, 100%) • Malaria (n = 14, 31%) • Measles (n = 14, 31%) |
| Enwonwu, 2005 [114] | Study type: Case control Location: Nigeria n: 91 noma cases |
• Respondents mean age 2.6 years (SD 1.0) • Malnutrition (median height for age z-score noma group -3.74, control group 1–1.41, control group 2 0.85) |
| Phillips, 2005 [7] | Study type: Case control Location: Nigeria n: 68 noma acute cases |
• Biological markers suggestive of malnutrition (lower plasma levels of vitamin A (p<0.001), vitamin C (p<0.05) and zinc (p<0.001)) • Marked reductions (p<0.001) in albumin and blood haemoglobin |
| Chidzonga, 2008 [96] | Study type: Retrospective chart review of charts between 2002 and 2006 Location: Zimbabwe n: 48 acute noma cases, all HIV positive (by design) |
• Respondents aged <16 years (n = 11, 64.7%) • Gender (female n = 31, 64.6%) |
| Millogo, 2012 [122] | Study type: Retrospective chart review from 1988 to 2007 Location: Burkina Faso n: 212 patients (n = 14, 6.6% had HIV) |
• Respondents mean age 15.3 years for HIV group, 4.7 years for non-HIV infected group • Concurrently HIV-infected patients had higher mortality (38% vs 6%) |
| Baratti-Mayer, 2013 [111] | Study type: Case control Location: Niger n: 82 cases and 327 controls |
• Respondents aged 0–12 years • Severe stunting (Height-for-age Z score ≤3 SD) (Odds Ratio) (OR) 4.87, 95% Confidence Interval (CI) 2.35–10.09) • Wasting (WHZ ≤3 SD) (OR 2.45, CI 1.25–4.83), • High number of previous pregnancies in the mother (OR 1.16, CI 1.04–1.31) • Presence of respiratory disease, diarrhoea or fever in the 3 months prior to data collection (OR 2.70, CI 1.35–5.40) • Absence of chickens at home (OR 1.90, CI 0.93–3.88) |
| Konsem, 2014 [104] | Study type: Chart review 2003 to 2012 Location: Burkina Faso n: 55 acute noma cases |
• Respondents mean age 7.64 years • Concomitant Bronchopneumonitis (n = 20, 36.4%) • Malaria (n = 14, 25.4%) • HIV (n = 11, 20.0%) • Low standard of living (n = 21, 38.2%) • Anaemia (n = 14, 25.4%) |
| Braimah, 2017 [147] | Study type: Retrospective chart review from 1999 to 2011 Location: Nigeria n: 159 acute noma cases |
• Mean age was 3.34 ± 2.2. • Measles 75 (47.2%), followed by • Protein-energy-malnutrition 67 (42.1%). |
| Adeniyi, 2019 [107] | Study type: Retrospective chart review from 1999 to 2011 Location: Nigeria n: 159 acute noma cases |
• Respondents aged between 1–5 years (n = 139, 87.4%) • Concurrent disease at presentation or in the 3 months preceding their presentation at the hospital (n = 148, 93.1%) • Measles (n = 75, 47.2%) • Protein-energy malnutrition (n = 67, 42.1%) |
| Farley, 2019 [143] | Study type: Case control study Location: Nigeria n: 74 cases and 222 controls |
• Respondents median age 5 (IQR 3, 15) • Vaccination coverage documented on vaccination cards for polio and measles was below 7% in both groups • Child being fed pap every day (OR 9.8; CI 1.5, 62.7); • Potential protective factors including: ○ the mother being the primary caretaker (OR 0.08; CI 0.01, 0.5) ○ the caretaker being married (OR 0.006; CI 0.0006, 0.5) ○ colostrum being given to the baby (OR 0.4; CI 0.09, 2.09) |
WHZ- weight-for-height Z score; OR = Odds Ratio; SD = Standard Deviation; CI = Confidence Interval; WHO = World Health Organisation; HIV = human immunodeficiency virus
Other studies have hypothesized further risk factors for noma development including household variables, such as proximity of livestock to living areas and poor sanitation [100], which is thought to lead to possible contamination of water and food sources and consequently increasing the risk of infections [159,160]. However; caution is needed when interpreting these findings as they are based on the proportions of cases vs controls having these risk factors and more robust evidence is needed to validate these findings.
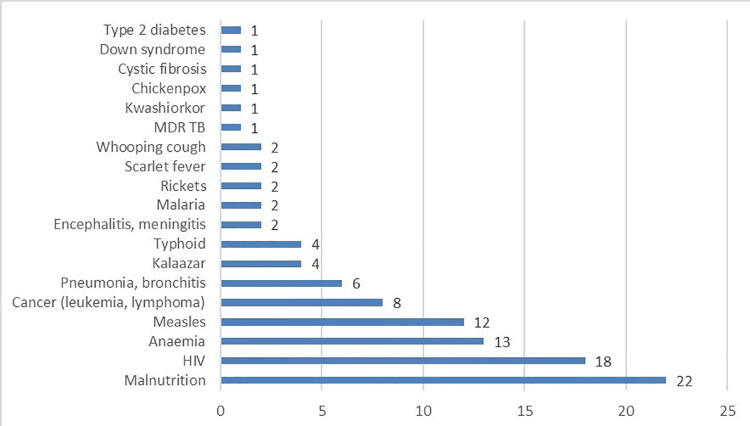
Reported comorbidities in the primary studies (case control, cohort, retrospective chart reviews) include malnutrition [11,75,107,111,114,118], respiratory disease [104,111], diarrhoea [11,111], HIV [96,104,122], malaria [104,118] and vaccine preventable diseases, specifically measles [11,107,118]. Most of the case reports and case series (n = 68) list at least one comorbidity (103 comorbidities listed in these case reports and case series). The most widely reported comorbidities in the case reports and case series included in this review are malnutrition [14,22,26,36,37,44,56,78,83,100,103,106], HIV [56,60,82,87,90,150], anaemia [8,96,106,112] and measles [4,39,49,161] (Fig 3). As this information is based solely on case reports and case series, primarily reported from health care centres, no causal link or strength of association can be measured. Infections are usually the product of a compromised host and a single offending agent or multiple offending agents. Due to challenges with conducting scientifically robust risk factor analysis for noma, it is difficult to separate comorbidities from predisposing conditions and true causative factors.
Fig 3. Comorbidities associated with noma in case reports and case series (N = 103).
One theory for the higher incidence of noma in children aged two to five years, is that this is the teething age when deciduous teeth are formed [41]. This formation slows down the circulatory flow to the gums due to compression, leaving the oral cavity more susceptible to infections [41]. A Zambian study postulated that during the weaning period from breastfeeding, children eat more solid food, which was less nutritious and less sterile than breast milk, and this placed them at potential risk for noma development [15]. Another study showed that if weaning foods are prepared under unhygienic conditions, they are frequently contaminated with pathogens and are a major factor in the cause of diarrhoeal diseases [162], a further reported risk factor for noma [163].
Studies that attempted to identify risk factors for noma were hampered by the retrospective nature of case ascertainment limiting the kinds and standardization of risk factor data collected [11,15,75,96,104,107,118,122]. The absence of a suitable control group precluded the ability to find associations between noma and potential risk factors in some studies [11,15,41,96,164]. In other studies there was no statistically robust examination of risk factors using proven statistical methods such as multivariable regression (which would adjust for confounders), limiting the validity and reliability of results [17,41,51,75].
Aetiology
The pathogenesis of noma is poorly understood. Strikingly, this quote from a paper written in 1893 still partly reflects the current debated nature of the pathogenesis of noma “There must surely be a specific organism and a combination of predisposing causes, not poverty alone, but poverty plus a sickly habit of body” [12].
A range of organisms have been identified in the oral flora of noma patients, but none have been consistently present, casting doubt on a specific organism’s role in the development of noma [7,17,38,40,98,111,165]. Other studies have noted that the characteristics of noma are similar to that of an opportunistic infection, implicating a change in the equilibrium of commensal bacteria due to a derailment of host defences [11,96,98,166]. Evidence that supports the understanding of noma being an opportunistic infection rests in the fact that most cases have concurrent infections or occur in immunocompromised individuals [11,15,17,41,51,75,104,107,111,114,118,122,147]. Table 2 below offers a summary of the etiologic studies included in this review and the organisms identified, the details of each study and limitations of the study methods.
Table 2. Microorganisms found in the oral flora of noma patients by year.
| Study | Study details | Organism | Limitations |
|---|---|---|---|
| Falkler, 1999 [38] | Study type: Cross-sectional study Location: Nigeria n: Eight cases Additional details: Cancrum oris lesions (present for six weeks to two years) were cultured for anaerobic microorganisms. |
Fusobacterium necrophorum and Prevotella intermedia were isolated from seven and six of the eight lesions, respectively. | Long duration of infection before testing (up to two years), small sample size, no healthy matched comparison group. |
| Phillips, 2005 [7] | Study type: Case control study Location: Nigeria n: 68 acute noma cases, 63 village and 45 urban controls Additional details: Cases were found over four years through house visits. Controls were matched by age and were children attending out-patient clinics and primary health care centres for routine checks, and had no recent history of any disease, fever and diarrhoea. Oral bacteria were studied by polymerase chain reaction on six cases. Excluded those treated with antibiotics or traditional medicine in last 48hrs. Excluded measles, HIV and malaria comorbid patients. |
Bacteria observed at the highest frequencies in noma lesions were Prevotella intermedia (83%), Tannerella for sythensis (83%), Porphyromonas gingivalis (50%), Campylobacter rectus (50%) and Treponema denticola (50%). | Control selection (children attending health care facility) could have biased results as these children were already accessing care. It is unknown how long each patient had noma for. The sample size for bacterial testing was small (n = 6). |
| Chidzonga, 2008 [96] | Study type: Retrospective chart review Location: Zimbabwe n: 48 acute noma cases, five cases had microbiologic investigations Additional details: All cases presented one to two weeks after onset of symptoms |
Staphylococcus aureus, Klebsiella species, group D Streptococcus, and group B hemolytic Streptococcus. | Small sample size, retrospective chart review, no control group. |
| Baratti-Mayer, 2013 [111] | Study type: Prospective matched, case-control study Location: Niger n: 82 acute noma cases, 327 matched controls Additional details: Study took place over six years. Exact stage of noma cases not defined. Controls matched on age and home village. Extracted total genomic deoxyribonucleic acid. Cases who received antibiotics or whose specimens deteriorated were excluded (n = 20), 117 microbial samples were processed from noma cases and 235 from controls. Multivariable model showed organisms associated with noma. |
A reduced proportion of Spirochaeta, Fusobacterium, Capnocytophaga, and Neisseria in the oral microbiota, but an increased proportion of Prevotella associated with noma. Controls had higher Fusobacterium genus levels raising doubts about previous findings. | Controls were significantly older than cases. 28% of observations in the analysis were excluded because of missing data for microbiological variables due to problems collecting data due to poor health. |
| Huyghe, 2013 [93] | Study type: Case control study Location: Niger n: 84 acute noma cases, 37 acute necrotizing gingivitis cases and 343 controls Additional details: Cases had no antibiotics, no dental cleaning and did not receive fortified food during the 3 previous months. Subjects with lesions older than 4 weeks were excluded. |
Compared to the healthy controls, a lower bacterial diversity was found in noma samples. Less Porphyromonadaceae, Tannerella spp., Capnocytophaga spp., Fusobacteria and Cetobacterium spp. were found in noma samples. Raises doubts about Fusobacterium necrophorum. | Authors state need for time series data and the utilization of high-throughput sequencing capacity to elucidate the aetiology of noma. |
Clinical progression
While the clinical manifestations and sequelae of noma in each case are unique, the infection invariably starts with inflammation of the gums, which then leads to ulceration and the rapid destruction (within weeks [1]) of the cheek and in some cases the jaw, lip, nose and/or the eye [15,148,167]. For the purposes of case detection, the World Health Organisation has classified noma into stages, the first stages (Stage 0 to 4) are the acute stages of noma lasting only a few weeks; Stage 0: simple gingivitis; Stage 1: acute necrotizing gingivitis; Stage 2: oedema; Stage 3: gangrene. Stage 4: scarring and Stage 5: sequelae [1]. Deaths in noma patients are primarily reported to be due to starvation, aspiration pneumonia, respiratory insufficiency or sepsis [13,128]. Even though noma primarily affects young children [2], noma cases in adults, mostly in conjunction with other severe infections (like HIV, cancer or oral myiasis) have been reported [76,82,95,150].
Treatment with antibiotics, wound debridement, and nutritional support in the early, reversible stages of the disease can reduce the duration and severity of the acute phase of noma and the extent of tissue damage, thus reducing mortality and morbidity of noma (discussed in detail below) [2]. Those who survive the acute stages will often have severe sequelae including difficulty eating, seeing and breathing [1,2,136]. Survivors often need complex surgical reconstruction to restore form and function [167]. Trismus (a restriction in mouth opening) is one of the most disabling sequelae [91,144] and can lead to complications such as aspiration, malnutrition, poor oral hygiene, speech deficits, a compromised airway, and pain [168].
The aesthetic and functional sequelae of noma are compounded by the psychological impacts of the disease, not only on the patient, but also on family members and caretakers. Studies have reported that noma has led to mental health issues due to the social isolation and shunning of survivors and their families, bullying, a lack of access to education, difficulties finding jobs, and limited marital prospects [16,102,141,156,163,169–172].
There have been no published reports of noma re-activating [163]. None of the literature included in this review provided evidence to suggest that noma was contagious [163]. Given the ethical and practical challenges of conducting studies to assess the progression of the disease among the hard-to-reach affected communities, information is lacking on the environmental, nutritional and physiological conditions that trigger the progression to the later gangrenous stages of the disease.
Treatment
Acute phase treatment (Stages 0 to 4)
Historically (1800’s and early 1900’s), noma cases presenting at medical institutions were managed with nutritional support with high protein foods (fruit, eggs, milk, meat [12,28,37]) alcohol (wine, brandy and whisky [10,12,28,31,32]) and wound cleaning using bicarbonate of soda [27,28], leeches, [27] and nitric acid [10,26,161]. It is difficult to know whether these methods were beneficial as all evidence was derived from case series and case reports. However, these treatments clearly point to an early appreciation of poor nutrition and hygiene being contributing factors to the progression of the disease.
More recently (later 1900’s and 2000’s), timely administration of broad-spectrum antibiotics [14,85,92,96,97], wound cleaning and debridement [9,17,73,78,84,97,106], and nutritional support [13,92,96,173] have shown to be effective in reducing the severity and sequelae of noma by arresting the acute phase of the infection in some patients. A range of antibiotics were reported in the included studies such as amoxicillin [78,84,97,106], metronidazole [8,9,38,84,103], lincomycin [80] and cefotaxime [8,81]. No studies comparing the relative efficacy of these antibiotics were identified.
The current WHO guidelines for the management of the acute stages of noma in clinical settings includes [1]: oral hygiene (mouth wash Chlorhexidine 0,2%, 10 ml), antibiotic treatment (amoxicillin and metronidazole), nutritional support (high protein), wound cleaning (compresses soaked in hydrogen peroxide) and dressing (honey for local dressing and for antibacterial action and regeneration) [1].
Sequelae treatment (Stage 5)
If the patient survives the acute illness, they can live into adulthood but often require extensive reconstructive surgery and intensive physiotherapy to improve the resulting structural and functional defects [174] that often require a number of surgical treatments [137]. Studies have highlighted the fact that the time between acute illness and surgical care can be decades [136,141,144]. The clinical manifestation of each noma case is unique, and as such, the surgical procedures used to treat each noma case differ [15,39,42,74,102,115–117,145,148]. Reported surgical techniques include pedicled supraclavicular flaps for the treatment of large unilateral facial defects [102,117]; myocutaneous submental artery flaps, bony and/or soft tissue trismus releases [109], forehead flaps [109,144] and lower lid ectropion release [109]. In one study, extra-articular ankylosis due to noma was treated using soft tissue reconstruction with large free flaps [116]. Trismus was treated using a bone distractor in one study [134], and in another mouth opening was performed by bone-bridge excision, sometimes associated with contralateral coronoidectomy [116]. In a further study, the reconstruction of an upper lip defect was conducted using Gillies fan flaps [89]. A 2006 book on noma surgical techniques includes information on the reconstruction of the lips and corner of the mouth using Abbe, Estlander and fan flaps; and the reconstruction of the cheek using temporo-parietal fascia and deltopectoral flaps; and the reconstruction of central defects using radial forearm and local turnover flaps [167]. Challenges with anaesthesia for noma survivors have been reported, particularly in patients with severe trismus [125,127].
Physiotherapy is an essential part of noma treatment, especially to prevent or minimise trismus [74] and can lead to improvements in eating, chewing and speaking [112].
Noma often leads to stigmatization and resultant social isolation of the patients and their family members from their communities [2,14,16,102,156]. Several studies have highlighted the importance of including social and psychological support for noma patients and their families [136,137,156,169].
Outcomes of noma treatment are difficult to ascertain due to inconsistent patient follow-up in most studies [91]. This is mostly due to the remote locations of the home villages of patients and difficulties in accessing health care facilities [13,14,113]. However, there have been evaluations of noma survivors after surgery which have shown that surgical treatment for noma survivors greatly improves their quality of life, even if functional improvements (specifically mouth opening) are not pronounced [14,102,136,141,156].The extent of long-term sequelae and their impact on quality of life of noma patients depends on the severity of the disease at initial presentation, efficacy of antibiotic treatment, wound debridement and the facial structures affected [91,92,96,97]. It was noted that a validated, standardized noma patient-reported outcome measurement tool would be helpful in standardizing outcome reporting after surgical treatment [136,141,175].
Traditional treatments
In Mali and Nigeria, traditional healers’ knowledge of noma was limited [126,146], however, several traditional healers in Nigeria reported treating different stages of the disease. In Nigeria, traditional treatments for noma include ground herbs, plants, ointments and piercing of the swollen cheek (in the oedema phase of noma) [126]. Traditional healers in Nigeria reported referring patients with the later stages of disease to (hospitals and clinics and being interested in assisting with referrals of noma patients, and attending trainings on the disease [126].
Mortality
There is limited and inconclusive evidence around the pathogenesis of noma leading to death. Factors that favour survival (apart from antibiotic treatment and wound debridement) are unknown. The mortality rate of noma depends on multiple factors and is poorly enumerated. The WHO (based on expert opinion and retrospective chart analyses) states that noma has a mortality rate of 90% within weeks after the onset of noma if left untreated [1]. The speed with which death occurs is also debated, some state death occurs in as little as two weeks from the onset of first symptoms [1] but it is unclear which symptoms these are. The clearest reported estimate is that death can occur in a matter of days after the onset of oedema [13]. What is certain is that when noma is identified and treated in a timely manner, mortality greatly decreases [176].
Table 3 explores the mortality rates reported in various studies included in this review. These estimates highlight the differences in mortality rates in groups who received no antibiotic treatment (49–94%), compared to those who had received drug therapies such as antibiotics (0–38%) (Table 3). It should be noted that these estimates are derived from case series and retrospective chart reviews; no standardized reporting of noma stage was used and the studies do not have the same follow-up periods. The evidence should be evaluated with these study design restrictions in mind, as they could over- or under-estimate the mortality rates of noma patients, particularly at the community level.
Table 3. Mortality reported in included studies.
| Study | Location | Study design | Cases | Mortality (%) | Treatment |
|---|---|---|---|---|---|
| Tourdes, 1848 [177] | Europe | Case series | 239 | 73% | No drug therapy |
| Barthez, 1855 [177] | Europe | Case series | 29 | 89% | No drug therapy |
| Ritchie, 1872 [4] | Europe | Case series | 8 | 63% | Iron with citric-acid, nutritional support |
| Springer, 1904 [177] | Europe | Case series | 88 | 94% | Wound debridement |
| Gupta, 1945 [44] | India | Case series | 79 | 49% | Pentavalent antimony (treatment of leishmaniasis), nutritional support, vitamins, blood transfusions, local antiseptic treatment |
| Jelliffe, 1952 [37] | Nigeria | Case series | 53 | 30% | Penicillin |
| Mehrotra, 1966 [49] | India | Case series | 20 | 15% | Antibiotics, multivitamins, high protein diet, sequestrectomy, plastic reconstructive surgeries |
| Adekeye and Ord, 1978–1982 [13] | Nigeria | Case series | 13 | 0% | Antibiotics |
| Bourgeois, 1981–93 [13] | Senegal | Case series | 73 | 10% | Drug therapy, kind of treatment not specified |
| Oginni, 1982–96 [13] | Nigeria | Case series | 133 | 0% | Drug therapy, kind of treatment not specified |
| Nath, 1998 [15] | Zambia | Retrospective chart review | 117 | 20% | Nutrition, wound care |
| Chidzonga, 1996 [48] | Zimbabwe | Case series | 8 | 38% | Antibiotics, wound debridement, removed mobile teeth, irrigated wounds |
| Millogo, 2012 [122] | Burkina Faso | Retrospective chart review | 212 | HIV 38%; non- HIV patients 6% | Antibiotics, anti-retroviral therapy |
| Konsem, 2014 [104] | Burkina Faso | Retrospective chart review | 55 | 15% | Antibiotics |
| Braimah, 2017 [147] | Nigeria | Retrospective chart review | 159 | 25% | Antibiotics |
Discussion
There is a dearth of research and literature on noma. The date of the first study included in this review was 1843, and since this time, an average of one publication has been written on the disease per year (calculated based on the studies included in this review). Despite significant progress in scientific methods since the first study, the literature remains predominantly populated with case reports and case series. More scientifically robust studies are needed. The reasons behind this neglect include the lack of knowledge about the disease by healthcare workers, in part due to noma not being included in medical curricula leading to under-reporting and misdiagnosis of cases [92,140], the hypothesized low prevalence of the disease [1], which may, in part, be due to inconsistent surveillance and reporting on the disease [107], the relative inaccessibility of the affected communities and the rapid progression of the disease, high mortality, stigmatization and isolation of noma survivors [2,16,102,156,176]. There is appreciation among the small community of clinicians and researchers involved in noma care and research that this lack of awareness impacts on the ability to develop and implement sound evidence-based policies and public health initiatives aimed at eradicating noma from communities that continue to be afflicted by this ancient disease. Several studies have stated that these gaps in research could be filled with better awareness about the disease and call for the inclusion of noma in the WHO list of neglected tropical diseases which would highlight noma in the global health arena [178–180]. It is likely that addressing the causes and conditions contributing to noma will lead to wide ranging benefits.
Based on this literature review, some of the main gaps in knowledge are enumerating the burden of disease (both incidence and prevalence); describing the true mortality rate and pathogenic cause(s) of noma and the role of different comorbidities (specifically measles and HIV) play the development of noma, a finding similar to other reviews [2,13,154,165,174,180–182]. Factors that influence prognosis and the long-term outcomes after care (surgical and non-surgical) [88], including the most effective antibiotic treatment protocols [91], need to be assessed. The knowledge of health care workers about noma in high risk areas, the number of medical school and tropical medicine curriculums that include noma; and the role the varying healthcare actors could play in prevention [183] need to be explored. An additional area for future studies would be to compare prevention methods and messaging [92] to identify the most effective mechanisms. Efforts to eliminate extreme poverty may lead to a reduction in the number of cases of noma, and potentially even eliminate this disease.
There were several limitations to this review. Given the inclusion time period of this review (from the 1800’s to present) it is likely that some manuscripts (especially in the earlier years) were not available on current indexing systems and hence not included in this review. We used Google Translate to translate non-English papers, which could have led to some misinterpretation, as it is not an official academic translating service. The inclusion of published manuscripts only and not books and other grey literature could have limited the amount of information identified.
In summary, noma is a preventable disease that affects young children in the most vulnerable and impoverished communities. It is a devastating and often fatal disease that requires urgent and intensive clinical and surgical care, often difficult to access as most cases of noma occur in resource-limited settings. Noma has been reported in the literature for hundreds of years; however major gaps in knowledge about the disease still exist. What is clear from the literature is the wide geographical spread of noma, and the need for further studies to gain an understanding of the burden and distribution of disease; the true mortality rate, and the pathogenic cause(s) and the factors that influence prognosis and outcomes after treatment. Filling these gaps in knowledge will help with the development of effective targeted interventions to reduce the burden of noma in the most affected populations.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors are grateful to all of the all of the authors whose work is cited in this review. A big thank you to Wendy Smith for helping to source the articles through the University of Cape Town medical library.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization Regional Office for Africa. Information brochure for early detection and management of noma. 2017. Available from https://apps.who.int/iris/handle/10665/254579; 2017. Available: https://apps.who.int/iris/handle/10665/25457979%0AAdditional
- 2.Ashok N, Tarakji B, Darwish S, Rodrigues JC, Altamimi MA. A Review on Noma: A Recent Update. Glob J Health Sci. 2016;8: 53–59. doi: 10.5539/gjhs.v8n4p53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MD, Horsley T, Weeks L HS. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. 2018;169: 467–73. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 4.Ritchie P. Observations on the inflammations of the mouth in children, with an illustrative case. Trans Edinburgh Obstet Soc. 1871; 418–435. [PMC free article] [PubMed] [Google Scholar]
- 5.Marck K. Cancrum oris and noma: Some etymological and historical remarks. Br J Plast Surg. 2003;56: 524–527. doi: 10.1016/s0007-1226(03)00224-8 [DOI] [PubMed] [Google Scholar]
- 6.Adedoja D, Kabue M, Sahila P. Cancrum oris in HIV infected children in Lesotho: report of two cases. East Afr Med J. 2002;79: 499–501. doi: 10.4314/eamj.v79i9.9124 [DOI] [PubMed] [Google Scholar]
- 7.Phillips R, Enwonwu C, Falkler W. Pro- versus anti-inflammatory cytokine profile in African children with acute oro-facial noma (cancrum oris, noma). Eur Cytokine Netw. 2005;16: 70–77. Available: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=2ahUKEwinyvHfhv3iAhU1ThUIHVfkDy0QFjABegQIAhAC&url=https%3A%2F%2Fpdfs.semanticscholar.org%2F460f%2Fcbb9fedf21767224f88769afcfea06717b06.pdf&usg=AOvVaw3HkJ7rGfulyI5kHmz31cIA [PubMed] [Google Scholar]
- 8.Yuca K, Yuca S, Çankaya H, Çaksen H, Calka O, Kir M. Report of an infant with Noma (Cancrum Oris). J Dermatol. 2004;31: 488–491. doi: 10.1111/j.1346-8138.2004.tb00539.x [DOI] [PubMed] [Google Scholar]
- 9.Oji C. Cancrum oris: its incidence and treatment in Enugu, Nigeria. Br J Oral Maxillofac Surg. 2002;40: 406–409. doi: 10.1016/S0266-4356(02)00192-4 [DOI] [PubMed] [Google Scholar]
- 10.Keiller A. On Cancrum Oris. Edinb Med J. 1862;VII: 919–934. [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus D. Cancrum oris—a 35-year retrospective study. S Afr Med J. 1997;87: 1379–1382. [PubMed] [Google Scholar]
- 12.Fleming A. A Case of Cancrum Oris, with a short critique on the disease. Trans Med Chir Soc Edinb. 1892; 251–259. [PMC free article] [PubMed] [Google Scholar]
- 13.Baratti-Mayer D, Pittet B, Montandon D, Bolivar I, Bornand JE, Jaquinet A, et al. Noma: an “infectious” disease of unknown aetiology. Lancet Infect. 2003;3: 419–431. doi: 10.1016/S1473-3099(03)00670-4 [DOI] [PubMed] [Google Scholar]
- 14.Srour L, Watt B, Phengdy B, Khansoulivong K, Harris J, Bennett C, et al. Noma in Laos: Stigma of severe poverty in rural Asia. Am J Trop Med Hyg. 2008;78: 539–542. [PubMed] [Google Scholar]
- 15.Nath S, Jovic G. Cancrum Oris: Management, Incidence, and Implications of Human Immunodeficiency Virus in Zambia. Plast Reconstr Surg. 1998;102: 350–357. doi: 10.1097/00006534-199808000-00008 [DOI] [PubMed] [Google Scholar]
- 16.Farley E, Lenglet A, Abubakar A, Bil K, Fotso A, Oluyide B, et al. Language and beliefs in relation to noma: a qualitative study, northwest Nigeria. PLoS Negl Trop Dis. 2020;14: 1–15. doi: 10.1371/journal.pntd.0007972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enwonwu C, Falkler W, Idigbe E, Afolabi B, Ibrahim M, Onwujekwe D, et al. Pathogenesis of cancrum oris (noma): Confounding interactions of malnutrition with infection. Am J Trop Med Hyg. 1999;60: 223–232. doi: 10.4269/ajtmh.1999.60.223 [DOI] [PubMed] [Google Scholar]
- 18.Kajiru I, Nyimbi I. The impact of myths, superstition and harmful cultural beliefs against albinism in Tanzania: A human rights perspective. Potchefstroom Electron Law J. 2020;23: 1–27. doi: 10.17159/1727-3781/2020/V23I0A8793 [DOI] [Google Scholar]
- 19.Stewart G. Royal Hospital for Sick Children, Edinburgh. Case of cancrum oris; recovery. Lancet. 1868;92: 601. doi: 10.1016/S0140-6736(02)72116-8 [DOI] [Google Scholar]
- 20.Yates P, Kingsford EC. Cancrum oris and its successful treatment by the local application of corrosive sublimate. Lancet. 1889;133: 880–882. doi: 10.1016/S0140-6736(01)91067-0 [DOI] [Google Scholar]
- 21.Kingsford E. Cancrum oris. Lancet. 1891;138: 607–608. doi: 10.1016/S0140-6736(02)01478-2 [DOI] [Google Scholar]
- 22.Usher S, Ross D. A case of cancrum oris following typhoid fever with plastic repair. Can Med Assoc J. 1931; 446–449. [PMC free article] [PubMed] [Google Scholar]
- 23.Locomotor ataxia with cancrum oris as a fatal complication. JAMA J Am Med Assoc. 1899;XXXIII: 234. doi: 10.1001/jama.1899.02450560054015 [DOI] [Google Scholar]
- 24.Gould F. A case of broncho-pneumonia, followed by cancrum oris and subsequent necrosis of jaw; recovery. Lancet. 1872;100: 150. doi: 10.1016/S0140-6736(02)52159-0 [DOI] [Google Scholar]
- 25.Duncan JF. On the administration of mercury in cases of cancrum oris. Dublin Q J Med Sci. 1852;14: 265–273. doi: 10.1007/BF02943899 [DOI] [Google Scholar]
- 26.Fayrer J. A case of acute malarial poisioning, enteric fever ensuing, complicated by brain and lung symptoms, also by extensive cancrum oris. Lancet. 1889: 296–300. [PMC free article] [PubMed] [Google Scholar]
- 27.Gray J. Cancrum Oris. Glas Med J. 1856; 16–21. [PMC free article] [PubMed] [Google Scholar]
- 28.O’Gorman P. Cancrum oris in an European soldier: enlarged spleen; pancreatic disease. Post mortem results. Ind Med Gaz. 1882; 14–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Cousins J. Cancrum oris followed by extensive ulceration of the cheek and ankylosis of the jaw: recovery. Br Med J. 1893; 739–740. doi: 10.1136/bmj.1.1684.739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elder G. Case of Cancrum Oris. Trans Medico-Chirurgical Soc Edinburgh. 1893;12: 259–262. Available: http://www.ncbi.nlm.nih.gov/pubmed/29584330 [PMC free article] [PubMed] [Google Scholar]
- 31.Barker H. Case of cancrum oris with necrosis of a large portion of the inferior maxillary bone, followed by recovery. Prov Med Surg J. 1852; 611–612. doi: 10.1136/bmj.s1-16.24.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt H. Remarks on cancrum oris, and the gangrenous erosion of the cheek of Mr Dease and Mr Underwood, and more particularly on the efficacy of the chlorate of potash in the treatment of those diseases. Med Chir Trans. 1843; 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Layman PR. Bypassing a problem airway. Anaesfhesia. 1983;38: 478–480. doi: 10.1111/j.1365-2044.1983.tb14035.x [DOI] [PubMed] [Google Scholar]
- 34.Hickey B. Cancrum Oris. Br Med J. 1949; 367. doi: 10.1136/bmj.1.4599.367-c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackay D. Cancrum oris amoung african natives. Br Me. 1949; 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hefferman L. Cancrum oris treated by excision and subsequent tube grafting. Ind Med Gaz. 1923; 434–437. [PMC free article] [PubMed] [Google Scholar]
- 37.Jelliffe D. Infective gangrene of the mouth (Cancrum Oris). Pediatrics. 1952;9: 1–9. [PubMed] [Google Scholar]
- 38.Falkler W, Enwonwu C, Idigbe E. Isolation of fusobacterium necrophorium from cancrum oris (noma). Am J Trop Med Hyg. 1999;60: 150–156. doi: 10.4269/ajtmh.1999.60.150 [DOI] [PubMed] [Google Scholar]
- 39.Cressy A. Cancrum oris successfully treated by excision of the cautery. Ann Surg. 1901; 288–290. doi: 10.1097/00000658-190107000-00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falkler W, Enwonwu C, Idigbe E. Microbiological understandings and mysteries of noma (cancrum oris). Oral Dis. 1999;5: 150–155. doi: 10.1111/j.1601-0825.1999.tb00081.x [DOI] [PubMed] [Google Scholar]
- 41.Ndiaye F, Bourgeois D, Leclercq M, Berthe O. Noma: public health problem in Senegal and epidemiological surveillance. Oral Dis. 1999;5: 163–166. doi: 10.1111/j.1601-0825.1999.tb00083.x [DOI] [PubMed] [Google Scholar]
- 42.Marck K. Noma: the Sokoto approach. Eur J Plast Surg. 1998;21: 277–280. [Google Scholar]
- 43.Findlay GM. Oral aureimycin in the treatment of tropical ulcers and cancrum oris. Trans R Soc Trop Med Hyg. 1950;44: 307–310. doi: 10.1016/0035-9203(50)90057-5 [DOI] [PubMed] [Google Scholar]
- 44.Gupta P, Chakravarty N. Penicillin in Cancrum Oris Complicating Kala-Azar. Ind Med Gaz. 1945; 542–545. [PMC free article] [PubMed] [Google Scholar]
- 45.Bene M, Amadei F, Petrolati M, Rovati L, Confalonieri P, Caronni E. Radial forearm fasciocutaneous free flap as a solution in case of noma. Microsurgery. 1999;19: 3–6. doi: [DOI] [PubMed] [Google Scholar]
- 46.Kumar M, Sen S, Zachariah N, Thomas R, Mammen K. Recurrent parotid abscess formation 8 years after an episode of cancrum oris. Pediatr Surg Int. 1998;13: 162. doi: 10.1007/s003830050275 [DOI] [PubMed] [Google Scholar]
- 47.Nath S, Jovic G. Total loss of upper and lower lips: challenges in reconstruction. Br J Oral A4axrllofacial Surg. 1998;36: 460–461. doi: 10.1016/s0266-4356(98)90464-8 [DOI] [PubMed] [Google Scholar]
- 48.Chidzonga M. Noma (cancrum oris) in human immunodeficiency virus acquired/ immune deficiency syndrome patients: report of eight cases. J Oral Maxillofac Surg. 1996;54: 1056–60. doi: 10.1016/s0278-2391(96)90159-7 [DOI] [PubMed] [Google Scholar]
- 49.Mehrotra M. Cancrum oris. A clinical survey of 20 cases. J Indian Med Assoc. 1966;147: 555–7. Available: https://www.ncbi.nlm.nih.gov/pubmed/5980942 [PubMed] [Google Scholar]
- 50.Griffiths W. Dermatology in Shiraz, Iran. Trans St Johns Hosp Dermatol Soc. 1975;61: 92–9. [PubMed] [Google Scholar]
- 51.Osuji O. Necrotizing ulcerative gingivitis and Cancrum Oris (noma) in Ibadan, Nigeria. J Periodontol. 1990;61: 769–772. doi: 10.1902/jop.1990.61.12.769 [DOI] [PubMed] [Google Scholar]
- 52.Shrand H. A case of cancrum oris; survival after treatment with penicillin. Clin Proc. 1947;6: 197–203. [PubMed] [Google Scholar]
- 53.Malden N. An interesting case of adult facial gangrene (from Papua, New Guinea). Oral Surg Oral Med Oral Pathol. 1985;59: 279–81. doi: 10.1016/0030-4220(85)90167-7 [DOI] [PubMed] [Google Scholar]
- 54.O’Donoghue JJ. A case of cancrum oris. Lancet. 1931;218: 1075. doi: 10.1016/S0140-6736(00)99272-9 [DOI] [Google Scholar]
- 55.Diamond JS. A case of noma (cancrum oris) complicating non-specific ulcerative colitis. Am J Dig Dis Nutr. 1935;2: 698–706. doi: 10.1007/BF03000985 [DOI] [Google Scholar]
- 56.Ghosh K. Use of penicillin in cancrum oris. Indian J Pediatr. 1947;14: 171–172. doi:https://link.springer.com/article/10.1007%2FBF02812636?LI=true [Google Scholar]
- 57.Griffin J, Bach D, Nespeca J, Marshall K. Noma: Report of two cases. Oral Surgery, Oral Med Oral Pathol. 1983;56: 605–607. doi: 10.1016/0030-4220(83)90077-4 [DOI] [PubMed] [Google Scholar]
- 58.Rotbart HA, Levin MJ, Jones JF, Hayward AR, Allan J, McLane MF, et al. Noma in children with severe combined immunodeficiency. J Pediatr. 1986;109: 596–600. doi: 10.1016/s0022-3476(86)80219-0 [DOI] [PubMed] [Google Scholar]
- 59.Stassen LF, Batchelor AG, Rennie JS, Moos KF. Cancrum oris in an adult Caucasian female. Br J Oral Maxillofac Surg. 1989;27: 417–22. doi: 10.1016/0266-4356(89)90083-1 [DOI] [PubMed] [Google Scholar]
- 60.Barrios T, Aria A, Brahney C. Cancrum oris in an HIV-positive patient. J Oral Maxillofac Surg. 1995;53: 851–5. https://www.ncbi.nlm.nih.gov/pubmed/7595806 doi: 10.1016/0278-2391(95)90349-6 [DOI] [PubMed] [Google Scholar]
- 61.McCURDY SL. Cancrum oris. JAMA J Am Med Assoc. 1907;XLIX: 1441. doi: 10.1001/jama.1907.25320170035004 [DOI] [Google Scholar]
- 62.Linenberg WB, Schmitt J, Harpole HJ. Noma. Report of a case. Oral Surg Oral Med Oral Pathol. 1961;14: 1138–41. doi: 10.1016/0030-4220(61)90508-4 [DOI] [PubMed] [Google Scholar]
- 63.Majumder D. A case note on cancrum oris following pneumonia treated by prontosoil. Ind Med Gaz. 1938;October: 614. [PMC free article] [PubMed] [Google Scholar]
- 64.Swain V. Sketches of surgical cases drawn in 1884–87 at the East London Hospital for Children, Shadwell. Prog Pediatr Surg. 1986;20: 265–80. https://www.ncbi.nlm.nih.gov/pubmed/3095879 doi: 10.1007/978-3-642-70825-1_20 [DOI] [PubMed] [Google Scholar]
- 65.Kaimenyi J, Guthua S. Residual facial deformity resulting from cancrum oris: a case report. East Afr Med J. 1994;71: 476–8. [PubMed] [Google Scholar]
- 66.STARK S. Noma or gangrenous stomatitis; report of a case. Oral Surg Oral Med Oral Pathol. 1956;9: 1076–9. doi: 10.1016/0030-4220(56)90070-6 [DOI] [PubMed] [Google Scholar]
- 67.Cameron R. Cancrum oris with its common complications. Lancet. 1901;158: 1730–1731. doi: 10.1016/S0140-6736(01)74321-8 [DOI] [Google Scholar]
- 68.Laslett EE. A case of cancrum oris affecting both sides. Lancet. 1902;159: 513–514. doi: 10.1016/S0140-6736(01)89488-5 [DOI] [Google Scholar]
- 69.King H. Noma (cancrum oris) in an adult, with report of a case. J Am Med Assoc. 1911;LVI: 1449. doi: 10.1001/jama.1911.02560200017011 [DOI] [Google Scholar]
- 70.Smith EC. Cancrum oris in a case of leukæmia. Trans R Soc Trop Med Hyg. 1926;19: 394–396. doi: 10.1016/S0035-9203(26)90506-3 [DOI] [Google Scholar]
- 71.Limongelli WA, Clark MS, Williams AC. Nomalike lesion in a patient with chronic lymphocytic leukemia. Review of the literature and report of a case. Oral Surg Oral Med Oral Pathol. 1976;41: 40–51. doi: 10.1016/0030-4220(76)90250-4 [DOI] [PubMed] [Google Scholar]
- 72.Weinstein RA, Choukas NC, Wood WS. Cancrum oris-like lesion associated with acute myelogenous leukemia. Oral Surg Oral Med Oral Pathol. 1974;38: 10–4. doi: 10.1016/0030-4220(74)90305-3 [DOI] [PubMed] [Google Scholar]
- 73.Ghose A. A case of cancrum oris as a complication of bacillary dysentery. Ind Med Gaz. 1937; 419–420. [PMC free article] [PubMed] [Google Scholar]
- 74.Mukerji S. A case of stiff jaw after cancrum oris—surgical interference-cure. Ind Med Gaz. 1927; 387. [PMC free article] [PubMed] [Google Scholar]
- 75.Oginni F, Oginni A, Ugboko V, Otuyemi O. A survey of cases of cancrum oris seen in Ile-Ife, Nigeria. Int J Paediatr Dent. 1999;9: 75–80. doi: 10.1046/j.1365-263x.1999.00110.x [DOI] [PubMed] [Google Scholar]
- 76.Lathigara M. An unusual case of cancrum oris. Indian J Pediatr. 1935; 626–627. [PMC free article] [PubMed] [Google Scholar]
- 77.Asai T, Matsumoto H, Shingu K. Awake tracheal intuba- tion through the intu- bating laryngeal mask. CAN J ANESTH. 1999;46: 182–184. doi: 10.1007/BF03012555 [DOI] [PubMed] [Google Scholar]
- 78.Weledji E, Njong S. Cancrum Oris (noma): the role of nutrition in management. J Am Coll Clin Wound Spec. 2016;7: 50–52. doi: 10.1016/j.jccw.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madabhavi I, Revannasiddaiah S, Sarkar M. Cancrum oris (noma): An early sign of acute lymphoblastic leukemia relapse. Indian J Dermatology, Venerelogy Leprol. 2018;84: 373–6. doi: 10.4103/ijdvl.IJDVL_1038_14 [DOI] [PubMed] [Google Scholar]
- 80.Lembo S, Leonibus C, Francia M, Lembo C, Ayala F. Cancrum oris in a boy with Down syndrome. J Am Dermatology. 2011;64: 1200–1202. doi: 10.1016/j.jaad.2009.08.048 [DOI] [PubMed] [Google Scholar]
- 81.Evans L, Lane H, Jones M. Cancrum oris in a Caucasian male with Type 2 diabetes mellitus. Diabet Med. 2001;18: 246–248. doi: 10.1046/j.1464-5491.2001.00375.x [DOI] [PubMed] [Google Scholar]
- 82.Koech K. Cancrum oris in an adult with human immunodeficiency virus infection: case report. East Afr Med J. 2010;87: 38–40. doi: 10.4314/eamj.v87i1.59953 [DOI] [PubMed] [Google Scholar]
- 83.Chiandussi S, Luzzati R, Tirelli G, Lenarda R, Biasotto M. Cancrum oris in developed countries. Aging Clin Exp Res. 2009;21: 475–477. doi: 10.1007/BF03327447 [DOI] [PubMed] [Google Scholar]
- 84.Behanan A, Auluck A, Pai K. Cancrum oris. Br J Oral Maxillofac Surg. 2004;42: 267–269. doi: 10.1016/j.bjoms.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 85.Resident S, Vaidya S, Agrawal A. Cancrum oris: a case report. Indian J Otolaryngol Head Neck Surg. 2006;58: 411–413. doi: 10.1007/BF03049618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woon C, Wei-ee K, Tan B, Lee S. Case report: Journey of a Noma Face. Open access J Plast Surg. 2010;10: 412–418. [PMC free article] [PubMed] [Google Scholar]
- 87.Sykes L, Essop R. Combination intraoral and extraoral prosthesis used for rehabilitation of a patient treated for cancrum oris: A clinical report. J Prosthet Dent. 2000; 613–616. [PubMed] [Google Scholar]
- 88.Huijing M, Marck K, Combes J, Mizen K, Fourie L, Demisse Y, et al. Facial reconstruction in the developing world: a complicated matter. Br J Oral Maxillofac Surg. 2011;49: 292–296. doi: 10.1016/j.bjoms.2009.08.044 [DOI] [PubMed] [Google Scholar]
- 89.Bello S. Gillies fan flap for the reconstruction of an upper lip defect caused by noma: case presentation. Clin Cosmet Investig Dent. 2012; 17–20. doi: 10.2147/CCIDEN.S31190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chidzonga M. HIV/AIDS orofacial lesions in 156 Zimbabwean patients at referral oral and maxillofacial surgical clinics. Oral Dis. 2003;9: 317–322. doi: 10.1034/j.1601-0825.2003.00962.x [DOI] [PubMed] [Google Scholar]
- 91.Bisseling P, Bruhn J, Erdsach T, Ettema AM, Sautter R, Berge SJ. Long-term results of trismus release in noma patients. Int J Oral Maxillofac Surg. 2010;39: 873–877. doi: 10.1016/j.ijom.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 92.Ahlgren M, Funk T, Marimo C, Ndiaye C. Management of noma: practice competence and knowledge among healthcare workers in a rural district of Zambia. Glob Health Action. 2017;10: 1–9. doi: 10.1080/16549716.2017.1340253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huyghe A, Franc P, Mombelli A, Tangomo M, Girard M, Baratti-Mayer D, et al. Microarray analysis of microbiota of gingival lesions in noma patients. PLoS Negl Trop Dis. 2013;7. doi: 10.1371/journal.pntd.0002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuhnel T, Dammer R, Dunzl B, Beule AG, Strutz J. New split scar cheek flap in reconstruction of noma sequelae. Br Assoc Plast Surg. 2003;56: 528–533. doi: 10.1016/s0007-1226(03)00225-x [DOI] [PubMed] [Google Scholar]
- 95.Aguiar M, Enwonwu C, Pires F. Noma (cancrum oris) associated with oral myiasis in an adult. Oral Dis. 2003;9: 158–159. doi: 10.1034/j.1601-0825.2003.03942.x [DOI] [PubMed] [Google Scholar]
- 96.Chidzonga M, Mahomva L. Noma (Cancrum Oris) in Human Immunodeficiency Virus Infection and Acquired Immunodeficiency Syndrome (HIV and AIDS): Clinical Experience in Zimbabwe. J Oral Maxillofac Surg. 2008;66: 475–485. doi: 10.1016/j.joms.2007.09.024 [DOI] [PubMed] [Google Scholar]
- 97.Masipa J, Baloyi A, Khammissa R, Altini M, Lemmer J, Feller L. Noma (Cancrum Oris): A report of a case in a young AIDS patient with a review of the pathogenesis. Head Neck Pathol. 2013;7: 188–192. doi: 10.1007/s12105-012-0393-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whiteson K, Lazarevic V, Tangomo-bento M, Girard M, Maughan H, Pittet D, et al. Noma affected children from Niger have distinct oral microbial communities based on high-throughput sequencing of 16S rRNA gene fragments. PLOS NTD. 2014;8: 1–13. doi: 10.1371/journal.pntd.0003240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Konsem T, Millogo M, Gare J, Ouedraogo D, Ouoba K. Noma et maladie de Burkitt, une association exceptionnelle à propos de trois observations vues au centre hospitalier universitaire Yalgado Ouedraogo (Burkina Faso). Bull Soc Pathol Exot. 2014;107: 146–150. doi: 10.1007/s13149-014-0358-5 [DOI] [PubMed] [Google Scholar]
- 100.Barrera J, Connor M. Noma in an Afghani child: A case report. Int J Pediatr Otorhinolaryngol. 2012;76: 742–744. doi: 10.1016/j.ijporl.2012.01.034 [DOI] [PubMed] [Google Scholar]
- 101.Madsen T, Medina C, Jespersen S, Wejse C, Hønge B. Noma in an HIV infected patient in Guinea—Bissau: a case report. Infection. 2017;45: 897–901. doi: 10.1007/s15010-017-1034-z [DOI] [PubMed] [Google Scholar]
- 102.Shaye D, Winters R, Rabbels J, Adentunje A, Magee A, Vo D. Noma Surgery. Laryngoscope. 2019;129: 96–99. doi: 10.1002/lary.27230 [DOI] [PubMed] [Google Scholar]
- 103.Maley A, Desai M, Parker S. Noma: A disease of poverty presenting at an urban hospital in the United States. JAAD Case Reports. 2015;1: 18–20. doi: 10.1016/j.jdcr.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Konsem T, Millogo M, Assouan C, Ouedraogo D. Evoluting form of cancrum oris, about 55 cases collected at the Academic Hospital Yalgado Ouedraogo of Ouagadougou. Bull Soc Pathol Exot. 2014;107: 74–78. doi: 10.1007/s13149-014-0338-9 [DOI] [PubMed] [Google Scholar]
- 105.Goetz A, Giessler M, Andreas B, Schmidt M. Noma: Experiences with a Microvascular Approach under West African Conditions. Plast Reconstr Surg. 2003; 947–954. doi: 10.1097/01.PRS.0000076217.58995.E2 [DOI] [PubMed] [Google Scholar]
- 106.Auluck A, Pai K. Noma: life cycle of a devastating sore—case report and literature review. J Can Dent Assoc. 2005;71: 757a–e. [PubMed] [Google Scholar]
- 107.Adeniyi S, Awosan K. Pattern of Noma (Cancrum Oris) and its risk factors in northwestern Nigeria: a hospital-based retrospective study. Ann Afr Med. 2019;18: 17–22. doi: 10.4103/aam.aam_5_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaidyanathan S, Tullu M, Lahiri K, Deshmukh C. Pseudomonas sepsis with noma: an association? Indian J Med Sci. 2005;59: 357–361. doi: 10.4103/0019-5359.16653 [DOI] [PubMed] [Google Scholar]
- 109.Saleh D, Fourie L, Mizen K. Reconstruction of complex oro-facial defects using the myocutaneous sub-mental artery flap. J Cranio-Maxillofacial Surg. 2014;42: 668–673. doi: 10.1016/j.jcms.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 110.Goff M, Jammet P, Breton-Torres I. Rééducation des séquelles du noma Rehabilitation of noma sequelae. Kinésithérapie, la Rev. 2015;15: 41–48. doi: 10.1016/j.kine.2014.10.010 [DOI] [Google Scholar]
- 111.Baratti-Mayer D, Gayet-Ageron A, Hugonnet S, François P, Pittet-Cuenod B, Huyghe A, et al. Risk factors for noma disease: a 6-year, prospective, matched case-control study in Niger. Lancet Glob Heal. 2013;1: 87–96. doi: 10.1016/S2214-109X(13)70015-9 [DOI] [PubMed] [Google Scholar]
- 112.García-Guilarte F, Urcelay R, Frohner B, Meli G, González D, Celada E. Mandibular ankylosis: a Noma frequent sequel. Cirugía Plástica Ibero-Latinoamericana -. 2009;35: 2–6. [Google Scholar]
- 113.Eip N, Neuhoefer E, La Rosee G, Choudhrie R, Samman N, Kreusch T. Case report Submental intubation for cancrum oris: a case report. Pediatr Anesth. 2005;15: 1009–1012. doi: 10.1111/j.1460-9592.2005.01573.x [DOI] [PubMed] [Google Scholar]
- 114.Enwonwu C, Phillips R, Ferrell C. Temporal relationship between the occurrence of fresh noma and the timing of linear growth retardation in Nigerian children. Trop Med Int Heal. 2005;10: 65–73. doi: 10.1111/j.1365-3156.2004.01351.x [DOI] [PubMed] [Google Scholar]
- 115.Marck K; van der Lei B; Spijkervet F, Adeniyi S; Mixner J; Bruijn H. The prefabricated superficial temporal fascia flap in noma surgery. Eur J Plast Surg. 2000;23: 188–191. [Google Scholar]
- 116.Ruegg E, Baratti-Mayer D, Jaquinet A, Montandon D, Pittet-Cuenod B. The surgical management of extra-articular ankylosis in noma patients. Int J Oral Maxillofac Surg. 2018;47: 1527–1533. doi: 10.1016/j.ijom.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 117.Hartman E, Van Damme P, Sauter H, Suominen S. The use of the pedicled supraclavicular flap in noma reconstructive surgery. J Plast Reconstr Aesthetic Surg. 2006;59: 337–342. doi: 10.1016/j.bjps.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 118.Denloye O, Aderinokun G, Lawoyin J, Bankole O. Reviewing trends in the incidence of cancrum oris in Ibadan, Nigeria. West Afr J Med. 2003;22: 26–29. doi: 10.4314/wajm.v22i1.27974 [DOI] [PubMed] [Google Scholar]
- 119.Naidoo S, Chikte UM. Noma (cancrum oris): case report in a 4-year-old HIV-positive South African child. SADJ. 2000;55: 683–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/12608242 [PubMed] [Google Scholar]
- 120.Gupta A, Sardana K, Gautam RK. An unusual case of noma caused by Klebsiella pnuemoniae and its management. Trop Doct. 2018;48: 230–232. doi: 10.1177/0049475518754720 [DOI] [PubMed] [Google Scholar]
- 121.Zawar V, Pawar M, Kumavat S, Shah M, Chaddha C. A progressive ulcer in immunocompetent man: cancrum oris. Trop Doct. 2018;48: 60–62. doi: 10.1177/0049475517709876 [DOI] [PubMed] [Google Scholar]
- 122.Millogo M, Konsem T, Ouedraogo D, Ouoba K, Zwetyenga N. HIV and noma in Burkina Faso. Rev Stomatol Chir Maxillofac. 2012;113: 433–6. doi: 10.1016/j.stomax.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 123.Chidzonga MM, Mahomva L. Recurrent noma (cancrum oris) in human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV and AIDS): report of a case. J Oral Maxillofac Surg. 2008;66: 1726–30. doi: 10.1016/j.joms.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 124.Adeola DS, Obiadazie AC. Protocol for managing acute cancrum oris in children: an experience in five cases. Afr J Paediatr Surg. 2009;6: 77–81. doi: 10.4103/0189-6725.54767 [DOI] [PubMed] [Google Scholar]
- 125.Sund GC, Muvunyi P, Harling MJ. Airway Management Through a Facial Defect Resulting From Noma (Orofacial Gangrene): A Case Report. A&A Pract. 2020;14: e01319. doi: 10.1213/XAA.0000000000001319 [DOI] [PubMed] [Google Scholar]
- 126.Farley E, Muhammad H, Lenglet A, Mehta U, Abubakar N, Samuel J. ‘I treat it but I don’t know what this disease is ‘: a qualitative study on noma (cancrum oris) and traditional healing in northwest Nigeria. Int Health. 2019; 1–8. doi: 10.1093/inthealth/ihy082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Braun U, Wiese KG, Merten HA, Timmermann A. Anaesthetic care for noma (cancrum oris)—the disease, the airway and how to provide anaesthetic care without a clinical safety infrastructure. Trends Anaesth Crit Care. 2020;31: 16–20. doi: 10.1016/j.tacc.2020.02.002 [DOI] [Google Scholar]
- 128.Silva K, Twaddell S, Powers D. A 40-year-old man with a perforated cheek. Am J Med Sci. 2011;341: 399–403. doi: 10.1097/MAJ.0b013e3181e2ed3e [DOI] [PubMed] [Google Scholar]
- 129.Baniulyte G, Mcallister P, Shafi A, Mnsell M, Thomson E. Atypical orofacial necrosis of unknown aetiology: A case report with features similar to noma. Int J Oral Maxillofac Pathol. 2019;10. [Google Scholar]
- 130.Traore H, Sogodogo E, Coulibaly A, Toure A, Thiocary S, Sidibé MD, et al. Case report: a rare case of NOMA (cancrum oris) in a Malian woman. New Microbes New Infect. 2021;42: 100907. doi: 10.1016/j.nmni.2021.100907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ding X, Wang QQ, Zhou Y, Xu JC. Case report: Malignant transformation of noma: Repair by forearm flap. Am J Trop Med Hyg. 2020;103: 1697–1699. doi: 10.4269/ajtmh.19-0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bouassalo KM, Mossi EK, Padaro E, Gunepin M, Weber E. Chronic lymphocytic leukemia revealed by a rare complication: Noma. First description from Togo. J Oral Med Oral Surg. 2019;25: 31. doi: 10.1051/mbcb/2019017 [DOI] [Google Scholar]
- 133.Putri IL, Agustina W, Hutagalung MR. Columella reconstruction using double nasolabial flap and costal cartilage: A case report. Ann Med Surg. 2021;64: 102213. doi: 10.1016/j.amsu.2021.102213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Holle J, Kubiena H, Issa OH. Distraction Therapy to Correct Trismus Following Noma. J Craniofac Surg. 2020;31: 488–491. doi: 10.1097/SCS.0000000000006082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bello SA, Adeoye JA, Oketade I, Akadiri OA. Estimated incidence and prevalence of noma in north central Nigeria, 2010–2018: A retrospective study. PLoS Negl Trop Dis. 2019;13: 2010–2018. doi: 10.1371/journal.pntd.0007574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rickart AJ, Rodgers W, Mizen K, Merrick G, Wilson P, Nishikawa H, et al. Facing Africa: Describing noma in Ethiopia. Am J Trop Med Hyg. 2020;103: 613–618. doi: 10.4269/ajtmh.20-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shafi’u I, Amirtharajah M, Farley E, Semiyu Adetunji A, Samuel J, Oluyide B, et al. Model of care, Noma Children’s Hospital, northwest Nigeria. Trop Med Int Heal. 2021; 1–10. 10.1111/tmi.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu L, Wei W, Ge X, Wan S, Yu J, Zhu X. Noma in a boy with septic shock: A case report. BMC Pediatr. 2019;19: 1–5. doi: 10.1186/s12887-018-1376-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fieger A, Marck KW, Busch R, Schmidt A. An estimation of the incidence of noma in north-west Nigeria. Trop Med Int Heal. 2003;8: 402–407. doi: 10.1046/j.1365-3156.2003.01036.x [DOI] [PubMed] [Google Scholar]
- 140.Brattström-Stolt L, Funk T, Sié A, Ndiaye C, Alfvén T. Noma-knowledge and practice competence among primary healthcare workers: a cross-sectional study in Burkina Faso. Int Health. 2019;11: 290–296. doi: 10.1093/inthealth/ihy088 [DOI] [PubMed] [Google Scholar]
- 141.Farley E, Amirtharajah M, Winters R, Taiwo A, Oyemakinde M, Fotso A, et al. Outcomes at 18 mo of 37 noma (cancrum oris) cases surgically treated at the Noma Children’s Hospital, Sokoto, Nigeria. Trans R Soc Trop Med Hyg. 2020;0: 1–8. doi: 10.1093/trstmh/traa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Farley E, Oyemakinde MJ, Schuurmans J, Ariti C, Saleh F, Uzoigwe G, et al. The prevalence of noma in northwest Nigeria. BMJ Glob Heal. 2020;5: 1–15. doi: 10.1136/bmjgh-2019-002141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farley E, Lenglet A, Ariti C, Jiya NM, Adetunji AS, van der Kam S, et al. Risk factors for diagnosed noma in northwest Nigeria: A case-control study, 2017. PLoS Negl Trop Dis. 2018;12: 1–11. doi: 10.1371/journal.pntd.0006631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ghorui T, Ray A. Release of Extra Articular Ankylosis of Jaws as a Sequelae of Cancrum Oris with Extensive Gingival Myasis in a Scoliosis Patient: A Rare Case Report. Indian J Otolaryngol Head Neck Surg. 2019;71: 734–736. doi: 10.1007/s12070-018-1526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Long N, Phong H, Truong D, Tung N, Phun N, Cuong M. Rehabilitation of Severe Maxillary Noma Defect with Zygomatic Implants: A case report. J Implant Adv Clin Dent. 2019;11: 16–23. [Google Scholar]
- 146.Baratti-Mayer D, Daou MB, Gayet-Ageron A, Jeannot E, Pittet-Cuénod B. Sociodemographic characteristics of traditional healers and their knowledge of noma: A descriptive survey in three regions of Mali. Int J Environ Res Public Health. 2019;16: 1–11. doi: 10.3390/ijerph16224587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Braimah R, Adeniyi A, Taiwo A, Ibikunle A, Gbotolorun M, Aregbesola S, et al. Risk factors and mortality rate of acute cancrum oris (noma) in Sokoto North-West Nigeria: A 13-year survey. J Pediatr Dent. 2017;5: 1. doi: 10.4103/jpd.jpd_58_16 [DOI] [Google Scholar]
- 148.Adeniyi S, Taiwo A, Ibikunle A, Braimah R, Gbotolorun O, Ogbeide M, et al. Pattern of tissue destruction among patients diagnosed with cancrum oris (Noma) at a Northwestern Nigerian Hospital, Sokoto. Saudi J Oral Sci. 2017;4: 101. doi: 10.4103/sjos.sjoralsci_55_16 [DOI] [Google Scholar]
- 149.Rowe D, McKerrow N, Uys A, Winstanley T. Cancrum oris (noma) in a malnourished HIV-positive child from rural Kwazulu-Natal. South African J HIV Med. 2004;16: 45–46. [Google Scholar]
- 150.Pedro K, Smit D, Morkel J. Cancrum Oris (noma) in an HIV-positive adult: a case report and literature review. SADJ. 2016;71: 248–252. [Google Scholar]
- 151.Ogbureke K, Ogbureke E. NOMA: A Preventable “scourge” of African children. Open Dent J. 2010;4: 201–206. doi: 10.2174/1874210601004010201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Enwonwu CO, Falklerz W, Idigbe EO, Falkler WA, Idigbe EO. Oro-facial gangrene (noma/cancrum oris): pathogenic mechanisms. Crit Rev Oral Biol Med. 2000;11: 159–171. doi: 10.1177/10454411000110020201 [DOI] [PubMed] [Google Scholar]
- 153.World Health Organisation (WHO) and Hilfsaktion Noma. Promoting Oral Health in Africa: Prevention and control of oral diseases and noma as part of essential noncommunicable disease interventions. 2016. Available: http://www.aho.afro.who.int/en/news/5236/official-launch-who-manual-“promoting-oral-health-africa”
- 154.Enwonwu C, Falkler W, Idigbe E, Savage K. Noma (cancrum oris): questions and answers. Oral Dis. 1999;5: 144–149. doi: 10.1111/j.1601-0825.1999.tb00080.x [DOI] [PubMed] [Google Scholar]
- 155.World Health Organization, OMS. World Health Report Life in the 21st century A vision for all Report of the Director-General. 51st World Heal Assem. 1998; 1–226. Available: https://www.who.int/whr/1998/en/whr98_en.pdf?ua=1
- 156.Lafferty N. Changing the face of Africa. Estimating the burden of noma in rural Ethiopia and identifying options for prevention and improvement in its diagnosis and management. Liverpool School of Tropical Medicine. 2012. doi: 10.1146/annurev.soc.28.110601.140938 [DOI] [Google Scholar]
- 157.Bourgeois DM, Diallo B, Frieh C, Leclercq MH. Epidemiology of the incidence of oro-facial noma: A study of cases in Dakar, Senegal, 1981–1993. Am J Trop Med Hyg. 1999;61: 909–913. doi: 10.4269/ajtmh.1999.61.909 [DOI] [PubMed] [Google Scholar]
- 158.Bourgeois D, Leclercq M. The World Health Organization initiative on noma. Oral Dis. 1999;5: 172–174. doi: 10.1111/j.1601-0825.1999.tb00085.x [DOI] [PubMed] [Google Scholar]
- 159.Garrett V, Ogutu P, Mabonga P, Ombeki S, Mwaki A, Aluoch G, et al. Diarrhoea prevention in a high-risk rural Kenyan population through point-of-use chlorination, safe water storage, sanitation, and rainwater harvesting. Epidemiol Infect. 2008;136: 1463–1471. doi: 10.1017/S095026880700026X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tumwine J, Thompson J, Katua-katua M, Mujwajuzi M, Johnstone N, Wood E, et al. Diarrhoea and effects of different water sources, sanitation and hygiene behaviour in East Africa. Trop Med Int Heal. 2002;7: 750–756. [DOI] [PubMed] [Google Scholar]
- 161.Fleming R. Case of Cancrum Oris. Trans Med Chir Soc Edinb. 1892; 259–262. [PMC free article] [PubMed] [Google Scholar]
- 162.Motarjemi Y, Kaferstein F, Moy G, Quevedo F. Contaminated weaning food: a major risk factor for diarrhoea and associated malnutrition. Bull World Health Organ. 1993;71: 79–92. [PMC free article] [PubMed] [Google Scholar]
- 163.Feller L, Altini M, Chandran R, Khammissa RAG, Masipa JN, Mohamed A, et al. Noma (cancrum oris) in the South African context. J Oral Pathol Med. 2014;43: 1–6. doi: 10.1111/jop.12079 [DOI] [PubMed] [Google Scholar]
- 164.Pithon M. Importance of the control group in scientific research. Dental Press J Orthod. 2013;18: 13–14. doi: 10.1590/s2176-94512013000600003 [DOI] [PubMed] [Google Scholar]
- 165.Feller L, Khammissa RAG, Altini M, Lemmer J. Noma (cancrum oris): An unresolved global challenge. Periodontol 2000. 2019;80: 189–199. doi: 10.1111/prd.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kimura T. An oro-facial disease ‘noma (cancrum oris)’ in a Japanese monkey (Macaca fuscata): clinical signs, clinicopathological features, and response to treatment. J Med Primatol. 2008;37: 217–222. doi: 10.1111/j.1600-0684.2008.00312.x [DOI] [PubMed] [Google Scholar]
- 167.Marck K and Bos K. The surgical treatment of Noma. Bathgate R, van Knippenberg M, editors. Amsterdam, The Netherlands: Mart Spruijt bv, Amsterdam; 2006. [Google Scholar]
- 168.Walker M, Burns K. Trismus: diagnosis and management considerations for the speech pathologist. American Speech-Language-Hearing Association. 2006. pp. 1–55. [Google Scholar]
- 169.Wali IM, Regmi K. People living with facial disfigurement after having had noma disease: A systematic review of the literature. J Health Psychol. 2016;September: 1243–1255. doi: 10.1177/1359105315624751 [DOI] [PubMed] [Google Scholar]
- 170.World Health Organisation (WHO), World Health Organization. A step-by-step guide to develop national action plans for noma prevention and control in priority countries. 2020. https://apps.who.int/iris/handle/10665/337203
- 171.Human Rights Council Advisory Committee. Study of the Human Rights Council Advisory Committee on severe malnutrition and childhood diseases with children affected by noma as an example, including annexed. “Human rights Princ Guidel to Improv Prot Child risk or Affect by malnutrition, specifically risk or Affect by noma” UN Doc A/HRC/19/73 24 Febr 2012. Available: https://documents-dds-ny.un.org/doc/UNDOC/GEN/G12/102/15/PDF/G1210215.pdf?OpenElement
- 172.Yunusa M, Obembe A. Prevalence of psychiatric morbidity and its associated factors among patients facially disfigured by cancrum oris in Nigeria a controlled study. Niger J Med. 2012;21: 277–281. Available: https://pubmed.ncbi.nlm.nih.gov/23304920/ [PubMed] [Google Scholar]
- 173.Barmes D, Enwonwu C, Leclercq M, Bourgeois D, Falkler W. The need for action against oro-facial gangrene. Trop Med Int Heal. 1997;2: 1111–1114. doi: 10.1046/j.1365-3156.1997.d01-220.x [DOI] [PubMed] [Google Scholar]
- 174.Enwonwu C, Falkler W, Phillips R. Noma (cancrum oris). Lancet. 2006;368: 147–156. doi: 10.1016/S0140-6736(06)69004-1 [DOI] [PubMed] [Google Scholar]
- 175.Speiser S, Langridge B, Birkl MM, Kubiena H, Rodgers W. Update on Noma: systematic review on classification, outcomes and follow-up of patients undergoing reconstructive surgery after Noma disease. BMJ Open. 2021;11: e046303. doi: 10.1136/bmjopen-2020-046303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Srour L, Marck K, Baratti-Mayer D. Noma: neglected, forgotten and a human rights issue. Int Health. 2015;7: 149–150. doi: 10.1093/inthealth/ihv001 [DOI] [PubMed] [Google Scholar]
- 177.Weaver G and Tunnicliff R. Noma: (gangrenous stomatitis; water cancer; scorbutic cancer; gangrena oris; gangrene of the mouth.). J Infect Dis. 1907;4: 8–35. [Google Scholar]
- 178.Farley E, Ariti C, Amirtharajah M, Kamu C, Oluyide B, Muhammad S, et al. Noma, a neglected disease: A viewpoint article. PLoS Negl Trop Dis. 2021;15: 1–5. doi: 10.1371/journal.pntd.0009437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Leila Srour M, Baratti-Mayer D. Why is noma a neglected-neglected tropical disease? PLoS Negl Trop Dis. 2020;14: 1–4. doi: 10.1371/journal.pntd.0008435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Farley E. Noma in northwest Nigeria: a neglected disease in neglected populations. University of Cape Town. 2020. https://open.uct.ac.za/handle/11427/32757?show=full [Google Scholar]
- 181.Enwonwu C. Noma: a neglected scourge of children in sub-Saharan Africa. Bull World Health Organ. 1995;73: 541–545. [PMC free article] [PubMed] [Google Scholar]
- 182.Marck K. Noma the face of Poverty. 1st ed. Hannover: MIT-Verlag GmbH; 2003. [Google Scholar]
- 183.Tapsoba H, Deschamps J. Use of medicinal plants for the treatment of oral diseases in Burkina Faso. 2006;104: 68–78. doi: 10.1016/j.jep.2005.08.047 [DOI] [PubMed] [Google Scholar]