Abstract
Background
Antibody-based strategies for COVID-19 have shown promise in prevention and treatment of early disease. COVID-19 convalescent plasma (CCP) has been widely used but results from randomized trials supporting its benefit in hospitalized patients with pneumonia are limited. Here, we assess the efficacy of CCP in severely ill, hospitalized adults with COVID-19 pneumonia.
Methods
We performed a randomized control trial (PennCCP2), with 80 adults hospitalized with COVID-19 pneumonia, comparing up to 2 units of locally sourced CCP plus standard care versus standard care alone. The primary efficacy endpoint was comparison of a clinical severity score. Key secondary outcomes include 14- and 28-day mortality, 14- and 28-day maximum 8-point WHO ordinal score (WHO8) score, duration of supplemental oxygenation or mechanical ventilation, respiratory SARS-CoV-2 RNA, and anti–SARS-CoV-2 antibodies.
Results
Eighty hospitalized adults with confirmed COVID-19 pneumonia were enrolled at median day 6 of symptoms and day 1 of hospitalization; 60% were anti–SARS-CoV-2 antibody seronegative. Participants had a median of 3 comorbidities, including risk factors for severe COVID-19 and immunosuppression. CCP treatment was safe and conferred significant benefit by clinical severity score (median [MED] and interquartile range [IQR] 10 [5.5–30] vs. 7 [2.75–12.25], P = 0.037) and 28-day mortality (n = 10, 26% vs. n = 2, 5%; P = 0.013). All other prespecified outcome measures showed weak evidence toward benefit of CCP.
Conclusion
Two units of locally sourced CCP administered early in hospitalization to majority seronegative participants conferred a significant benefit in clinical severity score and 28-day mortality. Results suggest CCP may benefit select populations, especially those with comorbidities who are treated early.
Trial Registration
ClinicalTrials.gov NCT04397757.
Funding
University of Pennsylvania.
Keywords: COVID-19, Clinical Trials
Keywords: Adaptive immunity
Introduction
Since the identification of the first SARS-CoV-2 infections in late 2019, the COVID-19 pandemic has caused more than 200 million cases and 4.5 million deaths worldwide (1). Prevention strategies are of paramount importance, but effective treatment approaches are needed for individuals who become infected. SARS-CoV-2 infection leads to widely variable outcomes, with a subset of infected individuals developing severe pneumonia requiring hospitalization. Substantial morbidity and mortality remain for patients with COVID-19 who are hospitalized with pneumonia, and few efficacious therapies exist.
Early in the COVID-19 pandemic, convalescent COVID-19 plasma (CCP) was recognized as a potentially promising intervention. Use of convalescent plasma in other infectious diseases (2–5) and previous coronavirus pandemics (6, 7) provided biological plausibility, and early observational studies suggested possible benefit (8–10). In the setting of limited treatments and desperate clinical need, CCP was widely used in hospitalized patients with COVID-19 in the United States via an expanded access program (EAP) or emergency use authorization (EUA; refs. 3, 11). These mechanisms enabled access to CCP by more than 500,000 hospitalized individuals, with up to 40% of US inpatients with COVID-19 receiving CCP in the fall of 2020 (12). Observational analyses of subcohorts of hospitalized CCP recipients from the US FDA’s EAP suggested possible benefit in recipients of early, high-titer plasma (13). Yet, results from randomized controlled trials of efficacy are mixed or demonstrate limited benefit (14–19). Here, we report results of a single health system, randomized controlled study of 80 severely ill, hospitalized patients with COVID-19 pneumonia treated with up to 2 units of CCP and standard of care versus standard of care alone.
Results
Participant demographics.
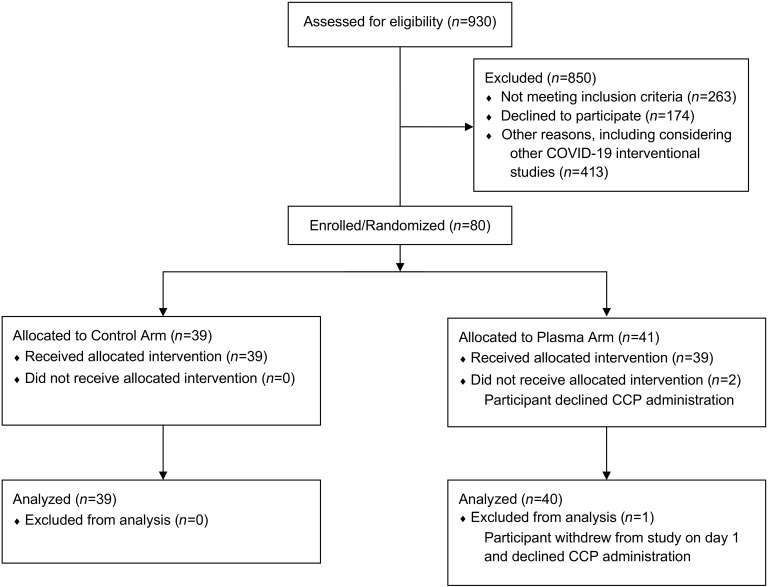
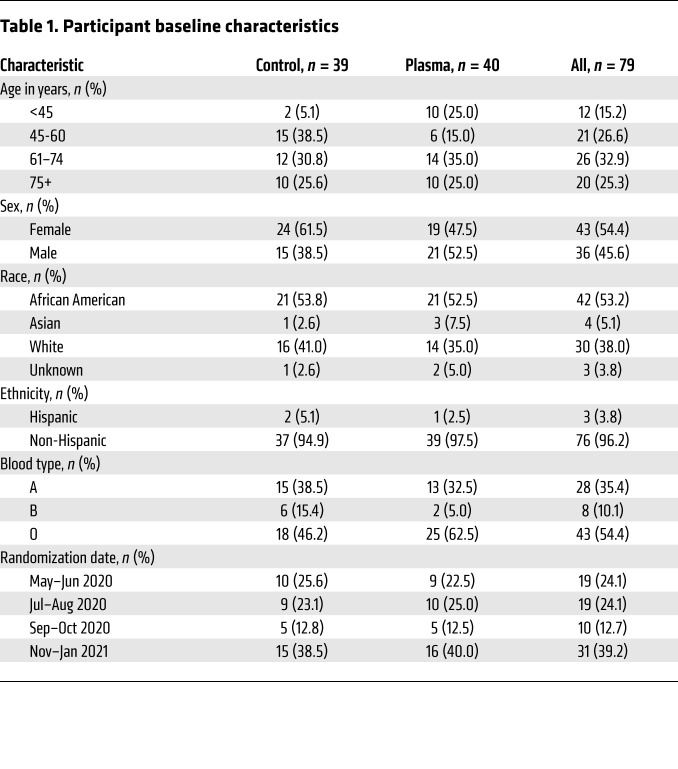
Between May 18, 2020, and January 8, 2021, we enrolled 80 participants, of whom 41 were randomized to the treatment and 39 to the control arm (Figure 1). Two participants in the treatment arm declined CCP administration. One participant who withdrew from the study on day 1 was not included in analyses, while the other was retained in the intent-to-treat analyses. Baseline characteristics of the 79 analyzed participants are described in Table 1.
Figure 1. Consort diagram.
Table 1. Participant baseline characteristics.
Participants’ median age was 63 years (IQR 52–74), with 58% over 60 years old and 25% over 75 years old. Participants were 54% female and 46% male, with 53% identifying as African American, 5% as Asian, 38% as White, and 4% as Hispanic. Enrollment fluctuated over the 8-month study period following the local epidemic and hospital admissions, with higher enrollment rates during May and June 2020 and November 2020 through January 2021.
Baseline clinical characteristics.
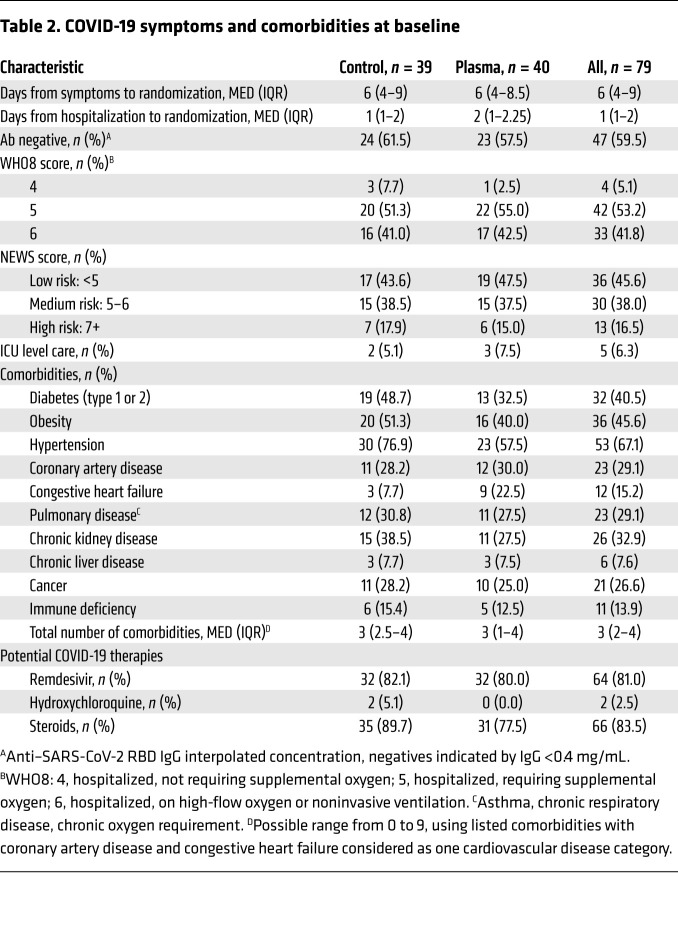
Participants’ baseline clinical characteristics are described in Table 2. Participants were enrolled early in their disease course, at a median of 6 days (IQR 4–9) from COVID-19 symptom onset and 1 day (IQR 1–2) from hospital admission. Sixty percent of participants were SARS-CoV-2 antibody seronegative at study enrollment.
Table 2. COVID-19 symptoms and comorbidities at baseline.
Baseline clinical severity was similar across study arms. The median maximum 8-point WHO ordinal score (WHO8) score was 5 (hospitalized, requiring supplemental oxygen) (IQR 5–6). No participants required mechanical ventilation at enrollment. National Early Warning Severity (NEWS; ref. 20) scores also indicated a range in clinical severity at enrollment.
Participants had a high frequency of baseline comorbidities, with a median of 3 (IQR 2–4) per participant. We noted a high prevalence of disease states associated with poor COVID-19 outcomes, including diabetes, obesity, hypertension, and cardiovascular and pulmonary disease (21, 22), as well as conditions associated with immunosuppression, including chronic kidney and liver disease, cancer, and immunodeficiency (23). Participants had frequent use of COVID-19 therapies at the time of enrollment, including remdesivir (81%) and steroids (84%).
Safety.
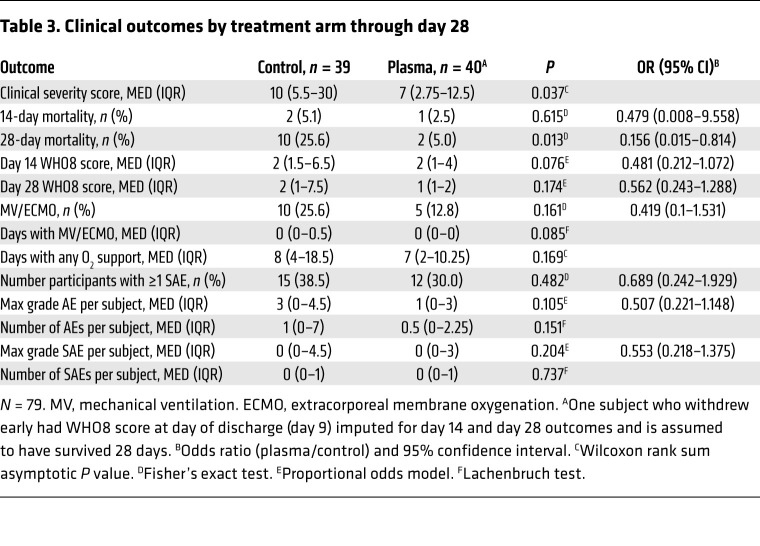
CCP administration was generally safe and well-tolerated. There were few serious adverse events (SAEs). Median (MED) and interquartile range (IQR) was 0 (0–1) per participant in both control and treatment arms, with 15 (38%) control and 12 (30%) plasma recipients with at least 1 SAE (Table 3). There were 3 treatment-related adverse events (AEs) (nausea, pruritis, and an acute allergic reaction; all grade 2). As shown in Table 3, there was weak evidence to suggest a greater number of total AEs (P = 0.151) and higher maximum severity of AEs (OR 0.507, P = 0.105) per participant in control versus treatment arms.
Table 3. Clinical outcomes by treatment arm through day 28.
Clinical efficacy.
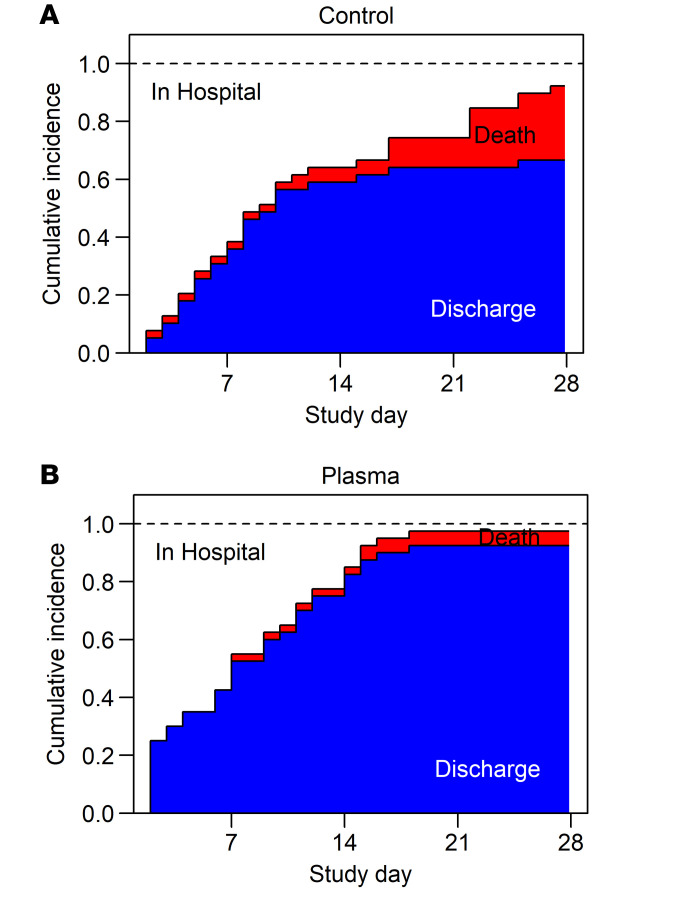
Comparing the clinical severity score (CSC) between study arms, CCP-treated participants ranked significantly better (lower severity) than controls (P = 0.037 by Wilcoxon rank-sum test), with a median clinical severity score of 7 (IQR 2.75–12.25) in the treatment arm versus 10 (IQR 5.5–30) in the control arm. Figure 2 shows cumulative incidence curves for discharge and mortality by treatment arm, censored at 28 days. While there were limited differences in time to discharge or mortality within the first 2 weeks, the curves diverge in the second 2 study weeks for both discharges (more in treatment) and deaths (more in control). The log rank test comparing survival and the cause-specific hazard ratio for discharge were also significant (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/JCI155114DS1).
Figure 2. Stacked cumulative incidence curves.
Incidence curves for the competing risks of remaining hospitalized, death, or discharge are shown over time, censored at 28 days for the control (A) and treatment arm (B) of the 79 participants of the ITT cohort. Deaths are shaded red and discharges blue. One participant who withdrew at day of discharge (day 9) is assumed to have survived 28 days.
CCP treatment showed a significant mortality benefit at day 28, OR 0.156, P = 0.013, with 5% (2 of 40) and 25.6% (10 of 39) mortality in treated versus control participants, respectively. Consistent with the overall lower severity score, several other prespecified secondary efficacy endpoints provided weak evidence (0.05 < P < 0.20) of benefit of CCP treatment, including WHO8 scores at day 14 and 28, any use of mechanical ventilation or extracorporeal membrane oxygenation (ECMO), duration of mechanical ventilation or ECMO use, and duration of supplemental oxygen use (Table 3).
In exploratory analyses, we examined whether the observed treatment benefit for mortality could be explained by imbalances between study arms at baseline by fitting a series of Cox proportional hazards model for mortality adjusting for treatment and one of the following baseline factors: randomization date, sex, age, race, SARS-CoV-2 Ab seropositivity, blood type, obesity, hypertension, diabetes, congestive heart failure, chronic kidney disease, cancer, immune deficiency, number of comorbidities, steroid use, and anti-thrombotic use (Supplemental Table 1). For steroid use, models were degenerate as there were no deaths in participants who were not receiving steroids at study enrollment. Otherwise, adjustment for the explored factors did not appreciably change the effect size or significance of the found treatment benefit and no additional independent predictors of mortality were identified (Supplemental Table 2). We conducted a sensitivity analysis with linear regression models for the CSC ranks, adjusting the treatment effect for the same baseline factors. Only baseline seropositive status and age were associated with CSC. Adjusted treatment effect sizes were similar to unadjusted and the significance of treatment generally remained in the adjusted models, except with adjustment for hypertension and having 2 or more comorbidities (P = 0.06; Supplemental Table 3).
Antibody measures.
Anti–SARS-CoV-2 RBD IgG levels were assessed in donor plasmas and in recipients at baseline (before plasma administration) on study day 1, and longitudinally throughout the study using a validated in-house assay shown to discriminate between seasonal betacoronavirus infection and correlate with neutralization titers (24, 25). All donor plasmas had IgG > 0.48 au/mL, with median levels of 3.69 (IQR 1.61–8.56). A total of 76 units of plasma from 53 unique donors were used in the study. Of the 40 participants randomized to receiving plasma in the ITT cohort, 37 received 2 units, 2 received 1 unit, and 1 received 0 units due to participant refusal. The median combined titer of antibody (total the units administered to each recipient) was 8.180 au/mL (IQR 4.195–20.980; Supplemental Figure 2).
In exploratory analyses, we used a distinct set of 22 donor plasmas and compared our assay with 2 commercial assays currently approved for certifying “high-titer” plasma by the FDA. We found that our anti-RBD IgG assay, which uses a quantitative titration-based read-out, correlated closely with the chemiluminescence-based Beckman Coulter RBD IgG immunoassay and the Euroimmun IgG S1 ELISA (Pearson correlations of 0.960 and 0.890, respectively) (Supplemental Figure 3). If we extrapolate from the log-linear relationship between our assay and the 2 commercial assay standards and the established cut-offs for high titer (3.3 on Beckman-Coulter and 3.5 on Euroimmun), we estimate that 24 (62%) plasma recipients (using Beckman Coulter levels) and 33 (85%) plasma recipients (Euroimmun levels) received at least 1 unit of high-titer plasma (Supplemental Figure 3).
At baseline, 60% (47 of 79) of participants were seronegative, with IgG levels ranging from 0.5 to 19.84 au/mL in seropositive participants (Supplemental Figure 4). At study days 3 through 60, CCP-treated and control participants appear to have similar antibody levels, though these analyses are limited by increasing numbers of missing samples and the potentially nonrandom pattern of missing samples. Missing data occurred with increasing frequency at later study days, as participants were either unwilling or unable to provide samples after discharge. Notably, there were not appreciable differences in longer-term humoral responses in sampled treated versus control participants at day 60 (n = 35).
SARS-CoV-2 quantification of respiratory samples.
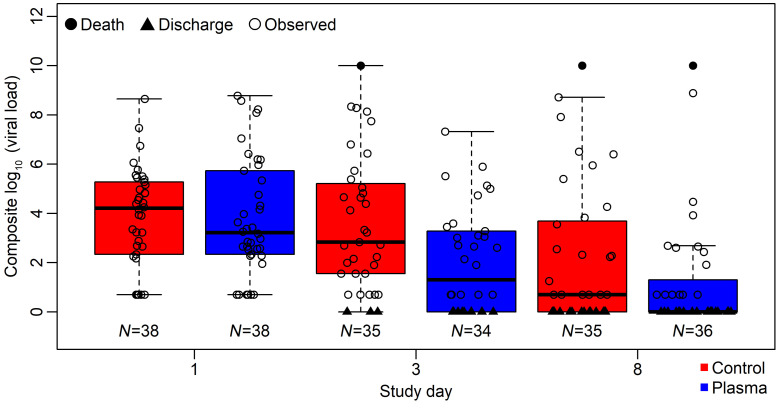
Quantification of SARS-CoV-2 levels in oropharyngeal swab–derived respiratory samples were assessed by RT-PCR at baseline and longitudinally. At baseline, 77 participants had evaluable samples. Eighty-three percent (n = 64) had detectable virus, with 44% (n = 34) having high-titer (>4 log10 copies) virus levels. To compare viral loads, we considered a composite score of viral load and clinical status, in which those discharged were assigned the lowest score, deaths the highest score, and those in-hospital the observed viral load. Plasma recipients had a lower composite score at day 3 (P = 0.0128 by Wilcoxon rank sum test; Figure 3).
Figure 3. Composite endpoint assessing respiratory sample viral load and clinical status, in which those who were discharged had the lowest score and those who died had the highest.
Control (red) and plasma (blue) arms are shown for baseline (prior to plasma administration) and study days 3 and 8. Imputed values are shown in filled symbols and measured virus levels are shown in open circles. Values were not significantly different at baseline and were significantly lower in treatment arm at day 3 (P = 0.0128 by Wilcoxon rank sum test).
Discussion
Antibody-based strategies for COVID-19 have shown promise in prevention and treatment of early disease (26–28), but data supporting benefit in hospitalized patients with pneumonia are more limited. Observational analyses of a subcohort of hospitalized CCP recipients from the US FDA’s EAP suggested possible benefit in recipients of early, high-titer plasma (13). More recently, reports from larger, randomized controlled trials suggest CCP is not efficacious when given broadly to hospitalized patients with COVID-19 (14, 17–19).
In this open-label, randomized controlled trial, we assessed the impact of early administration of multiple units of locally sourced CCP in hospitalized individuals with COVID-19 pneumonia. We found that CCP treatment was safe and conferred significant benefit as measured by our clinical severity score and 28-day mortality. In exploratory analyses, we found a reduction in a composite respiratory virus and clinical status score at study day 3 in plasma recipients. In all other prespecified outcome measures, including ordinal WHO8 scale at days 14 and 28, 14-day mortality, use and duration of oxygen and mechanical ventilation, and number and maximum grade of AE, we found weak evidence toward a benefit of CCP treatment.
Given recent large, randomized studies that have not shown benefit in general hospitalized cohorts, it is important to put the positive result of our study in context. This study has several unique characteristics that may have contributed to the demonstrated benefit, including the early administration of 2 units of locally sourced plasma in a highly comorbid, majority antibody-seronegative population (29, 30). In addition, we employed a sensitive primary outcome measure enabling a composite characterization of clinical status (31). First, we posit that relatively early treatment distinguished this study from many others, as we enrolled and administered CCP within a median of day 6 of symptoms and 1 day of hospitalization, in participants in whom 60% were seronegative at entry. Many other reported randomized controlled trials enrolled participants later in disease course, as determined by seropositivity and days since symptoms onset. For example, reports describe a median 30 days since symptom onset in the Wuhan study (32), median 10 days of symptoms and 63% seropositivity in RECOVERY (18), 83% seropositivity in PLACID (14), median 10 days of symptoms and 79% seropositivity in CONCOVID (15), median 8 days of symptoms in PlasmAR (17), and median 8 days of symptoms in CONCOR-1 (19). Benefit from earlier treatment with antibody-based interventions has also been reported, with early treatment with CCP in some high-risk outpatient cohorts (28, 33) and early treatment with monoclonal antibodies (26, 27). Though potentially confounded and requiring cautious interpretation, multiple subgroup analyses of earlier treated participants also suggest possible benefit (16, 34–36).
Second, we enrolled a highly comorbid population. Our study was conducted within tertiary care referral centers that serve highly complex patient populations. In our experience, the safety profile and permissive entry criteria of this study compared with competing COVID-19 clinical trials led to increased enrollment of higher risk individuals, in terms of both severe COVID-19 outcomes and immunodeficiency. Whereas our participants had a median of 3 comorbidities, and just 4% (3/79) had no reported comorbidities, many studies enrolled high proportions of participants without comorbidities (e.g., RECOVERY enrolled 44% of participants with no comorbidities and PlasmAR enrolled 35% with no comorbidities; refs. 17, 18). Further, we enrolled substantial numbers of participants with cancer (27%) and immunodeficiency (14%), both of which have high mortality from COVID-19 (23, 37, 38) and have been reported to incur benefit from antibody-based therapies (39–41). Thus, we suspect that early CCP treatment of a higher-risk, highly comorbid population may have conferred benefit in a way not seen in later-treated, more general hospitalized populations. The hypothesis that baseline clinical characteristics of plasma recipients and timing of CCP administration could substantially impact CCP efficacy is being more formally assessed in large, collaborative studies of treatment benefit index (35, 42).
We propose that our CSC primary endpoint (31) is well suited to detect more subtle distinctions in disease course, which mortality and duration of hospitalization outcomes alone may miss. We prespecified this validated clinical severity outcome, given the heterogeneity of disease outcomes in patients with COVID-19, the proposed mechanism of antibody-based treatments, an expected modest efficacy of CCP, and the smaller size of our study. Others have advocated for the use of similar disease severity scores in settings where participants may experience multiple outcomes and disease course is heterogenous with a spectrum of disease severity (37, 43). Further, continuous outcomes that consider time to recovery are advocated in COVID-19 as more robust in detecting differences than an ordinal score at a fixed time point because of the potential mismatch between the chosen time point of analysis and actual timing of patient recovery (44). Our sensitive severity score measure enabled us to detect an improvement in clinical disease progression not well detected by the WHO8 score at discrete time points. This outcome is also supported by a statistically significant 28-day mortality benefit.
Our study found a significant difference in mortality at 28 days, but less distinction between study arms earlier. Indeed, at day 14 we had fewer events: either discharges or deaths to distinguish between study arms. We note other trials have identified differences in 28-day mortality, with or without substantial earlier outcomes (16, 45).
High-titer antibodies in donor plasma have also been associated with improved outcomes (12). Our donor and recipient plasmas were tested by a validated, quantitative in-house assay (24), thus titers are not directly comparable to commercial assays currently used in assessment of clinically relevant titer. While our exploratory analyses have limitations, they suggest that more than two-thirds of participants received at least one unit of high-titer plasma and between 20% and 44% received 2 units of high-titer plasma (Supplemental Figure 3).
Our study has several limitations. It was smaller, open label, and performed at just 2 hospitals within a single health system. Use of ABO-compatible plasma limited enrollment for some blood types. Over the 8 months of study enrollment, the local epidemic shifted in severity and affected populations, approved and emergency use treatments changed, and standard practices for the treatment and infection control of COVID-19 evolved. Strengths of the study included its randomized nature, use of 2 units of locally sourced plasma, early enrollment, and permissive entry criteria. We note the inclusion of pregnant and lactating individuals, and the successful enrollment of 3 pregnant participants.
In summary, our randomized controlled study found that CCP conferred a significant benefit in clinical severity score and 28-day mortality. Results support the heterogeneity of COVID-19, and suggest CCP may benefit select populations, especially those with comorbidities who are treated early.
Methods
Trial design and oversight.
This open-label, controlled trial assessed the safety and efficacy of CCP in severely ill, hospitalized participants with pneumonia due to COVID-19 (ClinicalTrials.gov NCT04397757). This study enrolled adults 18 years old and older, including pregnant women. The study was conducted at 2 hospitals (Hospital of the University of Pennsylvania [HUP] and Penn Presbyterian Medical Center [PPMC]) within the University of Pennsylvania Health System in Philadelphia, Pennsylvania, USA.
Study participants.
The study enrolled hospitalized adults with RT-PCR–confirmed SARS-CoV-2 infection, radiographic documentation of pneumonia, and abnormal respiratory status, defined as room air saturation of oxygen (SaO2) less than 93%, or requiring supplemental oxygen, or tachypnea with a respiratory rate greater than or equal to 30. Participants were excluded if they had a contraindication to transfusion, were participating in other clinical trials of investigational COVID-19 therapy, if there was clinical suspicion that the etiology of acute illness was primarily due to a condition other than COVID-19, or if ABO-compatible CCP was unavailable.
Intervention and assessments.
A total of 80 eligible participants were randomized to receive either 2 units of CCP and standard of care (treatment arm) versus standard of care alone (control arm). Participants were assigned to treatment or control in a 1:1 ratio using randomization stratified on the use of remdesivir and mechanical ventilation at entry using block randomization with variable block size. Participants in the treatment arm received up to 2 units of convalescent plasma on study day 1 in addition to standard of care. Participants were assessed on all study days while hospitalized through day 29, and after discharge as outpatients on study days 15, 22, 29, and 60. Blood samples were collected at baseline (prior to CCP administration on study day 1), study days 3, 8, 15, 29, and 60. Respiratory samples (oropharyngeal swabs in nonintubated participants or endotracheal aspirates in intubated participants) were collected on study days 1, 3, 5, 8, 11, and 15. The protocol is available in the Supplemental Material.
COVID-19 convalescent plasma.
Between April 16 and July 6, 2020, the HUP apheresis unit collected donor plasma that was further manufactured into Penn CCP by the hospital blood bank/transfusion service. CCP was collected from individuals who would otherwise qualify as blood donors (per FDA), were diagnosed with SARS-CoV-2 by RT-PCR testing during acute COVID-19 infection, and were at least 28 days from symptoms. In addition to standard blood donor infectious disease tests, female donors were screened for the presence of anti-HLA antibodies, which disqualified plasma donation. CCP was then tested for the presence of anti–SARS-CoV-2 antibodies by ELISA (24). For each study participant randomized to treatment, 2 units ABO-compatible CCP with detectable antibodies were randomly selected, with a preference for use of CCP from 2 different donors when available.
Study objectives and outcomes.
The overall objectives of the study were to evaluate the safety and explore the efficacy of CCP in hospitalized participants with confirmed COVID-19 pneumonia. The primary efficacy outcome was a CSC, which could effectively rank patients by their disease severity by taking into account multiple endpoints in a prioritized manner, following a procedure similar to one previously described (31). Clinical severity was determined by a participant’s survival time, time to recovery, and disease course while in the hospital (considering WHO8, use of supplemental oxygen, and AEs; ref. 46). Detailed CSC methods are in the Supplemental Material. The composite severity score outcome was chosen as primary over a single mortality outcome to enhance power and in recognition that deaths could follow an initial recovery so time to recovery alone was anticipated to inadequately summarize outcomes. Key secondary and exploratory efficacy outcomes include 14- and 28-day mortality, 14- and 28-day WHO8 score, duration of supplemental oxygenation, use and duration of mechanical ventilation, presence and quantity of SARS-CoV-2 RNA in respiratory samples, and anti–SARS-CoV-2 antibody levels. Sample sizes were determined by desire to estimate safety and to provide a preliminary idea of efficacy. We estimated that 40 participants in the CCP arm enabled an 80% chance of observing at least 1 individual with an AE if the underlying AE rate is 4%. We approximated the power for the CSC primary efficacy comparison by considering the power of the Win Ratio (43) statistic. For 40 matched experimental-control pairs, we had over 80% power to reject the null proportion of 50% if the experimental treatment is associated with an 80% or higher probability of having better severity than a control participant.
Plasma anti–SARS-CoV-2 antibody testing.
To quantitate anti–SARS-CoV-2 IgG in donor plasma (CCP) and in participants, enzyme-linked immunosorbent assays (ELISAs) were completed using plates coated with recombinant receptor-binding domain and full-length SARS-CoV-2 spike protein, as previously described (24).
SARS-CoV-2 quantification in respiratory samples.
Oropharyngeal swabs were collected for all nonintubated participants and endotracheal aspirates were collected for intubated participants. From each sample, SARS-CoV-2 RNA was quantified by RT-PCR (47).
Statistics.
The primary safety endpoint was cumulative incidence of SAEs at day 29, calculated separately by arm as the percentage of individuals who had at least 1 SAE by day 29. The SAE rate, treatment-related AE rate, and the number and maximum grade of all AEs at day 29 were also calculated.
For the primary efficacy outcome, the Wilcoxon rank-sum test was used to assess the difference between arms. This type of prioritized outcome severity score can be interpreted as a weighted average of the log-rank type test statistic for survival. Binary secondary outcomes were analyzed with Fisher’s exact, ordinal endpoints by the proportional odds model, and the 28-day censored survival time by the Peto-Peto log-rank (see Supplemental Material). The cumulative incidence of discharge was estimated and the treatment effect on time-to-discharge assessed using a cause-specific proportional hazards model, with death as a competing risk.
Study approval.
The trial was sponsored by the University of Pennsylvania and approved by its institutional review board, located in Philadelphia, Pennsylvania, USA. All participants provided informed consent prior to participation in the study.
Author contributions
KJB, PAS, GHC, NA, AF, MC, JLP, MAE, IF, SEH, DLS, and PT designed the clinical trial. KJB, PAS, GHC, NA, AF, HSC, LG, JS, MA, MM, CA, GF, MD, MB, MC, JG, AW, MAM, FM, EL, AMM, HB, AP, LI, RT, RAE, FD, JLP, WRS, MAE, JB, NJM, KOD, IF, DLS, and PT conducted the clinical trial. KJB, LG, AW, MAM, FM, EL, SG, ETLP, SEH, and DLS conducted experiments. KJB, PAS, GHC, JBY, and PT analyzed data. KJB, PAS, GHC, MC, and PT wrote the manuscript.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Daniel S. Herman, Nancy Mayer, Ping Wang, Theodore Rutledge, Joyce Gonzalez, Andrew Gaano, and Wenzhao Meng for valuable assistance with sample coordination and assay completion, and John Rogan for assistance of the clinical study team. The authors thank Jeffrey Lurie, Joel Embiid, Joshua Harris, and David Blitzer for philanthropic support. The authors acknowledge support from the Penn Center for AIDS Research, P30 AI 045008, and COMPILE Continuous Monitoring of Pooled International Trials of Convalescent Plasma for COVID-19 Hospitalized Patients. All authors vouch for the accuracy and completeness of the data and analyses and the fidelity of the trial to the respective protocol. There was no commercial support for this trial.
Version 1. 11/17/2021
In-Press Preview
Version 2. 12/15/2021
Electronic publication
Footnotes
Conflict of interest: JLP reports consultancy fees from Pfizer; WRS reports consultancy fees from Viiv, Gilead, and Janssen; IF reports consultancy fees from Gilead and Merck; SEH reports consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck; PT reports consultancy fees from Merck, Gilead, Janssen, and Viiv.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(24):e155114.https://doi.org/10.1172/JCI155114.
Contributor Information
Katharine J. Bar, Email: BarK@pennmedicine.upenn.edu.
Pamela A. Shaw, Email: shawp@pennmedicine.upenn.edu.
Grace H. Choi, Email: ghchoi@pennmedicine.upenn.edu.
Nicole Aqui, Email: aqui@mail.med.upenn.edu.
Andrew Fesnak, Email: Andrew.Fesnak@uphs.upenn.edu.
Jasper B. Yang, Email: jasper.yang@pennmedicine.upenn.edu.
Haideliza Soto-Calderon, Email: Haideliza.Soto-Calderon@pennmedicine.upenn.edu.
Lizette Grajales, Email: glizette@sas.upenn.edu.
Julie Starr, Email: jstarr@pennmedicine.upenn.edu.
Michelle Andronov, Email: michelle.andronov@pennmedicine.upenn.edu.
Miranda Mastellone, Email: Miranda.Mastellone@pennmedicine.upenn.edu.
Chigozie Amonu, Email: chigozie.amonu@pennmedicine.upenn.edu.
Geoff Feret, Email: Geoff.Ferret@pennmedicine.upenn.edu.
Maureen DeMarshall, Email: demarshm@pennmedicine.upenn.edu.
Marie Buchanan, Email: mab212@comcast.net.
Maria Caturla, Email: caturm@upenn.edu.
James Gordon, Email: james.gordon58@gmail.com.
Alan Wanicur, Email: awanicur@pennmedicine.upenn.edu.
M. Alexandra Monroy, Email: amonroy515@gmail.com.
Felicity Mampe, Email: felicitymampe@gmail.com.
Emily Lindemuth, Email: Emily.Lindemuth@pennmedicine.upenn.edu.
Sigrid Gouma, Email: sigridgouma@gmail.com.
Anne M. Mullin, Email: Anne.Mullin@Pennmedicine.upenn.edu.
Holly Barilla, Email: hbarilla@upenn.edu.
Anastasiya Pronina, Email: anastasiyapronina318@gmail.com.
Leah Irwin, Email: Leah.Irwin@pennmedicine.upenn.edu.
Raeann Thomas, Email: Raeann.Thomas@pennmedicine.upenn.edu.
Risa A. Eichinger, Email: Risa.Eichinger@Pennmedicine.upenn.edu.
Faye Demuth, Email: enjmom@comcast.net.
Jose L. Pascual, Email: jose.pascual@pennmedicine.upenn.edu.
William R. Short, Email: William.Short@pennmedicine.upenn.edu.
Michal A. Elovitz, Email: elovitz@pennmedicine.upenn.edu.
Jillian Baron, Email: baronji@pennmedicine.upenn.edu.
Nuala J. Meyer, Email: Nuala.Meyer@pennmedicine.upenn.edu.
Kathleen O. Degnan, Email: kathleen.degnan3@pennmedicine.upenn.edu.
Ian Frank, Email: franki@pennmedicine.upenn.edu.
Scott E. Hensley, Email: hensley@pennmedicine.upenn.edu.
Donald L. Siegel, Email: siegeld@pennmedicine.upenn.edu.
Pablo Tebas, Email: Pablo.Tebas@pennmedicine.upenn.edu.
References
- 1. Worldometers.info. https://www.worldometers.info/coronavirus/ Updated November 1, 2021. Accessed November 1, 2021.
- 2.Casadevall A, et al. The principles of antibody therapy for infectious diseases with relevance for COVID-19. mBio. 2021;12(2):e03372-20. doi: 10.1128/mBio.03372-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyner MJ, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripoll JG, et al. Convalescent plasma for infectious diseases: historical framework and use in COVID-19. Clin Microbiol Newsl. 2021;43(4):23–32. doi: 10.1016/j.clinmicnews.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun M, et al. A potentially effective treatment for COVID-19: A systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease. Int J Infect Dis. 2020;98:334–346. doi: 10.1016/j.ijid.2020.06.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soo YO, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan K, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng QL, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with Coronavirus Disease 2019. J Infect Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen C, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020; 323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Senefeld JW, et al. Program and patient characteristics for the United States Expanded Access Program to COVID-19 convalescent plasma [preprint]. Posted on medRxiv April 10, 2021. [DOI]
- 12.Casadevall A, et al. Convalescent plasma use in the USA was inversely correlated with COVID-19 mortality. Elife. 2021;10:e69866. doi: 10.7554/eLife.69866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyner MJ, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384(11):1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gharbharan A, et al. Convalescent plasma for COVID-19. A randomized clinical trial. Nat Commun. 2020;12:3189 [Google Scholar]
- 16.O’Donnell MR, et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest. 2021;131(13):150646. doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397(10289):2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begin P, et al. Convalescent plasma for adults with acute COVID-19 respiratory illness (CONCOR-1): study protocol for an international, multicentre, randomized, open-label trial. Trials. 2021; 22(1):323. doi: 10.1186/s13063-021-05235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 Updated December 19, 2017. Accessed November 1, 2021.
- 21.Harrison SL, et al. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020; 17(9):e1003321. doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenthal N, et al. Risk factors associated with in-hospital mortality in a US National sample of patients With COVID-19. JAMA Netw Open. 2020;3(12):e2029058–e2029058. doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sormani MP, et al. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: An international cohort study. Mult Scler. doi: 10.1177/13524585211035318. [published online July 30, 2021]. [DOI] [PubMed] [Google Scholar]
- 24.Flannery DD, et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5(49):eabd5709. doi: 10.1126/sciimmunol.abd5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson EM, et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184(7):1858–1864. doi: 10.1016/j.cell.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Brien MP, et al. Subcutaneous REGEN-COV antibody combination in early SARS-CoV-2 infection [preprint]. Posted on medRxiv June 14, 2021. [DOI]
- 27.Weinreich DM, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libster R, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunze KL, et al. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat Commun. 2021;12(1):4864. doi: 10.1038/s41467-021-25113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korper S, et al. Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19. J Clin Invest. 2021;131(20):e152264. doi: 10.1172/JCI152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw PA, Fay MP. A rank test for bivariate time-to-event outcomes when one event is a surrogate. Stat Med. 2016;35(19):3413–3423. doi: 10.1002/sim.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korley FK, et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med. doi: 10.1056/nejmoa2103784. [published online August 18, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klassen SA, et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin Proc. 2021;96(5):1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petkova E, et al. Pooling data from individual clinical trials in the COVID-19 era. JAMA. 2020;324(6):543–545. doi: 10.1001/jama.2020.13042. [DOI] [PubMed] [Google Scholar]
- 36.Briggs N, et al. Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19. PLoS One. 2021;16(7):e0254453. doi: 10.1371/journal.pone.0254453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assaad S, et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans SR, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR) Clin Infect Dis. 2015;61(5):800–806. doi: 10.1093/cid/civ495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gharbharan A, et al. Effects of treatment of COVID-19 with convalescent plasma in 25 B-cell depleted patients. Clin Infect Dis. doi: 10.1093/cid/ciab647. [published online July 22, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson MA, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. doi: 10.1001/jamaoncol.2021.1799. [published online June 17, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodionov RN, et al. Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19. Lancet Microbe. 2021;2(4):e138. doi: 10.1016/S2666-5247(21)00030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldfeld KS, et al. Prospective individual patient data meta-analysis: Evaluating convalescent plasma for COVID-19. Stat Med. 2021;40(24):5131–5151. doi: 10.1002/sim.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pocock SJ, et al. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2011;33(2):176–182. doi: 10.1093/eurheartj/ehr352. [DOI] [PubMed] [Google Scholar]
- 44.Dodd LE, et al. Endpoints for randomized controlled clinical trials for COVID-19 treatments. Clin Trials. 2020;17(5):472–482. doi: 10.1177/1740774520939938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avendano-Sola C, et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021;131(20):e152740. doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization. COVID-19 Treatment Trial Design Master Protocol Synopsis. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf Updated February 18, 2020. Accessed November 1, 2021.
- 47.Everett J, et al. SARS-CoV-2 Genomic Variation in Space and Time in Hospitalized Patients in Philadelphia. mBio. 2021;12(1):e03456-20. doi: 10.1128/mBio.03456-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.