Abstract
Although napping is commonly used as a strategy to improve numerous physical and cognitive performances, the efficacy of this strategy for improving postural balance has not yet been elucidated. Thus, the aim of this study was to conduct a comprehensive examination of the effect of a 60 min nap opportunity (N60) on different components of postural control. Ten highly active individuals (age = 27 ± 3.5 y, height = 1.75 ± 0.52 m, weight = 66.02 ± 8.63 kg) performed, in a randomized order, two afternoon test sessions following no nap (NN) and N60. Postural balance was assessed using the sensory organisation test (SOT), the unilateral stance test (UST), and the limits of Stability Test performed on NeuroCom® Smart Balance Master. The subjective rating of sleepiness before and after the nap conditions was also assessed. Compared to NN, N60 improved the composite balance score (p < 0.05, ES = 0.75, Δ = 5.3%) and the average and maximum percentage balance in the most challenging postural conditions of the SOT (p < 0.05 for SOT-4 and 5 and p < 0.0005 for SOT-6; ES range between 0.58 and 1.1). This enhanced postural balance in N60 was accompanied with improved visual (p < 0.05; ES = 0.93; Δ = 8.9%) and vestibular (p < 0.05; ES = 0.81; Δ = 10.5%) ratios and a reduced level of sleepiness perception (p < 0.001, ES = 0.87). However, no significant differences were found in any of the UST and LOS components’ scores (p > 0.05). Overall, a 60 min post lunch nap opportunity may be viable for improving static balance, although further work, involving larger samples and more complex motor activities, is warranted.
Keywords: Balance, Nap, Athletes, Students, Motor skills, Sensory systems
INTRODUCTION
Sleep is a vital process with beneficial impacts on physical development, emotional regulation, and cognitive functions [1], and plays an important role in molecular mechanism regulation [2] and metabolic homeostasis [3]. Waterhouse et al. [4] asserted that a night of good sleep is recuperative, produces an improvement in cognitive ability and removes the feelings of fatigue. Concordantly, Leeder et al. [5] reported that an adequate amount of sleep is crucial to achieve optimal performance and recovery. In contrast, night time sleep disturbance has been suggested to negatively affect endurance [6, 7] and short-term maximal intensity [9, 10] performances as well as reaction time, alertness and mood [8].
Widely considered to be essential for optimal physical performance [11], human balance and postural control have been suggested to be negatively affected by sleep disturbance [12, 13]. Indeed, sleep deprivation has been previously shown to destabilize various sensory and motor systems involved in maintaining postural control [14], with more pronounced effects in the middle of the day (between 10 am and 2 pm) [15]. In this context, Patel et al. [12] observed that postural control in proprioceptive stimulation conditions (vibration of the calves with open eyes (EO) and closed eyes (EC)) was negatively affected after 24 h of sleep deprivation. Similarly, one night of total sleep deprivation has been shown to negatively impact cognitive performance and result in slower elaboration of visual inputs during the postural control process [16].
Collectively, adequate sleep represents a fundamental prerequisite for optimal, waking, functioning in humans. Indeed, at least 7 h of regular sleep has previously been recommended for healthy adults [17], with an additional ~2 h sleep per night may be needed for athletes and students [18, 19]. However, previous reports indicate that sleep disturbances are frequent amongst athletes [20] and students, with ~ 70% of university students reporting sleep deprivation (6 to 6.9 h/night) [19]. Thus, it is essential for these populations to find an effective strategy to counteract the negative effect of sleep deprivation. In this context, napping has been suggested as a safe and non-invasive countermeasure to alleviate the consequences of nocturnal sleep deprivation [9] and overcoming the cognitive and physical deteriorations caused by sleep loss [21]. Nevertheless, studies investigating the effect of nap opportunity after normal (i.e., prophylactic naps) or disturbed (i.e., replacement naps) sleep on physical and cognitive performance have yielded inconclusive results. Several studies have reported that various physical and cognitive performances, such as repeated-sprint [9, 22], jumping [21], endurance [24], sports specific skills [25], reaction time [23], attention [21, 26], and short term memory [27], were improved by daytime napping. However, other reports failed to show similar efficacy [28, 29].
Surprisingly, despite that postural control plays a key role in the performance of daily tasks, and contributes to achieve peak performance in many athletics activities (e.g., shooting accuracy, skating speed, precision, efficiency of martial art-specific techniques) [30], the effects of napping on postural control has not yet been elucidated. Therefore, ascertaining the impact of diurnal napping on the ability to maintain body balance represents an important consideration [31]. Accordingly, the present study sought to investigate the effect of 60 min nap opportunity on different components of postural control (e.g., double leg and unilateral standing balance, sensory organization and limit of stability) during stable and challenging postural conditions.
MATERIALS AND METHODS
Participants
Ten highly active sports science students (age = 27 ± 3.5 years, height = 1.75 ± 0.52 m, weight = 66.02 ± 8.63 kg, training experience ≥ 3 years) volunteered to participate in this study. All participants had normal or corrected-to-normal vision, and they reported no history of musculoskeletal or neurological disease, falls, dizziness, or complaints of vertigo. After receiving a detailed explanation about the study design, aims, and benefits, participants gave their written informed consent to participate. The protocol was approved by the local review board and the study was conducted according to the declaration of Helsinki.
The required sample size was calculated a priori using the G* power software (version 3.1.9.2; Kiel University, Kiel, Germany). Values for α were set at 0.05 and power at 0.80. Based on the study of Boukhris et al. [26] and discussions between the authors, effect size was estimated to be 0.86. The required sample size was therefore 10 participants.
Study design
Participants performed, in randomized order, two afternoon test sessions following no nap (NN) and a 60-min nap (N60) prior the measurement of postural stability, respectively. A minimum period of 48 h was observed between sessions. For the N60 condition, participants came to the laboratory at 12:45 p.m. and they attempted to take a 60-min nap from 1 p.m. to 2 p.m. in a quiet and darkened room. To dissipate sleep inertia after nap opportunity, postural stability test was performed 60 min following wake up [21, 22]. For NN, participants spent the time between 1 p.m. and 3 p.m. reading books, watching videos on television, or playing video games in a comfortable armchair. To minimize the effect of diurnal biological variations, tests of postural balance occurred at same time of day (3 p.m.) in both conditions [32, 33]. The night before each experimental test, participants were asked to adhere to their normal sleep duration (~7 h).
Measurements
Sleepiness and sleep quality
The subjective rating of sleepiness before and after the nap conditions were assessed using the Stanford Sleepiness Scale (SSS). The SSS is a 7-point scale ranging from “1” (high activeness) to “7” (high tiredness) [34]. After completing the nap, participants were also asked about their subjective sleep quality using a scale ranging from 0” (no sleep), “5” (some sleep with some interruptions) to “10” (uninterrupted, deep sleep throughout) [26].
Postural balance
Postural balance was assessed using the Smart Balance Master (NeuroCom® International, Inc., USA) instrumented platform system. The device consists of a dynamic force plate, visual surround, overhead attachment for a safety harness strap and computer with software. Both the force plate and visual surround are moveable. During all three measurements (i.e., sensory organisation, unilateral stance) and limits of stability tests), participants were asked to stand unshod on the force plate with hands resting on the iliac crests.
Sensory organisation test
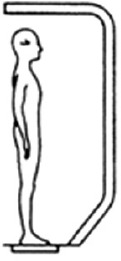
The Sensory Organisation Test (SOT) was employed to identify the sensory input influence during postural balance. The abilities as well as strategies to balance the posture were measured during six different conditions, which cause a suppression of the inputs from the inaccurate sensory system, and the participants generate appropriate motor and postural response strategies. This test compromises six conditions (SOT1-SOT6) as shown in Table 1. Sway reference involve an anteroposterior rotation of the visual surround or platform (or both). Three trials were applied for each condition with a duration of 20 seconds/trial.
TABLE 1.
The six measurement conditions of the Sensory Organisation Test.
| Test Condition | Eyes | Surroundings | Platform | Representation |
|---|---|---|---|---|
| SOT 1 | Open | Fixed | Fixed |
|
| SOT 1 | Closed | NA | Fixed |

|
| SOT 3 | Open | Sway referenced | Fixed |
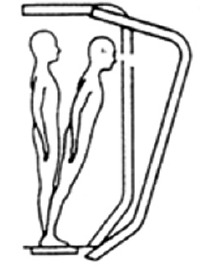
|
| SOT 4 | Open | Fixed | Sway referenced |
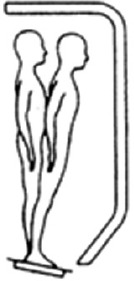
|
| SOT 5 | Closed | NA | Sway referenced |
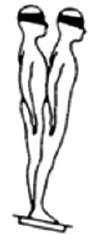
|
| SOT 6 | Open | Sway referenced | Sway referenced |
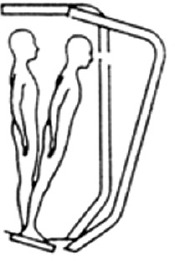
|
Note: SOT: The Sensory Organisation Test; Fixed = Stable; Sway Referenced = unstable; NA –not applicable; Representation: https://doi.org/10.1371/journal.pone.0091230. g001
Scores obtained after each condition were used to generate 4 scores associated with postural control: composite balance, and somatosensory, visual and vestibular ratios. These scores were calculated as follows:
The SOT’s composite balance score = ((Average (SOT-1)+Average (SOT-2)+Trial_1(SOT-3)+Trial_2(SOT-3)+Trial_3(SOT-3)+Trial_1(SOT4)+Trial_2(SOT-4)+rial_3(SOT-4)+Trial_1(SOT-5)+Trial_2(SOT-5)+Trial_3(SOT-)+Trial_1(SOT-6)+Trial_2(SOT-6)+Trial_3(SOT-6)) / (3 trials(SOT-3) + 3 trials(SOT-4) + 3 trials (SOT-5) + 3 trials (SOT-6) + 2 trials(SOT-1 and SOT -2)).
The SOT’s somatosensory ratio = Average (SOT–2)/Average (SOT–1)
The SOT’s visual ratio = Average (SOT–4)/Average (SOT–1)
The SOT’s vestibular ratio = Average (SOT–5)/Average (SOT–1)
Unilateral stance test
The Unilateral stance test (UST) test was performed to quantify the ability to maintain postural stability while standing either on the right or left leg on a firm surface (i.e., force plate) with eyes open and eyes closed [35]. This test compromises four conditions: (1) standing on left leg with eyes open; (2) standing on left leg with eyes closed; (3) standing on right leg with eyes open; and (4) standing on right leg with eyes closed.
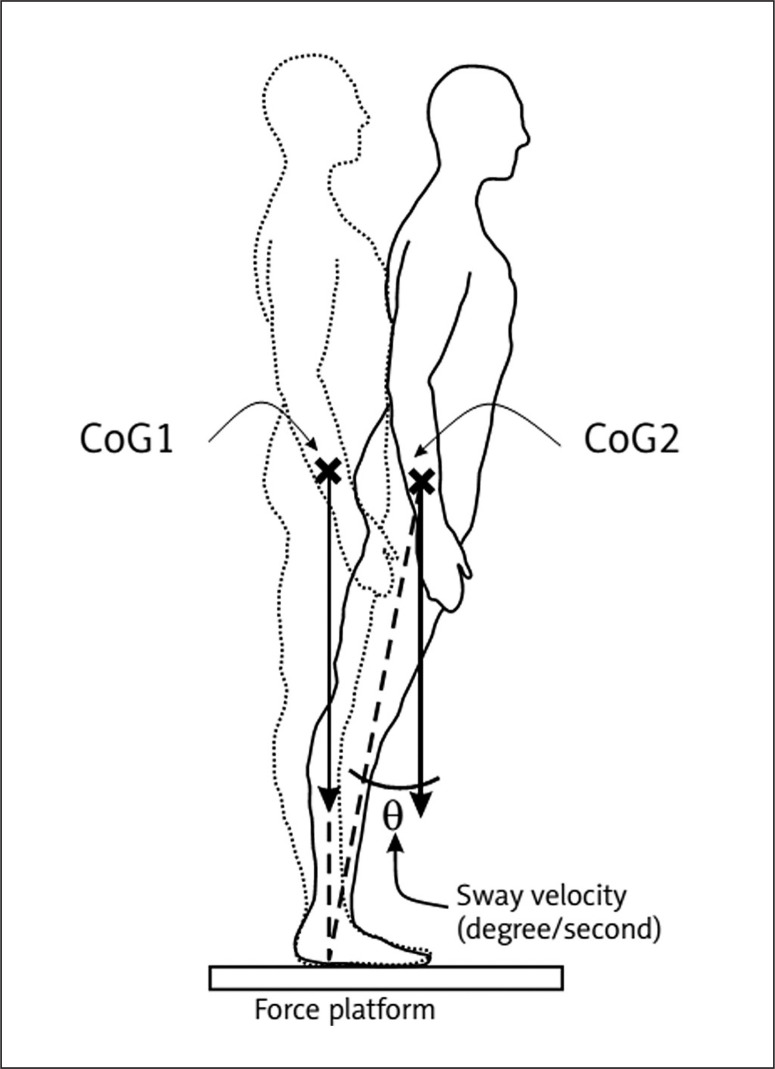
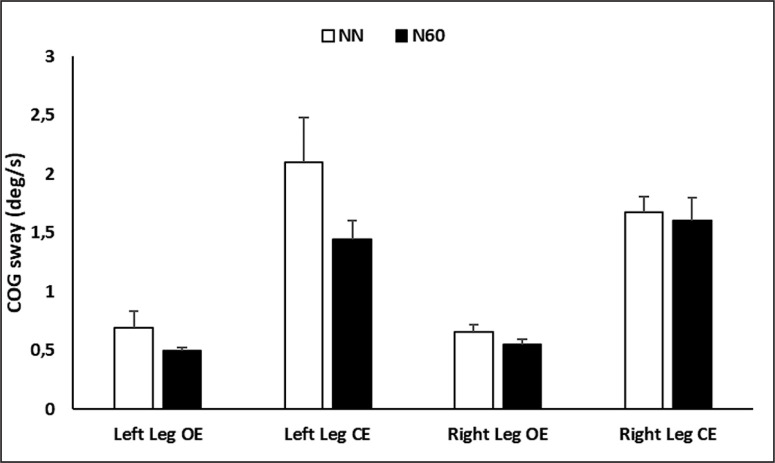
In each condition, three trials of 10-second/trial were performed. The right (condition 1 and 2) or the left (condition 3 and 4) foots was lifted to a standard height of 10 cm. During each trial, the center of gravity (COG) sway velocity (described as swapped degrees (θ) per second, figure 1) was calculated, which is the ratio of the distance travelled by the COG to the time of the trial (10 s). The UST enhances the observational testing of single leg stance performance by providing an objective measure of patient sway velocity for each of the four task conditions.
FIG. 1.
Sway velocity during the unilateral stance test
The Limits of Stability Test
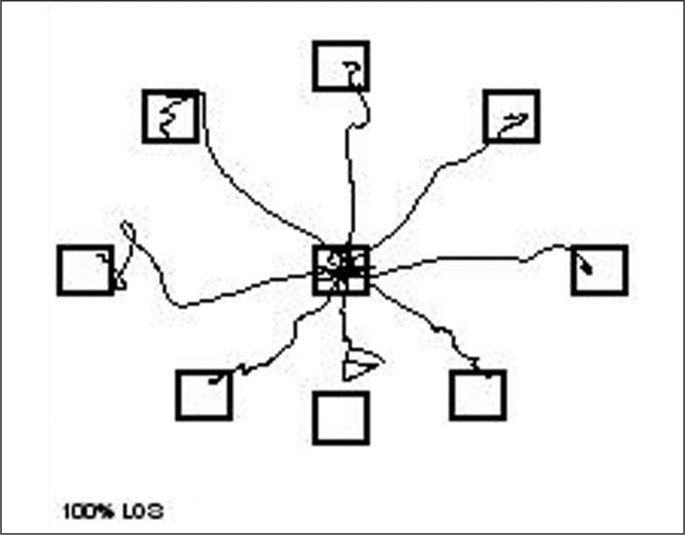
The Limits of Stability Test (LOS) assesses the participant’s ability to intentionally displace the COG in eight directions (four cardinal and four diagonal directions, Figure 2) and to concurrently maintain stability at those positions without losing balance [36]. Participants performed the test while watching a real time display of their COG. They were instructed as follows:” When you hear the tone and see the circle, shift your weight as fast as you can to move the icon quickly toward the target and maintain your position until you hear the second tone”. They were allowed to use any movement strategy they chose as long as they did not move or lift their feet. All trials started with participant in the center square. The first target is in the forward position and the test proceeds in a clockwise direction. The LOS measures movement react time (RT), Movement velocity (MVL), endpoint excursions (EPE), maximum excursions (MXE), and directional control (DCL). As defined by the NeuroCom® VSR Sport, RT was assessed as time from the signal (tone) to the COG sway exceeds the random range revealing that voluntary movement has started. MVL is the average speed of the COG shift to the target. EPE is a measurement of the COG’s traveled distance on the primary attempt to get to the target. MXE is the furthest COG’s traveled distance in a given trial. DCL was evaluated as the amount of movement in the desired direction minus amount of movement away from the intended axis.
FIG. 2.
Limits of Stability Test.
Statistical analysis
The Statistica software (StatSoft, version 12, Paris, France) was utilized to carry out statistical analysis. Data were presented as mean ± standard deviation (SD). The normality of distributions was checked through the Shapiro-Wilk test, and when normality of distribution was confirmed (i.e., maximal balance percentage during SOT’s conditions 2, 3, 5 and 6; average balance percentage during SOT’s conditions 2, 3, 4, 5 and 6; total SOT’s balance score; SOT’s visual ratio; SOT’s vestibular ratio; the MVL, EPE and DCL components of LOS; postural sway velocity during UST), a paired simple t test was conducted to compare between NN and N60 conditions. For non-normally distributed data (i.e., maximal balance percentage during SOT’s condition 1 and 4; average balance percentage during SOT’s condition 1; SOT’s somatosensory ratio, RT and MXE components of LOS), the Wilcoxon signed-rank test was utilized. Friedman nonparametric analysis of variance (ANOVA) was used for sleepiness perception, and pairwise comparisons were conducted using a Wilcoxon test. Effect sizes were calculated using Cohen’s d for all parameters, except the sleepiness perception, where the effect size was estimated by the Kendall’s coefficient of concordance. For all analysis, significance was set, a priori, at p < 0.05. The difference between the values registered during the NN and N60 conditions was calculated in % and was presented as Δ%
RESULTS
Sensory Organisation Test (SOT)
The balance percentages during the 6 conditions are presented in Table 2. There was no significant difference between NN and N60 for both average and maximal postural balance from condition 1 to condition 3 (p > 0.05). However, the average and maximal postural balance increased after N60 compared to NN during the condition 4, 5 and 6 (p < 0.05).
TABLE 2.
Balance percentages during the SOT’s six conditions recorded after the no-nap condition (NN) and the 60-min (N60) nap opportunity.
| Balance/ Condition | Average (3 trials) balance percentage | Max balance percentage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NN | N60 | P value | Cohen’s d | Δ% | NN | N60 | P value | C ohen’s d | Δ% | |
| SOT-1 | 95.0 ± 1.8 | 94.8 ± 1.7 | 0.44 | 0.11 | -0.2 | 96.2 ± 0.9 | 96.7 ± 0.8 | 0.20 | 0.58 | 0.5 |
| SOT-2 | 93.2 ± 1.7 | 92.8 ± 2.5 | 0.66 | 0.18 | -0.4 | 94.5 ± 1.4 | 94.5 ± 1.8 | 1.00 | 0.00 | 0.0 |
| SOT-3 | 91.9 ± 2.5 | 91.5 ± 4.0 | 0.76 | 0.11 | -0.6 | 93.3 ± 2.1 | 93.9 ± 3.0 | 0.52 | 0.23 | 0.6 |
| SOT-4 | 81.6 ± 11.3 | 89.3 ± 4.4 | 0.03 | 0.89 | 8.8 | 88.4 ± 7.9 | 92.0 ± 3.5 | 0.03 | 0.58 | 4.0 |
| SOT-5 | 67.3 ± 12.4 | 74.8 ± 6.2 | 0.03 | 0.79 | 10.3 | 76.3 ± 7.8 | 81.8 ± 6.5 | 0.03 | 0.76 | 6.5 |
| SOT-6 | 68.6 ± 11.1 | 77.2 ± 10.3 | < 0.0005 | 0.80 | 11.3 | 75.3 ± 8.8 | 84.5 ± 7.8 | < 0.0005 | 1.10 | 11.0 |
SOT-1: Sensory Organisation Test-condition 1; SOT-2: Sensory Organisation Test-condition 2; SOT-3: Sensory Organisation Test-condition 3; SOT-4: Sensory Organisation Test-condition 4; SOT-5: Sensory Organisation Test-condition 5; SOT-6: Sensory Organisation Test-condition 6.
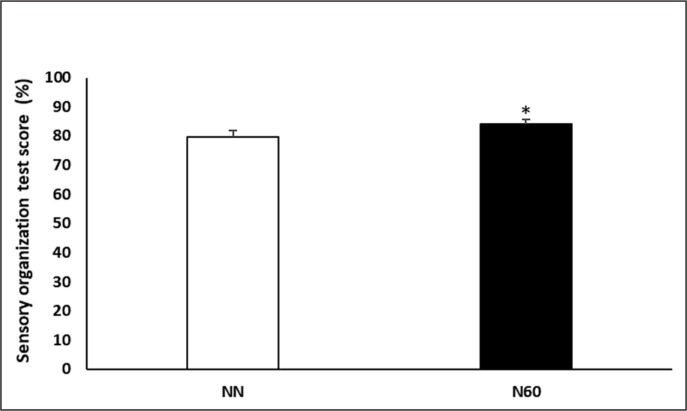
Concerning the SOT’s composite balance score (Figure 3), a significant increase of 5.3% was recorded during the N60, compared to NN condition (p < 0.05; Cohen’s d = 0.75).
FIG. 3.
Sensory organisation test score recorded after the no-nap condition (NN) and the 60-min (N60) nap opportunity. * Significant difference compared to NN.
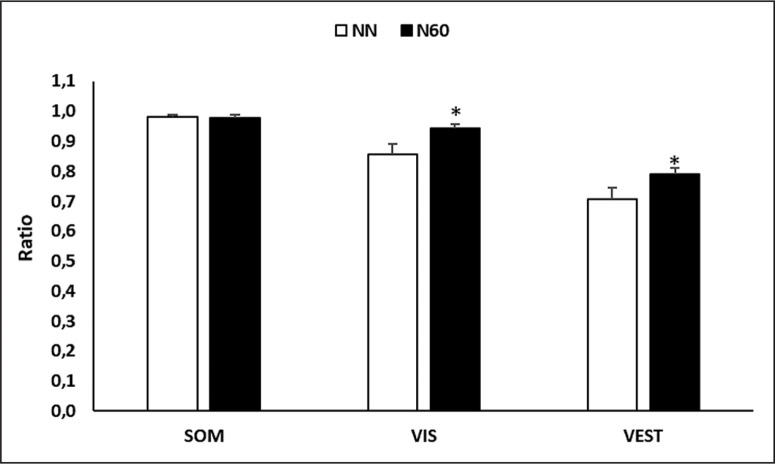
Regarding the influence of the sensory systems on postural balance (Figure 4), statistical analysis indicated that there was no significant difference between NN and N60 in somatosensory (p > 0.05; Cohen’s d = 0.07) system. However, the ratio of visual and vestibular systems were more dominant during N60, compared to NN (p < 0.05; Cohen’s d = 0.93; Δ = 8.9% for the visual ratio; p < 0.05; Cohen’s d = 0.81; Δ = 10.5% for vestibular ratio).
FIG. 4.
Representation of each sensory influence calculation recorded after the no-nap condition (NN) and the 60-min (N60) nap opportunity. SOM: somatosensory; VIS: visual; VEST: vestibular; * Significant difference compared to NN.
Unilateral stance test
As shown in Figure 5, there was no significant difference between NN and N60 in the postural sway velocity at any of the UST’s conditions (p > 0.05).
FIG. 5.
Center of gravity (COG) sway in eyes open and closed conditions recorded after the no-nap condition (NN) and the 60-min (N60) nap opportunity.
Limits of stability test
Limits of stability scores are presented in table 3. Statistical analysis indicated no significant difference between NN and N60 in RT, MVL, EPE, MXE and DCL (p > 0.05).
TABLE 3.
Limits of stability test scores recorded after the no-nap condition (NN) and the 60-min (N60) nap opportunity.
| Limits of stability | Average (8 directions) | ||||
|---|---|---|---|---|---|
| NN | N60 | P value | Cohen’s d | Δ% | |
| RT (s) | 0.55 ± 0.19 | 0.52 ± 0.20 | 0.54 | 0.15 | -6.6 |
| MVL (grad/s) | 5.9 ± 1.7 | 6.5 ± 2.8 | 0.18 | 0.25 | -8.0 |
| EPE (%) | 78.3 ± 8.0 | 83.3 ± 7.7 | 0.06 | 0.63 | 5.8 |
| MXE (%) | 93.5 ± 3.5 | 95.8 ± 2.8 | 0.16 | 0.72 | 2.4 |
| DCL (%) | 77.3 ± 5.8 | 78.0 ± 9.2 | 0.78 | 0.09 | 0.0 |
RT: Reaction time; MVL: Movement velocity; EPE: Endpoint excursions; MXE: Maximum excursions; DCL: Directional control.
Sleepiness perception
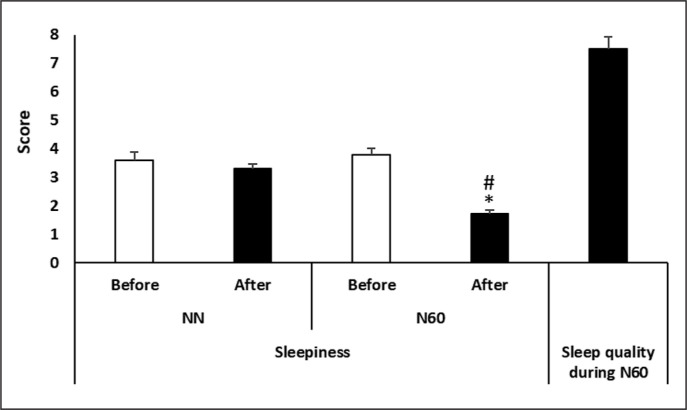
A Friedman test conducted on sleepiness perception demonstrated a significant main effect of nap condition (test = 23.52, p < 0.0005, Kendall’s W = 0.78).
Sleepiness perception was lower after N60 (p = 0.005, −55.2%) compared to NN (Figure 6). In addition, sleepiness perception recorded after N60 was lower than after NN (p = 0.005; Δ = -48.3%).
FIG. 6.
Subjective measurements of sleepiness scale recorded after the no-nap condition (NN) and the 60-min (N60) nap opportunity, and of sleep quality during the N60. * Significant difference compared to NN; # Significant difference compared to before.
DISCUSSION
Although napping is commonly used as a strategy to supplement nighttime sleep in order to improve human physical and cognitive performances [21, 22, 26], to the authors’ knowledge, the present study is the first to evaluate the effectiveness of napping (i.e., N60) on different components of postural balance. Accordingly, the main findings of the current study were that N60 improved the composite balance score, and the average and the maximum percentage balance in the most challenging postural conditions (SOT4-6), as well as the visual and the vestibular sensory systems during the SOT. However, this napping opportunity only generated minor, non-significant, improvements in the postural sway velocity during the UST and in the RT, and MVL components of the LOS test.
The complex integration of the central nervous system with the visual, vestibular, proprioceptive, and musculoskeletal systems is widely considered as the basis for maintaining postural control and achieving or restoring a state of balance in everyday functional tasks [13]. Under stable conditions, balance ability mainly rely on the somatosensory system, with a high relative weight of 70% [37]. However, on unstable surfaces, individual sensory dependence is altered, with increases in the dependency on vestibular (60%) and visual (30%) information, concomitant to decreases in surface somatosensory inputs for postural orientation [37]. Importantly, under more challenging conditions that result in inter-sensory conflict (e.g., both the surface and visual environments are unstable), the dominance of the vestibular system is more pronounced [37]. Taken together, the ability to maintain postural control in challenging sensory contexts (i.e., changeable conditions with redundant inputs) seems to be dependent on the ability of the central nervous system (CNS) to quickly reweight sensory dependence and select the appropriate inputs to rely on to generate an appropriate motor response [38]. Therefore, it has been posited that interventions or strategies which yielded a beneficial effect on brain functions, such as daytime napping [39], would also likely improve postural control.
Indeed, using different balance conditions, under stable and unstable support surfaces, with stable and/or sway visual surround, the present findings confirm this hypothesis, and indicate that N60 had a significant beneficial effect on postural balance during SOT’s condition 4, 5 and 6. Moreover, these findings revealed that the visual and vestibular systems are positively affected by N60 and suggest that these systems have higher relative sensory dependence weights compared to the somatosensory systems during the more challenging conditions (SOT4-6). The results of the sensory systems ratios confirm these suggestions and showed significantly higher visual and vestibular ratios during N60 compared to NN. Regarding the overall SOT score, the present findings also showed an improved SOT’s composite balance score during N60. This enhanced balance performance, compared to NN, may be attributed to a better integration of the central nervous system with the visual, vestibular and musculoskeletal systems [13] during the 3-last conditions (SOT4-6) of the SOT performed following N60.
Indeed, it has been previously demonstrated that there is an interaction between the sleeping or waking state of the brain and all sensory systems (i.e., visual, auditory, vestibular, somesthetic and olfactory) [40]. Particularly, the sensory information entered through the receptor may change the sleep-wake physiology, and conversely, the sleeping brain imposes rules on the incoming information [40]. In this context, it has been reported that the reduction in vestibular system efficiency and/or the integration of the various sensory inputs could explain the perturbation of postural sway after sleep restriction [41], which confirmed the reciprocal interactions between sleep and balance. Additionally, worse postural control performance has been previously reported as a consequence of bad sleep quality [42]. In the present study, the level of sleepiness perception was lower after napping (i.e., N60) compared to NN. Indeed, these findings support previous studies which reported that napping was efficacious in reducing daytime sleepiness [26, 27], and thereby improving postural balance. Regarding the underlying mechanism, the effectiveness of N60 on postural balance is most likely due to the occurrence of the slow wave sleep (SWS) –also known as deep non-rapid eye movement (NREM) sleep –during the nap. Indeed, NREM sleep has a vital role with restorative benefits for cognition [43] and is also associated with memory consolidation, learning of motor skills [25, 44] and improved physical [9, 21, 22, 24] and cognitive performance [21, 26, 27] performances. Additionally, SWS is thought to play an important role in cerebral restoration and recovery [45, 46] through a notable release of growth hormone [47], restoration of physical damage (i.e., stress to bones, muscles, tissues, and organs) and reduction of stress and anxiety [26]. Therefore, it is not surprising to find an enhanced balance performance following N60, and plausible that the SWS contained in N60 would facilitate the better integration of the central nervous system with the visual, vestibular, and musculoskeletal systems [13] during SOT.
Concerning UST and LST, the current study did not observe a significant positive effect of napping on the tested abilities. Particularly, N60 only generated minor improvements in the postural sway velocity during the UST and in the RT, and MVL components of the LOS test. The present findings suggest that N60 did not yield enough of an effect to significantly improve movement velocity and speed of decision making during more complex motor activities. Indeed, these two tests require more concentration and attentional resource, and present more difficulties than the SOT. Specifically, the LST necessitates dynamicity, muscular efficiency, and require a highly flexible postural control to cope with unpredictable perturbations. The absence of a significant effect of N60 on UST and LST components could be related to the absence or the lower duration of rapid eyes movement (REM) sleep during the 60 minutes nap opportunity. In fact, REM sleep was previously shown to enhance muscular efficiency [26], which represents a key factor in both UST and LST tests. Additionally, a complete sleep cycle (NREM + REM) has been suggested to reduce the severity of sleep inertia, since REM sleep is a lighter sleep state and waking up from this sleep stage is easier [48]. Concordantly, recent studies have reported that longer naps elicited a stronger effect on enhancing physical performance [9, 26, 49]. Thus, longer nap durations may be more suitable to generate significant improvements in postural balance during complex motor activities recurring high muscular efficiency. In this context, a 90 min nap has been reported to reduce sleep inertia, to a greater extent than shorter naps, as they permit a complete sleep cycle (NREM –REM) to occur [50]; accordingly, future research should consider varying durations of napping in order to discern the optimal outcome for UST and LST scores. In this context, future studies should investigate the effect of 90 min nap on postural control following normal sleep. Additionally, the effect of napping duration (i.e., longer vs. shorter durations) on postural control should be tested after restricted night sleep which was a common situation during COVID-19 home confinement [51].
Limitations
To the authors’ knowledge, the present study is the first to investigate the effect the effectiveness of 60 min napping opportunity (i.e., N60) on different components of postural balance in healthy trained student.
However, the absence of an objective measurement of the sleep quality and the exact nap quantity (e.g., polysomnography) could limit the final conclusion of the present study. Moreover, although novel, the present study consisted of a small sample size, which precluded out ability to accurately detect small differences, despite the presence of moderate to large effect sizes in some cases. Indeed, given the COVID-19 related restrictions and burden [52], the data of the present study has been collected on only 10 participants. Therefore, it could not be systematically generalized to athletics or students’ populations and larger studies still needed to confirm the present findings. However, we present encouraging results that can be used a as platform to further understand the efficacy of napping on postural control.
CONCLUSIONS
The present study showed that, following a normal night sleep, a post lunch nap opportunity (i.e., N60) improved static postural control during challenging SOT’s conditions under stable/unstable support surface with stable and/or sway visual surround. This enhanced postural balance was accompanied with improvements in the visual and vestibular sensory systems. However, N60 showed less efficacy on vigorous balance during UST and LST, which require more muscular efficiency and highly flexible postural control. Consequently, N60, or potentially longer napping durations (i.e., N90 allowing a complete sleep cycle), may be introduced before afternoon training sessions or late afternoon competition, in order to improve postural control which is a fundamental skill required to perform most of daily and sports activities. However, the veracity of these findings must be avowed in further studies.
REFERENCES
- 1.Watson AM. Sleep and athletic performance. Curr. Sports Med Rep. 2017;16(6):413–418. doi: 10.1249/JSR.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 2.Abe Y, de Kernier N. Sleep Disturbances from the Viewpoint of Suicidality: Implications for Future Psychosocial Interventions for Youngsters. Int Medical J. 2013;20(5) [Google Scholar]
- 3.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterhouse J, Fukuda Y, Morita T. Daily rhythms of the sleep-wake cycle. J Physiol Anthropol. 2012;31(1):1–14. doi: 10.1186/1880-6805-31-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeder J, Glaister M, Pizzoferro K, Dawson J, Pedlar C. Sleep duration and quality in elite athletes measured using wristwatch actigraphy. J Sports Sci. 2012;30(6):541–545. doi: 10.1080/02640414.2012.660188. [DOI] [PubMed] [Google Scholar]
- 6.Keramidas ME, Siebenmann C, Norrbrand L, Gadefors M, Eiken O. A brief pre-exercise nap may alleviate physical performance impairments induced by short-term sustained operations with partial sleep deprivation–A field-based study. Chronobiol Int. 2018;35:1464–70. doi: 10.1080/07420528.2018.1490316. [DOI] [PubMed] [Google Scholar]
- 7.Romdhani M, Hammouda O, Smari K, Chaabouni Y, Mahdouani K, Driss T, et al. Total Sleep Deprivation and Recovery Sleep Affect the Diurnal Variation of Agility Performance: The Gender Differences. J Strength Cond Res. 2021;35:132–140. doi: 10.1519/JSC.0000000000002614. [DOI] [PubMed] [Google Scholar]
- 8.Romdhani M, Hammouda O, Chaabouni Y, Mahdouani K, Driss T, Chamari K, et al. Sleep deprivation affects post-lunch dip performances, biomarkers of muscle damage and antioxidant status. Biol Sport. 2019;36:55–65. doi: 10.5114/biolsport.2018.78907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romdhani M, Souissi N, Chaabouni Y, Mahdouani K, Driss T, Chamari K, et al. Improved Physical Performance and Decreased Muscular and Oxidative Damage With Postlunch Napping After Partial Sleep Deprivation in Athletes. Int J Sports Physiol Perform. 2020:1–10. doi: 10.1123/ijspp.2019-0308. [DOI] [PubMed] [Google Scholar]
- 10.Souissi N, Chtourou H, Aloui A, Hammouda O, Dogui M, Chaouachi A, et al. Effects of time-of-day and partial sleep deprivation on short-term maximal performances of judo competitors. J Strength Cond Res. 2013;27:2473–80. doi: 10.1519/JSC.0b013e31827f4792. [DOI] [PubMed] [Google Scholar]
- 11.Paillard T. Relationship between sport expertise and postural skills. Front Psychol. 2019;10:1428. doi: 10.3389/fpsyg.2019.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel M, Gomez S, Berg S, Almbladh P, Lindblad J, Petersen H, et al. Effects of 24-h and 36-h sleep deprivation on human postural control and adaptation. Exp Brain Res. 2008;185(2):165–173. doi: 10.1007/s00221-007-1143-5. [DOI] [PubMed] [Google Scholar]
- 13.Montesinos L, Castaldo R, Cappuccio FP, Pecchia L. Day-to-day variations in sleep quality affect standing balance in healthy adults. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-36053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robillard R, Prince F, Boissonneault M, Filipini D, Carrier J. Effects of increased homeostatic sleep pressure on postural control and their modulation by attentional resources. Clin Neurophysiol. 2011;122(9):1771–1778. doi: 10.1016/j.clinph.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Bougard C, Davenne D. Effects of sleep deprivation and time-of-day on selected physical abilities in off-road motorcycle riders. Eur J Appl Physiol. 2012;112(1):59–67. doi: 10.1007/s00421-011-1948-6. [DOI] [PubMed] [Google Scholar]
- 16.Fabbri M, Martoni M, Esposito MJ, Brighetti G, Natale V. Postural control after a night without sleep. Neuropsychologia. 2006;44(12):2520–2525. doi: 10.1016/j.neuropsychologia.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Heal. 2015;1:233–43. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Mah CD, Mah KE, Kezirian EJ, Dement WC. The Effects of Sleep Extension on the Athletic Performance of Collegiate Basketball Players. Sleep. 2011;34:943–50. doi: 10.5665/SLEEP.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershner SD, Chervin RD. Causes and consequences of sleepiness among college students. Nat Sci Sleep. 2014;6:73. doi: 10.2147/NSS.S62907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta L, Morgan K, Gilchrist S. Does Elite Sport Degrade Sleep Quality? A Systematic Review. Sport Med. 2017;47:1317–33. doi: 10.1007/s40279-016-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsouna H, Boukhris O, Abdessalem R, Trabelsi K, Ammar A, Shephard RJ, et al. Effect of different nap opportunity durations on short-term maximal performance, attention, feelings, muscle soreness, fatigue, stress and sleep. Physiol Behav. 2019;211:112673. doi: 10.1016/j.physbeh.2019.112673. [DOI] [PubMed] [Google Scholar]
- 22.Boukhris O, Abdessalem R, Ammar A, Hsouna H, Trabelsi K, Engel FA, et al. Nap opportunity during the daytime affects performance and perceived exertion in 5-m shuttle run test. Front Physiol. 2019;10:1–8. doi: 10.3389/fphys.2019.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daaloul H, Souissi N, Davenne D. Effects of Napping on Alertness, Cognitive, and Physical Outcomes of Karate Athletes. Med Sci Sports Exerc. 2019;51:338–45. doi: 10.1249/MSS.0000000000001786. [DOI] [PubMed] [Google Scholar]
- 24.Blanchfield AW, Lewis-Jones TM, Wignall JR, Roberts JB, Oliver SJ. The influence of an afternoon nap on the endurance performance of trained runners. Eur J Sport Sci. 2018;18:1177–84. doi: 10.1080/17461391.2018.1477180. [DOI] [PubMed] [Google Scholar]
- 25.Morita Y, Ogawa K, Uchida S. Napping after complex motor learning enhances juggling performance. Sleep Sci. 2016;9:112–6. doi: 10.1016/j.slsci.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boukhris O, Trabelsi K, Ammar A, Abdessalem R, Hsouna H, Glenn JM, et al. A 90 min Daytime Nap Opportunity Is Better Than 40 min for Cognitive and Physical Performance. Int J Environ Res Public Health. 2020;17(13):4650. doi: 10.3390/ijerph17134650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterhouse J, Atkinson G, Edwards B, Reilly T. The role of a short post-lunch nap in improving cognitive, motor, and sprint performance in participants with partial sleep deprivation. J Sports Sci. 2007;25:1557–66. doi: 10.1080/02640410701244983. [DOI] [PubMed] [Google Scholar]
- 28.Petit E, Mougin F, Bourdin H, Tio G, Haffen E. A 20-min nap in athletes changes subsequent sleep architecture but does not alter physical performances after normal sleep or 5-h phase-advance conditions. Eur J Appl Physiol. 2014;114:305–15. doi: 10.1007/s00421-013-2776-7. [DOI] [PubMed] [Google Scholar]
- 29.Abdessalem R, Boukhris O, Hsouna H, Trabelsi K, Ammar A, Taheri M, et al. Effect of napping opportunity at different times of day on vigilance and shuttle run performance. Chronobiol Int. 2019;36(10):1334–1342. doi: 10.1080/07420528.2019.1642908. [DOI] [PubMed] [Google Scholar]
- 30.Hrysomallis C. Balance Ability and Athletic Performance. Sports Med. 2011;41:221–232. doi: 10.2165/11538560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Borzucka D, Kręcisz K, Rektor Z, Kuczyński M. Differences in static postural control between top level male volleyball players and non-athletes. Scientific Reports. 2020;10(1):1–7. doi: 10.1038/s41598-020-76390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ammar A, Chtourou H, Trabelsi K, Padulo J, Turki M, El Abed K, et al. Temporal specificity of training: intra-day effects on biochemical responses and Olympic-Weightlifting performances. J Sports Sci. 2015;33(4):358–68. doi: 10.1080/02640414.2014.944559. [DOI] [PubMed] [Google Scholar]
- 33.Ammar A, Chtourou H, Souissi N. Effect of time-of-day on biochemical markers in response to physical exercise. J Strength Cond Res. 2017;31(1):272–282. doi: 10.1519/JSC.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 34.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhry H, Findley T, Quigley KS, Bukiet B, Ji Z, Sims T, Maney M. Measures of postural stability. J Rehabil Res Dev. 2004 Sep;41(5):713–20. doi: 10.1682/jrrd.2003.09.0140. [DOI] [PubMed] [Google Scholar]
- 36.Lininger MR, Leahy TE, Haug EC, Bowman TG. Test-retest reliability of the limits of stability test performed by young adults using neurocom® vsr sport. Int J Sports Phys Ther. 2018;13(5):800–807. [PMC free article] [PubMed] [Google Scholar]
- 37.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002 Sep;88(3):1097–118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 38.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Cacchione PZ, Hodgson N, Riegel B, Keenan BT, Scharf MT, et al. Afternoon Napping and Cognition in Chinese Older Adults: Findings from the China Health and Retirement Longitudinal Study Baseline Assessment. J Am Geriatr Soc. 2017;65(2):373–380. doi: 10.1111/jgs.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VELLUTI R. Interactions between sleep and sensory physiology. J Sleep Res. 1997;6(2):61–77. doi: 10.1046/j.1365-2869.1997.00031.x. [DOI] [PubMed] [Google Scholar]
- 41.Souissi N, Zouita A, Abedelmalek S, Trabelsi K, Clark CC, Dziri K, et al. Partial sleep restriction impairs static postural control in elite judo athletes. Biol Rhythm Res. 2020:1–12. [Google Scholar]
- 42.Furtado F, Gonçalves BDSB, Abranches ILL, Abrantes AF, Forner-Cordero A. Chronic low quality sleep impairs postural control in healthy adults. PLoS One. 2016;11(10):e0163310. doi: 10.1371/journal.pone.0163310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 44.Davenne D. Sleep of athletes - Problems and possible solutions. Biol Rhythm Res. 2009;40:45–52. [Google Scholar]
- 45.Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5 [PMC free article] [PubMed] [Google Scholar]
- 46.Wisor JP, Rempe MJ, Schmidt MA, Moore ME, Clegern WC. Sleep slow-wave activity regulates cerebral glycolytic metabolism. Cereb Cortex. 2013;23:1978–87. doi: 10.1093/cercor/bhs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Donnell S, Bird S, Jacobson G, Driller M. Sleep and stress hormone responses to training and competition in elite female athletes. Eur J Sport Sci. 2018;18:611–8. doi: 10.1080/17461391.2018.1439535. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara M, De Gennaro L. The sleep inertia phenomenon during the sleep-wake transition: Theoretical and operational issues. Aviat Sp Environ Med. 2000;71(8):843–848. [PubMed] [Google Scholar]
- 49.Hammouda O, Romdhani M, Chaabouni Y, Mahdouani K, Driss T, Souissi N. Diurnal napping after partial sleep deprivation affected hematological and biochemical responses during repeated sprint. Biol Rhythm Res. 2018;49:927–939. [Google Scholar]
- 50.Davies DJ, Graham KS, Chow CM. The effect of prior endurance training on nap sleep patterns. Int J Sports Physiol Perform. 2010;5:87–97. doi: 10.1123/ijspp.5.1.87. [DOI] [PubMed] [Google Scholar]
- 51.Trabelsi K, Ammar A, Masmoudi L, Boukhris O, Chtourou H, Bouaziz B, et al. Globally altered sleep patterns and physical activity levels by confinement in 5056 individuals: ECLB COVID-19 international online survey. Biol Sport. 2021;38(4):495–506. doi: 10.5114/biolsport.2021.101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ammar A, Trabelsi K, Brach M, Chtourou H, Boukhris O, Masmoudi L, et al. Effects of home confinement on mental health and lifestyle behaviours during the COVID-19 outbreak: insights from the ECLB-COVID19 multicentre study. Biol Sport. 2021;38(1):9–21. doi: 10.5114/biolsport.2020.96857. [DOI] [PMC free article] [PubMed] [Google Scholar]