Abstract
Background
On April 15, 2021, the Surveillance, Epidemiology, and End Results (SEER) database released the latest lung cancer follow-up data. We selected 922,317 lung cancer patients diagnosed from 2000 to 2017 for survival analysis to provide updated data for lung cancer researchers.
Research Question
This study explored the latest trends of survival time in terms of gender, race, nationality, age, income, address, histological type and primary site.
Study Design and Methods
The SEER database covers 27.8% of the US population. We used life table, Kaplan–Meier, log-rank, Breslow and Tarone-Ware tests to calculate survival rate, time, and curve and to compare differences in survival distribution. We performed univariate and multivariate Cox proportional hazards analyses.
Results
The median survival time of all lung cancer patients diagnosed in 2017 increased by 41.72% compared to 2000. Median survival time of female patients diagnosed in 2017 increased by 70.94% compared to 2000. Median survival time of those diagnosed in 2017 for different primary sites was as follows: right middle lobe was the longest, then left lower lobe, right upper lobe, right lower lobe, and left upper lobe. Lung cancer patients older than 75 years had a significantly shorter median survival time. Patients living in metropolitan areas of 250,000 to 1 million had a longer median survival time. Median survival time in the adenocarcinoma group was significantly greater than other patients. Median survival of Asian and other races diagnosed in 2017 was 97.87% higher than those diagnosed in 2000. Survival rate of lung cancer increased gradually with the year of diagnosis.
Interpretation
The rapid improvement of the prognosis of female and young lung cancer patients contributes to the improvement of the overall prognosis. Primary lung cancer in the right middle lobe has the best prognosis.
Keywords: Cox regression analysis, Kaplan Meier survival analysis, lung cancer, prognosis, SEER
Introduction
The Surveillance, Epidemiology, and End Results (SEER) database released the most recent lung cancer follow-up data on 15 April 2021 (https://www.cancer.gov). Although previous articles1–3 analyzed patient survival,4–6 these predated this release and therefore the data were not up to date. Our study therefore aimed to provide lung cancer researchers with accurate and updated survival data.
SEER is an authoritative source of cancer statistics in the United States and the SEER Program provides statistics on the cancer burden among the US population. The SEER database collects and publishes cancer incidence and survival data from population-based cancer registries. These data are collected on every cancer case reported from 18 US geographic areas. Because these areas are representative of the entire US population, SEER can account for diverse populations. SEER also reports mortality data, which are provided by the National Center for Health Statistics.7
Our study analyzed the survival of 922,317 lung cancer patients diagnosed from 2000–2017 in detail. Grouped by gender, race, ethnicity, age, income, address, histologic type, and primary site, respectively, our study found large variations in survival times and conditions among different groups of lung cancer patients by factors, and this variation has further expanded in recent years. And we tabulated the hazard ratios corresponding to each group in each item in detail.
We consider our study meaningful in being able to inform researchers and policy makers on the survival differences of lung cancer patients from different perspectives and to support the latest data in research and policy. It may help public health authorities and policy makers to identify and monitor public health problems and focus interventions to reduce potential excess deaths in these areas.8
Methods
Data Sources
We selected 922,317 lung cancer cases from the latest available data from the SEER database on April 15, 2021. Incidence - SEER Research Data, 18 Registries, Nov 2020 Sub (2000–2018). SEER 18 covers approximately 27.8% of the US population (based on the 2010 census). Geographic areas (registries) covered San Francisco-Oakland SMSA, Connecticut, Detroit (Metropolitan), Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, Atlanta (Metropolitan), San Jose-Monterey, Los Angeles, Alaska Natives, Rural Georgia, California excluding, Kentucky, Louisiana, New Jersey, and Greater Georgia. We selected 13 entries including ID, survival months, status, sex, age, year of diagnosis, race recode (White, Black, American Indian/Alaska Native, Asian or Pacific Islander), origin recode the National Health Insurance Authority (Hispanic, Non-Hispanic), primary site-labeled, laterality, median household income inflation adjusted to 2019, the Rural-Urban Continuum Code, and ICD-O-3 (International Classification of Disease for Oncology-3) histologic type, The specific type of histologic type is shown in Table S1, but all lung tumors designed for this study refer to malignancies of the lung. Epithelial neoplasms including small cell carcinoma. Unless otherwise indicated, all text within the National Cancer Institute (NCI) products is free of copyright and may be reused without permission. Credit the NCI as the source. Each entry is integrated and grouped, and the specific grouping is shown in Table 1.
Table 1.
Basic Characteristics of Patients and Survival Analysis According to Different Factors
| Number of Patients | Percentage of Total Patients (%) | 3-Year Survival Rate (%) | Probability Density | 5-Year Survival Rate (%) | Probability Density | 10-Year Survival Rate (%) | Probability Density | Median Survival Time (Months) | Standard Error | 95.0%, CI | Mean Survival Time (Months) | Standard Error | 95.0%, CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||||||||||
| Total | 9,22,317 | 100 | 19 | 0.003 | 14 | 0.002 | 8 | 0.001 | 9 | 0.021 | 8.959 | 9.041 | 32.793 | 0.066 | 32.663 | 32.922 |
| Sex | ||||||||||||||||
| Female | 4,32,762 | 46.9 | 23 | 0.004 | 18 | 0.002 | 10 | 0.001 | 11 | 0.039 | 10.923 | 11.077 | 38.805 | 0.107 | 38.595 | 39.015 |
| Male | 4,89,555 | 53.1 | 16 | 0.003 | 12 | 0.002 | 6 | 0.001 | 8 | 0.025 | 7.952 | 8.048 | 27.522 | 0.08 | 27.365 | 27.68 |
| Age | ||||||||||||||||
| < 45 years | 15,701 | 1.7 | 32 | 0.003 | 28 | 0.001 | 24 | 0.001 | 16 | 0.284 | 15.443 | 16.557 | 67.082 | 0.763 | 65.586 | 68.579 |
| 45–54 years | 74,518 | 8.1 | 24 | 0.003 | 20 | 0.001 | 15 | 0.001 | 12 | 0.088 | 11.827 | 12.173 | 46.308 | 0.29 | 45.74 | 46.876 |
| 55–64 years | 1,98,128 | 21.5 | 23 | 0.003 | 18 | 0.002 | 12 | 0.001 | 11 | 0.051 | 10.9 | 11.1 | 41.111 | 0.165 | 40.788 | 41.433 |
| 65–74 years | 3,00,953 | 32.6 | 21 | 0.004 | 16 | 0.002 | 8 | 0.001 | 11 | 0.041 | 10.92 | 11.08 | 34.554 | 0.115 | 34.33 | 34.779 |
| 75–84 years | 2,54,341 | 27.6 | 15 | 0.003 | 10 | 0.002 | 3 | 0.001 | 7 | 0.034 | 6.933 | 7.067 | 23.075 | 0.087 | 22.904 | 23.246 |
| ≥ 85 years | 78,676 | 8.5 | 7 | 0.002 | 4 | 0.001 | 1 | 0 | 3 | 0.034 | 2.933 | 3.067 | 12.349 | 0.091 | 12.171 | 12.527 |
| Race | ||||||||||||||||
| White | 7,65,078 | 83 | 19 | 0.003 | 14 | 0.002 | 8 | 0.001 | 9 | 0.023 | 8.955 | 9.045 | 32.726 | 0.072 | 32.585 | 32.867 |
| Black | 1,00,450 | 10.9 | 17 | 0.003 | 12 | 0.002 | 7 | 0.001 | 9 | 0.06 | 8.882 | 9.118 | 29.497 | 0.191 | 29.124 | 29.871 |
| Asian and others (a) | 56,789 | 6.2 | 23 | 0.004 | 17 | 0.002 | 11 | 0.001 | 12 | 0.124 | 11.757 | 12.243 | 40.002 | 0.32 | 39.375 | 40.629 |
| Origin recode NHIA | ||||||||||||||||
| Non-Spanish-Hispanic-Latino | 8,72,814 | 94.6 | 19 | 0.003 | 14 | 0.002 | 8 | 0.001 | 9 | 0.021 | 8.958 | 9.042 | 32.686 | 0.068 | 32.553 | 32.818 |
| Spanish-Hispanic-Latino | 49,503 | 5.4 | 20 | 0.003 | 15 | 0.002 | 9 | 0.001 | 9 | 0.094 | 8.816 | 9.184 | 35.001 | 0.321 | 34.373 | 35.629 |
| Median household income inflation adj to 2019 | ||||||||||||||||
| < $35,000 | 19,711 | 2.1 | 15 | 0.003 | 11 | 0.001 | 5 | 0.001 | 7 | 0.111 | 6.783 | 7.217 | 25.872 | 0.405 | 25.079 | 26.665 |
| $35,000-$44,999 | 76,395 | 8.3 | 16 | 0.003 | 12 | 0.002 | 6 | 0.001 | 8 | 0.064 | 7.875 | 8.125 | 28.092 | 0.219 | 27.663 | 28.521 |
| $45,000-$54,999 | 1,52,943 | 16.6 | 17 | 0.003 | 13 | 0.002 | 6 | 0.001 | 8 | 0.049 | 7.905 | 8.095 | 28.94 | 0.151 | 28.645 | 29.235 |
| $55,000-$64,999 | 2,18,082 | 23.6 | 18 | 0.003 | 14 | 0.002 | 8 | 0.001 | 9 | 0.042 | 8.917 | 9.083 | 31.782 | 0.132 | 31.522 | 32.041 |
| $65,000-$74,999 | 1,95,430 | 21.2 | 20 | 0.003 | 15 | 0.002 | 8 | 0.001 | 9 | 0.05 | 8.901 | 9.099 | 33.927 | 0.147 | 33.639 | 34.215 |
| $75,000+ | 2,59,669 | 28.2 | 22 | 0.004 | 16 | 0.002 | 9 | 0.001 | 11 | 0.045 | 10.911 | 11.089 | 36.832 | 0.133 | 36.571 | 37.92 |
| Others (b) | 87 | 0 | 13 | 0.01 | 10 | 0.002 | 0 | 0 | 14 | 2.287 | 9.517 | 18.483 | 25.118 | 3.734 | 17.799 | 32.438 |
| Rural-Urban Continuum Code | ||||||||||||||||
| Counties in metropolitan areas greater than 1 million population | 5,24,354 | 56.9 | 20 | 0.003 | 15 | 0.002 | 9 | 0.001 | 10 | 0.031 | 9.939 | 10.061 | 34.716 | 0.091 | 34.537 | 34.895 |
| Counties in metropolitan areas of 250,000 to 1 million population | 1,76,075 | 19.1 | 19 | 0.003 | 14 | 0.002 | 8 | 0.001 | 9 | 0.047 | 8.907 | 9.093 | 32.469 | 0.151 | 32.172 | 32.765 |
| Counties in metropolitan areas of less than 250 thousand population | 81,081 | 8.8 | 18 | 0.003 | 13 | 0.002 | 7 | 0.001 | 9 | 0.068 | 8.867 | 9.133 | 29.798 | 0.205 | 29.396 | 30.199 |
| Nonmetropolitan counties adjacent to a metropolitan area | 80,876 | 8.8 | 16 | 0.003 | 12 | 0.002 | 6 | 0.001 | 8 | 0.064 | 7.875 | 8.125 | 28.298 | 0.2 | 27.906 | 28.689 |
| Nonmetropolitan counties not adjacent to a metropolitan area | 58,676 | 6.4 | 16 | 0.003 | 11 | 0.002 | 6 | 0.001 | 8 | 0.07 | 7.863 | 8.137 | 27.212 | 0.231 | 26.76 | 27.664 |
| Others (c) | 1255 | 0.1 | 14 | 0.003 | 11 | 0.001 | 5 | 0 | 9 | 0.475 | 8.069 | 9.931 | 25.974 | 1.478 | 23.078 | 28.87 |
| Primary site | ||||||||||||||||
| Right upper lobe | 2,44,392 | 26.5 | 23 | 0.004 | 17 | 0.002 | 10 | 0.001 | 12 | 0.052 | 11.898 | 12.102 | 38.607 | 0.136 | 38.341 | 38.873 |
| Right middle lobe | 38,374 | 4.2 | 26 | 0.004 | 20 | 0.002 | 12 | 0.001 | 13 | 0.151 | 12.705 | 13.295 | 43.843 | 0.381 | 43.097 | 44.589 |
| Right lower lobe | 1,23,735 | 13.4 | 22 | 0.004 | 17 | 0.002 | 9 | 0.001 | 11 | 0.072 | 10.859 | 11.141 | 37.296 | 0.191 | 36.922 | 37.671 |
| Left upper lobe | 1,95,234 | 21.2 | 22 | 0.004 | 16 | 0.002 | 9 | 0.001 | 11 | 0.053 | 10.896 | 11.104 | 36.099 | 0.149 | 35.807 | 36.39 |
| Left lower lobe | 99,281 | 10.8 | 23 | 0.004 | 18 | 0.002 | 10 | 0.001 | 11 | 0.078 | 10.848 | 11.152 | 37.904 | 0.221 | 37.471 | 38.337 |
| Main bronchus | 43,133 | 4.7 | 8 | 0.002 | 6 | 0.001 | 4 | 0 | 5 | 0.055 | 4.892 | 5.108 | 17.515 | 0.211 | 17.102 | 17.927 |
| Over lapping lesion of lung | 10,731 | 1.2 | 17 | 0.003 | 13 | 0.001 | 7 | 0.001 | 7 | 0.164 | 6.678 | 7.322 | 28.642 | 0.562 | 27.541 | 29.743 |
| Lung, NOS | 1,64,777 | 17.9 | 7 | 0.002 | 4 | 0.001 | 2 | 0 | 4 | 0.026 | 3.949 | 4.051 | 13.678 | 0.092 | 13.497 | 13.859 |
| Others (d) | 2660 | 0.3 | 8 | 0.002 | 5 | 0.001 | 2 | 0.001 | 5 | 0.22 | 4.569 | 5.431 | 15.838 | 0.668 | 14.527 | 17.148 |
| Histologic type (ICD-O-3) | ||||||||||||||||
| Neoplasia, NAS | 67,189 | 7.3 | 6 | 0.002 | 4 | 0.001 | 2 | 0 | 2 | 0.027 | 1.946 | 2.054 | 12.431 | 0.156 | 12.126 | 12.736 |
| Epithelial Neoplasms, NAS | 2,94,702 | 32 | 9 | 0.002 | 6 | 0.001 | 3 | 0 | 6 | 0.024 | 5.954 | 6.046 | 17.939 | 0.074 | 17.794 | 18.084 |
| Squamous cell neoplasms | 1,79,942 | 19.5 | 20 | 0.004 | 15 | 0.002 | 7 | 0.001 | 11 | 0.055 | 10.893 | 11.107 | 32.733 | 0.136 | 32.468 | 32.999 |
| Adenoma and adenocarcinomas | 3,47,366 | 37.7 | 28 | 0.004 | 22 | 0.002 | 13 | 0.001 | 15 | 0.061 | 14.88 | 15.12 | 46.591 | 0.132 | 46.333 | 46.849 |
| Cystic, mucinous and serous neoplasms | 12,070 | 1.3 | 29 | 0.004 | 23 | 0.002 | 14 | 0.001 | 15 | 0.377 | 14.261 | 15.739 | 48.995 | 0.762 | 47.5 | 50.489 |
| Acinous cell neoplasm | 7044 | 0.8 | 67 | 0.005 | 55 | 0.004 | 34 | 0.002 | 86 | 2.44 | 81.217 | 90.783 | 102.005 | 1.719 | 98.636 | 105.373 |
| Complex epithelial neoplasms | 10,523 | 1.1 | 26 | 0.004 | 20 | 0.002 | 11 | 0.001 | 14 | 0.319 | 13.375 | 14.625 | 42.256 | 0.671 | 40.94 | 43.571 |
| Others (e) | 3481 | 0.4 | 30 | 0.003 | 26 | 0.002 | 19 | 0.001 | 12 | 0.566 | 10.891 | 13.109 | 56.854 | 1.541 | 53.834 | 59.874 |
| Year of diagnosis | ||||||||||||||||
| 2000 | 48,893 | 5.3 | 15 | 0.003 | 11 | 0.001 | 6 | 0.001 | 8 | 0.071 | 7.862 | 8.138 | 27.008 | 0.228 | 26.56 | 27.455 |
| 2001 | 49,520 | 5.37 | 15 | 0.003 | 11 | 0.001 | 6 | 0.001 | 8 | 0.07 | 7.863 | 8.137 | 26.954 | 0.223 | 26.517 | 27.391 |
| 2002 | 49,658 | 5.38 | 15 | 0.003 | 11 | 0.001 | 6 | 0.001 | 8 | 0.07 | 7.863 | 8.137 | 26.672 | 0.216 | 26.249 | 27.095 |
| 2003 | 50,123 | 5.43 | 16 | 0.003 | 12 | 0.001 | 7 | 0.001 | 8 | 0.075 | 7.854 | 8.146 | 26.974 | 0.211 | 26.56 | 27.389 |
| 2004 | 49,890 | 5.41 | 16 | 0.003 | 12 | 0.002 | 7 | 0.001 | 8 | 0.076 | 7.851 | 8.149 | 27.146 | 0.206 | 26.741 | 27.55 |
| 2005 | 50,749 | 5.5 | 17 | 0.003 | 13 | 0.002 | 7 | 0.001 | 8 | 0.082 | 7.839 | 8.161 | 27.358 | 0.2 | 26.966 | 27.751 |
| 2006 | 51,223 | 5.55 | 18 | 0.003 | 13 | 0.002 | 7 | 0.001 | 9 | 0.085 | 8.833 | 9.167 | 27.555 | 0.194 | 27.175 | 27.935 |
| 2007 | 51,598 | 5.59 | 18 | 0.003 | 14 | 0.002 | 7 | 0.001 | 9 | 0.089 | 8.826 | 9.174 | 27.275 | 0.184 | 26.915 | 27.636 |
| 2008 | 51,869 | 5.62 | 19 | 0.003 | 14 | 0.002 | 8 | 0.001 | 9 | 0.086 | 8.831 | 9.169 | 26.995 | 0.175 | 26.653 | 27.338 |
| 2009 | 52,587 | 5.7 | 19 | 0.003 | 14 | 0.002 | N/A | N/A | 9 | 0.087 | 8.829 | 9.171 | 26.466 | 0.164 | 26.145 | 26.787 |
| 2010 | 51,355 | 5.57 | 20 | 0.003 | 15 | 0.002 | N/A | N/A | 9 | 0.087 | 8.829 | 9.171 | 25.787 | 0.155 | 25.483 | 26.091 |
| 2011 | 50,918 | 5.52 | 21 | 0.003 | 15 | 0.002 | N/A | N/A | 10 | 0.101 | 9.803 | 10.197 | 25.461 | 0.145 | 25.177 | 25.745 |
| 2012 | 51,591 | 5.59 | 20 | 0.003 | 15 | 0.002 | N/A | N/A | 10 | 0.099 | 9.806 | 10.194 | 23.689 | 0.129 | 23.435 | 23.943 |
| 2013 | 51,787 | 5.61 | 21 | 0.003 | 16 | 0.002 | N/A | N/A | 10 | 0.102 | 9.8 | 10.2 | 22.55 | 0.115 | 22.324 | 22.776 |
| 2014 | 52,469 | 5.69 | 23 | 0.004 | N/A | N/A | N/A | N/A | 11 | 0.104 | 10.796 | 11.204 | 21.215 | 0.1 | 21.019 | 21.411 |
| 2015 | 52,702 | 5.71 | 24 | 0.004 | N/A | N/A | N/A | N/A | 11 | 0.124 | 10.757 | 11.243 | 19.492 | 0.083 | 19.33 | 19.654 |
| 2016 | 52,642 | 5.71 | N/A | N/A | N/A | N/A | N/A | N/A | 12 | 0.135 | 11.736 | 12.264 | 16.725 | 0.064 | 16.601 | 16.85 |
| 2017 | 52,743 | 5.72 | N/A | N/A | N/A | N/A | N/A | N/A | 13 | 0.152 | 12.703 | 13.297 | 12.966 | 0.042 | 12.883 | 13.05 |
| Summary stage | ||||||||||||||||
| Distant | 4,80,617 | 52.1 | 5 | 0.002 | 3 | 0.001 | 2 | 0 | 4 | 0.013 | 3.974 | 4.026 | 12.89. | 0.047 | 12.798 | 12.982 |
| Localized | 1,69,955 | 18.4 | 51 | 0.006 | 40 | 0.004 | 24 | 0.002 | 49 | 0.258 | 48.494 | 49.506 | 78.295 | 0.22 | 77.863 | 78.726 |
| Regional | 2,03,809 | 22.1 | 28 | 0.005 | 21 | 0.003 | 11 | 0.001 | 18 | 0.076 | 17.851 | 18.149 | 45.042 | 0.154 | 44.739 | 45.344 |
| Unknown/unstaged | 67,936 | 7.4 | 10 | 0.003 | 7 | 0.001 | 3 | 0 | 6 | 0.07 | 5.863 | 6.137 | 19.61 | 0.186 | 19.245 | 19.976 |
Notes: (a) included Asian or Pacific Islander and American Indian/Alaska Native. (b) Others included unknown/missing/no match/Not 1990–2018. (c) Others included Unknown/missing/no match (Alaska or Hawaii - Entire State) and Unknown/missing/no match/Not 1990–2018. (d) Others included Only one side - side unspecified, Bilateral, single primary and Paired site, but no information concerning laterality. (e) Others included all the histological types of lung cancer except the above seven types.
Data Processing and Statistical Analysis
Because the data volume and analysis items were too large and limited to the length of the paper, the focus was briefly described in the results, and detailed and specific contents are detailed in the tables, figures, and other supplementary materials. We used frequency function statistics, and statistical analysis was performed with SPSS v. 24 (IBM). We used GraphPad Prism 8 to plot the trend of median survival time in different subgroups. We performed life table analysis, Kaplan Meier survival analysis, univariate and multivariate Cox proportional hazards analysis to analyze patient data.
Log rank (Mantel-Cox), Breslow (generalized Wilcoxon) and Tarone-Ware tests were used to compare the distribution of survival data between groups. To explore the factors influencing survival time (survival speed) and predict survival probability, we used univariate and multivariate Cox proportional hazard analysis using the backward Wald method. Taking the first group of each project as the comparison object, the confidence interval of the Hazard Ratio (HR) was 95%, the step probability of entering was 0.05, the step probability of going out was 0.10, and the maximum number of iterations was 20.
Results
The median survival time of all lung cancer patients diagnosed in 2017 (14.030 months) increased by 41.72% compared with 2000 (9.900 months). Table 1 describes the 3-, 5-, and 10-year survival rates, median survival time, and mean survival time according to the variables defined in the Methods section. Table 2 describes the chi square and P values of the three test methods of population comparison and pairwise comparison in Kaplan Meier survival analysis. Figure 2 shows the survival curves according to primary lung cancer site and patient demographics. Table 3 describes the univariate and multivariate Cox proportional HR, for which the first group of each item is taken as the comparison object.
Table 2.
Overall Comparison and Pairwise Comparison of Each Group in Kaplan–Meier Survival Analysis
| Comparison Type | Comparative Factor | Log Rank (Mantel-Cox) | Breslow (Generalized Wilcoxon) | Tarone-Ware | |||
|---|---|---|---|---|---|---|---|
| Chi Square | Sig. | Chi Square | Sig. | Chi Square | Sig. | ||
| Overall comparison | Sex | 3,119.588 | 0.000 | 2,577.577 | 0.000 | 2,934.977 | 0.000 |
| Age | 36,486.760 | 0.000 | 33,627.846 | 0.000 | 34,681.780 | 0.000 | |
| Race | 1,300.355 | 0.000 | 1,210.942 | 0.000 | 1,311.907 | 0.000 | |
| Origin recode NHIA | 10.506 | 0.001 | 0.624 | 0.429 | 0.323 | 0.570 | |
| Median household income inflation adj to 2019 | 3,119.588 | 0.000 | 2,577.577 | 0.000 | 2,934.977 | 0.000 | |
| Rural-Urban Continuum Code | 1,952.945 | 0.000 | 1,397.391 | 0.000 | 1,696.331 | 0.000 | |
| Primary site | 56,749.667 | 0.000 | 57,432.600 | 0.000 | 59,443.510 | 0.000 | |
| Histologic type (ICD-O-3) | 82,913.123 | 0.000 | 79,928.300 | 0.000 | 84,548.100 | 0.000 | |
| Year of diagnosis | 7,532.042 | 0.000 | 5,692.115 | 0.000 | 6,834.910 | 0.000 | |
| Summary stage | 240,670.146 | 0.000 | 219,186.158 | 0.000 | 239,656.003 | 0.000 | |
| Pairwise comparison | Sex | ||||||
| Female vs Male | 9,473.321 | 0.000 | 7,344.132 | 0.000 | 8,697.481 | 0.000 | |
| Age | |||||||
| < 45 years vs 45–54 years | 658.146 | 0.000 | 451.420 | 0.000 | 535.237 | 0.000 | |
| < 45 years vs 55–64 years | 1,186.997 | 0.000 | 755.752 | 0.000 | 891.847 | 0.000 | |
| < 45 years vs 65–74 years | 2,134.621 | 0.000 | 1,209.475 | 0.000 | 1,450.388 | 0.000 | |
| < 45 years vs 75–84 years | 5,280.355 | 0.000 | 3,281.758 | 0.000 | 3,847.412 | 0.000 | |
| < 45 years vs ≥ 85 years | 9,405.387 | 0.000 | 7,374.587 | 0.000 | 8,275.701 | 0.000 | |
| 45–54 years vs 55–64 years | 168.537 | 0.000 | 131.890 | 0.000 | 125.492 | 0.000 | |
| 45–54 years vs 65–74 years | 1,135.723 | 0.000 | 766.972 | 0.000 | 783.114 | 0.000 | |
| 45–54 years vs 75–84 years | 7,332.664 | 0.000 | 5,949.599 | 0.000 | 6,199.769 | 0.000 | |
| 45–54 years vs≥ 85 years | 16,522.957 | 0.000 | 16,185.395 | 0.000 | 16,484.200 | 0.000 | |
| 55–64 years vs 65–74 years | 734.202 | 0.000 | 495.365 | 0.000 | 520.272 | 0.000 | |
| 55–64 years vs 75–84 years | 9,978.407 | 0.000 | 8,503.476 | 0.000 | 8,906.353 | 0.000 | |
| 55–64 years vs≥ 85 years | 21,880.320 | 0.000 | 21,656.557 | 0.000 | 22,006.761 | 0.000 | |
| 65–74 years vs 75–84 years | 7,052.078 | 0.000 | 6,213.807 | 0.000 | 6,581.952 | 0.000 | |
| 65–74 years vs≥ 85 years | 19,673.925 | 0.000 | 19,042.105 | 0.000 | 19,640.948 | 0.000 | |
| 75–84 years vs ≥ 85 years | 6,915.700 | 0.000 | 6,358.064 | 0.000 | 6,757.770 | 0.000 | |
| Race | |||||||
| White vs Black | 278.487 | 0.000 | 106.505 | 0.000 | 189.853 | 0.000 | |
| White vs Asian and other races | 918.554 | 0.000 | 1,032.227 | 0.000 | 1,028.928 | 0.000 | |
| Black vs Asian and other races | 1,325.507 | 0.000 | 1,162.642 | 0.000 | 1,310.095 | 0.000 | |
| Origin recode NHIA | |||||||
| Non-Spanish-Hispanic-Latino vs Spanish-Hispanic-Latino | 10.506 | 0.001 | 0.624 | 0.429 | 0.323 | 0.570 | |
| Median household income inflation adj to 2019 | |||||||
| < $35,000 vs $35,000-$44,999 | 42.062 | 0.000 | 33.672 | 0.000 | 40.737 | 0.000 | |
| < $35,000 vs $45,000-$54,999 | 85.564 | 0.000 | 68.062 | 0.000 | 81.767 | 0.000 | |
| < $35,000 vs $55,000-$64,999 | 230.631 | 0.000 | 172.090 | 0.000 | 206.247 | 0.000 | |
| < $35,000 vs $65,000-$74,999 | 431.627 | 0.000 | 337.420 | 0.000 | 396.074 | 0.000 | |
| < $35,000 vs $75,000+ | 811.362 | 0.000 | 684.642 | 0.000 | 774.900 | 0.000 | |
| < $35,000 vs Others | 2.197 | 0.138 | 4.895 | 0.027 | 4.144 | 0.042 | |
| $35,000-$44,999 vs $45,000-$54,999 | 16.965 | 0.000 | 12.927 | 0.000 | 15.542 | 0.000 | |
| $35,000-$44,999 vs $55,000-$64,999 | 214.341 | 0.000 | 144.686 | 0.000 | 176.486 | 0.000 | |
| $35,000-$44,999 vs $65,000-$74,999 | 588.760 | 0.000 | 440.256 | 0.000 | 520.033 | 0.000 | |
| $35,000-$44,999 vs $75,000+ | 1,464.530 | 0.000 | 1,219.404 | 0.000 | 1,378.889 | 0.000 | |
| $35,000-$44,999 vs Others | 1.005 | 0.316 | 3.174 | 0.075 | 2.395 | 0.122 | |
| $45,000-$54,999 vs $55,000-$64,999 | 176.365 | 0.000 | 107.494 | 0.000 | 134.260 | 0.000 | |
| $45,000-$54,999 vs $65,000-$74,999 | 636.716 | 0.000 | 461.262 | 0.000 | 549.033 | 0.000 | |
| $45,000-$54,999 vs $75,000+ | 1,882.057 | 0.000 | 1,547.162 | 0.000 | 1,755.248 | 0.000 | |
| $45,000-$54,999 vs Others | 0.699 | 0.403 | 2.703 | 0.100 | 1.938 | 0.164 | |
| $55,000-$64,999 vs $65,000-$74,999 | 177.901 | 0.000 | 155.032 | 0.000 | 175.937 | 0.000 | |
| $55,000-$64,999 vs $75,000+ | 1,064.955 | 0.000 | 995.599 | 0.000 | 1,088.148 | 0.000 | |
| $55,000-$64,999 vs Others | 0.219 | 0.640 | 1.752 | 0.186 | 1.068 | 0.301 | |
| $65,000-$74,999 vs $75,000+ | 314.574 | 0.000 | 308.995 | 0.000 | 328.720 | 0.000 | |
| $65,000-$74,999 vs Others | 0.007 | 0.933 | 0.987 | 0.321 | 0.450 | 0.502 | |
| $75,000+ vs Others | 0.160 | 0.690 | 0.267 | 0.605 | 0.029 | 0.864 | |
| Rural-Urban Continuum Code | |||||||
| Counties in metropolitan areas greater than 1 million population vs Counties in metropolitan areas of 250,000 to 1 million population | 154.789 | 0.000 | 89.246 | 0.000 | 118.829 | 0.000 | |
| Counties in metropolitan areas greater than 1 million population vs Counties in metropolitan areas of less than 250 thousand population | 489.541 | 0.000 | 339.405 | 0.000 | 414.594 | 0.000 | |
| Counties in metropolitan areas greater than 1 million population vs Nonmetropolitan counties adjacent to a metropolitan area | 898.955 | 0.000 | 628.695 | 0.000 | 771.331 | 0.000 | |
| Counties in metropolitan areas greater than 1 million population vs Nonmetropolitan counties not adjacent to a metropolitan area | 1,002.371 | 0.000 | 758.392 | 0.000 | 899.192 | 0.000 | |
| Counties in metropolitan areas greater than 1 million population vs Others | 22.407 | 0.000 | 9.820 | 0.002 | 15.310 | 0.000 | |
| Counties in metropolitan areas of 250,000 to 1 million population vs Counties in metropolitan areas of less than 250 thousand population | 132.516 | 0.000 | 103.077 | 0.000 | 119.878 | 0.000 | |
| Counties in metropolitan areas of 250,000 to 1 million population vs Nonmetropolitan counties adjacent to a metropolitan area | 343.469 | 0.000 | 256.190 | 0.000 | 307.174 | 0.000 | |
| Counties in metropolitan areas of 250,000 to 1 million population vs Nonmetropolitan counties not adjacent to a metropolitan area | 471.405 | 0.000 | 381.350 | 0.000 | 441.650 | 0.000 | |
| Counties in metropolitan areas of 250,000 to 1 million population vs Others | 12.395 | 0.000 | 4.685 | 0.030 | 7.959 | 0.005 | |
| Counties in metropolitan areas of less than 250 thousand population vs Nonmetropolitan counties adjacent to a metropolitan area | 36.154 | 0.000 | 24.804 | 0.000 | 31.550 | 0.000 | |
| Counties in metropolitan areas of less than 250 thousand population vs Nonmetropolitan counties not adjacent to a metropolitan area | 102.205 | 0.000 | 84.179 | 0.000 | 98.169 | 0.000 | |
| Counties in metropolitan areas of less than 250 thousand population vs Others | 3.187 | 0.074 | 0.355 | 0.551 | 1.314 | 0.252 | |
| Nonmetropolitan counties adjacent to a metropolitan area vs Nonmetropolitan County not adjacent to a metropolitan area | 21.335 | 0.000 | 21.435 | 0.000 | 22.933 | 0.000 | |
| Nonmetropolitan counties adjacent to a metropolitan area vs Others | 0.529 | 0.467 | 0.090 | 0.764 | 0.022 | 0.882 | |
| Nonmetropolitan counties not adjacent to a metropolitan area vs Others | 0.031 | 0.860 | 1.436 | 0.231 | 0.617 | 0.432 | |
| Primary site | |||||||
| Right upper lobe vs Right middle lobe | 162.922 | 0.000 | 80.685 | 0.000 | 116.572 | 0.000 | |
| Right upper lobe vs Right lower lobe | 54.723 | 0.000 | 68.757 | 0.000 | 64.783 | 0.000 | |
| Right upper lobe vs Left upper lobe | 168.685 | 0.000 | 76.857 | 0.000 | 122.258 | 0.000 | |
| Right upper lobe vs Left lower lobe | 19.496 | 0.000 | 15.767 | 0.000 | 19.963 | 0.000 | |
| Right upper lobe vs Main bronchus | 10,216.526 | 0.000 | 10,257.262 | 0.000 | 10,769.433 | 0.000 | |
| Right upper lobe vs Over lapping lesion of lung | 595.338 | 0.000 | 829.226 | 0.000 | 770.682 | 0.000 | |
| Right upper lobe vs Lung, NOS | 37,093.583 | 0.000 | 36,323.045 | 0.000 | 38,235.231 | 0.000 | |
| Right upper lobe vs Others | 699.934 | 0.000 | 612.111 | 0.000 | 668.653 | 0.000 | |
| Right middle lobe vs Right lower lobe | 263.986 | 0.000 | 173.472 | 0.000 | 217.803 | 0.000 | |
| Right middle lobe vs Left upper lobe | 383.715 | 0.000 | 183.845 | 0.000 | 276.207 | 0.000 | |
| Right middle lobe vs Left lower lobe | 203.992 | 0.000 | 112.926 | 0.000 | 158.356 | 0.000 | |
| Right middle lobe vs Main bronchus | 6,517.198 | 0.000 | 5,704.728 | 0.000 | 6,423.992 | 0.000 | |
| Right middle lobe vs Over lapping lesion of lung | 779.740 | 0.000 | 877.108 | 0.000 | 882.311 | 0.000 | |
| Right middle lobe vs Lung, NOS | 13,575.417 | 0.000 | 11,318.686 | 0.000 | 13,058.521 | 0.000 | |
| Right middle lobe vs Others | 827.004 | 0.000 | 682.774 | 0.000 | 765.542 | 0.000 | |
| Right lower lobe vs Left upper lobe | 13.780 | 0.000 | 0.580 | 0.446 | 2.000 | 0.157 | |
| Right lower lobe vs Left lower lobe | 4.529 | 0.033 | 10.831 | 0.001 | 6.940 | 0.008 | |
| Right lower lobe vs Main bronchus | 7,741.953 | 0.000 | 7,473.661 | 0.000 | 8,027.688 | 0.000 | |
| Right lower lobe vs Over lapping lesion of lung | 446.655 | 0.000 | 621.232 | 0.000 | 581.078 | 0.000 | |
| Right lower lobe vs Lung, NOS | 23,928.595 | 0.000 | 22,385.509 | 0.000 | 24,235.716 | 0.000 | |
| Right lower lobe vs Others | 609.686 | 0.000 | 513.797 | 0.000 | 571.712 | 0.000 | |
| Left upper lobe vs Left lower lobe | 33.598 | 0.000 | 8.632 | 0.003 | 17.905 | 0.000 | |
| Left upper lobe vs Main bronchus | 8,554.529 | 0.000 | 8,966.598 | 0.000 | 9,271.170 | 0.000 | |
| Left upper lobe vs Over lapping lesion of lung | 419.865 | 0.000 | 684.012 | 0.000 | 599.494 | 0.000 | |
| Left upper lobe vs Lung, NOS | 30,093.214 | 0.000 | 30,515.243 | 0.000 | 31,760.602 | 0.000 | |
| Left upper lobe vs Others | 604.525 | 0.000 | 551.023 | 0.000 | 591.460 | 0.000 | |
| Left lower lobe vs Main bronchus | 7,605.617 | 0.000 | 7,473.549 | 0.000 | 7,956.885 | 0.000 | |
| Left lower lobe vs Over lapping lesion of lung | 487.337 | 0.000 | 692.059 | 0.000 | 636.294 | 0.000 | |
| Left lower lobe vs Lung, NOS | 21,655.850 | 0.000 | 20,479.488 | 0.000 | 22,098.264 | 0.000 | |
| Left lower lobe vs Others | 639.715 | 0.000 | 558.856 | 0.000 | 611.053 | 0.000 | |
| Main bronchus vs Over lapping lesion of lung | 574.941 | 0.000 | 374.213 | 0.000 | 491.592 | 0.000 | |
| Main bronchus vs Lung, NOS | 482.626 | 0.000 | 590.283 | 0.000 | 555.427 | 0.000 | |
| Main bronchus vs Others | 1.595 | 0.207 | 8.097 | 0.004 | 5.825 | 0.016 | |
| Over lapping lesion of lung vs Lung, NOS | 1,416.129 | 0.000 | 1,047.110 | 0.000 | 1,270.005 | 0.000 | |
| Over lapping lesion of lung vs Others | 122.072 | 0.000 | 55.546 | 0.000 | 83.040 | 0.000 | |
| Lung, NOS vs Others | 52.256 | 0.000 | 91.285 | 0.000 | 79.609 | 0.000 | |
| Right upper lobe vs Other single lobes | 71.499 | 0.000 | 53.158 | 0.000 | 66.958 | 0.000 | |
| Right middle lobe vs Other single lobes | 284.671 | 0.000 | 149.788 | 0.000 | 212.357 | 0.000 | |
| Right lower lobe vs Other single lobes | 21.601 | 0.000 | 43.991 | 0.000 | 32.919 | 0.000 | |
| Left upper lobe vs Other single lobes | 174.418 | 0.000 | 57.640 | 0.000 | 108.889 | 0.000 | |
| Left lower lobe vs Other single lobes | 0.963 | 0.326 | 1.070 | 0.301 | 1.432 | 0.231 | |
| Main bronchus vs Single lobes | 11,026.346 | 0.000 | 11,283.718 | 0.000 | 11,727.875 | 0.000 | |
| Over lapping lesion of lung vs Single lobes | 547.887 | 0.000 | 786.542 | 0.000 | 722.280 | 0.000 | |
| Lung, NOS vs Single lobes | 50,039.338 | 0.000 | 51,295.080 | 0.000 | 52,602.027 | 0.000 | |
| Histologic type (ICD-O-3) | |||||||
| Neoplasia, NAS vs Epithelial neoplasms, NAS | 3,757.214 | 0.000 | 7,408.176 | 0.000 | 5,986.605 | 0.000 | |
| Neoplasia, NAS vs Squamous cell neoplasms | 20,217.196 | 0.000 | 27,516.738 | 0.000 | 25,146.774 | 0.000 | |
| Neoplasia, NAS vs Adenoma and adenocarcinomas | 35,445.959 | 0.000 | 40,577.637 | 0.000 | 39,259.802 | 0.000 | |
| Neoplasia, NAS vs Cystic, mucinous and serous neoplasms | 6,149.905 | 0.000 | 6,029.979 | 0.000 | 6,450.057 | 0.000 | |
| Neoplasia, NAS vs Acinous cell neoplasm | 11,950.640 | 0.000 | 10,107.204 | 0.000 | 11,760.036 | 0.000 | |
| Neoplasia, NAS vs Complex epithelial neoplasms | 4,939.508 | 0.000 | 5,491.534 | 0.000 | 5,632.441 | 0.000 | |
| Neoplasia, NAS vs Others | 1,961.725 | 0.000 | 1,547.515 | 0.000 | 1,799.985 | 0.000 | |
| Epithelial neoplasms, NAS vs Squamous cell neoplasms | 18,051.629 | 0.000 | 18,642.122 | 0.000 | 19,723.395 | 0.000 | |
| Epithelial neoplasms, NAS vs Adenoma and adenocarcinomas | 52,956.139 | 0.000 | 43,604.909 | 0.000 | 49,887.906 | 0.000 | |
| Epithelial neoplasms, NAS vs Cystic, mucinous and serous neoplasms | 3,920.902 | 0.000 | 2,832.710 | 0.000 | 3,530.938 | 0.000 | |
| Epithelial neoplasms, NAS vs Acinous cell neoplasm | 9,902.939 | 0.000 | 8,306.628 | 0.000 | 9,832.557 | 0.000 | |
| Epithelial neoplasms, NAS vs Complex epithelial neoplasms | 2,880.374 | 0.000 | 2,512.595 | 0.000 | 2,904.016 | 0.000 | |
| Neoplasia, NAS vs Complex epithelial neoplasms | 4,939.508 | 0.000 | 5,491.534 | 0.000 | 5,632.441 | 0.000 | |
| Epithelial neoplasms, NAS vs Others | 1,183.182 | 0.000 | 575.683 | 0.000 | 841.398 | 0.000 | |
| Squamous cell neoplasms vs Adenoma and adenocarcinomas | 4,324.050 | 0.000 | 2,002.280 | 0.000 | 2,974.305 | 0.000 | |
| Squamous cell neoplasms vs Cystic, mucinous and serous neoplasms | 526.311 | 0.000 | 232.401 | 0.000 | 355.225 | 0.000 | |
| Squamous cell neoplasms vs Acinous cell neoplasm | 5,171.203 | 0.000 | 4,982.770 | 0.000 | 5,383.737 | 0.000 | |
| Squamous cell neoplasms vs Complex epithelial neoplasms | 246.850 | 0.000 | 171.523 | 0.000 | 210.048 | 0.000 | |
| Squamous cell neoplasms vs Others | 211.562 | 0.000 | 15.811 | 0.000 | 64.762 | 0.000 | |
| Adenoma and adenocarcinomas vs Cystic, mucinous and serous neoplasms | 9.479 | 0.002 | 4.511 | 0.034 | 6.611 | 0.010 | |
| Adenoma and adenocarcinomas vs Acinous cell neoplasm | 3,429.157 | 0.000 | 3,748.082 | 0.000 | 3,805.019 | 0.000 | |
| Adenoma and adenocarcinomas vs Complex epithelial neoplasms | 14.960 | 0.000 | 0.000 | 0.991 | 2.697 | 0.101 | |
| Adenoma and adenocarcinomas vs Others | 10.571 | 0.001 | 7.914 | 0.005 | 0.424 | 0.515 | |
| Cystic, mucinous and serous neoplasms vs Acinous cell neoplasm | 2,508.321 | 0.000 | 3,023.062 | 0.000 | 2,957.332 | 0.000 | |
| Cystic, mucinous and serous neoplasms vs Complex epithelial neoplasms | 24.420 | 0.000 | 2.074 | 0.150 | 8.732 | 0.003 | |
| Cystic, mucinous and serous neoplasms vs Others | 0.961 | 0.327 | 12.862 | 0.000 | 3.988 | 0.046 | |
| Acinous cell neoplasm vs Complex epithelial neoplasms | 2,915.827 | 0.000 | 3,292.872 | 0.000 | 3,306.154 | 0.000 | |
| Acinous cell neoplasm vs Others | 1,432.131 | 0.000 | 2,382.199 | 0.000 | 2,110.827 | 0.000 | |
| Complex epithelial neoplasms vs Others | 20.792 | 0.000 | 6.543 | 0.011 | 0.027 | 0.871 | |
| Summary stage | |||||||
| Distant vs Localized | 189,234.197 | 0.000 | 157,689.763 | 0.000 | 180,494.909 | 0.000 | |
| Distant vs Regional | 95,182.979 | 0.000 | 89,552.783 | 0.000 | 97,474.996 | 0.000 | |
| Distant vs Unknown/unstaged | 2,281.394 | 0.000 | 1,319.592 | 0.000 | 1,873.692 | 0.000 | |
| Localized vs Regional | 22,646.800 | 0.000 | 24,779.500 | 0.000 | 25,260.467 | 0.000 | |
| Localized vs Unknown/unstaged | 54,328.909 | 0.000 | 60,221.013 | 0.000 | 59,752.077 | 0.000 | |
| Regional vs Unknown/unstaged | 15,840.215 | 0.000 | 19,402.780 | 0.000 | 18,494.477 | 0.000 | |
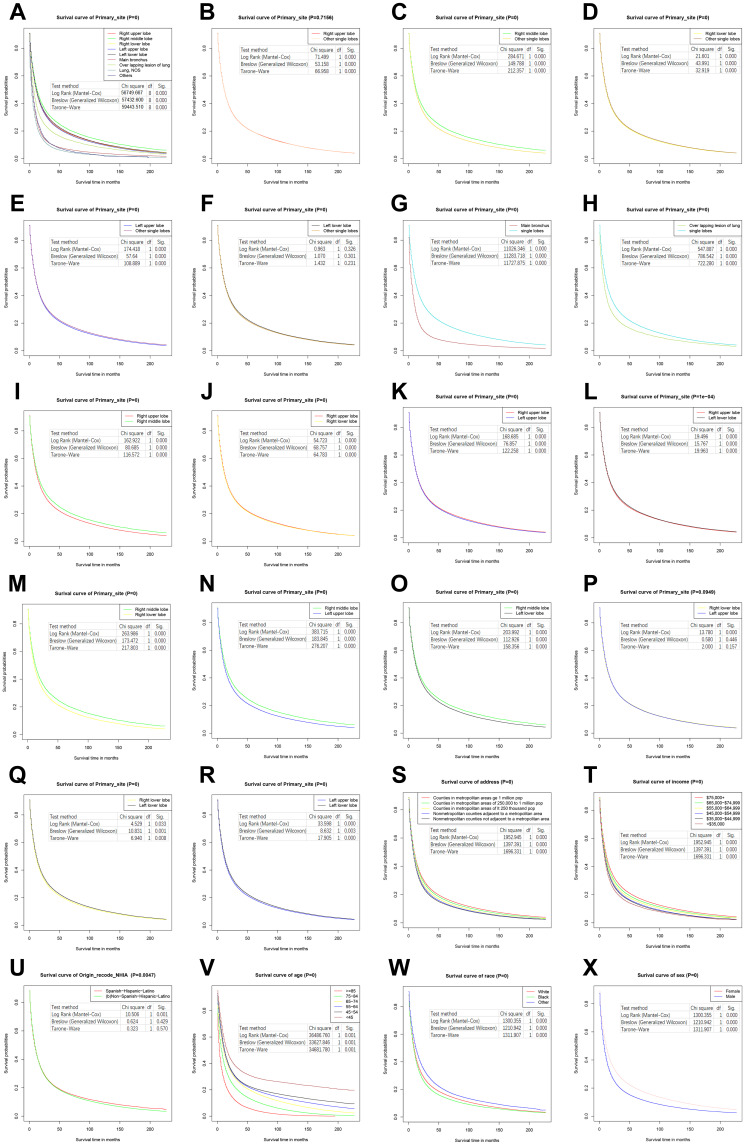
Figure 2.
Kaplan–Meier survival curves of lung cancer patients according to primary site and demographics. (A) All primary site groups. (B) Right upper lobe. (C) Right middle lobe. (D) Right lower lobe. (E) Left upper lobe. (F) Left lower lobe. (G) Main bronchus. (H) Overlapping lesion of lung. (I) Right upper lobe and right middle lobe. (J) Right upper lobe and right lower lobe. (K) Right upper lobe and left upper lobe. (L) Right upper lobe and left lower lobe. (M) Middle lobe and right lower lobe. (N) Right middle lobe and left upper lobe. (O) Middle lobe and left lower lobe. (P) Right lower lobe and left upper lobe. (Q) Right lower lobe and left lower lobe. (R) Left upper lobe and left lower lobe. (S) Address groups. (T) Income groups. (U) Ethnic groups. (V) Age groups. (W) Race groups. (X) Sex groups.
Table 3.
Univariate and Multivariate Cox Proportional Hazards Analysis of Lung Cancer Based on SEER Database
| Types of Cox Analysis | Comparing Factors | B | SE | Wald | df | Sig. | HR | 95.0% HR, CI | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Univariate | Primary site | 51,359.75 | 8 | 0.000 | |||||
| Right middle lobe vs Right upper lobe | −0.075 | 0.006 | 152.810 | 1 | 0.000 | 0.927 | 0.916 | 0.939 | |
| Right lower lobe vs Right upper lobe | 0.027 | 0.004 | 49.306 | 1 | 0.000 | 1.027 | 1.019 | 1.035 | |
| Left upper lobe vs Right upper lobe | 0.041 | 0.003 | 151.521 | 1 | 0.000 | 1.042 | 1.035 | 1.048 | |
| Left lower lobe vs Right upper lobe | 0.017 | 0.004 | 16.670 | 1 | 0.000 | 1.017 | 1.009 | 1.025 | |
| Main bronchus vs Right upper lobe | 0.528 | 0.005 | 9,373.596 | 1 | 0.000 | 1.696 | 1.678 | 1.714 | |
| Over lapping lesion of lung vs Right upper lobe | 0.247 | 0.010 | 557.362 | 1 | 0.000 | 1.280 | 1.254 | 1.306 | |
| Lung, NOS vs Right upper lobe | 0.654 | 0.004 | 34,847.930 | 1 | 0.000 | 1.923 | 1.910 | 1.937 | |
| Others (a) vs Right upper lobe | 0.511 | 0.020 | 626.507 | 1 | 0.000 | 1.666 | 1.601 | 1.734 | |
| Sex | |||||||||
| Male vs Female | 0.215 | 0.002 | 8,886.971 | 1 | 0.000 | 1.240 | 1.234 | 1.245 | |
| Age | 33,386.410 | 5 | 0.000 | ||||||
| 45–54 years vs < 45 years | 0.277 | 0.010 | 724.156 | 1 | 0.000 | 1.319 | 1.293 | 1.346 | |
| 55–64 years vs < 45 years | 0.343 | 0.010 | 1,225.613 | 1 | 0.000 | 1.409 | 1.382 | 1.436 | |
| 65–74 years vs < 45 years | 0.426 | 0.010 | 1,941.140 | 1 | 0.000 | 1.532 | 1.503 | 1.561 | |
| 75–84 years vs < 45 years | 0.658 | 0.010 | 4,590.031 | 1 | 0.000 | 1.931 | 1.894 | 1.968 | |
| 85+ years vs < 45 years | 1.001 | 0.010 | 9,588.206 | 1 | 0.000 | 2.722 | 2.668 | 2.777 | |
| Race | 1,222.947 | 2 | 0.000 | ||||||
| Black vs White | 0.059 | 0.004 | 263.022 | 1 | 0.000 | 1.061 | 1.053 | 1.068 | |
| Others (b) vs White | −0.144 | 0.005 | 867.837 | 1 | 0.000 | 0.865 | 0.857 | 0.874 | |
| Origin recode NHIA | |||||||||
| Non-Spanish-Hispanic-Latino vs Spanish-Hispanic-Latino | 0.016 | 0.005 | 9.888 | 1 | 0.002 | 1.016 | 1.006 | 1.027 | |
| Median household income inflation adj to 2019 | 2,931.738 | 6 | 0.000 | ||||||
| $35,000–$44,999 vs <$35,000 | −0.054 | 0.009 | 38.994 | 1 | 0.000 | 0.948 | 0.932 | 0.964 | |
| $45,000–$54,999 vs <$35,000 | −0.072 | 0.008 | 78.900 | 1 | 0.000 | 0.930 | 0.915 | 0.945 | |
| $55,000–$64,999 vs <$35,000 | −0.119 | 0.008 | 220.252 | 1 | 0.000 | 0.888 | 0.874 | 0.902 | |
| $65,000–$74,999 vs <$35,000 | −0.163 | 0.008 | 407.928 | 1 | 0.000 | 0.850 | 0.836 | 0.863 | |
| $75,000+ vs <$35,000 | −0.219 | 0.008 | 756.831 | 1 | 0.000 | 0.803 | 0.790 | 0.816 | |
| Others (c) vs <$35,000 | −0.173 | 0.123 | 1.967 | 1 | 0.161 | 0.841 | 0.661 | 1.071 | |
| Rural-Urban Continuum | 1,835.107 | 5 | 0.000 | ||||||
| Counties in metropolitan areas of 250,000 to 1 million pop vs Counties in metropolitan areas of greater than 1 million pop | 0.036 | 0.003 | 146.527 | 1 | 0.000 | 1.037 | 1.031 | 1.043 | |
| Counties in metropolitan areas of less than 250 thousand pop vs Counties in metropolitan areas of greater than 1 million pop | 0.087 | 0.004 | 462.909 | 1 | 0.000 | 1.091 | 1.083 | 1.100 | |
| Nonmetropolitan counties adjacent to a metropolitan area vs Counties in metropolitan areas of greater than 1 million pop | 0.118 | 0.004 | 848.592 | 1 | 0.000 | 1.126 | 1.117 | 1.135 | |
| Nonmetropolitan counties not adjacent to a metropolitan vs Counties in metropolitan areas of greater than 1 million pop | 0.144 | 0.005 | 946.941 | 1 | 0.000 | 1.155 | 1.144 | 1.166 | |
| Others (d) vs Counties in metropolitan areas of greater than 1 million pop | 0.139 | 0.030 | 21.202 | 1 | 0.000 | 1.149 | 1.083 | 1.220 | |
| Histologic type (ICD-O-3) | 74,262.360 | 7 | 0.000 | ||||||
| Epithelial neoplasms, NAS vs Neoplasia, NAS | −0.296 | 0.005 | 3,638.861 | 1 | 0.000 | 0.744 | 0.737 | 0.751 | |
| Squamous cell neoplasms vs Neoplasia, NAS | −0.700 | 0.005 | 18,147.780 | 1 | 0.000 | 0.497 | 0.492 | 0.502 | |
| Adenoma and adenocarcinomas vs Neoplasia, NAS | −0.900 | 0.005 | 33,303.950 | 1 | 0.000 | 0.406 | 0.403 | 0.410 | |
| Cystic, mucinous and serous neoplasms vs Neoplasia, NAS | −0.933 | 0.011 | 6,687.317 | 1 | 0.000 | 0.393 | 0.385 | 0.402 | |
| Acinous cell neoplasm vs Neoplasia, NAS | −2.005 | 0.021 | 9,539.132 | 1 | 0.000 | 0.135 | 0.129 | 0.140 | |
| Complex epithelial neoplasms vs Neoplasia, NAS | −0.859 | 0.012 | 5,433.430 | 1 | 0.000 | 0.424 | 0.414 | 0.433 | |
| Others (e) vs Neoplasia, NAS | −0.962 | 0.020 | 2,265.927 | 1 | 0.000 | 0.382 | 0.367 | 0.398 | |
| Year of diagnosis | 7,025.014 | 17 | 0.000 | ||||||
| 2001 vs 2000 | −0.006 | 0.007 | 0.709 | 1 | 0.400 | 0.994 | 0.982 | 1.007 | |
| 2002 vs 2000 | −0.006 | 0.007 | 0.912 | 1 | 0.339 | 0.994 | 0.981 | 1.007 | |
| 2003 vs 2000 | −0.022 | 0.007 | 11.283 | 1 | 0.001 | 0.978 | 0.966 | 0.991 | |
| 2004 vs 2000 | −0.038 | 0.007 | 33.250 | 1 | 0.000 | 0.963 | 0.951 | 0.975 | |
| 2005 vs 2000 | −0.054 | 0.007 | 68.099 | 1 | 0.000 | 0.947 | 0.935 | 0.960 | |
| 2006 vs 2000 | −0.077 | 0.007 | 136.769 | 1 | 0.000 | 0.926 | 0.914 | 0.938 | |
| 2007 vs 2000 | −0.088 | 0.007 | 181.709 | 1 | 0.000 | 0.915 | 0.904 | 0.927 | |
| 2008 vs 2000 | −0.104 | 0.007 | 252.221 | 1 | 0.000 | 0.901 | 0.889 | 0.913 | |
| 2009 vs 2000 | −0.117 | 0.007 | 315.202 | 1 | 0.000 | 0.890 | 0.878 | 0.901 | |
| 2010 vs 2000 | −0.131 | 0.007 | 388.059 | 1 | 0.000 | 0.877 | 0.866 | 0.889 | |
| 2011 vs 2000 | −0.162 | 0.007 | 586.987 | 1 | 0.000 | 0.850 | 0.839 | 0.861 | |
| 2012 vs 2000 | −0.156 | 0.007 | 540.690 | 1 | 0.000 | 0.855 | 0.844 | 0.867 | |
| 2013 vs 2000 | −0.182 | 0.007 | 724.595 | 1 | 0.000 | 0.833 | 0.822 | 0.844 | |
| 2014 vs 2000 | −0.220 | 0.007 | 1,029.316 | 1 | 0.000 | 0.803 | 0.792 | 0.814 | |
| 2015 vs 2000 | −0.271 | 0.007 | 1,512.721 | 1 | 0.000 | 0.762 | 0.752 | 0.773 | |
| 2016 vs 2000 | −0.311 | 0.007 | 1,876.193 | 1 | 0.000 | 0.733 | 0.722 | 0.743 | |
| 2017 vs 2000 | −0.348 | 0.008 | 2,105.528 | 1 | 0.000 | 0.706 | 0.696 | 0.717 | |
| Summary stage | 205,903.400 | 3 | 0 | ||||||
| Localized vs Distant | −1.426 | 0.004 | 165,939.800 | 1 | 0 | 0.240 | 0.239 | 0.242 | |
| Regional vs Distant | −0.884 | 0.003 | 90,444.560 | 1 | 0 | 0.413 | 0.411 | 0.415 | |
| Unknown/unstaged vs Distant | −0.256 | 0.005 | 2,733.579 | 1 | 0 | 0.774 | 0.767 | 0.782 | |
| Multivariate | Primary Site | 30,355.820 | 8 | 0.000 | |||||
| Right middle lobe vs Right upper lobe | −0.045 | 0.006 | 55.071 | 1 | 0.000 | 0.956 | 0.944 | 0.967 | |
| Right lower lobe vs Right upper lobe | 0.038 | 0.004 | 100.117 | 1 | 0.000 | 1.039 | 1.031 | 1.047 | |
| Left upper lobe vs Right upper lobe | 0.028 | 0.003 | 71.195 | 1 | 0.000 | 1.028 | 1.022 | 1.035 | |
| Left lower lobe vs Right upper lobe | 0.031 | 0.004 | 55.730 | 1 | 0.000 | 1.031 | 1.023 | 1.040 | |
| Main bronchus vs Right upper lobe | 0.446 | 0.005 | 6,583.468 | 1 | 0.000 | 1.562 | 1.545 | 1.579 | |
| Over lapping lesion of lung vs Right upper lobe | 0.257 | 0.010 | 603.009 | 1 | 0.000 | 1.293 | 1.267 | 1.320 | |
| Lung, NOS vs Right upper lobe | 0.519 | 0.004 | 20,830.791 | 1 | 0.000 | 1.681 | 1.669 | 1.693 | |
| Others (f) vs Right upper lobe | 0.424 | 0.020 | 431.055 | 1 | 0.000 | 1.528 | 1.468 | 1.590 | |
| Sex | |||||||||
| Male vs Female | 0.197 | 0.002 | 7,283.054 | 1 | 0.000 | 1.217 | 1.212 | 1.223 | |
| Age | 29,870.587 | 5 | 0.000 | ||||||
| 45–54 years vs < 45 years | 0.261 | 0.010 | 639.066 | 1 | 0.000 | 1.299 | 1.272 | 1.325 | |
| 55–64 years vs < 45 years | 0.348 | 0.010 | 1,254.460 | 1 | 0.000 | 1.417 | 1.389 | 1.444 | |
| 65–74 years vs < 45 years | 0.459 | 0.010 | 2,223.302 | 1 | 0.000 | 1.582 | 1.552 | 1.613 | |
| 75–84 years vs < 45 years | 0.688 | 0.010 | 4,960.363 | 1 | 0.000 | 1.990 | 1.952 | 2.029 | |
| 85+ years vs < 45 years | 0.960 | 0.010 | 8,635.988 | 1 | 0.000 | 2.611 | 2.559 | 2.665 | |
| Race | 849.583 | 2 | 0.000 | ||||||
| Black vs White | 0.095 | 0.004 | 642.820 | 1 | 0.000 | 1.099 | 1.091 | 1.108 | |
| Others (g) vs White | −0.062 | 0.005 | 153.648 | 1 | 0.000 | 0.939 | 0.930 | 0.949 | |
| Origin recode NHIA | |||||||||
| Non-Spanish-Hispanic-Latino vs Spanish-Hispanic-Latino | 0.018 | 0.005 | 12.181 | 1 | 0.000 | 1.018 | 1.008 | 1.029 | |
| Median household income inflation adj to 2019 | 1,279.382 | 6 | 0.000 | ||||||
| $35,000–$44,999 vs <$35,000 | −0.055 | 0.009 | 37.947 | 1 | 0.000 | 0.946 | 0.930 | 0.963 | |
| $45,000–$54,999 vs <$35,000 | −0.082 | 0.009 | 87.256 | 1 | 0.000 | 0.921 | 0.905 | 0.937 | |
| $55,000–$64,999 vs <$35,000 | −0.126 | 0.009 | 194.220 | 1 | 0.000 | 0.881 | 0.866 | 0.897 | |
| $65,000–$74,999 vs <$35,000 | −0.165 | 0.009 | 316.049 | 1 | 0.000 | 0.848 | 0.833 | 0.864 | |
| $75,000+ vs <$35,000 | −0.203 | 0.009 | 479.380 | 1 | 0.000 | 0.816 | 0.802 | 0.831 | |
| Others (h) vs <$35,000 | −0.773 | 0.127 | 36.796 | 1 | 0.000 | 0.462 | 0.360 | 0.593 | |
| Rural-Urban Continuum | 83.075 | 5 | 0.000 | ||||||
| Counties in metropolitan areas of 250,000 to 1 million pop vs Counties in metropolitan areas of greater than 1 million pop | 0.013 | 0.003 | 19.031 | 1 | 0.000 | 1.014 | 1.007 | 1.020 | |
| Counties in metropolitan areas of less than 250 thousand pop vs Counties in metropolitan areas of greater than 1 million pop | 0.001 | 0.004 | 0.068 | 1 | 0.795 | 1.001 | 0.992 | 1.010 | |
| Nonmetropolitan counties adjacent to a metropolitan area vs Counties in metropolitan areas of greater than 1 million pop | −0.005 | 0.005 | 0.897 | 1 | 0.344 | 0.995 | 0.986 | 1.005 | |
| Nonmetropolitan counties not adjacent to a metropolitan vs Counties in metropolitan areas of greater than 1 million pop | 0.008 | 0.006 | 1.923 | 1 | 0.166 | 1.008 | 0.997 | 1.020 | |
| Others (i) vs Counties in metropolitan areas of greater than 1 million pop | 0.243 | 0.032 | 59.247 | 1 | 0.000 | 1.275 | 1.199 | 1.357 | |
| Histologic type ICDO3 | 42,139.807 | 7 | 0.000 | ||||||
| Epithelial neoplasms, NAS vs Neoplasia, NAS | −0.053 | 0.005 | 106.351 | 1 | 0.000 | 0.949 | 0.939 | 0.958 | |
| Squamous cell neoplasms vs Neoplasia, NAS | −0.428 | 0.005 | 6,132.065 | 1 | 0.000 | 0.652 | 0.645 | 0.659 | |
| Adenoma and adenocarcinomas vs Neoplasia, NAS | −0.557 | 0.005 | 11,534.349 | 1 | 0.000 | 0.573 | 0.567 | 0.579 | |
| Cystic, mucinous and serous neoplasms vs Neoplasia, NAS | −0.578 | 0.012 | 2,506.946 | 1 | 0.000 | 0.561 | 0.549 | 0.574 | |
| Acinous cell neoplasm vs Neoplasia, NAS | −1.553 | 0.021 | 5,656.729 | 1 | 0.000 | 0.212 | 0.203 | 0.220 | |
| Complex epithelial neoplasms vs Neoplasia, NAS | −0.533 | 0.012 | 2,045.116 | 1 | 0.000 | 0.587 | 0.574 | 0.601 | |
| Others (j) vs Neoplasia, NAS | −0.619 | 0.020 | 928.675 | 1 | 0.000 | 0.539 | 0.518 | 0.560 | |
| Year of diagnosis | 2,414.036 | 17 | 0.000 | ||||||
| 2001 vs 2000 | −0.010 | 0.007 | 2.319 | 1 | 0.128 | 0.990 | 0.977 | 1.003 | |
| 2002 vs 2000 | −0.014 | 0.007 | 4.348 | 1 | 0.037 | 0.986 | 0.974 | 0.999 | |
| 2003 vs 2000 | −0.034 | 0.007 | 27.497 | 1 | 0.000 | 0.966 | 0.954 | 0.979 | |
| 2004 vs 2000 | −0.050 | 0.007 | 58.507 | 1 | 0.000 | 0.951 | 0.939 | 0.963 | |
| 2005 vs 2000 | −0.061 | 0.007 | 86.634 | 1 | 0.000 | 0.941 | 0.929 | 0.953 | |
| 2006 vs 2000 | −0.079 | 0.007 | 142.858 | 1 | 0.000 | 0.924 | 0.913 | 0.936 | |
| 2007 vs 2000 | −0.083 | 0.007 | 159.809 | 1 | 0.000 | 0.920 | 0.908 | 0.932 | |
| 2008 vs 2000 | −0.092 | 0.007 | 193.962 | 1 | 0.000 | 0.912 | 0.900 | 0.924 | |
| 2009 vs 2000 | −0.099 | 0.007 | 223.976 | 1 | 0.000 | 0.906 | 0.894 | 0.918 | |
| 2010 vs 2000 | −0.100 | 0.007 | 224.238 | 1 | 0.000 | 0.905 | 0.893 | 0.917 | |
| 2011 vs 2000 | −0.110 | 0.007 | 267.803 | 1 | 0.000 | 0.896 | 0.884 | 0.907 | |
| 2012 vs 2000 | −0.100 | 0.007 | 218.197 | 1 | 0.000 | 0.905 | 0.893 | 0.917 | |
| 2013 vs 2000 | −0.115 | 0.007 | 283.473 | 1 | 0.000 | 0.891 | 0.880 | 0.903 | |
| 2014 vs 2000 | −0.141 | 0.007 | 418.512 | 1 | 0.000 | 0.868 | 0.857 | 0.880 | |
| 2015 vs 2000 | −0.176 | 0.007 | 631.345 | 1 | 0.000 | 0.838 | 0.827 | 0.850 | |
| 2016 vs 2000 | −0.200 | 0.007 | 762.693 | 1 | 0.000 | 0.819 | 0.808 | 0.831 | |
| 2017 vs 2000 | −0.231 | 0.008 | 918.224 | 1 | 0.000 | 0.794 | 0.782 | 0.806 | |
| Summary stage | 184,737.000 | 3 | 0.000 | ||||||
| Localized vs Distant | −1.385 | 0.004 | 149,948.100 | 1 | 0.000 | 0.250 | 0.248 | 0.252 | |
| Regional vs Distant | −0.854 | 0.003 | 82,171.650 | 1 | 0.000 | 0.426 | 0.423 | 0.428 | |
| Unknown/unstaged vs Distant | −0.533 | 0.005 | 11,191.940 | 1 | 0.000 | 0.587 | 0.581 | 0.593 | |
Notes: (a) Others included Only one side - side unspecified, Bilateral, single primary and Paired site, but no information concerning laterality. (b) Others included Asian or Pacific Islander and American Indian/Alaska Native. (c) Others included unknown/missing/no match/Not 1990–2018. (d) Others included Unknown/missing/no match (Alaska or Hawaii - Entire State) and Unknown/missing/no match/Not 1990–2018. (e) Others included all the histological types of lung cancer except the above seven types. (f) Others included Only one side - side unspecified, Bilateral, single primary and Paired site, but no information concerning laterality. (g) Others included Asian or Pacific Islander and American Indian/Alaska Native (h) Others included unknown/missing/no match/Not 1990–2018 (i) Others included Unknown/missing/no match (Alaska or Hawaii - Entire State) and Unknown/missing/no match/Not 1990–2018. (j) Others included all the histological types of lung cancer except the above seven types.
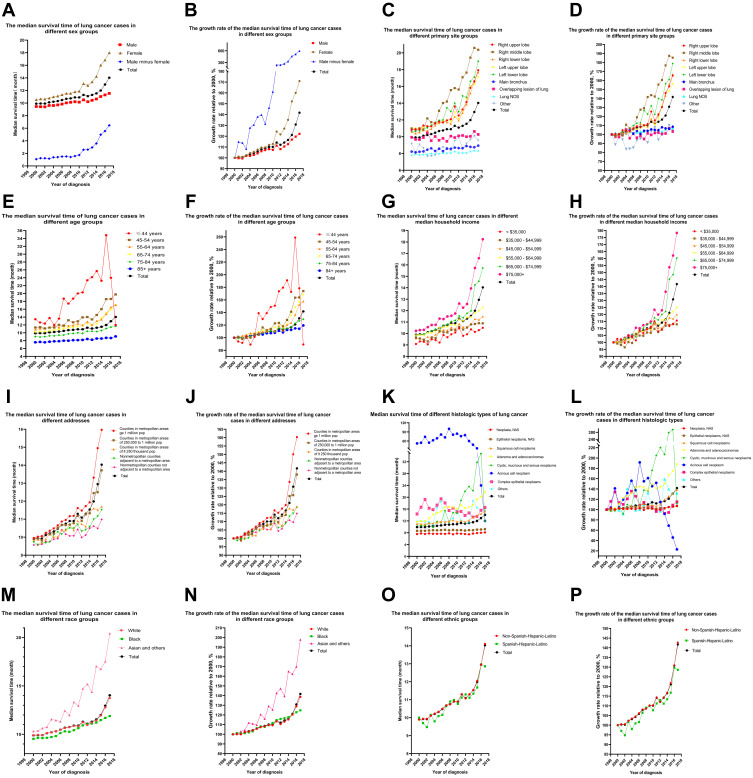
Sex
The median survival time of female patients increased faster than in males (Figure 1A and B). The median survival time of female patients diagnosed in 2017 (18.000 months, Table S2) increased by 70.94% (Table S3) compared with 2000 (10.530 months, Table S1). In males, the trend was similar although the increase was smaller: from 9.450 months in 2000 to 11.540 months in 2017 (Table S2), an increase of 22.12% (Table S3). Surprisingly, the difference in median survival time between female and male patients increased from 1.08 months to 6.46 months (Table S2). The 3-, 5-, and 10-year survival rates for female patients were 23%, 18%, and 10%, respectively; and for male patients these were 16%, 12%, and 6%, respectively (Table 1). The log rank (Mantel-Cox) for the overall comparison of females with males was 3,119.588, Breslow (generalized Wilcoxon) was 2,577.577, and Tarone-Ware was 2934.977. For the pairwise comparison, the log rank (Mantel-Cox) was 9,473.321, Breslow (generalized Wilcoxon) was 7,344.132, and Tarone-Ware was 8697.481. All had P < 0.001 (Table 2). The HR for univariate Cox analyses of male:female was 1.240 (1.234–1.246), P < 0.001. The HR for multivariate Cox analyses of male:female was 1.217 (1.212–1.223), P < 0.001 (Table 3).
Figure 1.
Median survival time. Survival and its growth rate in lung cancer patients from 2000 to 2017. (A and B) According to sex. (C and D) According to primary site. (E and F) According to age. (G and H) According to median household income (inflation adjusted to 2019). (I and J) According to address. (K and L) According to histologic type. (M and N) According to race. (O and P) According to ethnic group.
Primary Site
The median survival time when the primary site was in one lobe was greater than in patients whose primary site was in the main bronchus with an overlapping lesion in the lung (Figure 1C and D). Patients diagnosed in 2017 with a primary site in the right middle lobe had the longest median survival time (20.370 months), then in the left lower lobe (19.000 months), right upper lobe (17.930 months), right lower lobe (17.690 months), and left upper lobe (17.120 months, Table S4). The survival time of patients with single-lobe cancer increased markedly since 2000: the number of patients diagnosed in 2017 at the primary site of the right middle lobe, left lower lobe, right lower lobe, right upper lobe, and left upper lobe increased by 85.35%, 78.91%, 66.89, 65.56%, and 55.92%, respectively (Table S5). The specific values of the 3-year, 5-year, and 10-year survival rates of lung cancer patients of different primary sites were shown in Table 1.
The log rank (Mantel-Cox) for the overall comparison was 56,749.667, the Breslow (generalized Wilcoxon) was 57,432.600, and Tarone-Ware was 59,443.510, with P < 0.001 for all (Table 2). The specific values of univariate and multivariate hazard ratios (HRs) for different primary sites were shown in Table 3.
Age
Younger patients had a longer median survival time (Figure 1E and F), but survival time was almost equal between the 55–64 and 65–74 age groups, and the changes were synchronous (Tables S6 and S7). The sudden decrease in the median survival time of patients with lung cancer diagnosed in 2016 and 2017 in the group less than or equal to 44 years may be related to the small number of patients at onset and cannot be counted in the changing trend that the median survival time of patients with lung cancer diagnosed in the group less than or equal to 44 years increased overall. The specific values of the 3-year, 5-year, and 10-year survival rates of lung cancer patients of different ages were shown in Table 1. The log rank (Mantel-Cox) for the overall comparison was 36,486.760, the Breslow (generalized Wilcoxon) was 33,627.846, and Tarone-Ware was 34,681.780, P < 0.001 for all (Table 2). The specific values of univariate and multivariate hazard ratios (HRs) for different ages were shown in Table 3.
Median Household Income
A longer median survival time was seen in patients with higher incomes (Figure 1G and H) and Table S8). The median survival time of the $75,000 + group (18.24 months) was 7.740 months longer than the $35,000 group (10.500 months). The median survival times of the $35,000–$44,999, $45,000–$54,999, $55,000–$64,999 and $65,000–$74,999 groups were 10.890 months, 11.490 months, 12.260 months, and 15.730 months, respectively. The fastest increase in median survival time was 78.30% and 60.35% for the $75,000+ and $65,000–$74,999 groups, respectively (Table S9). The specific values of the 3-year, 5-year, and 10-year survival rates of lung cancer patients of different incomes were shown in Table 1. The log rank (Mantel-Cox) for the overall comparison was 3,119.588, the Breslow (generalized Wilcoxon) was 2,577.577, and Tarone-Ware was 2,934.977, P < 0.001 for all (Table 2). The specific values of univariate and multivariate hazard ratios (HRs) for different median household incomes were shown in Table 3.
Address
A longer survival time was seen in a metropolitan population (Figure 1I and J) and Table S9). Survival in metropolitan areas of 1 million (15.970 months) exceeded 14.030 months (all lung cancer patients), while in metropolitan areas of 1 million this was 13.720 months. Times in other areas were: metropolitan areas of 250,000 (11.700 months), nonmetropolitan counties adjacent to a metropolitan area (11.520 months), and the nonmetropolitan counties not adjacent to a metropolitan area group (10.990 months) had less survival time than 14.030 months (Table S10). As can be seen from Table S10, the fastest increases in median survival time were 60.34% and 38.03% for metropolitan areas of 1 million and metropolitan areas of 1 million, respectively (Table S11). The specific values of the 3-year, 5-year, and 10-year survival rates of lung cancer patients of different addresses were shown in Table 1. The log rank (Mantel-Cox) for the overall comparison was 1,952.945, the Breslow (generalized Wilcoxon) was 1,397.391, and Tarone-Ware was 1,696.331, all P < 0.001 (Table 2). The specific values of univariate and multivariate hazard ratios (HRs) for different addresses were shown in Table 3.
Histologic Type ICD-O-3
Median survival time of lung cancer patients in the adenocarcinoma group (Diagnosed 2017) was significantly higher than all other patients, followed by the complex epithelial neoplasms and squamous cell neoplasms groups. (Figure 1K and L) and Table S12). The number of patients in the Adenoma and adenocarcinomas group diagnosed in 2017 increased by 87.35% compared to 2000. Survival time in the Squamous cell neoplasms group (15.270 months) was longer than 14.030 months (Figure 1K and L) and Table S12), and the number of patients diagnosed in 2017 increased by 38.44% compared to 2000. Median survival time in the other groups was as follows: Neoplasia, NAS (8.100 months), Epithelial neoplasms, NAS (9.200 months) and Cystic, mucinous and serous neoplasms (12.000 months). Each was shorter than 14.030 months (Table S12 and S13). The specific values of the 3-year, 5-year, and 10-year survival rates of lung cancer patients of different histologic types were shown in Table 1.
The log rank (Mantel-Cox) for the overall comparison was 82,913.123, the Breslow (generalized Wilcoxon) was 79,928.300, and Tarone-Ware was 84,548.100, all P < 0.001 (Table 2). The specific values of univariate and multivariate hazard ratios (HRs) for different histologic types were shown in Table 3.
Race
Median survival time of Asian and other races diagnosed in 2017 was 97.87% higher than in 2000 (Figure 1M and N), and median survival time of White patients increased by 38.61%. In contrast, Black patients only had a 24.77% increase, which is below average (Table S15). Among lung cancer patients diagnosed in 2017, median survival was 13.750 months for White patients, 11.890 months for Black patients, and 20.420 months for Asian and other patients (Table S14). The 3 -, 5 -, and 10-year survival rates for White, Black, Asian and other races were, in order: 19.00%, 14.00%, 8.00%; 17.00%, 12.00%, 7.00%; 23.00%, 17.00%, 11.00% (Table 1).
The log rank (Mantel-Cox) for the overall comparison was 1,300.355, the Breslow (generalized Wilcoxon) was 1,210.942, and Tarone-Ware was 1,311.907, all P < 0.001 (Table 2). Univariate HRs compared with White for Black, Asian and other races were, in order: 1.061 (1.053–1.068) and 0.865 (0.857–0.874), P < 0.001. Multivariate HRs were, in order: 1.099 (1.091–1.108), 0.939 (0.930–0.949), all P < 0.001 (Table 3). The specific values of univariate and multivariate hazard ratios (HRs) for different races were shown in Table 3.
Origin Recode the National Health Insurance Authority
Median survival time was 14.110 months for Non-Spanish-Hispanic-Latino patients diagnosed in 2017 and 12.860 months for Spanish-Hispanic-Latino patients (Figure 1O and P) and Table S16). Compared to patients diagnosed in 2000, survival in Non-Spanish-Hispanic-Latinos increased by 42.67% and in Spanish-Hispanic-Latinos by 28.60% (Table S17). The 3 -, 5 -, and 10-year survival rates for Non-Spanish-Hispanic-Latinos and Spanish-Hispanic-Latinos were, in order: 19.00%, 14.00%, 8.00%; 20.00%, 15.00%, 9.00% (Table 1).
The log rank (Mantel-Cox) for the pairwise and overall comparisons was 10.506, the Breslow (generalized Wilcoxon) was 0.624, and Tarone-Ware was 0.323. P values were < 0.001, 0.429, and 0.570, respectively (Table 2). Univariate HR compared with Spanish-Hispanic-Latino for Non-Spanish-Hispanic-Latino was 1.016 (1.006–1.027), P = 0.002. Multivariate HR was 1.018 (1.008–1.029), P < 0.001 (Table 3).
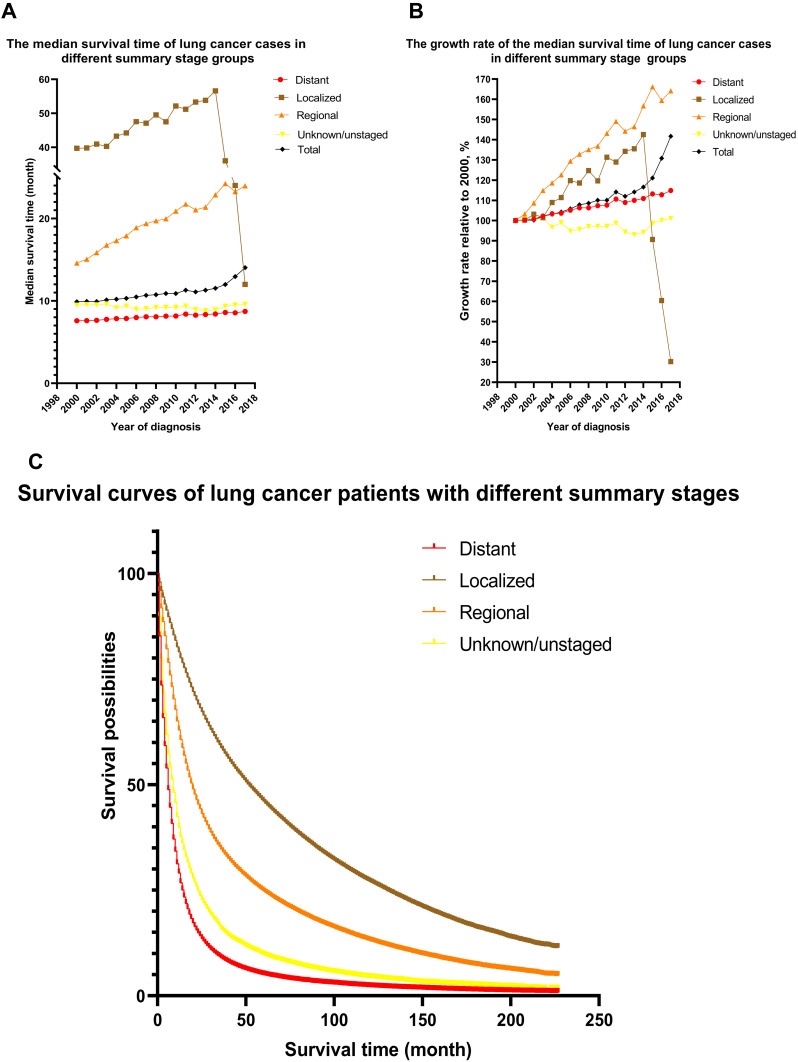
Summary Stage
The survival rates of lung cancer patients at different summary stages vary considerably, as detailed in Table 1. The survival difference of lung cancer patients with different summary stages was statistically significant (Table 2). The hrs of lung cancer patients with different summary stages differed significantly (Table 3). The survival curves and median survival times of lung cancer patients with different summary stages are shown in Figure 3.
Figure 3.
Survival curves of different summary stages and changes in median survival time of lung cancer patients from 2000 to 2017. (A) Median survival time of lung cancer patients at different summary stages from 2000 to 2017. (B) Increase in median survival time of lung cancer patients at different summary stages from 2000 to 2017. (C) Kaplan–Meier survival curves for different summary stages.
Discussion
According to Howlader and Forjaz,1 lung cancer mortality rate in the US has decreased significantly recently. Although lung cancer incidence has been described in multiple papers,9–12 studies with large samples, multiple sub items, multiple statistical analysis methods, and statistical details by year of diagnosis are not common13 and the data are mostly outdated.14–17 Therefore, we took advantage of the new data published by SEER on April 15, 2021, which allowed a detailed analysis of the survival of lung cancer patients in the US. After an in-depth study of 922,317 patients, we have several novel findings. The median survival time of all lung cancer patients diagnosed in 2017 (14.030 months) increased by 41.72% compared with 2000 (9.900 months).
Women’s median survival time and 3-year, 5-year, and 10-year survival rates were more significant and growing faster than men’s. Pilleron et al18 also found that gender was one of the most important factors influencing lung cancer survival time. The prognosis of female patients undergoing lobectomy/segmentectomy was significantly better than in male patients.19 Part of the reason may be that men have a higher smoking rate,20 and the pathobiology of adenocarcinoma in women may differ from that in men.21
The median survival time of patients with a single lobe primary site was the longest where this was in the right middle lobe, followed by the left upper lobe, right upper lobe, right lower lobe, and the shortest in left lower lobe. The rapidly increasing survival time may be due to the increase in early diagnosis of lung cancer22 and improved thoracoscopic lobectomy and segmentectomy techniques.23 In contrast, median survival rates where the primary site was in the main bronchus and over lapping lung did not significantly increase. These are independent predictors of lung cancer metastasis and worse outcomes.24
Although younger patients had a longer median survival time, interestingly, median survival time was almost the same in the 55–64 and 65–74 groups. This may be because there is little difference in the physical condition25 between the two age groups. The median survival time in those over 75 was significantly reduced, which may be related to the decline of the patient’s physical fitness or the increased likelihood of severe complications, which are associated with poor survival.26
Median survival time was longer for patients with higher incomes and there was also an association between family disposable income and survival.27 Low-income patients with lung cancer may have delays in diagnosis and treatment, requiring social intervention and care.28 Increased healthcare costs in the public sector were associated with lower cancer mortality.29
The farther the patient’s address is from a metropolitan area, the shorter the median survival time. In metropolitan areas with a population of more than 1 million, median survival time exceeded that of all other lung cancer patients, which may be related to the availability and timeliness of access to good medical care in these areas. The HR was highest in nonmetropolitan counties not adjacent to metropolitan areas. Singh and Siahpush found a widening life expectancy gap between urban and rural areas for lung cancer patients in the US between 1969–2009,30 and our results found that this gap has widened even further over the last decade. Routine tracking of lung cancer excess deaths through urban-rural county classification may help public health authorities and policy makers identify and monitor public health concerns and focus interventions to reduce potential excess deaths in these areas.8
Median survival time of lung cancer patients in the adenocarcinoma group (Diagnosed 2017) was significantly higher than all other patients, followed by the complex epithelial neoplasms and squamous cell neoplasms groups. Median survival time in the adenocarcinoma group was 6.35 months longer than in squamous cell carcinoma. This may be related to the improvement of minimally invasive surgery,31 chemotherapy,32 immunotherapy,33,34 molecular targeted therapy34 or other treatments for lung adenocarcinoma. The Epithelial neoplasms, NAS group was one of the worst groups, containing mainly large and small cell lung cancer. Although there are some new treatments,35–38 survival time has not improved significantly. The acinous cell neoplasm group had the longest median survival of lung cancer histology.
The median survival times and rates of Asian, Pacific Islanders and Native American Indians/Alaskans were significantly higher than of White and Black people, and the fastest growth rate was about 97.87%. In contrast, the growth rate in White people was only about 38.61% and in Black people was only about 24.76%. This may be due to different access to health care and the provision of recommended treatment.39 Efforts to ensure that all patients with lung cancer receive timely and appropriate treatment should reduce differences in survival between races.40 Median survival time was 14.110 months for Non-Spanish-Hispanic-Latino lung cancer patients (an increase of 42.67%) and 12.860 months for Spanish-Hispanic-Latino (an increase of only 28.60%). The univariate HR of non-Hispanic Latinos was higher than in Hispanic Latinos, which is contrary to a previous study41 but may be due to a difference in sample size. According to Soneji et al42 narrowing racial differences in lung cancer survival rates depends not only on equal opportunities for surgical resection, but also on better management and treatment of smoking-related complications and diseases.42
The later the year of diagnosis, the longer the median survival time and the lower the risk ratio. This showed that in the past 20 years, the treatment effect in the US has improved. The reason for the survival time of localized lung cancer patients is greater than that of Distant patients. This fully shows that early detection and early treatment are very important in the treatment of lung cancer.
Our study provides detailed insight into the relationship between patients’ sex, primary site, age, income, residential address, histological type, race, ethnicity, and survival thanks to the large sample size. However, we acknowledge that if patient data from other countries can be integrated, our study would be more representative. Incidence of lung cancer was not analyzed in detail so this could be further studied in subsequent papers. The SEER database still has some shortcomings, such as not collecting information on “smoking”.
Conclusions
After analyzing the data of 922,317 patients with lung cancer in the recently-published SEER database, we found large differences in survival time by gender, race and ethnicity, age, income, address, histological type, primary site and summary stage. This difference has grown in recent years. Government and society need to further strengthen policies to improve trends. We should increase the frequency and precision of lung cancer screening in the future.
Acknowledgments
Acknowledgment
We hereby thank the staff of the Department of Thoracic Surgery of the Second Affiliated Hospital of Nanchang University for their strong support for this study.
Abbreviations
HR, Hazard Ratio; ICD-O-3, International Classification of Disease for Oncology-3; NCI, The National Cancer Institute; SEER, The surveillance, epidemiology, and end results.
Ethics Approval and Informed Consent
Since SEER is an open database and all the data extracted from SEER are nonhuman studies, there is no need to obtain ethical approval from the Ethics Committee of the Second Affiliated Hospital of Nanchang University. Data published by the SEER database is publicly available and identifiable and therefore does not require patient informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in Lung-Cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi: 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolwin Y, Gillis R, Peled N. Gender and lung cancer-SEER-based analysis. Ann Epidemiol. 2020;46:14–19. doi: 10.1016/j.annepidem.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 3.Houston KA, Henley SJ, Li J, White MC, Richards TB. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer. 2014;86(1):22–28. doi: 10.1016/j.lungcan.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henley SJ, Richards TB, Underwood JM, Eheman CR, Plescia M, McAfee TA. Lung cancer incidence trends among men and women–United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 2014;63(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Song CK, Guo ZX, Shen XY, et al. Prognostic factors analysis and nomogram Construction of dual primary lung cancer: a population study. Biomed Res Int. 2020;2020:7206591. doi: 10.1155/2020/7206591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia B, Zheng Q, Wang J, et al. A nomogram model to predict death rate among non-small cell lung cancer (NSCLC) patients with surgery in surveillance, epidemiology, and end results (SEER) database. BMC Cancer. 2020;20(1):666. doi: 10.1186/s12885-020-07147-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149 [DOI] [PubMed] [Google Scholar]
- 8.Garcia MC, Rossen LM, Bastian B, et al. Potentially excess deaths from the five leading causes of death in metropolitan and nonmetropolitan counties - United States, 2010–2017. MMWR Surveill Summ. 2019;68(10):1–11. doi: 10.15585/mmwr.ss6810a1 [DOI] [PubMed] [Google Scholar]
- 9.Coleman NC, Burnett RT, Ezzati M, Marshall JD, Robinson AL, Pope CA 3rd. Fine particulate matter exposure and cancer incidence: analysis of SEER cancer registry data from 1992–2016. Environ Health Perspect. 2020;128(10):107004. doi: 10.1289/ehp7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–953. doi: 10.2147/cmar.S187317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Zhang G, Song S, Zheng Z. Surgery for small cell lung cancer: a Surveillance, Epidemiology, and End Results (SEER) survey from 2010 to 2015. Medicine. 2019;98(40):e17214. doi: 10.1097/md.0000000000017214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng XQ, Huang JF, Lin JL, et al. Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study. Transl Lung Cancer Res. 2019;8(4):367–379. doi: 10.21037/tlcr.2019.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang W, He J, Shen Y, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol. 2017;35(11):1162–1170. doi: 10.1200/jco.2016.67.5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19(11):1511–1521. doi: 10.1093/neuonc/nox077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122(19):3075–3086. doi: 10.1002/cncr.30164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meza R, Meernik C, Jeon J, Cote ML. Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS One. 2015;10(3):e0121323. doi: 10.1371/journal.pone.0121323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ten Haaf K, van Rosmalen J, de Koning HJ. Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiol Biomarkers Prev. 2015;24(1):154–161. doi: 10.1158/1055-9965.Epi-14-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilleron S, Maringe C, Charvat H, Atkinson J, Morris E, Sarfati D. Age disparities in lung cancer survival in New Zealand: the role of patient and clinical factors. Lung Cancer. 2021;157:92–99. doi: 10.1016/j.lungcan.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 19.Sachs E, Sartipy U, Jackson V. Sex and survival after surgery for lung cancer: a Swedish nationwide cohort. Chest. 2021;159(5):2029–2039. doi: 10.1016/j.chest.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- 21.Chang JW, Asamura H, Kawachi R, Watanabe S. Gender difference in survival of resected non-small cell lung cancer: histology-related phenomenon? J Thorac Cardiovasc Surg. 2009;137(4):807–812. doi: 10.1016/j.jtcvs.2008.09.026 [DOI] [PubMed] [Google Scholar]
- 22.Cainap C, Pop LA, Balacescu O, Cainap SS. Early diagnosis and screening in lung cancer. Am J Cancer Res. 2020;10(7):1993–2009. [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg. 2016;50(6):1060–1066. doi: 10.1093/ejcts/ezw189 [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Wang S, Gerber DE, et al. Main bronchus location is a predictor for metastasis and prognosis in lung adenocarcinoma: a large cohort analysis. Lung Cancer. 2018;120:22–26. doi: 10.1016/j.lungcan.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganti AK, Shostrom V, Alorabi M, et al. Early stage non-small-cell lung Cancer in octogenarian and older patients: a SEER database analysis. Clin Lung Cancer. 2016;17(4):285–291. doi: 10.1016/j.cllc.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 26.Yang CC, Fong Y, Lin LC, et al. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg. 2018;53(1):235–240. doi: 10.1093/ejcts/ezx215 [DOI] [PubMed] [Google Scholar]
- 27.Sachs E, Jackson V, Sartipy U. Household disposable income and long-term survival after pulmonary resections for lung cancer. Thorax. 2020;75(9):764–770. doi: 10.1136/thoraxjnl-2019-214321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nwagbara UI, Ginindza TG, Hlongwana KW. Health systems influence on the pathways of care for lung cancer in low- and middle-income countries: a scoping review. Global Health. 2020;16(1):23. doi: 10.1186/s12992-020-00553-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruthappu M, Watkins J, Noor AM, et al. Economic downturns, universal health coverage, and cancer mortality in high-income and middle-income countries, 1990–2010: a longitudinal analysis. Lancet. 2016;388(10045):684–695. doi: 10.1016/s0140-6736(16)00577-8 [DOI] [PubMed] [Google Scholar]
- 30.Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S. 1969–2009. Am J Prev Med. 2014;46(2):e19–29. doi: 10.1016/j.amepre.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Ma X, Shen X, et al. Surgery for pre- and minimally invasive lung adenocarcinoma. J Thorac Cardiovasc Surg. 2020. doi: 10.1016/j.jtcvs.2020.11.151 [DOI] [PubMed] [Google Scholar]
- 32.Drake JA, Sullivan JL, Weksler B. Adjuvant chemotherapy improves survival in patients with completely resected T3N0 non-small cell lung cancer invading the chest wall. J Thorac Cardiovasc Surg. 2018;155(4):1794–1802. doi: 10.1016/j.jtcvs.2017.11.091 [DOI] [PubMed] [Google Scholar]
- 33.Guo D, Wang M, Shen Z, Zhu J. A new immune signature for survival prediction and immune checkpoint molecules in lung adenocarcinoma. J Transl Med. 2020;18(1):123. doi: 10.1186/s12967-020-02286-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology. 2015;20(3):370–378. doi: 10.1111/resp.12490 [DOI] [PubMed] [Google Scholar]
- 35.Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1):47. doi: 10.1186/s13045-019-0736-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17(5):300–312. doi: 10.1038/s41571-019-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farago AF, Yeap BY, Stanzione M, et al. Combination Olaparib and temozolomide in relapsed small-cell lung cancer. Cancer Discov. 2019;9(10):1372–1387. doi: 10.1158/2159-8290.Cd-19-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Ye C, Mansmann UR. Comparing patient-derived xenograft and computational response prediction for targeted therapy in patients of early-stage large cell lung cancer. Clin Cancer Res. 2016;22(9):2167–2176. doi: 10.1158/1078-0432.Ccr-15-2401 [DOI] [PubMed] [Google Scholar]
- 39.Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of race on treatment and survival among U.S. veterans with early-stage lung cancer. J Thorac Oncol. 2016;11(10):1672–1681. doi: 10.1016/j.jtho.2016.05.030 [DOI] [PubMed] [Google Scholar]
- 40.Richards TB, Henley SJ, Puckett MC, et al. Lung cancer survival in the United States by race and stage (2001–2009): findings from the Concord-2 study. Cancer. 2017;123 Suppl 24(Suppl 24):5079–5099. doi: 10.1002/cncr.31029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klugman M, Xue X, Ginsberg M, Cheng H, Rohan T, Hosgood HD 3rd. Hispanics/Latinos in the Bronx have improved survival in non-small cell lung cancer compared with non-Hispanic whites. J Racial Ethn Health Disparities. 2020;7(2):316–326. doi: 10.1007/s40615-019-00660-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and ethnic disparities in early-stage lung cancer survival. Chest. 2017;152(3):587–597. doi: 10.1016/j.chest.2017.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]