Abstract
Neurotrophins influence growth and survival of sympathetic and sensory neurons through activation of their receptors, Trk receptor tyrosine kinases. Previously, we identified Src homology 2-B (SH2-B) and APS, which are structurally similar adapter proteins, as substrates of Trk kinases. In the present study, we demonstrate that both SH2-B and APS exist in cells as homopentamers and/or heteropentamers, independent of Trk receptor activation. Structure-function analyses revealed that the SH2-B multimerization domain resides within its amino terminus, which is necessary for SH2-B-mediated nerve growth factor (NGF) signaling. Overexpression of SH2-B enhances both the magnitude and duration of TrkA autophosphorylation following exposure of PC12 cells to NGF, and this effect requires the amino-terminal multimerization motif. Moreover, the amino terminus of SH2-B is necessary for TrkA/SH2-B-mediated morphological differentiation of PC12 cells. Together, these results indicate that the multimeric adapters SH2-B and APS influence neurotrophin signaling through direct modulation of Trk receptor autophosphorylation.
Neurotrophins are a family of neurotrophic factors that influence differentiation, survival, and plasticity of neurons. Cell surface receptors for neurotrophins are members of the Trk family of receptor tyrosine kinases (RTKs) (16) and a structurally distinct receptor, p75 (1). While the function of p75 remains unclear, Trk receptors appear to be the major mediators of neurotrophin signaling. TrkA is the signal-transducing receptor for the prototypic neurotrophin nerve growth factor (NGF) (9, 11).
The interaction between NGF and TrkA results in receptor homodimerization, which leads to autophosphorylation of TrkA on multiple tyrosine residues. Signaling molecules containing Src homology 2 (SH2) and/or phosphotyrosine binding domains (14), such as Shc and phospholipase C-γ, interact with tyrosine-phosphorylated TrkA and mediate activation of distinct signaling pathways (6, 10). For example, Shc binds to TrkA phosphotyrosine 490 and mediates NGF induction of Ras signaling and morphological differentiation of PC12 cells (21, 22).
We previously showed that the adapter proteins SH2-B and APS associate with tyrosine-phosphorylated Trk receptors and serve as substrates of Trk kinases in neurons. SH2-B and APS are highly related proteins with a very similar domain structure (15). Each contains a C-terminal SH2 domain, a pleckstrin homology (PH) domain, and several proline-rich motifs. In addition to binding to tyrosine-phosphorylated Trk receptors, SH2-B and APS also associate through their SH2 domains with the high-affinity immunoglobulin E (IgE) receptor FcɛRI (13), the nonreceptor tyrosine kinase JAK2 (20), c-kit kinase (24), and the insulin receptor (12). Moreover, both SH2-B and APS show sequence homology with a previously described protein, Lnk, which associates with the T-cell receptor complex (7). Thus, members of this family of adapters have the capacity to interact directly with RTKs, nonreceptor tyrosine kinases, and tyrosine kinase substrates through their highly conserved SH2 domains.
SH2-B and APS can associate with all three of the phosphorylated Trk receptors TrkA, -B, and -C and are sufficient to support Trk-mediated morphological differentiation of PC12 cells. Overexpression of SH2-B enhances NGF-induced neurite outgrowth, while overexpressing of an SH2-B mutant that cannot bind to TrkA prevents morphological differentiation of PC12 cells (15, 19). Moreover, disruption of SH2-B function in sympathetic neurons, which require NGF for survival, leads to axonal degeneration and death of these neurons (15). Together, these data support the idea that SH2-B and APS are important intracellular mediators of NGF/TrkA signaling in neurons. However, the mechanisms by which SH2-B and APS contribute to RTK signaling are not well understood. In this report, we provide evidence that SH2-B and APS exist in cells as oligomers through their amino-terminal association domains and that these multimerization domains are critical for at least some SH2-B and APS functions during RTK signaling.
MATERIALS AND METHODS
Cell lines and primary neuron cultures.
HEK293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin, and streptomycin. PC12nnr5 cells were kindly provided by Lloyd Greene and cultured in DMEM containing 10% FBS, 5% horse serum, penicillin, and streptomycin. Primary cortical neurons obtained from embryonic day 18 rat cortex were cultured as described (17).
Generation of DNA constructs.
Mammalian expression vectors encoding full-length rat TrkA and the TrkA mutant F8 were provided by Phil Barker and Naoyuki Inagaki (8), respectively. The full coding regions of rat APS and SH2-B fused to a Myc epitope-tagged sequence at the N terminus were subcloned into the mammalian expression vector pRK5 (3). Various deletions and mutations of SH2-B were amplified by PCR and cloned into the pRK5 vector.
HEK293T cell transfection, immunoprecipitation, and immunoblot.
HEK293T cells were transfected with expression vectors using Lipofectamine (Gibco-BRL). Two days after transfection, cells were treated as described and then lysed in an NP-40 lysis buffer (1% NP-40, 10% glycerol, 140 mM NaCl, 10 mM Tris, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM sodium orthovanadate plus 1 μg of aprotinin and 1 μg of leupeptin per ml [pH 7.4]). All subsequent steps were done at 4°C. Lysates were clarified by centrifugation at 16,000 × g for 20 min, and supernatants were subjected to immunoprecipitations using an anti-Myc monoclonal antibody (9E10). For immunoprecipitations from lysates prepared from PC12 cells or primary neurons, extracts were prepared as above. Extracts were subjected to immunoprecipitation with the indicated antibodies, and the immune complexes were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted with antibodies as indicated. Anti-SH2-B and anti-APS antisera were raised in rabbits as described (15) and used at dilutions of 1:200 for immunoprecipitation and 1:1,000 for immunoblotting. A phosphorylation site-specific polyclonal antibody directed against phosphorylated tyrosine 490 of TrkA (pTrkA490) was obtained from New England Biolabs, Inc., and used at a 1:1,000 dilution for immunoblotting (2). Polyclonal anti-Trk (C-14) and anti-human APS were from Santa Cruz Biotechnology, Inc., and used at dilutions of 1:200 and 1:1,000, respectively, for immunoblotting. Monoclonal antihemagglutinin (anti-HA; Boehringer Mannheim) was used at 1 μg/ml, and antitubulin (Sigma) was used at dilution of 1:10,000 for immunoblotting. Secondary antibodies were either anti-mouse or anti-rabbit Ig-horseradish peroxidase conjugates (Amersham). Signals were detected using ECL Plus (Amersham) and quantified using an Image Storm 860 analyzer (Molecular Dynamics, Inc.).
Purification of 6×His-SH2-B.
A six-histidine (6×His) tag sequence was introduced into the amino terminus of the SH2-B gene in the pRK5 expression vector to facilitate purification of SH2-B. HEK293T cells expressing 6×His-SH2-B were lysed in a hypotonic buffer (10 mM Tris, 1 mM PMSF plus 1 μg of aprotinin and 1 μg of leupeptin per ml [pH 8.0]). Extracts were prepared and incubated with Ni-nitrilotriacetic acid (NTA) resin (Qiagen) for 30 min at room temperature. The resin was washed extensively with washing buffer (15 mM imidazole, 10% glycerol, 300 mM NaCl, 2.5 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 1 mM PMSF plus 1 μg of aprotinin and 1 μg of leupeptin per ml [pH 8.0]) and then eluted into a buffer containing imidazole (250 mM [pH 6.0]). The eluent was dialyzed against phosphate-buffered saline (PBS) for 48 h at 4°C and then concentrated using Centron microconcentrators (Micron).
Size exclusion chromatography.
Nondenatured cell extracts from either cortical neurons or HEK293T cells transiently expressing SH2-B or APS were prepared as described above and applied to a size exclusion column (Sepharose 12; Pharmacia). Samples were eluted at a flow rate of 0.5 ml/min, and fractions were collected at 30-s intervals. Proteins in each fraction were resolved by SDS-PAGE and immunoblotted with antibodies directed against the Myc epitope, SH2-B, or APS.
Generation of stable PC12 cell lines expressing full-length SH2-B and M8 mutant.
PC12 cells were transfected with expression vector encoding either Myc-tagged full-length SH2-B or M8 together with the pCEP4 expression vector, which encodes the hygromycin resistance gene. Two days after the transfection, medium containing hygromycin (200 μg/ml) was added to the cells, and hygromycin-containing medium was replaced every 3 days. Individual colonies were clonally isolated and expanded and then tested for the expression of Myc-SH2-B or M8. Multiple PC12 cell clones expressing either full-length SH2-B or M8 were isolated.
PC12nnr5 cell transfection and NGF-induced neurite outgrowth.
PC12nnr5 cells were grown on 35-mm plates coated with poly-d-lysine. Cells were transfected with the indicated plasmids together with a cDNA encoding the green fluorescent protein (GFP) (pEGFP-Cl; Clontech). NGF was applied immediately following transfection to induce morphological differentiation of PC12nnr5 cells. Three days after NGF application, cells were fixed, and GFP-positive cells were scored for the presence of neurites. Cells with processes longer than two times the diameter of the cell body were considered positive. Similarly, PC12 cells stably expressing full-length SH2-B or M8 were treated with a range of NGF concentrations for 3 days and then fixed and scored for cells with neurites.
RESULTS
SH2-B and APS exist as homo- and heteromeric complexes in neurons.
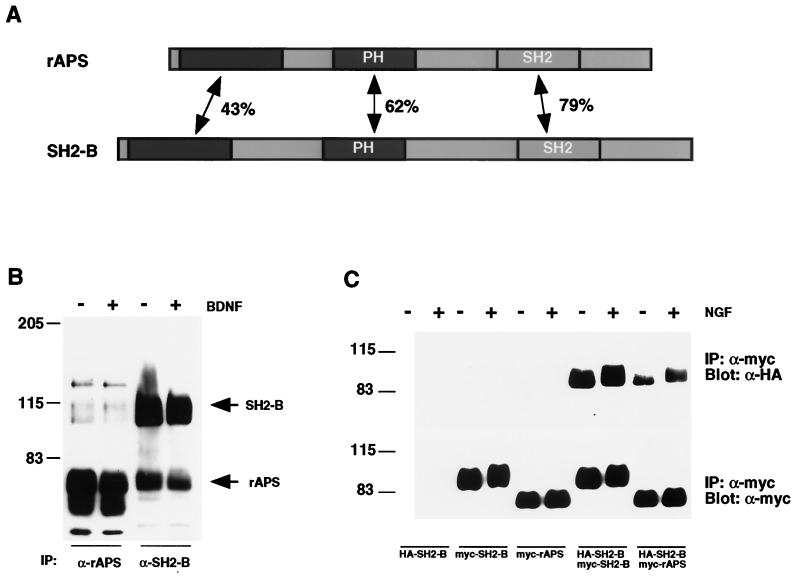
We recently identified SH2-B and APS, which are closely related SH2 and PH domain-containing proteins (Fig. 1A), as substrates of Trk receptors (15). SH2-B and APS are expressed in several populations of developing neurons. In the process of characterizing expression of SH2-B and APS in neurons, we found that SH2-B and APS were efficiently coimmunoprecipitated from lysates of cultured cells (Fig. 1B). For these experiments, SH2-B and APS antibodies, which were raised against divergent regions of SH2-B and APS and do not cross-react with APS and SH2-B proteins, respectively (15) (data not shown), were used for immunoprecipitations from extracts of cultured cortical neurons. Antibodies that recognize both APS and SH2-B were used for Western blotting of immune complexes. To further demonstrate that SH2-B can associate with APS and to ask whether SH2-B and/or APS form homomeric complexes, we transiently expressed Myc- and HA-tagged SH2-B and APS in HEK293T cells together with full-length TrkA and performed immunoprecipitation and immunoblot experiments. HA-tagged SH2-B was efficiently coprecipitated with both Myc-SH2-B and Myc-APS (Fig. 1C), indicating that SH2-B can form both homo- and heteromeric complexes. The association between SH2-B and APS is independent of Trk receptor activation. When cortical neurons or HEK293T cells expressing TrkA receptors were treated with brain-derived neurotrophic factor (BDNF) or NGF, respectively, the efficiency of coprecipitation of SH2-B and APS was not affected, although the stimulation caused mobility shifts and tyrosine phosphorylation of both SH2-B and APS (Fig. 1 and data not shown). Therefore, SH2-B and APS form homo- and heteromeric complexes in cortical neurons and when transiently expressed in HEK293T cells.
FIG. 1.
SH2-B and APS exist as homomultimers or heteromultimers in neurons. (A) Molecular structure of SH2-B and APS. SH2-B and APS were previously identified as Trk interactors (15) which contain several well-conserved domains, including SH2 and PH domains. Percent amino acid identity is indicated. (B) SH2-B heteromultimerizes with APS in neurons. Cortical neurons from E18 rats were untreated or treated with BDNF (50 ng/ml, 10 min), which activates TrkB. Cell lysates were prepared and subjected to immunoprecipitation (IP) using either SH2-B or APS antibodies. These antibodies do not cross-react (data not shown). Immune complexes were then resolved by SDS-PAGE and immunoblotted with an anti-human APS antibody (Santa Cruz) which recognizes both APS and SH2-B. (C) SH2-B forms homomultimers and heteromultimerizes with APS. Myc- and HA-tagged SH2-B and APS were transiently expressed in HEK293T cells. Cells were unstimulated or stimulated with NGF (100 ng/ml, 10 min). Cell lysates were prepared and subjected to immunoprecipitation (IP) using an anti-Myc antibody. Immune complexes were resolved by SDS-PAGE and subjected to immunoblotting with either anti-HA (top) or anti-Myc (bottom) antibodies. Sizes are shown in kilodaltons in this and subsequent figures. rAPS, rat APS.
SH2-B homomultimerizes through an amino-terminal association domain.
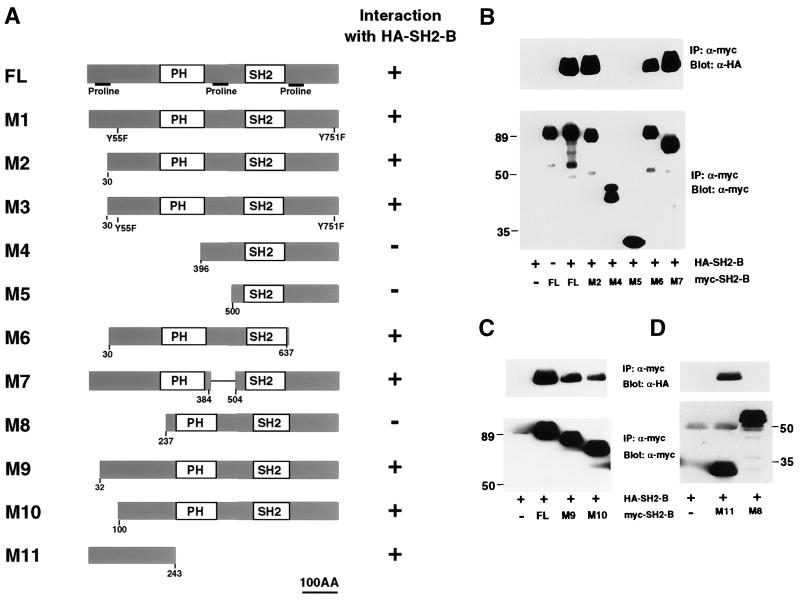
To identify the region(s) responsible for homomultimerization of SH2-B, a series of SH2-B truncation mutants containing an amino-terminal Myc epitope tag were employed (depicted in Fig. 2A). The SH2-B mutants were individually coexpressed with HA-tagged full-length SH2-B in HEK293T cells and then immunoprecipitated under nondenaturing conditions with an anti-Myc antibody. The immunoprecipitates were then immunoblotted with the anti-HA antibody to assess the presence of full-length HA-SH2-B in the immune complex. Although efficiently expressed in HEK293T cells, mutants M4, M5, and M8, which lack the amino-terminal region of SH2-B but do contain the PH and SH2 domains, did not form complexes with HA-SH2-B (Fig. 2B and D). These results suggest that neither the PH domain nor the SH2 domain is sufficient for SH2-B multimerization, but that the amino terminus is required for such multimerization. In fact, the amino terminus of SH2-B is also sufficient for multimerization. M11, which contains only the amino-terminal 243 amino acids of SH2-B, can effectively associate with HA-SH2-B (Fig. 2D).
FIG. 2.
SH2-B homomultimerizes through an amino-terminal association domain. (A) Schematic diagram of SH2-B deletion and tyrosine mutants. Full-length SH2-B and mutants containing an amino-terminal Myc epitope tag were cloned into mammalian expression vector pRK5. AA, amino acids. (B, C, and D) The amino-terminal region of SH2-B is both necessary and sufficient for SH2-B multimerization. Myc-tagged SH2-B mutants depicted in panel A were coexpressed with HA-tagged SH2-B in HEK293T cells. Cell extracts were subjected to immunoprecipitation (IP) with anti-Myc antibody. The immune complexes were then resolved by SDS-PAGE and immunoblotted using anti-Myc or anti-HA antibodies.
More subtle mutations that deleted 30 (M2 and M3), 32 (M9), and 100 (M10) amino acids of the amino terminus of Myc-SH2-B did not abolish association with HA-SH2-B (Fig. 2C). However, the amount of association between both M9 and M10 and HA-SH2-B was decreased by more than threefold. Taken together, these results suggest that the amino terminus of SH2-B is both required and sufficient for SH2-B multimerization.
SH2-B and APS exist as pentamers in neurons.
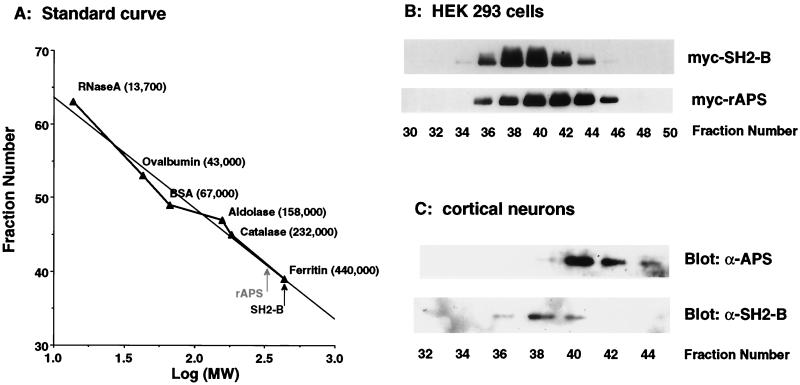
To determine the molecular composition of SH2-B and APS complexes, we performed experiments to assess the molecular mass of these complexes using fast protein liquid chromatography size exclusion chromatography (Sepharose 12; Pharmacia). Extracts prepared from HEK293T cells expressing either Myc-SH2-B or Myc-APS were fractioned by size exclusion chromatography, and samples of each fraction were immunoblotted with the anti-Myc antibody (Fig. 3B). The SH2-B and APS complexes eluted in fractions that corresponded to molecular masses of approximately 440 and 350 kDa, respectively. Similar results were obtained in experiments designed to assess the molecular masses of endogenous SH2-B and APS complexes found in cortical neurons (Fig. 3C). Importantly, endogenous and transiently expressed SH2-B and APS complexes eluted in single peaks; no SH2-B or APS was detected in other fractions (data not shown). The SH2-B mutant M8, which lacks the amino-terminal 237 amino acids and is incapable of forming a homomultimer (Fig. 2D), eluted in fraction 52, which corresponds to a molecular mass of 60 kDa (data not shown). These data corroborate the results of coprecipitation experiments that indicated that M8 exists in cells as a monomer.
FIG. 3.
SH2-B and APS exist as large complexes, as determined by size exclusion chromatography. (A) Standard molecular weight (MW) curve for size exclusion chromatography (Sepharose 12; Pharmacia). BSA, bovine serum albumin. (B) Lysates of HEK293T cells expressing either Myc-SH2-B or Myc-APS were fractionated by size exclusion chromatography, and samples from fractions were resolved by SDS-PAGE and immunoblotted using the anti-Myc antibody. (C) Nondenatured cell lysates prepared from primary cortical neurons were fractionated, and fractions were resolved by SDS-PAGE and immunoblotted with either anti-APS or anti-SH2-B antibodies. The SH2-B and APS complexes elute in fractions 36 to 42 and 38 to 44, which correspond to molecular masses of approximately 440 and 350 kDa, respectively. rAPS, rat APS.
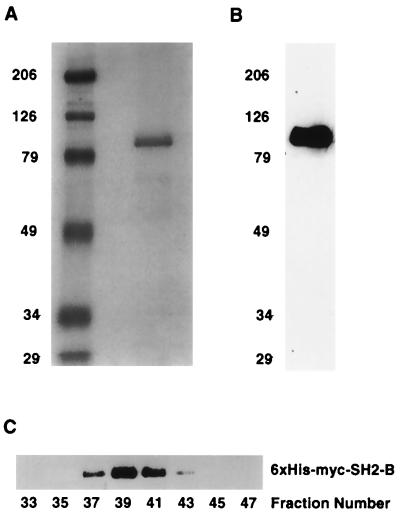
The 440-kDa SH2-B complex detected in the experiments shown in Fig. 3B could consist of SH2-B homomultimers and/or SH2-B complexed with an unidentified endogenous protein(s). To distinguish between these possibilities, we performed size exclusion chromatography experiments on purified SH2-B. A 6×His tag sequence was introduced into the amino terminus of SH2-B cDNA to facilitate purification of SH2-B. 6×His-tagged SH2-B expressed in HEK293T cells was purified using Ni-NTA chromatography under nondenaturing conditions. SH2-B is the major component of the purified complex, as determined by SDS-PAGE followed by Coomassie staining and immunoblotting (Fig. 4A and B). Importantly, the purified 6×His-SH2-B complex has a molecular mass of approximately 440 kDa, as determined by size exclusion chromatography (Fig. 4C). Thus, SH2-B itself is the major, if not sole, component of the 440-kDa SH2-B complex. Since the calculated molecular masses of monomer SH2-B and APS are 87 and 70 kDa, respectively, these results suggest that SH2-B and APS exist in cells as pentamers.
FIG. 4.
SH2-B is the major component of the SH2-B-containing complex. Cell lysates prepared from HEK293T cells expressing 6×His-Myc-SH2-B were prepared and incubated with Ni-NTA resin. After incubation, the 6×His-Myc-SH2-B complex was eluted with a buffer containing imidazole (250 mM, pH 6.5). The eluent was dialyzed against PBS and concentrated. The purified 6×His-Myc-SH2-B was resolved by SDS-PAGE and either stained with Coomassie blue (A) or immunoblotted with anti-Myc (B). The purified 6×His-Myc-SH2-B complex was fractionated by size exclusion chromatography, and fractions were resolved by SDS-PAGE and immunoblotted with anti-Myc (C).
SH2-B modulates the kinetics of TrkA phosphorylation.
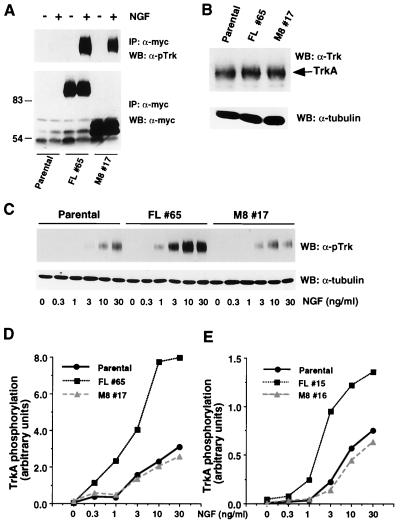
We next asked whether the amino terminus of SH2-B contributes to Trk signaling. Stable PC12 cell lines expressing either Myc-tagged full-length SH2-B or the SH2-B mutant M8, which lacks the amino-terminal multimerization domain, were established. To control for differences due to variations in PC12 cell clones, multiple independent clones were isolated and used for each experiment. We first asked whether M8, like full-length SH2-B, is a substrate of the TrkA receptor. PC12 cells expressing SH2-B and M8 were stimulated with NGF. Cell lysates were prepared and subjected to immunoprecipitation using the Myc antibody. Similar amounts of full-length SH2-B and M8 were detected in the cell lysates (Fig. 5A, lower panel). Phosphorylated TrkA receptor was coimmunoprecipitated with both full-length SH2-B and M8, as determined by immunoblotting using an antibody that specifically recognizes the tyrosine 490-phosphorylated TrkA receptor (pTrk) (Fig. 5A, upper panel). Reprobing the same blot with a phosphotyrosine antibody demonstrated that NGF stimulated tyrosine phosphorylation of M8 as well as full-length SH2-B in PC12 cells (data not shown). These results indicate that M8, like full-length SH2-B, associates with TrkA and is a substrate of the TrkA kinase. Furthermore, neither full-length SH2-B nor M8 overexpressed in PC12 cells changed the level of TrkA receptor expression, as determined by immunoblotting of cell extracts from these stable PC12 cell lines with the pan-Trk C-14 antibody (Fig. 5B).
FIG. 5.
SH2-B enhances NGF-induced tyrosine phosphorylation of TrkA in PC12 cells. (A) Parental PC12 cells or PC12 cells stably expressing either full-length SH2-B or the truncated M8 mutant were unstimulated or stimulated with NGF (100 ng/ml, 10 min). Cell lysates were prepared and subjected to immunoprecipitation (IP) using a Myc antibody. The immune complexes were resolved by SDS-PAGE and Western blotted (WB) using the pTrk antibody (upper panel) or anti-Myc (lower panel). (B) Cell lysates prepared from parental PC12 cells or PC12 cells stably expressing either full-length SH2-B or M8 were separated by SDS-PAGE and Western blotted (WB) with either a pan-Trk antibody (upper panel) or an antitubulin antibody. (C) Parental PC12 cells or PC12 cells stably expressing either full-length SH2-B (FL #65) or M8 (M8 #17) were stimulated with NGF at a range of concentrations as indicated. Cell extracts were resolved by SDS-PAGE and Western blotted (WB) with the pTrk antibody, and the blot was stripped and reprobed with antitubulin. The phosphorylated TrkA and tubulin signals were quantified using Image Storm analysis and the phospho-TrkA and tubulin levels illustrated in panel D. Similar experiments were performed using another set of stable PC12 clones expressing either full-length SH2-B (FL #15) or M8 (M8 #16). Quantitation of the level of phosphorylation of TrkA receptor normalized to amounts of tubulin is shown in panel E.
Since the amino-terminal oligomerization domain is distinct from the SH2 domain, which mediates association with TrkA, we postulated that oligomerization of SH2-B could facilitate oligomerization or clustering of autophosphorylated TrkA. Thus, we asked whether expression of SH2-B affects NGF-induced tyrosine phosphorylation of TrkA. Parental PC12 cells or PC12 cells stably expressing either full-length SH2-B or M8 were stimulated with medium containing a range of NGF concentrations. The extent of phosphorylation of TrkA was determined by immunoblotting the cell extracts with the pTrk antibody. Interestingly, NGF-induced tyrosine phosphorylation of TrkA was enhanced in PC12 cells overexpressing full-length SH2-B compared to parental PC12 cells. In contrast, there was no potentiation of NGF-induced TrkA phosphorylation in cells expressing M8 (Fig. 5C). Quantitative data obtained from experiments using multiple clonal cell lines are shown in Fig. 5D and E. The differences in the responses of the clonal cells are not due to different levels of expression of full-length SH2-B and M8 because comparable amounts of full-length SH2-B and M8 were detected in the cell lysates (Fig. 5A). Furthermore, similar results were also obtained in transient-transfection experiments (data not shown).
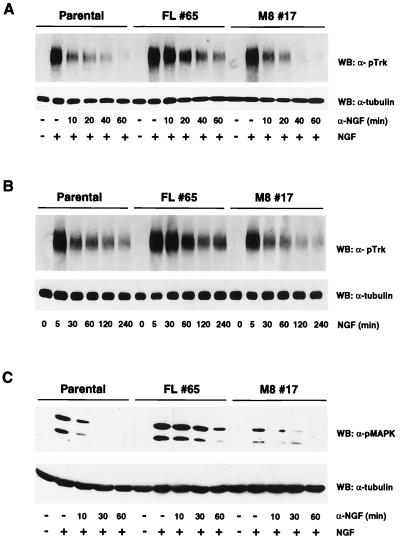
We also assessed the kinetics of NGF-mediated TrkA phosphorylation in parental, SH2-B, and M8 PC12 cells. Cells were exposed to a pulse of NGF (100 ng/ml, 10 min), and then fresh medium containing a neutralizing NGF antibody was added to the culture medium. The kinetics of TrkA dephosphorylation were determined by immunoblotting the cell extracts with the pTrk antibody. Both the magnitude and duration of TrkA phosphorylation were increased in cells expressing full-length SH2-B compared to parental PC12 cells and cells expressing M8. For example, TrkA phosphorylation remains high in cells expressing full-length SH2-B, but not in parental or M8 cells, 60 min after NGF withdrawal (Fig. 6A). Similar results were seen in other PC12 cell clones expressing full-length SH2-B or M8 (data not shown).
FIG. 6.
SH2-B modulates the kinetics of NGF-induced phosphorylation of both TrkA and MAP kinase (MAPK) in PC12 cells. Parental PC12 cells or PC12 cells stably expressing either full-length SH2-B or M8 were stimulated with a pulse of NGF (100 ng/ml, 10 min [A] or 3 ng/ml, 10 min [C]). Then, medium was replaced with fresh medium containing a function blocking NGF antibody (A and C). In the experiments shown in B, cells were exposed to NGF (100 ng/ml) continuously for the indicated times. Cell extracts were prepared and resolved by SDS-PAGE and Western blotted (WB) with the indicated antibodies. Similar results were observed with experiments using other SH2-B and M8 clones (data not shown).
Since TrkA phosphorylation is transient during continuous exposure of PC12 cells to NGF, we also compared the kinetics of TrkA phosphorylation during constant exposure of parental PC12, SH2-B, and M8 cells to NGF. Similar to the experiments discussed above, PC12 cells expressing SH2-B exhibited more pronounced phosphorylation of TrkA after various durations of NGF stimulation. For example, after 4 h of NGF treatment, TrkA phosphorylation was much greater (55% of peak) in cells expressing full-length SH2-B than in the parental cells or cells expressing M8 (less than 10% of peak) (Fig. 6B and data not shown). We further assessed whether SH2-B expression affects downstream signals of Trk receptor, such as phosphorylation and activation of the ERK1 and ERK2 mitogen-activated protein (MAP) kinases. We observed enhanced phosphorylation of ERK kinases in cells expressing full-length SH2-B compared to both parental cells and cells expressing M8 (Fig. 6C). Together, these results demonstrate that expression of SH2-B augments both the magnitude and duration of phosphorylation and activation of TrkA. Importantly, full-length SH2-B but not M8 enhanced phosphorylation of TrkA, suggesting that the amino-terminal multimerization domain is critical for the ability of SH2-B to promote phosphorylation of TrkA.
SH2-B multimerization domain is critical for NGF-induced morphological differentiation of PC12 cells.
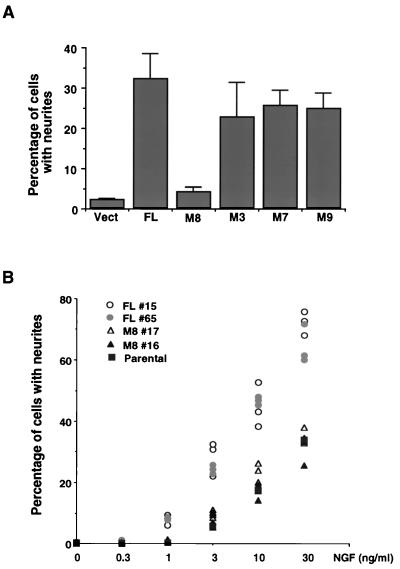
The TrkA mutant F8, which lacks all conserved tyrosine residues except those in the catalytic loop, cannot mediate NGF induction of morphological differentiation of PC12nnr5 cells (5, 15). However, NGF can induce outgrowth of neurites in PC12nnr5 cells that coexpress F8 and SH2-B (15). Similar transient-transfection experiments were performed to assess the role of the amino-terminal multimerization domain of SH2-B in promotion of morphological differentiation of PC12nnr5 cells. As seen previously, co expression of the TrkA mutant F8 and full-length SH2-B confers NGF-sensitive neurite outgrowth in PC12nnr5 cells. In contrast, M8 did not support differentiation of PC12nnr5 cells when coexpressed with the TrkA mutant F8. M7, an SH2-B mutant that can form homomultimers (Fig. 2B) but can no longer interact with Grb2 (15), does support morphological differentiation (Fig. 7A). Also, M3 and M9, which lack the amino-terminal 29 amino acids (M9) as well as two conserved tyrosines (M3), could support neurite outgrowth. Together, these data indicate that the amino-terminal multimerization domain of SH2-B and not the extreme amino terminus, the conserved NPXY motif, or the Grb2 association motif is necessary for SH2-B-mediated morphological differentiation of PC12nnr5 cells.
FIG. 7.
Amino terminus of SH2-B modulates NGF-mediated morphological differentiation of PC12 cells. (A) The SH2-B amino-terminal domain is necessary for NGF-induced differentiation of PC12nnr5 cells. Either full-length SH2-B or one of several SH2-B mutants was transiently expressed in PC12nnr5 cells along with the TrkA mutant F8 and GFP. Cells were treated with NGF (100 ng/ml) for 3 days after transfection. Cells were then fixed, and neurite outgrowth of GFP-positive cells was scored. The TrkA mutant F8 has mutations in all conserved tyrosines except Y670 and Y674/5 (8). This mutant cannot bind to Shc and phospholipase C-γ and cannot support morphological differentiation of PC12nnr5 cells unless coexpressed with SH2-B or APS (15). Vect, vector. (B) PC12 cells expressing full-length SH2-B are more responsive to NGF than either parental PC12 cells or PC12 cells expressing M8. Parental PC12 cells or PC12 cell clones stably expressing either full-length SH2-B (FL #15 and FL #65) or M8 (M8 #16 and M8 #17) were stimulated with the indicated concentrations of NGF for 3 days and then fixed and scored for neurite outgrowth. The results shown were from three independent experiments.
We also monitored neurite outgrowth in our stable PC12 clones, which express either SH2-B or M8. Previous work has shown that overexpression of full-length SH2-B enhances NGF induction of outgrowth of neurites of PC12 cells (19). Interestingly, in the experiments shown in Fig. 7B, NGF-induced neurite outgrowth was more robust in PC12 cells expressing full-length SH2-B than in either parental PC12 cells or PC12 cells expressing M8. For example, NGF at 1 ng/ml promoted neurite outgrowth in more than 7% of the cells expressing full-length SH2-B, whereas fewer than 1% of the cells expressing M8 differentiated in response to NGF. When exposed to NGF at 30 ng/ml, approximately 70% of the cells expressing full-length SH2-B had neurites by day 3, whereas only about 30% of parental cells or cells expressing M8 had neurites. Taken together, these results indicate that the amino-terminal multimerization domain of SH2-B facilitates NGF-dependent TrkA phosphorylation and downstream signaling events that support morphological differentiation of PC12 cells.
DISCUSSION
We previously identified SH2-B and APS as substrates of Trk kinases. Both proteins associate with tyrosine-phosphorylated Trk receptors and mediate neurotrophin signaling (15). In the present study, we provide evidence that both SH2-B and APS exist in cells as homomultimers and/or heteromultimers. SH2-B and APS likely exist as homo- and/or heteropentamers, as determined by size exclusion chromatography experiments. The SH2-B multimerization domain is located in its amino terminus; the amino-terminal 243 amino acids are both necessary and sufficient for multimerization of SH2-B. In support of the idea that the multimerization of SH2-B contributes to TrkA signaling, the amino-terminal multimerization domain of SH2-B is necessary for SH2-B-mediated augmentation of NGF-dependent TrkA autophosphorylation as well as morphological differentiation of PC12 cells.
It has recently been shown that SH2-Bβ, a splice variant of SH2-B, interacts with JAK2 and is a potent activator of JAK2 kinase (18). We found that overexpression of SH2-B does not activate TrkA kinase in the absence of its ligand, NGF. Rather, SH2-B dramatically enhances NGF activation of autophosphorylation of TrkA. Furthermore, the duration of TrkA phosphorylation is prolonged in cells stably expressing SH2-B compared to parental PC12 cells and cells expressing M8, which cannot multimerize. In accordance with these observations, SH2-B, when coexpressed with a TrkA mutant, F8, supports NGF induction of morphological differentiation of PC12nnr5 cells. F8 itself cannot support morphological differentiation of the cells (15).
Our study provides evidence that the amino-terminal multimerization domain of SH2-B is critical for NGF signaling. Although overexpression of full-length SH2-B enhances both the magnitude and duration of NGF activation of phosphorylation of TrkA, we observed no enhancement of NGF-induced TrkA kinase activity in PC12 cells expressing a truncated SH2-B mutant, M8, which lacks the amino-terminal multimerization domain. Moreover, only SH2-B mutants that can form multimers confer NGF-induced morphological differentiation of PC12nnr5 cells when coexpressed with F8. The simplest interpretation of these data is that SH2-B multimerization is required for NGF signaling. An alternative possibility is that some function of the amino-terminal motif other than multimerization contributes to SH2-B-mediated signaling. For example, both a proline-rich motif and a tyrosine residue (Y55) lie within the context of a putative phosphotyrosine binding site, an N/HPXY motif, in the amino-terminal region of SH2-B. Deletion of the SH2-B amino terminus, such as in M8, may disrupt potential interactions between SH2-B and putative signaling molecules that associate with these motifs. However, SH2-B mutants carrying either a deletion of the amino-terminal proline-rich motif (M9) or Y55 mutated to phenylalanine (M3) still mediate NGF-induced neurite outgrowth of PC12nnr5 cells when coexpressed with F8 (Fig. 7A). No other obvious protein-binding motifs are readily identified in the amino-terminal multimerization domain of SH2-B. Therefore, the multimeric nature of SH2-B may be critical for SH2-B-mediated augmentation of TrkA autophosphorylation and signaling.
The mechanism by which SH2-B and APS homo- or heteromultimers potentiate TrkA kinase activation remains unclear. At least three possibilities exist. One possibility is that by interacting with TrkA via the tyrosine residues within the kinase core domain, which are critical for activation and maintenance of TrkA kinase activity (4), SH2-B and APS can protect these critical tyrosine residues from being dephosphorylated by tyrosine phosphatase(s) and thus enhance and prolong TrkA kinase activity. We have previously shown that SH2-B and APS interact with a TrkA variant lacking all the conserved tyrosines except the three phosphotyrosine residues within the catalytic loop of the TrkA kinase domain (15). SH2-B was also found to be a substrate of the insulin receptor (IR), and the interaction between SH2-B and IR occurs between the SH2 domain of SH2-B and phosphotyrosines within the catalytic loop of the IR (12). The amino acids surrounding the catalytic loop tyrosines in Trk and IR kinases are very similar. Interestingly, the JAK-binding protein JAB, a protein that binds via its SH2 domain to a phosphotyrosine within the catalytic loop of the kinase domain of JAK2, has been shown to regulate JAK2 kinase activity (23). However, we do not favor the idea that SH2-B functions by preventing receptor dephosphorylation because our data indicate that M8, which effectively interacts with phospho-TrkA (Fig. 5A and data not shown), fails to potentiate NGF-dependent TrkA kinase activity and fails to promote morphological differentiation of PC12 cells. A second possibility is that SH2-B and APS multimers, when associated with activated TrkA receptors, can stabilize TrkA receptor dimers and prevent them from dissociating, thus prolonging TrkA autophosphorylation. A third possibility is that SH2-B and APS pentamers, by interacting with multiple phosphorylated TrkA receptor dimers, induce clustering of receptor dimers. Either of the latter two models is consistent with the observation that mutant M8, which cannot multimerize, fails to support enhancement of NGF-induced TrkA autophosphorylation and signaling. Moreover, these latter two possibilities are not mutually exclusive.
In summary, we have demonstrated that SH2-B and APS exist in cells as homomultimer and/or heteromultimer complexes and that an amino-terminal SH2-B domain that is necessary and sufficient for multimerization is critical for SH2-B function. Determination of the precise mechanism by which SH2-B and APS multimers contribute to autophosphorylation of TrkA should provide insight into NGF signaling in developing neurons.
ACKNOWLEDGMENTS
We thank Alex Kolodkin, Anirvan Ghosh, Richard Huganir, and members of the Ginty laboratory for discussions and suggestions and Ravi Misra, Bonnie Lonze, and Sohyun Ahn for critical reading of the manuscript. We thank Lloyd Greene for PC12nnr5 cells, Nayouki Inagaki for TrkA mutants, and Phil Barker for TrkA expression vectors.
This work was supported by NIH grant N534814 and a Pew Scholar Award (D.D.G.). D.D.G. is an Assistant Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Barker P. Nerve growth factor and the low-affinity neurotrophin receptor. In: Sieber-Blum M, editor. Neurotrophins and the neural crest. Boca Raton, Fla: CRC Press; 1998. pp. 59–93. [Google Scholar]
- 2.Binder D K, Routbort M J, McNamara J O. Immunohistochemical evidence of seizure-induced activation of trk receptors in the mossy fiber pathway of adult rat hippocampus. J Neurosci. 1999;19:4616–4626. doi: 10.1523/JNEUROSCI.19-11-04616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brakeman P R, Lanahan A A, O'Brien R, Roche K, Barnes C A, Huganir R L, Worley P F. Homer, a protein that selectively binds to metabotrophic glutamate receptors. Nature. 1997;386:221–223. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham M E, Stephens R M, Kaplan D R, Greene L A. Autophosphorylation of activation loop tyrosines regulates signaling by the TRK nerve growth factor receptor. J Biol Chem. 1997;272:10957–10967. doi: 10.1074/jbc.272.16.10957. [DOI] [PubMed] [Google Scholar]
- 5.Green S H, Rydel R E, Connolly J L, Greene L A. PC12 cell mutants that possess low-but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J Cell Biol. 1986;102:830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene L A, Kaplan D R. Early events in neurotrophin signalling via Trk and p75. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Li Y, Tanaka K, Moore K G, Hayashi J L. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1992;92:11618–11622. doi: 10.1073/pnas.92.25.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki N, Thoenen H, Lindholm D. TrkA tyrosine residues involved in NGF-induced neurite outgrowth of PC12 cells. Eur J Neurosci. 1995;7:1125–1133. doi: 10.1111/j.1460-9568.1995.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan D R, Hempstead B, Martin-Zanca D, Chao M V, Parada L F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;242:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan D R, Stephens R M. Neurotrophin signal transduction by the trk receptor. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Jing S, Nanduri V, O'Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 12.Kotani K, Wilden P, Pillay T S. SH2-Bα is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem J. 1998;335:103–109. doi: 10.1042/bj3350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne M A, Dalton S, Kochan J P. The yeast tribrid system— genetic detection of trans-phosphorylated ITAM-SH2-interactions. Biotechnology. 1995;13:1474–1478. doi: 10.1038/nbt1295-1474. [DOI] [PubMed] [Google Scholar]
- 14.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 15.Qian X, Riccio A, Zhang Y, Ginty D D. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron. 1998;21:1017–1029. doi: 10.1016/s0896-6273(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 16.Reichardt L F, Farinas I. Neurotrophic factors and their receptors: roles in neuronal development and function. In: Cowan W M, Jessel T M, Zipursky S L, editors. Molecular and cellular approaches to neural development. New York, N.Y: Oxford University Press; 1997. pp. 220–263. [Google Scholar]
- 17.Rosen L B, Ginty D D, Weber M J, Greenberg M E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 18.Rui L, Carter-Su C. Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA. 1999;96:7172–7177. doi: 10.1073/pnas.96.13.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rui L, Herrinton J, Carter-Su C. SH2-B is required for nerve growth factor-induced neuronal differentiation. J Biol Chem. 1999;274:10590–10594. doi: 10.1074/jbc.274.15.10590. [DOI] [PubMed] [Google Scholar]
- 20.Rui L, Mathews L S, Hotta K, Gustafson T A, Carter-Su C. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol. 1997;17:6633–6644. doi: 10.1128/mcb.17.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segal R A, Greenberg M E. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 22.Stephens R M, Loeb D M, Copeland T D, Pawson T, Greene L A, Kaplan D R. Trk receptors use redundant signal transduction pathways involving Shc and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 23.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle J N, Yoshimura A. The JAK-binding protein JAB inhibits Janus kinase tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokouchi M, Suzuki R, Masuhara M, Komiya S, Inoue A, Yoshimra A. Cloning and characterization of APS, an adaptor molecule containing PH and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene. 1997;15:7–15. doi: 10.1038/sj.onc.1201163. [DOI] [PubMed] [Google Scholar]