Abstract
Systemic sclerosis (SSc), the most lethal of rheumatologic conditions, is the cause of death in >50% of SSc cases, led by pulmonary fibrosis followed by pulmonary hypertension and then scleroderma renal crisis (SRC). Multiple other preventable and treatable SSc-related vascular, cardiac, gastrointestinal, nutritional and musculoskeletal complications can lead to disability and death.
Vascular injury with subsequent inflammation transforming to irreversible fibrosis and permanent damage characterizes SSc. Organ involvement is often present early in the disease course of SSc, but requires careful histories and vigilance in screening to detect. Inflammation is potentially reversible provided that treatment intensity quells inflammation and other immune mechanisms. In any SSc phenotype, opportunities for early treatment are prone to be under-utilized, especially in slowly progressive phenotypes that indolently accrue irreversible organ damage resulting in later-stage life-limiting complications such as pulmonary hypertension, severe ILD, cardiac involvement and malnutrition.
A single SSc patient visit often requires much more physician and staff time, organization, vigilance and direct management for multiple organ systems compared to other rheumatic or pulmonary diseases. Efficiency and efficacy of SSc care enlists trending symptoms and bio-data; and can be sustained financially by understanding insurance reimbursement policies. Sharing care between scleroderma centers and local cardiology/pulmonary/rheumatology/gastroenterology colleagues may prevent complications and poor outcomes, while providing support to local specialists.
As scleroderma specialists, we offer a practical framework with tools to facilitate an approach to optimal, comprehensive and sustainable care in SSc. We anticipate this framework to remain relevant in the assessment, care and prevention of disease and treatment complications of this complex disease.
Keywords: interstitial lung disease, pulmonary fibrosis, renal crisis, pulmonary hypertension, disability, scleroderma, systemic sclerosis, symptom burden, quality of life, survival, mortality, health systems
I. INTRODUCTION
Systemic sclerosis (SSc), the most lethal of rheumatologic conditions, is the direct cause of death in >50% of SSc cases; led by pulmonary fibrosis, pulmonary hypertension and then scleroderma renal crisis (SRC). Multiple other preventable and treatable SSc-related vascular, cardiac, gastrointestinal, nutritional and musculoskeletal complications also lead to disability and death.
SSc is characterized by vascular injury and disrepair that incites systemic progressive inflammatory transformation to fibrosis at widely variable rates and intensities. Inflammation is a reversible phenomenon provided the intensity of treatment matches that of the inflammation. End-stage fibrosis is permanent and irreversible. Organ involvement is present early in the SSc disease course, requiring ongoing screening and careful patient questioning to detect. Reduction of disability and mortality hinges on prevention of vascular and fibrotic damage which is directly dependent upon early recognition of active disease, even in the indolent disease phenotypes, with initiation of appropriate treatment to prevent fibrotic transformation.
Delayed diagnosis is common in autoimmune diseases and disproportionately frequent in those of African and Hispanic descent, for whom these diseases tend to be more severe and deadly.1–6 Importantly, slowly progressive phenotypes indolently accruing irreversible structural changes and organ damage are less prone to receive treatment, resulting in end-stage SSc complications such as pulmonary hypertension, cardiac involvement and malnutrition. Diagnostic delays, misdiagnoses and complication oversights are likely underpinned by preferential reliance on laboratory data in a clinical setting that is hurried and where authentic empathetic listening, careful history-taking and physical exam performance may be impaired.
Efficiency and efficacy of SSc care that meets the health-related quality of life (HRQoL) and survival needs of patients requires trending symptoms and bio-data over time; it also requires multiple streams of management that are sustained by understanding visit reimbursement policies. A single SSc patient visit commonly involves extensive investigation, coordination and direct management for multiple organ systems, exacting physician and staff time and effort beyond other diseases. Sharing care between scleroderma centers and local specialists provides robust patient-centered management and patient skill-building for self-management of this complex disorder.
As scleroderma specialists, we offer an abbreviated reference manual and practical framework, that we hope supports clinicians and patients, with informational summaries on symptoms, manifestations and complications with tools and templates for screening, assessment, documentation, risk stratification, counselling and anticipatory guidance, and discussions surrounding clinician sustainability.
II. PATHOLOGIC DRIVERS IN SSC THAT IMPACT TREATMENT DECISIONS
A. Inflammation-Fibrosis Axis: From Preventable to Irreversible Damage
Beyond the widely heterogeneous nature of SSc presentation, progression and potential organ involvement, a major challenge impeding SSc care is the ability to distinguish between states of active progressive disease and its subsequent fibrotic damage. Inflammation-fibrosis transformation is a progressive process with an advancing front of potentially reversible inflammatory assault. Inflammatory tissue left untreated is damaged with increasing expanses of fibrosis. Inflammation and fibrosis are often coexistent, but with increasing fibrotic expanse leads to worsening irreversible disability and, possibly, death over time. Though currently difficult to distinguish with certainty, even in the absence of ESR or CRP elevation and regardless of coexistent fibrosis, concern for any degree of inflammation i.e. progression, should prompt consideration to initiate systemic immunomodulatory therapy.
Symptoms and impairment burden dynamically relate to the extent of either inflammation, fibrosis or a combination thereof (Figure 1). Symptoms worsen with extent of involvement; but potential symptom reduction or reversal with systemic treatment requires some degree of active tissue inflammation to be present. For example, progressive ILD, can manifest by dropping forced vital capacity (FVC), dry inspiratory cough, and breathlessness that improves after systemic treatment.7–10 Whereas, residual inactive fibrotic damage resulting from prior inflammation is now unresponsive to immunosuppression.
Figure 1.
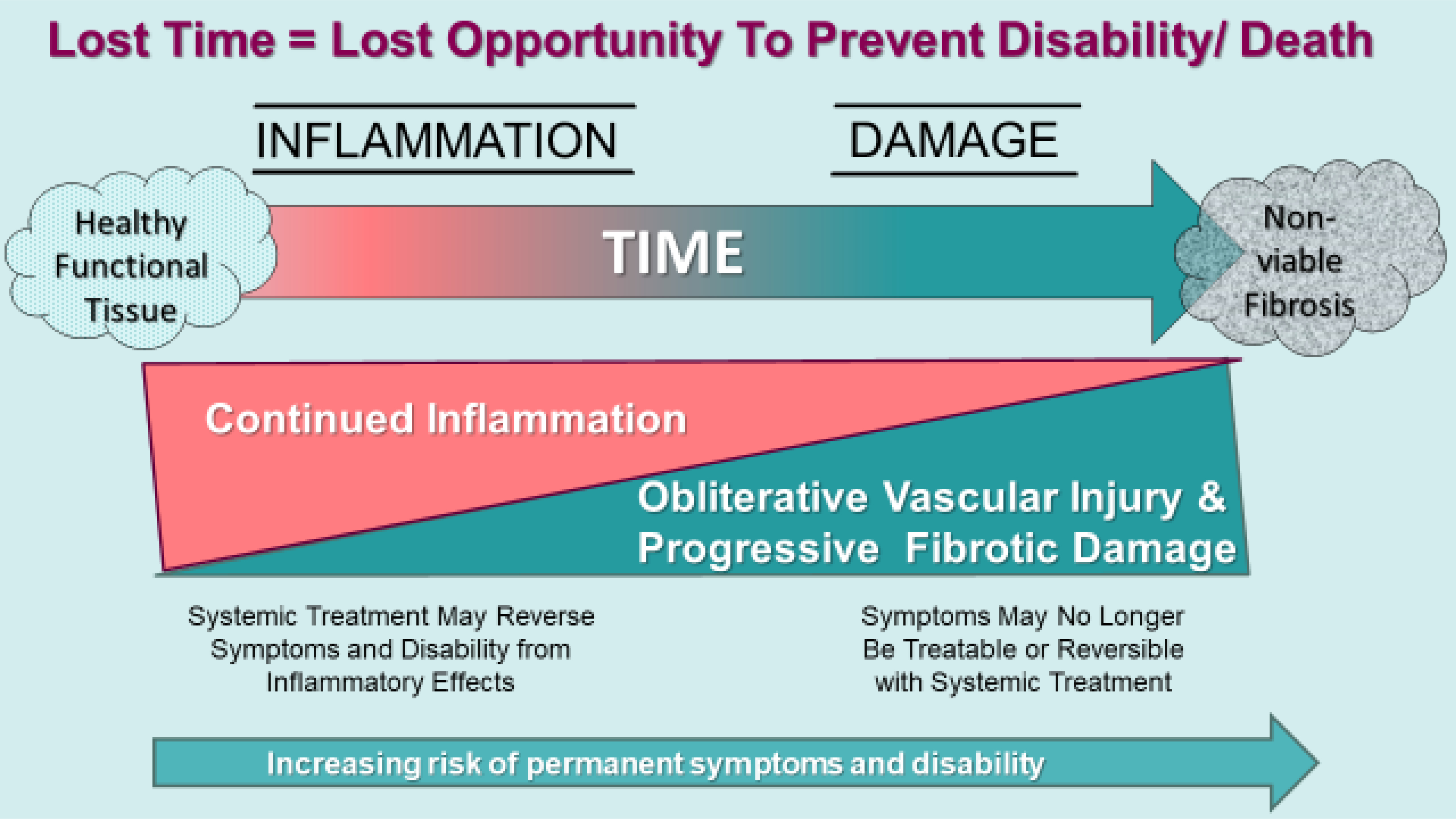
SSc involved tissue, of which the lung is one example, experiences transition from healthy tissue to fibrosis as inflammation is incited and progressively extends within resident organs. Vascular injury with tissue hypoxia is an important factor to the development of tissue fibrosis. Symptoms and disability can be transient with active inflammation with systemic treatment. Over time untreated inflammation irreparably injures effected tissue resulting in scarring and fibrosis. Fibrosis is irreversible and results in permanent organ-related disability. (Courtesy of LA Saketkoo, rights reserved)
B. Circulation and Mechanisms of Disease
Vasculopathy, vascular injury with tissue hypoxia and pathologic circulation interplay with and are drivers of inflammation and fibrosis. The earliest hallmark of SSc disease is vascular injury, dysfunction and disrepair, without evidence of inflammatory infiltration i.e. not vasculitis.11,12 Vascular dysfunction and Raynaud’s phenomenon (RP) symptoms predominantly predate non-RP symptoms by several years. In the genetically predisposed host, vascular injury may incite immune system activation through upregulation of adhesion cells and perivascular migration of immune cells, including macrophages, which may have a direct role in fibroblast stimulation.
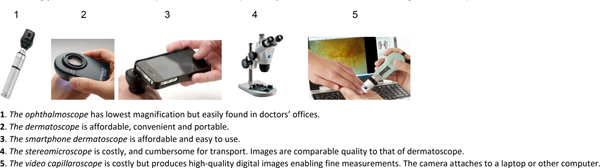
The presence of abnormal capillaroscopy predicts the development of connective tissue disease (CTD) in patients with RP; and ANA positivity heightens that predictive power.13 SSc nailfold capillaroscopy patterns are well-described reflecting the vasculature struggling against the pathologic progression of the disease. 14(Figure 2). The presence of abnormal nailfold capillaries contributes >20% toward SSc classification criteria15 and predicts16 the development of a CTD 17and SSc;13,16,18,19 making capillaroscopy, with at least a handheld device, an essential assessment tool in rheumatologic care (Figure 3).
Figure 2.
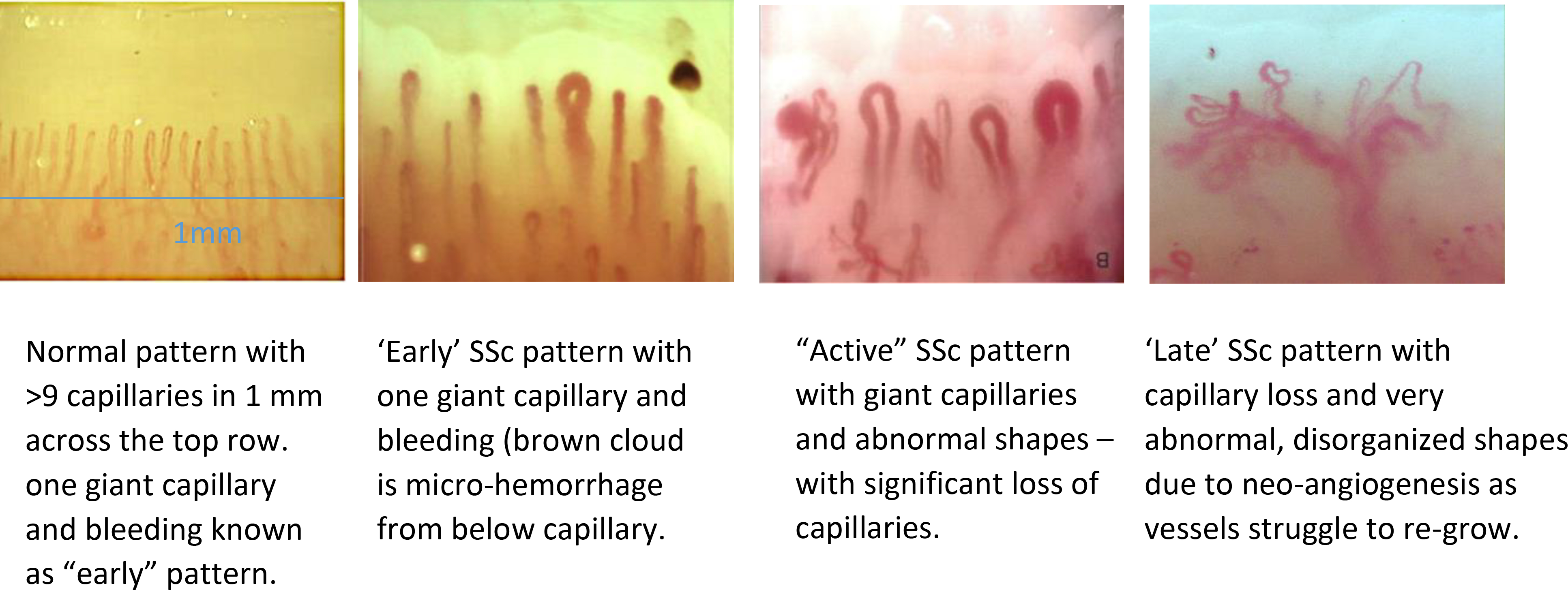
Demonstration of ‘normal’ and various SSc patterns on nail fold video capillaroscopy. (Images courtesy of Vanessa Smith; University of Ghent, Belgium.)
Figure 3. Capillaroscopy is an essential rheumatologic service.
An abnormal capillaroscopy satisfies >20% of SSc criteria and confers 96% predictive power for development of CTD; making it an essential part of the rheumatologic exam. With any method capillaries become increasingly easier to visualize with practice over time. (Courtesy of T Frech & LA Saketkoo, rights reserved)
A normal nailfold bed demonstrates long thin hairpin loops resembling the abundance of wheat fields. In the ‘early’ and ‘active’ SSc patterns, the capillaries dilate and giant loops occur, as well as microhemorrhages, ballooning above the injured vessels. Later in the course of SSc, capillaries ‘drop-out’ leading to a rarefaction of the capillary network. Edematous ‘puffy fingers’ or diffuse infiltrative fibrosis sometimes make nailfold capillaries difficult to visualize. 19–25 The ‘late’ pattern is characterized by marked rarefaction and often reflects the vasculature’s struggles to repair itself, albeit ineffectively despite high levels of circulating pro-angiogenic factors, creating a network of thin, matted vessels inefficient for supporting healthy tissue. This can be seen also in GI and skin i.e. GAVE and telangiectasias.
Lethal vascular complications such as PH and cardiac involvement correlate with other circulatory phenomena e.g. digital ulcers (DU), telangiectasias,20,21 osseous vascular complications e.g. radiographic calcinosis, and acro-osteolysis,22 and with inflammation-predominant complications e.g. arthritis and muscle involvement. These associations suggest a deep-rooted interplay between systemic inflammation, autoimmunity, fibrosis and vasculopathy.
Systemic autoimmune, inflammatory drivers influencing SSc vascular complications is a major current consideration in research and patient care.23–27 SSc-specific autoantibodies help predict the potential clinical course and phenotypes in SSc patients, however, only functional antibodies not specific to SSc, such as the anti-endothelial cell antibody, demonstrate a direct pathogenic role; although reports are conflicting.28,29 Healing of non-friction DUs upon initiation of systemic treatment e.g. mycophenolate mofetil (MMF), and subsequent DU re-emergence upon immunosuppression discontinuation, are anecdotally noted by SSc experts. Potential influence of immunosuppressants on improved outcomes in SSc-PH are increasingly being investigated.23–27
III. CONSIDERATIONS THAT DRIVE MANAGEMENT IN SSC
A. Goals of SSc Management
Preventing death and permanent disability in SSc is accomplished with early and appropriate treatment.
SSc is an extensively complex disease often with delayed diagnosis. By the time patients receive expert management, most will have permanently lost some degree of physical function and have diminished well-being, eroding one’s ability to sustain the crucial life areas and personal satisfactions of family, intimate and social interactions including financial solvency. Recent data suggest initiating early treatment may prevent development of complications such as ILD.30
SSc is associated with significant unemployment, worker absenteeism, decreased worker productivity.31 Preventable SSc-related work impairment results in substantial economic burden and diminished HRQoL32 with loss of work, lost income, and loss of health insurance and healthcare. Working closely with patients and their employers to attain appropriate modifications to their work environment and situation may improve functioning and improve productivity.31–36
B. Risk Awareness in SSc
The risk for and the actual rate of disease progression, guides the level of systemic treatment intended to quell inflammation and prevent further organ damage. They also identify patients with rapidly progressive disease potentially benefitting from hematopoietic stem cell transplantation (HSCT) before end-organ damage occurs. While there is no formal SSc risk stratification tool, certain factors put patients with SSc at even greater risk of death, disability and rapidly progressive disease (Table 1, 2). Sensitizing clinicians to these risk factors heightens vigilance for treatable lethal and/or permanently disabling disease.
Table 1.
Risk Factors for Death, Disability and Rapidly Progressive Disease
| Risk Factor | Clinical measures | Indication of Rapidly Progressive SSc or Severe Disease |
|---|---|---|
| Diffuse skin involvement | Modified Rodnan total Skin thickness Score (mRSS) | Increasing diffuse skin thickness, mRSS > 29 |
| Tendon Friction Rub | Palpable presence on exam | Palpable presence on exam |
| Anti-topoisomerase I | See measures for ILD, dcSSc, renal crisis, and cardiac fibrosis | |
| Interstitial lung disease | PFT: spirometry PFT: DLCO HRCT: Extent of ground-glass opacity and honeycombing fibrosis |
FVC<70% DLCO<70% >20% extent of disease on HRCT |
| Pulmonary arterial hypertension (PAH) | Echocardiography | Estimated sPAP >40 mmHg Right atrial or ventricular enlargement Septal flattening |
| Right heart catheterization | mPAP>20mmHg | |
| WHO / NYHA Classification | PVR ≥ 3 Wood units Class III / IV |
|
| Cardiac Involvement | ECG Echocardiography Cardiac MRI |
ECG arrhythmia, heart block, valve disease, Diastolic dysfunction >grade 2 left ventricular ejection fraction <45% |
| Digital ulcers, gangrene | Nailfold capillaroscopy | Severe capillary loss, with fibrotic infiltration |
| Scleroderma renal crisis | Hypertension | Abnormal or an unusually elevated value for patient Normotensive possible if on prednisone, vasodilators or anti-hypertensive |
| Serum biomarkers | Rising serum creatinine Anti-RNA polymerase III |
|
| GAVE | Gastric bleeding Anemia |
Frank blood on inspection Hb < 9.6 g/dL |
| Severe malabsorption | Weight loss Muscle atrophy Stool frequency Electrolytes Albumin/Pre-albumin |
|
| Polyarthritis | HAQ-DI DAS-28 |
HAQ-DI >2.00 |
| General health status | Weight loss/BMI Serum biomarkers |
Weight loss > 10% Low albumin, Low Hb |
| Comorbidities | Presence of : COPD, malignancy, diabetes mellitus | Anti-polymerase III in relation to malignancy |
GAVE: gastric antral vascular ectasia, ILD: Interstitial lung disease; PAS: estimated pulmonary artery systolic pressure by Doppler echo; HAQ-DI : Health Assessment Questionnaire-Disability Index
Table 2.
| Organ manifestation | Risk factors with Associated Findings |
|---|---|
| Heart | Diffuse cutaneous SSc Elevated ultra-sensitive CRP Myocardial fibrosis on CMR Anti-topoisomerase 1 antibody Male gender Pericarditis Arrhythmia Right bundle branch block (RBBB) Left ventricular dysfunction Myopathy Tendon friction rubs |
| Kidney, (renal crisis) | Diffuse cutaneous SSc Rapid skin progression in the first year of the onset Presence of anti-RNA polymerase III autoantibodies Medium or high dose glucocorticoid therapy, i.e. >10mg prednisone daily Significant cardiac manifestation Joint contractures Tendon friction rubs |
| Interstitial lung disease (ILD) | African ancestry Male gender High mRSS Diffuse cutaneous SSc Anti-topoisomerase I antibody (Scl-70) Anti-Th/To antibody Anti-U11/U12 (RNPC) antibody Increased ESR or CRP FVC<70 %, DLCO<70 % |
| Progressive ILD | Active polyarthritis Increased ESR or CRP Disease onset over 55 years High mRSS Reflux (GERD) NYHA III-IV heart disease Decreased SpO2 during 6MWT Progressive drop in %FVC corroborated by HRCT and symptoms Advanced ILD (traction bronchiectasis, honeycombing) within 5 years of disease onset |
| Pulmonary arterial hypertension | Disease onset over 55 years Long disease duration African ancestry for early onset Skin telangiectasia (increased number and size) Isolated DLCO decrease FVC/DLCO ratio > 1.6 Severe Raynaud’s Severe digital ulcers Decreased capillary density by nail fold capillaroscopy Increased serum uric acid Presence of anti-nucleolar (anti-Th/To, and anti-U3 RNP) autoantibodies |
| Gastrointestinal | Disease duration Anti-U3-RNP Dysbiosis (microbiome composition) End-stage vasculopathy features such as DU, calcinosis Dysphagia Frequent food regurgitation Small Intestinal Bacterial Overgrowth and related chronic diarrhea Chronic intestinal pseudo-obstruction Fecal soiling Weight loss Low albumin/pre-albumin |
| Digital ulcers | Diffuse cutaneous SSc High mRSS Male gender Polyarthritis Early non-Raynaud’s first symptom Increased capillary loss by capillary-microscopy |
| Arthritis, contractures, tendon friction rubs | Early manifestation in diffuse cutaneous SSc DAS-28 Presence of overlap SSc Presence of anti-RNA Polymerase III and anti-Scl-70 (anti-Topoisomerase I) autoantibodies |
6MWT: 6-minute walk test, CMR: Cardiac MRI, CRP: C-reactive protein, DAS-28: Disease Activity Score-28, DLCO: diffusion capacity of the lung for carbon monoxide, ESR: erythrocyte sedimentation rate, FVC: forced vital capacity, GERD: gastroesophageal reflux disorder, mRSS: modified Rodnan Skin Score, NYHA: New York Heart Association; SpO2: blood oxygen saturation; WHO: World Health Organization
It should be clarified that both limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) carry an increased risk of death. The terms diffuse and limited cutaneous are descriptors of skin thickness distribution only; and provide crude sub-typing of an extremely complex disease. However, limited sub-type may carry a higher risk of PAH, dcSSc carries higher risk for progressive ILD; and early dcSSc with rapid increases in skin thickening is associated new internal organ involvement.37,38, 19,39–49 Both sub-types can develop ILD and PH, and malnutrition from severe GI involvement.
Autoantibodies are also helpful for predicting outcome, particularly anti-centromere predicting PAH, Scl-70 predicting ILD and RNA polymerase III predicting renal crisis (Figure 4). Race and ethnicity are also associated with increased risk of severe disease. Black race, compared to whites, independently predicts more rapid progression and higher mortality, more severe disease at a younger age of onset, and with higher risk of early and concomitant ILD and PH. These racial differences may be associated with distinct antibody and genetic profiles supporting that early aggressive intervention in blacks with ILD may offset mortality.5,32 Hispanic and Asian ancestry also portends higher severity than whites.50–52 Male sex, early diffuse cutaneous disease or presence of tendon friction rubs also confer increased risk of mortality.
Figure 4. Clinical-Serologic Classification and Internal Organ Associations.
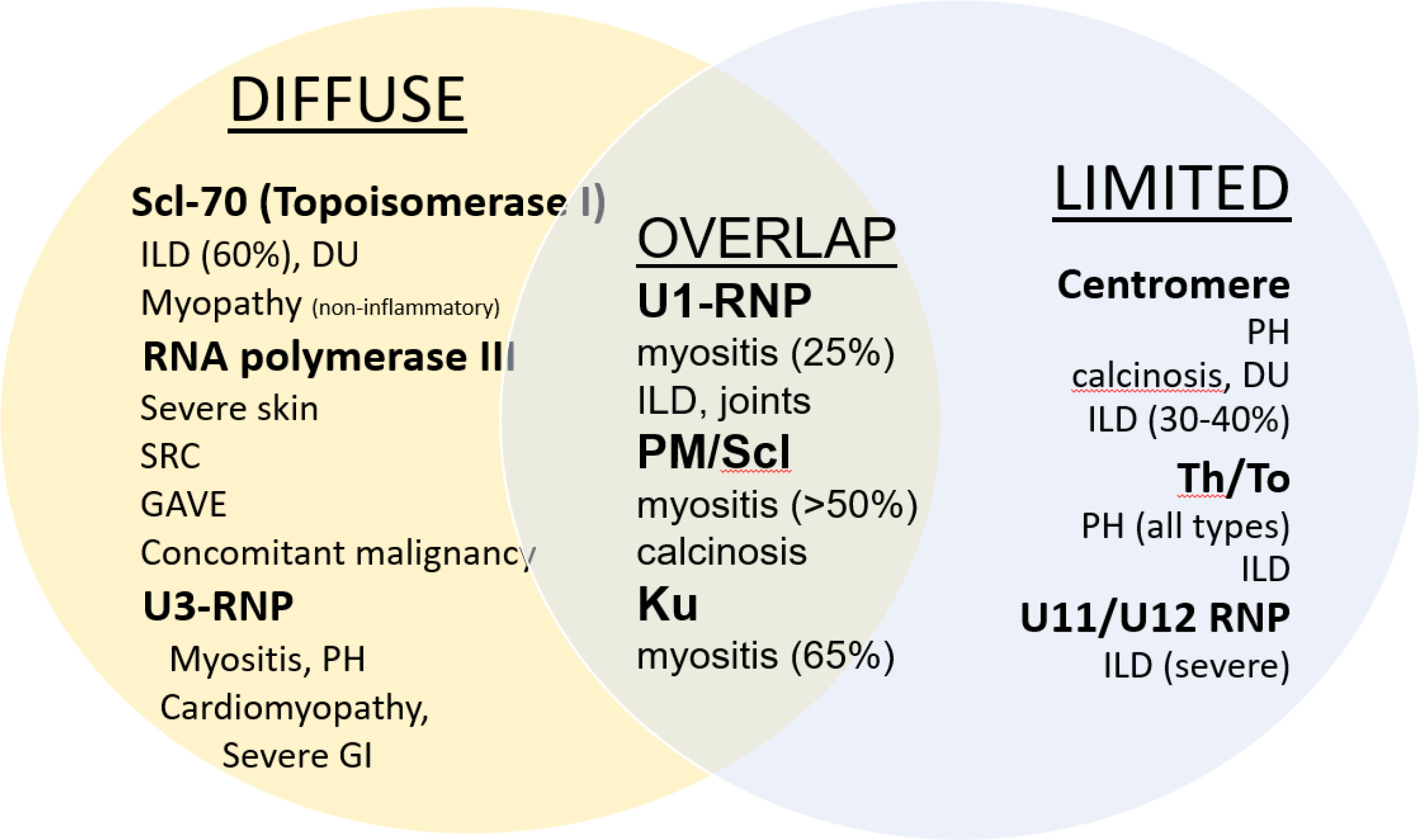
(Courtesy of RT Domsic, rights reserved)
ILD = interstitial lung disease; DU = digital ulcers; SRC = scleroderma renal crisis; PH = pulmonary hypertension
C. Tracking Symptoms and Metrics for Recognition and Intervention
Any type of organized framework containing SSc domains and sub-domains that tracks changes in clinical features, symptomatology, complications and bio-data of multiple manifestations over time, facilitates a comprehensive and efficient care continuum. This also enables communication of important details across specialties. Such documentation captures SSc manifestations as they newly emerge, improve, resolve, stabilize or worsen, and creates an overview that depicts treatment responsiveness, potentially sparking consideration for new, additive or change in treatment approach. Tables 3–6 and the resource list provide example tools.
Table 3.
Domain Organization for Clinical Assessment and Documentation in SSc. Each sub-domain is often characterized by onset, coincident intervention, and changes over time.
| Domains | Sub-Domains | Assessment Considerations |
|---|---|---|
| Background | Biological sex | |
| Ethnicity and race | ||
| Environmental exposure history | e.g. chemicals via occupation or proximity | |
| Cardiovascular history | Especially noting hypertension | |
| Disease Duration | Raynaud’s phenomenon onset (month/year) | |
| What was 1st non-Raynaud’s phenomenon symptom | ||
| Onset of 1st non-Raynaud symptom (month/year) | ||
| Physician diagnosis of SSc (month/year) | ||
| Skin Thickening | Onset month/year | |
| Distribution (mRSS) | ||
| Pruritus | ||
| Pigmentation disturbances | e.g. hypo-, hyer- or poikiloderma | |
| Telangiectasia and Calcinosis | Recorded here or under the vascular domain | |
| Vascular Manifestations Please see and incorporate components of Table 7 for document template | ||
| HEENT | Facial Changes | Oral aperture |
| Eyes | Dry Eyes | |
| Oral | Tooth loosening, Chewing difficulty Oral pain Dry mouth Dental caries |
|
| Naso-pharyngeal | Post Nasal Drip (lung irritant) Hoarseness of voice (vocal cord fibrosis or acid injury) |
|
| Cardiopulmonary | History of symptoms Dyspnea/Cough / Exercise Intolerance NYHA Symptom Category |
1st noticed symptoms to now Tables 9–10 for contextualizing history taking Although a categorical variable that limits utility, a worsening NYHA classification marks significant clinical worsening |
| Cardiac symptoms including lower extremity edema, orthopnea | Arrhythmias/conduction disturbances, heart failure | |
|
Gastrointestinal Consider following SCTC-GIT or Geissen tools for overall GI impact |
Swallowing difficulty | Proximal Distal Choking, coughing |
| Acid related | Heartburn Hoarseness Cough, timing e.g. morning |
|
| Gastric | History of GAVE Early satiety Regurgitation of food Emesis of food Bloating / distension / pain |
|
| Biliary | History of primary biliary cholangitis Itching, jaundice, pruritus, but may be asymptomatic Bilirubin and transaminase profiles, possible anti-mitochondrial antibody presence |
|
| Small bowel | Diarrhea, pain, weight loss, malabsorption Cramping Bloating |
|
| Large bowel | Constipation Fecal soiling |
|
| Muscular | Atrophy, Muscle strength, Muscle endurance, Aerobic capacity (submaximal test) Hand grip and pinch strength |
MMT-8 TST/30-sec CST^ FI-2/FI-3 ^ Ebbeling treadmill test* Astrand cycle test* 6 MWT* Jamar or Grippit dynamometer* Pinch meter* |
| Joint | AROM upper extremity, AROM/PROM hands/fingers |
FSA* Goniometer* HAMIS* Cochin Hand Function Scale* DAS-28 |
6MWT: 6 minute walk test for distance, CST: Chair-Stands Test, DAS-28: Disease Activity Scale-28, FI-2: Functional Index 2, FI-3: Functional Index 3, FSA: Function Shoulder Assessment, HAMIS: Hand Mobility in Scleroderma, mRSS: modified Rodnan Skin Score, NYHA: New York Heart Association
implemented routinely by OT, PT
implemented by PT, OT but can be performed in clinic by physician or staff
Table 6.
Key Physical Exam Assessments in SSc Courtesy of T Frech & LA Saketkoo, rights reserved.
| Category | Assessment Area | Observed Finding | Comment |
|---|---|---|---|
|
| |||
| CONSTITUTIONAL | Nutrition | Weight | |
| Fit of clothes | |||
| Temporal muscle atrophy | |||
| Overall mobility | Observation into room, seating, reaching for coat, bag etc Use of assist device for ambulation |
||
|
| |||
| HEMATOLOGICAL | Pallor | Observation | Anemia can occur from GAVE, medication effect, SRC |
| Lymph nodes | Palpation | ||
|
| |||
| HEENT | |||
| Facial appearance | General facial structural features | Lip thinning | Most facial changes are difficult to track |
| *Telangiectasia | See below | May indicate increasing vasculopathy | |
| Eyes | Dryness | ||
| Conjunctival pallor | |||
| Oropharyngeal | Oral Cavity | Dryness | |
| Sublingual pallor | |||
| Dentition/crowding | |||
| Oral aperture | Aperture diameter in mm | ||
| *Telangiectasia | See below | Often the first location to appear | |
| Naso-pharyngeal | Signs of post-nasal drip (PND), i.e. erythema, ‘cobble-stoning’ | PND and Reflux are micro-aspirated and irritate sensitive lung tissue causing parenchymal inflammation and possibly worsening ILD. | |
|
| |||
| VASCULAR | |||
| *Circulation/RP | - color - coolness - location |
||
|
*Capillaroscopy - morphology: Drop-out Hemorrhage Dilated (giant) Tortuous Disorganized |
Positive morphology contributes to diagnosis. Ophthalmoscope or dermatoscope easily identify morphologic changes. Nailfold video capillaroscope can mark detailed changes over time. | ||
| *Digital ulcers | - number - location - depth - ‘true’ vs friction - drainage - infection^ |
||
| *Pitting | - number - location - tenderness |
||
| Calcinosis | - number - location - consistency (solid v paste) - tenderness - infection^ |
Size, draining or not | |
| (Acro)-Osteolysis | Presence of distal to proximal: - Digital shortening - Nailbed tapering from sides - Nailbed blunting from tip |
||
| *Telangiectasias | - count - location (inner lip, face, chest, palms) - matted v non-matted |
- used for diagnostic purposes - followed over time |
|
|
| |||
| CARDIOPULMONARY | |||
| Cardiac | Observation | Jugular venous distension | |
| Lower extremity edema | |||
| Positional chest pain | Pericarditis | ||
| Auscultation | Rhythm, presence of gallop, rub | Pericarditis can occur in early phase dcSSc | |
| Aerobic capacity | 6MWT | ||
| Pulmonary | Observation | Respiratory rate | |
| Depth of inhalation | Patients with ILD/PF often ‘splint’ to protect from coughing | ||
| Cough with inhalation | Possible ILD/PH | ||
| Auscultation | From apices to bases, from beginning of inhalation to end of exhalation Listening for crackles, absent breath sounds |
If not hearing breath sounds, instruct patient during exam. Splinting occurs commonly in ILD to avoid inspiratory cough. Otherwise, consider pleural effusion | |
| Inspiratory cough | |||
| Oximetry | SpO2%/Pulse oximetry, at rest and exertion–e.g. walk to exam room. 6MWT | Preferably ear or forehead oximetry Finger may display results not reflective of true SpO2 |
|
| Aerobic capacity | 6MWT for distance | Musculoskeletal involvement may impact results, but overall 6MWT can reliably tend exercise tolerance | |
|
| |||
| GASTROINTESTINAL | Nutrition | As above | |
| Abdomen | Observable, palpable distension | ||
|
| |||
| MUSCULOSKELETAL | |||
| Articular/Peri-articular | Joint extension | To 180 degrees | PIPs, MCPs, wrists, elbows, shoulders, knees, hips, ankle joints |
| Joint flexion | Fixed contracture (yes/no) | ||
| Finger-to-palm | |||
| Tenderness +/− swollen joints | Palpation especially PIPs, MCPs, wrists | Synovitis is even more difficult to appreciate in SSc than other CTDs | |
| Tendon Friction Rubs | Localization for documentation | ||
|
|
|||
| Muscle | Observation | Mobility | Muscle involvement is: - common in SSc - of variable and combined pathology: atrophy, inflammatory, necrotic, fibrotic - associated with SSc cardiac involvement |
| Atrophy | |||
| Strength/Endurance | MMT 5 or 8† | ||
| Functional Index-2 (FI-2)^ FI-3-2†^ |
Endurance is a more revealing assessment and more problematic for SSc patients than isometric strength. Usually performed by physiotherapist. | ||
|
|
|||
| Functional capacity | TST^ or 30-sec CST†^ | ||
|
| |||
| SKIN | General Appearance | Pigmentation: - Hyper- - Hypo- - Poikiloderma Sheen: - Across chest Telangiectasias (here or detailed in ‘vascular’ domain) |
|
| Breakdown | Ulceration - Digital - Other areas Pitting |
||
| *Thickness: Extent and Degree | mRSS† | Skin thickness may also impair ROM | |
| Phase of Thickness | - Edematous v Bound-down - Initial signs of edematous phase often include puffy fingers; before skin thickening occurs |
Edematous phase can cause diffuse pain and itching and often mistaken as fibromyalgia. | |
| Stretching may reduce inflammation, edema, contractures and skin tightness of hands, fingers, shoulders, chest, hamstrings and hips; as well as increase ROM. | |||
Indicates SSc classification criteria marker
Infection = assessing for redness and purulence
see corresponding photo/s
please see resource list for instructional content
implemented by PT, OT but can be performed in clinic by physician or staff ‡
IV. MULTI-FACTORIAL SYMPTOMATOLOGY
This section addresses common SSc symptoms that have multiple or combined causes, approaches to distinguishing cause(s), and where applicable, therapeutic intervention. SSc being a disease of inciting vascular injury, special attention is given to RP in this section, though not a multi-factorial symptom, as it is pervasive and often not straightforward to diagnosis.
A. Cold in SSc, and Raynaud’s Phenomenon specifically, is the most common symptom and highest ranked SSc-specific symptom diminishing HRQoL. Without preventive and palliative intervention, RP can lead to other vascular complications such as DUs, acro-osteolysis and calcinosis.20,53–56 (Table 7) RP affects glabrous skin regions (fingers, toes, nipples, ears, toes). Glabrous skin’s unique vascular structure contains large numbers of cutaneous arteriovenous connections. RP in SSc, triggered by stress or cold has variable duration and severity, generally lasts <20 minutes upon trigger removal, but can endure hours or days, or establish a new baseline severity upon which exacerbations occur.
Table 7.
Vascular history, physical, counseling, therapeutic considerations. (Table courtesy of T Frech and LA Saketkoo, rights reserved)
| Manifestation | Initial History | Current & Past Symptoms | Physical Function/Self Esteem | Exam | Counseling Considerations | Therapeutic Considerations |
|---|---|---|---|---|---|---|
| Raynaud(RP) | - 1st RP recollection - Provoking factors - Location - Frequency - Pain - Duration of attack -Medication use - History of: Gangrene, Surgical amputation, Sympathectomy, Botox injections |
- Pain sensation quality and intensity (numbness, tingling, burning stinging, pain) - Location (ears, nose, fingers, nipples, toes) - Frequency - Color changes |
-Impact on social life -Impact on employment |
-Acro-osteolysis | - Stress management - Warming measures - Discontinue exacerbating medications - Avoid tobacco |
-See table below for medications |
| Digital Ulcers | -Location* -Number -Concurrent infection or gangrene - Duration |
Severity of Pain -Infection -Size - Location - Frequency - Duration |
Impact on social life -Impact on employment |
-Number -Location -Size -Infection - Gangrene |
-Identifying critical digital ischemia | - OT -Wound care -Salves -See table below for medications -See table below for medications - RP Prevention *Ulcers can appear in other locations |
| Pitting | -Location -Pain |
- Pain - Numbness - Location - Frequency |
Impact on social life -Impact on employment |
-Number -Size |
Protective measures | |
| Calcinosis | -Location -Pain -Drainage |
-Pain -Drainage -Location -Surgical needs |
Impact on social life -Impact on employment - Impact on joint function or contractures |
-Number -Size -Location - Attachment to tendons, ligaments, muscle planes |
Protective measures from trauma to site Surgical options |
- RP treatment - RP prevention - Trauma prevention - Surgical removal - Possible IV prostacyclin |
| Telangiectasia | -Location -Change in number |
-Location -Treatment |
Impact on social life -Impact on employment |
-Number -New lesions for last exam -Location |
Cosmetic options | -Laser beam therapies |
| Erectile Dysfunction (ED) | Impact on self-esteem, intimate life | Aerobic exercise may help, attention to cold prevention may help | Referral to ED specialist, aerobic exercise | |||
| GAVE | -See below | |||||
| PH | - See below |
The classic tri-phasic episodes of RP, more noticeable in lesser pigmented populations, demonstrates discoloration with distinct demarcation lines of blanching (white), cyanosis (blue/purple) and then erythematous (red) phase with rewarming, which can be the most painful phase. Not all individuals experience tri-phasic attacks, but some degree of blanching which may be difficult to notice in highly pigmented patients, supports a RP diagnosis.
While Primary RP may affect healthy individuals or be familial, SSc-RP vascular patterns are uniquely associated with vascular injury and vasculopathy. As previously mentioned, ANA presence with abnormal capillaroscopy predict CTD occurrence.13 Similarly, puffy fingers, SSc-specific antibodies and abnormal capillaroscopy are highly predictive for development of SSc57
The impact of RP events on vital organ vasculature or hastening PAH, is lesser known, but patients report that episodes can result in systemic symptoms of whole-body heat loss, debilitating fatigue, headache in addition to worsening pain of DUs and calcinosis.55,56,58 Thus, RP worsens diffuse, diverse disability, making recurrent preventive counselling imperative, with non-pharmacological therapy, e.g. electrical heated gloves and treat-to-target pharmacological therapy often required.54,56,58–60
B. Pain in SSc is often multi-factorial requiring careful discernment to address coinciding diverse, modifiable causes. SSc-pain can be an overwhelming prospect for the clinician resulting in inaccurate ‘fibromyalgia’ diagnoses.61 Careful characterization of each pain type the patient is experiencing is critical towards determining the most appropriate treatment (Table 8). For example, inflammatory pain can manifest as either diffuse subcutaneous edematous tenderness, or skin-tightening, often with accompanying pruritus, neuropathic pain from small nerve fiber disruption, or as joint tenderness, stiffness or aching, or even myalgias possibly requiring systemic treatment(x).56,62 While fibrous shoulder tendinopathy might require targeted physical therapy.
Table 8.
Modifiable causes and treatment of fatigue and pain in SSc. (Table courtesy of LA Saketkoo, rights reserved.)
| Symptom | System/Origin | Potential Causes | Team Involvement/Interventions |
|---|---|---|---|
| Fatigue | Anemia | GI loss, chronic inflammatory disease | |
| Cardiac | PH, diastolic HF, CAD, physical deconditioning | PT and PR teach adapted aerobic and muscular exercises, and breath pattern training OT teaches energy conservation strategies such as pacing, prioritizing and accommodating devices | |
| Respiratory | PH, ILD, OSA | OT, PT, PR as for cardiac | |
| Muscular | Low muscle endurance, muscle strength or reduced aerobic capacity | MT, MMM, PR-PTr, PT for Aerobic exercises, muscle strengthening and endurance exercises, education | |
| Systemic inflammation | Effects on hypothalamic axis, causing systemic malaise, effects on muscle | Immunosuppression, exercise | |
| Psychological | Anxiety, depression, fear, impact of reduced self-esteem and self-image | MT, MMM, PR-PTr, PT, OT, Breath pattern training, Psychologist, Social Worker | |
| Neurological | Pain: ischemic, edematous skin, articular, restless leg syndrome | Assess treatable causes, MT MMM, PR | |
| Malnutrition | Weight loss, malabsorption | Dietary and nutritional counselling | |
| Sleep-related | OSA, nocturnal pain, pruritus, GI symptoms, depression, anxiety, steroid or opioid use | SH, RSS, MMM, MT | |
| Medication-Related | Methotrexate, MMF, nintedanib etc. | ||
| Pain/Dysesthesia | Vascular | Raynaud | EC preventive strategies, MT, vasodilators, PT for aerobic exercise to improve blood flow |
| Sympathectomy for critical ischemia | |||
| Digital ulcers | EC wound care, protective dressing, anesthetics, OT for daily activities, MT, PT as for RP | ||
| Calcinosis | As above, UTPRM: soaking for relief | ||
| Infected digital ulcers/calcinosis | EC red flags, Aerobic exercise to improve circulation | ||
| Dermal | Skin tightening | PT, ST, OT for stretching and manipulation | |
| Subcutaneous edema and pressure | MT,ST, OT as above | ||
| Pruritus | MT, SH, ST, opioid receptor blocker, phototherapy | ||
| Musculoskeletal | Myopathy/Myalgias | MMM, OT, PT, PR-PTr, for strength, endurance and anti-inflammatory effects of exercise | |
| Fibrous tendinopathy | MMM, OT, PT, THE as above | ||
| Inflammatory arthropathy/tendinopathy | MMM, OT, PT, ST, local injections, muscle strengthening, stretching, targeted hand exercises | ||
| Secondary fibromyalgia | MMM, PR-PTr, SH, education | ||
| Gastrointestinal | Heartburn | EC, RH, NH, anti-acid and PPI | |
| Abdominal cramping | See below | ||
| Abdominal bloating | |||
| Genitourinary | Dyspareunia | Pelvic floor therapies, sometimes systemic treatment | |
| Vaginal dryness | Lubricants, topical estrogen | ||
| Erectile dysfunction | Vasodilators, PT for aerobic exercise, specialist referral | ||
Abbreviations: AG = anticipatory guidance, ATT = assessment with targeted treatment, EC = education/counselling, DHS = dental hygiene strategies, ILD= interstitial lung disease, MMM = mindful movement modalities (e.g. gentle yoga, tai chi etc), MT= mindfulness training strategies, OSA= obstructive sleep apnea, OT= occupational therapy, NH = nutrition hygiene (EC on attention to selection, volume, texture, preparation, combination strategies of foods), PAH= pulmonary arterial hypertension, PPI = proton pump inhibitors, POS = practical organizational strategies, PT = physiotherapy, RH = reflux hygiene (including head of bed elevation), RHS = refer to hand specialist, RME = refer to motility expert, THE= targeted home exercises, PR = pulmonary rehabilitationist, PR-EC = pulmonary rehabilitation educational component, PR-PTr = PR physical training component, RSS = refer to sleep specialist, SH = sleep hygiene, SR = specialist referral, ST = systemic treatment, UTPRM = untested patient-reported management[10]
The presence of tendon friction rubs (TFRs), another source of pain from inflammation and tendon sheath irritation, indicates active cutaneous or inflammatory disease that without appropriate treatment, portends a poor prognosis including worsening skin and risk for SRC.38,62,63 Thus, careful tendon examination is necessary; and ultrasound can helpful to assess for active joint inflammation and risk for disability.63
Vascular complications such as ischemic RP, ischemic digital ulcers and calcinosis cause significant, and sometimes constant, pain even at rest.55 Increased intensity of pain and local tenderness may also signal concomitant infection, however, calcinotic lesions are frequently painful in the absence of infection depending on location. Large lesions can occasionally lead to nerve impingement resulting in neuropathic pain symptoms. Pain and discomfort related to the GI system in SSc is diverse. Dry mouth, oral thrush, odynophagia from esophageal candidiasis, abdominal pain and cramping from obstipation or distention are common pain sources that patients experience. Opioid analgesics requires careful consideration for worsening SSc-symptoms e.g. sicca, GI motility; with initiation of preventive regimens being important.
C. Fatigue in SSc, another potentially overwhelming clinical consideration, impacts all areas of daily living, work, parenting, and social participation. There are many types of fatigue: mental/cognitive, motivational, physical, muscular, general, etc. Although non-specific symptom, fatigue can be evidence of several serious SSc complications such as GI bleeding, ILD or PH. Fatigue may also reflect worsening inflammatory disease, malnutrition, poor sleep quality, gastroesophageal reflux (GERD) or the burden of decreased physical function. Further, dyspnea and cough episodes with longer recovery times are exhausting symptoms with high calorie demand and psychological burden. An organized approach to assessing and addressing fatigue can guide investigation.
Sleep disordered breathing is significantly elevated in SSc and beyond fatigue likely impacts cardiopulmonary health(ref).64,65 Epworth Sleepiness Scale and the STOP-BANG questionnaire help identify those patients at risk for OSA and qualify for a sleep study. If warranted, CPAP use improves fatigue and potentially prevents SSc cardiopulmonary and esophageal complications.66 However, as with breathlessness, fatigue in SSc can result from commonplace co-morbidities requiring investigation, such as hypothyroidism and coronary artery disease.
D. Breathlessness and Exercise Intolerance in SSc is often multi-factorial and can be related to myriad, sometimes severe, complications beyond cardiopulmonary involvement, and like fatigue requires thorough investigation. Breathlessness is the most common symptom of ILD, PH and myocardial disease. However, its development is often quite subtle, and patients may not recognize or explicitly complain of dyspnea. Careful questioning of patients’ activity and changes in activity over time is necessary to determine if there has been a significant change (Table 9). Careful historical probing may reveal a history of decreased exercise tolerance, changes in the intensity and duration of daily activities and an unconscious slowing of movement. Further these changes may be apparent to patients’ loved ones when not overtly apparent to the patient themselves. Therefore, screening requires physicians asking appropriate questions and patients recognizing changes to determine if dyspnea is present. Dyspnea or coughing with deep inspiration or activities that engage deeper inspiration such as laughing, sneezing walking-talking suggest a restrictive process like ILD.67–70 ILD, PH, anemia, heart involvement, physical deconditioning and anxiety are each common causes of dyspnea in SSc and are not mutually exclusive. (Table 10)
Table 9.
Screening questions to help patients reflect on potential onset and changes in dyspnea and cough. Courtesy of LA Saketkoo, rights reserved.
| DYSPNEA Screening | COUGH Screening for ILD |
|---|---|
| Do you notice being more short-winded now than one month ago, six months ago, last year while doing activates (consider activities likely for the patient)? | Have you been coughing? More in the past 3/6 months? |
| Do you notice it takes you longer to vacuum, mop, make the bed, mowing the lawn? | Do you cough when taking a deep breath in? |
| Do you notice you are more short of breath when vacuuming, making the bed, mowing the lawn? | Do you cough with laughing or sneezing? |
| Are you able to keep up with family members / peers when walking? Do you feel they slow their pace for you? Do you find it difficult to walk and talk at the same time? |
Do you cough while talking? |
| Do you feel that bending over takes your breath away? | Does coughing make you feel short-winded? |
Table 10.
Common causes of dyspnea and cough in SSc. Courtesy of LA Saketkoo & MB Scholand, rights reserved.
| DYSPNEA | COUGH |
|---|---|
| ILD | ILD – dry inspiratory |
| Pulmonary Hypertension – any or any combination of the following: Groups I, II, III, IV | PND – possible drip sensation, often in morning, sore throat |
| Bronchiectasis* | Bronchiectasis* |
| Cardiac dysfunction or arrhythmia | Heart failure |
| Anemia | GERD – can be ‘wet’ cough / gastroparesis |
| Physical deconditioning | |
| Intrinsic or extrinsic myopathy e.g. restrictive truncal skin involvement (carapace chest), accessory muscle myopathy | |
| General population considerations: CAD, COPD | |
| Disordered breath patterns |
Bronchiectasis can be either traction (extrinsic pulling and distortion of the bronchioles often seen in pulmonary fibrosis on HRCT) or cylindrical (laxity of the bronchiole wall either due to infection or perhaps CTD itself, creating a stasis environment for bacteria cough is often productive)
E. Cough in SSc, the second most common symptom of ILD, is associated with increased ILD severity and worse health-related quality of life (HRQoL).7,8 Cough, though, is often multi-factorial and requires careful historical assessment to differentiate the causes e.g. ILD, reflux, post-nasal drip (PND) or sinus problems. A dry, inspiratory cough limiting inspiratory depth is often ILD-related; and can trigger frightening, embarrassing, exhausting and inconvenient episodes dyspneic coughing that usually have prolonged recovery phases.67–70 Patients often restrict inspiration to prevent this from happening.67–70 The quality of cough varies in patients with SSc-ILD with >50% of patients reporting a cough productive of sputum.8,71
Dysphagia and GERD with micro- or macro-aspiration may produce a wet, post-prandial or early morning cough that often clears or lessens during the day, but recurs at night. However, a dry cough related to GERD can also occur from pulmonary irritation. SSc-ILD patients with GERD reported cough significantly more frequently than SSc-ILD patients without GERD.8 PND can also cause a wet or throat-irritating cough. Cylindrical bronchiectasis, weakening of bronchiole walls creating mucous stasis and sub-acute infection (as opposed to traction bronchiectasis, an extrinsic force causing bronchial distortion often seen on HRCT in ILD) is not uncommon in CTDs often occurring with productive cough that comes and goes, and often improves with antibiotic therapy.
V. SYSTEM-BASED SYMPTOMATOLOGY AND MANAGEMENT
A. Gastrointestinal System manifestations occur in virtually all SSc patients from the oral cavity through the lower GI tract and anus (Fig. 5). Gastrointestinal symptoms are associated with higher patient-perceived disease severity and lower HRQoL, when compared to traditional SSc severity measures (PH, ILD, renal and cardiac).54,56 Multiple and diffuse morphological and functional GI abnormalities result in high degrees of symptom distress, life disruption and diminished HRQoL. These destructive changes are hypothesized to result from progressive sub-/mucosal inflammatory-fibrotic infiltration and vascular insufficiency, leading to neuronal dysfunction, and subsequently to dys-/non-motility.
Figure 5. Depiction of the diffuse nature of gastrointestinal involvement in SSc.
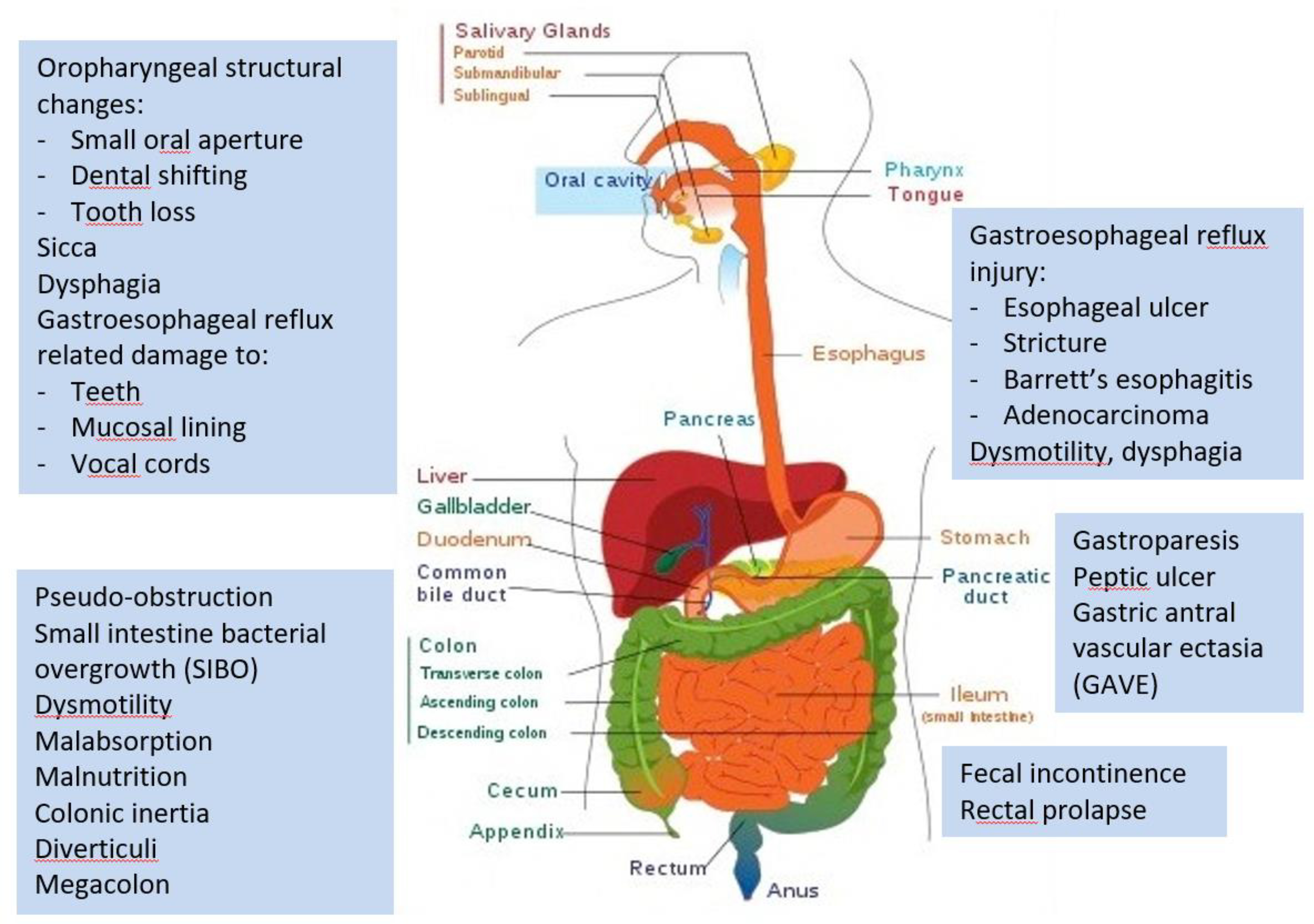
Courtesy of T Frech, rights reserved.
Oro-maxillary and pharyngeal structural changes with painful or difficult mastication and swallowing; esophageal dysmotility with dysphagia; malnutrition from malabsorption or decreased intake; gastroparesis with bloating, nausea/emesis; colonic inertia with constipation; bacterial overgrowth with bloating, abdominal distension and diarrhea; and loss of anal sphincter tone resulting in fecal incontinence. Dysmorphic surface vessels, vulnerable to abrasion, such as arteriovenous malformations and gastric antral vascular ectasia (GAVE), may cause symptomatic anemia with dyspnea/fatigue due to slow or rapid blood loss.
Patients express frustration that despite extent and severity of GI manifestations in SSc, rheumatologists generally avoid GI-related discussion. Anecdotal clinical evidence and patient discussions support that systemic treatment in early SSc disease – as with ILD - may prevent or reverse GI symptom progression.
While esophageal involvement is the most common aspect of GI involvement, weight loss, diarrhea, and fecal soilage can indicate the presence of small bacterial overgrowth requiring treatment.72 Additionally, micronutrient deficiency and malnutrition is a concern in SSc and patients’ appetite and dietary intake should be assessed.43 Working closely with a dietician and gastroenterologist to help guide diagnostic and therapeutic interventions can help with the management of SSc GI involvement.73
Gastroesophageal Reflux Disorder (GERD), a manifestation with far-reaching detrimental effects on the esophagus and the lung, demands dedicated robust attention. The ongoing injury caused to the esophageal mucosa puts patients with SSc at higher risk of pre-malignant and malignant injury, as well as structural abnormalities such as webbing, scarring and the development of strictures. The injury to associated neuromuscular complexes results in dysphasia and poor acid clearance. The absence of heartburn or regurgitation are often discordant with endoscopic findings of esophageal injury and pH testing. Prior to proton pump inhibitor (PPI) introduction, inability to eat from severe esophageal dysfunction, was a major cause of malnutrition and mortality. The advent of PPI use, effected a significant decrease in esophageal strictures. Further, extent of ILD and lung parenchymal inflammation is associated with degree of GERD and uncontrolled GERD, and hypothetically PND poses a similar concern. Chronic GERD or PND can cause hoarse voice or dysphonia. Guideline-based care highlights the value of a multidisciplinary approach and the role for diagnostic testing.74
Severe GERD may not be symptomatic, as early stages require significant neuronal recruitment and in later stages nerves may be dysfunctional to pain perception – but ongoing injury will still occur. The SSc specialist community is largely of the opinion that benefit of empiric PPI use in SSc-GERD outweighs the risks. Often, standard dosing of PPIs may require increased frequency and possibly addition of other agents such as histamine-2-blockers (H2-blockers) e.g. famotidine, or coating agents such as sucralfate. Use of these therapies may require attention to timing of administration to avoid drug-drug interactions.75
However, it is essential that anti-reflux measures are thoroughly explained and strictly practiced. This includes: elevation of the head of the bed to 60 degrees by wedge pillow, mattress elevation, bricked bed legs or automatic adjustable bed; and avoidance of right-side sleeping as gastric contents will spill back toward esophagus. For patients using CPAPs, we underscore that adherence can help to suppress reflux.66
B. Cardiopulmonary Involvement
Pulmonary Involvement in SSc
ILD and PH are the leading causes of SSc-related death. Identifying these entities early and initiating early appropriate treatment prolongs survival.2,9,10 Initial screening in all SSc patients with pulmonary function testing (PFTs) including diffusion capacity of lung for carbon monoxide (DLCO) and high resolution CT scan (HRCT), exercise tolerance and PFTs are key to detecting important changes reflecting developing cardiopulmonary involvement (Diagram 1).
Diagram 1.
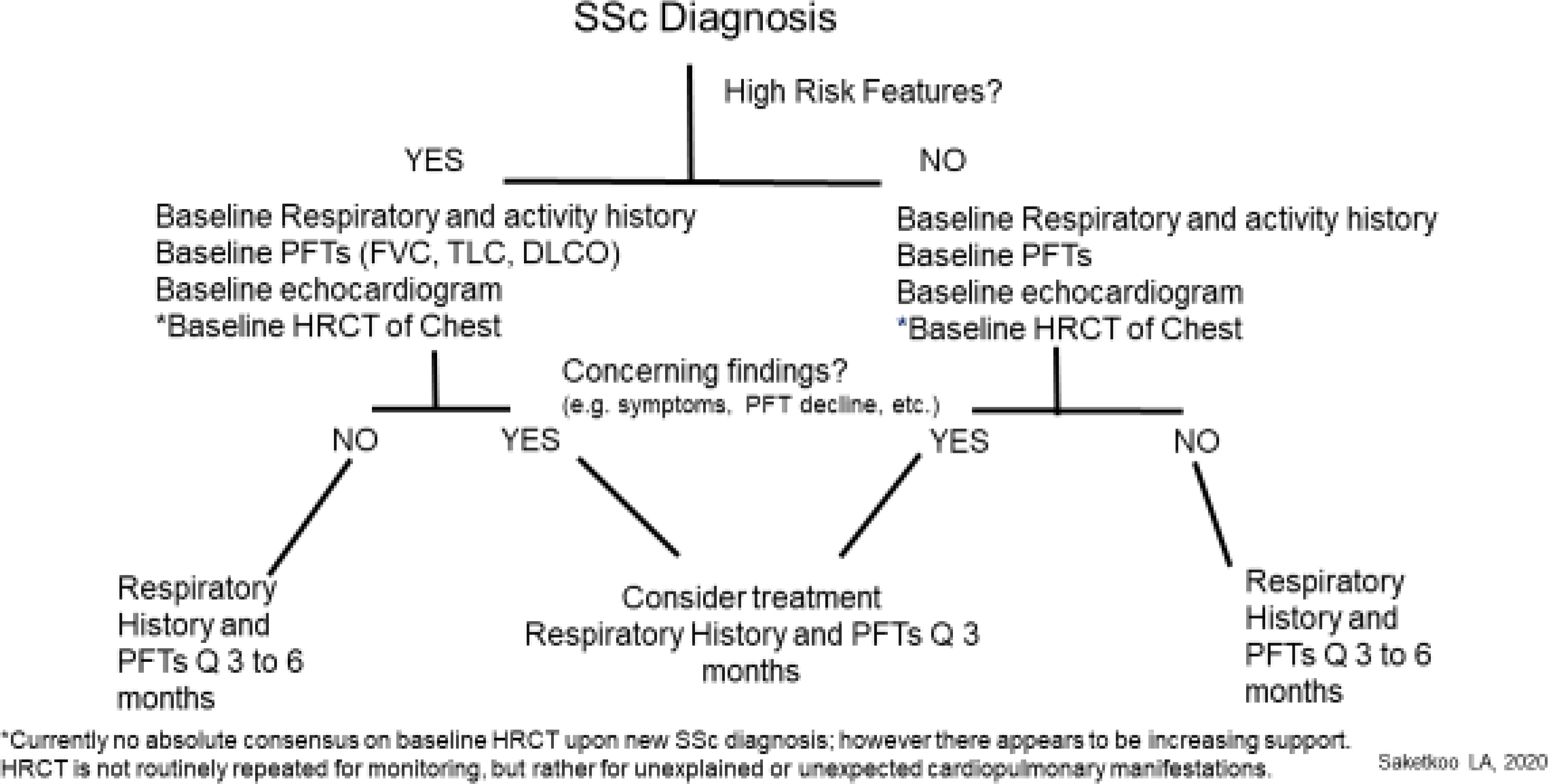
Proposed Screening and Monitoring Algorithm for Clinically Significant SSc-ILD. Courtesy of LA Saketkoo, rights reserved.
It is essential in SSc care to recognize that: 1. ILD behavior is variable across patients (e.g. stable, slowly progressive, rapidly progressive), can change over time and requires individualized and vigilant approach, 2. ILD and PH often coexist, combined PH/ILD occurs much earlier in patients of African descent, 3. Patients with SSc are vulnerable to developing: a) either PH WHO Group 1, 2, 3 or 4, each requiring different therapeutic approaches b). coexistent PH group types (e.g. combined WHO Groups 1 PAH and 2 diastolic dysfunction, combined WHO Groups 1 PAH and 3 ILD), 4. Screening, detection, characterization of PH Group type, and initiation of appropriate treatment demands adherence to clinical diagnostic algorithms and tracking of patient symptoms (Table 11).
Table 11.
Screening and characterization of pulmonary hypertension in SSc. Courtesy of LA Saketkoo, rights reserved.
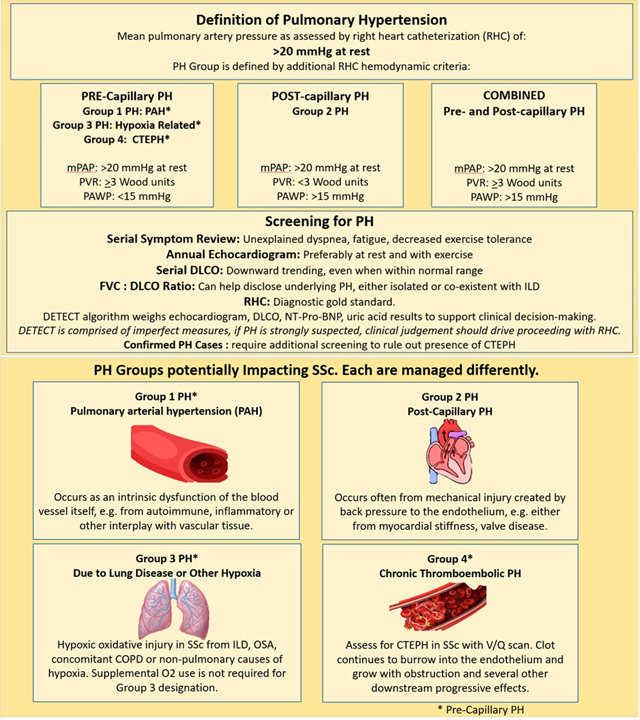
|
Though, without formal consensus amongst SSc specialists, HRCT is the gold standard for screening for ILD in SSc. Numerous studies demonstrate that PFTs are inadequate in detecting ILD in this population, particularly early in the stages.71 However, insurance constraints may limit the ability to obtain this study in a limited cutaneous, asymptomatic patient with normal PFTs. Follow-up PFTs with careful trending are crucial. Repeat HRCT is indicated for unexplained symptom changes (dyspnea, cough), PFT worsening (drop ≥10% in FVC or 5–10% fall in FVC with ≥15% decrease in DLCO) to investigate co-existent infection or malignancy versus progressive ILD. Bronchoscopy is reserved for co-existent concern of infection or malignancy. Lung biopsy is not warranted for diagnosing SSc-ILD in patients with SSc with a typical HRCT pattern i.e. usual or non-specific interstitial pneumonitis (UIP or NSIP).
Documenting serial PFT data along with temporally coincident medication dosing and any contextual factors that might explain an aberrant PFT performance on that day (e.g. sinusitis, allergies etc) is an essential investment in the care of SSc patients. Charting the trajectory beginning from first available PFTs affords insights into disease behavior, e.g. rapidly progressive vs. stable vs. slowly progressing ILD.76 Furthermore, it protects the clinician from overlooking progressing disease in the context of normal range values, as a 5% decrease in FVC over 6 months (or 10% annually) despite normal values warrants investigation and possible changes to or additions to treatment.
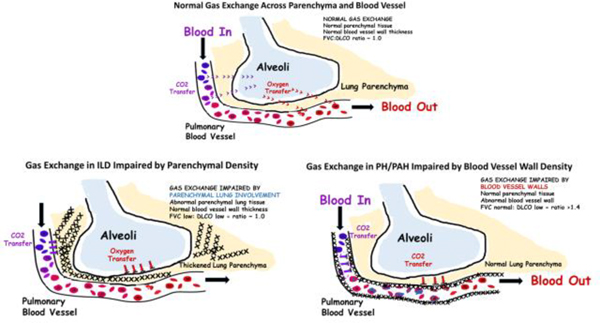
Though serial FVC is considered a reliable reflection of restrictive lung disease, DLCO can be a key differentiator between parenchymal versus vascular lung disease, and provide an early detection mechanism for pulmonary hypertension. While FVC reflects restriction related to parenchymal lung disease, DLCO reflects the ability of gas to cross from airspace to bloodstream which requires gas to diffuse across two barriers: the lung parenchyma and also the blood vessel wall (Figure 6). If either or both are resistant to permeable gas, as can occur in SSc-ILD or SSc-PH this will cause reduction in DLCO. In parenchymal disease the FVC and DLCO commonly trend downward in parallel; while in vascular disease the DLCO has a much steeper decline than FVC (Figure 7). However, early in the course of SSc-ILD, the FVC may be normal, while the DLCO is often decreased. Over the course of SSc, the FVC:DLCO ratio may help distinguish pulmonary vascular disease from progression of SSc-ILD with a higher ratio suggesting a predominant pulmonary vascular process(x).77,78 Therefore, in addition to yearly screening echocardiogram at rest and with exercise, DLCO is an important indicator of pulmonary vascular involvement.
Figure 6.
Diffusion Capacity of the Lung for Carbon Monoxide Measures the Ability of Gas Transfer (illustrations courtesy of LA Saketkoo, rights reserved.)
Figure 7. DLCO behavior in ILD versus PH Predominance.
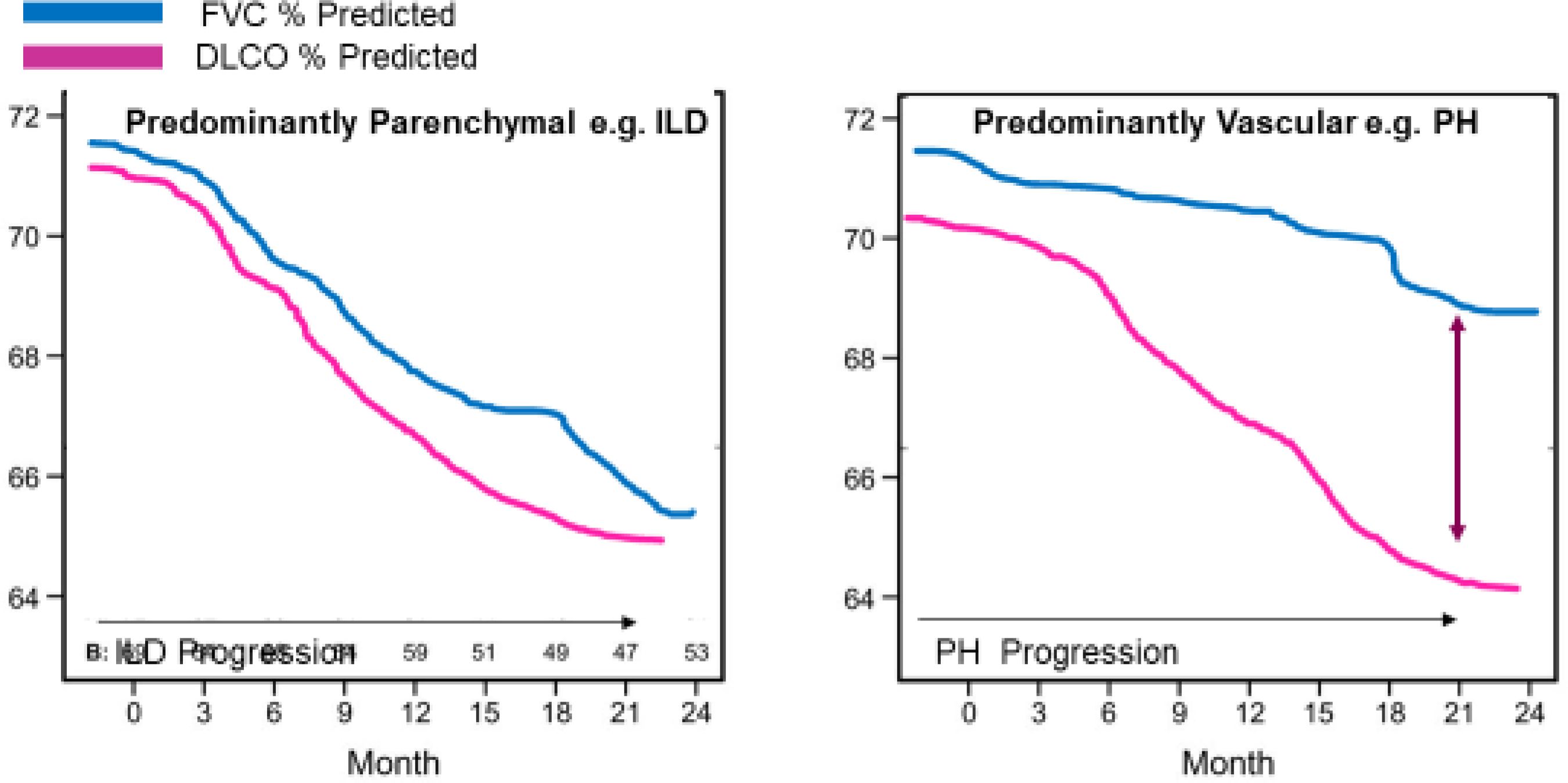
The closer the ratio of FVC:DLCO is to 1 the more likely abnormal changes are related to restrictive lung disease. (illustration courtesy of LA Saketkoo)
Cardiac Involvement in SSc may result from microvascular insufficiency, or inflammatory-fibrotic infiltration of the myocardium, causing arrhythmias, diastolic or systolic dysfunction, pericarditis, or myocarditis which are managed similarly to non-SSc cardiac complications. SSc-specific treatment is yet unclear, and likely depends on suspected disease activity. Baseline/annual echocardiogram serves as a comparison should cardiac problems or PH develop later. Non-contrast cardiovascular magnetic resonance (CMR) demonstrates 45% prevalence of myocardial fibrosis unexplained by other causes and often associated with diffuse skin involvement and elevated ultra-sensitive CRP; CMR may play a role in early diagnosis. 79–81 Additional serum biomarkers that are commonly followed as predictors of onset and worsening are NT-Pro-BNP and uric acid, of which NT-Pro-BNP has demonstrated reliably properties.
Routine cardiovascular risk reduction with blood pressure monitoring and lipid screening is encouraged in SSc patients. Cardiac involvement was often found to be associated with SSc-myopathy in several studies.79,82–85
When to Consider Transplantation:
Despite prior misconceptions of worse outcomes for patients with SSc (for ILD, PH, or both) compared to those with non-SSc lung disease, lung transplantation in SSc is safe, with similar survival outcomes. Lung transplantation is reserved for patients whose lung disease progresses despite maximal systemic therapy. (Table 12). Early referral for transplant evaluation permits time for patients and caregivers to become familiar with the transplant process, make informed unhurried decisions, and adjust to psychosocial and financial pressures related to transplant.
Table 12:
When to Consider Referral for Lung Transplant
| Diagnosis | Indications for Referral |
|---|---|
| Interstitial lung disease | • Radiographic or biopsy proven disease • FVC <= 80% • Need for supplemental oxygen |
| Pulmonary hypertension | • Severe functional limitation with NYHA functional class III or IV • Rapid decline in functional status • Decreasing 6MWT • Increasing oxygen requirements • Need for intravenous therapies |
| Myocardial disease | • RV failure without evidence of RV infarction (isolated RV failure related to pulmonary hypertension [any WHO class] recovers after lung transplant) • Irreversible LV involvement, heart-lung transplant evaluation may be warranted |
Abbreviations: 6MWT, 6-minute walk test; FVC, forced expiratory volume; LV, left ventricle; NYHA, New York Heart Association; RV, right ventricular; WHO, World Health Organization
Most common barriers to lung transplant in patients with SSc can be overcome (Table 13). Physical conditioning is an important factor in transplant selection and successful post-transplant recovery. Early referral gives patients who are deconditioned an opportunity to engage in healthy lifestyle changes and home fitness practices supported by pulmonary rehabilitation.
Table 13:
Common Challenges of Lung Transplant Evaluation in Patients with Systemic Sclerosis
| Challenge | Considerations |
|---|---|
| Obesity | • BMI <35, preferably <30 and transplant center dependent • Early counseling regarding healthy weight |
| Age | • Highly variable and transplant center dependent |
| Frailty/Deconditioning/Post-transplant rehabilitation potential | • Pulmonary rehab participation • Frailty Assessment Score • Chronic pain/Advanced osteoporosis |
| Active substance abuse/dependence | • 6 months sobriety with only rare exceptions • Participation in counseling |
| Esophageal dysmotility | • Full evaluation of esophagus • GJ tube for full nutritional support may be recommended, assessment for willingness and compliance |
| History of malignancy | • Time free from malignancy is multifactorial and transplant center dependent |
| Social support | • Identify 24-hour caregiver for at least 3 months • Some transplant centers also require a committed back-up caregiver • Caregivers will be evaluated for appropriateness |
| Finances | • Financial counseling to establish ability to afford transplant • Fundraising may be required/recommended • Insurance clearance required before evaluation is initiated |
Abbreviations: BMI, body mass index; GJ, gastrostomy-jejunostomy
Potential transplant candidates with SSc undergo extensive testing to identify needed interventions for SSc manifestations that might overtime injure the allograft, e.g., Nissen fundoplication for severe GERD or heart-lung transplantation with coexistent irreversible myocardial disease. Severe esophageal dysmotility or GERD (lower esophageal sphincter incompetency) lead to chronic aspiration which poses significant risk for acute and chronic allograft rejection, may also warrant GI tube for nutrition posttransplantation.
C. Muscle Involvement in SSc is under-recognized and multi-factorial and ranging from atrophy, inflammatory, vasculopathic, fibrotic to necrotic pathology. Both muscle strength and endurance in proximal muscles are commonly reduced, especially in patients with significant lung disease.86 Systemic treatment and exercise can improve SSc myopathy, with physical therapy targeting strengthening and prevention of large joint contracture, particularly in the shoulders. (Table 14)
Table 14.
Common Therapeutic and Surgical Referrals Resourced in SSc Care. Cultivating referral relationships with colleagues who are interested in SSc may have best outcomes for people living with SSc
| THERAPEUTIC/SURGICAL | INDICATIONS |
|---|---|
| Occupational Therapy | For hand, face and oral health Joint and skin mobility Self-management, breath pattern training Home and work adaptations |
| Hand Surgery Vascular Surgery |
Early referral (for physician/patient familiarization) for patients at high risk for vascular or wound complications including: Critical ischemia DUs/calcinosis complicated by infection Calcinosis complicated by nerve entrapment Macrovascular occlusion For procedures including sympathectomy, botulin toxin injections |
| Physiotherapy | For building muscle strength, muscle endurance and aerobic capacity Increasing physical capacity and activity Balance, joint/skin mobility Education on fatigue and pain |
| Pulmonary Rehabilitation | For enhancing aerobic capacity, endurance and education on cardiopulmonary efficiency Includes Singing, Yoga, Dance for Lung Health programs |
| Dental care / Oral surgery | At least twice yearly Access to pediatric is a consideration Dry mouth care Preservation of dentition |
| Speech | For swallowing, exercises for mouth strength and speech production |
| Nutrition / Dietetic Care | To enhance calorie intake, detailed counselling on gastroparesis and food tolerance strategies |
| Wound Care | Management of DUs, calcinosis |
| Hyperbaric Therapy | For DUs, avascular necrosis, general wound healing |
| Psychological Support and Counselling | For managing anxiety, depression, impact of changing appearance on body image and self-esteem Developing coping skills to manage changing ability, uncertainty |
Consideration of medication-related myopathy culprits, such as statins, steroids and hydroxychloroquine, is a mainstay of investigation. SSc-myopathy predicts SS-related cardiac involvement.79,82–85
D. Hands in SSc are especially subject to diffuse morphological changes, impairment and pain due to inflammation, vasculopathy and fibrosis. These pathological processes result in bony, periarticular and cutaneous destruction with infection, ulceration, calcinosis, acro-osteolysis, flexion contractures, synovitis, tendinopathy and amputation. Arthritis, contractures, tendon friction rubs come early during the disease course, therefore require early intervention. The role of hand exercises in SSc is critically important. Exercise improves circulation, healthy vascular and skin repair, increases warmth, reduces local inflammation and stiffness, and very importantly, increases muscle strength and hand function. Preventive strategies to maintain hand warmth may help to prevent further vascular injury (Table 14).
E. Renal Involvement in SSc: prior to the availability of Angiotensin Converting Enzyme (ACE) inhibitors renal crisis was the leading cause of death in SSc. Early intervention with ACE inhibitor therapy and rapid control of blood pressure may abort a “crisis” and minimize renal damage. However, with close monitoring of high risk patients including prednisone use >10 mg/day, abrupt and severe BP elevation, presence of anti-RNA polymerase III, we can identify and aggressively treat SRC. Late recognition, delayed or inappropriate therapy persist and result in renal failure and other complications of malignant hypertension. Despite progression to end-stage disease, with continued treatment with ACE inhibitors, renal function may return months after initiating dialysis. Educating patients at higher SRC risk on warning signs and plan of action is crucial to improving outcomes, including consideration of home blood pressure monitoring, and providing the “renal crisis prevention card” (Fig 9) on a patient’s first visit for use in emergent situations.87
Figure 9.
The Renal Crisis Prevention Card may help patients direct emergency healthcare providers to abort a crisis and avoid adverse outcomes.83
VI. IMPORTANT NON-PHARMACOLOGICAL THERAPEUTIC CONSIDERATIONS
A. Exercise as an Essential Multi-Modal Disease-Modifying Medicine
Physical function and activity are key predictors of HRQoL and survival. Available evidence on exercise strongly supports diverse and diffuse benefits of physical activity as a potential cornerstone to SSc management88. (Table 15) Exercise reduces inflammation and increases circulation (and body heat), which are essential drivers of SSc symptoms, in addition to enhancing mobility through improving strength, stiffness, endurance and aerobic capacity. Physical activity is critical for all levels of ability and for modulating the biochemical impact of depression/anxiety, stress and physical pain – while improving self-esteem in a disease notorious for diminished self-image. Exercise’s muscle and vascular benefits likely contribute to its beneficial impact on sleep and fatigue.89 Increasing physical activity and reduction of a sedentary lifestyle in SSc is crucial to self-management, even in mild pulmonary involvement.90 Patients with SSc desire physician counselling and augment their physical activity accordingly. A routine visit should document patients physical activity, counsel on the medicinal effects of exercise and advise that exercise be pleasurable, working up to ≥30-minutes/day, 5 days weekly; with hand, face and feet exercises to increase circulation, mobility and anti-inflammatory profiles regularly reviewed. (See resource list) Further, a long-term physically active lifestyle improves GI motility, and favorable enhancement of gut flora.91
Table 15.
Essential counseling on exercise in SSc. Note: in many countries, physiotherapists also teach breathing exercises to optimise breath patterns and strength of breath. General exercise applications are also a part of cardiopulmonary rehabilitation programs.
| EXERCISE GENERAL APPLICATION | STRETCHING | RESISTANCE | AEROBIC | MONITOR |
|---|---|---|---|---|
| Both upper and lower extremities with dedicated focus on areas with impaired range of motion (e.g. shoulders/pectorals, calves, hamstrings and external rotators in hip). Easier to do when warm, after exercise or sauna |
Ideally >15 reps for 3 sets at least twice a week To gain muscle mass To preserve overall muscle strength |
Warm-up and cool-down important esp PAH, treadmill, ergometer cycle Ideally 30 min, 3 days/week |
At least initially with PAH and/or ILD: SpO2, heart rate, blood pressure. Dyspnea and muscle tiredness with Borg CR-10 and exertion with Borg RPE | |
| OROFACIAL | ||||
| Emphasize -mouth opening -facial grimaces such as smile, pucker lips, etc |
Isometric-hold positons - see resources |
N/A | -use ruler to monitor - see resources section |
|
| HANDS | ||||
| Emphasize -Flexion MCP and IP joints -Extension PIP joints -First commissure) -Finger web spaces (interdigit) -Wrist flexion and extension |
-Squeezing foam, dough, putty) -Finger extension – rubber bands/putty as resistance -Rolling out dough/putty with finger -Pinch with foam, dough, putty (finger tips to thumb, thumb to side of index finger) |
N/A | Hand tracings: - In extension - In fist Grip and pinch strength - see resources section |
|
| Heat modalities prior to stretching enhances practice (e.g. paraffin wax, warm water, etc) | ||||
Abbreviations: N/A = not applicable, MCP = metacarpophalangeal joint, PIP = proximal interphalangeal joint
B. Anticipatory and Preventive Education
Keys to Patient-Centered Outcomes
Counselling and education (Table 16) on a model of shared decision-making (SDM) (Box. 1) provide patients with insight into this complex disease as pertains to their circumstances, cultivates clinician-patient partnership, and increases patient trust, adherence, self-efficacy, and mental health – all essential to patient outcomes. SDM is an ongoing process requiring time to ensure clinicians understand and address the patient’s perceptions, priorities and self-management activities of their disease experience. SDM enables effective palliation and protection against disease progression and complications. Patients with SSc often feel fearful, scared and isolated especially as families, friends, and other health care providers are not familiar with SSc. SDM and providing resources on self-management strategies and support groups are imperative.92,93
Table 16.
Key Elements of Recurrent Counseling (Courtesy of LA Saketkoo, rights reserved)
| Category | Sub-Category | Item | Advisements for Patients |
|---|---|---|---|
| VASCULAR | Raynaud | Prevention is key | - Related complications include DUs, calcinosis, osteolysis and core temperature loss - Initiate protective measure in anticipation of and upon noticing a cold atmosphere, before allowing oneself to ‘feel’ cold - Immediate action can result in decreased recovery time, pain and the sequela associated with loss of core warmth (fatigue, headache, incapacity etc.) - Avoid extreme temperature changes, e.g. from cold to warmth - Anticipate cold environments, e.g. air conditioning in summer, grocery store freezer aisle, hospitals etc. |
| Core Temperature | - Exercise / movement increases circulation and body heat - Clothes layering and use of insulated vests |
||
| Peripheral | - Gloves / socks always at hand - Should allow for a thin space to trap a warming layer of air - Pocket hand warmers, can be placed in pockets, gloves, socks, undergarments - Heated gloves / insoles/shoes |
||
| Digital Ulcers / Calcinosis | Protection | Cushioned bandages for high friction areas Waterproof gloves for washing or handling wet items Bandage and gloves for handling dry household items potentially snagging healing ulcers and to protect from bacteria and chemical irritants Exercise gloves for use of gym equipment |
|
| Pain management | - Protection as above - Topical lidocaine - Cleansing routine |
||
| Signs of infection | - Increased pain/tenderness - Redness - Purulence |
||
| Prevention | As much as possible avoid: - Cold exposure - Trauma Topical antibiotics with signs of infection |
||
| Additional calcinosis | Advisement | - Avoid digging to prevent infection - If intolerable can try repeated soaking in warm Epsom salt water - Topical antibiotics |
|
| Erectile dysfunction | - Increased physical activity may help protect circulatory and neuronal function - Preventive measures as for RP might have a protective effect |
||
| NUTRITION | Calorie intake | Nutritious | - Avocado - Nuts, nut butters - Cheeses, butter - Potatoes, rice - Olive and other oils |
| Food Tolerance | Nutritious | - Pureed foods (soups, dips, stews) - Smaller amounts of a food - Foods softened (marinated) with small amounts of citrus or vinegar - Mobility after eating to increase motility |
|
| HEENT | Oro-facial | Facial Exercises and Massage for skin tightness, mobility and circulation | |
| Oral | High risk for dental complications: - Essential follow-up with a dental clinician sensitive to SSc care or perhaps pediatric dentist - Proactive dental care - Keeping mouth moist - Adapted and powered devices for teeth and oral care |
||
| SICCA | Wetting and pro-salivation products Possibly singing, humming, chanting and exercise |
||
| CARDIOPULMONARY | Graded exercise essential to health | ||
| Control of GERD and PND to avoid lung injury from micro-aspiration | |||
| Vaccination for prevention of infection | |||
| PH and Cardiac | Monitor for symptoms of heart failure | Daily weights as needed; recording of post-void morning weight Alert MD of new onset lower extremity edema |
|
| GASTROINTESTINAL | |||
| GERD | Esophageal Injury & Lung Risks | Reflux in SSc is a serious issue of which related injury can lead to multiple complications that impact mortality. - Often exists without pain - Pain not equate severity - Esophagitis - Esophageal cancer - Dysphagia and potential loss of swallow function - Strictures & Webbing - Need for esophageal stretching - Acid aggravates lung disease |
|
| Medications | - PPI daily or twice daily, especially with esophagitis or esophageal ulcer - Adding PRN or OTC agents (e.g. sucralfate, H2 blockade) -- it is perceived that in SSc the benefits of PPIs greatly outweigh associated risks |
||
| Sleep Essentials | - Head of Bed Elevation (wedge pillow, leveraging mattress, bricks/books under bed legs) - Avoid right side lying |
||
| Reflux hygiene | - Smaller, more frequent meals - Avoid meals 2–3 hours before lying - Avoid sphincter relaxants at end of day e.g. alcohol, chocolate, caffeine, mint etc. |
||
| Gastroparesis | - Sleep and hygiene as for GERD - Exercise / walking may help - Gravity strategies for passive digestion - upright position - attention to food consistency e.g. thinner foods - Gastroparesis dietary suggestions for food tolerance |
||
| Bloating | - Exercise for motility - Small frequent meals |
||
| Nausea | SSc or Medication related | - Mobility / exercise to decrease nausea - Ginger sweets, drink - Sucking candies - Cold pops - Instruction on PRN anti-emetics |
|
| Diarrhea | SSc or Medication related | Logistics until controlled: change of clothes, time planning Medication use: risks / benefits / when |
|
| MEDICATION | See Appendix of Medications | ||
| VACCINES | See Table 17 | Pneumococcal immunizations per CDC guidelines Influenza annually Herpes zoster (killed only i.e. Shingrix) COVID-19 |
|
| EXERCISE | Improves: - Circulation and vascular responsiveness - Body warmth - Sleep - Self-Esteem - Breathlessness - Joint mobility stiffness and lubrication - Skin function - GI function - Possibly erectile function - Nausea - Salivation - Respiratory performance - Cognitive clarity Decreases: - inflammation - Pain (anywhere) - Joint stiffness - Possibly contractures - Possibly skin tightness - Depression - Stress - Fatigue |
||
| WOMEN OF CHILD-BEARING AGE | Medication toxicity | - Use of contraception essential with specific IS and PAH medications - Discontinuation of specific IS or PAH medications prior to conception |
|
| Conception | - Must be a planned - Medication washout pre-conception - Discuss assessing extent of ILD, PH, cardiac or renal involvement in light of safe pregnancy |
||
| Care of children | -Adaptations for child care - Strategies to manage fatigue |
||
| PSYCHOLOGICAL | Advocacy / Education Groups Local support groups |
||
| Online self-management program (see resources) | |||
Box 1. Checklist to Support Shared Decision-Making (Courtesy of LA Saketkoo, rights reserved).
Shared Decision-Making Checklist
Name the patient’s items of concern as presented by the patient and if possible which are highest priority
Ascertain patient’s thoughts on the potential underlying cause/s
Name the items of concern from the clinical perspective including short and long-term (e.g. potential progressive damage, associated abrupt complications etc)
Respond to patient’s perceptions of potential cause in support of & clarifying divergence from patient perceptions. Remain transparent in what is known, unknown, yet to be known and that which requires researching by the clinician.
Name the treatment options available, including any non-pharmacological with particular attention those suggested by the patient
Discuss safety, side effects and efficacy (including anticipated onset) of available therapies and those suggested by the patient.
Assess Patient Expectations of treatment
Set Treatment Expectations including prognosis, anticipated degree of symptom/impairments resolution, cure versus slowing progression, disease activity versus damage
Routine Health Maintenance
The medical complexity of SSc often overshadows the importance of routine health maintenance (RHM). RHM addresses preventive strategies (table 17) directly related to SSc complications. Vaccinations prevent severe pneumonia and influenza in ILD/PH. Age-appropriate cancer screening becomes increasingly significant given the higher malignancy risk in SSc particularly with anti-polymerase III positivity. Screening for cardiovascular disease and OSA may prevent worse outcomes in those already with cardiopulmonary and circulatory impairment. SSc portends a higher risk of osteoporosis94,95 and fractures, and lower vitamin D absorption with chronic PPI use.
Table 17.
Routine Health Maintenance in SSc. Courtesy of N Sandorfi, rights reserved.
| Immunization | HPV (ages 16–26), Influenza (yearly), Hepatitis B, Pneumococcus (at any age with immune disease), Diphtheria/Tetanus/Pertussis (ages 19–64), Varicella Zoster killed (ages 50 or older), (*) COVID-19 |
| Age, sex and risk factor based cancer screening | Gynecological, prostate, gastrointestinal, skin (*) Higher risk exists around the time of disease onset for those with anti-RNA polymerase III positivity (with consideration of breast, lung, prostate and tongue cancer) |
| General | Hypertension, diabetes cholesterol, sexually transmitted diseases (*) |
| Osteoporosis | Women ages 65< or earlier if risk factors exist (special considerations in SSc: malabsorption, corticosteroid use, prolonged use of proton pump inhibitors) (*) |
| Ophthalmology | Special considerations: sicca symptom related complications, hydroxychloroquine associated toxicity (**) |
| Dental | Routine exam and sicca symptom related complications (***) |
| Psychological | Chronic disease related psychological conditions (depression/anxiety) (****) |
| Laboratory | Tuberculosis, hepatitis C/B screening related to pharmacological therapies |
Other essential pre-treatment RHM include infectious hepatitis and tuberculosis screening, consideration of antibiotic prophylaxis, and recurrent pregnancy planning with females on immunosuppression. As with exercise, keeping RHM as part routine documentation framework will protect health outcomes in SSc.
VII. PRACTICAL CONSIDERATIONS FOR PATIENT AND CLINICIAN SUPPORT
A. Pre-Visit Preparations
Patients require time to be heard and time to hear important concepts related the condition they are living with. Protecting one’s attention and time to address pivotal patient care issues is crucial to outcomes when caring for people with multi-organ system disease with multiple debilitating manifestations. Attention to patient environment and comfort, anticipatory scheduling to consolidate medical appointments and employing operational throughputs e.g medical record attainment and chart review that support pre-visit data collection, scheduling realistic visit appointments (with appropriate billing) facilitate a greater ease of communication for patient and clinician and protects one’s dedicated time with the patient. (Tables 18–19 and resources).
Table 18.
Patient-Centered Visit Preparations
| Environment | |
| Temperature | Cold can be injurious in SSc Control clinic temperature exposure via either: - thermostat adjusted to >72F/22C - or with blankets for dedicated patient use Patients should be advised to bring clothing layers to maintain warmth for areas outside of clinic control |
| Fragrance-free | A perfume-free policy maintains a safe environment for patients, family members and clinic staff: - fragrance can trigger dyspneic coughing episodes in patients with ILD - fragrance can impede PFT performance for that patient and other nearby patients or those who subsequently in the suite Advisement occurs prior to visit, during scheduling. |
| Supplemental Oxygen Availability | Many patients with SSc use supplemental oxygen tanks that hold a limited oxygen supply. Upon patient arrival, switching their tank to a clinic tank: - assures sufficient supply for their visit - preserves supply for the patient’s journey home If this can’t be done, patients should be advised of anticipated length of time at the facility for which they will need to have sufficient supply. |
| Wait Times | Multiple procedures on a single day can be exhaustive and unanticipated. Helping patients and families anticipate their needs with the following advisements creates a more comfortable (and safe) experience: |
| Nutrition Needs | - Bringing snacks and lunch |
| Hydration | - Having water or preferred beverages available |
| Down time | - Reading materials etc. to pass time waiting between tests and visits |
| Visit Times | Assessment, intervention and counselling in SSc that is sufficient to reduce SSc-related symptoms and complications requires time. |
| New Patients | ≥90 minutes but can require 3 hours, depending on disease extent, complications and initiating management |
| Established Patients | 45 to 90 minutes as even stable patients with SSc requires multi-organ assessment and SSc-specific counselling that is beyond a usual visit for most other conditions |
| Chart Review | SSc management is often time-sensitive and relies upon diagnostic testing and symptom history trended overtime. New patients often require extensive data organization; while Interval history is often dense for established patients. Appropriate chart review requires pre-visit attainment of past and interval medical records. Documentation of serial data points prior to visit facilitates proactive management, freeing up clinician attention for meaningful patient-centered discussions. Real-time interventions and counselling may result in: - fairly immediate relief of some disabling symptoms - prevention of disease progression Chart review is reimbursable in the US, whether on same day or other day. See resources list for medical record intake template and other clinic support documents. |
| Consolidating Testing | Consolidating SSc diagnostic testing in as few visits as possible reduces travel burden, employment impact on patient and family |
| Out-patient | Past records, pre-visit labs, PFTs, HRCT or echo, ordered prior to scheduled visit, with results forwarded for clinician review, expedites management during the visit. Same day SSc-diagnostic testing, prior to clinician visit: - Common approach at SSc centers: coordinating SSc testing to occur same day, prior to scheduled visit |
| In-patient | An alternate approach for new and established patients at SSc centers in some countries with: - Approximate 2 day hospital admission - All routine SSc diagnostic testing and any further testing as indicated by - Evaluation and teaching by PT/OT - Management with counselling over one or both days Patients also visit as out-patients for interim monitoring, repeat testing |
Table 19.
Quality healthcare provision sufficient to address the most pressing and essential aspects of SSc care and other complex multi-system disorders, demands time. Accounting for time usage is foundational for reimbursement or, especially for countries and provinces where complexity is not accounted for, an advocacy metric for policy revision. Using the 2021 policies from U.S. Centers for Medicare & Medicaid Services is used as an example below, similar constructs may exist in other countries.
| Discrete Care Events | Stipulations for billing in the US | Coding | ||
|---|---|---|---|---|
| Chart Review/Pre-visit Charting/Post-visit Charting | If occurs same day as patient clinic visit | See below | Tabulate in the time-based visit coding using prolonged codes | |
| If occurs on a day other than actual clinic visit If occurs as a non-visit consultation |
Stand alone codes | - 99358 for 31 to 74 minutes on non-direct patient care - 99359 for each additional 15–30 minutes i.e. added for 75–90 min and again for 104 –120 minutes (maximum) |
||
| Clinic Visit |
No direct patient time requirement Primary code to which other codes are added e.g. prolonged clinician or staff codes |
Complexity-based | See below | |
| Time-based | ||||
| Patient-initiated queries | Telephone or internet-based communication with patient that is unrelated to a visit 7 days prior nor leads to visit in next 24 hours | Time-based for communication and tasks related to query | 99441 5 –10 min 99442 11–20 min 99443 21–30 min |
|
| Clinic Staff Time | Added to clinic visit code, for prolonged services by staff beyond the typical time | 99415 45 – 74 min 99416, each additional 15–30 min |
||
| Clinic Visit Breakdown for Time-based Coding | ||||
| New Patient | Established Patient | |||
| 99202 15–29 minutes | 99212 10–19 minutes | |||
| 99203 30–44 minutes | 99213 20–29 minutes | |||
| 99204 45–59 minutes | 99214 30–39 minutes | |||
| 99205 60–74 minutes | 99215 40–54 minutes | |||
| Prolonged Coding | ||||
| 99205 or 99215 need to be fulfilled then | ||||
| Codes that may be obsolete in 2021 | 99354 additional 30–74 minutes 99355 for each further 15–30 minute increments |
Possible new 2021 codes Added for each additional 15 min increment |
CMS Code: G212 AMA Code 99417 |
|
B. Implementing the Plan
SSc is a health condition whereby timely treatment initiation of active disease is key to reversibility of impairment and prevention of permanent damage; and whereby even palliative intervention of irreversible damage can immediately optimize HRQoL, workability, nutrition, mental health and physical well-being and function. Patient education on self-management and engagement with SSc education and advocacy organizations such as the Scleroderma Foundation, Scleroderma and Raynaud’s UK (SRUK), Federation of European Scleroderma Associations (FESCA), Scleroderma Australia and Scleroderma India, are central to successful care.
Delays occur in scheduling diagnostic procedures, therapist and consultant referrals, and treatment initiation greatly impacts patient outcomes. Enlisting an ‘extended team’ of scheduling contacts in other hospital areas who understand the precarious nature of SSc, facilitates teamwork in proactive timely and patient-centered scheduling and prior-authorizations.
Prior Authorizations (PAs):
Advocacy for streamlining PAs96 is essential to improve patient survival and outcomes. Further, denials lead to higher insurance company costs,97 as most clinicians finally attain authorization.98 An organized approach can expedite most procedures and medication authorizations, including an appeal, within 72 hours in the US. Correspondence logs to track initiation, requests for additional information and appeals (see Appendix) streamline response times. In the US, any licensed health professional (medical assistant, nurse) are considered ‘peers’ in a ‘peer-to-peer’ review; and can obtain approval with increasing efficiency overtime.
The Scleroderma Foundation attained Medicare approval for MMF as a first-line therapy in SSc. However important treatments, e.g. rituximab, may not initially receive authorization. However, adding ‘co-existent diagnoses’ that satisfy authorization requirements is reasonable from insurance peer reviewers’ perspective, e.g. ‘seronegative rheumatoid arthritis’ or ‘lupus’, as long as manifestations (e.g. inflammatory arthritis, ANA positivity) can be supported with documentation99 that states ‘clinical features consistent with____’ (co-existent diagnosis). Insurance company requirements for TNFinhibitors failure before rituximab or tocilizumab, are easily refuted explaining contraindication in patients high risk for fibrotic lung disease. Conveying SSc statistics e.g. 50% mortality and ensuing disability without appropriate treatment is generally effective.
Proactive Procedure Scheduling:
Again, consolidating procedures and clinic appointments enhances overall HRQoL. Appointments can be costly to patients and their families in terms of travel expenditures and time, work productivity and income loss100 but also over-medicalizes patients’ lives.
Referral Fulfillment:
Clinician-to-clinician communication is key to conveying the expeditious nature of clinical concerns. ‘Extended teams’, mentioned above, serve to expedite timely scheduling for therapy and specialist consultations.
C. Benefits of Concurrent Care between SSc Specialists and General/Local Specialists
Concurrent care, mutual decision-making and close communication between a recognized SSc-center and a patients’ local rheumatologists, pulmonologists and other specialists, benefit SSc patients. SSc remains a complex disease, with professional education disproportionately represented by industry’s easily misinterpreted therapeutic messaging. Patient volume at SSc-centers habituates attention to the subtleties and complexities of SSc care and therapeutics. SSc-centers offer specialty and experimental treatments, availability of clinical trials, registries, and consultation with specialty PT/OT/nutritionists.
D. Future of SSc Care
Telehealth offsets the frequency of travel burden for patients and family (e.g. time, logistics, financial, work) with its immediacy possibly expediting initiation of appropriate care. During the COVID-19 pandemic, supplementing history to target physical exam findings and guiding patients in physical exam elements has proven helpful to establish degree of vascular, cutaneous and musculoskeletal complications. Home spirometry, may also support expansion of telehealth visits.101
Patient self-management are a critical aspect of patient self-management with well-being strategies and therapeutic practices that influence inflammation, fibrotic mechanisms, respiratory function, fatigue and pain such as home exercise practices, therapeutic singing,.102–104
Practice Points:
Serial screening detects potentially lethal complications and is key to decreasing disability and mortality in SSc
Organ-based documentation optimizes comprehensive assessment, treatment and counselling. Documenting serial patient metrics, such as testing and symptoms, detects ominous early trends of progressive disease that warrants aggressive intervention, such as dropping PFTs despite normal values
Coexistent ILD and PH is common. Co-existent WHO PH groups is also common. Clinical tools exist to help detect and distinguish pulmonary involvement in SSc
SSc is very heterogeneous and requires an individualized approach to patient care
Concurrent care between SSc centers and general/local rheumatologists and pulmonologists is an increasingly common care model that may enhance patient health outcomes
Patient-centered and outcome-centered care in SSc demands time. Optimizing clinical infrastructure and appropriate billing can help protect clinical time with SSc patient
Optimal health outcomes in SSc demands a multi-specialty multi-disciplinary approach
Exercise has multiple-organ based benefits in SSc and may be a disease modifying intervention
Research Agenda:
Investigate the impact of standardized clinical data collection using customizable open source interfaces like OpenEMR105 on health outcomes and health disparities in SSc and other complex multi-organ diseases
Investigate health economics, health outcomes when clinicians optimize appropriate billing to protect clinician-patient visits time related to optimized in SSc
Investigate the potential of early systemic treatment as preventive in the development of severe gastrointestinal SSc disease (and other manifestations association with disease duration)
Characterize the degree and dosage of exercise on SSc local and systemic effects.
Assess the use of pre-visit intake apps (compared to no app), on patients’ clinic visit experiences and outcomes.
VIII. SUMMARY
SSc is a devastating multi-system disease, requiring extensive, thoughtful assessment, organized documentation and management of multiple organ-related manifestations that can directly impact a patient’s survival and HRQoL. An optimal approach coordinates healthcare services and empowers access to disease-related education and resources. The future of SSc care depends on effective communication along with expeditious assessment and treatment with appropriate pharmacological and non-pharmacological therapeutics. Quality healthcare in SSc is reliant upon sustainable clinical operations and policy-making that optimize survival and HRQoL in SSc.
Table 4.
Snapshot Diagram of Common Diagnostic Testing in SSc
| VITALS | CARDIOPULMONARY | GASTROINTESTINAL as indicated by history and clinical findings | LABORATORY | |
|---|---|---|---|---|
| Weight | FVC | Consider smaller, softer mouth piece | ^pH Probe, 24 Hour monitoring | CBC |
| Blood Pressure | TLC | ^Esophagram / Swallow study | CMP | |
| Heart Rate | DLCO | ^Esophagogastroduodenoscopy | Inflammatory markers: ESR, CRP, albumin, platelets | |
| Respiratory Rate | 6 MWT for distance and saturation with forehead oximeter | ^Gastric Emptying Study | Muscle assessment: CK, aldolase, LDH | |
| Oxygen saturation | HRCT of chest | ^Stool for Ova, Parasite and Culture | Cardiac markers: BNP/NTproBNP, Troponin, Uric acid | |
| Echocardiogram | ^Colonoscopy | Tuberculosis/HBV if considering therapy with Rituximab or Tocilizumab | ||
| ^ECG | ^Glucose or Lactose H2 breath test for SIBO | 25-OH Vitamin D | ||
| ^Right Heart Catheterization | HIV for new PH diagnosis | |||
| ^VQ Perfusion Scan – should be considered at new PH diagnosis | ||||
As indicated by history or clinical findings
SIBO: small intestine bacteria overgrowth
Table 5.
Common laboratory abnormalities in SSc. Courtesy of JK Gordon & LA Saketkoo, rights reserved.
| Category | Specific Lab | Common Implications |
|---|---|---|
| Antibody Presence | ||
| ANA, **Anti-nucleolar pattern of any titer | Positive in 90–95% of cases. Perform by immunofluorescence. If negative, consider other fibrosing illnesses. | |
| *Anti-Scl-70 (Anti-topoisomerase I) | 70% diffuse SSc, 30% limited SSc, higher risk ILD and higher risk severe ILD | |
| *Anti-RNA Polymerase III | Higher risk diffuse SSc, rapidly progressive skin, musculoskeletal involvement, higher risk SRC, GAVE, concomitant malignancy; Raynaud may present later in disease course | |
| *Anti-centromere | Limited SSc, PAH | |
| **Anti-Fibrillarin (U3-RNP) | Severe ILD, PAH, cardiomyopathy, severe GI involvement, diffuse SSc, | |
| **Anti-Th/To | Limited skin involvement, PAH, ILD | |
| **Anti-PM-Scl | Myositis, overlap | |
| Anti-U11/U12 RNP | Limited skin, ILD | |
| Anti-U1-RNP | Myositis, MCTD/Overlap, ILD, PAH, arthritis | |
| Anti-Ku | Myositis, Overlap | |
| Anti-NOR 90 | Anecdotally associated with SSc, and other CTDs with RP; forgotten antibody | |
| Anti-Ro52 | ILD, overlap | |
| HEMATOLOGIC | ||
| Hemoglobin/Hematocrit | GI loss, Medication effect, active inflammation | |
| Schistocytes | Concern for SRC | |
| Platelets | Elevated: Active inflammation, Low: SRC, medication effect | |
| Erythrocyte Sedimentation Rate | active inflammation, infection, malignancy | |
| Serum Protein Electrophoresis | Hypergammaglobulinemia Associated with active disease, severe lung involvement, SSA antibody; More prevalent in African ancestry | |
| Chemistry | ||
| Creatinine | SRC related renal injury | |
| Transaminases (ALT/SGOT, AST/SGPT) | Medications, myopathy, | |
| Creatine Kinase | Myopathy, myocardial infarction | |
| Albumin | If low: Active inflammation, low nutrition status, malabsorption | |
| Troponin | Myocarditis | |
| C-Reactive Protein | Active inflammation, infection Prognostic indicator |
|
| Aldolase | Myopathy | |
| Uric Acid | Pulmonary hypertension predictor, cardiovascular disease | |
| Pro-NT-beta natriuretic protein / Beta-natriuretic protein | Pulmonary hypertension, heart failure | |
| Urine | ||
| Protein | Prognostic indicator SRC |
|
| Red cells | SRC | |
Indicates criteria marker
Indicates strong correlation with SSc diagnosis
Acknowledgments
Funding statement: Charles and Elizabeth Wetmore Foundation of Greater New Orleans (LAS), National Institute of Health Research UK (AMR), National Institutes of Health: L30 HL129466 (MRL) and R01 AR073270 (MH), National Heart, Lung, Blood Institute K23HL150237-02 (ERV); Promobilia Foundation (HA) Pulmonary Fibrosis Trust UK (AMR), Sarcoidosis Awareness Foundation of Louisiana (SAFOL) (LAS), Swedish Research Council (HA, HP), Swedish Rheumatism Association (HA, HP), The Victoria Porter Family Fund for Autoimmunity Research (LJC).
APPENDIX of MEDICATION TABLES
Please note medications marked with ‘^’ are either formally approved by drug agencies for use in SSc or part of national formularies e.g. MMF in the US.
Raynaud’s and Digital Ulcer Medications
| Drug Class | Drug Names | General Side Effects |
|---|---|---|
| Calcium Channel Blocker (CCB) | Hypotension, flushing, dizziness, and edema. | |
| Angiotensin Receptor Blocker (ARB) | Losartan Valsartan |
Dizziness, diarrhea, hypotension, muscle cramps, and headache. |
| Angiotensin Converting Enzyme (ACE) Inhibitors | Captopril, Enalapril, Quinapril, Ramipril, Lisinopril | |
| Alpha Blockers | Prazosin | Hypotension, dizziness, drowsiness |
| Nitrates | Topical Nitroglycerin 2% | Rash, headache, facial flushing, hypotension, dry mouth, tachycardia. |
| Phosphodiesterase-5 Inhibitors^ | Sildenafil Tadalafil |
Blurred vision, flushing, headache, hypotension, visual impairment, tachycardia. |
| Prostacyclin/prostacyclin analog^ | Epoprostenol Treprostinil Iloprost |
Hypotension, dizziness, muscle cramps, nausea and vomiting, edema, headache |
| Endothelial Receptor Blockers^ | Bosentan Ambrisentan Macitentan |
Hepatoxicity, headache, flushing, edema, fatigue, hypotension, pruritus, and weight gain. |
| Topical lidocaine | Avoid getting into eyes or sensitive areas | |
| Botulin Toxin Injections |
Analgesic Medications
| Drug Class | Drug names | Concerns for use in SSc |
|---|---|---|
| NSAIDS | ibuprofen, naproxen, | • Esophageal concerns • Gastritis / gastric bleeding • Hypertension • Kidney impairment • Gastric bleeding • Fluid Retention • Cardiac events with long-term use |
| Opioids | Hydrocodone Oxycodone Tramadol |
• Decreased motility in GI tract ○ Constipation in general population → may be exaggerated in scleroderma patients • Risk of respiratory depression • Fractionated sleep, impaired sleep • Pruritus |
| Anti-convulsants | Gabapentin Pregabalin |
• Dizziness • Somnolence • Swelling |
Pharmacological Therapy for Gastrointestinal Manifestations in SSc (Courtesy of Monika Lammi, rights reserved)
| Drug Class | Drug Name | Concerns for use/Comments |
|---|---|---|
| GERD | ||
| Proton Pump Inhibitors | Pantoprazole* Omeprazole* Lansoprazole* Esomeprazole* Rabeprazole* Dexlansoprazole |
*Bioavailability reduced if taken with food Take 30–60 min before breakfast. Decreased absorption of: Mg, B12, Fe Risks (prolonged use): renal insufficiency, osteoporosis, atypical fractures, pneumonia, and dementia. |
| Histamine 2 Antagonists | Famotidine Cimetidine |
Possible inhibition of cytochrome P450 with possible enhanced effects of drugs with P450 reliant metabolism |
| Antiacids | Calcium carbonate | Hyperkalemia, alkalosis and acute or chronic renal injury. |
| Aluminum hydroxide | Aluminum retention with neurotoxicity and anemia in renal failure. Hypophosphatemia. |
|
| Surface agents | Gaviscon +/− alginate | |
| Sucralfate | Can bind to other drugs if taken simultaneously. Hypophosphatemia. Combining with antiacids can amplify these side effects. |
|
| Promotility/LES | Metoclopramide | May increase gastric motility in patients with systemic sclerosis. FDA advised against use for more than 3 months SE: traditive dyskinesia, cardiac arrythmia (monitor with EKG) |
| Domperidone | Not FDA approved in US. Can be obtained with Investigational New Drug Application. SE: cardiac arrhythmia |
|
| Buspirone | Increases the lower esophageal sphincter pressure, amplitude of esophageal contractions. Appears more effective for GERD-related symptoms not esophageal hypomotility symptoms (dysphagia and chest pain). |
|
| Baclofen | Shown to augment lower esophageal sphincter pressure in patients with GERD. Not studied in patients with SSc. | |
| Gastroparesis | ||
| Promotility agents | Metoclopramide | May increase gastric motility in patients with systemic sclerosis FDA advised against use for more than 3 months Risk: traditive dyskinesia, cardiac arrythmia (monitor with EKG) FDA advised against use for more than 3 months |
| Domperidone | Not FDA approved in US. Can be obtained with Investigational New Drug Application. SE: cardiac arrhythmia (monitor with EKG) |
|
| Erythromycin | Not recommended long term: tachyphylaxis, may cause small bowel dysmotility. SE: cardiac arrhythmia (monitor with EKG) |
|
| Prucalopride | Improves gastric, small bowel and colonic transit. FDA approved for constipation. |
|
| Cisapride | Improves postprandial symptoms and gastric emptying. More potent acutely than metoclopramide. Withdrawn from the US market because of cardiac arrhythmia. |
|
| Antiemetics | Ondansetron | SE: Prolongs GI transit, headache, cardiac arrhythmia. |
| Granisetron | SE: constipation, headache, cardiac arrhythmias | |
| Prochlorperazine | SE: Sedation, tardive dyskinesia | |
| Promethazine | SE: Central, cardiac arrhythmia | |
| Dyspepsia | ||
| Neuromodulators | Buspirone | Improves gastric accommodation and symptoms of dyspepsia, but decreases gastric emptying of liquids. |
| Mirtazapine | Improves dyspepsia, sleep, depression SE: weight gain, drowsiness |
|
| Herbal | FDgard (caraway oil and I-menthol) | |
| SIBO | ||
| Antibiotics | Rifaximin Metronidazole Amoxicillin/clavulanic acid Norfloxacin |
Treat for 2 weeks. High risk of recurrence due to small bowel dysmotility. Cycle regimens to limit antibiotic resistance. May consider use of prokinetics, see below. Antibiotics, e.g. fluoroquinolones may contribute to clostridium difficile overgrowth |
| Chronic Intestinal pseudo-obstruction | ||
| Prokinetics | Metoclopramide Erythromycin Prucalopride |
See above. May also be considered for SIBO. |
| Cholinesterase inhibitor | Pyridostigmine | SE: bradycardia, excessive bronchial secretions, cholinergic crisis |
| Somatostatin analog | Octreotide | Used in patients who failed to respond to other prokinetic agents. Inhibits gastric motility. |
| Constipation | ||
| Bulk forming laxatives | Psyllium Methylcellulose |
Patients with gastric dysmotility and visceral hypersensitivity may not be able to tolerate. |
| Osmotic laxatives | Polyethylene glycol | SE: abdominal pain, distention, bloating. |
| Stimulant laxatives | Bisacodyl Glycerol |
|
| Guanylate cyclase-C receptor agonists | Linaclotide | Diarrhea, bloating. |
| Plecanatide | Improves gastric, small bowel and colonic transit. Diarrhea. |
|
| Chloride channel activator | Lubiprostone | Nausea, diarrhea. |
| Promotility agent | Prucalopride | Improves gastric, small bowel and colonic transit. FDA approved for constipation. |
Systemic Treatment/Immune Suppression / Anti-Fibrotics
| Drug | Side Effects | Monitoring/Counseling | Common Uses |
|---|---|---|---|
| Mycophenolate mofetil (MMF) mycophenolic acid ^ | Diarrhea, increased risk of infection, headache, fatigue, leukopenia, thrombocytopenia, teratogenic | CBC, serum electrolytes especially with ongoing diarrhea, drug interactions; REMS (pregnancy) If side effects occur, decrease dose to side effect free level, and keep at this dose for longer period before increasing again. Use of contraception |
Anti-fibrotic immunosuppressant First-line for progressive ILD Skin-tightening, Joint involvement |
| Cyclophosphamide | Increased risk of infection Hair loss, GI upset, decreased appetite, stomatitis, amenorrhea, interstitial cystitis, infertility, oligospermia/azoospermia, Stevens-Johnson syndrome, increased risk of bladder cancer |
CBC, urinalysis (monthly if on IV therapy) | Progressive ILD Progressive skin-tightening |
| Rituximab | Risk of infection Infusion reaction common Very rare, demyelinating disorders |
Progressive skin-tightness Progressive ILD Joint involvement, possibly PH |
|
| Tocilizumab ^ | Risk of Infection Transaminitis, hepatotoxicity Very rare, demyelinating disorders Rare risk of GI perforation Hyperlipidemia |
Serum lipids CBC Transaminases |
Skin-tightness Joint involvement Slowing down progressive ILD Possibly PH |
| Intravenous Immunoglobulin G | Headache, fatigue, renal dysfunction, transient ischemic episodes, cerebrovascular event, urticaria, flushing, hypertension, aseptic meningitis | Progressive SSc | |
| Hematopoietic Stem Cell Transplantation | Extreme immunosuppression High risk of infection and sepsis Heart failure, arrhythmia |
Per protocol | Progressive SSc prior to significant organ damage |
| Methotrexate | Nausea, diarrhea, hepatotoxicity, stomatitis, alopecia, myelosuppression, teratogenic Medication-induced pneumonitis, rare Increased risk of infection |
CBC, Serum creatinine, Transaminases, Concomitant use of folic acid Avoidance of alcohol Use of contraception |
Joint involvement |
| Azathioprine | GI upset, myalgia, leukopenia, thrombocytopenia, risk of infection, hepatotoxicity | Signs of bleeding, jaundice, change in color of stool; TPMT deficiency, drug interactions | Joint involvement |
| Glucocorticoids | Scleroderma Renal Crisis Amongst many other potentially detrimental side effects |
Opportunities to lower dose or discontinue | Restricted use of very low doses |
| Leflunomide | Hepatotoxicity Nausea, Diarrhea, Hypertension, Rash, Headache, Abdominal pain, Alopecia; Peripheral neuropathy |
CBC and transaminases, signs of infection Avoid alcohol, use of contraception |
Joint involvement |
| Sulfasalazine | Nausea, Diarrhea, Headache, Photosensitivity, Myelosuppression | CBC GI distress, SPF use |
Joint involvement |
| Hydroxychloroquine | Nausea, Diarrhea, Headache, vision changes Rarely myopathy Rarely myelosuppression |
Baseline eye exam Screening according to published protocols Visual changes at night or peripheral |
Joint involvement |
|
Anti-Fibrotic e.g. nintedanib^, pirfenidone |
Gastrointestinal distress, hepatotoxicity, fatigue, swelling | Transaminase levels Electrolytes with vomiting or diarrhea |
Slowing down lung progression. Uncertain regarding whether these have systemic effects, thus far there has been no demonstrated improvement on skin, joint, or quality of life. |
APPENDIX OF CLINICIAN and PATIENT RESOURCES
Clinical Skills Resources:
Functional Index-2: https://www.youtube.com/watch?v=qw4XvWKQErU
Manual Muscle Test 8 (MMT-8): https://www.niehs.nih.gov/research/resources/assets/docs/mmt8_grading_and_testing_procedures_for_the_abbreviated_8_muscle_groups_508.pdf
Modified Rodnan Skin Score: https://www.youtube.com/watch?v=Bl3EX_2PaUc
Timed Up and Go Test: https://youtu.be/auqAb_AWM1U
TImed sit to stand test: https://www.youtube.com/watch?v=puJhQXUlbdA
30-seconds Sit to Stand Test: https://www.youtube.com/watch?v=PzCTwkJVhWg
DETECT Algorithm for PH Screening: https://www.suspectpahctd.com/DETECT/
Examples of Clinic Operations Documents:
Medical Records Intake Form for Scheduling New Patients: https://www.dropbox.com/s/wapuv8p8dkoz2n3/PATIENT%20SCHEDULING%20INTERVIEW.docx?dl=0
New Patient Questionnaire: https://www.dropbox.com/s/jynfaq5ax3cyo8a/SSc%20Patient%20Intake%20Form.docx?dl=0
Infusion Order Record: https://www.dropbox.com/s/v1vkwiegpjhead4/INFUSION%20ORDER%20RECORD.docx?dl=0
Oral Medication Authorization Record: https://www.dropbox.com/s/algr06i6upndtmf/RECORD%20ORAL%20MEDS%20PRIOR%20AUTH.docx?dl=0
Patient Questionnaires:
StopBang Questionnaire Online Calculator: http://stopbang.ca/osa/screening.php
Epworth Sleepiness Scale: https://www.thecalculator.co/health/Epworth-Sleepiness-Scale-Calculator-905.html
http://epworthsleepinessscale.com/
Scleroderma Health Assessment Questionnaire: https://www.dropbox.com/s/gd9847e9bw82101/SHAQ%20-%20SF36%20-%20Cochin%20Hand%20Function.doc?dl=0
SF-36 form: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html
https://www.rand36calculator.com/
Cochin Hand Function Questionnaire: https://www.dropbox.com/s/gd9847e9bw82101/SHAQ%20%20SF36%20-%20Cochin%20Hand%20Function.doc?dl=0
Giessen GI Form: https://www.dropbox.com/s/o2i68d7f4ojvu4h/Giessen%20Gastrointestinal%20Questionnaire%20for%20Scleroderma.doc?dl=0
SSc-GIT: https://www.dropbox.com/s/yi5wzl3yezgmqn7/GIT%20Questionnaire%20-%20The%20Actual%20Survey.doc?dl=0
Patient Specific Functional Scale (PSFS) User Manual: https://www.physiopedia.com/Patient_Specific_Functional_Scale
Patient and Physician Education and Advocacy Resources:
Scleroderma Foundation: www.scleroderma.org
FESCA: www.fesca-scleroderma.eu/wordpress/
Scleroderma Australia: https://www.sclerodermaaustralia.com.au/
Scleroderma & Raynaud’s UK: https://www.sruk.co.uk/scleroderma/
Scleroderma Societies of Canada and Ontario: www.scleroderma.ca, https://www.sclerodermaontario.ca/, https://sclerodermie.ca/en/
Pulmonary Fibrosis Foundation: https://www.pulmonaryfibrosis.org/
Pulmonary Hypertension Association: https://phassociation.org/
Renal Crisis Card: https://ard.bmj.com/content/74/Suppl_2/1136.1
Educational Resources for Patients:
Oxygen Use (for patients in the U.S.): https://www.dropbox.com/s/3d8wyikb8204ira/What%20Patients%20Should%20Know%20About%20OXYGEN%20THERAPY%20-%208%20-2-2017.pdf?dl=0
Patient Information on Medications: www.rheuminfo.com
Janet Poole Hands / Face Instructional Links: https://www.youtube.com/watch?v=1F02FxdOgwI
https://www.youtube.com/watch?v=8MztM3zItik
https://www.youtube.com/watch?v=YwWP7mgcYhU
Stretching exercises for the hand and face. The Scleroderma Foundation, http://www.scleroderma.org/site/DocServer/Form_16c_low_res.pdf?docID=19809&AddInterest=1281
Taking Charge of Systemic Sclerosis (TOSS): an internet program for systemic sclerosis. https://www.selfmanagescleroderma.com/
Living Well: Heart, Lung, Muscle & Mind: A collection of videos dedicated to yoga rehab and dance rehab for heart, lung, muscle and autoimmune conditions https://www.youtube.com/channel/UCRgvkbyzep-Q3LGBiAksQZw/videos
3-3-1 Exercise Tutorial https://www.youtube.com/watch?v=zsBRxmkzAnM&t=2s
Move Towards Health: UMC CPHC Instructional Booklet on Safe Home-based Dance Practice https://doi.org/10.13140/RG.2.2.25576.49927
Sleep Booklet: https://www.dropbox.com/s/0axd782mi818smc/SF%20Arizona%20Conference%20%20-SLEEP%20-%20DOUBLE%20Booklet.docx?dl=0
Mindfulness Booklet: https://www.dropbox.com/s/mrpl33zxjsk20br/SF%20Arizona%20Conference%20%20-RESTORE%20YOURSELF-%20DOUBLE%20Booklet.docx?dl=0
Mindfulness in Scleroderma Videos: https://www.youtube.com/watch?v=pNK9RP4Abyw https://www.youtube.com/watch?v=lmQKOCDJ19Y
Footnotes
Conflict of interest:
None of the authors have conflicts of interest to report that are related to the reported content of this paper.
We dedicate this effort to people living with SSc and their grace and valor in the face of devastating disease. We also dedicate this collaborative work to Dr. Nadia Morgan a young, energetic, meticulous, creative and heartful SSc clinical scientist; her loss resounds in the SSc research community.
This work is endorsed by: Federation of European Scleroderma Associations (FESCA), Scleroderma Australia, Scleroderma Canada, Scleroderma & Raynaud’s UK (SRUK), and Scleroderma Foundation USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lim SS, Helmick CG, Bao G, et al. Racial Disparities in Mortality Associated with Systemic Lupus Erythematosus - Fulton and DeKalb Counties, Georgia, 2002–2016. MMWR Morb Mortal Wkly Rep. 2019;68(18):419–422. doi: 10.15585/mmwr.mm6818a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Pla A, Simms RW. Geographic disparity in systemic sclerosis mortality in the United States: 1999–2017. J Scleroderma Relat Disord. Published online August 2019. doi:doi: 10.1177/2397198319869566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelber AC, Manno RL, Shah AA, et al. Race and association with disease manifestations and mortality in scleroderma: a 20-year experience at the Johns Hopkins Scleroderma Center and review of the literature. Medicine (Baltimore). 2013;92(4):191–205. doi: 10.1097/MD.0b013e31829be125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore DF, Steen VD. Racial Disparities in Systemic Sclerosis. Rheum Dis Clin North Am. 2020;46(4):705–712. doi: 10.1016/j.rdc.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 5.Morgan ND, Shah AA, Mayes MD, et al. Clinical and serological features of systemic sclerosis in a multicenter African American cohort: Analysis of the genome research in African American scleroderma patients clinical database. Medicine (Baltimore). 2017;96(51):e8980. doi: 10.1097/MD.0000000000008980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Increased Morbidity and Mortality of Scleroderma in African Americans Compared to Non-African Americans - PubMed. Accessed May 6, 2021. https://pubmed.ncbi.nlm.nih.gov/30821906/ [DOI] [PubMed]
- 7.Theodore AC, Tseng C-H, Li N, Elashoff RM, Tashkin DP. Correlation of cough with disease activity and treatment with cyclophosphamide in scleroderma interstitial lung disease: findings from the Scleroderma Lung Study. Chest. 2012;142(3):614–621. doi: 10.1378/chest.11-0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tashkin DP, Volkmann ER, Tseng C-H, et al. Improved Cough and Cough-Specific Quality of Life in Patients Treated for Scleroderma-Related Interstitial Lung Disease: Results of Scleroderma Lung Study II. Chest. 2017;151(4):813–820. doi: 10.1016/j.chest.2016.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719. doi: 10.1016/S2213-2600(16)30152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–2666. doi: 10.1056/NEJMoa055120 [DOI] [PubMed] [Google Scholar]
- 11.Cutolo M, Soldano S, Smith V. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol. 2019;15(7):753–764. doi: 10.1080/1744666X.2019.1614915 [DOI] [PubMed] [Google Scholar]
- 12.Saketkoo LA, Distler O. Is there evidence for vasculitis in systemic sclerosis? Curr Rheumatol Rep. 2012;14(6):516–525. doi: 10.1007/s11926-012-0296-9 [DOI] [PubMed] [Google Scholar]
- 13.Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58(12):3902–3912. doi: 10.1002/art.24038 [DOI] [PubMed] [Google Scholar]
- 14.Avouac J, Lepri G, Smith V, Toniolo E, Hurabielle C, Vallet A, Amrouche F, Kahan A, Cutolo M, Allanore YS Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum. 2017;47((1):86–94.):(1):86–94. [DOI] [PubMed] [Google Scholar]
- 15.2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative - PubMed. Accessed May 6, 2021. https://pubmed.ncbi.nlm.nih.gov/24122180/
- 16.Smith V, Vanhaecke A, Herrick AL, et al. Fast track algorithm: How to differentiate a “scleroderma pattern” from a “non-scleroderma pattern.” Autoimmun Rev. 2019;18(11):102394. doi: 10.1016/j.autrev.2019.102394 [DOI] [PubMed] [Google Scholar]
- 17.Wollersheim H, Thien T, Hoet MH, Van Venrooy WJ. The diagnostic value of several immunological tests for anti-nuclear antibody in predicting the development of connective tissue disease in patients presenting with Raynaud’s phenomenon. Eur J Clin Invest. 1989;19(6):535–541. doi: 10.1111/j.1365-2362.1989.tb00271.x [DOI] [PubMed] [Google Scholar]
- 18.Snow MH, Saketkoo L-A, Frech TM, et al. Results from an American pilot survey among Scleroderma Clinical Trials Consortium members on capillaroscopy use and how to best implement nailfold capillaroscopy training. Clin Exp Rheumatol. 2019;37 Suppl 119(4):151. [PubMed] [Google Scholar]
- 19.Smith V, De Keyser F, Pizzorni C, et al. Nailfold capillaroscopy for day-to-day clinical use: construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann Rheum Dis. 2011;70(1):180–183. doi: 10.1136/ard.2010.132431 [DOI] [PubMed] [Google Scholar]
- 20.Mihai C, Landewé R, van der Heijde D, et al. Digital ulcers predict a worse disease course in patients with systemic sclerosis. Ann Rheum Dis. 2016;75(4):681–686. doi: 10.1136/annrheumdis-2014-205897 [DOI] [PubMed] [Google Scholar]
- 21.Hurabielle C, Avouac J, Lepri G, de Risi T, Kahan A, Allanore Y. Skin Telangiectasia and the Identification of a Subset of Systemic Sclerosis Patients With Severe Vascular Disease. Arthritis Care Res. 2016;68(7):1021–1027. doi: 10.1002/acr.22766 [DOI] [PubMed] [Google Scholar]
- 22.Avouac J, Guerini H, Wipff J, et al. Radiological hand involvement in systemic sclerosis. Ann Rheum Dis. 2006;65(8):1088–1092. doi: 10.1136/ard.2005.044602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saketkoo Lesley Ann, Lammi Matthew R, Fischer Aryeh, Molitor Jerry A, Steen Virginia D. Mycophenolate Mofetil (MMF) Use in Scleroderma Patients with Pulmonary Hypertension: Observations from the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma Cohort. In: Vol 1931.; 2014. https://acrabstracts.org/abstract/mycophenolate-mofetil-mmf-use-in-scleroderma-patients-with-pulmonary-hypertension-observations-from-the-pulmonary-hypertension-assessment-and-recognition-of-outcomes-in-scleroderma-cohort/ [Google Scholar]
- 24.Lammi MR, Saketkoo LA, Okpechi SC, et al. Microparticles in systemic sclerosis: Potential pro-inflammatory mediators and pulmonary hypertension biomarkers. Respirol Carlton Vic. 2019;24(7):675–683. doi: 10.1111/resp.13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lammi MR, Saketkoo LA, Gordon JK, Lauto P, Steen VD. Scleroderma Patients With Pulmonary Hypertension and Increased Pulmonary Capillary Wedge Pressure In The Pulmonary Hypertension Assessment and Recognition Of Outcomes In Scleroderma (PHAROS) Cohort. In: Vol 2589.; 2013. https://acrabstracts.org/abstract/scleroderma-patients-with-pulmonary-hypertension-and-increased-pulmonary-capillary-wedge-pressure-in-the-pulmonary-hypertension-assessment-and-recognition-of-outcomes-in-scleroderma-pharos-cohort/ [Google Scholar]
- 26.Zamanian RT, Badesch D, Chung L, et al. Safety and Efficacy of B-Cell Depletion with Rituximab for the Treatment of Systemic Sclerosis Associated Pulmonary Arterial Hypertension: A Multi-center, Double-blind, Randomized, Placebo-controlled Trial. Am J Respir Crit Care Med. Published online March 2, 2021. doi: 10.1164/rccm.202009-3481OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nihtyanova SI, Brough GM, Black CM, Denton CP. Mycophenolate mofetil in diffuse cutaneous systemic sclerosis--a retrospective analysis. Rheumatol Oxf Engl. 2007;46(3):442–445. doi: 10.1093/rheumatology/kel244 [DOI] [PubMed] [Google Scholar]
- 28.Berger M, Steen VD. Role of anti-receptor autoantibodies in pathophysiology of scleroderma. Autoimmun Rev. 2017;16(10):1029–1035. doi: 10.1016/j.autrev.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 29.Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis - PubMed. Accessed May 6, 2021. https://pubmed.ncbi.nlm.nih.gov/25181620/ [DOI] [PubMed]
- 30.Hoa S, Bernatsky S, Baron M, et al. ASSOCIATION BETWEEN IMMUNOSUPPRESSIVE THERAPY AND INCIDENT RISK OF INTERSTITIAL LUNG DISEASE IN SYSTEMIC SCLEROSIS. Chest. Published online June 18, 2021:S0012–3692(21)01124–7. doi: 10.1016/j.chest.2021.06.014 [DOI] [PubMed] [Google Scholar]
- 31.Morrisroe K, Sudararajan V, Stevens W, et al. Work productivity in systemic sclerosis, its economic burden and association with health-related quality of life. Rheumatol Oxf Engl. 2018;57(1):73–83. doi: 10.1093/rheumatology/kex362 [DOI] [PubMed] [Google Scholar]
- 32.Morrisroe K, Huq M, Stevens W, et al. Determinants of unemployment amongst Australian systemic sclerosis patients: results from a multicentre cohort study. Clin Exp Rheumatol. 2016;34 Suppl 100(5):79–84. [PubMed] [Google Scholar]
- 33.Sandqvist G, Hesselstrand R, Scheja A, Håkansson C. Managing work life with systemic sclerosis. Rheumatol Oxf Engl. 2012;51(2):319–323. doi: 10.1093/rheumatology/ker324 [DOI] [PubMed] [Google Scholar]
- 34.Sandqvist G, Hesselstrand R, Petersson IF, Kristensen LE. Work Disability in Early Systemic Sclerosis: A Longitudinal Population-based Cohort Study. J Rheumatol. 2015;42(10):1794–1800. doi: 10.3899/jrheum.150023 [DOI] [PubMed] [Google Scholar]
- 35.Poole JL, Anwar S, Mendelson C, Allaire S. Workplace barriers encountered by employed persons with systemic sclerosis. Work Read Mass. 2016;55(4):923–929. doi: 10.3233/WOR-162448 [DOI] [PubMed] [Google Scholar]
- 36.Mendelson C, Poole JL, Allaire S. Experiencing work as a daily challenge: the case of scleroderma. Work Read Mass. 2013;44(4):405–413. doi: 10.3233/WOR-2012-1420 [DOI] [PubMed] [Google Scholar]
- 37.Steen VD, Medsger TA. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43(11):2437–2444. doi: [DOI] [PubMed] [Google Scholar]
- 38.Steen VD, Medsger TA. The palpable tendon friction rub: an important physical examination finding in patients with systemic sclerosis. Arthritis Rheum. 1997;40(6):1146–1151. doi: [DOI] [PubMed] [Google Scholar]
- 39.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol Hoboken NJ. 2014;66(6):1625–1635. doi: 10.1002/art.38390 [DOI] [PubMed] [Google Scholar]
- 40.Sebastiani M, Manfredi A, Vukatana G, et al. Predictive role of capillaroscopic skin ulcer risk index in systemic sclerosis: a multicentre validation study. Ann Rheum Dis. 2012;71(1):67–70. doi: 10.1136/annrheumdis-2011-200022 [DOI] [PubMed] [Google Scholar]
- 41.Silva I, Almeida J, Vasconcelos C. A PRISMA-driven systematic review for predictive risk factors of digital ulcers in systemic sclerosis patients. Autoimmun Rev. 2015;14(2):140–152. doi: 10.1016/j.autrev.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 42.Avouac J, Huscher D, Furst DE, et al. Expert consensus for performing right heart catheterisation for suspected pulmonary arterial hypertension in systemic sclerosis: a Delphi consensus study with cluster analysis. Ann Rheum Dis. 2014;73(1):191–197. doi: 10.1136/annrheumdis-2012-202567 [DOI] [PubMed] [Google Scholar]
- 43.Hesselstrand R, Scheja A, Wuttge DM. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand J Rheumatol. 2012;41(1):39–43. doi: 10.3109/03009742.2011.610032 [DOI] [PubMed] [Google Scholar]
- 44.Patel S, Ross L, McKelvie P, Nikpour M. Constrictive pericarditis as the presenting manifestation of systemic sclerosis. Rheumatol Oxf Engl. 2019;58(4):732–734. doi: 10.1093/rheumatology/key404 [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Mayes MD, Pedroza C, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res. 2013;65(8):1375–1380. doi: 10.1002/acr.21968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Domsic RT, Rodriguez-Reyna T, Lucas M, Fertig N, Medsger TA. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis. 2011;70(1):104–109. doi: 10.1136/ard.2009.127621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cottrell TR, Wise RA, Wigley FM, Boin F. The degree of skin involvement identifies distinct lung disease outcomes and survival in systemic sclerosis. Ann Rheum Dis. 2014;73(6):1060–1066. doi: 10.1136/annrheumdis-2012-202849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lóránd V, Bálint Z, Komjáti D, et al. Validation of disease activity indices using the 28 joint counts in systemic sclerosis. Rheumatol Oxf Engl. 2016;55(10):1849–1858. doi: 10.1093/rheumatology/kew246 [DOI] [PubMed] [Google Scholar]
- 49.Lóránd V, Nagy G, Bálint Z, et al. Sensitivity to change of joint count composite indices in 72 patients with systemic sclerosis. Clin Exp Rheumatol. Published online March 17, 2021. [DOI] [PubMed] [Google Scholar]
- 50.Amoda O, Ravat V, Datta S, Saroha B, Patel RS. Trends in Demographics, Hospitalization Outcomes, Comorbidities, and Mortality Risk among Systemic Sclerosis Patients. Cureus. 2018;10(5):e2628. doi: 10.7759/cureus.2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung MP, Dontsi M, Postlethwaite D, et al. Increased Mortality in Asians With Systemic Sclerosis in Northern California. ACR Open Rheumatol. 2020;2(4):197–206. doi: 10.1002/acr2.11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaeger VK, Tikly M, Xu D, et al. Racial differences in systemic sclerosis disease presentation: a European Scleroderma Trials and Research group study. Rheumatol Oxf Engl. 2020;59(7):1684–1694. doi: 10.1093/rheumatology/kez486 [DOI] [PubMed] [Google Scholar]
- 53.Willems LM, Kwakkenbos L, Leite CC, et al. Frequency and impact of disease symptoms experienced by patients with systemic sclerosis from five European countries. Clin Exp Rheumatol. 2014;32(6 Suppl 86):S-88–93. [PubMed] [Google Scholar]
- 54.Jaeger VK, Distler O, Maurer B, et al. Functional disability and its predictors in systemic sclerosis: a study from the DeSScipher project within the EUSTAR group. Rheumatol Oxf Engl. 2018;57(3):441–450. doi: 10.1093/rheumatology/kex182 [DOI] [PubMed] [Google Scholar]
- 55.Christensen A, Khalique S, Cenac S, et al. SYSTEMIC SCLEROSIS RELATED CALCINOSIS: PATIENTS PROVIDE WHAT SPECIALISTS WANT TO LEARN. J La State Med Soc Off Organ La State Med Soc. 2015;167(3):158–159. [PubMed] [Google Scholar]
- 56.Saketkoo LA. Wildflowers abundant in the garden of systemic sclerosis research, while hopeful exotics will one day bloom. Rheumatol Oxf Engl. 2018;57(3):410–413. doi: 10.1093/rheumatology/kex420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Randone SB, Lepri G, Husher D, et al. OP0065 THE VERY EARLY DIAGNOSIS OF SYSTEMIC SCLEROSIS (VEDOSS) PROJECT: PREDICTORS TO DEVELOP DEFINITE DISEASE FROM AN INTERNATIONAL MULTICENTRE STUDY. Ann Rheum Dis. 2019;78(Suppl 2):104. doi: 10.1136/annrheumdis-2019-eular.7164 [DOI] [Google Scholar]
- 58.Pauling JD, Saketkoo LA, Matucci-Cerinic M, Ingegnoli F, Khanna D. The patient experience of Raynaud’s phenomenon in systemic sclerosis. Rheumatol Oxf Engl. 2019;58(1):18–26. doi: 10.1093/rheumatology/key026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pauling JD, Domsic RT, Saketkoo LA, et al. Multinational Qualitative Research Study Exploring the Patient Experience of Raynaud’s Phenomenon in Systemic Sclerosis. Arthritis Care Res. 2018;70(9):1373–1384. doi: 10.1002/acr.23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettersson H, Nordin A, Svenungsson E, Alexanderson H, Boström C. Experiences of physical activity and exercise in individuals with systemic sclerosis: A qualitative study. Musculoskeletal Care. 2020;18(2):150–160. doi: 10.1002/msc.1447 [DOI] [PubMed] [Google Scholar]
- 61.Ostuni P, Botsios C, Sfriso P, et al. [Prevalence and clinical features of fibromyalgia in systemic lupus erythematosus, systemic sclerosis and Sjögren’s syndrome]. Minerva Med. 2002;93(3):203–209. [PubMed] [Google Scholar]
- 62.Avouac J, Walker U, Tyndall A, et al. Characteristics of joint involvement and relationships with systemic inflammation in systemic sclerosis: results from the EULAR Scleroderma Trial and Research Group (EUSTAR) database. J Rheumatol. 2010;37(7):1488–1501. doi: 10.3899/jrheum.091165 [DOI] [PubMed] [Google Scholar]
- 63.Hubac J, Gilson M, Gaudin P, Clay M, Imbert B, Carpentier P. Ultrasound prevalence of wrist, hand, ankle and foot synovitis and tenosynovitis in systemic sclerosis, and relationship with disease features and hand disability. Joint Bone Spine. 2020;87(3):229–233. doi: 10.1016/j.jbspin.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 64.Sariyildiz MA, Batmaz I, Budulgan M, et al. Sleep quality in patients with systemic sclerosis: relationship between the clinical variables, depressive symptoms, functional status, and the quality of life. Rheumatol Int. 2013;33(8):1973–1979. doi: 10.1007/s00296-013-2680-9 [DOI] [PubMed] [Google Scholar]
- 65.Nokes BT, Raza HA, Cartin-Ceba R, et al. Individuals With Scleroderma May Have Increased Risk of Sleep-Disordered Breathing. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2019;15(11):1665–1669. doi: 10.5664/jcsm.8036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shoenut JP, Kerr P, Micflikier AB, Yamashiro Y, Kryger MH. The effect of nasal CPAP on nocturnal reflux in patients with aperistaltic esophagus. Chest. 1994;106(3):738–741. doi: 10.1378/chest.106.3.738 [DOI] [PubMed] [Google Scholar]
- 67.Lammi M, Baughman R, Birring S, et al. Outcome Measures for Clinical Trials in Interstitial Lung Diseases. Curr Respir Med Rev. 2015;11(2):163–174. doi: 10.2174/1573398X11666150619183527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saketkoo LA, Mittoo S, Huscher D, et al. Connective tissue disease related interstitial lung diseases and idiopathic pulmonary fibrosis: provisional core sets of domains and instruments for use in clinical trials. Thorax. 2014;69(5):428–436. doi: 10.1136/thoraxjnl-2013-204202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saketkoo LA, Mittoo S, Frankel S, et al. Reconciling healthcare professional and patient perspectives in the development of disease activity and response criteria in connective tissue disease-related interstitial lung diseases. J Rheumatol. 2014;41(4):792–798. doi: 10.3899/jrheum.131251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mittoo S, Frankel S, LeSage D, et al. Patient Perspectives in OMERACT Provide an Anchor for Future Metric Development and Improved Approaches to Healthcare Delivery in Connective Tissue Disease Related Interstitial Lung Disease (CTD-ILD). Curr Respir Med Rev. 2015;11(2):175–183. doi: 10.2174/1573398X11666150619182624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernstein EJ, Jaafar S, Assassi S, et al. Performance Characteristics of Pulmonary Function Tests for the Detection of Interstitial Lung Disease in Adults With Early Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol Hoboken NJ. 2020;72(11):1892–1896. doi: 10.1002/art.41415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pittman N, Rawn SM, Wang M, Masetto A, Beattie KA, Larché M. Treatment of small intestinal bacterial overgrowth in systemic sclerosis: a systematic review. Rheumatol Oxf Engl. 2018;57(10):1802–1811. doi: 10.1093/rheumatology/key175 [DOI] [PubMed] [Google Scholar]
- 73.Gyger G, Baron M. Systemic Sclerosis: Gastrointestinal Disease and Its Management. Rheum Dis Clin North Am. 2015;41(3):459–473. doi: 10.1016/j.rdc.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 74.Hansi N, Thoua N, Carulli M, et al. Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis. Clin Exp Rheumatol. 2014;32(6 Suppl 86):S-214–221. [PubMed] [Google Scholar]
- 75.Schaier M, Scholl C, Scharpf D, et al. Proton pump inhibitors interfere with the immunosuppressive potency of mycophenolate mofetil. Rheumatol Oxf Engl. 2010;49(11):2061–2067. doi: 10.1093/rheumatology/keq238 [DOI] [PubMed] [Google Scholar]
- 76.Volkmann ER. Natural History of Systemic Sclerosis-Related Interstitial Lung Disease: How to Identify a Progressive Fibrosing Phenotype. J Scleroderma Relat Disord. 2020;5(2 Suppl):31–40. doi: 10.1177/2397198319889549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steen VD, Graham G, Conte C, Owens G, Medsger TA. Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum. 1992;35(7):765–770. doi: 10.1002/art.1780350709 [DOI] [PubMed] [Google Scholar]
- 78.Chung L, Domsic RT, Lingala B, et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res. 2014;66(3):489–495. doi: 10.1002/acr.22121 [DOI] [PubMed] [Google Scholar]
- 79.Nie L-Y, Wang X-D, Zhang T, Xue J. Cardiac complications in systemic sclerosis: early diagnosis and treatment. Chin Med J (Engl). 2019;132(23):2865–2871. doi: 10.1097/CM9.0000000000000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez-Reyna TS, Morelos-Guzman M, Hernández-Reyes P, et al. Assessment of myocardial fibrosis and microvascular damage in systemic sclerosis by magnetic resonance imaging and coronary angiotomography. Rheumatol Oxf Engl. 2015;54(4):647–654. doi: 10.1093/rheumatology/keu350 [DOI] [PubMed] [Google Scholar]
- 81.Rodríguez-Reyna TS, Rosales-Uvera SG, Kimura-Hayama E, et al. Myocardial fibrosis detected by magnetic resonance imaging, elevated U-CRP and higher mRSS are predictors of cardiovascular complications in systemic sclerosis (SSc) patients. Semin Arthritis Rheum. 2019;49(2):273–278. doi: 10.1016/j.semarthrit.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 82.Follansbee WP, Zerbe TR, Medsger TA. Cardiac and skeletal muscle disease in systemic sclerosis (scleroderma): a high risk association. Am Heart J. 1993;125(1):194–203. doi: 10.1016/0002-8703(93)90075-k [DOI] [PubMed] [Google Scholar]
- 83.Ranque B, Authier F-J, Le-Guern V, et al. A descriptive and prognostic study of systemic sclerosis-associated myopathies. Ann Rheum Dis. 2009;68(9):1474–1477. doi: 10.1136/ard.2008.095919 [DOI] [PubMed] [Google Scholar]
- 84.Ranque B, Bérezné A, Le-Guern V, et al. Myopathies related to systemic sclerosis: a case-control study of associated clinical and immunological features. Scand J Rheumatol. 2010;39(6):498–505. doi: 10.3109/03009741003774626 [DOI] [PubMed] [Google Scholar]
- 85.West SG, Killian PJ, Lawless OJ. Association of myositis and myocarditis in progressive systemic sclerosis. Arthritis Rheum. 1981;24(5):662–668. doi: 10.1002/art.1780240506 [DOI] [PubMed] [Google Scholar]
- 86.Pettersson H, Boström C, Bringby F, et al. Muscle endurance, strength, and active range of motion in patients with different subphenotypes in systemic sclerosis: a cross-sectional cohort study. Scand J Rheumatol. 2019;48(2):141–148. doi: 10.1080/03009742.2018.1477990 [DOI] [PubMed] [Google Scholar]
- 87.Shapiro L, Saketkoo LA, Farrell J, Fligelstone K. AB0712 Development of a “Renal Crisis Prevention Card” as an Education Tool to Improve Outcomes in High Risk Patients with Systemic Sclerosis (SSC). Ann Rheum Dis. 2015;74(Suppl 2):1136. doi: 10.1136/annrheumdis-2015-eular.3605 [DOI] [Google Scholar]
- 88.Exercise as a Multi-modal Disease-Modifying Medicine in Systemic Sclerosis: An Introduction by The Global Fellowship on Rehabilitation and Exercise in Systemic Sclerosis (G-FoRSS). [DOI] [PMC free article] [PubMed]
- 89.Alexanderson H, Bergegård J, Björnådal L, Nordin A. Intensive aerobic and muscle endurance exercise in patients with systemic sclerosis: a pilot study. BMC Res Notes. 2014;7:86. doi: 10.1186/1756-0500-7-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Oliveira NC, Portes LA, Pettersson H, Alexanderson H, Boström C. Aerobic and resistance exercise in systemic sclerosis: State of the art. Musculoskeletal Care. 2017;15(4):316–323. doi: 10.1002/msc.1185 [DOI] [PubMed] [Google Scholar]
- 91.Allen JM, Mailing LJ, Niemiro GM, et al. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50(4):747–757. doi: 10.1249/MSS.0000000000001495 [DOI] [PubMed] [Google Scholar]
- 92.van der Vaart R, Repping-Wuts H, Drossaert CHC, Taal E, Knaapen-Hans HKA, van de Laar MAFJ. Need for online information and support of patients with systemic sclerosis. Arthritis Care Res. 2013;65(4):594–600. doi: 10.1002/acr.21875 [DOI] [PubMed] [Google Scholar]
- 93.Rubenzik TT, Derk CT. Unmet patient needs in systemic sclerosis. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2009;15(3):106–110. doi: 10.1097/RHU.0b013e31819dbe83 [DOI] [PubMed] [Google Scholar]
- 94.Yuen SY, Rochwerg B, Ouimet J, Pope JE. Patients with scleroderma may have increased risk of osteoporosis. A comparison to rheumatoid arthritis and noninflammatory musculoskeletal conditions. J Rheumatol. 2008;35(6):1073–1078. [PubMed] [Google Scholar]
- 95.Chen J, Lei L, Pan J, Zhao C. A meta-analysis of fracture risk and bone mineral density in patients with systemic sclerosis. Clin Rheumatol. 2020;39(4):1181–1189. doi: 10.1007/s10067-019-04847-0 [DOI] [PubMed] [Google Scholar]
- 96.Psotka MA, Singletary EA, Bleser WK, et al. Streamlining and Reimagining Prior Authorization Under Value-Based Contracts: A Call to Action From the Value in Healthcare Initiative’s Prior Authorization Learning Collaborative. Circ Cardiovasc Qual Outcomes. 2020;13(7):e006564. doi: 10.1161/CIRCOUTCOMES.120.006564 [DOI] [PubMed] [Google Scholar]
- 97.Wallace ZS, Harkness T, Fu X, Stone JH, Choi HK, Walensky RP. Treatment Delays Associated With Prior Authorization for Infusible Medications: A Cohort Study. Arthritis Care Res. 2020;72(11):1543–1549. doi: 10.1002/acr.24062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jew OS, Okawa J, Barbieri JS, McCaffrey J, Hayward E, Werth VP. Evaluating the effect of prior authorizations in patients with complex dermatologic conditions. J Am Acad Dermatol. 2020;83(6):1674–1680. doi: 10.1016/j.jaad.2020.06.998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Twiddy D. Beating the Prior Authorization Blues. Fam Pract Manag. 2016;23(5):15–19. [PubMed] [Google Scholar]
- 100.Hudson M, Steele R, Lu Y, Thombs BD, Canadian Scleroderma Research Group, Baron M. Work disability in systemic sclerosis. J Rheumatol. 2009;36(11):2481–2486. doi: 10.3899/jrheum.081237 [DOI] [PubMed] [Google Scholar]
- 101.Russell A-M, Maher TM, all authors. Daily Home Spirometry: A New Milestone in the Field of Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016;194(8):1034–1035. doi: 10.1164/rccm.201606-1238LE [DOI] [PubMed] [Google Scholar]
- 102.Saketkoo LA, Alexanderson H, Lammi MR, et al. An ode to the primal tonic of dance-congratulating the Life of Breath project. Lancet Respir Med. 2020;8(12):e90–e91. doi: 10.1016/S2213-2600(20)30466-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poole JL, Mendelson C, Skipper B, Khanna D. Taking charge of systemic sclerosis: a pilot study to assess the effectiveness of an internet self-management program. Arthritis Care Res. 2014;66(5):778–782. doi: 10.1002/acr.22192 [DOI] [PubMed] [Google Scholar]
- 104.Murphy SL, Barber MW, Homer K, Dodge C, Cutter GR, Khanna D. Occupational Therapy Treatment to Improve Upper Extremity Function in Individuals with Early Systemic Sclerosis: A Pilot Study. Arthritis Care Res. 2018;70(11):1653–1660. doi: 10.1002/acr.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zaidan AA, Zaidan BB, Al-Haiqi A, Kiah MLM, Hussain M, Abdulnabi M. Evaluation and selection of open-source EMR software packages based on integrated AHP and TOPSIS. J Biomed Inform. 2015;53:390–404. doi: 10.1016/j.jbi.2014.11.012 [DOI] [PubMed] [Google Scholar]