Abstract
Individuals with HIV have elevated risks for cardiovascular diseases (CVDs) ranging from myocardial infarction to heart failure. Our understanding of this heightened HIV-associated cardiovascular risk has evolved over the past 2 decades. In the early era of antiretroviral therapy (ART), concern existed that ART was the primary driver of cardiovascular risk. However, it has become increasingly apparent that HIV-related viremia, immune dysregulation, and inflammation are primary drivers of HIV-associated cardiovascular risk, along with traditional cardiovascular risk factors such as tobacco smoking. Indeed, early and effective ART blunts risk for CVDs among individuals with HIV. Despite these improvements in HIV-associated cardiovascular risk, questions remain regarding how to optimally predict, prevent, and treat CVDs among individuals with HIV. Efforts are underway to define more precisely which diagnostic and therapeutic strategies will be most effective in curbing HIV-associated CVDs.
Keywords: HIV, cardiovascular disease, CVD, inflammation, atherosclerosis, heart failure, myocardial infarction
Individuals with HIV have higher risks for cardiovascular diseases (CVDs), including myocardial infarction (MI), heart failure, and pulmonary hypertension, than individuals without HIV.1–5 As life expectancy has increased for individuals with HIV as a result of effective antiretroviral therapy (ART), the burden of diseases of aging, including CVDs, has increased among individuals with HIV.6–8
There are several reasons for elevated CVD risk among people with HIV that reflect the diverse pathophysiologies of CVDs. Diseases of the vasculature, such as MI, occur as a result of a combination of atherosclerosis and thrombosis. Meanwhile, heart failure reflects a final common pathway of different and often overlapping pathophysiologies, ranging from the sequelae of MI to the toxic effects of drugs on the myocardium. Our understanding of HIV-associated CVD risks has improved greatly over the past decade and a half due to seminal epidemiologic and mechanistic studies.
Epidemiology of HIV-Associated Cardiovascular Diseases
Studies in large cohorts comparing individuals with HIV with individuals without HIV have demonstrated approximately 50% higher risk for MI among individuals with HIV.3,9,10 This elevated risk remained after accounting for demographic and clinical confounding variables. Likewise, studies investigating heart failure have demonstrated 50% or higher increased risk among individuals with HIV than among individuals without HIV, even after adjustment for key confounding variables.2,4 Studies also suggest a mildly increased risk for stroke among individuals with HIV11 and a dramatically increased risk for pulmonary hypertension.12
In the early ART era, prolonged exposure to ART was hypothesized as a primary contributor to HIV-associated CVD risks. Indeed, there is some variability in the putative effects of individual antiretroviral drugs on CVD outcomes, including a potential modest effect of abacavir on increasing MI risk.13,14 Nevertheless, more recent data suggest that the effects of ART on CVD are relatively small,15 particularly compared with the problematic effects of uncontrolled HIV. Several studies from large cohorts indicate that HIV viremia and lower CD4+ cell counts are strongly associated with MI and heart failure risk.1–4 Taken together, along with clinical data suggesting that immediate (vs deferred) ART protects against MI,16,17 these data tilt the balance in favor of a net-cardioprotective effect of immediate and continuous ART.1 Of course, immediate and continuous ART remains the cornerstone of HIV therapy. Therefore, from both a CVD prevention standpoint and an overall HIV care standpoint, it is clear that early and continuous ART is essential to optimizing health outcomes for individuals with HIV.
In addition to poor HIV control (ie, with HIV viremia) and immunologic progression (marked by decreasing CD4+ cell count), there are other factors that may increase risk of CVD among in dividuals with HIV. Metabolic abnormalities, including atherogenic dyslipidemia and body composition changes, are common among individuals with HIV. In the current era, increases in dysfunctional subcutaneous fat and visceral fat have been observed among people with HIV; these changes, in turn, are associated with atherosclerotic plaque.18 Coinfection with hepatitis C virus or cytomegalovirus (CMV) reactivation may also contribute to HIV-associated CVD risk. Individuals with HIV smoke at a significantly higher rate than uninfected individuals; the MI attributable to smoking is considerably higher for individuals infected with HIV than those without.19 Finally, hypertension and high alcohol use may also contribute disproportionately to HIV-associated CVD risk.1
Mediators of Atherosclerosis and Thrombosis in HIV
Atherosclerosis and thrombosis play central roles in the development of vascular diseases and their clinical manifestations, including MI and stroke. Classically, MIs occur when lipidrich, inflammatory plaque ruptures or erodes acutely, triggering overlying thrombus and vessel occlusion. In addition to these classic type I MIs, individuals with HIV are at heightened risk for type II MIs, in which myocardial oxygen supply is inadequate relative to demand, triggering myocardial injury.
In this setting, investigators in several studies have observed increased levels of systemic and vascular inflammation, as well as increased platelet activation or aggregation, in individuals with HIV. A common finding across studies of individuals with HIV, as well as monkey models of HIV pathogenesis, is the centrality of inflammation and immune activation to HIV-related CVD risk. Individuals infected with HIV have heightened levels of systemic inflammation and more subclinical atherosclerosis than uninfected individuals.1 These include elevated levels of soluble CD163 and CD14, as well as common CVD-associated inflammatory markers such as interleukin-6, which are in turn associated with atherosclerosis and mortality. Treatment with ART reduces the levels of several of these markers, but some remain elevated despite ART. Likewise, individuals with HIV have elevated levels of coagulation markers and tissue factor-expressing inflammatory monocytes, creating a functionally procoagulant state that has been associated with clinical thrombosis in HIV.20
Mechanisms and Presentation of HIV-Associated Heart Failure
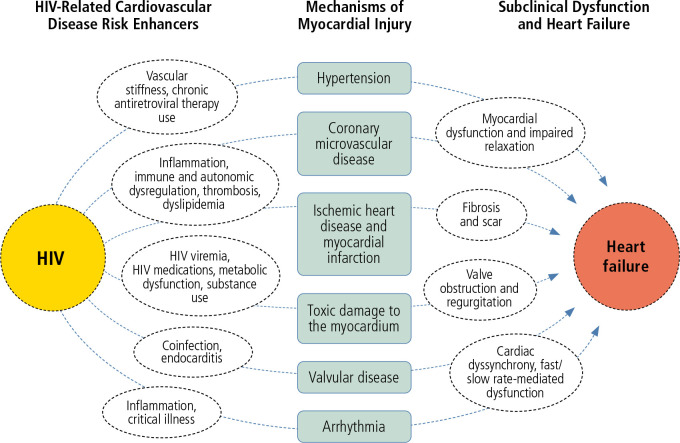
Causes and presentations of heart failure are heterogeneous in the general population and for individuals with HIV. In the pre-ART era, heart failure was a recognized complication of advanced HIV and marked by viremia and progressive immune compromise, along with global myocardial dysfunction and often inflammation. With widespread uptake of ART, the causes and manifestations of heart failure in individuals with HIV have likewise expanded (Figure 1).
Figure 1.
Conceptual model of the proposed mechanisms of heart failure in HIV. Adapted from Feinstein et al.1
Individuals with persistent HIV viremia, immune progression, and opportunistic infections may still experience HIV-associated cardiomyopathy marked by severe systolic dysfunction in the absence of obstructive coronary artery disease. Furthermore, viremia and immune progression still play a role in less overt cardiomyopathy and heart failure, as each are associated with diastolic dysfunction among individuals with HIV. Yet, with the increasing burden of coronary artery disease and MI among individuals with HIV, ischemic etiologies of heart failure, driven by post-MI myocardial scarring and potential microvascular dysfunction, have become increasingly common. Toxic etiologies of myocardial dysfunction and heart failure also play an outsized role in HIV-related heart failure given the higher rates of myocardial-toxic drug use (eg, methamphetamines) among individuals with HIV.
More subtle manifestations of heart failure in the absence of systolic dysfunction also occur in HIV, as individuals with HIV have higher risks for heart failure with preserved ejection fraction than individuals without HIV. This is likely due to a combination of factors, including but not limited to myocardial inflammation, fibrosis, microvascular ischemia, and myopericardial fat deposition. Indeed, several studies using cardiac magnetic resonance imaging, computed tomography, and positron emission tomography have demonstrated that individuals with HIV have more myocardial inflammation, fibrosis, and steatosis than matched control individuals without HIV.1 These subclinical changes in cardiac tissues are known to be associated with myocardial injury and dysfunction.
Cardiovascular Disease Risk Stratification, Prevention, and Therapy for Individuals with HIV
Epidemiologic and mechanistic studies have advanced our knowledge of the scope and causes of CVDs in individuals with HIV. However, there are relatively sparse data to practically guide cardiovascular risk stratification, prevention, and treatment for individuals with HIV who are effectively treated with ART. Despite the limitations of these data and absent large-scale trial data for CVD prevention and treatment during effective ART, there are certain consistent findings on HIV-associated CVD risk that may help inform practical approaches.
The clinical data on CVD-preventive stratagies among individuals with HIV are relevant in this regard. Over the past 2 decades, numerous small studies have evaluated the effects of statin use on various subclinical markers of inflammation and atherosclerosis for individuals with HIV.21,22 Results of these studies on arterial inflammation have not been consistent, although statins do appear to reduce select inflammatory markers and, as expected, reduce atherogenic lipid levels (eg, low density lipoprotein cholesterol) in individuals with HIV. Questions related to the efficacy of statins in preventing hard atherosclerotic coronary artery disease endpoints in individuals with HIV will be answered with more clarity by the REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) study. REPRIEVE randomly assigned individuals with HIV at low to moderate risk for atherosclerotic CVD to pitavastatin or placebo. Enrollment of more than 7500 individuals with HIV is complete and follow-up is ongoing.
Compared with statins, even less clarity exists regarding other potential CVD preventive stratagies for individuals with HIV. Ongoing studies (not yet powered for clinical endpoints) are evaluating the role of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for individuals with HIV. More data are likewise needed related to antithrombotic therapy among individuals with HIV, who have elevated risks for thrombosis. Mechanistic and biomarker studies suggest that aspirin may not be as effective an antiplatelet agent for individuals with HIV than for uninfected individuals, but further study and clinical correlations related to aspirin and other antithrombotic therapies in HIV are needed.
Other anti-inflammatory therapies for individuals with HIV are also of interest. These include therapies that target the gut to reduce microbial tranlocation and gut inflammation, which have not consistently affected biomarkers of inflammation among individuals with HIV. Other therapies tested include canakinumab, an interleukin-1-beta antagonist that reduced inflammatory markers and reduced arterial and bone marrow inflammation in a small study of individuals with HIV, and methotrexate, which did not affect inflammatory markers but modestly reduced CD8+ T-cell counts in individuals with HIV. The efficacy and ultimate clinical applicability of these options remain under investigation.
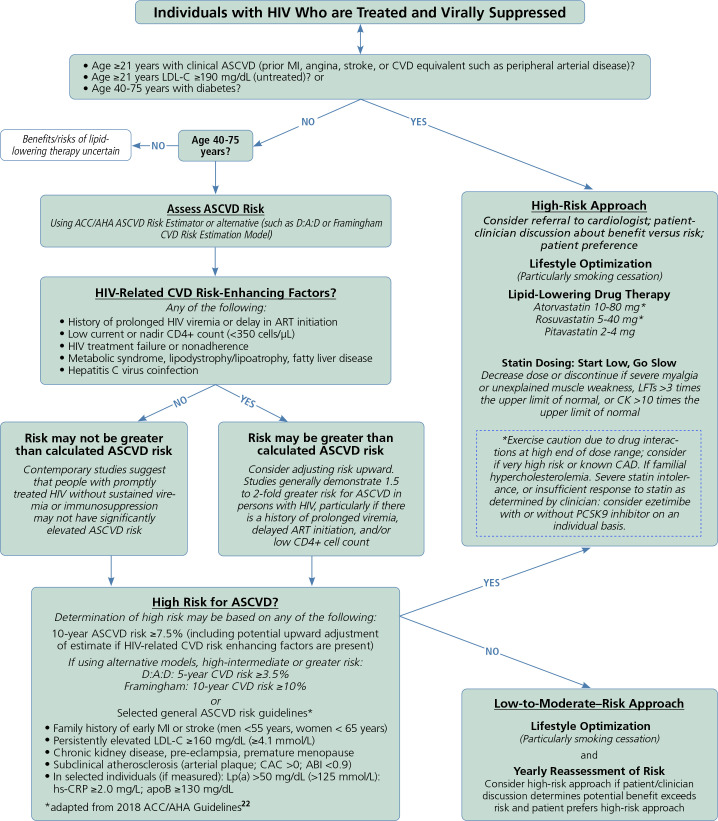
In the absence of large-scale clinical trial data powered for hard CVD endpoints, interim guidance related to CVD risk stratification, prevention, and treatment for individuals with HIV is based on extrapolation of clinical and mechanistic data. Regarding risk stratification, several studies indicate that traditional CVD risk estimation tools consistently underestimate CVD risk among individuals with HIV. This heightened risk is largely attributable to HIV-related CVD risk-enhancing factors such as prolonged HIV viremia, low current or nadir CD4+ cell count, coinfection (eg, with hepatitis C virus), and the presence of lipid distribution abnormalities. However, for those who are treated promptly and who do not experience these HIV-related risk enhancers, HIV-related increases in CVD risk are modest. Accordingly, the American Heart Association's scientific statement on HIV-1 recommended adjusting predicted CVD risk upwards by 1.5-fold to 2-fold for individuals with HIV who have HIV-related risk enhancers (Figure 2). In the absence of compelling data otherwise, risk-based approaches to CVD-preventive therapy for individuals with HIV is recommended, with the understanding that as CVD risk increases, the absolute and net benefit of statin therapy for CVD prevention likewise increases.
Figure 2.
A pragmatic approach to atherosclerotic CVD risk stratification, prevention, and therapy for individuals with HIV. Adapted from Feinstein et al1 and 2018 ACC/AHA guidelines.22 Abbreviations: ABI, ankle brachial index; ACC, American College of Cardiology; AHA, American Heart Association; apoB, apolipoprotein B-100; ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CK, creatine kinase; CVD, cardiovascular disease; D:A:D, Data collection on Adverse events of anti-HIV Drugs; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LFT, liver function test; Lp(a), lipoprotein (a); MI, myocardial infarction; PCSK9, proprotein convertase subtilisin/kexin type 9.
Conclusion
As life expectancy among individuals with HIV has increased, noncommunicable conditions such as CVDs have become more common for individuals aging with HIV. Several HIV-related factors increase CVD risk, with chronic inflammation and immune dysregulation playing a key role. Limited large-scale clinical trial data exist to guide HIV-specific CVD prevention and therapy, highlighting the importance of further clinical and mechanistic study in this area.
Footnotes
This article was based, in part, on a webcast presented by Dr Matthew J. Feinstein in June 2021: https://youtu.be/_CaWrGD9r2U. This article was prepared by Dr Feinstein in July 2021.
Financial affiliations with ineligible companies (formerly named “commercial interests” by the Accreditation Council for Continuing Medical Education [ACCME]) in the past 24 months: Dr Feinstein has served on an advisory board for Novartis AG. (Updated September 27, 2021)
References
- 1. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140(2):e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc. 2018;7(21):e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho JE, Hsue PY. Cardiovascular manifestations of HIV infection. Heart. 2009; 95(14):1193–1202. [DOI] [PubMed] [Google Scholar]
- 6. Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016; 117(2):214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palella FJ Jr., Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. JAIDS. 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- 8. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation. 2018;138(11):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. JAIDS. 2015;68(2): 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. JAIDS. 2012; 60(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS. 2008;22 Suppl 3:S35–S40. [DOI] [PubMed] [Google Scholar]
- 13. Sabin CA, Reiss P, Ryom L, et al. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D:A:D Study Group, Sabin CA, Worm SW, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371(9622): 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smit M, van Zoest RA, Nichols BE, et al. Cardiovascular disease prevention policy in human immunodeficiency virus: recommendations from a modeling study. Clin Infect Dis. 2018;66(5):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. [DOI] [PubMed] [Google Scholar]
- 17. Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348(8):702–710. [DOI] [PubMed] [Google Scholar]
- 18. Palella FJ Jr., McKibben R, Post WS, et al. Anatomic fat depots and coronary plaque among human immunodeficiency virus-infected and uninfected men in the Multicenter AIDS Cohort Study. Open Forum Infect Dis. 2016;3(2):ofw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasmussen LD, Helleberg M, May MT, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60(9): 1415–1423. [DOI] [PubMed] [Google Scholar]
- 20. Musselwhite LW, Sheikh V, Norton TD, et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS. 2011;25(6):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol. 2015; 115(12):1760–1766. [DOI] [PubMed] [Google Scholar]
- 22. Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, doubleblind, placebo-controlled trial. Lancet HIV. 2015;2(2):e52–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]