Abstract
Purpose
Few case reports and letters to the editor have been published regarding oral signs and symptoms in COVID-19 patients. The aim of the study therefore is to investigate different types of oral manifestations in COVID-19 patients as well as their prevalence.
Patients and Methods
The study is a cross-sectional study from a single medical center. A convenience sample was taken from all patients who were COVID-19 confirmed, symptomatic, and non-hospitalized. Demographic information, medical and travel history, general symptoms, and clinical examination results of the oral cavity were collected.
Results
This study included a total of 109 patients. Loss of taste was the most common oral manifestation of COVID-19 (43.4%), followed by erythema/desquamated gingivitis and coated tongue (7.3% each) and ulcers/blisters (6.4%). Loss of taste was the only symptom persisting for 10 days. Oral manifestations appeared as a single symptom (79.3%), and dorsum of tongue was the most common oral location (72.4%).
Conclusion
Loss of taste was the most prevalent specific reported oral manifestation. Other nonspecific oral lesions/symptoms are controversial. It has been suggested that oral examinations of COVID-19 patients should be conducted as part of routine examinations to investigate any possible correlation between the disease and the oral cavity.
Keywords: oral cavity, loss of taste, COVID-19, symptoms, prevalence
Introduction
The novel coronavirus disease (COVID-19) outbreak is still a pandemic persisting after about more than one year. It has affected almost 240 million people worldwide with more than 4.8 million deaths.1 The most commonly reported symptoms are fever, loss of taste, tiredness, and dry cough.2–5 The majority of cases were mild (80%), and 3% maximum of cases may become serious and critically ill. The total number of recovered/discharged cases was 97%.1
Updated research shows that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can infect human cells through ACE2 (angiotensin-converting enzyme 2) receptors.6,7 This process can in turn cause an inflammatory reaction in the mucosal membrane of the tongue, salivary gland tissues, and other affected organs and tissues.2,8 SARS-CoV-2 may also alter taste bud sensitivity.9 Therefore, COVID-19 could contribute to several oral signs and symptoms, such as taste loss, nonspecific ulcers, blisters, desquamative gingivitis, and co-infections with fungal infections such as candida,10,11 and few studies have been published regarding oral manifestations in specific.12–14 However, it is still controversial whether these symptoms/signs are specific to COVID-19 disease as a result of the SARS-CoV-2 infection or as a result of other underlying conditions and consequences, such as co-infections, immune system impairment caused by the virus, or adverse effects of COVID-19 medications.15
Moreover, the prevalence of clinical oral manifestations remains unclear. For this reason, oral manifestations of COVID-19 are a rising topic of interest in the field of oral health care, and more research with better study design is needed to clarify the association between COVID-19 and oral manifestations. Therefore, the aim of this study is to investigate the types of oral manifestations and their prevalence in patients with COVID-19 in a cross-sectional study.
Materials and Methods
A cross-sectional study was conducted between July 28th and October 5th, 2020, at King Fahad General Hospital, a COVID-19 center in Jeddah, Saudi Arabia. Ethical approval was obtained from the research and studies department, Jeddah Health Affairs, Ministry of Health, and registered at King Abdulaziz City for Science and Technology (KACST) IRB # 1317. The study was conducted in accordance with the Declaration of Helsinki. Patients completed a consent form to participate in the survey and the clinical examination.
Subjects and Settings
A convenience sample was taken from all COVID-19 confirmed cases by reverse transcription polymerase chain reaction (RT-PCR). The patients were symptomatic and non-hospitalized, and were present at the hospital in order to pick up their first COVID-19 medications. They were followed up until they showed negative test results. They were diagnosed with COVID-19 through a nasopharyngeal swab, which was transported through viral transporting media, and then an RT-PCR was conducted using the Logix Smart ™ COVID-19 Test kit. Exclusion criteria were as follows: underage patients <18 years or suspected patients without any definitive diagnosis.
Clinical Outcomes
Data were collected by two separate investigators (AA, MD) and reported on an electronic datasheet. The two investigators performed the data collection independently using a questionnaire and clinical examination. They were calibrated in terms of diffident potential oral lesions, which they may have encountered. The kappa statistics were between 0.74 and 0.83. This datasheet included demographic information, medical and travel history, self-reported co-morbidities, and general symptoms during the first two days of the disease (cough, fever, sore throat, runny nose, muscle pain, headaches, nausea, and diarrhea), date of COVID-19 diagnosis, and clinical oral examination results. The oral hygiene was classified based on the plaque index (PI) as good (PI=0, ie absence of plaque), fair (PI=0.1–1.9, ie presence of plaque) and poor (PI=2.0–3.0, ie plaque is obvious by naked eye).16,17
Sample Size and Statistical Methods
This was a pilot cross-sectional study. The sample size calculation was not applicable due to few published case reports, which are not sufficient to estimate the prevalence. All selected patients agreed to participate in the study. Primary outcomes were the type and prevalence of oral manifestations in patients with COVID-19. Secondary outcomes were time of duration and location of lesions. Data were collected in an Excel® datasheet. For quantitative variables, descriptive statistics were performed (mean and standard deviation were calculated), while frequency and percentage were used for qualitative variables. After a normality check, we used a Student’s t-test for quantitative variables and a Chi-squared test or a Fisher’s test for qualitative data. A level of p < 0.05 was considered statistically significant.
Results
The study included a total of 109 patients. Patient characteristics with or without oral manifestations are described in Table 1. The majority of the participants were married, males, non-healthcare workers, and non-smokers. Muscle pain was the most common general symptom (77.1%), followed by fever (67.9%) and cough (50%). Diarrhea and loss of smell were more likely to be associated with patients who had oral manifestations (p-value 0.042 and <0.001, respectively).
Table 1.
Sample Characteristics
| Variable | Overall | With Oral Symptoms N (%) | Without Oral Symptoms N (%) | p value** |
|---|---|---|---|---|
| N=109 | n=58 | n=51 | ||
| Age | 39.3±12.4 | 39.1±12.2 | 38.5±13.4 | 0.528 |
| Gender | 0.637 | |||
| Male | 73(67.0) | 40(69.0) | 33(64.7) | |
| Female | 36(33.0) | 18(31.0) | 18(35.3) | |
| Marital status | 0.076 | |||
| Single | 22(20.2) | 8(13.8) | 14(27.5) | |
| Married | 87(79.8) | 50(86.2) | 37(72.5) | |
| Occupation | 0.927 | |||
| Health care | 2(1.8) | 1(1.7) | 1(2.0) | |
| Non health care | 107 (98.2) | 57(98.3) | 50(98.0) | |
| Citizen | 0.285 | |||
| Saudi | 33(30.3) | 15(25.9) | 18(35.3) | |
| Non | 76(69.7) | 43(74.1) | 33(64.7) | |
| Oral hygiene | 0.762 | |||
| Fair | 28(275) | 4(7.8) | 6(11.8) | |
| Poor | 10(9.8) | 15(29.4) | 13(25.5) | |
| Good | 64(62.7) | 32(62.7) | 32(62.7) | |
| General symptoms | ||||
| Fever | 74(67.9) | 38(65.5) | 36(70.6) | 0.572 |
| Sore throat | 39(35.8) | 22(37.9) | 17(33.3) | 0.617 |
| Runny nose | 17(15.6) | 8 (13.8) | 9 (17.6) | 0.580 |
| Cough | 55(50.5) | 28 (48.3) | 27 (52.9) | 0.627 |
| Shortness of breath | 24(22.0) | 14 (22.4) | 11 (21.6) | 0.915 |
| Nausea | 22(20.2) | 10 (17.2) | 12 (23.5) | 0.414 |
| Vomiting | 10(9.2) | 7(12.1) | 3(5.9) | 0.331 |
| Headache | 54(49.5) | 33 (56.9) | 21 (41.2) | 0.101 |
| Muscle pain | 84(77.1) | 46(79.3) | 38(74.5) | 0.552 |
| Diarrhea | 34(31.2) | 23 (39.7) | 11 (21.6) | 0.042* |
| No smell | 48(44.0) | 35(60.3) | 13(25.5) | <0.001* |
| Co-morbidity | 10(18.3) | 10(17.2) | 10(19.6) | 0.750 |
| Diabetes | 11(10.1) | 8(13.8) | 3(5.9) | 0.171 |
| Hypertension | 8(7.3) | 3(5.2) | 5(9.8) | 0.470 |
| Asthma | 3(2.8) | 1(1.7) | 2(3.9) | 0.598 |
| Epilepsy and arthritis | 2(1.8) | 1 (1.7) | 1 (2.0) | 0.937 |
Notes: *p value < 0.05. **p value using Student’s t-test (age) and the remaining variables either Chi-squared test or a Fisher’s test.
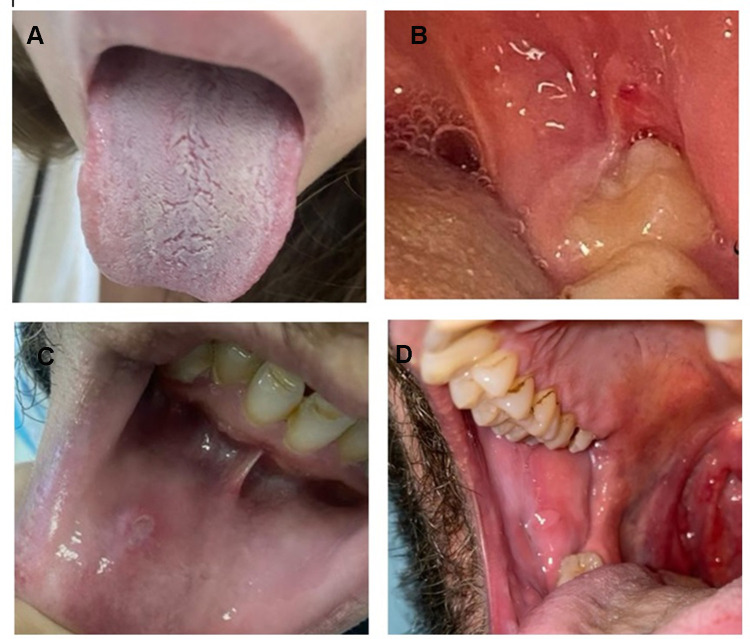
Loss of taste was the most common oral manifestation of COVID-19 (43.4%), followed by erythema/desquamated gingivitis and coated tongue (7.3% each), and ulcers and blisters (6.4%) (Table 2, Figure 1). Loss of taste was the only symptom that persisted for 10 days. Other symptoms persisted for seven days or less, and it took one to four days for these symptoms to be detected in 81% of the patients with oral manifestations (Table 2).
Table 2.
Types of Oral Manifestations and Duration of Each Symptom
| Type | Total | Duration of the Oral Symptom in Days (d) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N=109 | 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | 10 d | |
| n=19* | n=9 | n=12* | n=7* | n=3 | n=3 | n=2 | n=3 | ||
| N (%) | |||||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Loss of taste | 43(39.4) | 14(73.7) | 6(67.7) | 11(91.7) | 5(71.4) | 2(66.7) | 1(33.3) | 1(50) | 3(100) |
| Erythema and desquamated | 8 (7.3) | 7(36.8) | – | 1(8.3) | – | – | – | – | – |
| Coated tongue | 8 (7.3) | 1(5.3) | 1(11.1) | 3(25) | 1(14.3) | – | 1(33.3) | 1(50) | – |
| Ulcers and blisters | 7(6.4) | 1(5.3) | 2(22.2) | 1(8.3) | 2(28.6) | – | 1(33.3) | – | – |
| Pain and soreness | 3 (2.8) | 2(10.5) | – | 1(8.3) | – | – | – | – | – |
| Dry mouth | 1 (0.9) | – | – | – | – | 1(33.3) | – | – | – |
Note: *Some patients had more than one symptom.
Figure 1.
Forms of oral manifestations among patients with oral symptoms (N=58) (A) Coated tongue, (B) Gingival erythema, (C) Ulcer, and (D) blister.
Oral manifestations appeared as a single symptom (79.3%), followed by combined symptoms (19%) (Table 3). Dorsum of tongue was the most common oral location (72.4%), followed by vestibules (12.1%), and buccal mucosa, lips, and gingiva (8.6% each). The majority of participants were aware of these symptoms (84.5%) and were experiencing them for the first time (93.9%) (Table 3).
Table 3.
Characteristics of the Oral Symptoms
| Characteristics | N (%) |
|---|---|
| N=58 | |
| ● Number of the oral symptoms | |
| Three combined symptoms | 1(1.7) |
| Ulcers and blisters + Coated tongue + Loss of taste | 1(1.7) |
| Two combined symptoms | 11(19.0) |
| Loss of taste +coated tough | 4 (6.9) |
| Loss of taste + pain and soreness | 3(5.2) |
| Loss of taste +erythema | 2(3.4) |
| Pain and soreness +erythema | 1(1.7) |
| Ulcer and blister + dry mouth | 1(1.7) |
| Single symptom | 46(79.3) |
| Loss of taste | 33(56.9) |
| Ulcers and blisters | 5(8.3) |
| Erythema and desquamated | 5 (8.3) |
| Coated tongue | 3(5.2) |
| Dry mouth | 0 |
| Pain and soreness | 0 |
| ● Oral location* | |
| Dorsum of tongue | 42(72.4) |
| Vestibules | 7(12.1) |
| Buccal mucosa | 5(8.6) |
| Lips | 5(8.6) |
| Gingiva | 5(8.6) |
| Ventrum of tongue | 1(1.7) |
| Floor of mouth | 1(1.7) |
| ● Awareness of oral symptoms | |
| Yes | 49(84.5) |
| No | 9(15.7) |
| ● Onset of the symptoms | |
| 1st time | 46(93.9) |
| Recurrent | 3(6.1) |
Note: *Patient may has more than one location.
Discussion
The current study was conducted in response to the dramatic surge of COVID-19 cases, which prompted us to investigate the general effects of this disease on multiple sites/organs, including the oral cavity. The most common specific oral symptom in patients with COVID-19 was loss of taste.2 However, during the previous SARS-CoV and MERS-CoV pandemics, loss of taste was not among the reported symptoms.18 In this study, the prevalence of taste loss was similar to that of a recently published systematic review and meta-analysis reporting the overall prevalence to be 45%.2 This confirms that the loss of taste may be considered a specific COVID-19 symptom in Saudi Arabia as well.
Several hypotheses are explaining how the loss of taste may be related to COVID-19.9,12,13,19 One of these hypotheses suggests that the normal function of taste buds can be affected by rhinitis triggering a related local inflammatory response,19 although loss of taste may happen without any nasal mucosal inflammation.2 Another hypothesis states that the loss of taste could be attributed to COVID-19 drug treatment side effects.12,13 However, it still appeared in COVID-19 patients who were found to be drug-free.
Other oral symptoms have been suggested as potential manifestations of COVID-19 and published in few case reports and letters to the editor.14,20,21 These suggestions were controversial, as the symptoms may have been caused primarily by the SARS-CoV-2 or were a secondary manifestation.2,20 These symptoms included lesions in several oral locations, while this study showed the same nonspecific pattern of oral clinical features, such as ulcers, and blisters. A study published by Ansari et al found several ulcers in two COVID-19 patients. They attributed these ulcers to the effects of stress as a trigger factor.20,22 Meanwhile, Martín Carreras-Presas et al suggested COVID-19 as a possible cause of desquamative gingivitis, ulcers, oral pain, and blisters.10,11 These ulcers could be diagnosed as canker sores, fever blisters, aphthous ulcers or herpes type 1, which could be related indirectly to COVID-19 patients through stress, nervous habits, grinding, increased blood pressure, poor diet, type 2 diabetes.8,17,23–28 Moreover, COVID-19 medications were less likely to contribute to the oral manifestations, since patients did not use it and they came to the hospitals/clinics to pick up their medications.
A study by Vieira found that only severe cases of periodontitis may worsen COVID-19, while mild cases of COVID-19 may not show any oral manifestations, and vice versa.14 They suggested a possible link between oral manifestations and the inflammatory process, which can trigger the coagulation cascade and increase levels of fibrinogen degradation products (eg D-dimer).29 Moreover, Patel suggested a possible relationship between necrotizing periodontal disease (NPD) and COVID-19 due to bacterial co-infections occurring intraorally.21 An abnormally high bacterial read of the major etiological bacteria of NPD was detected frequently in those infected with SARS-CoV-2 using metagenomic analyses, in addition to several other acute periodontal bacteria species, such as Fusobacterium and Treponema. A recent review identified 18 studies with 25 cases. However, there were nonspecific symptoms, such as taste alteration, loss of taste, burning sensation in the mouth, mucosal ulceration, dry mouth and halitosis, or even pseudomembranous nodules, maculae or plaque mucosal lesions.30 Crusted lips, multiple rashes, targeted lesions, blisters and vesiculobullous lesions were reported in some cases.30
This study showed there were no specific oral manifestations related to COVID-19 patients. To our knowledge, few case reports and letters to the editor have been published on this topic, and this is the first study conducted in Saudi Arabia. However, all the results reported in this article should be interpreted with caution: most oral manifestations may go unnoticed and some variables were self-reported which may underestimate these variables, in addition to the difficulty in registering the oral findings, which can impose exposure and contamination risk during photographic image conduction. Moreover, loss of taste was measured based on patient reporting, which is a subjective method and may not accurately diagnose this condition. In addition, we need to classify the taste disorder to understand the effect of COVID-19 on gustatory buds. Lastly, all patients were examined only once. It would be better if the patients were examined daily, from day 1 to day 10. However, it is not feasible due to the nature of the disease.
Conclusion
Specific oral symptoms are not commonly observed in COVID-19 patients. However, taste loss was a highly reported oral manifestation in this study. Other nonspecific oral lesions/symptoms found are controversial, and could be due co-infections, immunity impairment, or adverse drug events. We recommend that oral examinations of COVID-19 patients should be conducted as a routine examination to investigate any possible correlation between the disease and the oral cavity.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Worldometers. Corona virus. 2021.
- 2.Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. 2021;100:141–154. doi: 10.1177/0022034520957289 [DOI] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95:1621–1631. doi: 10.1016/j.mayocp.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa KV, Carnaúba ATL, Rocha KW, Andrade KCL, Ferreira SMS, Menezes PL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86:781–792. doi: 10.1016/j.bjorl.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badran Z, Gaudin A, Struillou X, Amador G, Soueidan A. Periodontal pockets: a potential reservoir for SARS-CoV-2? Med Hypotheses. 2020;143:109907. doi: 10.1016/j.mehy.2020.109907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alshaeri HK, Natto ZS. A contemporary look at COVID-19 medications: available and potentially effective drugs. Eur Rev Med Pharmacol Sci. 2020;24:9188–9195. [DOI] [PubMed] [Google Scholar]
- 8.Ansari R, Gheitani M, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19). Oral Dis. 2020. doi: 10.1111/odi.13465 [DOI] [PubMed] [Google Scholar]
- 9.Cox MJ, Loman N, Bogaert D, O’Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1:e11. doi: 10.1016/S2666-5247(20)30009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikshit S. Fibrinogen degradation products and periodontitis: deciphering the connection. J Clin Diagn Res. 2015;9:ZC10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finsterer J, Stollberger C. Causes of hypogeusia/hyposmia in SARS-CoV2 infected patients. J Med Virol. 2020;92:1793–1794. doi: 10.1002/jmv.25903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo Cde B, Mimura MA, Sugaya NN. Psychological stress and recurrent aphthous stomatitis. Clinics (Sao Paulo). 2009;64:645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariz B, Brandão TB, Ribeiro ACP, Lopes MA, Santos-Silva AR. New insights for the pathogenesis of COVID-19-related dysgeusia. J Dent Res. 2020;99:1206. doi: 10.1177/0022034520936638 [DOI] [PubMed] [Google Scholar]
- 14.Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2020a. doi: 10.1111/odi.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. SARS-CoV-2 oral-associated lesions: discussion of elicited response. Oral Dis. 2020b. doi: 10.1111/odi.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID-19. Oral Dis. 2020. doi: 10.1111/odi.13462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellegrino R, Cooper KW, Di Pizio A, Joseph PV, Bhutani S, Parma V. Corona viruses and the chemical senses: past, present, and future. Chem Senses. 2020;45:415–422. doi: 10.1093/chemse/bjaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020a;10:1103–1104. doi: 10.1002/alr.22593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 2020b;42:1570–1576. doi: 10.1002/hed.26228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira AR. Oral manifestations in coronavirus disease 2019 (COVID-19). Oral Dis. 2020. doi: 10.1111/odi.13463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Mutawa SA, Shyama M, Al-Duwairi Y, Soparkar P. Oral hygiene status of Kuwaiti schoolchildren. East Mediterr Health J. 2011;17:387–391. doi: 10.26719/2011.17.5.387 [DOI] [PubMed] [Google Scholar]
- 22.Rodan R, Khlaifat F, Smadi L, Azab R, Abdalmohdi A. Prevalence and severity of gingivitis in school students aged 6–11 years in Tafilah Governorate, South Jordan: results of the survey executed by National Women's Health Care Center. BMC Res Notes. 2015;8:662. doi: 10.1186/s13104-015-1532-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natto ZS. Dental students’ knowledge and attitudes about electronic cigarettes: a cross-sectional study at one Saudi University. J Dent Educ. 2021;84:27–33. doi: 10.21815/JDE.019.162 [DOI] [PubMed] [Google Scholar]
- 24.Natto ZS, Alshaeri HK. Characteristics of first cases of Coronavirus disease 2019 and the effort to prevent the early spread of COVID-19 in Saudi Arabia. Risk Manag Healthc Policy. 2021;14:315–321. doi: 10.2147/RMHP.S278394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natto ZS, Alshaeri HK. Are Saudi healthcare students aware of COVID-19, and do they behave safely during viral outbreaks? Niger J Clin Pract. 2021;24:406–411. doi: 10.4103/njcp.njcp_259_20 [DOI] [PubMed] [Google Scholar]
- 26.Natto ZS, Parashis A, Steffensen B, Ganguly R, Finkelman MD, Jeong YN. Efficacy of collagen matrix seal and collagen sponge on ridge preservation in combination with bone allograft: a randomized controlled clinical trial. J Clin Periodontol. 2017;44:649–659. doi: 10.1111/jcpe.12722 [DOI] [PubMed] [Google Scholar]
- 27.ALHarthi SSY, Natto ZS, Midle JB, Gyurko R, O’Neill R, Steffensen B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol. 2019;90:16–25. doi: 10.1002/JPER.18-0183 [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam T, Nikalje MR, Jadhav S. Oral manifestations among COVID-19: an observational study of 713 patients. Dent Res J (Isfahan). 2021;18:67. doi: 10.4103/1735-3327.324026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzêda-E-Silva VD, de Sá IB, Martins J, Pedreira N, Vieira VPS, Silva BHM. Oral lesions associated with COVID-19: a systematic review. Stomatologija. 2021;23:3–8. [PubMed] [Google Scholar]
- 30.Surboyo MD, Ernawati DS, Budi HS. Oral mucosal lesions and oral symptoms of the SARS-CoV-2 infection. Minerva Dent Oral Sci. 2021;70:161–168. doi: 10.23736/S2724-6329.21.04493-9 [DOI] [PubMed] [Google Scholar]