Abstract
Microbial control of mosquitoes via the use of symbiotic or pathogenic microbes, such as Wolbachia and entomopathogenic fungi, are promising alternatives to synthetic insecticides to tackle the rapid increase in insecticide resistance and vector-borne disease outbreaks. This study evaluated the susceptibility and host responses of two important mosquito vectors, Ae. albopictus and Cx. pipiens, that naturally carry Wolbachia, to infections by entomopathogenic fungi. Our study indicated that while Wolbachia presence did not provide a protective advantage against entomopathogenic fungal infection, it nevertheless influenced the bacterial / fungal load and the expression of select anti-microbial effectors and phenoloxidase cascade genes in mosquitoes. Furthermore, although host responses from Ae. albopictus and Cx. pipiens were mostly similar, we observed contrasting phenotypes with regards to susceptibility and immune responses to fungal entomopathogenic infection in these two mosquitoes. This study provides new insights into the intricate multipartite interaction between the mosquito host, its native symbiont and pathogenic microbes that might be employed to control mosquito populations.
Author summary
Control of mosquitoes via the use of microbes is a promising alternative to synthetic insecticides and a potential solution to tackle the rapid evolution of insecticide resistance in mosquitoes. Recently, a parasitic microbe named Wolbachia has been found to render the mosquito resistant to virus infections and it is currently showing great promise in reducing dengue cases on tests conducted in the field. On the other side of the symbiotic spectrum, we have entomopathogenic fungi, who have evolved to naturally infect and kill insects, and offer a unique potential to control mosquito populations. In this study, we examined the effect that native Wolbachia can have on the mosquito susceptibility to fungal entomopathogens. Our findings show that while Wolbachia does not affect the action of entomopathogenic fungi on mosquitoes, it does influence the expression of important mosquito immune genes, suggesting that Wolbachia has a closer interaction with the mosquito response to microbial infections than previously reported. Furthermore, our study provides new records on the susceptibility of two important mosquito vectors in the USA (Aedes albopictus and Culex pipiens), with Cx. pipiens showing significant resistance to the action of one fungal entomopathogen tested. This article informs on the mosquito susceptibility and interaction with other microbes that will aid in the selection of fungal entomopathogens to control mosquitoes, especially those that carry native microbes such as Wolbachia.
Introduction
Despite concerted efforts to control vector-borne diseases, outbreaks around the world continue to increase in frequency and intensity [1–4]. In the absence of effective therapeutic drugs against vector borne pathogens, vector control remains the most important component of public health programs around the world [5]. However, the effectiveness of mosquito control, primarily based on the use of chemical pesticides, has been impacted by the rapid evolution of insecticide resistance [6–8]. Microbial and symbiotic control, using symbiotic or entomopathogenic microbes that kill or render the mosquito host less competent in transmitting pathogens, offer an alternative to tackle this increasingly important public health problem [5,9–12].
In this respect, the endosymbiotic α-proteobacterium Wolbachia is currently being adopted as a novel strategy to curb the transmission of arboviruses by mosquitoes [13–15]. For instance, transinfection of Wolbachia wMel strain from Drosophila melanogaster or wAlbB from Ae. albopictus into Aedes aegypti, impaired the mosquito’s ability to transmit dengue [9,16]. Although the main mechanism of Wolbachia-derived viral suppression is not clearly established [17,18], changes in the mosquito immune system [19, 20], Wolbachia-virus competition and Wolbachia modulation of the mosquito methylation patterns have been suggested as potential mechanisms of viral suppression [17,21–24]. In addition to interfering with the replication and transmission of several arboviruses [9], Wolbachia has been shown to reduce filarial [25] and Plasmodium infection in mosquitoes [9,26] and to interact with the host native microbiota [27], resulting in increased host fitness [28,29]. Furthermore, at least one study reported a Wolbachia-mediated protection in Drosophila melanogaster fruit fly against a B. bassiana fungal strain [30]. Hence, a plethora of research provides evidence that Wolbachia can influence the host susceptibility to pathogens [28]. In terms of natural Wolbachia infections, most Aedes albopictus and Culex pipiens mosquitoes are infected with Wolbachia, however each strain can have different characteristics or interactions with their mosquito hosts [31].
The use of entomopathogenic fungi to control mosquitoes is another strategy that is being considered to suppress mosquito populations [32,33]. Entomopathogenic fungi have the ability to infect its insect host on contact, quickly developing an infection peg and producing chitinases and proteinases that allow them to penetrate the insect cuticle [34,35]. Once inside the insect hemocoel, the fungal entomopathogen multiplies as single cell blastospores, disseminating throughout the mosquito body, up taking nutrients and eventually leading to host death [35]. Differences in host susceptibility to fungal entomopathogens are thought to be due to variations in fungal strain virulence and to host-specific antifungal responses [36,37]
In terms of host responses during multipartite interactions, the mosquito immune system is at the interface of symbiotic and pathogenic interactions with the mosquito host [38,39]. While Wolbachia modulation of the mosquito immune system is recognized in heterologous systems of Wolbachia transinfection [20,40], it is less clear what effect native Wolbachia infections might have on their host. Mosquito responses to Wolbachia transinfections, following the artificial transfer from other mosquito species or from other insects, indicates induction of several components of the mosquito immune defense. For instance, transinfections with wAlbB in the mosquito Ae. aegypti led to the activation of the immune signaling pathways Toll and Imd and were thought to protect the mosquito against bacteria and fungi [20]. In addition, transinfections with wMelPop and wMel strains of Wolbachia led to the upregulation of the melanization cascade. Lastly Wolbachia transinfections have been found to alter the host microbial flora [41].
Similar host response profiles have been observed during pathogenic interactions. For instance, during fungal entomopathogen-mosquito-microbiota interactions, fungal entomopathogens induce a range of mosquito responses, ranging from activation of canonical immune signaling pathways, antimicrobial effectors, oxidative stress and the melanization cascade [35,37,42]. Interestingly, entomopathogenic fungi also interact with the mosquito gut microbiota, creating an environment that leads to dysbiosis of the mosquito gut [36,43]. Furthermore, our studies with the mosquito Ae. aegypti have shown that the mosquito infection-responsive repertoire tends to display a level of compartmentalization with tissue-specific expression that is fungal strain-specific [36].
In this study we explored the effects of fungal entomopathogenic infections on the survival and immune responses of two important mosquito vectors, Ae. albopictus and Cx. pipiens in the context of native Wolbachia infections. Our study shows that while Wolbachia did not affect the susceptibility of either mosquito species to entomopathogenic fungi, it had significant influence on the microbial load and mosquito transcriptional responses to fungal infection. Such responses, though vastly modulated by fungal infection, were also affected by the presence of native Wolbachia, and in some cases, effects of fungal infection and Wolbachia interacted. This study expands our knowledge of fungal entomopathogenic susceptibility in two important mosquito species and provides a snapshot of the molecular interactions of natural Wolbachia infections with mosquitoes during a pathogenic infection process.
Materials and methods
Mosquito rearing
Aedes albopictus eggs were provided by collaborators at Tyson Research Center, Washington University, Eureka, MO and reared at the National Center for Agricultural Utilization Research in Peoria, IL. All eggs, larvae, pupae, and adults were housed at 28°C with 14:10 light:dark photoperiod. Eggs were allowed to hatch for 72 hours in a 12 x 10 x 3 photo developer tray with 1L of DI water and maintained on a mixture of rabbit food, fish food, and liver powder. Adults were provided with 10% sucrose solution and were provided with a blood meal at 3–6 days post emergence using an artificial membrane feeder and bovine blood (Hemostat). Culex pipiens were collected in Normal, IL and reared at Illinois State University, Normal, IL. Adults of the original Wolbachia(+) Cx. pipiens colony were housed in an insectary at ~25°C with a 16:8 light:dark photoperiod with a 2-hour dawn/dusk phase. To avoid cross-contamination between colonies with Wolbachia, adults of the Wolbachia-free Cx. pipiens colony were housed in a separate walk-in environmental room at 25°C, with a 14:10 light:dark photoperiod. Adults of both colonies were provided with 20% sucrose solution and blood fed during the dark phase from anesthetized laboratory mice (IACUC protocol #842043) placed on the screen top of the cage. Custom-made mesh magnetic cages were placed over feeding females after they settled on the mice to limit each mouse to fewer than 25 bites. To encourage synchronous egg laying and to provide ample water surface area, 5 days post-blood feeding 7.5 L buckets containing white oak leaf infusion were placed in the cages. Egg rafts from both colonies were removed the next day and placed in separate beakers with white oak leaf infusion. The hatched larvae were counted into cohorts of 500 larvae, placed into 3 L of 2 g/L white oak infusion in 7.5 L buckets, and transported to the National Center for Agricultural Utilization Research in Peoria, IL, where experimental larvae were reared to adulthood at 28°C with a 14:10 light:dark photoperiod. The water level was maintained at 3 L by adding DI water as needed. Larvae were given bovine liver powder daily and 3 g of timothy hay was added to the buckets after they reached 3rd instar. Experimental adults were provided with 10% sucrose solution, and adult females from both species entered experiments when they reached 3–5 days old.
Entomopathogenic fungal strains and infection bioassays
To evaluate the effect of natural Wolbachia infection in the mosquito susceptibility to fungal entomopathogens we used two entomopathogenic fungal strains: Beauveria bassiana (MBC076) and Beauveria brongniartii (MBC397). Infection bioassays were conducted as previously described [36]. Briefly, fungal cultures were grown on ¼ strength Sabouraud dextrose agar yeast extract (SDAY) medium and conidia oil formulations were prepared from 15-day old cultures using soybean oil as a carrier. Following homogenization, the mixture was filtered through cheese cloth and conidia counted using an improved Neubauer hemocytometer. The suspension was adjusted to a conidial concentration of 1 x 108 conidia/ml. Topical exposure was conducted by depositing 50.6 nl of the conidial suspension (equivalent to 50,600 conidia/mosquito) on the coxal region of cold-anesthetized mosquitoes via a Nanoject III micropipet. Mosquitoes from the control group were exposed to the same treatment and exposed to the same volume of soybean oil devoid of any fungal conidia. At least three independent experiments were conducted for survival assays and for analysis of gene expression. Each experiment was conducted with fresh batches of mosquitoes and fungal suspensions. Treated mosquitoes were transferred and maintained in insect-cup cages under standard insectary conditions and provided with 10% sucrose solution. All experimental mosquitoes were maintained solely on sucrose solution and at no time were allowed to blood-feed. Mosquito survival was monitored daily, and mosquito cadavers removed from the cage. Survival curves from each treatment were analyzed via Kaplan-Meier estimator with median survival time differences between each treatment compared via Log-rank test (GraphPad Prism9.0). The lethal time to 50% mortality (LT50) values were calculated by probit analysis.
Wolbachia clearance
Wolbachia clearance from both mosquito species was conducted via tetracycline treatment of the adult mosquitoes as previously specified [44]. Briefly, adult mosquitoes were separated at the time of emergence into control (Wolbachia (+)), and tetracycline-treated cohorts (Wolbachia (-)), and provided with either sugar alone or sugar meals laden with 1.25 mg/ml of tetracycline respectively. All sugar meals were replaced every other day and following blood meals. Wolbachia clearance was verified via qPCR using DNA extracted (DNeasy Blood and Tissue Kit, QIAGEN) and Wolbachia-specific primers (S1 Table) following the methodology from [45] from subsamples of 5–10 adult males and females randomly collected from each treated group. Five and three generations of tetracycline treatment were necessary to completely clear Wolbachia from Ae. albopictus and Cx. pipiens mosquitoes respectively. To conduct bioassays and once clearance of Wolbachia was confirmed, eggs from both treatment groups were hatched and larvae and adult mosquitoes were reared in the absence of tetracycline for the remainder of the experimental procedures. Wolbachia strain identification via PCR in Aedes albopictus mosquitoes determined that these mosquitoes were superinfected with wAlbA and wAlbB strains, while Cx. pipiens mosquitoes carried the wPip strain. All qPCR screening assays included samples randomly picked from the W+ cohorts to serve as positive controls for DNA extraction and qPCR-based Wolbachia detection.
Gene expression analyses
Gene expression analyses was conducted on pools of 5 mosquitoes collected at 6d post-infection (PI). The time point was selected based on our previous assays with the mosquito Ae. aegypti and it corresponds to the late stages of infection [36]. RNA from whole body homogenates were extracted using TRizol (Invitrogen) following the manufacturer’s instructions. RNA concentration and purity were assessed via Nanodrop (Thermo Scientific). RNA samples were normalized to 1μg and then used in cDNA synthesis using the QuantiTec reverse transcription kit with DNA Wipeout (Qiagen). Gene expression analysis was conducted using the PowerUp SYBR green Master mix qPCR kit (Qiagen) and gene-specific primers (S1 Table) in a 10 μl reaction using one microliter of cDNA. Primers used in this study were those available in the literature or designed for this study based on orthology via VectorBase, using the structural annotation version AaloF1.2 for Ae. albopictus and CpipJ2.5 for a representative of the Cx. pipiens complex, Cx. quinquefasciatus. VectorBase uses the OrthoMCL algorithm for homolog predictions [46]. The resulting protein gene sequences from the OrthoMCL’s ortholog groups were used with Clustal Omega to create a phylogenetic tree of the alignment for Ae. aegypti, Ae. albopictus, Cx. quinquefasciatus and Anopheles gambiae gene targets (See Sup document 1). We employed the RT-qPCR cycling conditions recommended for the master mix and it consisted of holding at 95.0°C for 10min and 40 cycles of 15 s at 95.0°C and 1min at 60°C. Melt curve analysis was included at the end of each qPCR run. Each sample was assayed in duplicate (technical replicates) and the reproducibility of the results evaluated with at least 3 independent experiments (2 to 4 biological replicates per experiment) conducted with fresh batches of mosquitoes and fungal suspensions. To normalize cDNA samples, we evaluated the expression of Rps7 and Rps3 for Ae. albopictus and Cx. pipiens, respectively. The RT-qPCR assays were conducted on an Applied Biosystems QuantStudio 6 Flex Real-time PCR system (ThermoFisher Scientific). Transcript abundance was evaluated post-run using the ΔΔCt method [47] with data from three independent experiments pulled in the analysis.
Phenoloxidase assays
Phenoloxidase activity (PO) as conducted as previously reported [36]. Briefly, mosquitoes were collected at six days post-infection, pairs of two cold-anesthetized mosquitoes from each factorial treatment were placed in a 2 mL tube with 50 μL of chilled 1x PBS and a 2.4 mm bead. Samples were macerated in a TissueLyserII (QIAGEN) for 30s at 30Hz. The homogenized samples were immediately snap-frozen in liquid nitrogen to prevent enzyme catalyzation, thawed on ice and centrifuged at 10000 rpm for 5 min at 4C. Here, 35 μL of the supernatant were transferred to a 1.5 mL centrifuge tube, frozen in liquid nitrogen and stored at -80°C until ready for active PO assay. All reactions were prepared on ice except when noted. In duplication, 15 μL of samples were added to a flat-bottomed 96-well plate well that also contained 140 μL chilled distilled water and 20 μL cold PBS. Two wells were filled with an additional 15 μL and no sample to serve as a blank for the non-enzymatic production of dopaquinone. To each well was added 20 μL of L-Dopa solution (4 mg per mL H2O; 3,4 dihydroxy-L-phenylalanine) and read with a spectrophotometer (Multiskan GO, Thermal Scientific). Change in absorbance was measured at 490nm for 30 min at 30°C and measured every 15s. Enzymatic activity was calculated as the slope (Vmax) of the reaction curve during its linear phase. At least three independent experiments were conducted with 10 samples per treatment and per experiments employed.
Statistical analyses
For qPCR data and PO data, outliers were identified via GraphPad statistical software and removed from the analysis. A total of 25 out of 1407 data points were identified as outliers in Ae. albopictus and 45 out of 1393 data points in Cx. pipiens. A 2-way ANOVA was performed within gene targets for each species using a generalized linear model (PROC GLIMMIX, SAS 9.4) with a Gamma distribution of error and a log link function. The SAS ILINK option was used to express least squares means and confidence intervals on the original scale. Significant effects were further analyzed by pairwise comparisons of the gamma distributed estimates for the main effects of fungal treatment (F), with 3 levels (B. bassiana, B. brongniartii, uninfected control) Wolbachia treatment (W) with 2 levels (W+, W-) and their interaction (F*W) with a Tukey adjustment for multiple comparisons. Graphical representation of the data was done using Graph-Pad Prism 9 (GraphPad). Analyses for fungal effects on Wolbachia load were done using only the Wolbachia infected groups in a one-way ANOVA, with the same 3 levels (B. bassiana, B. brongniartii, uninfected control), using a generalized linear model (PROC GLIMMIX, SAS 9.4) with a Gamma distribution of error and a log link function. Pairwise comparisons among means also used a Tukey adjustment.
Results
Clearance of natural Wolbachia does not affect mosquito susceptibility to fungal entomopathogens
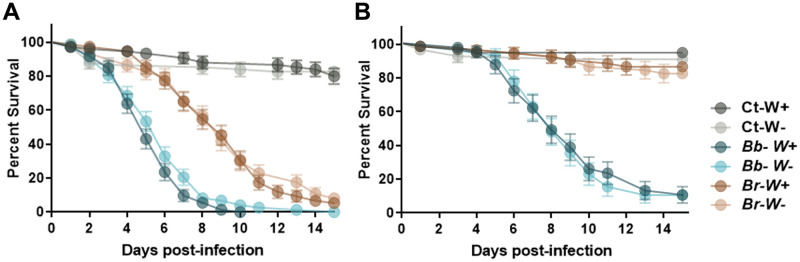
To evaluate whether the presence of Wolbachia affected the susceptibility of mosquitoes to fungal entomopathogens, Wolbachia-infected (W+) and Wolbachia-free (W-) mosquitoes maintained solely with sucrose solutions, were infected with either B. bassiana or B. brongniartii entomopathogenic fungi. Overall, mosquito survival post fungal infection differed significantly with each fungal strain and mosquito host. However, mosquito survival post-fungal infection did not differ between W+ and W- mosquitoes. This was observed in both Ae. albopictus infected with B. bassiana (log-rank Mantel-Cox test, χ2: 2.8, P = 0.0933) or B. brongniartii (log-rank Mantel-Cox test, χ2: 0.34, P = 0.5608), and in Cx. pipiens infected with either B. bassiana (log-rank Mantel-Cox test, χ2: 0.03, P = 0.8572) or B. brongniartii (log-rank Mantel-Cox test, χ2: 0.24, P = 0.6231) (Fig 1).
Fig 1. Survival curves of Wolbachia-infected (W+) and Wolbachia-free (W-).
Ae. albopictus (A) and Cx. pipiens (B) mosquitoes following challenge with either Beauveria bassiana (Bb) or Beauveria brongniartii (Br) fungal entomopathogens. Ct = control group exposed to soy oil carrier without fungal spores. Survival graphs represents five independent experimental replicates (total n = 75 individuals per treatment) for Ae. albopictus and four independent experimental replicates (total n = 40 individuals per treatment) for Cx. pipiens. Data was analyzed with Log-rank Mantel-Cox test.
Likewise, Probit analysis indicated no difference in LT50 values between W+ and W- mosquitoes when infected with either of the two entomopathogenic fungi. However, we observed a difference in susceptibility in these two mosquito species, with Ae. albopictus showing greater susceptibility to B. bassiana (LT50: 4.5 days; 95% CI: 3.6–5.5 days) and to B. brongniartii (LT50: 8.35 days; 95% CI: 4.8–11.9 days) than Cx pipiens. In fact, Cx. pipiens mosquitoes were less susceptible to B. bassiana spores (LT50: 7.9 days; 95%CI: 5.5–10.3 days) and highly resistant to B brongniartii infection. The LT50 for B. brongniartii-infected Cx. pipiens could not be determined due to mosquito survival in this cohort exceeding 50% at the end of the experiment.
We present our qPCR analyses in sets of genes with related functions. These sets are: Immune signaling genes (Rel 1, Rel 2, PGRP-LC, PGRP-S1); antimicrobial effector genes (CecA, DefC, LysE, Tep22, LysC); oxidative stress response genes (Duox, DuoxA); antioxidant defense genes (Catalase, CuZnSOD, GPX, OXR, GST); pro-phenoloxidase genes (PPO1-PPO9); and genes that indicate bacterial, fungal, and Wolbachia loads (16srRNA, 18srRNA, Wolbachia wsp, respectively). We also present a similar analysis of phenoloxidase activity level.
Expression of immune signaling pathways and antimicrobial effectors are more affected by fungal infection than Wolbachia infection status
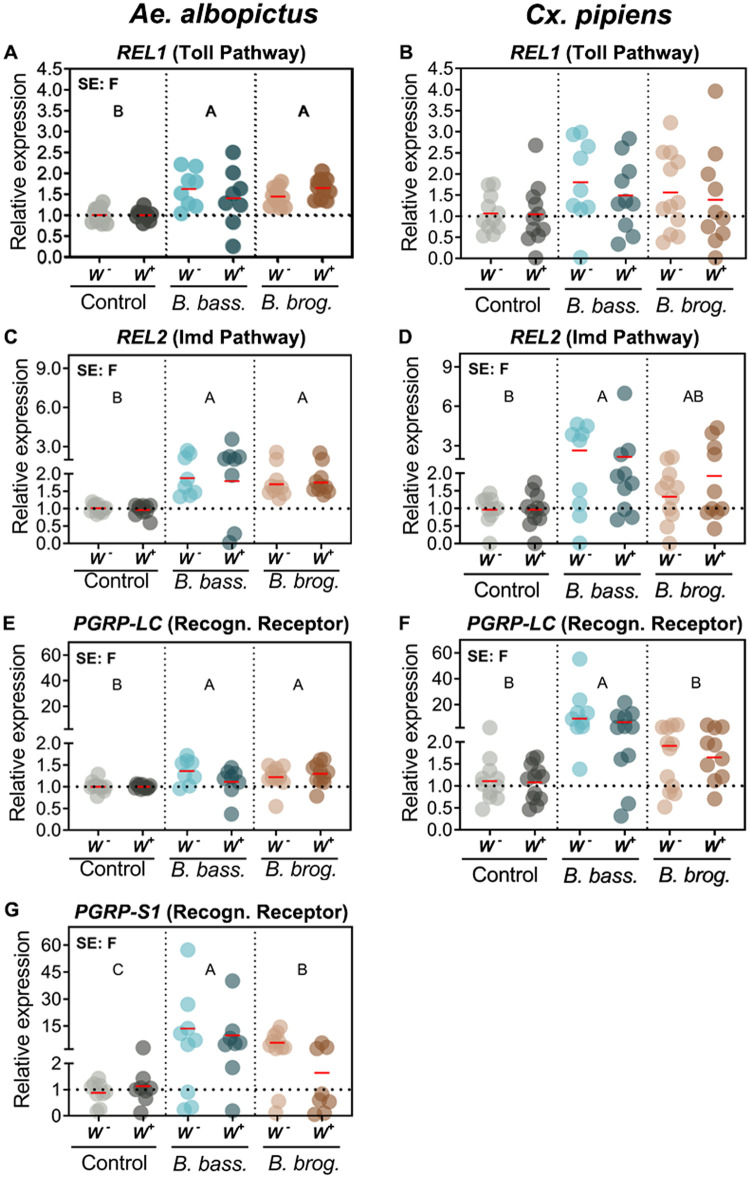
To further assess the interaction between Wolbachia infection status and fungal entomopathogenic infection, we evaluated the expression of key anti-fungal immune markers that have been shown to be important in the Ae. aegypti immune response to these same entomopathogenic fungi. Our analysis showed no significant interactive effect of fungal strain and Wolbachia presence, and no significant main effect of Wolbachia, on the immune signaling pathways of both Ae. albopictus and Cx. pipiens mosquitoes (Fig 2 and Table 1). However, there was a significant effect of fungal infection on pathogen recognition receptors PGRP-LC (p < 0.0013), PGRP-S1 (p < 0.0001), and transcription factors REL1 (Toll Pathway) (p < 0.0001) and REL2 (Imd pathway) (p < 0.0001) in the mosquito Ae. albopictus (Table 1). This significant increase in expression was observed independent of fungal strain, as we observed high significant induction when Ae. albopictus mosquitoes were infected with either B. bassiana or B. brongniartii compared to the uninfected controls (Fig 2).
Fig 2. Gene expression of mosquito immune signaling pathway components under the context of natural Wolbachia and fungal entomopathogenic infections.
Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. The red horizontal line indicates LS-means from eight to eleven biological replicates per treatment, originating from at least three independent experiments. Groups sharing the same letter are not significantly different at p<.05 based on differences of least-squares means. Uppercase letters refer to statistically significant fungal effects. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. See Table 1 for complete statistics from the Two-Way ANOVA.
Table 1. Interactive effects of 2-way ANOVA for microbial load, immune signaling pathways and AMPs (qPCR Type III Fixed effects).
Arrows indicate up or down-regulation of gene expression during fungal or Wolbachia infection.
| Target | Effect | Ae. albopictus | Cx. pipiens | |||||
|---|---|---|---|---|---|---|---|---|
| Num/Den DF | F Value | Pr>F | Num/Den DF | F Value | Pr>F | |||
| Imm. Sign. Pathways | PGRP-LC | F | 2/54 | ↑7.57 | 0.0013 | 2/57 | ↑45.38 | <0.0001 |
| W | 1/54 | 0.77 | 0.3831 | 1/57 | 1.21 | 0.2763 | ||
| F*W | 2/54 | 1.96 | 0.1509 | 2/57 | 0.38 | 0.6844 | ||
| PGRP-S1 | F | 2/47 | ↑23.56 | <0.0001 | ||||
| W | 1/47 | 2.31 | 0.1355 | |||||
| F*W | 2/47 | 2.31 | 0.1109 | |||||
| Rel1 | F | 2/54 | ↑18.72 | <0.0001 | 2/57 | 1.94 | 0.1525 | |
| W | 1/54 | 0.01 | 0.9078 | 1/57 | 0.31 | 0.5782 | ||
| F*W | 2/54 | 1.36 | 0.2662 | 2/57 | 0.07 | 0.936 | ||
| Rel2 | F | 2/51 | ↑12.33 | <0.0001 | 2/55 | ↑4.62 | 0.014 | |
| W | 1/51 | 0.03 | 0.8641 | 1/55 | 0.05 | 0.8214 | ||
| F*W | 2/51 | 0.04 | 0.9565 | 2/55 | 0.47 | 0.6293 | ||
| Antimicrobial Effectors | CecA | F | 2/54 | ↑36.64 | <0.0001 | 2/55 | ↑9.2 | 0.0004 |
| W | 1/54 | 2.47 | 0.1222 | 1/55 | ↓18.96 | <0.0001 | ||
| F*W | 2/54 | 0.55 | 0.5782 | 2/55 | 3.04 | 0.0557 | ||
| DefC | F | 2/52 | ↑55.37 | 0.0001 | 2/50 | ↑16.84 | <0.0001 | |
| W | 1/52 | 0.99 | 0.3248 | 1/50 | 0.66 | 0.4199 | ||
| F*W | 2/52 | 1.33 | 0.2735 | 2/50 | 4.68 | 0.0137 | ||
| Lys | F | 2/53 | ↑43.29 | <0.0001 | 2/55 | ↑5.15 | 0.0089 | |
| W | 1/53 | 0.23 | 0.6354 | 1/55 | 0.4 | 0.5275 | ||
| F*W | 2/53 | 0.38 | 0.6862 | 2/55 | 1.9 | 0.1588 | ||
| Tep22 | F | 2/52 | 0.59 | 0.5594 | 2/56 | ↑7.32 | 0.0015 | |
| W | 1/52 | ↑12.16 | 0.001 | 1/56 | ↓4.74 | 0.0337 | ||
| F*W | 2/52 | 3.73 | 0.0305 | 2/56 | 0.22 | 0.806 | ||
| Oxidative Stress | Duox | F | 2/53 | ↑4.21 | 0.02 | 2/59 | 0.03 | 0.973 |
| W | 1/53 | 2.59 | 0.1135 | 1/59 | 0.47 | 0.4955 | ||
| F*W | 2/53 | 2.49 | 0.0922 | 2/59 | 0.33 | 0.7234 | ||
| DuoxA | F | 2/55 | ↑3.39 | 0.0408 | ||||
| W | 1/55 | 1.31 | 0.2565 | |||||
| F*W | 2/55 | 0.05 | 0.9471 | |||||
| Antioxidant Defense | Catalase | F | 2/51 | 0.86 | 0.4283 | 2/56 | ↑6.56 | 0.0028 |
| W | 1/51 | ↓8.29 | 0.0058 | 1/56 | ↑15.46 | 0.0002 | ||
| F*W | 2/51 | 4.84 | 0.0119 | 2/56 | 3.82 | 0.0278 | ||
| GPX | F | 2/55 | ↑10.21 | 0.0002 | 2/55 | 0.28 | 0.758 | |
| W | 1/55 | 0.18 | 0.6705 | 1/55 | 0.5 | 0.4816 | ||
| F*W | 2/55 | 0.41 | 0.6662 | 2/55 | 1.47 | 0.2389 | ||
In contrast, our analysis of Cx. pipiens mosquitoes indicated a significant effect of fungal infection on PGRP-LC (p < 0.0001) and REL2 expression (p < 0.014); but not on REL1 (Table 1). This induction however was observed only when Cx. pipiens mosquitoes were infected with B. bassiana. Although B. brongniartii-infected Cx pipiens mosquitoes presented an increase in expression, it was not statistically significant (Fig 2). We did not assess Cx pipiens PGRP-S1, as repeated attempts designing a working primer set did not produce a unique PCR product and was not included in our qPCR assessment.
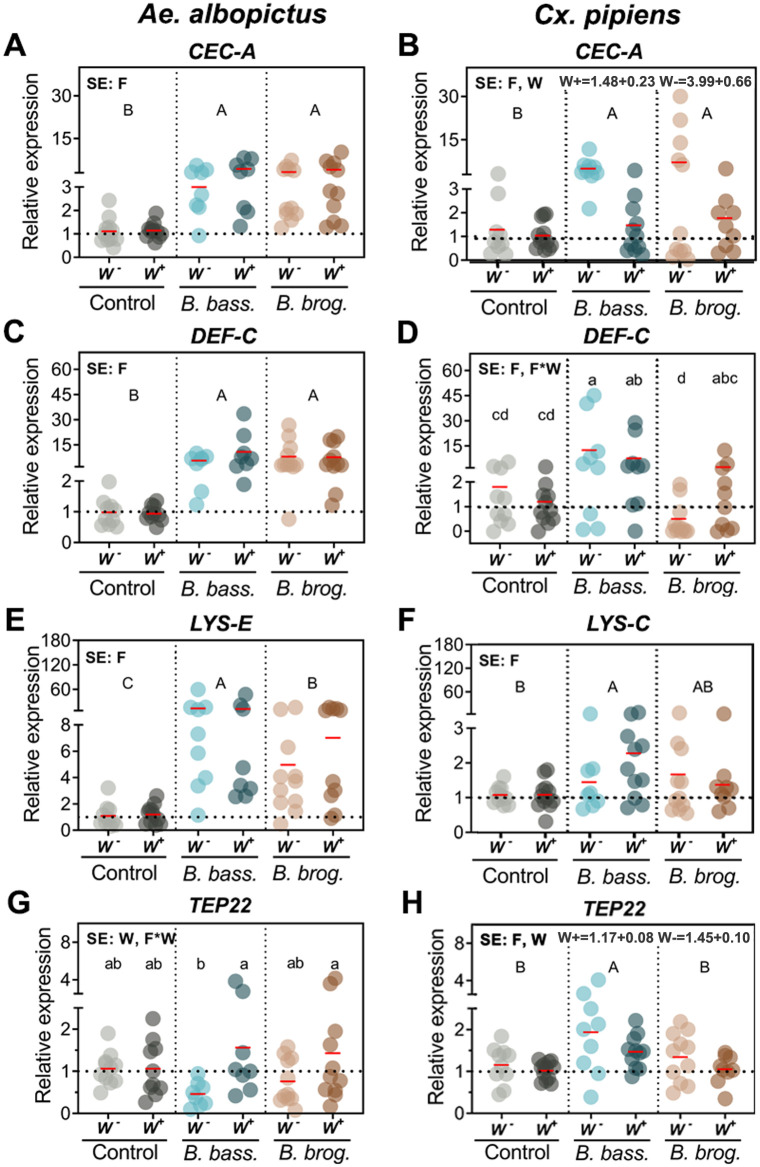
We further evaluated the gene expression of antimicrobial effectors whose orthologs in Ae. aegypti have been shown to be elicited upon fungal infection. We found significant interactive effects of fungal infection and Wolbachia only for TEP22 (p < 0.0305) for Ae. albopictus, and only for DEFC for Cx. pipiens (p < 0.0137) (Table 1). There were significant main effects of fungal infection on the expression of CECA (p < 0.0001), DEFC (p = 0.0001), LYSE (p < 0.0001) in Ae. albopictus and on CECA (p < 0.0004), DEFC (p < 0.0001), LYSC (p = 0.0089) and TEP22 (p = 0.0015) in Cx. pipiens (Table 1). Both entomopathogenic fungi, B. bassiana and B. brongniartii, significantly induced the expression of CECA in both mosquitoes, and Wolbachia presence yielded significantly lower CECA expression (p < 0.0001) in Cx. pipiens (Fig 3B).
Fig 3. Gene expression of anti-microbial effectors as a result of natural Wolbachia and fungal entomopathogenic infections.
Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. Lowercase letters indicate interactive effects (F*W), uppercase letters refer to fungal effects and any Wolbachia effect is represented by their mean and standard deviation on the upper right corner of the graph. The red horizontal line indicates LS-means from eight to eleven biological replicates per treatment, originating from at least three independent experiments. Groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. CecA, Cecropin A; Def-C, Defensin C; Lys-E, Lysozyme E; Lys-C, Lysozyme C; TEP22, Thioester-containing protein 22. See Table 1 for complete statistics from the Two-Way ANOVA.
Defensin expression had a profile similar to cecropin, with fungal infection inducing upregulation of DEFC in Ae. albopictus (p < 0.0001) and Cx pipiens (p < 0.0001) mosquitoes. While DEFC induction in Ae. albopictus was significantly upregulated during infection with either fungal entomopathogen (Fig 3C), in Cx. pipiens the interaction arose because both W- and W+ B. bassiana infected adults yielded mean expressions significantly greater than those for corresponding uninfected controls (Fig 3D), but both W- and W+ B. brongniartii infected adults yielded mean expressions not significantly greater than those for uninfected controls (Fig 3D). Further, W- adults had significantly greater DEFC expression when infected with B. bassiana than when infected B. brongniartii, but the difference between the two fungal species was not significant in W+ adults (Fig 3D).
The effect of fungal infection on lysozyme expression was highly significant but differed between mosquitoes and with fungal strain. In Ae. albopictus, LYSE expression was significantly higher (p < 0.0001) in B. bassiana and B. brongniartii-infected mosquitoes compared to the control groups. However, LYSE expression was significantly higher in B. bassiana-infected mosquitoes than in those infected with B. brongniartii (Fig 3E). In contrast, increased expression of LYSC in Cx. pipiens was only statistically significant for B. bassiana-infected mosquitoes (p = 0.0089) (Fig 3F). We also evaluated the expression of TEP22, whose ortholog in Ae. aegypti functions as a potent anti-fungal effector. For TEP22 in Ae. albopictus, mean expression was significantly greater for W+ than for W- adults when infected with B. bassiana, but this difference was not statistically significant when infected with B. brongniartii or when uninfected (Fig 3G). Differences in TEP22 expression between W- and W+ Ae. albopictus were similar in direction for both fungus infected groups (Fig 3G), with lower TEP22 expression in W- than in W+. Wolbachia infection affected Ae. albopictus and Cx. pipiens TEP22 expression differently, with TEP22 expression increasing in fungus infected W+ compared to W- Ae. albopictus, but decreasing significantly in W+ compared to W- Cx. pipiens regardless of infection (Compare Fig 3G and 3H).
Fungal infection alters the state of oxidative stress and induces the antioxidant defense in a Wolbachia and fungal strain dependent manner
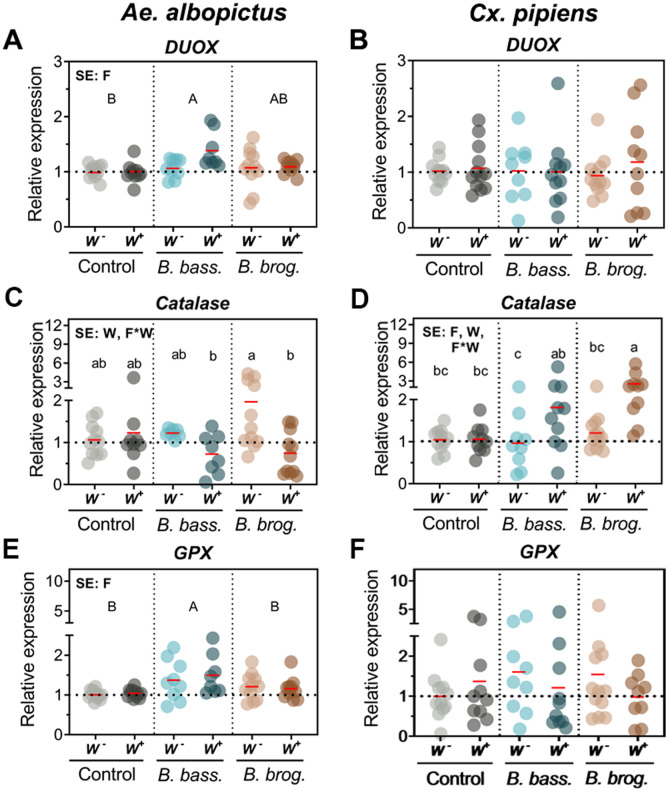
To understand the implication of the oxidative stress pathway and antioxidant defense system during a fungal entomopathogenic infection and Wolbachia presence, we evaluated the expression of several genes involved in oxidative stress and antioxidant defense. Our transcript abundance analysis indicated a significant effect of fungal infection on the expression of dual oxidase genes DUOX (p = 0.02) (Fig 4A) and DUOXA (p = 0.0408) (S1 Fig) in Ae. albopictus mosquitoes. This however was fungal strain dependent, with Ae. albopictus mosquitoes presenting a significant increase in DUOX expression only when challenged with B. bassiana but not when infected with B. brongniartii (Fig 4A). In comparison, the expression of a DUOX gene in Cx pipiens (DUOX) did not show any significant change (p = 0.973) in transcript abundance with either fungal or Wolbachia infection status. Further analysis of genes involved in the antioxidant defense indicated a significant effect of B. bassiana infection on GPX expression (p = 0.0002) only in Ae. albopictus mosquitoes (Fig 4E and Table 1).
Fig 4. Gene expression of oxidative stress and detoxification genes during natural Wolbachia and fungal entomopathogenic infections.
Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. Lowercase letters indicate interactive effects (F*W), uppercase letters refer to fungal effects and any Wolbachia effect is represented by their mean and standard deviation on the upper right corner of the graph. The red horizontal line indicates LS-means from eight to eleven biological replicates per treatment, originating from at least three independent experiments. Groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. See Table 1 for complete statistics from the Two-Way ANOVA.
We found significant interactions of fungal infection and Wolbachia for expression of the catalase gene in both mosquito species (Ae. albopictus, p = 0.0119) (Cx. pipiens, p = 0.0278) (Table 1). The direction and magnitude of catalase gene induction differed between these two mosquitoes. W- Ae. albopictus mosquitoes presented greater expression of catalase compared to their W+ counterpart when challenged with fungal infection and that difference was significant in pairwise comparisons for B. brongniartii, but not for B. bassiana infection (Fig 4C). In contrast, W- Cx. pipiens mosquitoes presented lesser expression of catalase compared to their W+ counterpart when infected with either of the fungal entomopathogens (Fig 4D). This differential response of W+ and W- mosquitoes was absent in the fungus-free controls (Fig 4C and 4D). We observed no change in expression for antioxidant defense genes CuZnSOD and OXR1 in Ae. albopictus and GST in Cx pipiens mosquitoes (S2 Fig and S2 Table).
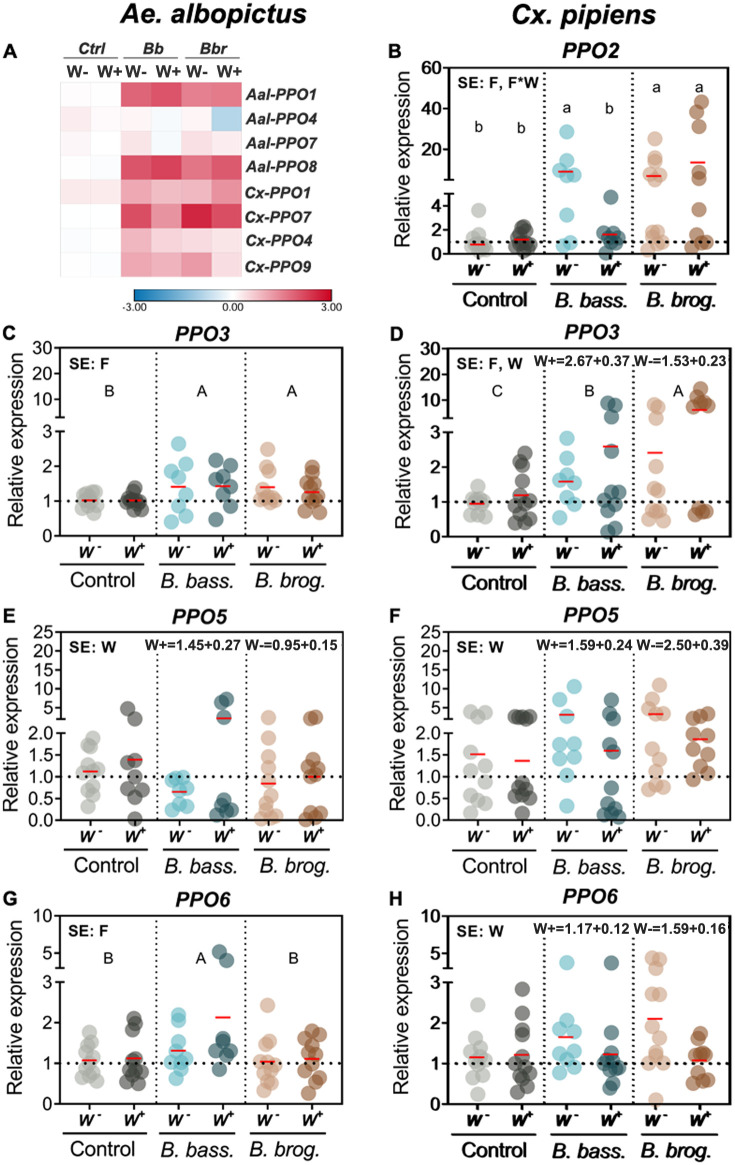
Pro-phenoloxidase genes are differentially elicited as a result of natural Wolbachia and fungal entomopathogenic infections
Given the important role that pro-phenoloxidase (PPO) cascade plays in the mosquito response to fungal infection, we assessed PPO genes in these two mosquitoes. Across all PPO genes, Cx. pipiens expression yielded consistently more effects of fungal infection, Wolbachia, and their interaction (Table 2). There was a significant interaction between Wolbachia and fungal infection only for PPO2 expression, and only in Cx. pipiens mosquitoes (p = 0.0005) (Table 2). Fungal infection significantly induced PPO2 expression (p < 0.0001) compared to control for W- Cx. pipiens infected with B. bassiana, and for both W+ and W- Cx. pipiens infected with B. brongniartii (Fig 5B). In contrast, W+ Cx. pipiens infected with B. bassiana did not show significant gene induction compared to control (Fig 5B).
Table 2. Interactive effects of 2-way ANOVA for PPO expression and phenoloxidase activity (qPCR Type III Fixed effects).
Arrows indicate up or down-regulation of gene expression during fungal or Wolbachia infection.
| Target | Effect | Ae. albopictus | Cx. pipiens | |||||
|---|---|---|---|---|---|---|---|---|
| Num/Den DF | F Value | Pr>F | Num/Den DF | F Value | Pr>F | |||
| Pro-phenoloxidase | PPO1 | F | 2/53 | ↑58.07 | <0.0001 | 2/59 | ↑3.86 | 0.0265 |
| W | 1/53 | 0.21 | 0.6523 | 1/59 | 0.07 | 0.7967 | ||
| F*W | 2/53 | 0.17 | 0.8444 | 2/59 | 0.61 | 0.5482 | ||
| PPO2 | F | 2/54 | ↑33.28 | <0.0001 | ||||
| W | - | 1/54 | 0.74 | 0.3949 | ||||
| F*W | 2/54 | 8.7 | 0.0005 | |||||
| PPO3 | F | 2/53 | ↑5.14 | 0.0091 | 2/56 | ↑13.85 | <0.0001 | |
| W | 1/53 | 0.11 | 0.7381 | 1/56 | 7.28 | 0.0092 | ||
| F*W | 2/53 | 0.17 | 0.8455 | 2/56 | 1.07 | 0.3484 | ||
| PPO4 | F | 2/54 | 1.25 | 0.2949 | 2/59 | ↑10.22 | 0.0002 | |
| W | 1/54 | 2.32 | 0.1335 | 1/59 | 0.97 | 0.3276 | ||
| F*W | 2/54 | 0.92 | 0.4033 | 2/59 | 0.96 | 0.3887 | ||
| PPO05 | F | 2/52 | 0.63 | 0.538 | 2/58 | 2.44 | 0.0961 | |
| W | 1/52 | ↑4.27 | 0.0437 | 1/58 | ↓4.23 | 0.0442 | ||
| F*W | 2/52 | 1.60 | 0.2118 | 2/58 | 0.66 | 0.5186 | ||
| PPO6 | F | 2/55 | ↑4.98 | 0.0103 | 2/59 | 1.08 | 0.3475 | |
| W | 1/55 | 2.75 | 0.1029 | 1/59 | ↓4.61 | 0.0359 | ||
| F*W | 2/55 | 1.26 | 0.2923 | 2/59 | 2.24 | 0.1155 | ||
| PPO7 | F | 2/53 | 1.39 | 0.2589 | 2/54 | ↑15.25 | <0.0001 | |
| W | 1/53 | 2.99 | 0.0898 | 1/54 | 1.32 | 0.2563 | ||
| F*W | 2/53 | 0.54 | 0.5845 | 2/54 | 0.41 | 0.6638 | ||
| PPO8 | F | 2/52 | ↑60.97 | <0.0001 | ||||
| W | 1/52 | 0.82 | 0.3693 | |||||
| F*W | 2/52 | 0.55 | 0.5815 | |||||
| PPO9 | F | 2/56 | ↑6.32 | 0.0034 | ||||
| W | 1/56 | 1.92 | 0.1712 | |||||
| F*W | 2/56 | 1 | 0.3726 | |||||
| Phenoloxidase | Phenoloxidase Activity | F | 2/18 | 1.62 | 0.2254 | 2/18 | ↑4.37 | 0.0284 |
| W | 1/18 | 0.25 | 0.6251 | 1/18 | 0.93 | 0.3475 | ||
| F*W | 2/18 | 1.94 | 0.1719 | 2/18 | 0.12 | 0.8843 | ||
Fig 5. Gene expression of pro-phenoloxidase genes during natural Wolbachia and fungal entomopathogenic infections.
(A) Heatmap of PPO genes affected only by fungal infection in both Ae. albopictus and Cx pipiens. (B-H) Varying effects of independent factors on the relative expression of PPO genes of both mosquito species. Heatmap represents the log2 LS-mean values, with red color indicating upregulation and blue color downregulation in comparison to the controls. Gene expression Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. Lowercase letters indicate interactive effects (F*W), uppercase letters refer to fungal effects and any Wolbachia effect is represented by their mean and standard deviation on the upper right corner of the graph. The red horizontal line indicates LS-means from eight to eleven biological replicates per treatment, originating from at least three independent experiments. Groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. PPO, pro-phenoloxidase. See Table 2 for complete statistics from the Two-Way ANOVA.
The main effect of fungal infection differentially affected the expression of PPO genes in these two mosquitoes. The fungal effect on the expression was significant for four (PPO1, PPO3, PPO6, PPO8) out of seven PPO genes in Ae. albopictus, and six (PPO1, PPO2, PPO5, PPO7, PPO4 and PPO9) out of eight PPO genes in Cx pipiens (Table 2). The greatest increase in PPO transcript expression (>2.1-fold relative to controls) due to fungal infection was observed only for two PPO genes (PPO1 and PPO8) in Ae. albopictus. In contrast, five PPO genes attained this level of transcript expression (PPO1, PPO2, PPO3, PPO7 and PPO9) in Cx. pipiens (Fig 5). In general, the magnitude and direction of gene expression was similar in both mosquitoes when infected with either B. bassiana or B. brongniartii (Fig 5 and S1 Fig). A few of the exceptions were PPO1, PPO6, and PPO7. For instance, the expression of PPO1 in Ae. albopictus was significantly higher (p < 0.0001) than controls, irrespective of fungal strain (S1 Fig), while in Cx. pipiens, only those mosquitoes infected with B. brongniartii were significantly higher than controls. Furthermore, while PPO6 expression was significantly enhanced relative to control only in B. bassiana-infected Ae. albopictus (p = 0.013), there was no effect of fungal infection in Cx. pipiens (p = 0.3475) (Fig 5G–5H).
The main effect of Wolbachia infection also differentially affected the expression of PPO genes in these two mosquitoes. The Wolbachia effect on expression was significant in one PPO gene (PPO5) in Ae. albopictus (p = 0.0437) and three PPO genes (PPO3, PPO5 and PPO6) in Cx. pipiens (p = 0.0092, p = 0.0442, p = 0.0359 respectively) (Table 2). Interestingly, the direction of the Wolbachia effect on PPO5 gene expression differed in Ae. albopictus and Cx. pipiens. Here, our bioassays show that while there was a slight but significant increase in PPO5 expression in W+ Ae. albopictus compared to their W- counterparts, a much greater significant decrease in PPO5 expression occurred in W+ compared to W- in Cx. pipiens (Fig 5E and 5F).
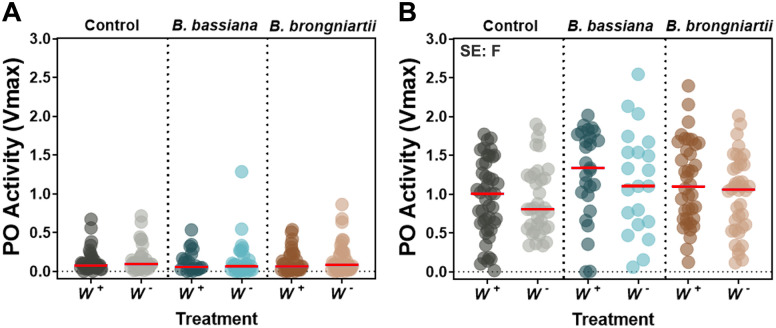
Fungal entomopathogenic infection increases phenoloxidase activity that is dependent on fungal strain and varies with mosquito species
To corroborate the PPO gene expression profile observed via qPCR, we also evaluated the whole body phenoloxidase (PO) enzymatic activity in these two mosquitoes under the context of Wolbachia and fungal infections. We found no effects of fungal infection, Wolbachia, or interaction on PO activity levels in Ae. albopictus, but a significant, though small, effect of only fungal infection in Cx. pipiens (p = 0.0284) (Table 2). Follow up tests yielded no significant pairwise differences among fungus treatments (Fig 6B). A notable observation was the low levels of PO activity in Ae. albopictus mosquitoes compared to Cx. pipiens (Fig 6). Repeated measures with different batches of Ae. albopictus mosquitoes produced the same results, indicating low basal levels of PO activity in Ae. albopictus compared to Cx. pipiens using this method (Fig 6 and S3 Fig).
Fig 6. Impact of natural Wolbachia and fungal entomopathogenic infections on phenoloxidase activity at 6d post-infection.
PO activity in Ae. albopictus (A) and Cx. pipiens (B) mosquitoes. Data represents 21 to 45 samples per treatment originating from three independent experiments. Each dot represents a pool of 2 mosquitoes (Ae. albopictus) or 1 mosquito (Cx. pipiens) and the horizontal bar indicates the median level of PO activity. Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. See Table 2 for complete statistics from the Two-Way ANOVA.
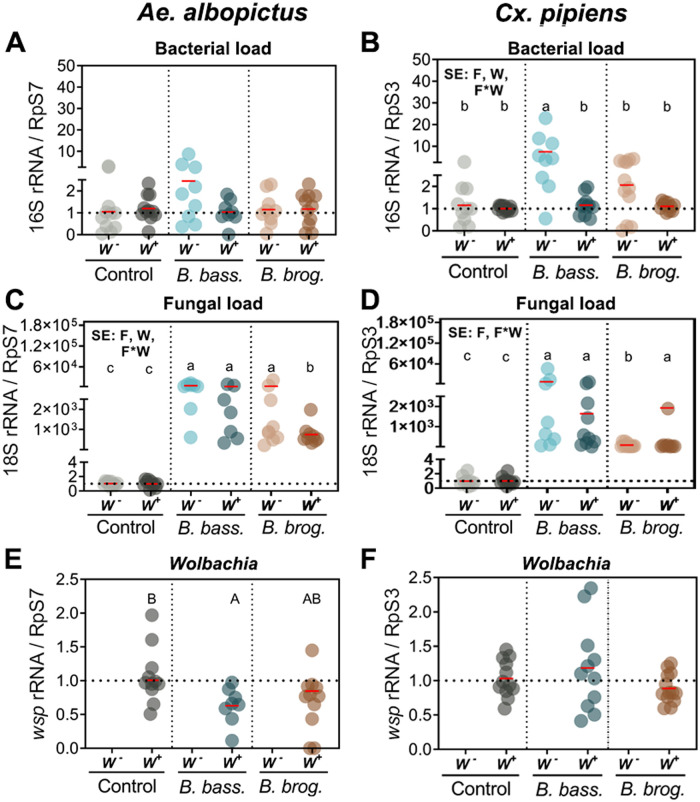
Fungal entomopathogenic infection alters the mosquito microbial load
Prior studies have shown that fungal infection leads to an increase in the microbial load of infected mosquitoes [36,43]. Hence, to evaluate any potential effects of Wolbachia presence/absence and fungal infection on the proliferation of mosquito bacteria and fungi we conducted a relative quantification of these two microbes via qPCR analysis of 16s rRNA (bacteria) and 18s rRNA (fungi). The analysis revealed no significant main effects or interactions on bacterial load (16s rRNA) in Ae. albopictus (p = 0.3944) but significant effects of Wolbachia-fungal infection interaction on bacterial load in Cx. pipiens (p < 0.0001) (Table 3). Bacterial load with B. bassiana infection in W- Cx. pipiens was both significantly greater than corresponding control, and greater than in B. bassiana-infected W+ Cx. pipiens (Fig 7B). Bacterial load with B. bassiana infection in W+ Cx. pipiens did not differ from corresponding control (Fig 7B). In contrast, infection with B. brongniartii led to a slight, but not significant, increase in bacterial load of W- Cx. pipiens, compared to either corresponding control or to W+ Cx. pipiens (Fig 7B).
Table 3. Interactive effects of 2-way factor ANOVA for microbial load (qPCR Type III Fixed effects).
Arrows indicate up or down-regulation of gene expression during fungal or Wolbachia infection.
| Target | Effect | Ae. albopictus | Cx. pipiens | |||||
|---|---|---|---|---|---|---|---|---|
| Num/Den DF | F Value | Pr>F | Num/Den DF | F Value | Pr>F | |||
| Microbial Load | 16S (Bacteria) | F | 2/53 | 0.95 | 0.3944 | 2/58 | ↓11.52 | <0.0001 |
| W | 1/53 | 1.14 | 0.2907 | 1/58 | 25.98 | <0.0001 | ||
| F*W | 2/53 | 1.88 | 0.1632 | 2/58 | 8.81 | 0.0005 | ||
| 18S (Fungi) | F | 2/47 | ↑587.65 | <0.0001 | 2/52 | ↑197.03 | <0.0001 | |
| W | 1/47 | ↓11.52 | 0.0014 | 1/52 | 2.05 | 0.1581 | ||
| F*W | 2/47 | 4.63 | 0.0146 | 2/52 | 15.21 | <0.0001 | ||
| Wolbachia (wsp) | F | 2/25 | 4.06 | 0.0298 | 2/33 | 2.05 | 0.1447 | |
| W | - | - | - | - | - | - | ||
| F*W | - | - | - | - | - | - | ||
Fig 7. Microbial load following infections with entomopathogenic fungi in mosquitoes with cleared or natural Wolbachia infections.
Bacterial, fungal and Wolbachia loads were measured via the relative quantification of bacterial 16s rRNA (A-B), Fungal 18s rRNA (C-D) and Wolbachia wsp (E-F) respectively. Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. Lowercase letters indicate interactive effects (F*W), uppercase letters refer to fungal effects and any Wolbachia effect is represented by their mean and standard deviation on the upper right corner of the graph. The red horizontal line indicates LS-means from eight to eleven biological replicates per treatment, originating from at least three independent experiments. Groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. See Table 3 for complete statistics from the Two-Way ANOVA. For Wolbachia relative abundance means associated with the same letter are not significantly different at p<0.05.
Fungal loads in both mosquitoes were significantly affected by the interaction of Wolbachia and fungal infection (Table 3). In Ae. albopictus, significantly higher fungal load was observed in all combinations of fungal infection–Wolbachia compared to control (p = 0.0146). Here, in B. bassiana-infected Ae. albopictus there was no difference between W+ and W- groups, whereas in B. brongniartii-infected Ae. albopictus the W+ group had a lower fungal load than did the W- group (Fig 7C). The W+ B. brongniartii-infected group also had a significantly lower fungal load than did the W+ B. bassiana-infected group (Fig 7C). The fungal infection-Wolbachia interaction effect was also significant in Cx. pipiens mosquitoes (p < 0.0001), but the direction of the difference in fungal load for W+ vs. W- groups was opposite of that observed in Ae. albopictus mosquitoes (compare Fig 7C and 7D).
Finally, we also evaluated the effect of fungal infection on Wolbachia relative abundance by measuring the Wolbachia wsp gene in these two mosquito populations. Our results indicate a significant effect of fungal infection on Wolbachia density for Ae. albopictus (One-way ANOVA, F2,25 = 4.03, p = 0.0304), but not for Cx. pipiens (One-way ANOVA, F2,33 = 2.03, p = 0.1469; Fig 7F). For Ae. albopictus, there was a slight but significant drop in Wolbachia density in mosquitoes infected with B. bassiana compared to control, but not with B. brongniartii (Fig 7E). The difference between Wolbachia density for Ae. albopictus infected with the two fungi was also not significant (Fig 7E).
Discussion
The outcome of entomopathogenic infections is often defined by pathogen virulence, and host genotype. However, other host-associated microbiota can have significant impact on host-pathogen interactions [48–50]. This can be particularly true with naturally occurring symbionts, such as Wolbachia, which have evolved to be intimately associated with their insect hosts [51]. Nevertheless, such multipartite interactions during an entomopathogenic infection process are not entirely understood. Thus, in this study, we evaluated the responses of two important mosquito species (Ae. albopictus and Cx. pipiens) when challenged with different fungal entomopathogens, with or without native Wolbachia infections.
First, we assessed whether natural Wolbachia infections could affect mosquito susceptibility to fungal entomopathogens. Our studies comparing Wolbachia-infected (W+) and Wolbachia-free (W-) mosquitoes indicate that clearance of Wolbachia does not affect mosquito susceptibility to fungal entomopathogens in Ae. albopictus and Cx. pipiens mosquitoes. However, it is plausible that different combinations of mosquitoes, Wolbachia and fungal entomopathogen genotypes could generate different outcomes. For instance, spider mite (Tetranychus urticae) populations naturally infected with Wolbachia presented variable effects of fungal infection when challenged with Metarhizium brunneum and B. bassiana. While neither of these fungal entomopathogens had any effect on one spider mite population, Wolbachia presence led to an increase in mortality when a different mite population was challenged with B. bassiana [48]. In addition, a study conducted with the fruit fly Drosophila melanogaster reported a protective effect of Wolbachia against infection with the entomopathogenic fungus Beauveria bassiana [30].
With regards to mosquito susceptibility to fungal entomopathogens, our bioassays suggest that while Ae. albopictus is susceptible to the strain of B. brongniartii we used in this assay, Cx. pipiens mosquitoes appear to be resistant to this fungal entomopathogen, independent of Wolbachia infection status.
Next, we evaluated the anti-fungal response repertoire of Ae. albopictus and C. pipiens to these two fungal entomopathogens by assessing key immune response markers. These results appear largely similar to that observed in Ae. aegypti [36,52,53], with fungal entomopathogenic infections presenting itself as a strong independent factor, engaging upstream pathogen recognition receptors such as PGRP-LC and PGRP-S1 and leading to the induction of canonical transcription factors REL1 and REL2, from the Toll and Imd pathway respectively. Previous studies have demonstrated the importance of these two immune pathways in the mosquito defense against entomopathogenic fungi [53–55]. Thus, except for REL1 in Cx. pipiens mosquitoes, our results suggest that similar immune signaling pathways are governing the main anti-fungal responses in these two mosquitoes.
Anti-fungal effectors, including antimicrobial peptides and thioester proteins, have been shown to increase in expression in response to entomopathogenic fungal infection [36,53,56]. Our study evaluating orthologs of three Ae. aegypti antimicrobial peptides (CECA, DEFC and LYS-C), indicates that distinct patterns of expression are occurring in Ae. albopictus and Cx. pipiens during fungal and Wolbachia infections. For instance, while fungal strain was the only factor significantly increasing CECA expression in Ae. albopictus, CECA expression in Cx. pipiens was significantly affected by both fungal and Wolbachia infections. Our data further suggests that Wolbachia is repressing the expression of CECA in Cx. pipiens during an entomopathogenic fungal infection given the significantly higher level of CECA expression in W- compared to its W+ counterpart. In a similar pattern, the expression of defensin appeared to be induced by fungal infection in both mosquito species but in Cx. pipiens there is a strong interactive effect of fungi and Wolbachia affecting DEFC expression. This was observed between W- and W+ Cx pipiens mosquitoes, with W+ cohorts exhibiting higher DEFC induction. Interestingly, while B. bassiana induced a higher DEFC expression in Wolbachia-free Cx. pipiens mosquitoes, the opposite was true in W- Cx. pipiens mosquitoes infected with B. brongniartii. This might suggest that while DEFC is important in the defense against B. bassiana, it is also employed in the interaction between the Cx. pipiens mosquito and Wolbachia. Alternatively, it might indicate that B. brongniartii can more efficiently suppress DEFC in Wolbachia-free Cx. pipiens. However, the lack of any detrimental effect of B. brongniartii on the survival of Cx. pipiens mosquitoes does not lend much support to this possibility. Nevertheless, these diverging patterns of AMP expression might indicate that their elicitation and function is different from its Ae. aegypti ortholog.
While lysozyme is induced by fungal infection in similar patterns in both mosquitoes, our results indicate that infections by B. bassiana elicit stronger responses than B. brongniartii. This phenotype is most likely reflective of the higher replicative nature of B. bassiana blastospores inside the mosquito during the infection stage, as demonstrated by our fungal load analysis and is comparable to what we observed in Ae. aegypti-entomopathogenic fungi interactions.
TEP22 is another important anti-fungal effector in Ae. aegypti, one that is elicited independent of the fungal entomopathogenic strain [36,56]. However, our assays show a divergence from this phenotype, with TEP22 expression in Ae. albopictus not affected by fungal infection alone but rather, its induction appears to be regulated by Wolbachia presence and dependent on the type of infecting fungal strain. In contrast, TEP22 expression in Cx pipiens is significantly induced with fungal infection, resembling partly what is observed in Ae. aegypti [36,56]. This most likely indicates that in Cx. pipiens mosquitoes TEP22 also functions as an integral part of the anti-fungal repertoire. The slight but significant increase in Cx. pipiens TEP22 expression when Wolbachia is absent, compared to present, could indicate that Wolbachia is also tightly, and negatively, regulating this important mosquito effector during an infection process, independent of the strain of infecting fungi.
Elicitation of the oxidative pathway and the corresponding antioxidant defense system are crucial components of the mosquito defense against microbial infections [57,58]. Previous studies with Ae. aegypti have found that fungal infections modulate the state of oxidative stress in the infected mosquito; one that is in turn dependent on the strain of infecting fungi [36]. Interestingly, our study indicates a slight but significant induction of the ROS-generating enzyme DUOX gene in Ae. albopictus but not in Cx. pipiens mosquitoes and only with infections with B. bassiana. This infection-induced ROS production by DUOX is likely reflective of the more virulent characteristics of B. bassiana, as observed by the higher mosquito mortality associated with B. bassiana than with B. brongniartii infection.
To prevent the overstimulation of the oxidative pathway and overgeneration of reactive oxygen species, a set of detoxifying enzymes are set in place to regulate this process and avoid cellular damage [58–60]. Our gene expression analysis of antioxidant defense genes indicate that the catalase gene is a critical component in the mosquito responses to fungal infections. However, the responses are drastically different in Ae. albopictus and Cx pipiens mosquitoes. The interactive effect of fungi and Wolbachia infection in Ae albopictus mosquitoes appear to suggest that while fungal infection by itself does not affect catalase elicitation, Wolbachia could be dampening catalase induction only under infections with a less pathogenic fungi such as B. brongniartii, which does not appear to induce DUOX. Alternatively, B. brongniartii could be eliciting other ROS-generating enzymes not evaluated in our study, which in turn might be inducing catalase expression when the mosquito is free from Wolbachia regulation in W- Ae. albopictus mosquitoes. In contrast, Cx pipiens display a diverging phenotype, with significant catalase elicitation only under the presence of Wolbachia and with stronger induction during infections with the less virulent B. brongniartii entomopathogenic fungi. Whether the high catalase induction observed in Cx pipiens mosquitoes is linked to the mosquito resistant phenotype against B. brongniartii infection remains to be elucidated; but our results indicate that Wolbachia is playing a dynamic role in the mosquito antioxidant responses to infections by fungal entomopathogens. In this context, while Wolbachia interactions with oxidative stress have been documented in transfected hosts, our data suggests that native Wolbachia might be involved in maintaining host redox homeostasis during a pathogenic infection process, as previously hypothesized [60].
The prophenoloxidase cascade is another important anti-fungal response mechanism that has been observed in several insects [37,53,61]. In Ae. aegypti, its expression is affected by fungal pathogenic strain and by the progression of fungal infection, with higher PPO gene expression observed at the later stages of infection [36,37]. In our assays, the absence of any interactive effect of Wolbachia presence and fungal entomopathogenic infection in Ae. albopictus mosquitoes demonstrates that these genes are tightly linked to either the anti-fungal response or Wolbachia symbiotic homeostasis. In contrast, our bioassays with Cx pipiens indicated a highly significant interactive effect of fungi and Wolbachia infection on the expression of one PPO gene (PPO2). In this interaction, while fungal infection elicited PPO2 expression, infections by the most lethal fungi B. bassiana failed to induce PPO2 when Wolbachia was present. Given the absence of this phenotype with B. brongniartii, our data might suggest that under the physiological conditions provided by the more virulent entomopathogenic fungus, Wolbachia is able to limit the action of a potent PPO2 and avoid damage to its host cell. Whether the same phenotype occurs with an Ae. albopictus PPO gene that we did not test remains to be seen, but our efforts to locate a PPO2 ortholog in Ae. albopictus were not successful.
Furthermore, the PPO cascade gene members in these two mosquito species show distinct expression profiles, potentially indicating that they are playing diverging roles in the response to fungal infection and Wolbachia homeostasis. For instance, while two PPO genes (PPO1 and PPO8) show the highest degree of gene expression in response to fungal infection in Ae. albopictus, five PPO genes showed higher transcript abundance in Cx. pipiens (PPO1, PPO2, PPO5, PPO7 and PPO9). Whether these genes are playing the same role in these two mosquitoes remains to be elucidated, but their expression profile might explain why Cx. pipiens is less susceptible to B. bassiana and especially resistant to infection by the fungal entomopathogen B. brongniartii. Furthermore, our assays conducted to corroborate our PO gene expression profiles, partly support this finding, with an increase in PO activity in Cx. pipiens mosquitoes infected with B. bassiana and no increase in PO activity levels with B. brongniartii. These results differ from the significant drop of PO activity that has been observed in Ae. aegypti when challenged with a range of fungal entomopathogenic strains [36,37]. While pre-infection levels of PO activity in Cx. pipiens could potentially determine this phenotype, our analysis on the basal levels of PO activity shows similar profiles between Ae. aegypti and Cx pipiens, indicating that the observable values are a true representation of their diverging responses to the same entomopathogenic fungal strains. Alternatively, it could also mean that Cx pipiens are much more resistant to the potential immune suppressive activity of these entomopathogenic fungi.
While our PO activity analysis in Ae. albopictus was inconclusive, our transcript abundance analysis indicates a dynamic gene expression for some PO cascade gene members. Repeated measures with different batches of Ae. albopictus mosquitoes produced the same results, potentially suggesting that Ae. albopictus maintains a low basal PO activity level, one that is maintained independent of fungal infection. Alternatively, our failure to successfully measure PO enzymatic activity in Ae. albopictus, could indicate an Ae. albopictus-derived inhibitor affecting our methodology rather than an absence of PO activity in this mosquito.
Our study also shows a potential Wolbachia interaction with its native host at the PPO cascade, given the Wolbachia modulation of two PPO genes in each mosquito species. This might suggest that some of these PO genes are involved in Wolbachia maintenance. In a study that included Drosophila melanogaster, D. simulans and Ae. aegypti, Thomas et al. [62] demonstrated an increase in melanization in all three dipterans infected with Wolbachia wMelPop; indicating an interaction between this symbiont and the insect melanization cascade. Further studies evaluating the role that the prophenoloxidase cascade plays in the maintenance of natural Wolbachia infections could add to our understanding of the tightly woven interaction between this endosymbiotic microbe and its mosquito host. Although, some studies have found no impact of Wolbachia on insect immunity [63,64], these studies did not evaluate the effects of Wolbachia under the context of an active coinfection with a microbial pathogen. For instance, Blagrove et al [65] showed no significant immune regulation when an Ae. albopictus Wolbachia-free line was transiently infected with wMelPop or wAlbB strains, or with heat-killed Escherichia coli. Blagrove et al [65] suggest that the absence of a robust immune induction during Wolbachia transinfection might be due to Ae. albopictus immunotolerance to Wolbachia. In turn, the lack of any significant immune induction with E. coli might be due to the nature of this microbe (heat-killed) not providing the same level of immune challenge of an actively replicating microbe. Thus, it is plausible that such impacts on immunity are much more apparent under stress (i.e., another infection) as has been previously suggested [60,66].
Finally, we evaluated the effects of entomopathogenic fungal infection and Wolbachia on components of the microbial community of these mosquitoes, given that prior studies have shown significant alterations on bacterial load during fungal entomopathogenic infections [36,43]. Our analysis revealed a significant interactive effect of Wolbachia presence and fungal entomopathogenic strain on bacterial load in Cx. pipiens mosquitoes but its absence in Ae. albopictus. This contrasting interactive effect on bacterial load might reflect the specific mosquito immune responses mounted against the different fungal strains and the Wolbachia strain-specific interaction with its host. Our data suggests that these responses might be driven by AMPs, especially by CECA and DEFC, given the significant effect of Wolbachia on CECA expression and the interactive effect of fungi-Wolbachia on DEFC expression observed in Cx. pipiens mosquitoes. In addition, it is possible that other antimicrobial peptides or genes governing gut microbiota homeostasis in mosquitoes are also being affected by this interaction. A plausible alternative explanation could be that the differences in bacterial load are due to the removal of native bacteria following tetracycline treatment of the parent pool. Given that this study only assessed bacterial load and not bacterial diversity, we are unable to determine with certainty if this is the case. However, other than Wolbachia, there is no other bacterium that is known to be transmitted vertically in Ae. albopictus and Cx. pipiens mosquitoes. The symbiotic bacterium Asaia spp. has been shown to infect different tissues and be present on egg surfaces of other mosquito species that do not harbor Wolbachia (Anopheles spp, and Aedes aegypti) but has not been found on eggs of Ae. albopictus or Cx. pipiens [67]. Furthermore, as part of the transition from larvae to adult, and in a process that involves microbiota encapsulation and excretion in the meconium, there is almost a complete removal of midgut bacteria in newly emerged adults [68]. Thus, it is not surprising that most of the mosquito core gut microbiota are acquired from the environment (larval habitat or from a sugar/plant source as adults) [69–71].
Our studies also show diverging results when we measured the total fungal load in these mosquitoes, potentially indicating that the infection-derived responses and fungal-Wolbachia interactions were having different effects on these two mosquito species. However, these different phenotypes were only observed under the context of B. brongniartii infections and suggests that B. bassiana proliferation are not affected by the presence of Wolbachia in any of the two mosquito species. Our study also indicates that entomopathogenic fungal infection has a detrimental effect on Wolbachia density, one that is dependent on fungal strain and mosquito host. This drop in Wolbachia loads in B. bassiana-infected Ae. albopictus mosquitoes might reflect the higher toxicity of this fungus and/or that many more tissues are compromised in this mosquito during B. bassiana infections. This might be supported by our data if we consider the earlier mortality observed in Ae. albopictus compared to Cx. pipiens during infections with B. bassiana.
In summary, our study shows complex interactions involving entomopathogenic fungal infections under the context of native Wolbachia infections. While some of the anti-fungal host responses from Ae. albopictus and Cx. pipiens are similar, there are distinct differences with regards to the direction and magnitude of expression observed post-fungal infection. This was true for gene members of important mosquito immune functions such as canonical signaling pathways, AMPs, oxidative/detoxification genes, and the PO cascade, known critical components of the mosquito’s anti-fungal repertoire. One potential limitation of our study is that it did not assess any potential genetic variation that might exists between Wolbachia-infected and its Wolbachia-free counterparts. It is plausible that slight genetic changes (i.e via genetic drift) may have occurred during the tetracycline treatment that could affect the interpretation of our results. Although, the Wolbachia-fungi interactive effects we observed does not appear to impact mosquito survival to entomopathogenic infections, they might influence other important vector biology parameters such as vector competence/capacity and mosquito reproduction. To our knowledge this is the first study to evaluate fungal entomopathogenic infections under the context of a native mosquito symbiont. Given the inclusion of Wolbachia in alternative methods of mosquito and mosquito-borne pathogen control, this study provides a snapshot of the mosquito susceptibility and immune responses when challenged with fungal entomopathogens and under the context of native Wolbachia infections.
Supporting information
(XLSX)
(XLSX)
Trees were built in VectorBase with the resulting protein gene sequences from their respective OrthoMCL’s ortholog groups.
(DOCX)
Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. The red horizontal line indicates LS-means. Uppercase letters refer to fungal effects and groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. See Table 3 for complete statistics from the Two-Way ANOVA.
(TIF)
Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. The red horizontal line indicates LS-means. Uppercase letters refer to fungal effects and groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. See S2 Table for complete statistics from the Two-Way ANOVA.
(TIF)
Data analyzed via single-factor ANOVA using PROC GLIMMIX with a gamma distribution in SAS. Species mean Vmax rates sharing the same letter are not significantly different at p<.05 based on differences of least-squares means.
(TIF)
Acknowledgments
We would like to thank Marie Claude Bon and the European Biological Control Laboratory (EBCL) for providing the fungal strains used in this study.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. The mention of firm names or trade products does not imply that they are endorsed or recommended by the USDA over other firms or similar products not mentioned. USDA is an equal opportunity provider and employer.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This work was supported in part by the U.S. Department of Agriculture, Agricultural Research Service Project Number 5010-22410-020-00D to JLR. SAJ and GDO were supported by National Institutes of Health grant 1R15AI124005-01 to SAJ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barzon L. Ongoing and emerging arbovirus threats in Europe. Journal of Clinical Virology. 2018;107:38–47. doi: 10.1016/j.jcv.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Murray KO. Dengue, West Nile virus, chikungunya, Zika—and now Mayaro? PLOS Neglected Tropical Diseases. 2017;11(8):e0005462. doi: 10.1371/journal.pntd.0005462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. Chikungunya virus: evolution and genetic determinants of emergence. Current Opinion in Virology. 2011;1(4):310–7. doi: 10.1016/j.coviro.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeller H, Van Bortel W, Sudre B. Chikungunya: Its History in Africa and Asia and Its Spread to New Regions in 2013–2014. The Journal of Infectious Diseases. 2016;214(suppl_5):S436–S40. doi: 10.1093/infdis/jiw391 [DOI] [PubMed] [Google Scholar]
- 5.Achee NL, Grieco JP, Vatandoost H, Seixas G, Pinto J, Ching-Ng L, et al. Alternative strategies for mosquito-borne arbovirus control. PLOS Neglected Tropical Diseases. 2019;13(1):e0006822. doi: 10.1371/journal.pntd.0006822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annual Review of Entomology. 2015;60(1):537–59. doi: 10.1146/annurev-ento-010814-020828 . [DOI] [PubMed] [Google Scholar]
- 7.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLOS Neglected Tropical Diseases. 2017;11(7):e0005625. doi: 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weetman D, Djogbenou LS, Lucas E. Copy number variation (CNV) and insecticide resistance in mosquitoes: evolving knowledge or an evolving problem? Current Opinion in Insect Science. 2018;27:82–8. doi: 10.1016/j.cois.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya and Plasmodium. Cell. 2009;139. doi: 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 10.Becker N, Lüthy P. Chapter 26—Mosquito Control With Entomopathogenic Bacteria in Europe A2—Lacey, Lawrence A. Microbial Control of Insect and Mite Pests: Academic Press; 2016. p. 379–92.
- 11.Blanford S, Chan BHK, Jenkins N, Sim D, Turner RJ, Read AF, et al. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308. doi: 10.1126/science.1108423 [DOI] [PubMed] [Google Scholar]
- 12.Bukhari T, Takken W, Koenraadt CJ. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit Vectors. 2011;4:23. doi: 10.1186/1756-3305-4-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. New England Journal of Medicine. 2021;384(23):2177–86. doi: 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffries CL, Walker T. Wolbachia biocontrol strategies for arboviral diseases and the potential influence of resident Wolbachia strains in mosquitoes. Curr Trop Med Reports. 2016;3. doi: 10.1007/s40475-016-0066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross PA. Designing effective Wolbachia release programs for mosquito and arbovirus control. Acta Tropica. 2021;222:106045. doi: 10.1016/j.actatropica.2021.106045 [DOI] [PubMed] [Google Scholar]
- 16.Caragata EP, Dutra HLC, Sucupira PHF, Ferreira AGA, Moreira LA. Wolbachia as translational science: controlling mosquito-borne pathogens. Trends in Parasitology. 2021. doi: 10.1016/j.pt.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Schultz MJ, Connor JH, Frydman HM. Group B Wolbachia Strain-Dependent Inhibition of Arboviruses. DNA and Cell Biology. 2018. doi: 10.1089/dna.2017.4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martins M, Ramos LFC, Murillo JR, Torres A, de Carvalho SS, Domont GB, et al. Comprehensive Quantitative Proteome Analysis of Aedes aegypti Identifies Proteins and Pathways Involved in Wolbachia pipientis and Zika Virus Interference Phenomenon. Frontiers in Physiology. 2021;12(199). doi: 10.3389/fphys.2021.642237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Wang Y, He K, Yang Q, Gong M, Ji M, et al. Wolbachia limits pathogen infections through induction of host innate immune responses. PLOS ONE. 2020;15(2):e0226736. doi: 10.1371/journal.pone.0226736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan X, Pike A, Joshi D, Bian G, McFadden MJ, Lu P, et al. The bacterium Wolbachia exploits host innate immunity to establish a symbiotic relationship with the dengue vector mosquito Aedes aegypti. The ISME Journal. 2018;12(1):277–88. doi: 10.1038/ismej.2017.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molloy JC, Sommer U, Viant MR, Sinkins SP, Schloss PD. Wolbachia Modulates Lipid Metabolism in Aedes albopictus Mosquito Cells. Applied and Environmental Microbiology. 2016;82(10):3109–20. doi: 10.1128/AEM.00275-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson KN. The Impact of Wolbachia on Virus Infection in Mosquitoes. Viruses. 2015;7(11):5705–17. doi: 10.3390/v7112903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamtchum-Tatuene J, Makepeace BL, Benjamin L, Baylis M, Solomon T. The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Current Opinion in Infectious Diseases. 2017;30(1):108–16. doi: 10.1097/QCO.0000000000000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terradas G, McGraw EA. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Current Opinion in Insect Science. 2017;22:37–44. doi: 10.1016/j.cois.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 25.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune Activation by Life-Shortening Wolbachia and Reduced Filarial Competence in Mosquitoes. Science. 2009;326(5949):134–6. doi: 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7. doi: 10.1371/journal.ppat.1002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caragata EP, Rancès E, Hedges LM, Gofton AW, Johnson KN, O’Neill SL, et al. Dietary Cholesterol Modulates Pathogen Blocking by Wolbachia. PLOS Pathogens. 2013;9(6):e1003459. doi: 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanaei E, Charlat S, Engelstädter J. Wolbachia host shifts: routes, mechanisms, constraints and evolutionary consequences. Biological Reviews. 2021;96(2):433–53. doi: 10.1111/brv.12663 [DOI] [PubMed] [Google Scholar]
- 29.Xie K, Lu Y-J, Yang K, Huo S-M, Hong X-Y. Co-infection of Wolbachia and Spiroplasma in spider mite Tetranychus truncatus increases male fitness. Insect Science. 2020;27(5):921–37. doi: 10.1111/1744-7917.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panteleev DIu GI, Andrianov BV, Reznik NL, Lazebnyĭ OE, Kulikov AM.. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Genetika. 2007;43(9):1277–80. [PubMed] [Google Scholar]
- 31.Atyame CM, Duron O, Tortosa P, Pasteur N, Fort P, Weill M. Multiple Wolbachia determinants control the evolution of cytoplasmic incompatibilities in Culex pipiens mosquito populations. Mol Ecol. 2011;20(2):286–98. doi: 10.1111/j.1365-294X.2010.04937.x . [DOI] [PubMed] [Google Scholar]
- 32.de Paula AR, Brito ES, Pereira CR, Carrera MP, Samuels RI. Susceptibility of adultAedes aegypti(Diptera: Culicidae) to infection byMetarhizium anisopliaeandBeauveria bassiana: prospects for Dengue vector control. Biocontrol Science and Technology. 2008;18(10):1017–25. doi: 10.1080/09583150802509199 [DOI] [Google Scholar]
- 33.Evans HC, Elliot SL, Barreto RW. Entomopathogenic fungi and their potential for the management of Aedes aegypti (Diptera: Culicidae) in the Americas. Memórias do Instituto Oswaldo Cruz. 2018;113:206–14. doi: 10.1590/0074-02760170369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butt TM, Coates CJ, Dubovskiy IM, Ratcliffe NA. Chapter Nine—Entomopathogenic Fungi: New Insights into Host–Pathogen Interactions. In: Brian L, Raymond JSL, editors. Adv Genet. Volume 94: Academic Press; 2016. p. 307–64. [DOI] [PubMed] [Google Scholar]
- 35.Lovett B, St. Leger RJ. The Insect Pathogens. Microbiol Spectr. 2017;5(2). doi: 10.1128/microbiolspec.FUNK-0001-2016 [DOI] [PubMed] [Google Scholar]
- 36.Ramirez JL, Dunlap CA, Muturi EJ, Barletta ABF, Rooney AP. Entomopathogenic fungal infection leads to temporospatial modulation of the mosquito immune system. PLOS Neglect Trop D. 2018;12(4):e0006433. doi: 10.1371/journal.pntd.0006433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez JL, Muturi EJ, Dunlap C, Rooney AP. Strain-specific pathogenicity and subversion of phenoloxidase activity in the mosquito Aedes aegypti by members of the fungal entomopathogenic genus Isaria. Scientific Reports. 2018;8(1):9896. doi: 10.1038/s41598-018-28210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosche KL, Sidak-Loftis LC, Hurtado J, Fisk EA, Shaw DK. Arthropods Under Pressure: Stress Responses and Immunity at the Pathogen-Vector Interface. Frontiers in Immunology. 2021;11(3920). doi: 10.3389/fimmu.2020.629777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrieli P, Caccia S, Varotto-Boccazzi I, Arnoldi I, Barbieri G, Comandatore F, et al. Mosquito Trilogy: Microbiota, Immunity and Pathogens, and Their Implications for the Control of Disease Transmission. Frontiers in Microbiology. 2021;12(633). doi: 10.3389/fmicb.2021.630438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyes JIL, Suzuki Y, Carvajal T, Muñoz MNM, Watanabe K. Intracellular Interactions Between Arboviruses and Wolbachia in Aedes aegypti. Frontiers in Cellular and Infection Microbiology. 2021;11(540). doi: 10.3389/fcimb.2021.690087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Audsley MD, Seleznev A, Joubert DA, Woolfit M, O’Neill Scott L, McGraw EA. Wolbachia infection alters the relative abundance of resident bacteria in adult Aedes aegypti mosquitoes, but not larvae. Molecular Ecology. 2018;27(1):297–309. doi: 10.1111/mec.14436 [DOI] [PubMed] [Google Scholar]
- 42.Lu HL, St Leger RJ. Insect Immunity to Entomopathogenic Fungi. Adv Genet. 2016;94:251–85. doi: 10.1016/bs.adgen.2015.11.002 . [DOI] [PubMed] [Google Scholar]
- 43.Wei G, Lai Y, Wang G, Chen H, Li F, Wang S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. P Natl Acad Sci USA. 2017;114(23):5994–9. doi: 10.1073/pnas.1703546114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joanne S, Vythilingam I, Yugavathy N, Doss JI. Modified technique of Wolbachia removal from Malaysian Aedes albopictus. Asian Pacific Journal of Tropical Biomedicine. 2014;4(7):557–60. doi: 10.12980/APJTB.4.2014APJTB-2014-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes GL. Wolbachia infections in Anopheles gambiae cells: transcriptomic characterization of a novel host-symbiont interaction. PLoS Path. 2011;7. doi: 10.1371/journal.ppat.1001296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer S, Brunk BP, Chen F, Gao X, Harb OS, Iodice JB, et al. Using OrthoMCL to Assign Proteins to OrthoMCL-DB Groups or to Cluster Proteomes Into New Ortholog Groups. Current Protocols in Bioinformatics. 2011;35(1):6.12.1–6.9. doi: 10.1002/0471250953.bi0612s35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 48.Zélé F, Altıntaş M, Santos I, Cakmak I, Magalhães S. Population-specific effect of Wolbachia on the cost of fungal infection in spider mites. Ecology and Evolution. 2020;10(9):3868–80. doi: 10.1002/ece3.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boucias DG, Zhou Y, Huang S, Keyhani NO. Microbiota in insect fungal pathology. Applied Microbiology and Biotechnology. 2018;102(14):5873–88. doi: 10.1007/s00253-018-9089-z [DOI] [PubMed] [Google Scholar]
- 50.Yang K, Chen H, Bing X-L, Xia X, Zhu Y-X, Hong X-Y. Wolbachia and Spiroplasma could influence bacterial communities of the spider mite Tetranychus truncatus. Experimental and Applied Acarology. 2021;83(2):197–210. doi: 10.1007/s10493-021-00589-4 [DOI] [PubMed] [Google Scholar]
- 51.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Path. 2010;6. doi: 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramirez JL, Muturi EJ, Flor-Weiler LB, Vermillion K, Rooney AP. Peptidoglycan Recognition Proteins (PGRPs) Modulates Mosquito Resistance to Fungal Entomopathogens in a Fungal-Strain Specific Manner. Frontiers in Cellular and Infection Microbiology. 2020;9(465). doi: 10.3389/fcimb.2019.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tawidian P, Rhodes VL, Michel K. Mosquito-fungus interactions and antifungal immunity. Insect Biochemistry and Molecular Biology. 2019;111:103182. doi: 10.1016/j.ibmb.2019.103182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramirez JL, Muturi EJ, Barletta ABF, Rooney AP. The Aedes aegypti IMD pathway is a critical component of the mosquito antifungal immune response. Developmental & Comparative Immunology. 2019;95:1–9. doi: 10.1016/j.dci.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 55.Dong Y, Morton JC Jr., Ramirez JL, Souza-Neto JA, Dimopoulos G. The entomopathogenic fungus Beauveria bassiana activate toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem Mol Biol. 2012;42(2):126–32. doi: 10.1016/j.ibmb.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y-H, Hu Y, Xing L-S, Jiang H, Hu S-N, Raikhel AS, et al. A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes. PLOS Pathog. 2015;11(6):e1004931. doi: 10.1371/journal.ppat.1004931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng C-C, Sofiyatun E, Chen W-J, Wang L-C. Life as a Vector of Dengue Virus: The Antioxidant Strategy of Mosquito Cells to Survive Viral Infection. Antioxidants. 2021;10(3):395. doi: 10.3390/antiox10030395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliveira JHM, Talyuli OAC, Goncalves RLS, Paiva-Silva GO, Sorgine MHF, Alvarenga PH, et al. Catalase protects Aedes aegypti from oxidative stress and increases midgut infection prevalence of Dengue but not Zika. PLOS Neglected Tropical Diseases. 2017;11(4):e0005525. doi: 10.1371/journal.pntd.0005525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen T-H, Tang P, Yang C-F, Kao L-H, Lo Y-P, Chuang C-K, et al. Antioxidant defense is one of the mechanisms by which mosquito cells survive dengue 2 viral infection. Virology. 2011;410(2):410–7. doi: 10.1016/j.virol.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 60.Zug R, Hammerstein P. Wolbachia and the insect immune system: what reactive oxygen species can tell us about the mechanisms of Wolbachia–host interactions. Frontiers in Microbiology. 2015;6(1201). doi: 10.3389/fmicb.2015.01201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin SW, Zou Z, Raikhel AS. A new factor in the Aedes aegypti immune response: CLSP2 modulates melanization. EMBO reports. 2011;12(9):938–43. doi: 10.1038/embor.2011.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas P, Kenny N, Eyles D, Moreira LA, O’Neill SL, Asgari S. Infection with the wMel and wMelPop strains of Wolbachia leads to higher levels of melanization in the hemolymph of Drosophila melanogaster, Drosophila simulans and Aedes aegypti. Developmental & Comparative Immunology. 2011;35(3):360–5. doi: 10.1016/j.dci.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 63.Bourtzis K, Pettigrew MM, O’Neill SL. Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Molecular Biology. 2000;9(6):635–9. doi: 10.1046/j.1365-2583.2000.00224.x [DOI] [PubMed] [Google Scholar]
- 64.Molloy JC, Sinkins SP. Wolbachia Do Not Induce Reactive Oxygen Species-Dependent Immune Pathway Activation in Aedes albopictus. Viruses. 2015;7(8):4624–39. doi: 10.3390/v7082836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blagrove MSC, Arias-Goeta C, Failloux A-B, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proceedings of the National Academy of Sciences. 2012;109(1):255–60. doi: 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zug R, Hammerstein P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological Reviews. 2015;90(1):89–111. doi: 10.1111/brv.12098 [DOI] [PubMed] [Google Scholar]
- 67.Rossi P, Ricci I, Cappelli A, Damiani C, Ulissi U, Mancini MV, et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasites & Vectors. 2015;8(1):278. doi: 10.1186/s13071-015-0888-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moll RM, Romoser WS, Modrakowski MC, Moncayo AC, Lerdthusnee K. Meconial Peritrophic Membranes and the Fate of Midgut Bacteria During Mosquito (Diptera: Culicidae) Metamorphosis. Journal of Medical Entomology. 2001;38(1):29–32. doi: 10.1603/0022-2585-38.1.29 [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Gilbreath TM, Kukutla P, Yan G, Xu J. Dynamic Gut Microbiome across Life History of the Malaria Mosquito Anopheles gambiae in Kenya. PLoS ONE. 2011;6. doi: 10.1371/journal.pone.0024767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pennington MJ, Prager SM, Walton WE, Trumble JT. Culex quinquefasciatus larval microbiomes vary with instar and exposure to common wastewater contaminants. Scientific Reports. 2016;6(1):21969. doi: 10.1038/srep21969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caragata EP, Otero LM, Tikhe CV, Barrera R, Dimopoulos G. Microbial Diversity of Adult Aedes aegypti and Water Collected from Different Mosquito Aquatic Habitats in Puerto Rico. Microbial Ecology. 2021. doi: 10.1007/s00248-021-01743-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Trees were built in VectorBase with the resulting protein gene sequences from their respective OrthoMCL’s ortholog groups.
(DOCX)
Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. The red horizontal line indicates LS-means. Uppercase letters refer to fungal effects and groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. See Table 3 for complete statistics from the Two-Way ANOVA.
(TIF)
Significant effects (SE) indicate whether the independent factors: Fungal entomopathogen (F), Wolbachia presence (W) or their interaction (F*W) were statistically significant. The red horizontal line indicates LS-means. Uppercase letters refer to fungal effects and groups sharing the same letter are not significantly different at p<0.05 based on differences of least-squares means. W-, Wolbachia-free; W+, Wolbachia-infected; B. bass., B. bassiana; B. brog., B. brongniartii. See S2 Table for complete statistics from the Two-Way ANOVA.
(TIF)
Data analyzed via single-factor ANOVA using PROC GLIMMIX with a gamma distribution in SAS. Species mean Vmax rates sharing the same letter are not significantly different at p<.05 based on differences of least-squares means.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.