Abstract
Circular RNAs (circRNAs) are a novel class of RNAs distinguished by their single-stranded, covalently-closed topology. Although initially perceived as rare byproducts of aberrant splicing, circRNAs are now recognized as ubiquitously expressed and functionally significant. These discoveries have led to a growing need for ways to model circRNAs in living cells to advance our understanding of their biogenesis, regulation, and function, and to adopt them as new technologies for application within research and medicine. In this review, we provide an updated summary of approaches used to produce circRNAs in vitro and in vivo, the latter of which has grown considerably in recent years. Given increased interest in the unique functions carried out by individual circRNAs, we further dedicate a section on how to customize synthesized circRNAs for specific biological roles. We focus on the most common applications, including designing circRNAs for protein delivery, to target miRNAs and proteins, to act as fluorescent reporters, and to modulate cellular immunity.
Keywords: CircRNA, RNA synthesis, Backsplicing, CircRNA minigene, In vitro transcription, CircRNA translation
1. Introduction
Circular RNAs (circRNAs) are a class of single-stranded RNAs distinguished by their covalently-closed topology. In the decades closely following their discovery, circRNAs were presumed to be rare, functionally inert byproducts of aberrant splicing [1]. However, there has been a fundamental shift in the past decade towards the perception of circRNAs as functionally significant molecules with pervasive roles in biology. This can largely be attributed to the emergence of RNA-sequencing (RNA-seq) technologies that revealed that circRNAs are ubiquitous and evolutionarily conserved throughout eukaryotes [2,3]. Within the transcriptomes of metazoans, thousands of circRNAs are produced from a sizeable proportion of actively transcribed genes [4,5]. Subsequent characterizations of eukaryotic circRNAs has uncovered general properties that are widely shared among them, such as their exonic composition, mid-range size (~0.2–1.5 kilobases, kb), resistance to exoribonuclease digestion, and longer half-lives (~24–48 h) [2].
Emerging work has also led to an appreciation of circRNA diversity, as exhibited by their cell-specific expression, cellular localization patterns, and attributed functions [3,6]. While most circRNAs are lowly expressed, there exist several examples of abundant circRNAs that comprise the predominant transcript isoform of their gene [4]. Some circRNAs are thought to compete with the protein-coding isoforms of their parental gene for transcription, while others have been identified to function downstream of their biogenesis by sponging miRNAs, antagonizing proteins, or by being translated [7–12]. These discoveries have positioned circRNAs as overlooked regulators of many biological pathways involved in cellular homeostasis, viral infection, the immune response, aging, and cancer [13–18]. This recognition has led to a surge in interest within the field to mine the functions of other endogenous circRNAs, and to harness the functional properties unique to circRNAs for applications within research and medicine.
There is a growing need for ways to model circRNA biology by engineering custom circRNAs with specific properties. In this review, we present current strategies to produce circRNAs both in vitro and in vivo. Here, “in vitro“ refers to outside of a living cell (i.e. “in a test tube”), whereas “in vivo” refers to production within living cells, including cultured cell lines and whole organism models. We also include a section devoted to approaches for functionalizing circRNAs for translation, to target miRNAs and proteins, to act as fluorescent reporters, and to stimulate or suppress cellular immunity.
2. CircRNA biogenesis
In metazoans, the spliceosome catalyzes the removal of intronic regions from pre-mRNA transcripts as delineated by splice site motifs flanking the 5′ and 3′ ends of each intron. This process conventionally involves an upstream donor splice site joining a downstream acceptor splice site, and frequently yields multiple spliced isoforms when distinct exons and introns are paired from the same pre-mRNA transcript (Fig. 1fig1A) [19]. In contrast, the vast majority of eukaryotic circRNAs are produced through a process known as “backsplicing” [3], a special form of alternative splicing defined by the cis-pairing of a downstream donor site with an upstream acceptor site [3,20]. In the prevailing model of backsplicing, exon circularization occurs directly from the nascent linear transcript as the flanking introns splice together in a toe-to-head fashion to excise the intervening region as an RNA circle (Fig. 1B) [21]. In addition, circularization can also proceed indirectly from an exon-containing lariat intermediate (produced by forward splicing) that undergoes an internal splicing reaction resulting in excision of the skipped exon as an RNA circle (Fig. 1C) [22]. Investigations into the factors that promote backsplicing over forward splicing have revealed that the former is favored when the ends of the sequence to be circularized are in close proximity [23,24]. This can occur when the flanking introns engage in base pairing or are brought together by dimerizing RNA-binding proteins (RBPs) that bind across the circularized region within the intronic sequences [25,26].
Fig. 1.
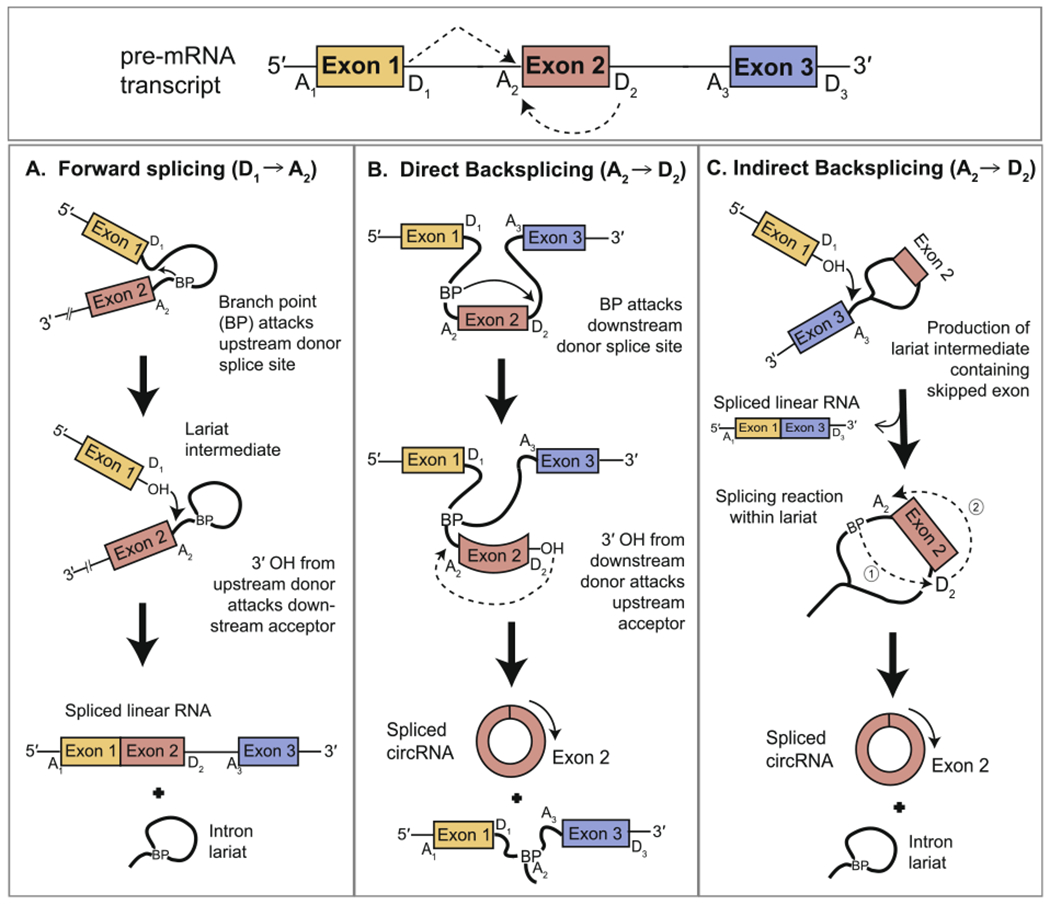
Alternative splicing of a pre-mRNA transcript can produce linear and circRNA isoforms. (A) Linear RNA is produced by a canonical (i.e. forward) splicing reaction in which a donor splice site is paired with a downstream acceptor splice site. Two sequential transesterification reactions occur to result in exon ligation and excision of the intervening region as a branched intron lariat. (B) CircRNA is produced by a noncanonical backsplicing reaction. The reaction chemistry remains the same as that of forward splicing, except that the donor splice site is now paired with an upstream acceptor. It is generally thought that most backsplicing reactions proceed directly from a linear pre-mRNA transcript [21]. (C) Backsplicing can also occur indirectly following a completed forward splicing reaction that releases a lariat intermediate containing a skipped exon [22]. An internal backsplicing reaction occurs within the lariat to excise the skipped exon as an exonic circRNA.
Beyond backsplicing, endogenous circRNAs can also be generated by the direct circularization of intronic sequences [27]. Some of these non-canonical, intronic circRNAs originate from intron lariat byproducts of pre-mRNA splicing as well [28]. For example, while the majority of intron lariats are readily degraded, some stable intronic sequences escape debranching, and can be trimmed to form unbranched RNA circles [28,29]. These intronic circRNAs are retained in the nucleus where they can regulate transcription of their parental gene. Other classes of intronic circRNAs can be generated independently of the spliceosome. Examples include the production of unbranched circular byproducts from self-splicing intron systems and the circularization of tRNA and rRNA introns by specialized host ligases [30–33]. These diverse endogenous mechanisms of circRNA production form the basis of several strategies currently used to generate circRNAs both in vitro and in vivo.
3. In vitro circRNA synthesis
The in vitro generation of a circRNA involves the synthesis of a linear RNA precursor followed by ligation of its ends to produce a covalently-closed loop. This can be achieved by a variety of chemical and enzymatic approaches that can be mixed-and-matched (chemical synthesis and enzymatic ligation or enzymatic synthesis and chemical ligation) depending on the desired circular product and the reagents and technologies available (Table 1tbl1).
Table 1.
Approaches for CircRNA Synthesis. Summary of the methods used to synthesize circRNAs and their advantages and disadvantages.
| Approach | Advantages | Disadvantages |
|---|---|---|
| Chemical synthesis of linear RNA precursor | High purity of the resulting RNA | Limited to short RNAs (<70–80 nucleotides) |
| Enzymatic synthesis of linear RNA precursor | High yield, longer RNAs (kb in length) | First nucleotide must be a guanosine for optimal yields; transcribed RNAs can exhibit sequence heterogeneity at 3′ end |
| Chemical ligation | Effective for very small circRNAs (2–100 nucleotides) | Competition from intermolecular ligations and formation of 2′-5′ phosphodiester bonds |
| Enzymatic ligation by DNA ligase 1 | Effective for larger circRNAs; ligation is sequence specific; agnostic to terminal nucleotide identity; can produce exact sequence mimic | Competition from intermolecular ligations; requires optimization of splint with each new substrate; higher amounts of enzyme needed due to low turnover |
| Enzymatic ligation by T4 RNA ligase 1 | Effective for larger circRNAs; higher efficiency; splint not required; easier to purify due to minimal reaction components; can produce exact sequence mimic | Efficiency of circularization highly dependent on structure of linear precursor; competition from intermolecular ligations; lower sequence specificity; strong nucleotide preference at the 3′ and 5′ ends |
| Enzymatic ligation by T4 RNA ligase 2 | Effective for larger circRNAs; greater substrate flexibility; splint not required; agnostic to terminal nucleotide identity; can produce exact sequence mimic | Efficiency of circularization highly dependent on structure of linear precursor; competition from intermolecular ligations |
| Permuted intron–exon (PIE) system with group I introns | Effective for various RNA sizes (100 nucleotides – 5 kb); can be applied both in vivo and in vitro | Retains portions of the exons from the native group I intron-containing gene; several splicing intermediates produced as byproducts |
| Permuted intron–exon (PIE) system with group II introns | Can be used to mimic an endogenous circRNA sequence precisely without any extraneous sequence; can be applied both in vivo and in vitro | Limited characterization |
| Hairpin ribozymes | Generates high yields of small circRNAs (~50–150 nucleotides) with sequence homogeneity | CircRNA contains sequences of ribozyme and cognate cleavage site with potential for ribozymatic activity |
| Repetitive ICS | Allows for generation of a circRNA mimic in vivo using endogenous gene locus harboring Alu intronic elements | Can generate undesired byproducts from competing splicing reactions in vivo; complementary, repetitive sequences may challenge experimental manipulation of construct in vitro |
| Non-repetitive ICS | Allows for generation of a circRNA mimic in vivo; uses shorter sequences to drive circularization, which can readily be designed in silico | Can generate undesired byproducts from competing splice reactions in vivo; complementary sequences may challenge experimental manipulation of construct in vitro |
| RBP motifs | Modularity: binding sites can be integrated into most pre-existing backbones | May require overexpression of the RBP |
| tricRNA/Tornado system | High circularization efficiency and yields; circRNA generated independently of the spliceosome and autocatalytic pathways | Pre-tRNA intronic sequence retained at ligation junction |
3.1. Methods for synthesis of a linear RNA precursor
3.1.1. Chemical strategies
Current techniques for the chemical synthesis of RNA rely on synthesizer machines that use derivatives of nucleotide triphosphates, known as phosphoramidites, as the building blocks of linear oligonucleotides (Fig. 2fig2A). Since natural nucleotide triphosphates contain reactive substituents such as hydroxyl and amino groups, they can participate in undesired reactions that preclude the formation of the desired 3′-5′ phosphodiester linkages during chemical synthesis. Nucleoside phosphoramidites use inert substituents to protect the reactive moieties and favor phosphodiester bond formation, leading to greater and more homogenous yields. Once synthesis is complete, these protecting groups are removed to generate oligonucleotides that have the same basic chemical structure as cellular RNA. The primary advantages of chemical synthesis are the high purity of the resulting RNA and the wide availability of commercial services specializing in custom synthesis. However, the RNA molecules generated are usually limited to less than 70–80 nucleotides in length; beyond this, yield significantly diminishes and costs become more prohibitive. This limitation can be circumvented to create midsized RNAs (less than 300 nucleotides) by the enzymatic ligation of multiple smaller RNA oligonucleotides, but the generation of larger RNA molecules is a significantly challenge.
Fig. 2.
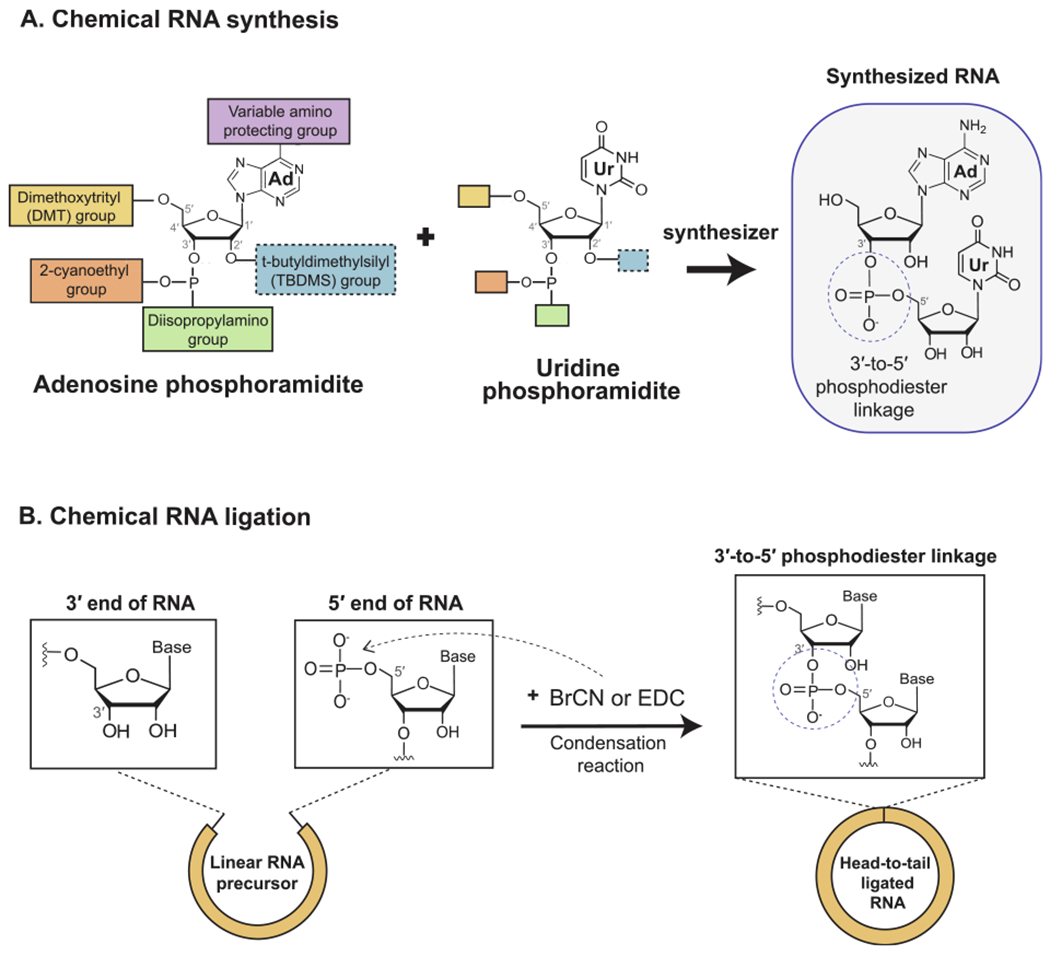
In vitro generation of circRNA by chemical means. (A) Chemical synthesis of RNA is performed using an automated synthesizer that executes chain assembly using specialized nucleotide derivatives known as phosphoramidites.The structures of adenine and uridine phosphoramidites are shown alongside the dinucleotide resulting from their joining by a 3′-5′ phosphodiester bond. (B) Chemical ligation of the distal ends of a linear RNA precursor can be achieved using cyanogen bromide (BrCN) or 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The ensuing condensation reaction results in ligation by a 3′-5′ phosphodiester bond.
3.1.2. Enzymatic strategies
More commonly, synthesis of the linear RNA precursor for circularization is carried out using phage RNA polymerases to perform in vitro transcription (IVT). The major components of an IVT reaction are the double-stranded DNA (dsDNA) template, ribonucleotide triphosphates, and a DNA-dependent RNA polymerase usually derived from the T7, SP6, or T3 bacteriophages (Fig. 3fig3A). The DNA template should contain the ~20 nucleotide cognate promoter sequence required for the polymerase to dock and catalyze downstream transcription. Depending on the polymerase used, this DNA template can be a double-stranded PCR product containing the promoter sequence at its 5′ end or a linearized plasmid containing the promoter directly upstream of the DNA sequence to be transcribed. The purity and amount of DNA template, and the incubation time are main factors affecting the yields, which can be in the milligram range.
Fig. 3.
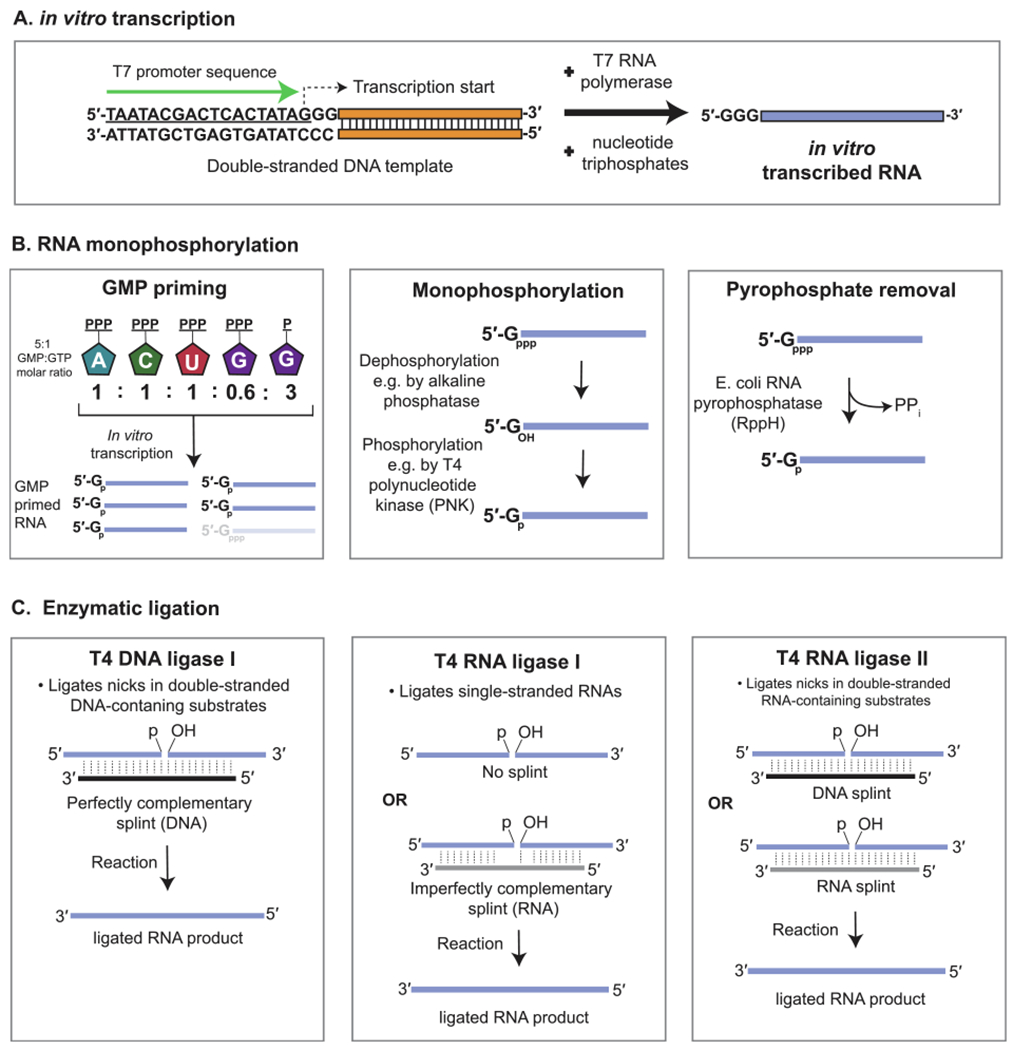
In vitro generation of circRNA by enzymatic means. (A) Enzymatic synthesis of RNA is performed using a phage RNA polymerase, such as T7, and a double-stranded DNA template containing the cognate promoter sequence. Nucleotide triphosphates used during in vitro transcription (IVT) result in a single-stranded RNA containing guanine nucleotide triphosphate (GTP) at its 5′ end. (B) Enzymatic intramolecular ligation of RNA in vitro requires the RNA to contain a monophosphate at its 5′ end. This can be achieved by using an excess molar ratio of GMP to GTP during the IVT reaction. Alternatively, if only GTP was used to prime transcription, monophosphorylation can be achieved by treatment of the RNA with a phosphatase followed by a kinase, or by treatment with a pyrophosphatase. (C) Enzymatic ligation of a monophosphorylated RNA can be achieved using T4 DNA ligase, T4 RNA ligase 1, or T4 RNA ligase 2. An RNA or DNA splint is often used to bring the terminal ends of the RNA together.
Compared to chemical synthesis, enzymatic synthesis allows for the generation of much larger linear RNAs (kb in length), but poses certain challenges if the goal is to produce a uniform RNA species precisely mimicking a sequence found in vivo. For example, the first nucleotide of the IVT transcript generated by T7, SP6, and T3 polymerases must be a guanosine to obtain optimal reaction yields [34]. This requirement can be met by permuting the linear precursor sequence such that the resulting circular sequence is left unchanged due to its lack of defined ends. A greater challenge persists from the run-off nature of phage polymerases, which can lead to the addition of 1–3 nucleotides to the end of the RNA transcript or to incomplete transcripts [35,36]. Fortunately, several strategies have been introduced to circumvent this limitation, including i) appending the hepatitis delta virus ribozyme sequence (~67 nucleotides long) to the end of the DNA template, which leads to precise ribozymatic cleavage at the 3′ end following transcription, ii) use of a PCR template containing 2′-O-methyl nucleotides at its 3′ end, which reduces non-templated nucleotide addition by T7, [37] and iii) choice of a ligation method that exhibits substrate specificity for the desired junction sequence (see section 3.2.2).
3.2. Methods for ligation of a linear RNA precursor
3.2.1. Chemical strategies
Following synthesis of the linear RNA, an intramolecular reaction between the first and the last nucleotides leads to circularization. The condensation reagents cyanogen bromide (BrCN) or 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) are most commonly used to activate RNA circularization by purely chemical means (Fig. 2B) [38]. Additional chemical strategies rely on substituting the native phosphate and hydroxyl groups at the ends of the linear RNA with other functional groups. Upon the addition of the appropriate activating reagent, these alternative reactive groups can undergo bond formation with higher efficiency and specificity, allowing for purer, larger yields of circularized RNA [39]. The drawback is that the circularizing bond is not a phosphodiester bond, and thus would not chemically mirror the back-splice junction found in vivo. These non-natural linkages can potentially impact the secondary structure and function of the prepared circRNA, and careful evaluation will be needed to assess their suitability for the experiment.
Regardless of the chemical strategy pursued, formation of a circularized product by an intramolecular reaction always competes with the generation of a concatenated linear product through an intermolecular reaction. Thus, additional strategies are warranted to bias circRNA generation. A low concentration of the input linear RNA can decrease the likelihood of concatemerization, but this will expectedly affect reaction yield [40]. Alternatively, the linear RNA precursor can be engaged in a secondary structure that favors circularization. This is most commonly achieved by bringing the 5′ and 3′ ends of the RNA molecule into close proximity using a short oligonucleotide splint anti-sense to both ends, or by installing base-pairing sequences at the ends of the linear RNA. Several variations of both strategies are routinely used to enhance both the purity and efficiency of the circularization reaction, and are reviewed in depth elsewhere [39,41]. Non-specific products also commonly result from the formation of a 2′-5′ phosphodiester bonds, leading to a mixture of circularized RNAs with 2′ or 3′ hydroxyls at the ligation junction. One strategy to prevent this involves the use of a 2′ deoxynucleotide at the 3′ end of the linear RNA precursor, which eliminates the reactive 2′ hydroxyl group [42].
3.2.2. Enzymatic strategies
More commonly, DNA and RNA ligases derived from T4 bacteriophage are used to enzymatically facilitate RNA ligation. These enzymes require the linear RNA substrate to harbor a 5′ monophosphate for circularization (Fig. 3B). If the RNA is produced by an IVT reaction using only nucleotide triphosphates, tandem dephosphorylation and phosphorylation reactions will remove the 5′ triphosphate and install a monophosphate. Alternatively, guanosine monophosphate (GMP) can be included at a higher molar ratio to guanosine triphosphate (GTP) during the IVT reaction to prime the RNA synthesis with GMP [43]. A third novel approach involves the treatment of the GTP-primed RNA with RNA 5′ Pyrophosphohydrolase (RppH), an E. coli enzyme capable of cleaving the beta and gamma phosphates from the 5′ end of triphosphorylated RNA [44,45].
In T4 bacteriophage, DNA ligase 1 catalyzes repair of nicks within double-stranded substrates containing DNA where there is perfect complementarity between the two strands at the ligation junction [46]. In vitro application of DNA ligase 1 for RNA ligation requires a short DNA oligonucleotide splint that hybridizes to both ends of the single-stranded RNAs, thus creating a local region of double-strandedness that is recognized by the enzyme (Fig. 3C) [47,48]. This strategy has been adapted for generating circRNAs by designing the splint to hybridize to 10–20 nucleotides on each end of the same RNA molecule [43,49]. Since T4 DNA ligase 1 has low tolerance for mismatches at the ligation junction, RNA exhibiting perfect complementarity to the DNA splint is ligated with highest efficiency [47,50]. This specificity is particularly desirable when the linear precursor RNA is synthesized through IVT, which results in considerable 3′ terminal heterogeneity [35,36]. Since the turnover efficiency of DNA ligase I on RNA substrates is low, a large amount of enzyme is often needed to achieve RNA ligation, particularly when compared to use of dedicated RNA ligases [47].
Compared to DNA ligase 1, T4 RNA ligase 1 works only on single-stranded substrates and exhibits lower reaction specificity [51]. The efficiency of RNA ligase I has been shown to vary considerably depending on the structure of the linear precursor. To favor intramolecular circularization, the linear RNA should be pre-oriented such that the termini are kept in close proximity [52]. If the RNA sequence does not naturally take on such a structure, pre-orientation can be achieved by adding extra base-pairing sequences to the ends of the precursor while still leaving the very terminal nucleotides unpaired [44,53,61]. Using this strategy, Carmona et al. (2019) devised an optimized “complement-reverse complement (CRC) motif” to enable efficient circularization upon incorporation into a variety of linear RNA sequences up to 4 kb in size [44]. Alternatively, if the RNA precursor sequence is to remain unchanged, helper oligonucleotides can be added to the reaction to ensure proper pre-orientation (Fig. 3C). To this end, various groups have reported increased circularization efficiency using an RNA splint binding to the ends of the precursor while leaving 2–3 nucleotides at the ligation junction unpaired, or when using a DNA oligonucleotide that hybridizes away from the reactive ends to be ligated, thus increasing their accessibility to the enzyme [41,52,54]. Beyond RNA secondary structure, the terminal nucleotides to be joined also affect the yield of a T4 RNA ligase 1 reaction, with cytidine and adenosine preferred at the 5′ and 3′ ends, respectively [41,55].
T4 RNA ligase 2 has also been successfully used to effect circularization of RNA [56]. It is compatible with both double-stranded and single-stranded substrates, but joins nicks within double-stranded RNA with highest efficiency (Fig. 3C) [41]. Petkovic et al. (2013) used RNA ligase 2 to circularize a 104 nucleotide single-stranded RNA in which the ends were held together through internal base pairing [57]. Intramolecular ligation can also be promoted by the use of a complementary DNA or RNA splint. Abe et al. (2018) observed that the efficiencies of T4 RNA ligase 2 and T4 DNA ligase 1 were comparable when used with a 20 nucleotide DNA splint to circularize a 252 nucleotide linear RNA [49]. Without the splint, however, the efficiency of the RNA ligase remained unchanged whereas that of the DNA ligase decreased. An original strategy for high-yield circularization using RNA ligase 2 without a splint was recently advanced by Chen et al. (2020) [58]. By permuting the sequence of the linear RNA precursor such that the 3′ terminus falls within a double-stranded region, circularization of different small circRNAs was achieved at a preparative scale with close to 100% selectivity [58]. Since this approach requires a minimum of 3 continuous base pairs at the 3′ terminus of the linear precursor, it can potentially be applied for circularization of RNAs widely diverse in sequence, structure, and size.
Independent of the type of ligase used, RNA concatemers formed by intermolecular ligation are major competing byproducts. A general strategy for minimizing intermolecular ligation is to maintain a low concentration of linear precursor in a relatively large reaction volume [40,47,58]. Following reaction completion, the product can be treated with exoribonuclease RNase R or exonuclease T for degradation of linear RNAs having a free 3′ end [58,59]. Size selection is routinely used in conjunction with enzymatic enrichment to further purify circRNA away from other reaction components [33,44,75,87,109,132]. Separation can be achieved by polyacrylamide gel electrophoresis (PAGE), as circRNAs exhibit different migration patterns from their linear counterparts of equal length on PAGE gels [60,61]. However, the properties of these gels limit them to isolation of relatively small circRNAs (less than 500–600 nucleotides) [62]. For resolution of larger RNAs, denaturing agarose gels are generally used, with the caveat that linear and circRNAs of the same size often have identical migration patterns on these gels. This can potentially be circumvented by treatment of the ligation products with E. coli poly(A) polymerase. Polymerization adds 100–200 adenosines to linear RNA to visibly reduce its electrophoretic mobility, while that of the circRNA remains unchanged due to its lack of a free 3′ end [44]. In cases where large circRNA yields of maximal purity are warranted, high performance liquid chromatography (HPLC) has been successfully employed to fractionate an RNA mixture based on differences in the molecular weights of individual species [44,63,64].
3.3. In vitro circRNA synthesis using ribozymes
Ribozymes are RNAs that perform the catalytic actions typical of protein enzymes. The use of certain ribozymatic sequences constitutes a third strategy for producing circRNAs in vitro. The most popular of these methods rely on the autocatalytic activity of group I introns naturally found within the rRNA, tRNA, and mRNA genes of bacteria and non-metazoan eukaryotes. Group I introns splice themselves out without assistance from the spliceosome or other proteins, and instead rely on magnesium and a free guanosine nucleotide to initiate the splicing reaction in vivo. This process results in joining of the flanking exons and circularization of the intervening intron to produce an intronic circRNA.
3.3.1. The PIE system
Puttaraju and Been (1992) were the first to demonstrate that the group I intron system can be adapted for generating a circRNA of interest from a linear intron-containing precursor [65]. The Anabaena pre-tRNALeu gene was used to design a permuted intron exon (PIE) construct in which the 5′ half of the group I tRNA intron was transferred to the tail of the tRNA exon and the remaining 3′ half of the intron was positioned at the head of the same exon (Fig. 4fig4A). IVT of this permuted template, supplemented with free guanosine, leads to spontaneous circularization of the intervening exonic sequence (Fig. 4B). Ford and Ares (1994) replicated this strategy by permuting the intron from the thymidylate synthase (td) gene of T4 bacteriophage to circularize the td exon [66]. Since then, the permuted T4 td and Anabaena pre-tRNA genes have become staple backbones in the field for circularization of a wide assortment of exonic sequences inserted between the group I intron halves [67–76]. Wesselhoeft et al. (2018) reported significant increases in circRNA yield when a luciferase sequence was transferred from a PIE construct with td introns to one containing Anabaena pre-tRNA introns [75], suggesting that choice of group I intron affects circularization efficiency. Using the Anabaena backbone, the authors generated a circRNA up to 5 kb in length, whereas chemical and enzymatic ligation strategies normally show reduced yields for RNAs greater than 1 kb [41,75].
Fig 4.
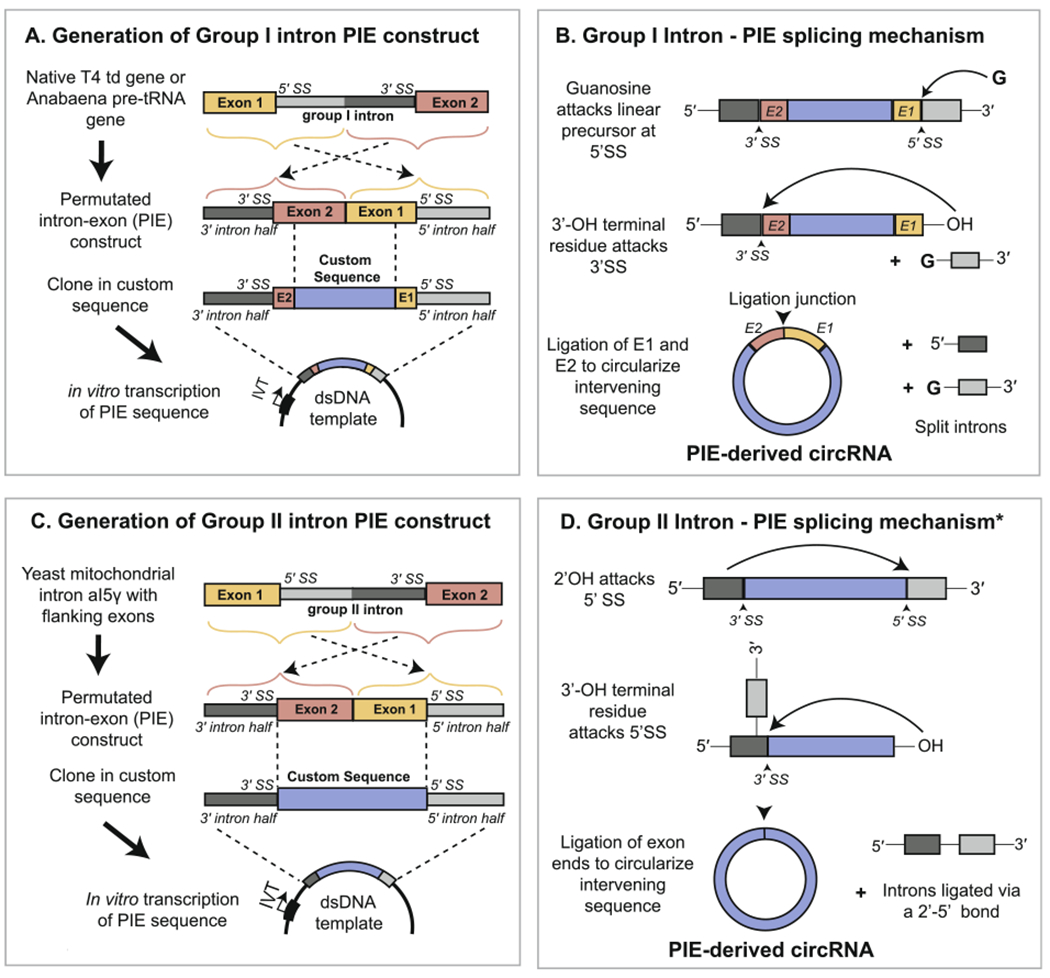
Generation of circRNA in vitro by the PIE method. (A) Generation of a PIE RNA construct from permutation of a native group I intron and insertion of a custom exonic sequence. (B) Upon in vitro transcription of the PIE sequence, circularization of the exonic region occurs auto-catalytically in the presence of free guanosine. A small portion of the flanking exonic sequence from the native group I intron-containing gene is retained at the ligation junction, which is formed from a 3′–5′ phosphodiester bond. (C) Generation of a PIE RNA construct from permutation of a native group II intron and insertion of a custom exonic sequence. Use of a Group II intron allows for the complete substitution of the native exonic sequence. (D) Upon in vitro transcription, circularization of the exonic region occurs auto-catalytically following release of the 5′ intron half (light gray). The asterisk signifies that the depicted mechanism, involving attack of the 2′ OH from the 5′ splice site, is one of multiple mechanisms by which exon release can occur. The resulting circRNA is ligated with a 3′-5′ bond and lacks remnants of the native group II intron-containing gene.
A critical limitation of the existing PIE method is that it requires retention of the terminal portions of the original phage or Anabaena exons at the ligation junction of the final circularized product (Fig. 4B). Rausch et al. (2021) published a novel strategy for overcoming this drawback that allowed for use of a td PIE backbone to make a perfect sequence mimic of circPVT1, a 410 nucleotide circRNA from the human PVT1 gene [77]. This was achieved via selective permutation of the linear circPVT1 precursor such that its ends contain sequences resembling the retained portions of the td exon that could function equivalently to drive circularization. To expand the utility of their strategy, the authors used RNA-seq to screen for alternative sequences from a synthetic RNA library, and identified some that even resulted in higher circularization efficiencies than the native td exonic sequence. The results of this screen were incorporated into a publicly available bioinformatics pipeline to aid prediction of the optimal permutation for any given circRNA sequence [77].
In addition to group I introns, group II introns have also been used to produce circRNAs in vitro. Group II introns constitute a ribozymatic self-splicing system with a mechanism similar to that of both group I introns and pre-mRNA introns. Mikheeva et al. (1997) demonstrated that a PIE construct capable of generating a human circRNA sequence in vitro could be made using a permuted group II intron from the yeast mitochondrial genome (Fig. 4C) [78]. Unlike the group I intron PIE system, use of a group II intron results in a splicing mechanism that does not require the native yeast exons, thus allowing for the production of a perfect sequence mimic (Fig. 4D). The removed group II intron and the circularized exon are ligated with 2′-5′ and 3′-5′ phosphodiester bonds, respectively, but the precise mechanisms by which this is achieved in vitro remain unclear [33,78,79]. Future work in this area is likely to clarify these mechanistic details and the overall applicability of this approach.
3.3.2. Use of ribozymes derived from subviral agents for ligation
The circRNA genomes of subviral agents, such as viroids and the hepatitis delta virus, have also served as a source of inspiration for directed synthesis of circRNAs in vitro. The genomes of these pathogens include ribozymatic elements, namely hairpin ribozymes (HPRs) and hammerhead ribozymes, that facilitate subviral genome replication in vivo through their intrinsic cleavage and ligation activities [80]. For application in vitro, Diegelman et al. (1998) demonstrated that the HPR element and its respective cleavage motif can be used to generate circRNAs from a circular, single-stranded DNA template [81,82]. Rolling circle transcription of this circular DNA by T7 or E. coli RNA polymerases generates a linear RNA concatemer capable of spontaneous self-cleavage and ligation of the resulting monomers to form RNA circles. Compared to previously mentioned methods for circRNA synthesis, this strategy is distinguished in its suitability for generating high yields of small circRNAs (~50–150 nucleotides) with sequence homogeneity at the ligation junction. However, this system has not seen wider use within the field likely due to the following drawbacks: i) the sequence of the HPR ribozyme and cognate cleavage site remain embedded in the final circRNA, ii) the resulting circRNA itself retains active ribozymatic activity causing vacillation between a cleaved (i.e. linear) and ligated (i.e. circular) conformation, and, (iii) upon introduction into cells, a circRNA containing these sequences can potentially behave in trans to cleave other RNAs, leading to off-target effects.
More recently, Petkovic et al. optimized the use of HPR ribozymes for circRNA production [39,57]. The authors observed that the circularized RNA state can be favored by using custom RNA sequences with an overall stabilizing secondary structure and certain co-factors that further support ligation [83,84]. Additional research in this area is likely to clarify the applicability of this ribozymatic strategy in comparison to other circRNA synthesis methods, as well as reveal additional ways ribozymatic sequences can be aptly customized for circRNA production in vitro.
4. In vivo circRNA synthesis
Custom circRNAs can be generated in living cells by the overexpression of a plasmid harboring a minigene sequence that is naturally circularized following transcription [85,86]. Since splicing is the primary mechanism for circularization in metazoans, most circRNA minigenes feature at least an exonic region containing the sequence to be circularized, and 5′ and 3′ flanking intronic sequences that contain the splicing motifs. Several minigene designs have been presented for circularization of virtually any sequence from 100 nucleotides to 5 kb in size by using different combinations of intronic cis-elements. These designs can be divided into four categories, namely those using (i) introns with complementary sequences, (ii) introns with binding motifs for certain regulatory proteins, (iii) introns with ribozymatic activity, and (iv) introns derived from metazoan tRNAs (Figures 5–6fig5,Fig. 6).
Fig 5.
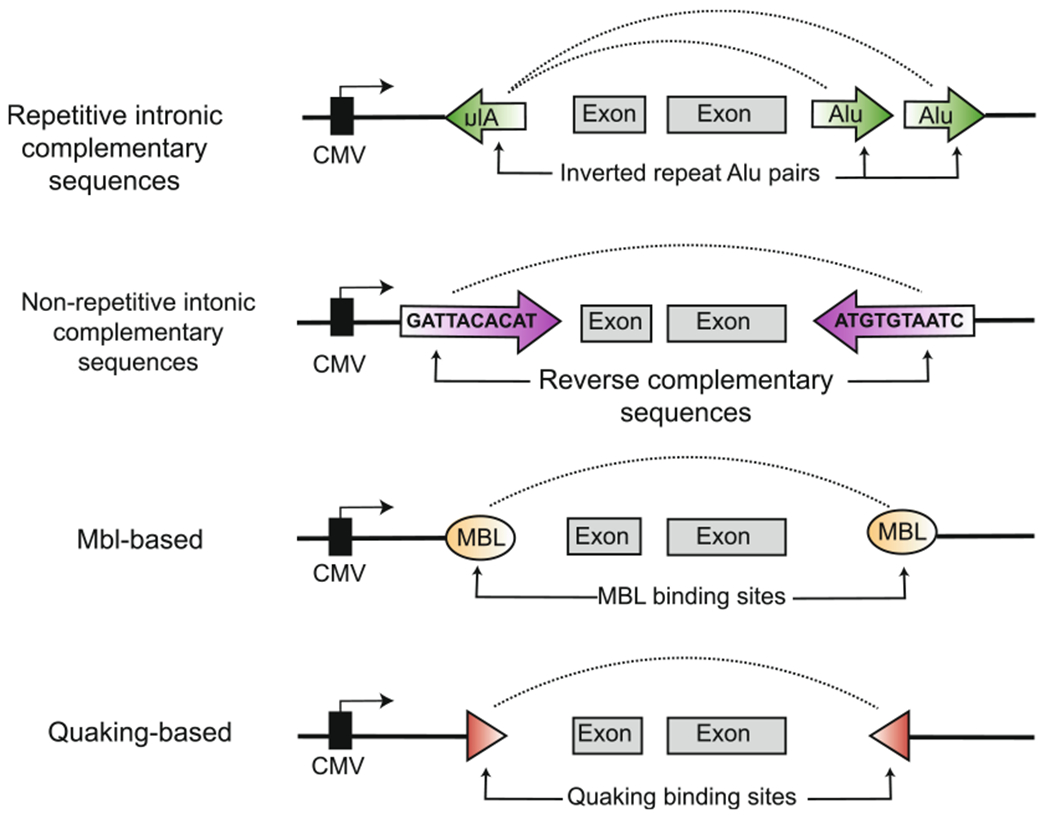
Plasmid minigene designs for producing circRNA in vivo. CircRNA production in cells can be achieved using pol II-based minigenes containing certain intronic elements. Introns are represented as thin black lines surrounding the exonic sequences. Inclusion of repetitive Alu elements, non-repetitive regions of reverse complementary sequence, and protein-binding sites for Muscleblind or Quaking have been shown to promote circRNA formation. These elements commonly work by bringing together the exon-flanking portions of the introns, containing the downstream donor splice site and upstream acceptor site, in close proximity.
Fig 6.
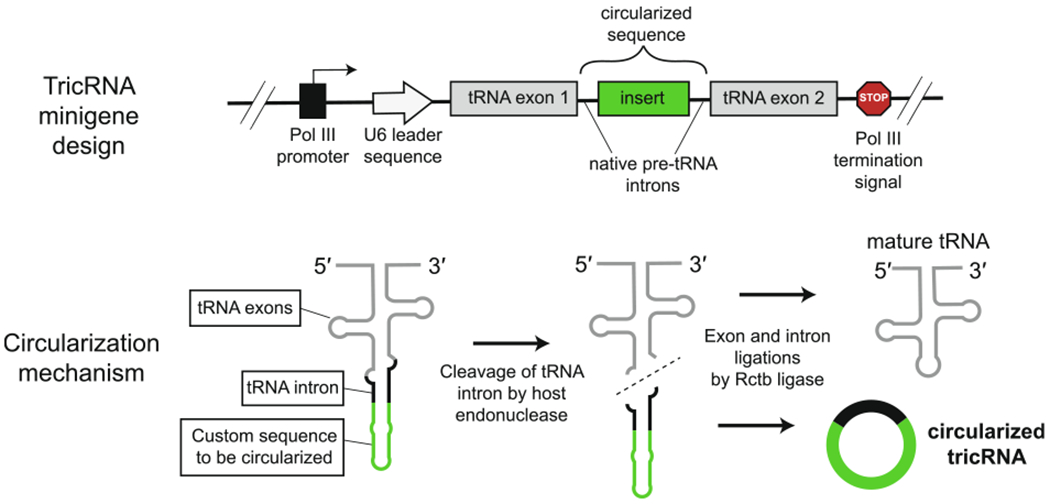
tRNA-based system for circRNA generation in vivo. Top: Structure of the minigene devised to produce customs circRNAs from a metazoan pre-tRNA backbone [30,106]. The circRNA sequence of interest is inserted within the intron of a tRNA gene and high copy-number expression is driven by use of a pol III promoter. Bottom: Circular intronic tRNAs (tricRNAs) are generated when the pre-tRNA transcript is processed by host enzymes that cleave the intron and subsequently ligate it. Portions of the tRNA intron are retained at the ligation site.
Compared to circRNAs produced entirely in vitro, the circRNAs produced in vivo from plasmids are usually preferred models for endogenous circRNAs due to their generation in situ. In vivo expression has proven particularly indispensable for obtaining insights into circRNA biogenesis and function. However, the usefulness of minigene systems for studying circRNAs remains undermined by specificity issues. Circularization vectors can still produce variable amounts of unwanted heterogenous cis-, trans-, and backspliced RNAs that cannot be readily identified nor purified away in vivo, and background circRNA production has been observed using plasmids lacking any elements known to enhance circularization [87,88]. As the degree of these non-specific effects is highly sequence and context dependent, determination of the optimal vector for a given experimental system is best performed empirically by rigorous testing from a panel of potential designs, as presented below (Table 1).
4.1. Minigenes using intronic complementary sequences (ICS)
Transcriptome-wide RNA-seq has revealed that the flanking introns of most endogenous circRNAs are enriched for sequences that are the reverse complement of each other [5,23]. Base-pairing between these flanking regions of reverse complementarity is postulated to create a local region of double-strandedness in the premature RNA transcript [89]. As a result, an upstream acceptor and downstream donor splice site are brought into close proximity in a way that promotes backsplicing. This endogenous mechanism for circRNA formation has been widely adapted to create circRNAs from custom RNA sequences by incorporating different types of complementary sequences into the flanking introns of plasmid minigenes. These intronic complementary sequences (ICS) can be repetitive or non-repetitive in nature, and derived entirely from endogenous genes or partially designed in silico.
4.1.1. Repetitive ICS
Repetitive ICS used to design circularization vectors are derived from endogenous DNA repeat elements that are ubiquitous in the genome under study. In humans and primates, the most common repetitive sequences are Alu elements, which are ~300 nucleotide conserved A-rich sequences belonging to a class of retroelements known as short interspersed repeat elements (SINEs). Alu elements are estimated to make up 11% of the human genome, and are particularly enriched within the introns of protein-coding genes, leading to their localization in pre-mRNA transcripts [90].
Nearly 90% of endogenous human circRNAs are flanked by complementary Alu elements [5,24,91,92]. These intronic Alu sequences situated across circularized exons are often oriented opposite to each other, suggesting their ability to form inverted repeated Alu (IRAlu) pairs [24]. Zhang et al. (2014) showed that generation of a Pol2ra minigene with a reverse Alu element in the upstream intron and two forward Alu elements in the downstream intron (thus imitating the endogenous gene locus) was sufficient to drive transcript circularization, and this phenotype was reversed upon deletion of putative IRAlu pairs (Fig. 5) [24]. Introns can be up to tens of kb in length, but the 500–800 nucleotide sequence most proximal to each side of the exon is usually sufficient to capture the IRAlu pairs needed to recapitulate circularization of most endogenous circRNAs [93].
As Alu elements are primate-specific, extension of this design strategy to non-primates requires identifying alternative repetitive elements native to those species. The HY_pMT circularization vector is a successful example of such a backbone that uses 150 nucleotides of the native intronic sequence from the Laccase2 gene in Drosophila [91]. These intronic fragments each contain a divergently oriented DNAR-EP1_DM transposon, which are found at unusually high copy numbers throughout the Drosophila genome [94]. Transfer of these repeats into the HY_pMT plasmid yielded a backsplicing efficiency comparable to the endogenous Laccase2 locus.
As an alternative to using the endogenous gene locus, one can also clone the sequence of interest into a vector containing introns derived from an orthogonal gene already known to circularize efficiently [86]. Vectors available for this purpose include those with introns from the Zkscan1, Hipk3, and Laccase2, among other genes [8,14,23,91]. These backbones are particularly useful if the sequence of interest is not naturally circularized or the endogenous determinants of circularization are not easily identified. The backsplicing efficiency will depend on the overall secondary structure of the pre-mRNA transcript, and thus varies with the pairing of different exonic sequences and intronic backbones.
4.1.2. Non-repetitive ICS
Circularization minigenes can also be made using endogenous ICS that are not repetitive. This includes all complementary sequences across flanking introns that are not due to the presence of known DNA repeat elements, such as SINEs (Fig. 5). The sex-determining region-Y (Sry) gene, which produces a highly abundant circRNA in mice, is a prototypical example of an endogenous locus containing introns with non-repetitive complementary sequences [89]. Dubin et al. (1995) observed that the circularized Sry exon is flanked by 15.5 kb of complementary sequences, and that nearly complete removal of either the upstream or downstream ICS completely abrogated circSry expression. Years later, profiling by RNA-seq further revealed a transcriptome-wide enrichment of these non-repetitive complementary sequences in the introns flanking human circRNAs [24]. Most of these endogenous non-repetitive ICS are significantly shorter than that of circSry, and it has been determined that even short 30–40 nucleotide stretches of reverse complementarity can be sufficient to recapitulate circularization [23]. This has facilitated incoporation of endogenous introns containing non-repetitive complementary sequences into diverse circularization minigene designs.
The introns containing complementary sequences can also be partially designed in silico. This is usually carried out by pairing an intronic sequence from any endogenous gene with an artificial second intron comprised of the exact reverse complement sequence. In some instances, such minigenes have been reported to promote circularization at higher rates than minigenes using entirely endogenous ICS [88,95]. However, the opposite has also been reported for other minigene systems, and the mechanisms determining circularization efficiencies are not yet fully understood [91]. This approach has been used to generate circular GFP and mCherry reporters for obtaining robust fluorescent readouts of backward and forward splicing ratios in human cells [88,95,96].
4.2. Minigenes using RBP motifs
Multiple RNA binding proteins have roles in enhancing backsplicing in vivo. As a result, their binding sites can be integrated into overexpression vectors to drive circularization of the ensuing transcript.
4.2.1. Musclebind
In Drosophila and humans, the RBP Muscleblind (Mbl) binds to conserved sites throughout the genome and increases backsplicing of RNAs from these loci [97]. These sites are specifically located in the introns flanking the circularized region, and their presence on both sides is thought to promote Mbl dimerization that brings the flanking introns into close proximity. Interestingly, dozens of these sites are concentrated in the introns flanking exon 2 of the Mbl gene itself. RNA immunoprecipitation experiments have confirmed that the Mbl transcript interacts with the Mbl protein, suggesting a feedback loop in which Mbl down-regulates further expression of its own protein-coding transcript by increasing expression of the circular, non-coding isoform.
Ashwal-Fluss et al. (2014) applied these findings to design circularization minigenes containing introns from the second exon of the Mbl gene (Fig. 5) [97]. Upon expression in Drosophila S2 cells, these MBL-backbone vectors were sufficient to drive robust backsplicing of orthogonal exonic sequences due to the ICS and Mbl binding sites in the introns. Co-transfection of the Mbl protein encoded on a separate plasmid further increased circRNA yields. Their Mbl-based circularization vectors were also highly effective in human cells, which express three homologs of Muscleblind (MBNL1, 2, 3).
4.2.2. Quaking
The RBP Quaking (QKI) is another trans-acting factor known to facilitate backsplicing. The role of QKI in regulating circRNA formation was discovered in an small interfering RNA (siRNA) screen for proteins that altered circular to linear splicing ratios from a minigene reporter [98]. QKI knockdown decreased production of the circular reporter without significantly impacting linear splicing. Follow-up investigations suggested a mechanism similar to Mbl: QKI bound directly to the reporter pre-mRNA at sites immediately flanking each side of the circularized region. Broader assessment revealed QKI knockdown also reduced the abundance of multiple endogenous circRNAs harboring QKI binding sites.
To conclusively demonstrate the ability of QKI to direct circRNA biogenesis, the authors generated minigenes from coding regions verified to solely produce linear transcripts. Upon adding QKI binding sites, in vivo circularization of these constructs could be observed (Fig. 5). Zhao et al. (2019) used QKI binding sites to enhance expression of a viral circRNA sequence from a plasmid containing genes from the parental human papilloma virus [99]. Enhancement was observed despite the lack of introns in the compact 8 kb HPV genome. These examples indicate that engagement of QKI-dependent circRNA biogenesis is achieved by including the binding site (ACTAACN1-20TAAC) within the minigene construct. Compared to the Mbl minigene system, this strategy does not require RBP co-transfection in human cells and is more suited for modular integration into pre-existing backbones.
Several other RBPs have since been discovered to regulate circRNA biogenesis by inducing dimerization of the introns flanking the exon(s) to be circularized (e.g. NF90/110, RBM20, Fus, DHX9), or by enhancing or silencing recognition of certain splice sites (e.g. hnRNPs and SR proteins) [26,96,100–104]. However, investigation into the impact of incorporating these protein binding sites into pre-existing circularization vectors is relatively limited [102]. Such work has the potential to expand our understanding of the diverse mechanisms by which RBPs promote circularization, as well as provide useful reagents to the community for the expression of custom circRNAs in vivo.
4.3. Minigenes using the PIE system
Among the in vitro strategies previously described, the PIE method is unique as it can also be applied for circRNA production in vivo. Once a plasmid containing the permuted group I template is generated, in vivo expression of the intervening circRNA sequence is achieved by transfection of the plasmid into cells. In contrast to minigene systems using pre-mRNA introns, plasmids containing a PIE sequence produce linear transcripts that circularize due to the self-splicing ribozymatic activity.
In vivo production of RNA circles by the PIE method was first performed in E. coli and yeast using plasmids containing the permuted td backbone [66,71]. By substitution of the exonic sequence, a wide variety of small to mid-sized circRNAs (71–1130 nucleotides) could be robustly produced in these organisms that resembled the splicing products obtained from IVT of the same PIE sequence. Since then, this strategy has been successfully adopted to produce functional circRNAs at high titers in bacteria and fungi [76,105].
The efficacy of this strategy in mammalian cells remained unclear for a long time, as there were no reports regarding its adoption for mammalian expression [41]. This vacancy was addressed upon publication that a plasmid containing circularization signals derived from permuted phage introns robustly stimulated innate immune signaling in HeLa cells, leading to the death of transfected cells [74]. A circRNA with the same exonic sequence but made from ZKSCAN1 introns did not have the same effect. Mechanistic investigation confirmed that intron identity can be an immunogenic determinant of exogenous circRNAs. CircRNAs produced by the endogenous spliceosome associate with certain RBPs that mark the RNA as self, but these proteins are absent from circRNAs produced independently by autocatalytic means. These findings highlight immunogenicity as a factor of worthwhile consideration when choosing between minigene systems that use a spliceosome-assisted vs spliceosome-independent (i.e. ribozymatic) method for in vivo circularization. The latter strategy is likely better suited for producing circRNAs in non-metazoans lacking mammalian pre-mRNA splicing and innate immune signaling systems.
Compared to the PIE system, other ribozymatic strategies for generating circRNAs have not yet been tested as extensively for application in vivo. Given similar mechanisms, it is plausible that these other methods would lead to robust circRNA production in bacteria and fungi but exhibit limited production in mammalian cells. However, more investigation is needed to determine the extent to which this occurs.
4.4. Minigenes using tRNA introns
A novel method for achieving high copy, in vivo expression of engineered circRNAs has been developed based on metazoan tRNA splicing. In 2015, analysis of Drosophila RNA-Seq data revealed a class of abundant, tRNA intron circular RNAs (tricRNAs) that are generated independently from the spliceosome but not produced in an auto-catalytic manner [30]. Instead, tricRNAs result from the cleavage of intron-containing pre-tRNAs by a set of highly conserved host enzymes, followed by intramolecular ligation of the excised introns by the host ligase RctB (Fig. 6). By insertion of custom RNA sequences between the intronic cleavage sites, this mechanism was adapted to generate “designer tricRNAs” from plasmids containing a Drosophila tRNA gene backbone (Fig 6) [30,106]. Further sequence elements are included to ensure transcription of the insert by RNA polymerase III (pol III), the polymerase responsible for abundant production of tRNAs and other small RNAs. Although endogenous pol III transcripts are generally small (less than 400 nucleotides), their system was able to efficiently generate circRNAs ranging in length from approximately 75 to 800 nucleotides upon expression in human and Drosophila cell lines.
A significant update to this system increased the circularization efficiency by replacing the tRNA backbone with self-cleaving ribozymatic sequences [107]. This new construct, dubbed the Tornado system, achieved micromolar concentrations of circRNAs comparable to other pol III transcripts without inducing cytotoxicity. The authors tested the Tornado system for circularization of a linear aptamer sequence that sequesters NF-κΒ to prevent its activation of immune gene transcription. Using a luciferase-based NF-κB reporter, the authors observed approximately 8-fold lower immune activation following stimulation of the cells with IL-1β. The Tornado system was further shown to be effective for producing functional circRNA aptamers that bound small molecule targets, underscoring its broad utility.
5. Functionalization of artificial circRNAs
Once a suitable strategy is chosen for synthesis, the next step is to consider whether any supplemental elements should be incorporated into the design of the artificial circRNA to facilitate downstream experimentation. This is particularly relevant if the goal is to enable the circRNA to carry out a specific role in cells or to study its functional properties. There remains much to be uncovered about the roles of endogenous circRNAs in vivo, and strategies for directed circRNA functionalization remain in their infancy as there exist few standardized approaches. In this section, we highlight some of the diverse strategies used to functionalize artificial circRNAs by focusing on the most commonly desired applications.
5.1. Designing circRNAs for translation
A major challenge to designing circRNAs for protein production is their lack of a 5′ cap and a 3′ poly(A) tail. Without these features, circRNAs cannot proceed through the canonical, cap-dependent mechanism used to initiate efficient translation of linear messenger RNAs. This fact and the results from early studies originally led to the classification of circRNAs as universally non-coding [5,108,109], but it is now recognized that a few endogenous circRNAs can be translated through cap-independent translation [110–116]. These mechanisms have been adopted to facilitate translation of synthetic circRNAs, and further strategies have evolved for maximizing protein production from circRNAs downstream of the initiation step.
5.1.1. Translation initiation of circRNAs
Translation of a synthetic circRNA was first reported of a construct generated by in vitro splint ligation that contained an internal ribosome entry site (IRES) from encephalomyocarditis virus (EMCV) [43]. An IRES is a structured RNA element upstream of the start codon that recruits the eukaryotic ribosome to initiate translation in the absence of a 5′ cap. Originally discovered in uncapped viral transcripts, IRES sequences can also be found within the 5′ UTR of select eukaryotic linear mRNAs to allow for their dynamic regulation during cellular stress [117]. Thus far, a handful of endogenous circRNAs have been reported to be translated due to the presence of ribosome entry sites found at various positions along their sequence [118]. These examples of endogenous circRNA-specific IRESes have not yet been applied for the design of protein-producing circRNAs due to their current rarity and heterogeneity. Instead, most engineered circRNAs for translation use a virally-derived IRES upstream of the circular sequence containing the ORF (Fig. 7fig7A). Since the initial demonstration using an in vitro translation system [43], the ability of the EMCV IRES to direct circRNA translation in mammalian cell lines and animal models has been established by multiple studies [74,75,88,119,120]. Although the EMCV IRES is the most popular choice, Wesselhoeft et al. (2018) examined translation of a synthetic circRNA using eight alternative viral IRESes, of which the coxsackievirus B3 IRES increased translation 3–4 fold compared to that of EMCV [75].
Fig 7.
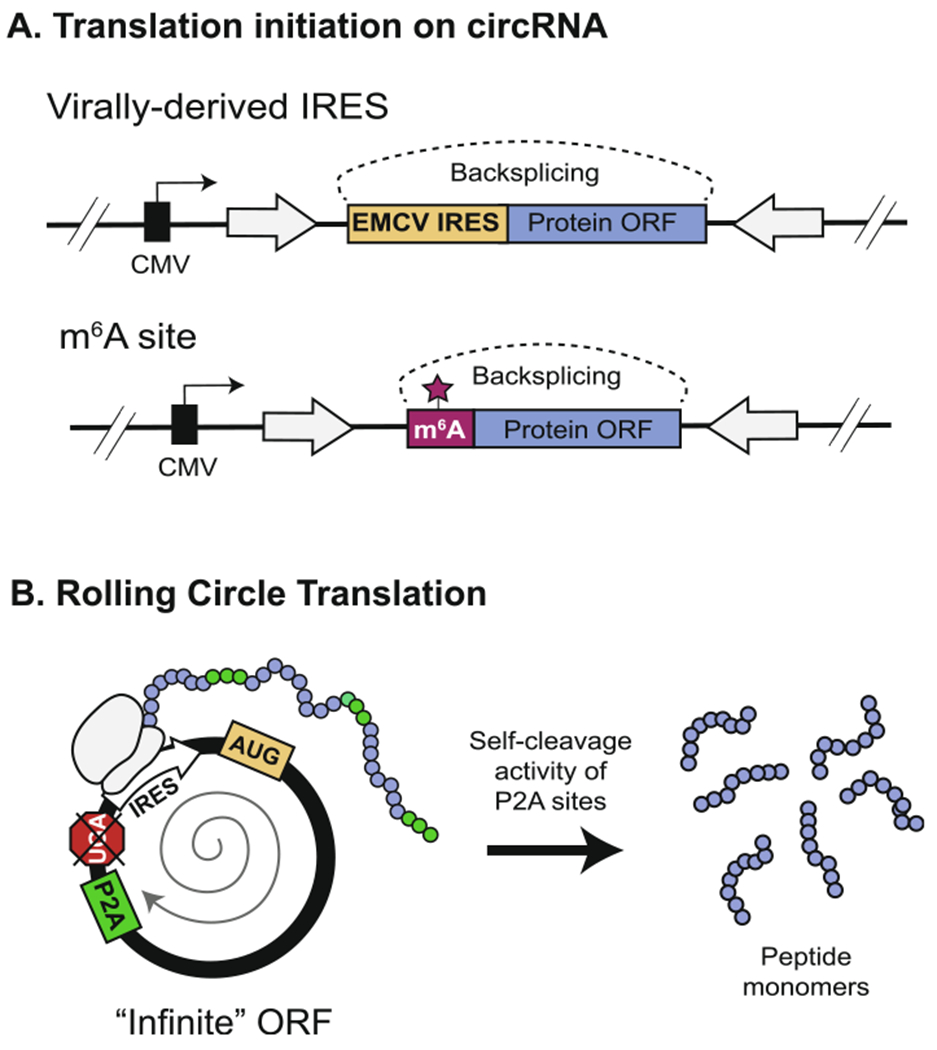
Strategies for circRNA translation. (A) As an alternative to cap-mediated translation, translation of a synthetic circRNA can be initiated by inclusion of a viral IRES sequence or an m6A modification site upstream of the protein open reading frame (ORF). (B) Translation is further enhanced by the removal of putative stop-codons from a circRNA sequence with a length divisible by 3 to create an “infinite” ORF (right). This allows the ribosome to continually circumnavigate the same circRNA, producing a long, repeating peptide. Inclusion of a P2A site allows for self-cleavage of the resulting protein into homogenous monomeric units [123].
The N6-methyladenosine (m6A) modification of the region immediately upstream of the start codon is another mechanism reported to enable cap-independent translation of endogenous circRNAs [121]. m6A is the most abundant internal mRNA modification in eukaryotes, and is responsible for controlling multiple aspects of RNA and subsequently cellular function [122]. m6A modification is dynamically regulated by a set of enzymes that install, remove, or recognize the modification on sites where the motif RRA6CH (R = G or A, H = A, C, or U) is present. Yang et al. (2017) demonstrated that the inclusion of a short 19 nucleotide fragment containing 1–2 copies of an m6A motif (“GGACU”) was sufficient to drive translation of a circular GFP sequence in human cells (Fig. 7A) [121]. This was reversed upon mutation of putative modification sites, and the reader protein YTHDF3 was found to be essential for m6A-mediated translation of circRNAs through its recruitment of the noncanonical translation initiation factor eIF4G2. More recently, m6A modification was also observed to be necessary for viral protein production from the HPV E7 circRNA, suggesting that the origin of the circRNA does not have to be eukaryotic [99]. Costello et al. (2019) applied these findings to create a translatable circRNA using an m6A motif as an “IRES-like sequence” for production of secreted erythropoietin from Chinese Hamster Ovarian cells [123]. Similar studies are needed to establish how broadly generalizable this strategy is for different circRNA expression constructs. Compared to viral IRESs, the m6A modification offers a desirable alternative particularly when construct size is a concern.
5.1.2. Enhancing circRNA translation post-initiation
Once the barrier to translation initiation is overcome, circRNAs have the potential to produce significantly more protein compared to their linear counterparts [75]. In addition to having longer half-lives, the covalently-closed topology of circRNAs allows them to engage the ribosome in rolling circle translation (Fig. 7B) [43,71,119,124]. During translation, the ribosome can traverse the length of the circRNA even after approaching a stop codon because the continuous nature of the RNA template poises the ribosome for reassociation, thus lowering the barrier for translation re-initiation. Complete removal of the stop codon from a circRNA with a length divisible by 3 generates an “infinite open reading frame” for translation of several copies of the protein sequence in the form a long, repeating peptide [124]. This strategy maximizes protein production, with the drawback being that the concatemerized protein may not necessarily be functional or homogenous. To overcome this, Costello et al. (2019) created a translatable circRNA template containing a 2A self-cleaving peptide (P2A) sequence that induces co-translational separation of the protein concatemer into functionally active, monomeric units (Fig. 7B) [123]. This strategy retains the productivity of the infinite open reading frame without sacrificing the quality of the final protein product.
Since the poly(A) tail of linear RNAs enhances their translation through the activity of poly(A) binding proteins, it has also been tested whether the addition of a poly(A) tract can enhance circRNA translation [125]. Wang et al. (2014) observed decreased translation upon addition of a 40 nucleotide poly(A) spacer to a circRNA minigene containing ICS, whereas Wesselhoeft et al. (2018) found that inclusion of such a spacer within an in vitro PIE construct increased translation [75,88]. These differing results suggest that the efficacy of a poly(A) spacer will likely depend on the specific construct design.
Lastly, it is worth noting that any approach that significantly boosts RNA expression from a circularization minigene in vivo will likely also increase translation of the ensuing circRNA. Numerous strategies developed for enhancing ectopic overexpression of linear RNAs from plasmids can be applied to meet this end. For example, several CMV-driven expression vectors contain a chimeric intron sequence immediately downstream of the promoter to engage intron mediated enhancement, by which inclusion of certain intronic sequences significantly enhances transcription of any gene containing that intron [126]. Mo et al. (2019) reported that inclusion of the commonly used β-globin/IgG chimeric intron in an ICS-based circularization vector increased both RNA and protein expression from the encoded circRNA [127].
5.2. Designing circRNAs for sponging miRNAs and proteins
Several circRNA-related functions have been attributed to their abilities to physically associate with miRNA or proteins. In many of these scenarios, the circRNA is thought to sponge its miRNA or protein target to prevent it from carrying out its normal function on a known mRNA substrate, and thus participate in a competitive endogenous RNA network [10,11,128]. These findings establish circRNAs as ubiquitous regulators of many cellular processes and suggest their utility for therapeutic applications. As a result, there has been growing interest in developing artificial circRNAs that recapitulate the RNA and protein binding abilities of certain endogenous circRNAs.
5.2.1. Circular miRNA sponges
The eukaryotic circRNA CDR1as/ciRS-7, which contains over 70 binding sites for miRNA-7 (miR-7), was among the earliest circRNAs to be functionally characterized. CDR1as actively sequesters miR-7 without being degraded by the associated RNA-induced silencing complex (RISC), and thus suppresses targeting of mRNAs with miR-7 seed sites [9,129]. The authors further demonstrated that another tissue-specific circRNA in mice from the Sry gene locus contains 16 binding sites for sponging miR-138 [9]. This pioneering work has inspired some groups to engineer artificial circRNAs that can function as sponges for suppression of the activity of pathogenic host miRNAs.
Jost et al. (2018) designed a circRNA sponge targeting miR-122, a host miRNA essential for replication of Hepatitis C virus (HCV) (Fig. 8fig8A) [61,130]. miR-122 directly binds to the 5′ end of the HCV genome, where it protects the viral RNA from degradation. CircRNA sponge for miR-122 (ciRS-122) was designed by concatenating 8 miR-122 binding sites that were each 22 nucleotides long and separated by 4 nucleotide spacers. The authors compared using a perfectly complementary miR-122 binding site to a bulged binding site containing a few mismatched nucleotides in the center. These constructs were transcribed in vitro and could both precipitate miR-122 upon transfection into cells, indicating that perfect complementarity to an miRNA target does not always lead to significant degradation as was previously reported for CDR1as [9]. Of note, a number of binding sites on the order of circRNAs CDR1as (~70 sites) or Sry (16 sites) was not needed to reduce viral protein levels in their cell culture model of HCV infection.
Fig 8.
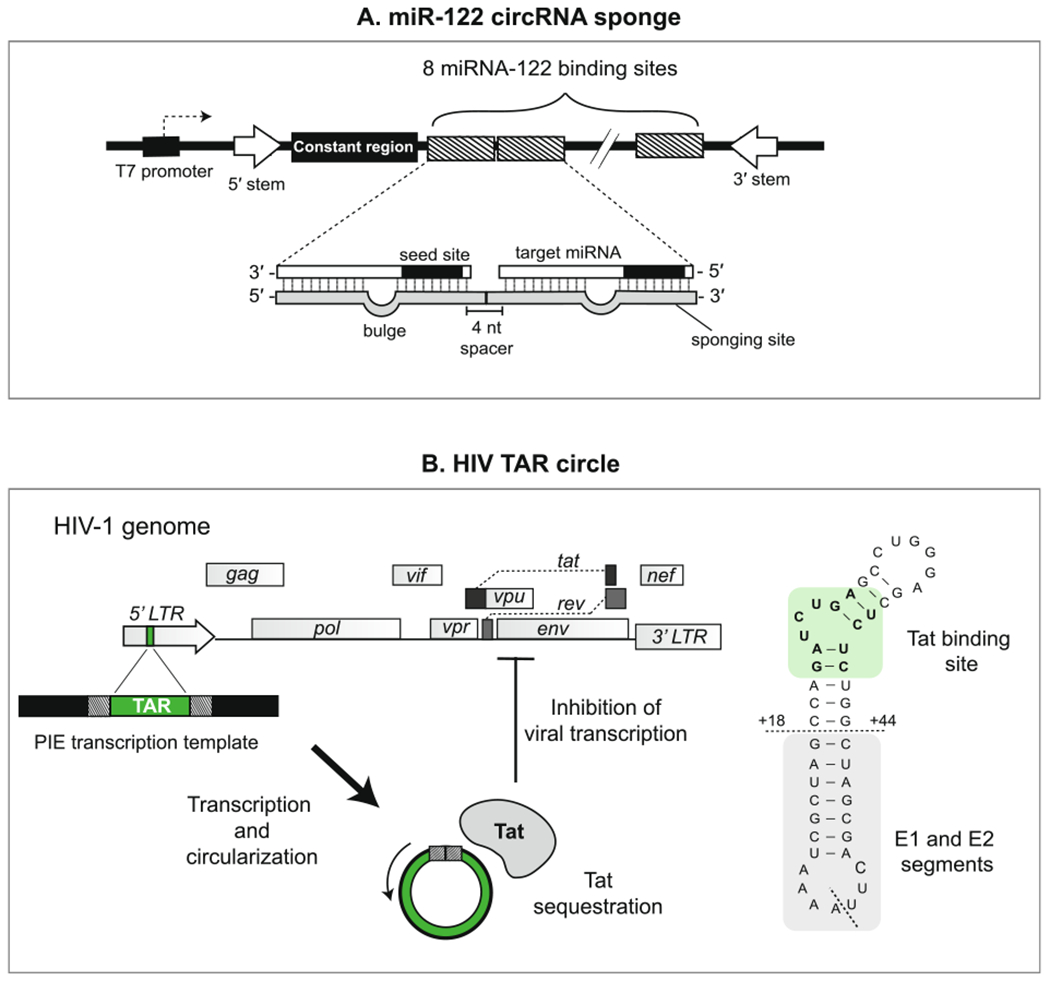
Design examples of miRNA- and protein-binding circRNAs. (A) Design of the minigene used to create a circular miR-122 sponge (ciRS-122) by Jost et al. (2018) [61,130]. 8 miR-122 sponge sites were concatenated, each exhibiting imperfect binding to miR-122 through introduction of a “bulged” region in the middle nucleotides. A constant region was included in the circularized sequence to facilitate detection of the circRNA sponge. Base-pairing sequences in the introns flanking the circularized region promote a stem-like secondary structure that facilitates circularization in vitro. (B) Design of the earliest protein-binding synthetic circRNA by Puttaraju and Been et al. (1992) [65,68,70]. The TAR sequence, found within the LTR promoter region of the HIV-1 genome, was incorporated into a PIE template to generate a synthetic RNA circle capable of binding the viral protein Tat. The sequence of the TAR circle (left) includes the Tat binding site (highlighted) and exonic sequences from the native group I intron (below horizontal dashed line). The ligation junction is indicated with a diagonal dashed line.
Subsequent efforts to create synthetic circRNA sponges exemplify the use of alternative design strategies. To suppress the activity of miRNAs implicated in cardiac hypertrophy, Lavenniah et al. (2020) engineered a circRNA containing 12 alternating binding sites (6 each) for miR-132 and miR-212 [131]. Using a luciferase reporter, an optimal spacer length of 12 nucleotides between adjacent miRNA binding sites was chosen from lengths ranging between 6 and 72 nucleotides. Two versions of the sponge containing either binding sites with perfect complementarity or bulged complementarity were compared, and the rescue activity of the former was significantly less due to its increased degradation. To underscore therapeutic application, the bulged construct was integrated into an adeno-associated viral vector and was able to reduce cardiac hypertrophy in a mouse model. The authors of this paper also generated a version of the sponge in vitro, but the effect was less potent than the plasmid-based expression system due to lower titers.
5.2.2. Protein-binding circRNAs
Several endogenous circRNAs have been functionally categorized as protein scaffolds, decoys or sponges due to their abilities to bind to and modulate specific protein targets. Likewise, various efforts have been put forth to engineer artificial circRNAs with similar protein-binding abilities. For example, upon discovery that endogenous circRNAs bind and suppress the double-stranded RNA sensor protein kinase R (PKR), Liu et al. (2019) were able to recapitulate this phenomenon by plasmid overexpression of circPOL2RA and circCAMSAP1, two circRNAs with double-stranded regions [132]. The therapeutic utility of this strategy was demonstrated when these circRNA overexpression plasmids were introduced into peripheral blood mononuclear cells from patients with systemic lupus erythematosus and resulted in reduced PKR autoactivation and expression of type I interferon.
A similar yet distinct approach to engineering protein-binding circRNAs was put forth by Puttaraju & Been (1992), who applied the PIE system in vitro to make a 48 nucleotide circRNA containing the TAR sequence from the HIV genome (Fig. 8B) [65,68,70]. Similar to the native TAR sequence, the synthetic TAR circle bound a peptide from the RNA binding domain of Tat, an HIV protein critical for transcription of viral genes. In cell-free assays, the TAR circle exhibited a longer half-life compared to its linear counterpart (20 min vs 12 h) and effectively inhibited viral transcription likely through sequestration of Tat. The authors subsequently applied this strategy to make another circRNA comprised of the Rev-response element from the HIV genome, which successfully bound the viral protein Rev critical for nuclear export of viral RNAs [70]. Similar to that of Liu et al. (2019), this work also provides proof-of-principle evidence that endogenous protein-binding motifs can be used to design inhibitory circRNAs for viral infection.
Alternatively, synthetic RNA aptamer sequences can also be used to design protein-binding circRNAs. Aptamer sequences are usually selected through screening a large library of randomized, linear RNA sequences for their abilities to optimally bind a specific target. Umekage et al. (2009) used this approach to produce an 83 nucleotide circular aptamer by transferring the sequence of a linear streptavidin-binding RNA into an optimized PIE construct [73,133]. Circularization was achieved in vitro and in vivo using E. coli. More recently, Litke et al. (2019) devised the Tornado system for production of circular aptamers in mammalian cells using an optimized backbone that combines elements of pre-tRNA splicing and ribozymatic processing [107]. The authors demonstrated that the Tornado system could produce circular aptamers at high copy number and efficiencies, thus facilitating physiological modulation of protein and small molecule targets in living cells. The Tornado system was tested by Schreiner et al. (2020) for in vivo expression of a circRNA sponge containing multiple synthetic binding sites for the splicing factor hnRNP L. Overexpression of the circRNA sponge resulted in strong cytoplasmic translocation of hnRNP L protein, and sequencing analyses revealed global changes in gene expression and alternative splicing comparable to those observed with siRNA knockdown of HNRNPL mRNA [134].
5.3. Designing circRNAs as fluorescent reporters
Artificial circRNAs can also be functionalized as fluorescent reporters to enable detection by imaging or flow cytometry. This can be achieved directly, by having the circRNA molecule itself yield a fluorescent signal, or indirectly, by coupling the circRNA to the production of another molecule that then yields the signal. Several designs of these reporter systems have been successfully used to gain visual and quantitative insights into circRNA turnover, movement, translation, and interaction networks.
5.3.1. Methods using fluorescent proteins
The most common method for generating a fluorescent reporter circRNA is by having it encode the sequence of a known fluorescent protein. For in vitro production of such a reporter, a detailed procedure by Wesselhoeft et al. (2018) was published using an optimized PIE construct containing the Gaussian luciferase sequence [75]. Following IVT, circularization of the luciferase sequence is driven by Anabaena pre-tRNA introns. Upon transfection of the circRNA into cells, use of an IRES derived from coxsackievirus B3 led to maximum fluorescence from their construct compared to the commonly used EMCV IRES.
Alternatively, a fluorescent circRNA reporter can also be produced in vivo using a circularization plasmid containing the fluorescent protein ORF downstream of an IRES sequence. Wang et al. (2014) exemplified this approach using a minigene design in which the GFP sequence was split such that a portion of the C-terminal ORF is placed upstream of the N-terminal sequence (Fig. 9fig9A) [88]. A protein with an intact ORF is produced if backsplicing occurs to restore the C-terminal sequence downstream of the IRES and N-terminal sequence. In this way, the fluorescent signal maintains some specificity for circRNA expression, as unspliced linear byproducts would not produce the intact GFP ORF. Meganck et al. (2018) demonstrated that this split-GFP transgene can be packaged into a recombinant adeno-associated virus vector for delivery into and expression in mice [120]. Multiple groups have also expanded on this work by creating dual expression vectors that encode two different fluorescent proteins to allow for concomitant detection of forward and backsplicing events (Fig. 9B) [96,135].
Fig 9.
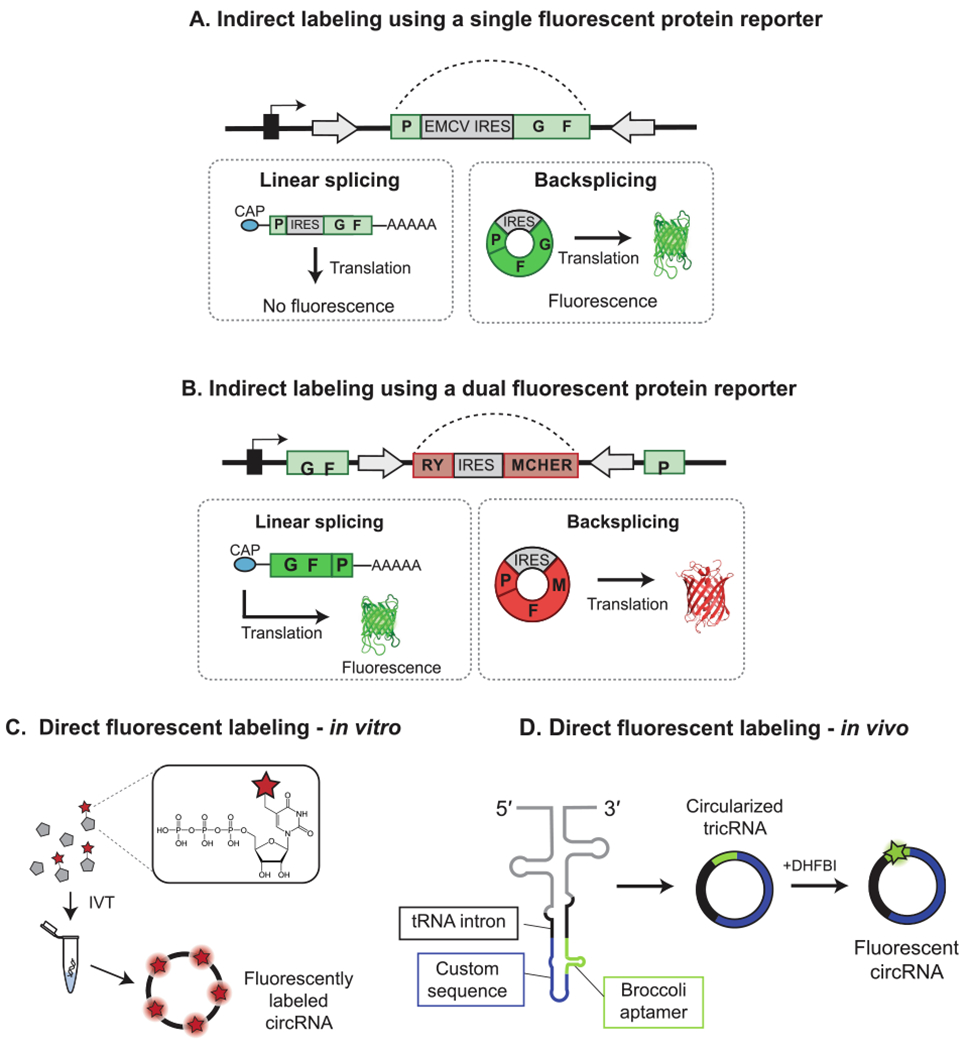
Design examples of fluorescent circRNA reporters. (A) Design of the split-GFP circRNA used to study circRNA backsplicing and translation by Wang et al. (2014) [88]. The N-terminus of GFP is positioned downstream of the C-terminus and IRES sequence. Backsplicing leads to restoration of the GFP ORF to lead to fluorescence, while linear monomeric byproducts do not result in an intact ORF and thus generate no signal. (B) A version of the circRNA fluorescent protein reporter designed by Li et al. (2017) in which the product of forward splicing produces a linear RNA encoding GFP, and backsplicing produces a circRNA encoding mCherry [96]. (C) Direct labeling of circRNAs in vitro is achieved by the inclusion of fluorescent nucleotides during in vitro transcription. (D) Direct labeling of circRNAs in vivo is achieved by the inclusion of fluorogenic aptamer sequence in the circRNA sequence [107]. The incorporation of the sequence for the GFP-like Broccoli aptamer in a tricRNA minigene is shown. Addition of the small molecule substrate DHFBI to transfected cells allows for visualization of cells actively expressing the circRNA.
5.3.2. Methods for direct fluorescent labeling
One important consideration in using fluorescent protein-based reporters is that they are direct reporters of circRNA translation but not circRNA transcription. Furthermore, these constructs are not ideal for characterizing sequence-specific properties of a particular circRNA since the sequence to be circularized necessarily encodes the fluorescent protein. To address these limitations, a strategy can be used in which the circRNA itself is directly labeled using fluorescent nucleotides. Chen et al. (2017) generated Cy3-labeled circRNA in vitro by including Cy3-UTP nucleotides during the in vitro transcription reaction (Fig. 9C) [74]. Cells that were successfully transfected with the Cy3-labeled circRNA could then be readily visualized by flow cytometry. As an alternative to enzymatic labeling, RNAs can also be fluorescently labeled by a variety of chemical means [136]. Dye-labeled nucleoside phosphoramidites are used to achieve site-specific labeling during automated chain assembly of an RNA oligonucleotide. For labeling post-synthesis, the RNA is first synthesized with unlabeled nucleotides that harbor specific reactive functional groups which can then be chemically conjugated to dye derivatives. Care should be taken to ensure that the in vitro labeling method is not incompatible with the circularization strategy: for example, RNAs synthesized using dye-labeled phosphoramidites present at the ends cannot be joined by T4 DNA ligase, which only recognizes specific end groups [136]. Following successful generation of the labeled circRNA, PAGE or HPLC purification is usually performed to remove unlabeled RNAs and excess dye.
To generate a direct circRNA fluorescent reporter in vivo, Litke et al. (2019) used the Tornado system to express a circularized version of the fluorogenic aptamer Broccoli within cells (Fig. 9D) [107]. The Broccoli RNA sequence binds the small molecule 3,5-difluoro-4-hydroxybenzyliene imidazolinone (DFHBI), causing the fluorescence of DFHBI to significantly increase to detectable levels. Compared to the linear aptamer, the circularized Broccoli sequence was reported to be nearly 200 times as bright without inducing significant immune activation or cytotoxicity despite micromolar accumulation. The authors further demonstrated that the 49 nucleotide Broccoli sequence could be appended to various functional RNA sequences encoded on the same circRNA, thus allowing for visual tracking of the whole circRNA molecule.
5.4. Modulating circRNA immunogenicity
CircRNA immunity is an emerging topic within the field due to its novelty and high potential for translational impact. There exists interest in ways to suppress the immunogenicity of circRNAs for purposes of protein delivery, as well as interest in how circRNA immunogenicity can be enhanced for the stimulation of anti-tumor and anti-viral immunity. Multiple published examples suggest that endogenous circRNAs are not intrinsically immunostimulatory, and may even serve immunosuppressive roles within cells through their interactions with various immune factors: (i) Li et al. (2017) reported that disassociation of the RBPs NF90/NF110 from circRNAs allow them to bind to viral RNAs and inhibit viral replication [96]; (ii) Liu et al. (2019) reported that endogenous circRNAs inhibit PKR, and during viral infection RNase L degrades these circRNAs to stimulate an antiviral immune response [132], and (iii) Xia et al. 2018 reported that the circRNA, cia-cGAS binds to the DNA sensor cGAS (cyclic GMP-AMP synthase) to prevent its activation by nuclear dsDNA and generation of type I interferon in hematopoietic stem cells [13].
In contrast to the existing consensus on endogenous circRNAs, the tendency for exogenously synthesized circRNAs to stimulate an immune response is less clear. Current reports suggest that the immunogenicity of exogenous circRNAs resembles that of synthetic linear RNAs in that it can be aptly modulated by selection of particular synthesis, purification, and chemical modification strategies (Fig. 10fig10).
Fig 10.
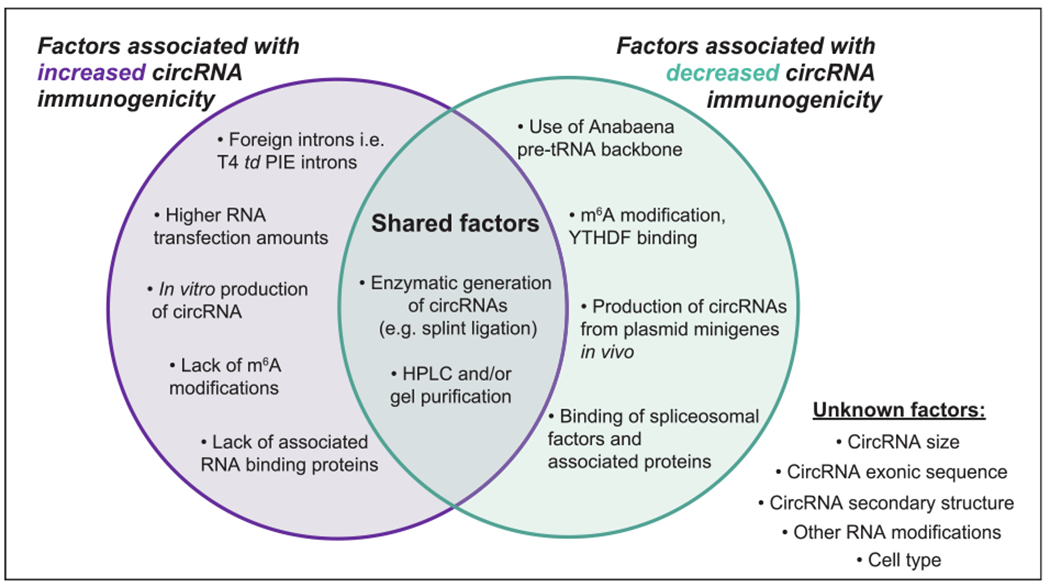
Factors associated with circRNA immunogenicity. Several factors are noted that have been found to either increase or decrease the immunostimulatory potential of exogenous circRNAs upon introduction into living cells [63,74,137]. In some cases, the same factor has been found to do both. There still remains several other properties of synthetic circRNAs whose effects on immunogenicity have yet to be determined.
5.4.1. Factors associated with increased circRNA immunogenicity
Chen et al. (2017) demonstrated that intron identity is a major determinant of circRNA immunogenicity [74]. They observed that use of a PIE construct based on the T4 group I intron to generate a circRNA either in vitro or in vivo consistently resulted in upregulated mRNA expression of a panel antiviral genes, including retinoic acid like gene I (RIG-I), in transfected HeLa cells. Mechanistic investigation revealed that the human splicing machinery and associated processes lead to the decoration of circRNAs with proteins that mark it as self and impede its detection by the RNA sensor RIG-I. For constructs produced by self-splicing, these marks are absent. This work suggests that immunogenic circRNAs are best prepared using the td PIE backbone, as opposed to a plasmid minigene system with pre-mRNA introns that would generate circRNAs via the normal backsplicing pathway. The authors were also able to confirm that generation of circRNA by splint ligation using T4 DNA ligase also resulted in immune stimulation, further suggesting that processing through endogenous splicing is needed to license circRNAs as “self”. Application of their findings to a mouse B16-OVA model showed that their foreign circRNA preparations were successful as tumor vaccine adjuvants capable of boosting CD8+ T-cell responses and slowing cancer progression.
Further mechanistic analysis into factors that contribute to the immunogenicity of PIE-derived circRNAs revealed an important role for m6A marks. Chemical modifications have long been recognized to regulate the immunogenicity of synthetic linear RNAs by interfering with their direct detection by cellular RNA sensors. Using an immunoprecipitation-based m6A-sequencing approach, Chen et al. (2019) found that their PIE-derived circRNA exhibited fewer and distinct m6A methylation marks compared to a similar circRNA generated using the ZKSCAN1 minigene backbone [137]. Abrogating mutations of the m6A motifs in the PIE-derived circRNA further increased its ability to stimulate antiviral immune genes, while enzymatic addition of m6A modified nucleotides during in vitro transcription of the construct had the opposite effect.
5.4.2. Factors associated with reduced circRNA immunogenicity
Wesselhoeft et al. (2019) expanded on this work by developing a novel system for generating synthetic circRNAs with reduced immunogenicity [63]. These circRNAs were also generated in vitro by the PIE method and were efficiently translated when introduced into mammalian cells. Compared to the work of Chen et al. (2017), several key differences exist in the design and experimental treatment of this PIE-derived circRNA that likely contributed to its reduced immunogenic potential, and may serve as guidelines for those wanting to produce immunogenicity-free circRNAs.
Wesselhoeft et al. (2019) produced circRNAs in vitro using a PIE-backbone based on a modified version of the Anabaena pre-tRNA group I intron system. Although a direct comparison between the immunogenicity of the T4 td and Anabaena pre-tRNA backbone was not performed, the authors did note that using the latter system resulted in higher circularization efficiencies of an exonic luciferase sequence, which they attributed to a more favorable secondary structure. Likewise, it remains plausible that differences in the sequence and secondary structure of these two backbones could lead to differences in the ability of the resulting circRNA to trigger certain immune sensors.
In addition to differences in design, Wesselhoeft et al. (2019) emphasize their post-synthesis purification strategy as key to minimizing immunogenicity of their circRNAs. They note that self-splicing of their PIE derived constructs produced several highly immunostimulatory, non-circular byproducts such as double-stranded RNAs and linear triphosphate RNAs. Application of a multi-layer purification strategy consisting of i) size purification by agarose gel electrophoresis or HPLC, ii) phosphatase treatment, and iii) RNase R digestion led to significant decrease in the ability of their circRNAs to stimulate cytokine release in A549 cells. Of note, Chen et al. (2019) later employed a similar purification strategy resembling that of Wesselhoeft et al. (2019), yet still found the resulting size-selected circRNAs to retain their immunogenicity in HeLa cells [137].
Additional investigation into the effects of RNA modifications by Wesselhoeft et al. (2019) found that N1-methylpseudoruridine did not alter the immunogenicity of circRNAs produced and purified in vitro using their system [63]. However, detrimental effects on circularization efficiency and translation were observed, suggesting that in vitro RNA modification strategies may not be feasible for all circRNA constructs.
Beyond those already mentioned, there remain several additional factors that are also important to consider when evaluating the immunogenic potential of a circRNA, including choice of: i) cell line model, ii) RNA delivery method, iii) RNA transfection amount, (iv) type of linear RNA used for baseline comparisons, and iv) the nature of the immune markers used as readouts (e.g. expression of antiviral mRNAs or protein cytokines). The pioneering work by Chen et al. and Wesselhoeft et al. highlight interesting strategies for altering these key variables and emphasize the need for more research to systematically characterize the factors determining circRNA immunogenicity in cells. To this end, the extensive body of research available on the immunogenic potential of synthetic linear RNAs may serve as a useful guide.
6. Conclusion
Many of the earliest efforts to generate artificial circRNAs relied on in vitro strategies grounded in biochemistry. In more recent years, several novel approaches based in molecular biology have emerged for creating circRNAs directly in living cells. These latter methods are mostly inspired by the diverse endogenous mechanisms now known to be responsible for natural circRNA production, and have been used in a reciprocal manner to gain additional insights into circRNA biogenesis. We anticipate that more optimized and standardized approaches for the generation of artificial circRNAs will arise out of continued work in this area. The use of high-throughput RNA-seq to screen libraries of RNA sequences can accelerate this process and circumvent the limitations of current strategies relying largely on endogenously derived sequences. The development of advanced models for circRNA production will further enable critical investigations into outstanding questions of circRNA function, and the translation of these insights into novel circRNA-based technologies.
Acknowledgements
This work was supported by the National Institute of Health (5K12CA215110), the American Cancer Society (IRG17-172-57), the Yale Cancer Center Pilot Grant, and the Rita Allen Foundation to Y.G.C, and the National Institute of Health (T32AI07019) to P.O.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B, Scrambled exons, Cell 64 (3) (1991) 607–613, 10.1016/0092-8674(91)90244-S. [DOI] [PubMed] [Google Scholar]
- [2].Jeck WR, Sharpless NE, Detecting and characterizing circular RNAs, Nat. Biotechnol 32 (5) (2014) 453–461, 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wilusz JE, A 360° view of circular RNAs: from biogenesis to functions, Wiley Interdiscip. Rev. Rna 9 (2018), e1478, 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO, Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types, PLoS ONE 7 (2012), e30733, 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE, Circular RNAs are abundant, conserved, and associated with ALU repeats., RNA New York N Y. 19 (2012) 141–57. 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO, Cell-type specific features of circular RNA expression, Plos Genet. 9 (2013), e1003777, 10.1371/journal.pgen.l003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Conn VM, Hugouvieux V, Nayak A, Conos SA, Capovilla G, Cildir G, Jourdain A, Tergaonkar V, Schmid M, Zubieta C, Conn SJ, A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation, Nat Plants 3 (2017) 17053, 10.1038/nplants.2017.53. [DOI] [PubMed] [Google Scholar]
- [8].Liang D, Tatomer DC, Luo Z, Wu H, Yang L, Chen L-L, Cherry S, Wilusz JE, The output of protein-coding genes shifts to circular RNAs when the Pre-mRNA processing machinery is limiting, Mol Cell. 68 (5) (2017) 940–954.e3, 10.1016/j.molcel.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N, Circular RNAs are a large class of animal RNAs with regulatory potency, Nature 495 (7441) (2013) 333–338, 10.1038/naturell928. [DOI] [PubMed] [Google Scholar]
- [10].Panda AC, Circular RNAs act as miRNA Sponges, Adv. Exp. Med. Biol 1087 (2018) 67–79, 10.1007/978-981-13-1426-l_6. [DOI] [PubMed] [Google Scholar]
- [11].Huang A, Zheng H, Wu Z, Chen M, Huang Y, Circular RNA-protein interactions: functions, mechanisms, and identification, Theranostics 10 (2020) 3503–3517, 10.7150/thno.42174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shi Y, Jia X, Xu J, The new function of circRNA: translation, Clin Transl Oncol Official Publ Fed Span Oncol Soc National Cancer Inst Mexico. (2020) 1–8. 10.1007/s12094-020-02371-1. [DOI] [PubMed] [Google Scholar]
- [13].Xia P, Wang S, Ye B, Du Y, Li C, Xiong Z, Qu Y, Fan Z, A circular RNA protects dormant hematopoietic stem cells from DNA sensor cGAS-mediated exhaustion, Immunity 48 (4) (2018) 688–701.e7, 10.1016/j.immuni.2018.03.016. [DOI] [PubMed] [Google Scholar]
- [14].Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, Huang S, Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs, Nat Commun. 7 (2016) 11215, 10.1038/ncommsll215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR, Lai EC, Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation, Cell Reports. 9 (5) (2014) 1966–1980, 10.1016/j.celrep.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lei M, Zheng G, Ning Q, Zheng J, Dong D, Translation and functional roles of circular RNAs in human cancer, Mol Cancer. 19 (2020) 30, 10.1186/s12943-020-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, Wu Y-M, Dhanasekaran SM, Engelke CG, Cao X, Robinson DR, Nesvizhskii AI, Chinnaiyan AM, The landscape of circular RNA in cancer, Cell 176 (4) (2019) 869–881.e13, 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weigelt CM, Sehgal R, Tain LS, Cheng J, Eßer J, Pahl A, Dieterich C, Grönke S, Partridge L, An Insulin-sensitive circular RNA that regulates lifespan in drosophila, Mol Cell. 79 (2) (2020) 268–279.e5, 10.1016/j.molcel.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kornblihtt AR, Schor IE, Alió M, Dujardin G, Petrillo E, Muñoz MJ, Alternative splicing: a pivotal step between eukaryotic transcription and translation, Nat. Rev. Mol. Cell Bio 14 (2013) 153–165, 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- [20].Braun S, Domdey H, Wiebauer K, Inverse splicing of a discontinuous pre-mRNA intron generates a circular exon in a HeLa cell nuclear extract, Nucleic Acids Res. 24 (1996) 4152–4157, 10.1093/nar/24.21.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cocquerelle C, Mascrez B, Hétuin D, Bailleul B, Mis-splicing yields circular RNA molecules, Faseb J. 7 (1) (1993) 155–160, 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- [22].Barrett SP, Wang PL, Salzman J, Circular RNA biogenesis can proceed through an exon-containing lariat precursor, Elife. 4 (2015), e07540, 10.7554/elife.07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liang D, Wilusz JE, Short intronic repeat sequences facilitate circular RNA production, Gene Dev. 28 (20) (2014) 2233–2247, 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang X-O, Wang H-B, Zhang Y, Lu X, Chen L-L, Yang L, Complementary sequence-mediated exon circularization, Cell 159 (1) (2014) 134–147, 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- [25].Pasman Z, Been MD, Garcia-Bianco MA, Exon circularization in mammalian nuclear extracts, Rna New York N Y. 2 (1996) 603–610. [PMC free article] [PubMed] [Google Scholar]
- [26].Chen L-L, The expanding regulatory mechanisms and cellular functions of circular RNAs, Nat. Rev. Mol. Cell Bio (2020) 1–16, 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- [27].Robic A, Kühn C, Beyond back splicing, a still poorly explored world: non-canonical circular RNAs, Genes-Basel. 11 (2020) 1111, 10.3390/genesll091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Y, Zhang X-O, Chen T, Xiang J-F, Yin Q-F, Xing Y-H, Zhu S, Yang L, L.-L. Chen, Circular intronic long noncoding RNAs, Mol Cell. 51 (6) (2013) 792–806, 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- [29].Gardner EJ, Nizami ZF, Talbot CC, Gall JG, Stable intronic sequence RNA (sisRNA), a new class of noncoding RNA from the oocyte nucleus of Xenopus tropicalis, Gene Dev. 26 (2012) 2550–2559. 10.1101/gad.202184.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lu Z, Filonov GS, Noto JJ, Schmidt CA, Hatkevich TL, Wen Y, Jaffrey SR, Matera AG, Metazoan tRNA introns generate stable circular RNAs in vivo, Rna New York N Y. 21 (9) (2015) 1554–1565, 10.1261/rna.052944.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Salgia SR, Singh SK, Gurha P, Gupta R, Two reactions of Haloferax volcanii RNA splicing enzymes: Joining of exons and circularization of introns, RNA 9 (2003) 319–330, 10.1261/rna.2118203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grabowski PJ, Zaug AJ, Cech TR, The intervening sequence of the ribosomal RNA precursor is converted to a circular RNA in isolated nuclei of tetrahymena, Cell 23 (2) (1981) 467–476, 10.1016/0092-8674(81)90142-2. [DOI] [PubMed] [Google Scholar]
- [33].Murray HL, Mikheeva S, Coljee VW, Turczyk BM, Donahue WF, Bar-Shalom A, Jarrell KA, Excision of group II introns as circles, Mol Cell. 8 (1) (2001) 201–211, 10.1016/S1097-2765(01)00300-8. [DOI] [PubMed] [Google Scholar]
- [34].Rong M, He B, McAllister WT, Durbin RK, Promoter specificity determinants of T7 RNA polymerase, Proc. National Acad. Sci 95 (1998) 515–519, 10.1073/pnas.95.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC, Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates, Nucleic Acids Res. 15 (21) (1987) 8783–8798, 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gholamalipour Y, Karunanayake Mudiyanselage A, Martin CT, 3′ end additions by T7 RNA polymerase are RNA self-templated, distributive and diverse in character—RNA-Seq analyses, Nucleic Acids Res. 46 (2018) gky796-, 10.1093/nar/gky796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kao C, Zheng M, Rüdisser S, A simple and efficient method to reduce nontemplated nucleotide addition at the 3′ terminus of RNAs transcribed by T7 RNA polymerase, RNA 5 (1999) 1268–1272, 10.1017/S1355838299991033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dolinnaya NG, Sokolova NI, Ashirbekova DT, Shabarova ZA, The use of BrCN for assembling modified DNA duplexes and DNA-RNA hybrids; comparison with water-soluble carbodiimide, Nucleic Acids Res. 19 (11) (1991) 3067–3072, 10.1093/nar/19.ll.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Müller S, Appel B, In vitro circularization of RNA, Rna Biol. 14 (8) (2017) 1018–1027, 10.1080/15476286.2016.1239009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Petkovic S, Müller S, Circular RNAs methods and protocols – synthesis and engineering of circular RNAs, Methods Mol. Biol. Clifton N J 1724 (2018) 167–180, 10.1007/978-l-4939-7562-4_14. [DOI] [PubMed] [Google Scholar]
- [41].Petkovic S, Müller S, RNA circularization strategies in vivo and in vitro, Nucleic Acids Res. 43 (2015) 2454–2465, 10.1093/nar/gkv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang S, Kool ET, Circular RNA oligonucleotides. Synthesis, nucleic acid binding properties, and a comparison with circular DNAs, Nucleic Acids Res. 22 (12) (1994) 2326–2333, 10.1093/nar/22.12.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen C, Sarnow P, Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs, Science 268 (5209) (1995) 415–417, 10.1126/science:7536344. [DOI] [PubMed] [Google Scholar]
- [44].Carmona EM, Circular RNA: Design Criteria for Optimal Therapeutic Utility, 2019. [Google Scholar]
- [45].Deana A, Celesnik H, Belasco JG, The bacterial enzyme RppH triggers messenger RNA degradation by 5′ pyrophosphate removal, Nature 451 (7176) (2008) 355–358, 10.1038/nature06475. [DOI] [PubMed] [Google Scholar]
- [46].Lohman GJS, Tabor S, Nichols NM, Current Protocols in Molecular Biology - DNA ligases, Curr Protoc Mol Biology Ed Frederick M Ausubel Et Al. Chapter 3 (2011) 3.14.1–3.14.7. 10.1002/0471142727.mb0314s94. [DOI] [Google Scholar]
- [47].Moore MJ, Query CC, [7] Joining of RNAs by splinted ligation, Methods Enzymol. 317 (2000) 109–123, 10.1016/s0076-6879(00)17009-0. [DOI] [PubMed] [Google Scholar]
- [48].Kershaw CJ, O’Keefe RT, Splint ligation of RNA with T4 DNA ligase – recombinant and in vitro RNA synthesis, methods and protocols, Methods Mol. Biol. Clifton N J 941 (2012) 257–269, 10.1007/978-l-62703-113-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Abe N, Kodama A, Abe H, Circular RNAs, methods and protocols – preparation of circRNA in vitro, Methods Mol. Biol. Clifton N J 1724 (2018) 181–192, 10.1007/978-l-4939-7562-4_15. [DOI] [PubMed] [Google Scholar]
- [50].Moore M, Sharp P, Site-specific modification of pre-mRNA: the 2′-hydroxyl groups at the splice sites, Science 256 (1992) 992–997, 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
- [51].Uhlenbeck OC, Gumport RI, T4 RNA ligase, Enzyme 15 (1982) 31–58, 10.1016/sl874-6047(08)60274-7. [DOI] [Google Scholar]
- [52].Wang L, Ruffner DE, Oligoribonucleotide circularization by ‘template-mediated’ ligation with T4 RNA ligase: Synthesis of circular hammerhead ribozymes, Nucleic Acids Res. 26 (10) (1998) 2502–2504, 10.1093/nar/26.10.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Beadudry D, Perreault J-P, An efficient strategy for the synthesis of circular RNA molecules, Nucleic Acids Res. 23 (15) (1995) 3064–3066, 10.1093/nar/23.15.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lang K, Micura R, The preparation of site-specifically modified riboswitch domains as an example for enzymatic ligation of chemically synthesized RNA fragments, Nat Protoc. 3 (9) (2008) 1457–1466, 10.1038/nprot.2008.135. [DOI] [PubMed] [Google Scholar]
- [55].McLaughlin LW, Romaniuk E, Romaniuk PJ, Neilson T, The effect of acceptor oligoribonucleotide sequence on the T4 RNA ligase reaction, Eur. J. Biochem 125 (1982) 639–643. 10.1111/j.1432-1033.1982.tb06730.x. [DOI] [PubMed] [Google Scholar]
- [56].Yin S, Kiong Ho C, Miller ES, Shuman S, Characterization of bacteriophage KVP40 and T4 RNA ligase 2, Virology 319 (1) (2004) 141–151, 10.1016/j.virol.2003.10.037. [DOI] [PubMed] [Google Scholar]
- [57].Petkovic S, Müller S, RNA self-processing: formation of cyclic species and concatemers from a small engineered RNA, Febs Lett. 587 (2013) 2435–2440, 10.1016/j.febslet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- [58].Chen H, Cheng K, Liu X, An R, Komiyama M, Liang X, Preferential production of RNA rings by T4 RNA ligase 2 without any splint through rational design of precursor strand, Nucleic Acids Res. 48 (2020) e54–e54. 10.1093/nar/gkaal81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xiao M-S, Wilusz JE, An improved method for circular RNA purification using RNase R that efficiently removes linear RNAs containing G-quadruplexes or structured 3′ ends, Nucleic Acids Res. 47 (2019) 8755–8769, 10.1093/nar/gkz576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tabak HF, Van der Horst G, Smit J, Winter AJ, Mul Y, Koerkamp GMJA, Discrimination between RNA circles, interlocked RNA circles and lariats using two-dimensional polyacrylamide gel electrophoresis, Nucleic Acids Res. 16 (14) (1988) 6597–6605, 10.1093/nar/16.14.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Breuer J, Rossbach O, Production and purification of artificial circular RNA sponges for application in molecular biology and medicine, Methods Protoc. 3 (2020) 42, 10.3390/mps3020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Petrov A, Wu T, Puglisi EV, Puglisi JD, Chapter seventeen RNA purification by preparative polyacrylamide gel electrophoresis, Methods Enzymol. 530 (2013) 315–330, 10.1016/b978-0-12-420037-l.00017-8. [DOI] [PubMed] [Google Scholar]
- [63].Wesselhoeft RA, Kowalski PS, Parker-Hale FC, Huang Y, Bisaria N, Anderson DG, RNA circularization diminishes immunogenicity and can extend translation duration in vivo, Mol Cell. 74 (3) (2019) 508–520.e4, 10.1016/j.molcel.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Weissman D, Pardi N, Muramatsu H, Karikó K, Synthetic messenger RNA and cell metabolism modulation, methods and protocols, Methods Mol. Biol 969 (2012) 43–54, 10.1007/978-l-62703-260-5_3. [DOI] [Google Scholar]
- [65].Puttaraju M, Been M, Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons, Nucleic Acids Res. 20 (20) (1992) 5357–5364, 10.1093/nar/20.20.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ford E, Ares M, Synthesis of circular RNA in bacteria and yeast using RNA cyclase ribozymes derived from a group I intron of phage T4, Proc. National Acad. Sci 91 (8) (1994) 3117–3121, 10.1073/pnas.91.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Puttaraju M, Perrotta AT, Been MD, A circular trans-acting hepatitis delta virus ribozyme, Nucleic Acids Res. 21 (18) (1993) 4253–4258, 10.1093/nar/21.18.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Puttaraju M, Been MD, Generation of nuclease resistant circular RNA decoys for HIV-Tat and HIV-Rev by autocatalytic splicing, Nucl. Acid S (1995) 49–51. [PubMed] [Google Scholar]
- [69].Puttaraju M, Been MD, Circularizing ribozymes and decoy-competitors by autocatalytic splicing in vitro and in vivo, Saas Bulletin Biochem. Biotechnol 9 (1996) 77–82. [PubMed] [Google Scholar]
- [70].Bohjanen PR, Colvin RA, Puttaraju M, Been MD, Garcia-Bianco MA, A small circular TAR RNA decoy specifically inhibits Tat-activated HIV-1 transcription, Nucleic Acids Res. 24 (1996) 3733–3738, 10.1093/nar/24.19.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Perriman R, Ares M, Circular mRNA can direct translation of extremely long repeating-sequence proteins in vivo, RNA 4 (1998) 1047–1054, 10.1017/sl35583829898061x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ochi A, Umekage S, Kikuchi Y, Non-enzymatic in vitro production of circular hammerhead ribozyme targeting the template region of human telomerase RNA, Nucl Acid S. 53 (1) (2009) 275–276, 10.1093/nass/nrpl38. [DOI] [PubMed] [Google Scholar]
- [73].Umekage S, Kikuchi Y, In vitro and in vivo production and purification of circular RNA aptamer, J. Biotechnol 139 (4) (2009) 265–272, 10.1016/j.jbiotec.2008.12.012. [DOI] [PubMed] [Google Scholar]
- [74].Chen YG, Kim MV, Chen X, Batista PJ, Aoyama S, Wilusz JE, Iwasaki A, Chang HY, Sensing self and foreign circular RNAs by intron identity, Mol Cell. 67 (2) (2017) 228–238.e5, 10.1016/j.molcel.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wesselhoeft RA, Kowalski PS, Anderson DG, Engineering circular RNA for potent and stable translation in eukaryotic cells, Nat. Commun 9 (2018) 2629, 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rostain W, Shen S, Cordero T, Rodrigo G, Jaramillo A, Engineering a circular riboregulator in Escherichia coli, Biodesign Res. 2020 (2020) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rausch JW, Heinz WF, Payea MJ, Sherpa C, Gorospe M, Le Grice SFJ, Characterizing and circumventing sequence restrictions for synthesis of circular RNA in vitro, Nucleic Acids Res. (2021), 10.1093/nar/gkaal256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mikheeva S, Hakim-Zargar M, Carlson D, Jarrell K, Use of an engineered ribozyme to produce a circular human exon, Nucleic Acids Res. 25 (1997) 5085–5094, 10.1093/nar/25.24.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jarrell KA, Inverse splicing of a group II intron, Proc. National Acad. Sci 90 (1993) 8624–8627. 10.1073/pnas.90.18.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Branch A, Robertson H, A replication cycle for viroids and other small infectious RNA’s, Science 223 (4635) (1984) 450–455, 10.1126/science:6197756. [DOI] [PubMed] [Google Scholar]
- [81].Diegelman AM, Kool ET, Generation of circular RNAs and trans -cleaving catalytic RNAs by rolling transcription of circular DNA oligonucleotides encoding hairpin ribozymes, Nucleic Acids Res. 26 (1998) 3235–3241, 10.1093/nar/26.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Diegelman AM, Daubendiek SL, Kool ET, Generation of RNA ladders by rolling circle transcription of small circular oligodeoxyribonucleotides, Biotechniques 25 (5) (1998) 754–758, 10.2144/98255bm01. [DOI] [PubMed] [Google Scholar]
- [83].Vauléon S, Müller S, External regulation of hairpin ribozyme activity by an oligonucleotide effector, ChemBioChem 4 (2-3) (2003) 220–224, 10.1002/cbic.v4:2/310.1002/cbic.200390035. [DOI] [PubMed] [Google Scholar]
- [84].Strohbach D, Novak N, Müller S, Redox-active riboswitching: allosteric regulation of ribozyme activity by ligand-shape control, Angew. Chem. Int. Ed 45 (2006) 2127–2129, 10.1002/anie.200503820. [DOI] [PubMed] [Google Scholar]
- [85].Tatomer DC, Liang D, Wilusz JE, Methods in molecular biology – inducible expression of eukaryotic circular RNAs from plasmids, Methods Mol. Biol. Clifton N J 1648 (2017) 143–154, 10.1007/978-l-4939-7204-3_ll. [DOI] [PubMed] [Google Scholar]
- [86].Liu D, Conn V, Goodall GJ, Conn SJ, Methods in molecular biology – a highly efficient strategy for overexpressing circRNAs, Methods Mol. Biol. Clifton N J 1724 (2018) 97–105, 10.1007/978-l-4939-7562-4_8. [DOI] [PubMed] [Google Scholar]
- [87].Ho-Xuan H, Glažar P, Latini C, Heizler K, Haase J, Hett R, Anders M, Weichmann F, Bruckmann A, Van den Berg D, Hüttelmaier S, Rajewsky N, Hackl C, Meister G, Comprehensive analysis of translation from overexpressed circular RNAs reveals pervasive translation from linear transcripts, Nucleic Acids Res. (2020) gkaa704-, 10.1093/nar/gkaa704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang Y, Wang Z, Efficient backsplicing produces translatable circular rnRNAs, Rna New York N Y. 21 (2) (2015) 172–179, 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dubin RA, Kazmi MA, Ostrer H, Inverted repeats are necessary for circularization of the mouse testis Sry transcript, Gene 167 (1-2) (1995) 245–248, 10.1016/0378-1119(95)00639-7. [DOI] [PubMed] [Google Scholar]
- [90].Deininger P, Alu elements: know the SINEs, Genome Biol. 12 (12) (2011) 236, 10.1186/gb-2011-12-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kramer MC, Liang D, Tatomer DC, Gold B, March ZM, Cherry S, Wilusz JE, Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins, Gene Dev. 29 (20) (2015) 2168–2182, 10.1101/gad.270421.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N, Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals, Cell Reports 10 (2) (2015) 170–177, 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- [93].Welden JR, Pawluchin A, van Doom J, Stamm S, Use of alu element containing minigenes to analyze circular RNAs, J. Vis. Exp 157 (2020), 10.3791/59760. [DOI] [PubMed] [Google Scholar]
- [94].Kapitonov VV, Jurka J, Molecular paleontology of transposable elements in the Drosophila melanogaster genome, Proc. National Acad. Sci 100 (11) (2003) 6569–6574, 10.1073/pnas.0732024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang Y, Xue W, Li X, Zhang J, Chen S, Zhang J-L, Yang L, Chen L-L, The biogenesis of nascent circular RNAs, Cell Reports 15 (3) (2016) 611–624, 10.1016/j.celrep.2016.03.058. [DOI] [PubMed] [Google Scholar]
- [96].Li X, Liu C-X, Xue W, Zhang Y, Jiang S, Yin Q-F, Wei J, Yao R-W, Yang L, L.-L. Chen, Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection, Mol Cell. 67 (2) (2017) 214–227.e7, 10.1016/j.molcel.2017.05.023. [DOI] [PubMed] [Google Scholar]
- [97].Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S, circRNA biogenesis competes with Pre-mRNA splicing, Mol. Cell 56 (1) (2014) 55–66, 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- [98].Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ, The RNA binding protein quaking regulates formation of circRNAs, Cell 160 (6) (2015) 1125–1134, 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- [99].Zhao J, Lee EE, Kim J, Yang R, Chamseddin B, Ni C, Gusho E, Xie Y, Chiang C-M, Buszczak M, Zhan X, Laimins L, Wang RC, Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus, Nat Commun. 10 (2019) 2300, 10.1038/s41467-019-10246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Fei T, Chen Y, Xiao T, Li W, Cato L, Zhang P, Cotter MB, Bowden M, Lis RT, Zhao SG, Wu Q, Feng FY, Loda M, He HH, Liu XS, Brown M, Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing, Proc. National Acad Sci 114 (2017) E5207–E5215, 10.1073/pnas.l617467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Khan MAF, Reckman YJ, Aufiero S, van den Hoogenhof MMG, van der Made I, Beqqali A, Koolbergen DR, Rasmussen TB, van der Velden J, Creemers EE, Pinto YM, RBM20 regulates circular RNA production from the titin gene, Circ. Res 119 (9) (2016) 996–1003, 10.1161/CIRCRESAHA.116.309568. [DOI] [PubMed] [Google Scholar]
- [102].Errichelli L, Modigliani SD, Laneve P, Colantoni A, Legnini I, Capauto D, Rosa A, Santis RD, Scarfò R, Peruzzi G, Lu L, Caffarelli E, Shneider NA, Morlando M, Bozzoni I, FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons, Nat. Commun 8 (2017) 14741, 10.1038/ncommsl4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhao Q, Liu J, Deng H, Ma R, Liao J-Y, Liang H, Hu J, Li J, Guo Z, Cai J, Xu X, Gao Z, Su S, Targeting mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output, Cell (2020), 10.1016/j.cell.2020.08.009. [DOI] [PubMed] [Google Scholar]
- [104].Aktaş T, Avşar Ilık İ, Maticzka D, Bhardwaj V, Pessoa Rodrigues C, Mittler G, Manke T, Backofen R, Akhtar A, DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome, Nature 544 (7648) (2017) 115–119, 10.1038/nature21715. [DOI] [PubMed] [Google Scholar]
- [105].Umekage S, Uehara T, Fujita Y, Suzuki H, Kikuchi Y, Innovations in Biotechnology: In Vivo Circular RNA Expression by the Permuted Intron-Exon Method, (2012). 10.5772/28220. [DOI] [Google Scholar]
- [106].Noto JJ, Schmidt CA, Matera AG, Engineering and expressing circular RNAs via tRNA splicing, Rna Biol. 14 (8) (2017) 978–984, 10.1080/15476286.2017.1317911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Litke JL, Jaffrey SR, Highly efficient expression of circular RNA aptamers in cells using autocatalytic transcripts, Nat Biotechnol. 37 (6) (2019) 667–675, 10.1038/s41587-019-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Guo JU, Agarwal V, Guo H, Bartel DP, Expanded identification and characterization of mammalian circular RNAs, Genome Biol. 15 (2014) 409, 10.1186/sl3059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Schneider T, Hung L-H, Schreiner S, Starke S, Eckhof H, Rossbach O, Reich S, Medenbach J, Bindereif A, CircRNA-protein complexes: IMP3 protein component defines subfamily of circRNPs, Sci. Rep.-Uk 6 (2016) 31313, 10.1038/srep31313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S, Translation of CircRNAs, Mol Cell. 66 (1) (2017) 9–21.e7, 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Xia X, Li X, Li F, Wu X, Zhang M, Zhou H, Huang N, Yang X, Xiao F, Liu D, Yang L, Zhang N, A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1, Mol. Cancer 18 (2019) 131, 10.1186/s12943-019-1056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z, Xu B, Wu C, Zhou Q, Hu W, Wu C, Jiang J, A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling, Mol Cancer. 18 (2019) 47, 10.1186/sl2943-019-1010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhang M, Huang N, Yang X, Luo J, Yan S, Xiao F, Chen W, Gao X, Zhao K, Zhou H, Li Z, Ming L, Xie B, Zhang N, A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis, Oncogene 37 (13) (2018) 1805–1814, 10.1038/s41388-017-0019-9. [DOI] [PubMed] [Google Scholar]
- [114].Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, Huang N, Yang X, Zhao K, Zhou H, Huang S, Xie B, Zhang N, Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis, Jnci J. National Cancer Inst 110 (2017) 304–315, 10.1093/jnci/djxl66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I, Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis, Mol Cell. 66 (1) (2017) 22–37.e9, 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Liang W-C, Wong C-W, Liang P-P, Shi M, Cao Y, Rao S-T, Tsui S-K-W, Waye M-M-Y, Zhang Q, Fu W-M, Zhang J-F, Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway, Genome Biol. 20 (2019) 84, 10.1186/sl3059-019-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hellen CUT, Sarnow P, Internal ribosome entry sites in eukaryotic mRNA molecules, Gene Dev. 15 (2001) 1593–1612, 10.1101/gad.891101. [DOI] [PubMed] [Google Scholar]
- [118].Diallo LH, Tatin F, Florian D, Godet A-C, Zamora A, Prats A-C, Garmy-Susini B, Lacazette E, How are circRNAs translated by non-canonical initiation mechanisms? Biochimie 164 (2019) 45–52, 10.1016/j.biochi.2019.06.015. [DOI] [PubMed] [Google Scholar]
- [119].Abe N, Matsumoto K, Nishihara M, Nakano Y, Shibata A, Maruyama H, Shuto S, Matsuda A, Yoshida M, Ito Y, Abe H, Rolling circle translation of circular RNA in living human cells, Sci. Rep.-Uk 5 (2015) 16435, 10.1038/srepl6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Meganck RM, Borchardt EK, Rivera RMC, Scalabrino ML, Wilusz JE, Marzluff WF, Asokan A, Tissue-dependent expression and translation of circular RNAs with recombinant AAV vectors in vivo, Mol. Ther. – Nucleic Acids 13 (2018) 89–98, 10.1016/j.omtn.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen L-L, Wang Y, Wong CCL, Xiao X, Wang Z, Extensive translation of circular RNAs driven by N6-methyladenosine, Cell Res. 27 (5) (2017) 626–641, 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang C, Fu J, Zhou Y, A review in research progress concerning m6A methylation and immunoregulation, Front. Immunol 10 (2019) 922, 10.3389/fimmu.2019.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Costello A, Lao NT, Barron N, Clynes M, Continuous translation of circularized mRNA improves recombinant protein titer, Metab. Eng 52 (2019) 284–292, 10.1016/j.ymben.2019.01.002. [DOI] [PubMed] [Google Scholar]
- [124].Abe N, Hiroshima M, Maruyama H, Nakashima Y, Nakano Y, Matsuda A, Sako Y, Ito Y, Abe H, Rolling circle amplification in a prokaryotic translation system using small circular RNA, Angew. Chem. Int. Ed 52 (27) (2013) 7004–7008, 10.1002/anie.201302044. [DOI] [PubMed] [Google Scholar]
- [125].Kahvejian A, Svitkin YV, Sukarieh R, M’Boutchou M-N, Sonenberg N, Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms, Gene Dev. 19 (2005) 104–113, 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Gallegos JE, Rose AB, The enduring mystery of intron-mediated enhancement, Plant Sci. 237 (2015) 8–15, 10.1016/j.plantsci.2015.04.017. [DOI] [PubMed] [Google Scholar]
- [127].Mo D, Li X, Raabe CA, Cui D, Vollmar J-F, Rozhdestvensky TS, Skryabin BV, Brosius J, A universal approach to investigate circRNA protein coding function, Sci. Rep.-Uk 9 (2019) 11684, 10.1038/s41598-019-48224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Mitra A, Pfeifer K, Park K-S, Circular RNAs and competing endogenous RNA (ceRNA) networks, Transl, Cancer Res. 7 (2018) S624–S628, 10.21037/tcr.2018.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J, Natural RNA circles function as efficient microRNA sponges, Nature 495 (7441) (2013) 384–388, 10.1038/naturell993. [DOI] [PubMed] [Google Scholar]
- [130].Jost I, Shalamova LA, Gerresheim GK, Niepmann M, Bindereif A, Rossbach O, Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges, Rna Biol. (2018) 1–8, 10.1080/15476286.2018.1435248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lavenniah A, Luu TDA, Li YP, Lim TB, Jiang J, Ackers-Johnson M, Foo RY, Engineered circular RNA sponges act as miRNA inhibitors to attenuate pressure overload induced cardiac hypertrophy, Mol Ther. 28 (6) (2020) 1506–1517, 10.1016/j.ymthe.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Liu C-X, Li X, Nan F, Jiang S, Gao X, Guo S-K, Xue W, Cui Y, Dong K, Ding H, Qu B, Zhou Z, Shen N, Yang L, Chen L-L, Structure and degradation of circular RNAs regulate PKR activation in innate immunity, Cell 177 (4) (2019) 865–880.e21, 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- [133].Umekage S, Kikuchi Y, Production of circular streptavidin RNA aptamer in vivo, Nucl Acid S. 51 (1) (2007) 391–392, 10.1093/nass/nrml96. [DOI] [PubMed] [Google Scholar]
- [134].Schreiner S, Didio A, Hung L-H, Bindereif A, Design and application of circular RNAs with protein-sponge function, Nucleic Acids Res. 48 (2020) gkaa1085-, 10.1093/nar/gkaal085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Borchardt EK, Meganck RM, Vincent HA, Ball CB, Ramos SBV, Moorman NJ, Marzluff WF, Asokan A, Inducing circular RNA formation using the CRISPR endoribonuclease Csy4, RNA 23 (5) (2017) 619–627, 10.1261/rna.056838.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Solomatin S, Herschlag D, Chapter 3 Methods of Site-Specific Labeling of RNA with Fluorescent Dyes, Methods Enzymol. 469 (2009) 47–68. 10.1016/s0076-6879(09)69003-0. [DOI] [PubMed] [Google Scholar]
- [137].Chen YG, Chen R, Ahmad S, Verma R, Kasturi SP, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B, Hur S, Chang HY, N6-methyladenosine modification controls circular RNA immunity, Mol. Cell 76 (1) (2019) 96–109.e9, 10.1016/j.molcel.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


