Abstract
We generated mice carrying a STAT3 allele amenable to Cre-mediated deletion and intercrossed them with Mx-Cre transgenic mice, in which the expression of Cre recombinase can be induced by type I interferon. Interferon-induced deletion of STAT3 occurred very efficiently (more than 90%) in the liver and slightly less efficiently (about 70%) in the bone marrow. Analysis of the induction of liver acute-phase genes in response to bacterial lipopolysaccharide unequivocally identifies STAT3 as a fundamental mediator of their induction. The different degrees of defectiveness displayed by the various genes allowed us to differentiate them into three separate groups according to their degree of dependence on STAT3. Induction was totally defective for group I genes, defective at 24 h but almost normal at earlier time points for group II genes, and only slightly defective for group III genes. This division was in good agreement with the known structures of the respective promoters. We also found that the overall induction of the transcription factors C/EBPβ and -δ was only minimally defective in the absence of STAT3. Finally, even though corticosterone levels and action were found to be normal in the conditional-mutant mice, production of both proinflammatory and antiinflammatory cytokines was increased and prolonged, probably as a result of STAT3 deletion in macrophages.
The acute-phase (AP) proteins are liver plasma proteins whose levels of expression are either positively or negatively regulated by cytokines during inflammation, chiefly through the regulation of the activities of their cognate genes (13). Interleukin 1 (IL-1) and IL-6 are the main inflammatory mediators involved in this transcriptional induction (27), acting synergistically to activate a subset of AP genes known as class I. In contrast, class II genes are solely responsive to IL-6-type cytokines. In addition, corticosteroid hormones are induced by inflammatory cytokines and participate in the induction of most AP genes, being required for their optimal induction and in turn exerting an inhibitory effect on cytokine production, thus activating a negative-feedback loop (3, 4, 35). Two main kinds of cytokine-responsive elements have been characterized on the promoters of AP genes (recently reviewed in reference 36). Type I IL-6-responsive elements (IL-6REs) are binding sites for CAAT/enhancer binding protein (C/EBP) transcription factors, which can mediate transcriptional induction by both IL-6 and IL-1, and have been identified on the promoters of most class I genes, such as those for haptoglobin (HP) (25), α1-acid glycoprotein (AGP) (38, 47), hemopexin (Hpx) (37), complement component 3 (C3) (24), C-reactive protein (CRP) (34), and serum amyloids A (SAA) (21) and P (SAP) (34). On the other hand, class II genes, such as α-2 macroglobulin (α2M) (45) and fibrinogens (FBs) (10, 32, 50), appear to be mainly regulated by type II IL-6REs, which are binding sites for members of the signal transducers and activators of transcription (STAT) family of transcription factors and particularly for STAT3-APRF (45). Type II IL-6RE–STAT3 sites have also been identified on class I gene promoters.
IL-6-type cytokines, which share the receptor signaling subunit gp130, are known to elicit the activation of two major signaling pathways through the activation of kinases belonging to the JAK family: tyrosine phosphorylation and activation of STAT factors, mainly STAT3 (19, 41), and activation of the mitogen-activated protein kinase (MAPK) pathway through recruitment of the SH2-containing protein tyrosine phosphatase 2 (SHP-2) as a molecular adapter (14, 26). Both pathways lead to the activation of transcription factors involved in the regulation of AP genes: STAT3-APRF is activated directly by JAK family kinases, while C/EBPβ and -δ, the two C/EBP family members that are induced during inflammation, are activated through the MAPK pathway (see reference 36 and references therein). However, on the basis of a number of in vitro data, STAT3 has been proposed to be the main mediator of AP gene induction downstream of IL-6 and other gp130 cytokines (29, 30), and indeed, in IL-6-deficient turpentine-treated mice, failure to activate the AP genes correlated with defective STAT3 activation (2, 12). On the other hand, the analysis of C/EBPβ-deficient mice has failed to reveal any dramatic defect in the activation of AP genes, although a final conclusion on the overall role of C/EBPs in the regulation of AP promoters cannot be drawn from these data, since C/EBPδ might well be able to compensate for the absence of C/EBPβ (8).
STAT3 can be activated by many cytokines and growth factors in addition to gp130 cytokines (see reference 1 for a recent review), and indeed, STAT3 inactivation by gene targeting leads to early embryonic lethality (44). Cre-mediated inactivation of STAT3 in different cell types has been recently described, demonstrating important roles for this factor in mediating functions of IL-6, IL-2, epidermal growth factor (EGF), and prolactin (9, 40, 42, 43). We describe here the independent generation of conditional STAT3 mutant mice in which inactivation of the gene can be induced in several tissues by treatment with synthetic double-stranded RNA [poly(I · C)], which triggers the expression of an interferon (IFN)-inducible Cre recombinase (28). Although STAT3 deletion obtained through repeated poly(I · C) treatments eventually triggers the development of a fulminant form of ulcerative colitis (T. Alonzi et al., unpublished data), short-term treatment causes preferential deletion in the liver and macrophages. We made use of this model to directly assess in vivo the role of STAT3 in regulating transcription of AP genes in response to treatment with recombinant IL-6 or bacterial lipopolysaccharide (LPS). We demonstrate that STAT3 is indeed essential for the induction of all tested genes downstream of IL-6. On the other hand, STAT3 also plays an important role in the activation of most genes in response to LPS, which triggers the production of a much more complex repertoire of inflammatory cytokines, including IL-6, IL-1, and tumor necrosis factor alpha (TNF-α). In this case, however, its relative functional relevance varied in agreement with the structures of the different promoters. Moreover, we show that STAT3 is only marginally involved in the transcriptional induction of C/EBPβ and -δ and that corticosterone (CS) production is normal in STAT3 conditional-mutant mice.
MATERIALS AND METHODS
Generation of the targeting vector and of the targeted ES cells and mice.
A genomic library from the 129/SV mouse strain (Stratagene Cloning Systems, La Jolla, Calif.) was screened with a cDNA clone for the mouse STAT3, and several overlapping positive clones were identified. A SalI-StuI fragment of approximately 10 kb, containing exons 6 to 14, was subcloned into a Bluescript plasmid. An 88-bp fragment containing a single loxP site, engineered to contain an EcoRV restriction site, was obtained by PCR from the plasmid pGEM-30 (18) and cloned into the EcoRI site in intron 14. A SalI-XbaI fragment containing a pMC1/Neo expression cassette flanked by loxP sites from the plasmid pL2-Neo (17) was inserted into the EcoRI site of intron 11. Finally, a pMC1 herpes simplex virus thymidine kinase cassette (33) was inserted downstream of the 3′ homology region to generate the targeting vector. This was linearized with SalI and electroporated into E14 embryonic stem (ES) cells (20) according to standard protocols. Clones resistant to both G418 and ganciclovir were screened for homologous recombination by Southern blotting of EcoRV-digested genomic DNA and probed with a cDNA fragment containing exons 15 to 24, not included in the region of homology. The predicted fragment sizes were as follows: wild-type, allele, 15 kb; replaced allele, 11 kb. By using a 5′ probe (not shown), correct recombination was confirmed to have also occurred at the 5′ side. Three targeted clones were transiently transfected with pMC1-Cre (18) to delete the Neo cassette. Clones which had became sensitive to G418 were further analyzed by Southern blotting upon digestion with EcoRV and hybridization with a 2.1-kb EcoRV/EcoRI fragment from intron 11 (upstream of the Neo insertion) as a probe. The predicted sizes for the different alleles were as follows: wild type, 11 kb; replaced, 5.2 kb; floxed, 4.1 kb; deleted, 2.2 kb. Four STAT3 clones carrying the floxed allele, derived from two distinct replaced clones, were microinjected into CB6F1 (C57BL/6 × BALB/c) blastocysts to generate chimeric mice. Germ line transmission was obtained from all of them. STAT3fl/+ mice derived from two distinct clones were then intercrossed to obtain mice homozygous for the floxed mutation (STAT3fl/fl). After verifying that both STAT3fl/fl lines were viable and fertile, the one derived from clone 138 was chosen for further studies.
Animals and treatments.
STAT3fl/fl mice generated as described above were crossed with MX-Cre mice (28). MX+ (i.e., deletable) and MX− (i.e., nondeletable littermate controls) STAT3fl/fl mice for the experiments were generated by mating MX+ STAT3fl/fl males with MX− STAT3fl/fl females. Genetic screening for the Cre transgene was performed by PCR using the following oligonucleotides: CRE1, 5′-AGGCGTTTTCTGAGCATACC-3′; CRE10, 5′-TAGCTGGCTGGTGGCAGATG-3′.
The mice were bred and maintained under specific-pathogen-free conditions in our facility under a 12-h light-dark cycle and provided irradiated food and autoclaved water ad libitum. Procedures involving animals and their care were conducted in conformity with national (Home Office) and international laws and policies and were approved by the Faculty Ethical Committee. Eight- to 10-week-old mice were used for the experiments. MX+ and MX− STAT3fl/fl mice were injected intraperitoneally (i.p.) once with 250 μg of poly(I · C) 4 days before LPS treatment. LPS (Escherichia coli serotype O26:B6; Sigma Chemical Co., St. Louis, Mo.) was resuspended in sterile pyrogen-free saline solution and injected i.p. at a dose of 1 mg/kg of body weight. Human recombinant IL-6 was injected at a dose of 10 μg/mouse as described previously (2), and dexamethasone (Decadron Shock Pack; MSD, Brussels, Belgium) (750 μg/mouse) was injected i.p. 30 min prior to LPS treatment. The mice were sacrificed by CO2 asphyxiation, blood was collected by cardiac puncture, and the livers were immediately removed.
Total liver protein extraction and Western blot analysis.
The frozen livers were lysed by homogenization in a buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM NaF, 1 mM sodium vanadate, and a 40-μg/ml protease inhibitor cocktail (Sigma) and cleared by centrifugation. The protein concentration was determined by Bradford assay (Bio-Rad Laboratories, Hercules, Calif.). Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. The antibodies used were as follows: anti-STAT3 monoclonal antibody directed against the amino-terminal part of the protein and anti-STAT1 polyclonal serum (Signal Transduction Laboratories, San Diego, Calif.) and anti-phospho-STAT3 (Tyr 705), anti-phospho-p44/42 MAPK, and anti-p44/42 MAPK (New England Biolabs, Beverly, Mass.).
Liver nuclear extracts and electrophoretic mobility shift assays (EMSAs).
Nuclear extracts were prepared after LPS or saline treatment from freshly removed livers as described previously (16) with modifications. Briefly, 1 g of liver was homogenized in 2.5 ml of homogenization buffer (10 mM HEPES [pH 7.6], 15 mM KCl, 2 mM EDTA, 2 M sucrose, 10% glycerol, 0.5 mM spermidine, 0.15 mM spermine, 0.5 mM dithiothreitol, 0.5 mM PMSF, and 1% aprotinin) using a glass-Teflon Dounce homogenizer. The nuclei were pelleted by ultracentrifugation over a cushion of the same buffer and directly lysed in 10 mM HEPES (pH 7.9), 400 mM NaCl, 0.1 mM EGTA, 5% glycerol, 0.5 mM dithiothreitol, 0.5 mM PMSF, and 1% aprotinin at 4°C for 30 min. The lysates were cleared by centrifugation and frozen in liquid nitrogen. The protein concentration was determined by Bradford assay.
EMSAs were carried out by incubating 6 μg of each extract in a 20-μl final volume of a solution of 20 mM HEPES (pH 7.9), 50 mM NaCl containing 3 μg of poly(dI-dC), and 2 μg of salmon sperm DNA for 10 min on ice. 32P-labeled double-stranded oligonucleotides (2 × 104 cpm) were added, and the mixture was incubated for 15 min at room temperature. DNA-protein complexes were separated by electrophoresis on a 6% polyacrylamide gel in 0.25× TBE buffer (25 mM Tris, 25 mM boric acid, 0.6 mM EDTA) and visualized by autoradiography. Antibodies for supershift experiments were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.) and added to the preincubation mixture for 30 min on ice prior to addition of the probe.
The double-stranded oligonucleotides were labeled by filling in with Klenow polymerase. The sequences of the upper strands were as follows: NF-κB binding site, 5′-GATCCGCTGGGGACTTTCCAGGCG-3′; STAT site, 5′-GATCGATTTCCCCGAAAT-3′; C/EBP site, 5′-GGGCATAGTGGCGCAAACTCCCTTACTG-3′.
Slot blot analysis.
Total RNA was prepared from frozen livers using a Qiagen kit according to the manufacturer's instructions. Five or 20 μg, respectively, of total RNA was analyzed by slot blotting or by Northern blotting as previously described (12). The different cDNA probes used have been previously described (8, 12) and were labeled by random priming. The relative abundances of the different mRNAs were measured by phosphorimager analysis and normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Cytokine and CS measurements.
Cytokines were measured by enzyme-linked immunosorbent assay (ELISA) using kits purchased from PharMingen (San Diego, Calif.) according to the manufacturer's instructions. IL-1β levels were assayed by a two-sided sandwich ELISA using antibody pairs purchased from R&D Systems (Minneapolis, Minn.). CS was measured by radioimmunoassay using a kit from ICN (Costa Mesa, Calif.) according to the manufacturer's instructions.
Statistical analysis.
Results were analyzed by the analysis of variables test using the Statview computer program (Abacus Concept). A P value of <0.05 was considered statistically significant.
RESULTS
Generation of a mouse line amenable to Cre-mediated conditional inactivation of STAT3.
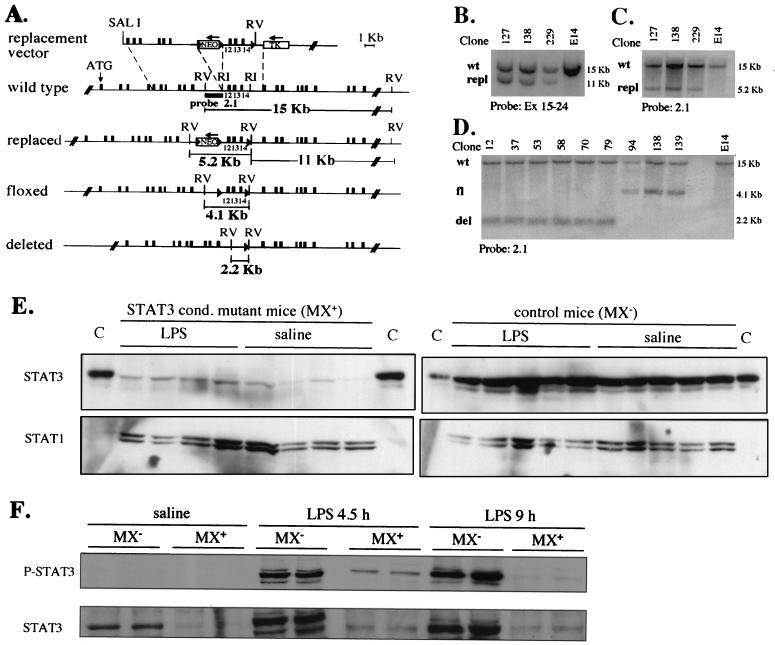
A targeting vector was constructed in which the region corresponding to exons 12 to 14 (encoding the putative DNA binding domain) was flanked with two loxP sites, while a loxP-Neo cassette was inserted into intron 11 (Fig. 1A). Homologous recombinant E14 embryonic stem cell clones were identified by Southern blot analysis (Fig. 1B and C) and subjected to transient transfection with a Cre-encoding plasmid. Colonies which had lost resistance to G418 were amplified and analyzed by Southern blotting, thus identifying those clones which had the Neor cassette but not the exonic region deleted (Fig. 1D). The resulting STAT3 floxed allele (STAT3fl) was expected to be functional but amenable to inactivation by Cre-mediated recombination through removal of the region corresponding to exons 12 to 14 and generation of a STAT3 “deleted” (Δ) allele. Transcription from this STAT3Δ allele generates a shorter, frameshifted mRNA that should be unable to encode a functional protein. Embryonic stem cells carrying a floxed allele were injected into recipient blastocysts, and the resulting chimeras were crossed to BALBC/A mice to generate mice carrying the STAT3fl allele in their germ line. STAT3fl/fl mice were obtained from heterozygous matings at a Mendelian ratio and were phenotypically indistinguishable from wild-type or heterozygous littermates, indicating that the STAT3fl allele is functional.
FIG. 1.
Generation of STAT3fl/+ ES cells and of MX+ or MX− STAT3fl/fl mice. (A) The structure of the replacement targeting vector along with the structures of the wild-type, replaced, floxed, and deleted alleles, the position of the probe used, and the predicted sizes of the restriction fragments are depicted. The replaced allele was detected by Southern blotting on EcoRV-digested genomic DNA using as a probe a cDNA fragment spanning exons 15 to 24. Cre-mediated deletion was expected to generate STAT3 floxed and deleted alleles and was diagnosed by Southern blotting using the intronic probe 2.1. (B, C, and D) Southern blot analysis of genomic DNA from targeted ES cells either before (B and C) or after (D) Cre-mediated deletion. The probes used and the relevant alleles detected are indicated (wt, wild type; repl, replaced; fl, floxed; del, deleted). (E and F) Western blot of liver extracts from poly(I · C)-treated mice. MX+ or MX− STAT3fl/fl mice were injected once i.p. with poly(I · C) followed 4 days later by either LPS or apyrogenic saline solution. The livers from the indicated mice were collected after 4.5 and 9 h (F) or after 24 h (E), and total extracts were subjected to Western blot analysis with anti-STAT3 (E) or anti-phospho-(Tyr 705) STAT3 (P-STAT3) (F) antibodies. The blots were stripped and reprobed with anti-STAT1 (E) or anti-STAT3 (F) antibodies. C, STAT3-enriched control extract.
Inducible STAT3 inactivation.
In order to generate animals in which the STAT3 gene could be inactivated in an inducible way, STAT3fl/fl mice were bred to Mx-Cre transgenic mice, which express the Cre recombinase under the control of the IFN-responsive Mx-1 promoter (28), thus generating MX+ (i.e., where the STAT3 allele is deletable) or MX− (control) STAT3fl/fl mice. Cre expression was induced in adult mice by a single injection of a synthetic double-stranded RNA [poly(I · C)], which is known to induce a strong and transient production of type I IFN. The efficiency of Cre-mediated deletion in different tissues was evaluated by Southern blot analysis of genomic DNA extracted 2 days after the treatment and was found to be almost 100% in the liver and 70 to 80% in the adipose tissue and bone marrow (not shown). Western blot analysis of liver protein extracts 4 (not shown) and 5 (Fig. 1E) days after poly(I · C) treatment confirmed that liver STAT3 protein levels were greatly reduced, indicating that the deletion allele is unable to encode a detectable protein. Therefore, MX+ STAT3fl/fl mice that have been treated with poly(I · C) as described above will hereafter be referred to as STAT3 conditional-mutant mice. STAT3 levels were slightly induced by LPS treatment in both kinds of mice (Fig. 1E and F). In addition, STAT3 activation as detected in liver nuclear extracts by specific anti-phosphotyrosine STAT3 antibodies was strongly induced by LPS in the MX− control mice at both 4.5 and 9 after LPS treatment (Fig. 1F). In contrast, STAT3 phosphorylation was barely detectable at 4.5 h and not at all by 9 h in the STAT3 conditional-mutant mice (Fig. 1F), thus confirming that very low levels of active STAT3 were present even under the inflamed conditions.
Analysis of the AP response in the STAT3 conditional-mutant mice.
In order to compare the induction of AP mRNAs in the presence and absence of STAT3 in the liver, MX+ and MX− STAT3fl/fl mice were treated once with poly(I · C) to trigger STAT3 inactivation and injected with LPS after 4 days. Liver and blood samples were then collected at different times, and total RNA from the liver was subjected to slot blot analysis with cDNA probes directed to different AP mRNAs. The results were quantified and normalized to GAPDH as an internal control, and the normalized arbitrary levels are plotted in Fig. 2. It is worth noticing that poly(I · C) treatment did not increase the basal levels of the different AP mRNAs tested, which were equivalent in the saline-treated mice injected and not injected with poly(I · C) (not shown). All mRNAs tested were increased by severalfold in the control mice, although with slightly different time courses. Induction of all tested AP mRNAs was affected in the STAT3 conditional-mutant mice, and the respective genes could be divided into three groups. Group I comprised genes whose activation was always totally defective in the absence of STAT3, including those for SAP, one of the major AP reactant in the mouse, and FBα and -γ. Group II comprised genes whose activation was comparable to that of the controls at early time points but declined much faster and was not detectable at 24 h, including those for HP, AGP, and FBβ. Finally, group III comprised genes whose activation was only slightly defective and whose mRNAs were induced at levels comparable to those of the controls 6 or 12 h after LPS treatment and remained only slightly defective at 24 h. The SAA, Hpx, and C3 genes belonged to this category. The SAA probe used in this experiment recognized all three major forms of SAA, which in the mouse are encoded by three homologous genes, SAA1, -2, and -3. To study the specific contributions of the different SAA forms to the total SAA RNA level, probes preferentially recognizing each of the three isoforms were used. As shown at the bottom of Fig. 2, SAA3 induction was the least affected by the absence of STAT3, being normal at 6 and 12 h and only reduced by about 50% at 24 h. In contrast, SAA1 induction was almost totally defective, and the SAA2 mRNA showed an intermediate behavior, being induced at 6 h but failing to increase any further.
FIG. 2.
LPS induction of AP mRNAs as measured by slot blot analysis on STAT3 conditional-mutant (open bars) and control (solid bars) mice. The mice were injected with either LPS or saline solution 4 days after poly(I · C) treatment, and their livers were collected after 0, 1.5, 6, 12, and 24 h, as indicated. Total RNA was extracted, denatured, transferred to nylon membranes by slot blotting, and hybridized with the indicated cDNA probes. The results, shown as mean values + standard errors of the mean of at least five mice per group, were plotted after normalization with GAPDH as an internal control. All values obtained after saline injection were uniform and were therefore pooled and shown collectively as time zero, representing the steady-state value.
Although the data reported above clearly show a critical role for STAT3 in the induction of most AP mRNAs, LPS leads to systemic production of several inflammatory cytokines, and STAT3 in our model is not inactivated exclusively in the liver. The possibility therefore exists that some of the effects we are assessing are due to indirect modes of action caused by alterations of the systemic response rather than by a direct role of STAT3 in gene transcription in the liver. In order to ensure that this is not the case and to provide a direct test of the transcriptional role of STAT3 downstream of gp130 cytokines, we have analyzed the induction of AP mRNAs upon treatment with recombinant IL-6. Induction of all tested mRNAs was dramatically impaired in the conditional-mutant mice (Fig. 3), thus providing a striking confirmation of the crucial role of STAT3 in mediating IL-6 induction of AP genes. Interestingly, although most mRNAs were totally unresponsive to IL-6 in the absence of STAT3, some (for HP, AGP, and Hpx) did show a slight activation which followed the same trend as in the LPS treatments, i.e., it occurred early but not late. Moreover, under these experimental conditions, C3 did not appear to be induced by IL-6 in either kind of mouse.
FIG. 3.
IL-6 induction of AP mRNAs as measured by slot blot analysis on STAT3 conditional-mutant (open bars) and control (solid bars) mice. The mice (three per group) were injected with either recombinant IL-6 or saline solution 4 days after poly(I · C) treatment, and their livers were collected after 0, 1.5, 6, and 24 h, as indicated. The methods and symbols are described in the legend to Fig. 2.
Defective AP mRNA induction is not due to lack of C/EBPβ or -δ activation.
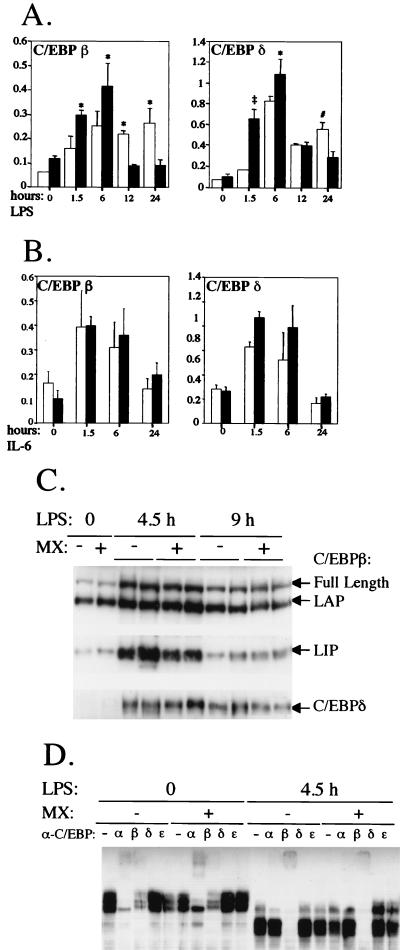
Transcription factors belonging to the C/EBP family, and in particular C/EBPβ and -δ, are believed to play an important role in mediating the cytokine inducibility of most AP genes (36). In addition, transcription of both factors is activated during inflammation and in response to IL-6 both in vivo and in vitro, and this activation has been proposed, at least for C/EBPδ, to be dependent on STAT3 (7). We therefore analyzed the induction of both C/EBPβ and -δ mRNAs in the livers of the STAT3 conditional-mutant and control mice upon treatment with either LPS or recombinant IL-6. As shown in Fig. 4A and B, the overall inductions of both genes were similar in the conditional-mutant and control mice upon both LPS and IL-6 treatment. This suggests that STAT3 is not strictly required for transcriptional induction of C/EBPβ or -δ in the liver during inflammation and that the defective expression of AP mRNAs in the conditional-mutant mice is directly due to lack of STAT3 function and is not mediated by defective C/EBP induction. Interestingly, however, induction of C/EBPβ and C/EBPδ by LPS was slightly but significantly blunted at 1.5 and 6 h after LPS treatment but then increased and was still apparent at 24 h, when it had almost returned to basal levels in the control mice. In contrast, the inductions of both mRNAs were completely comparable in response to recombinant IL-6. These observations suggest that STAT3 may indeed play a role in mediating full induction of both genes at early time points but apparently not in response to IL-6. It is worth noting that the mRNA levels of C/EBPα, a family member thought not to be involved in the regulation of AP genes and whose transcription is actually reduced by LPS and IL-6, were equivalent in untreated STAT3 conditional-mutant and control mice and decreased to similar extents upon LPS or IL-6 treatment (data not shown).
FIG. 4.
Induction of C/EBPβ and -δ as measured by Northern blot, Western blot, and EMSA analyses. (A and B) STAT3 conditional-mutant (open bars) and control (solid bars) mice were treated for the indicated times as described in the legend to Fig. 2 with either LPS (A) or recombinant IL-6 (B) and analyzed by Northern blotting. The data are shown as mean values + standard errors of five (A) or three (B) mice per group. The values were normalized and plotted as described in the legend to Fig. 2. The symbols indicate statistically significant differences between the two groups of mice at each time point: #, P < 0.03; ∗, P < 0.01; ‡, P < 0.0001. (C) Western blot analysis of C/EBPβ and C/EBPδ. Mice were treated with LPS as described above for 4.5 or 9 h, and nuclear extracts were analyzed by Western blotting with specific antisera. The different polypeptides detected are indicated. −, negative; +, positive. (D) EMSA analysis of C/EBP binding activities. Nuclear extracts from mice either untreated or treated with LPS for 4.5 h were used in an EMSA with a double-stranded oligonucleotide carrying a C/EBP binding site. Where indicated, antisera against different C/EBP proteins were preincubated with the extracts.
In order to verify that the levels of C/EBPβ and -δ proteins and their DNA binding capacities reflected the abundance of the respective mRNAs, liver nuclear extracts were analyzed by Western blotting. As shown in Fig. 4C, the levels of both C/EBPβ and -δ were strongly increased 4.5 h after LPS treatment and slightly decreased by 9 h, and the overall abundances of the two proteins were comparable in the conditional-mutant and control mice. C/EBPβ occurs in three different forms, the full-length form, a slightly shorter form called LAP, and a truncated form termed LIP, which lacks the activation domain and is thought to act as a dominant negative (11). Interestingly, while the intermediate form, LAP, was already abundant in the liver extracts of untreated mice and was slightly increased only at 4.5 h, the other two forms were strongly induced at 4.5 h and still well above basal levels at 9 h. Finally, we have analyzed by EMSA the C/EBP DNA binding activities present in the liver nuclear extracts from mice either untreated or treated with LPS for 4.5 h, when the levels of both the C/EBPβ and -δ proteins were highest. Using a double-stranded oligonucleotide carrying a C/EBP binding site, the DNA-protein complexes formed with the extracts from untreated mice were equivalent in the conditional-mutant and control mice (Fig. 4D). In both cases, most complexes were supershifted by antibodies against either C/EBPα or C/EBPβ, suggesting that under untreated conditions, C/EBPα and -β homo- and heterodimers are responsible for the vast majority of the detected C/EBP DNA binding activities. Upon LPS treatment, the mobilities of the complexes were greatly increased, and no C/EBPα binding activity was detected anymore by supershift analysis. In contrast, all complexes detected appeared to contain C/EBPβ, since everything was supershifted by anti-C/EBPβ antibodies. Again, no difference was detected between extracts from STAT3 conditional-mutant mice and controls. Surprisingly, we were unable to detect any C/EBPδ DNA binding activity by supershift analysis despite having attempted it with two different sources of anti-C/EBPδ antibodies expected to possess supershifting activity, including those that readily detected the protein by Western blotting. Perhaps the proportion of C/EBPβ isoforms present in the extracts is too high for C/EBPδ to detectably bind, at least in vitro, under these conditions.
Hyperproduction of cytokines and susceptibility to endotoxic shock in the STAT3 conditional-mutant mice.
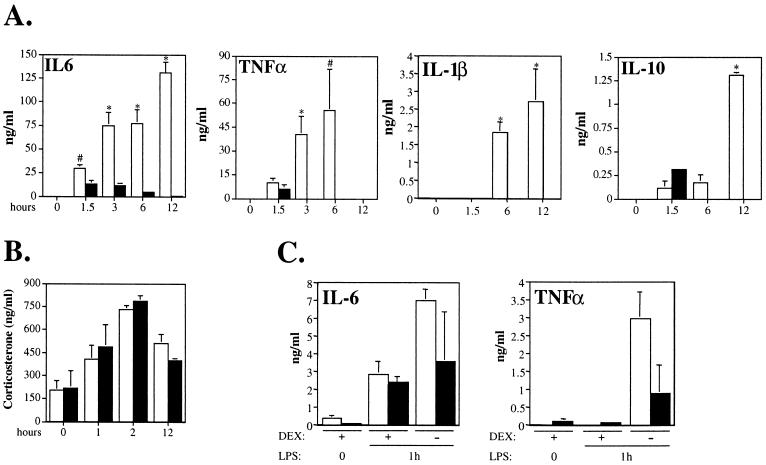
As already mentioned, STAT3 inactivation induced by poly(I · C) was not limited to the liver but occurred at good efficiency in other compartments as well, and most notably in the bone marrow (data not shown) and in bone marrow-derived macrophages (Alonzi et al., unpublished data). Since macrophages are the major cytokine-producing inflammatory cells, it was important to assess their functionality by measuring the levels of circulating inflammatory cytokines produced upon LPS injection in the STAT3 conditional-mutant mice compared to those in the controls. Interestingly, the induction of all proinflammatory cytokines tested was found to be stronger and more sustained in the STAT3 conditional mutants (Fig. 5A). IL-6 was still increasing between 6 and 12 h after injection, at a time when it was reduced to basal levels in the control mice. IL-1β followed a very similar pattern of induction, although its levels were below the detection limits of our assay in the control mice. Similarly, TNF-α was still increasing at 6 h, while it had become undetectable after 3 h in the control mice. Taken together, these data indicate that the defective activation of AP genes detected in the conditional-mutant mice in response to LPS is most definitely not due to impaired cytokine production, and if anything they emphasize the fundamental role of STAT3, which is absolutely required for the induction of most AP genes even in the presence of abnormally high levels of cytokines.
FIG. 5.
(A and B) Serum cytokine and CS levels measured in LPS-treated STAT3 conditional-mutant (open bars) and control (solid bars) mice. The mice were treated as described in the legend to Fig. 2. Blood was collected at the indicated time points, and the cytokine content of the serum was measured by ELISA (A) or the CS content was measured by radioimmunoassay (B). The data are shown as mean values + standard errors of the mean of at least five (A) or three (B) mice per group. The symbols indicate statistically significant differences between the two groups of mice at each time point: #, P < 0.01; ∗, P < 0.001. (C) Cytokine levels upon pretreatment with dexamethasone. Mice were treated with either dexamethasone or apyrogen saline solution 30 min prior to LPS injection, and blood was collected 1 h later and subjected to ELISA analysis. +, present; −, absent.
Despite the low dosage used, which was well tolerated by all control mice, most conditional-mutant mice were found to be almost moribund 24 h after LPS injection, most likely due to the endotoxic shock caused by TNF-α hyperproduction. Our results are in agreement with those reported by S. Akira and colleagues in mice in which STAT3 had been inactivated specifically in macrophages and granulocytes (42) and can be explained by their observation that STAT3 is required for responsiveness to IL-10, a cytokine known for its potent deactivating effects on macrophages. IL-10, is also known to exert an inhibitory effect on its own synthesis, and apparently this action is also dependent on STAT3, since IL-10 levels in the conditional-mutant mice were abnormally high 12 h after LPS treatment (Fig. 5A).
CS production and action are normal in the STAT3 conditional-mutant mice.
The activation of the hypothalamic-pituitary-adrenal axis by inflammatory cytokines and the consequent production of glucocorticoid hormones play an important dual role in the inflammatory response by participating as coactivators in the transcriptional induction of several AP genes (3) while at the same time providing negative feedback for cytokine production (4, 35). STAT3 deletion occurring in tissues other than the liver may therefore interfere either with cytokine-induced CS production or with the action of CS itself on target cells. We have therefore measured CS levels in the serum of mice treated with LPS for different lengths of time and found no differences between the STAT3 conditional mutant and the control mice (Fig. 5B), suggesting that the activation of the hypothalamic-pituitary-adrenal axis is not compromised. Moreover, administration of dexamethasone 30 min prior to LPS injection was able to significantly reduce IL-6 and TNF-α levels in the blood of both the control and the conditional-mutant mice (Fig. 5C), thus demonstrating that glucocorticoid hormone action is not impaired by STAT3 inactivation.
Increased NF-κB and STAT1 activation in the livers of the STAT3 conditional-mutant mice.
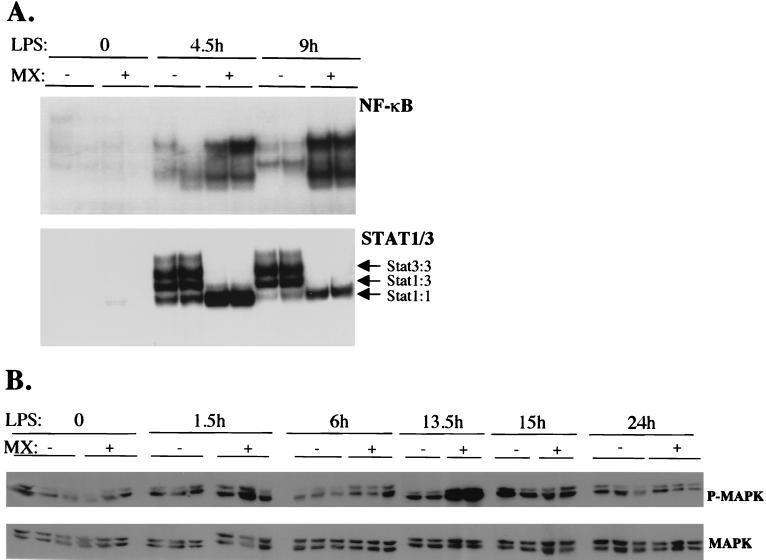
As shown in Fig. 4, the overall induction of both C/EBPβ and -δ in the conditional mutant mice was comparable to that in the control mice, and accordingly, C/EBP DNA binding activities in liver nuclei before and after LPS injection were also equivalent. Other transcription factors that are activated by LPS and that may play a role in the induction of AP genes are NF-κB and STAT1. NF-κB is the main target of TNF-α and IL-1, while STAT1 is activated along with STAT3 by IFN-γ and gp130 cytokines. LPS injection triggered the activation of NF-κB DNA binding activity, which was abundant after 4.5 h and slightly decreased after 9 h in the livers of the control mice (Fig. 6A, top). The same complexes, only much more abundant, were also formed by using nuclear extracts from the STAT3 conditional-mutant mice, suggesting a stronger and more sustained NF-κB activation.
FIG. 6.
EMSAs and anti-MAPK detection with liver extracts from STAT3 conditional-mutant and control mice. The mice were treated as described in the legend to Fig. 2. (A) Livers were collected 4.5 and 9 h after LPS injection, and nuclear extracts were prepared immediately. Total nuclear extracts were incubated with 32P-labeled double-stranded oligonucletides carrying binding sites for NF-κB (from the human immunodeficiency virus 3′ long terminal repeat) or STAT3-STAT1 (corresponding to the sis-inducible element on the c-fos promoter). (B) To detect MAPK phosphorylation, total extracts from mice treated with LPS for the indicated lengths of time were subjected to Western blot analysis with anti-phospho-(p44/42) MAPK antibodies (P-MAPK). The blots were stripped and reprobed with anti-MAPK antibodies (MAPK). +, positive; −, negative.
Three different DNA-protein complexes are formed using liver nuclear extracts from LPS-treated mice with a STAT binding site as a probe and are known to correspond to STAT3 homodimers, STAT3-STAT1 heterodimers, and STAT1 homodimers. All three complexes were detected when liver nuclear extracts from the LPS-treated control mice were used (Fig. 6A, bottom). No STAT3 DNA binding activity was detected in the extracts from the conditional-mutant mice, thus confirming that the low levels of tyrosine-phosphorylated STAT3 present in the livers of these mice (Fig. 1F) were not sufficent to allow detectable binding. However, the complex corresponding to STAT1 homodimers was considerably more intense at both time points analyzed.
The inactivation of STAT docking sites on gp130 has been proposed to prolong MAPK activation (30), and this might in turn partly explain the slightly prolonged induction observed for both the C/EBPβ and -δ mRNAs. Indeed, phosphorylation of ERK1 and ERK2 was more elevated in the absence of STAT3, although by 15 h after LPS treatment the activation levels were equivalent to those found in the control mice, and by 24 h phosphorylation levels were back to their basal values in both cases (Fig. 6B).
DISCUSSION
Our previous observation that in IL-6-deficient mice impaired and normal induction of AP genes in response to different stimuli correlated with defective and normal activation of STAT3, respectively (2, 12), pointed towards a fundamental role for STAT3 in the induction of AP genes in vivo. Indeed, we found that deletion of STAT3 in the livers of adult mice virtually abolished the ability of IL-6 to induce all AP mRNAs measured. More importantly still, AP gene induction was also defective upon LPS injection, thus largely confirming the pivotal role of STAT3 even under conditions that mimic a systemically induced inflammatory reaction and lead to the production of a broad variety of cytokines.
Promoter structure correlates with functional predominance of STAT3 for induction of AP genes.
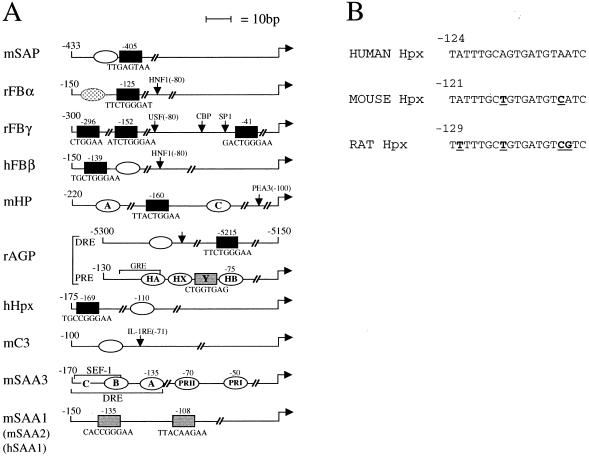
A functional analysis of the known structure of the corresponding promoters, whose schematic representation is shown in Fig. 7, indicates a surprisingly good correlation between known functional promoter organization and the differential role played by STAT3 in their induction. The scheme focuses particularly on C/EBP and STAT sites, which are the two main regulatory elements common to most AP genes, but other sites characterized as functionally important are indicated as well. Where functional data were not available for the mouse promoters, either the rat or the human promoter is shown.
FIG. 7.
(A) Structure of the analyzed AP promoters from the corresponding mouse (m), rat (r), or human (h) genes. Open ovals, C/EBP–type I IL-6RE sites; solid rectangles, STAT–type II IL-6RE sites; shaded rectangles, proposed STAT sites which have not been functionally characterized; dotted oval on the rat FBα promoter, potential C/EBP site that was found not to be functional. The position and sequence of each STAT element is shown, and previous nomenclature is also given when appropriate. The coordinates of each promoter are indicated, and elements identified on the promoters but not involved in cytokine responsiveness are named and indicated by arrows. Both the distal (DRE) and the Proximal (PRE) regulatory regions of the rat AGP gene are shown. The glucocorticoid responsive element (GRE) contained within the PRE is required for optimal induction. References are given in the text. (B) Sequence comparison of the C/EBP sites on the human, mouse, and rat Hpx promoters. Bases mutated in the mouse and rat promoters compared to the human sequence are in boldface and underlined.
According to this analysis, the mouse AP genes can be divided into three groups: genes whose promoters have been shown to only carry STAT3 sites as cytokine-responsive elements appeared to be totally dependent on STAT3 for their induction (group I; FBα and -γ) (32, 50); genes carrying both STAT and C/EBP sites of equivalent functional importance on their promoters, whose induction was also totally defective at 24 h but was almost normal at earlier time points (group II, including HP [25], AGP [38, 47], and FBβ [25]); and finally, genes with no characterized STAT site on their promoters, which were only minimally affected by the absence of STAT3 (group III, including SAA3 [21] and C3 [24, 46]). The only two exceptions to this rule involved the SAP and Hpx genes, each of which carries one STAT and one C/EBP site (22, 34) and yet was totally or minimally defective, respectively. In both cases however, one of the sites was found to be dominant over the other (34, 37), which suggests that perhaps in vivo this functional predominance may be more stringent. Particularly in the case of Hpx, while the C/EBP site has been shown to be dominant in the human promoter (37), the reverse is true for the rat promoter (23). Although no functional data are available for the mouse gene, sequence comparison indicates that the C/EBP site on the mouse promoter is more similar to the human site than to the rat site, and in contrast to the rat site, it can be predicted to be a strong binding site for C/EBP proteins (Fig. 7B) (F. Altruda, personal communication). The relative independence from STAT3 shown by the SAA3 and C3 genes is in agreement with previous data obtained with C/EBPβ-deficient mice, where in contrast, Hpx induction was completely uncompromised (8). This could perhaps reflect a dominant role for C/EBPδ rather than -β in the induction of this gene. Some weak and therefore not-yet-characterized STAT element might likewise be present on the mouse SAA3 and C3 promoters, since their induction is slightly but reproducibly impaired in the STAT3 conditional-mutant mice. However, these two promoters appear to be functionally different, since SAA3 was strongly induced by recombinant IL-6 while C3 was not.
The regulation of the SAA family revealed some unexpected characteristics. C/EBP and NF-κB sites have been shown to play a major role in the transcriptional induction of all characterized SAA genes (mouse SAA3 [21], rabbit SAA [39], rat SAA1 [31], and human SAA2 [5]), which therefore would be expected not to be affected by the absence of STAT3. As discussed above, this was indeed the case for SAA3, whose induction by LPS was only partially reduced in the STAT3 conditional-mutant mice. In contrast, induction of both SAA1 and SAA2 was profoundly impaired. Although the mouse SAA1 and -2 gene promoters have not been characterized, computer-assisted alignment including the human SAA1 promoter revealed two conserved potential STAT elements but no C/EBP or NF-κB sites (6, 48), which is in good agreement with the substantially defective activation of these two genes in the absence of STAT3. It is worth noting that, although SAA3 induction was only partially impaired by STAT3 inactivation, IL-6-dependent induction was totally defective, suggesting that the portion of SAA3 induction due to IL-6 action is dependent on STAT3. On the other hand, the low but reproducible two- to threefold induction displayed by the Hpx gene in response to IL-6 seems to suggest that IL-6 is indeed capable of inducing certain AP genes via a STAT3-independent pathway, most likely involving C/EBPβ and -δ activation, which was normal in the STAT3 conditional-mutant mice.
C/EBPβ and C/EBPδ induction are not dependent on STAT3.
STAT3 has been proposed to play an important role in the induction of C/EBPδ by IL-6, and C/EBPβ has been shown to carry a functionally active STAT binding site (7). It was therefore conceivable that the defective AP gene induction in the absence of STAT3 might be at least partly mediated by impaired induction of these two C/EBP family members. Our data indicate that STAT3 may play a marginal role in the induction of both C/EBPβ and -δ mRNAs, mostly at early time points, but suggest that its function is not strictly required, since overall induction was only partially blunted. Moreover, both protein levels and DNA binding activities were comparable in STAT3 conditional-mutant and control mice, thus confirming that defective C/EBP activity could not be an indirect cause of defective AP gene induction. The reason why C/EBP factors alone are not able to at least weakly activate genes such as those for AGP, HP, and FBβ while they are apparently able to do so for Hpx, C3, and SAA3 is at present not clear. Possibly this difference originates from the structures of the respective promoters. For example, efficient induction of a given AP gene by C/EBPβ or -δ might require cooperation with at least another type of cytokine-inducible factor. Indeed, in addition to the C/EBP site(s), the C3 promoter carries an NF-κB-like IL-1RE (24), and the murine SAA3 promoter carries a binding site for SEF-1 (SAA enhancer factor), which is also required for induction (21). In contrast, only C/EBP and STAT sites are involved in the induction of the HP, AGP, and FBβ gene promoters (10, 25, 38, 47).
Cytokine overproduction and prolonged C/EBP and MAPK activation.
The reason why induction of C/EBPβ and -δ is prolonged in the absence of STAT3 will require further study. One possible cause could be the prolonged and increased production of inflammatory cytokines that occurs as a likely consequence of the lack of responsiveness to IL-10 of STAT3-deficient macrophages (42). This could also explain the stronger and prolonged NF-κB and ERK activation detected in the conditional-mutant mice. On the other hand, abnormally prolonged activation of the MAPK pathway, involved in the induction of C/EBP factors, has already been shown to occur in vitro when a mutant form of gp130 unable to activate STAT factors is used (30). This has been proposed to occur through physical interference of STAT3, bound to its membrane-proximal docking site, with the association of SHP-2 to its own docking site, which is situated in close proximity. It is tempting to speculate that, in analogy to the dual role of SHP-2 as activator of ERKs and down-regulator of the STAT activity, STAT3 activation might in turn exert an inhibitory effect on the MAPK pathway, the absence of which could explain the prolonged induction of the C/EBP factors.
Finally, the observation that the HP, AGP, and FBβ genes were activated in the absence of STAT3 6 h after LPS treatment but were totally defective after 24 h raises the question of which other transcription factor(s) might be involved in the early induction. Even though IL-6REs are exquisitively responsive to STAT3, some have been shown to be able to respond to STAT1 as well, although at a lower efficiency (49). It is therefore tempting to propose that perhaps the abnormally high STAT1 levels present in the conditional-mutant mice, particularly at earlier time points (4.5 h), might be able to partially compensate for the absence of STAT3 and could account for the initial burst of AP gene activation, perhaps in conjunction with C/EBP factors. It has been shown that two of the four gp130 YXXQ domains that act as docking sites for STAT factors are specific for STAT3 while two can recruit both STAT3 and STAT1 with similar affinities (15). It is therefore likely that the absence of STAT3 releases competition for the docking site and favors recruitment and activation of STAT1.
ACKNOWLEDGMENTS
We are grateful to W. Müller, U. Betz, and K. Rajewsky for providing the Mx-Cre transgenic mice; to H. Baumann, S. Maeda, U. Müller-Eberhard, J. Sipe, W. Liao, K. Yamamoto, S. McKnight, and J. E. Darnell for the gift of plasmids; to H. van der Putten for providing the E14 ES cells; to F. Altruda for sharing unpublished sequences; to Ian Newton for technical help; and to L. Malone and V. Murray-Tait for expert mouse care. STAT3 targeted mice were generated at the Istituto Ricerche di Biologia Molecolare. Angeletti by V.P. and T.A.
This work was supported by the Wellcome Trust (Senior Research Fellowship to V.P.). T.A. and B.G. were the recipients of EC Marie Curie fellowships.
REFERENCES
- 1.Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. doi: 10.1038/sj.onc.1203478. [DOI] [PubMed] [Google Scholar]
- 2.Alonzi T, Fattori E, Cappelletti M, Ciliberto G, Poli V. Impaired stat3 activation following localized inflammatory stimulus in IL-6-deficient mice. Cytokine. 1998;10:13–18. doi: 10.1006/cyto.1997.0250. [DOI] [PubMed] [Google Scholar]
- 3.Baumann H, Richards C, Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987;139:4122–4128. [PubMed] [Google Scholar]
- 4.Besedovsky H, del Rey A, Sorkin E, Dinarello C A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 5.Betts J C, Cheshire J K, Akira S, Kishimoto T, Woo P. The role of NF-κB and NF-IL6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by interleukin-1 and interleukin-6. J Biol Chem. 1993;268:25624–25631. [PubMed] [Google Scholar]
- 6.Butler A, Whitehead A S. Structure of the mouse serum amyloid A 5 (Saa5) gene: relationship to other members of the serum amyloid A family. Scand J Immunol. 1997;45:160–165. doi: 10.1046/j.1365-3083.1997.d01-385.x. [DOI] [PubMed] [Google Scholar]
- 7.Cantwell C A, Sterneck E, Johnson P F. Interleukin-6-specific activation of the C/EBPδ gene in hepatocytes is mediated by Stat3 and SP1. Mol Cell Biol. 1998;18:2108–2117. doi: 10.1128/mcb.18.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappelletti M, Alonzi T, Fattori E, Libert C, Poli V. C/EBPβ is required for the late phases of acute phase gene induction in the liver and for tumour necrosis factor-α, but not interleukin-6, regulation. Cell Death Differ. 1996;3:29–35. [PubMed] [Google Scholar]
- 9.Chapman R S, Lourenco P C, Tonner E, Flint D J, Selbert S, Takeda K, Akira S, Clarke A R, Watson C J. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999;13:2604–2616. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalmon J, Laurent M, Courtois G. The human beta fibrinogen promoter contains a hepatocyte nuclear factor 1-dependent interleukin-6-responsive element. Mol Cell Biol. 1993;13:1183–1193. doi: 10.1128/mcb.13.2.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 12.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni F, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fey G H, Gauldie J. The acute phase response of the liver inflammation. In: Popper H A S, editor. Progress in liver diseases. Vol. 9. Philadelphia, Pa: W. B. Saunders; 1990. pp. 89–116. [PubMed] [Google Scholar]
- 14.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 15.Gerhartz C, Heesel B, Sasse J, Hemmann U, Landgraf C, Schneider-Mergener J, Horn F, Heinrich P C, Graeve L. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. I. Definition of a novel phosphotyrosine motif mediating STAT1 activation. J Biol Chem. 1996;271:12991–12998. doi: 10.1074/jbc.271.22.12991. [DOI] [PubMed] [Google Scholar]
- 16.Gorski K, Carneiro M, Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986;47:767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- 17.Gu H, Marth J D, Orban P C, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 18.Gu H, Zou Y R, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 19.Hemmann U, Gerhartz C, Heesel B, Sasse J, Kurapkat G, Grotzinger J, Wollmer A, Zhong Z, Darnell J E, Jr, Graeve L, Heinrich P C, Horn F. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. II. Src homology SH2 domains define the specificity of Stat factor activation. J Biol Chem. 1996;271:12999–13007. doi: 10.1074/jbc.271.22.12999. [DOI] [PubMed] [Google Scholar]
- 20.Hooper M, Hardy K, Handyside A, Hunter S, Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 21.Huang J H, Liao W S. Induction of the mouse serum amyloid A3 gene by cytokines requires both C/EBP family proteins and a novel constitutive nuclear factor. Mol Cell Biol. 1994;14:4475–4484. doi: 10.1128/mcb.14.7.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Immenschuh S, Nagae Y, Satoh H, Baumann H, Muller-Eberhard U. The rat and human hemopexin genes contain an identical interleukin-6 response element that is not a target of CAAT enhancer-binding protein isoforms. J Biol Chem. 1994;269:12654–12661. [PubMed] [Google Scholar]
- 23.Immenschuh S, Song D X, Satoh H, Muller-Eberhard U. The type II hemopexin interleukin-6 response element predominates the transcriptional regulation of the hemopexin acute phase responsiveness. Biochem Biophys Res Commun. 1995;207:202–208. doi: 10.1006/bbrc.1995.1173. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura N, Singer L, Wetsel R A, Colten H R. Cis-and trans-acting elements required for constitutive and cytokine-regulated expression of the mouse complement C3 gene. Biochem J. 1992;283:705–712. doi: 10.1042/bj2830705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Baumann H. The carboxyl-terminal region of STAT3 controls gene induction by the mouse haptoglobin promoter. J Biol Chem. 1997;272:14571–14579. doi: 10.1074/jbc.272.23.14571. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Baumann H. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol Cell Biol. 1999;19:5326–5338. doi: 10.1128/mcb.19.8.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koj A, Gauldie J, Baumann H. Biological perspectives of cytokine and hormone networks. In: Mackiewicz K A B, editor. Acute phase proteins: molecular biology, biochemistry, and clinical applications. Boca Raton, Fla: CRC Press; 1993. pp. 275–287. [Google Scholar]
- 28.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 29.Lai C F, Ripperger J, Morella K K, Wang Y, Gearing D P, Fey G H, Baumann H. Separate signaling mechanisms are involved in the control of STAT protein activation and gene regulation via the interleukin 6 response element by the box 3 motif of gp130. J Biol Chem. 1995;270:14847–14850. doi: 10.1074/jbc.270.25.14847. [DOI] [PubMed] [Google Scholar]
- 30.Lai C F, Ripperger J, Wang Y, Kim H, Hawley R B, Baumann H. The STAT3-independent signaling pathway by glycoprotein 130 in hepatic cells. J Biol Chem. 1999;274:7793–7802. doi: 10.1074/jbc.274.12.7793. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Liao W S L. Cooperative effects of C/EBP-like and NF-kB-like binding sites on rat serum amyloid A1 gene expression in liver cells. Nucleic Acids Res. 1992;20:4765–4772. doi: 10.1093/nar/20.18.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Fuller G M. Detection of a novel transcription factor for the A alpha fibrinogen gene in response to interleukin-6. J Biol Chem. 1995;270:7580–7586. doi: 10.1074/jbc.270.13.7580. [DOI] [PubMed] [Google Scholar]
- 33.Mansour S L, Thomas K R, Capecchi M R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 34.Ochrietor J D, Harrison K A, Zahedi K, Mortensen R F. Role of Stat3 and C/Ebp in Cytokine-dependent expression of the mouse serum amyloid P-component (Sap) and C-reactive protein (Crp) genes. Cytokine. 2000;12:888–899. doi: 10.1006/cyto.2000.0668. [DOI] [PubMed] [Google Scholar]
- 35.Parant M, Le Contel C, Parant F, Chedid L. Influence of endogenous glucocorticoid on endotoxin-induced production of circulating TNF-alpha. Lymphokine Cytokine Res. 1991;10:265–271. [PubMed] [Google Scholar]
- 36.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 37.Poli V, Mancini F P, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643–654. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- 38.Ratajczak T, Williams P M, DiLorenzo D, Ringold G M. Multiple elements within the glucocorticoid regulatory unit of the rat alpha 1-acid glycoprotein gene are recognition sites for C/EBP. J Biol Chem. 1992;267:11111–11119. [PubMed] [Google Scholar]
- 39.Ray A, Hannink M, Ray B K. Concerted participation of NF-kappa B and C/EBP heteromer in lipopolysaccharide induction of serum amyloid A gene expression in liver. J Biol Chem. 1995;270:7365–7374. doi: 10.1074/jbc.270.13.7365. [DOI] [PubMed] [Google Scholar]
- 40.Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl N, Boulton T G, Farruggella T, Ip N Y, Davis S, Witthuhn B A, Quelle F W, Silvennoinen O, Barbieri G, Pellegrini S, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 42.Takeda K, Clausen B E, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 44.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wegenka U M, Buschmann J, Lutticken C, Heinrich P C, Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993;13:276–288. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson D R, Juan T S, Wilde M D, Fey G H, Darlington G J. A 58-base-pair region of the human C3 gene confers synergistic inducibility by interleukin-1 and interleukin-6. Mol Cell Biol. 1990;10:6181–6191. doi: 10.1128/mcb.10.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Won K A, Baumann H. The cytokine response element of the rat alpha 1-acid glycoprotein gene is a complex of several interacting regulatory sequences. Mol Cell Biol. 1990;10:3965–3978. doi: 10.1128/mcb.10.8.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto K, Goto N, Kosaka J, Shiroo M, Yeul Y D, Migita S. Structural diversity of murine serum amyloid A genes. Evolutionary implications. J Immunol. 1987;139:1683–1688. [PubMed] [Google Scholar]
- 49.Yuan J, Wegenka U M, Lutticken C, Buschmann J, Decker T, Schindler C, Heinrich P C, Horn F. The signalling pathways of interleukin-6 and gamma interferon converge by the activation of different transcription factors which bind to common responsive DNA elements. Mol Cell Biol. 1994;14:1657–1668. doi: 10.1128/mcb.14.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Fuentes N L, Fuller G M. Characterization of the IL-6 responsive elements in the gamma fibrinogen gene promoter. J Biol Chem. 1995;270:24287–24291. doi: 10.1074/jbc.270.41.24287. [DOI] [PubMed] [Google Scholar]