Abstract
Objective
Fertility-sparing treatment for young women with atypical endometrial hyperplasia (AEH) and early endometrial cancer (EC) is a difficult challenge. Insulin resistance (IR) and metabolic syndrome (MetS) are two potentially crucial, but currently enigmatic factors in the recurrence of AEH and early EC patients. In this study we attempt to elucidate these factors.
Methods
A retrospective study was conducted from January 2010 to December 2019. Risk factors for recurrence and complete remission time after recurrence (RCR time) were investigated. ROC curves were built to estimate the accuracy of the metabolic characteristics and Kaplan–Meier (K–M) analysis was used to calculate recurrence-free survival (RFS) for patients with various IR or MetS statuses.
Results
A total of 111 AEH or early EC patients met the criteria and were enrolled in our study. Univariate analysis found that BMI ≥25 kg/m2 (OR = 2.7, 95% CI: 1.1–6.4, P = 0.03), IR (OR = 9.5, 95% CI: 3.3–27.0, P <0.001), MetS (OR = 4.9, 95% CI:1.5–15.5, P = 0.008), IR+ and MetS+ (OR = 21.0, 95% CI: 4.8–92.7, P <0.001), histological type (OR = 3.5, 95% CI: 1.5–7.9, P = 0.003), and maintenance treatment (OR = 0.3, 95% CI: 0.1–0.6, P = 0.005) were all significantly associated with recurrence and longer RCR time. Among these factors, IR and MetS were determined to be two independent risk factors for recurrence. Moreover, using IR and MetS as markers significantly improved the diagnostic accuracy of recurrence for fertility-sparing treatment patients (AUC = 0.818, P <0.05) and may play synergistic roles in suppressing treatment. K–M analysis indicated both metabolic features played important roles in RFS (P <0.05).
Conclusion
Both IR and MetS were significantly associated with recurrence and longer RCR time in AEH and early EC patients receiving fertility-sparing treatment.
Keywords: fertility-sparing treatment, atypical endometrial hyperplasia, insulin resistance, metabolic syndrome, recurrence
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries (1), with an extensively increasing number of cases in China, particularly among younger women (2). Approximately 3–14% of EC cases are reported in premenopausal women under 40 who want to preserve their fertility (3). EC diagnosed in this age group is increasing in frequency and are typically early-stage, well-differentiated, endometrioid type adenocarcinomas (4). As the number of young EC patients rises, fertility-preserving therapy is becoming one of the most important conservative methods for these women and for the corresponding national policy in China. To date, progestin therapy has been the most common type of fertility-preserving therapy for atypical endometrial hyperplasia (AEH) and early EC (5).
Although the majority of patients show complete remission (CR) to conservative treatment, the recurrence rate is high, between 16.7 and 62%, and this probability continually increases with time (6). A systematic review and meta-analysis of risk factors for recurrence found that the recurrence rate at 1 and 2 years were 9.6% and 29.2%, respectively. However, this study failed to evaluate possible risk factors for the relapse of the disease (7).
Metabolic features including insulin resistance (IR) and metabolic syndrome (MetS) have long been regarded as some of the most essential risk factors for EC (8).
IR is defined as the reduced biological effect of a specified amount of insulin after binding to the receptor and is manifested as the decreased use and increased output of peripheral glucose (9). Previous study has demonstrated that IR occurs early during the development from hyperplasia to cancer in the endometrium (10). MetS, comprising obesity, dyslipidemia, hypertension, and hyperglycemia, is a cluster of risk factors not only for cardiovascular disease but some common cancers as well, notably EC (11, 12).
There are many studies that focusing on the relationship between IR/MetS and EC. However, it remains unclear whether IR and MetS have an impact on fertility-sparing treatment in AEHfocusing and early EC, especially in a recurrent event. In this study, we conducted a retrospective study to investigate the relationship between these metabolic features and recurrent events of preservative therapy for AEH and early EC patients. Furthermore, we explore the potential of IR and MetS in improving diagnostic accuracy for recurrence and prognostic prediction. Utilizing these markers may help us to further understand the role of metabolism in fertility-sparing treatment and better prevent recurrence in patients.
Materials and Methods
Patients
We retrospectively analyzed the data of patients who received fertility-sparing therapy for AEH and early (Grade 1, Stage IA) endometrioid adenocarcinoma (G1EA) from January 2012 to December 2019 in Peking University People’s Hospital. Baseline and clinicopathological data as well as follow-up data were collected.
Indications for conservative therapy for AEH and G1EA were as follows: (i) the patients were younger than 45 years and strongly desired to preserve their fertility; (ii) endometrial tissue sampling for diagnosis was carried out by dilation and curettage (D&C); and (iii) patients who were diagnosed with G1EA and underwent pelvic magnetic resonance imaging (MRI) for staging, myometrial invasion, or any displayed extra uterine lesions were ruled out by institutional radiologists. All patients agreed and signed informed consent for the treatment.
The patients were followed up with by July, 2020. The clinicopathological data from patients were retrieved from electronical medical records. This study was approved by the Ethics Committees of the Peking University People’s Hospital (No. 2020PHB063-01).
Insulin Resistance and Metabolic Syndrome Evaluation
The homeostasis model assessment-insulin resistance (HOMA-IR) value was used to determine IR status. The HOMA-IR value was calculated as fasting blood glucose (FBG, mmol/L) × fasting insulin (FINS, μU/ml)/22.5. Patients with diabetes or whose HOMA-IR ≥2.95, were considered as insulin resistant (IR) (10). The MetS criteria were proposed by the Chinese Medical Association Diabetes Branch and defined as including three or four of the following criteria: 1) Overweight and/or obese, BMI (body mass index) is greater than 25.0 kg/m2. 2) High blood glucose, FBG is greater than 6.1 mmol/L and/or 2 h BG is greater than 7.8 mmol/L, and/or has been diagnosed with diabetes. 3) Hypertension, systolic/diastolic blood pressure was greater than 140/90 mmHg, and/or has been diagnosed with hypertension. 4) Dyslipidemia, blood TG is greater than 1.7 mmol/L, and/or fasting blood HDL <1.0 mmol/L (39 mg/dl).
Treatment and Relapse
Patients were scheduled to receive 250/500 mg of medroxyprogesterone acetate (MPA), 160 mg of megestrol acetate (MA) orally, or GnRH on a daily basis for 12 weeks. After the treatment, endometrial biopsy was performed by D&C to assess the efficacy of the therapy after CR. CR was defined as a normal endometrium without atypical hyperplasia. The patients were followed up with every 3 to 6 months. Ultrasound and endometrial biopsy were used to evaluate the endometrium. Recurrence was defined as the reappearance of a lesion that had initially regressed following treatment. The relapse time studied was the time interval from CR to relapse during follow-up. Recurrence-free survival (RFS) was defined as the time, in months, from the date of achieving CR to the date of recurrence. RCR time was defined as the time required for CR after the primary recurrence.
Statistical Analysis
Data are presented as mean ± SD or as counts with proportions. Possible risk factors associated with relapse or RCR time, including age, BMI, CA125, HOMA-IR, MetS, FBG, triglyceride, HDL, menstruation cycle, gestation, parity, family history (tumor history), hypertension, diabetes, Polycystic Ovarian Syndrome (PCOS), histological and progestin type, time to CR, and maintenance treatment after primary CR were investigated. Univariate and multivariate analyses were performed in the recurrence and non-recurrence groups using the mentioned risk factors by a logistic regression to determine the likelihood ratio and odds ratios (OR) were calculated along with 95% confidence intervals (95% CI). The rate of recurrence was analyzed using Kaplan–Meier (K–M) curve and compared between groups using a log-rank test. All of the analyses were performed with the statistical software packages of R version 3.4.3 (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A two-sided significance level of <0.05 was considered statistically significant.
Results
Patients’ Selection and Characteristics
During the study period, a total of 63 EAH and 48 G1EA patients who met the inclusion criteria were evaluated ( Figure 1 ). Baseline clinical characteristics between the recurrence-free and recurrence groups are shown in Table 1 . The average age and BMI at diagnosis were 31.3 ± 4.5 years old and 26.6 ± 4.9 kg/m2, respectively. The mean value of HOMA-IR in the two groups was 3.5 and 3.9. The median length of RFS was 46.2 ± 32.1 months for the recurrence-free group, compared to 20.9 ± 17.5 months for the recurrence group. A total of 63 (56.8%) patients were diagnosed as IR, including 30 recurrence-free women and 33 recurrence women. Of the 101 patients, 15 (13.5%) had MetS and 10 out of 15 of these belonged to recurrent cases. The most common type of conservative therapy administered was MPA 250 mg (N = 59, 53.2%), followed by MPA 500 mg (N = 21, 18.9%), MA (N = 17, 15.3%), GnRH (N = 7, 6.3%), and combination therapy (N = 7, 6.3%). There were 40.5% patients (N = 45) who took less than 3 months to become CR, 32.4% (N = 36) took 3–6 months, and the rest (N = 30, 27.1%) took longer than 6 months. The majority of patients underwent maintenance treatment (N = 55, 65.4%, in the recurrence-free group and 55.3%, N = 21 in the recurrence group).
Figure 1.
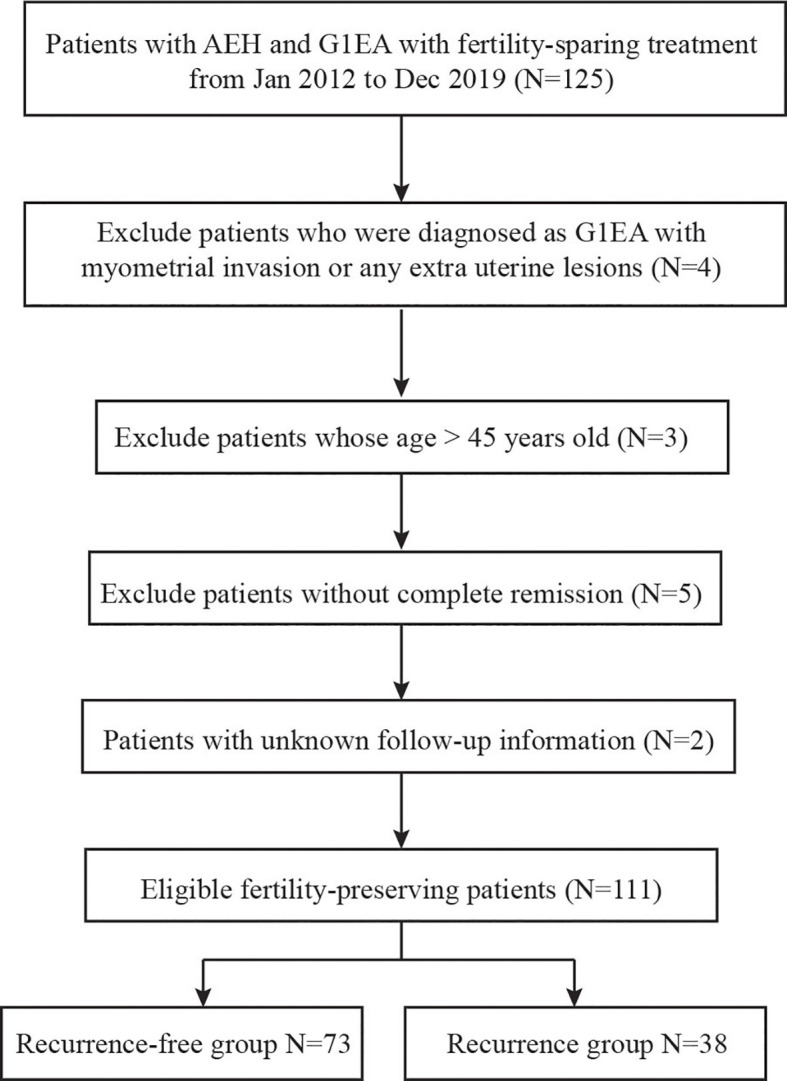
Flow diagram of inclusion criteria and exclusion criteria.
Table 1.
Clinicopathological characteristics and treatment in recurrence-free and recurrence group.
| Recurrence | Total | Recurrence | |
|---|---|---|---|
| Mean+SD | No (N=73) | Yes (N=38) | |
| Age at diagnosis (year) | 31.3 ± 4.5 | 31.5 ± 4.5 | 31.1 ± 4.6 |
| BMI (kg/m2) | 26.6 ± 4.9 | 26.5 ± 5.0 | 26.9 ± 4.6 |
| CA125 (U/mL) | 22.5 ± 21.1 | 20.8 ± 21.7 | 25.7 ± 19.5 |
| HOMA-IR | 3.6 ± 2.7 | 3.5 ± 3.2 | 3.9 ± 1.7 |
| Glucose (mmol/L) | 5.3 ± 3.5 | 5.4 ± 4.2 | 5.1 ± 1.1 |
| Triglyceride (mmol/L) | 1.6 ± 1.1 | 1.6 ± 1.1 | 1.6 ± 1.0 |
| HDL (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.3 |
| RCR time (month) | 3.8 ± 4.3 | NA | 7.3 ± 4.3 |
| RFS (month) | 37.5 ± 30.4 | 46.2 ± 32.1 | 20.9 ± 17.5 |
| IR | N (%) | N (%) | N (%) |
| No | 48 (43.2%) | 43 (58.9%) | 5 (13.2%) |
| Yes | 63 (56.8%) | 30 (41.1%) | 33 (86.8%) |
| MetS | |||
| No | 96 (86.5%) | 68 (93.2%) | 28 (73.7%) |
| Yes | 15 (13.5%) | 5 (6.8%) | 10 (26.3%) |
| Menstruation cycle | |||
| Regular | 56 (50.5%) | 38 (52.1%) | 18 (47.4%) |
| Irregular | 55 (49.5%) | 35 (47.9%) | 20 (52.6%) |
| Gestation | |||
| No | 63 (56.8%) | 36 (49.3%) | 27 (71.1%) |
| Yes | 48 (43.2%) | 37 (50.7%) | 11 (28.9%) |
| Parity | |||
| No | 92 (82.9%) | 57 (78.1%) | 35 (92.1%) |
| Yes | 19 (17.1%) | 16 (21.9%) | 3 (7.9%) |
| Family history | |||
| No | 98 (88.3%) | 67 (91.8%) | 31 (81.6%) |
| Yes | 13 (11.7%) | 6 (8.2%) | 7 (18.4%) |
| Hypertension | |||
| No | 103 (92.8%) | 69 (94.5%) | 34 (89.5%) |
| Yes | 8 (7.2%) | 4 (5.5%) | 4 (10.5%) |
| Diabetes | |||
| No | 87 (78.4%) | 60 (82.2%) | 27 (71.1%) |
| Yes | 24 (21.6%) | 13 (17.8%) | 11 (28.9%) |
| PCOS | |||
| No | 60 (54.1%) | 37 (50.7%) | 23 (60.5%) |
| Yes | 51 (45.9%) | 36 (49.3%) | 15 (39.5%) |
| Histological type | |||
| AEH | 63 (56.8%) | 49 (67.1%) | 14 (36.8%) |
| G1EA | 48 (43.2%) | 24 (32.9%) | 24 (63.2%) |
| Progestin type | |||
| MPA250mg | 59 (53.2%) | 36 (49.3%) | 23 (60.5%) |
| MPA500mg | 21 (18.9%) | 19 (26.0%) | 2 (5.3%) |
| MA | 17 (15.3%) | 8 (11.0%) | 9 (23.7%) |
| GnRH | 7 (6.3%) | 6 (8.2%) | 1 (2.6%) |
| ≥2 kinds | 7 (6.3%) | 4 (5.5%) | 3 (7.9%) |
| Time to CR (month) | |||
| <3 | 45 (40.5%) | 31 (42.5%) | 14 (36.8%) |
| 3-6 | 36 (32.4%) | 20 (27.4%) | 16 (42.1%) |
| >6 | 30 (27.1%) | 22 (30.1%) | 8 (21.1%) |
| Maintenance treatment after primary CR | |||
| No | 35 (31.5%) | 18 (34.6%) | 17 (44.7%) |
| Yes | 76 (68.5%) | 55 (65.4%) | 21 (55.3%) |
SD, standard deviation; BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance-insulin resistance; HDL, high-density lipoprotein; RCR time, complete remission time after recurrence; RFS, recurrence-free survival; IR, insulin resistance; MetS, metabolic syndrome; PCOS, polycystic ovarian syndrome; AEH, atypical endometrial hyperplasia; CR, complete remission.
Factors Associated With Recurrence and RCR Time
To investigate the relationship between clinicopahological indexes and recurrence, as well as explore the risk factors, a logistic regression analysis was conducted. Univariate analysis ( Table 2 ) showed that a BMI ≥25 kg/m2 (OR = 2.7, 95% CI: 1.1–6.4, P = 0.03), IR (OR = 9.5, 95% CI: 3.3–27.0, P <0.001), MetS (OR = 4.9, 95% CI:1.5–15.5, P = 0.008), IR+ and MetS+ (OR = 21.0, 95% CI: 4.8–92.7, P <0.001), histological type (OR = 3.5, 95% CI: 1.5–7.9, P = 0.003), and maintenance treatment (OR = 0.3, 95% CI: 0.1–0.6, P = 0.005) appeared to be positively associated with recurrence. Upon further investigation it was demonstrated that BMI ≥25 kg/m2 (OR = 2.3, 95% CI: 1.7–3.9, P = 0.006), IR (OR = 3.0, 95% CI: 1.5–4.5, P <0.001), MetS (OR = 6.2, 95% CI: 4.2–8.2, P <0.001), IR+ and MetS+ (OR = 7.6, 95% CI: 5.5–9.8, P <0.001), histological type (OR = 2.5, 95% CI: 1.5–4.0, P = 0.002), and maintenance treatment (OR = 0.4, 95% CI: 0.1–0.7, P = 0.016) were also significantly related with recurrence. Unlike with recurrence, diabetes (OR = 2.8, 95% CI: 1.1–4.7, P = 0.004) was found to be a risk factor for RCR time. No relationships were found between other factors and recurrence or RCR time (P >0.05).
Table 2.
Univariate analysis between clinicopathological characteristics and recurrence/RCR time.
| Recurrence | RCR time | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (year) | 1.0 (0.9, 1.1) | 0.689 | -0.1 (-0.2, 0.1) | 0.510 |
| BMI (kg/m2) | 1.0 (0.9, 1.1) | 0.679 | 0.1 (-0.1, 0.3) | 0.220 |
| CA125 (U/mL) | 1.0 (1.0, 1.0) | 0.266 | 0.0 (-0.0, 0.0) | 0.674 |
| HOMA-IR | 1.1 (0.9, 1.2) | 0.444 | 0.2 (-0.1, 0.5) | 0.283 |
| FBG (mmol/L) | 1.0 (0.8, 1.1) | 0.691 | -0.0 (-0.3, 0.2) | 0.799 |
| Triglyceride (mmol/L) | 1.0 (0.7, 1.4) | 0.972 | -0.0 (-0.8, 0.7) | 0.914 |
| HDL (mmol/L) | 1.0 (0.1, 1.8) | 0.270 | -1.8 (-4.1, 0.5) | 0.138 |
| BMI | ||||
| <25kg/m2 | 1.0 | 1.0 | ||
| ≥25kg/m2 | 2.7 (1.1, 6.4) | 0.029 | 2.3 (1.7, 3.9) | 0.006 |
| IR | ||||
| Yes | 1.0 | 1.0 | ||
| No | 9.5 (3.3, 27.0) | <0.001 | 3.0 (1.5, 4.5) | <0.001 |
| MetS | ||||
| Yes | 1.0 | 1.0 | ||
| No | 4.9 (1.5, 15.5) | 0.008 | 6.2 (4.2, 8.2) | <0.001 |
| IR+ and MetS+ | ||||
| IR- and MetS- | 1.0 | 1.0 | ||
| IR+ or MetS+ | 7.2 (2.4,21.1) | <0.001 | 1.6 (0.2, 3.0) | 0.029 |
| IR+ and MetS+ | 21.0 (4.8.92.7) | <0.001 | 7.6 (5.5, 9.8) | <0.001 |
| Menstruation cycle | ||||
| Regular | 1.0 | 1.0 | ||
| Irregular | 1.2 (0.6, 2.6) | 0.640 | 1.1 (-0.5, 2.7) | 0.189 |
| Gestation | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.4 (0.2, 0.9) | 0.030 | -1.4 (-3.0, 0.2) | 0.083 |
| Parity | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.3 (0.1, 1.1) | 0.074 | -1.7 (-3.8, 0.4) | 0.107 |
| Family history | ||||
| No | 1.0 | 1.0 | ||
| Yes | 2.2 (0.6, 7.2) | 0.212 | 0.5 (-2.0, 3.1) | 0.688 |
| Hypertension | ||||
| No | 1.0 | 1.0 | ||
| Yes | 2.0 (0.5, 8.6) | 0.337 | 1.9 (-1.2, 5.0) | 0.231 |
| Diabetes | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.9 (0.7, 4.7) | 0.180 | 2.8 (1.1, 4.7) | 0.004 |
| PCOS | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.7 (0.3, 1.5) | 0.325 | -1.2 (-2.8, 0.4) | 0.152 |
| Histological type | ||||
| AEH | 1.0 | 1.0 | ||
| G1EA | 3.5 (1.5, 7.9) | 0.003 | 2.5 (1.5, 4.0) | 0.002 |
| Progestin type | ||||
| MPA250mg | 1.0 | 1.0 | ||
| MPA500mg | 1.0 (0.3, 3.5) | 0.967 | -1.2 (-3.7, 1.3) | 0.347 |
| MA | 2.5 (0.8, 7.3) | 0.103 | 0.4 (-1.8, 2.7) | 0.717 |
| GnRH | 0.4 (0.0, 3.2) | 0.365 | -2.1 (-5.4, 1.2) | 0.217 |
| ≥2 types | 1.6 (0.3, 8.0) | 0.539 | 2.9 (-0.4, 6.2) | 0.088 |
| Time to CR (month) | ||||
| <3 | 1.0 | 1.0 | ||
| 3-6 | 1.1 (0.6, 2.0) | 0.621 | 0.8 (0.5, 1.5) | 0.518 |
| >6 | 2.0 (0.8, 5.1) | 0.375 | 0.9 (0.4, 1.8) | 0.857 |
| Maintenance treatment after primary CR | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.3 (0.1, 0.6) | 0.005 | 0.4 (0.1, 0.7) | 0.016 |
SD, standard deviation; BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance-insulin resistance; HDL, high-density lipoprotein; RCR time, complete remission time after recurrence; RFS, recurrence-free survival; IR, insulin resistance; MetS, metabolic syndrome; PCOS, polycystic ovarian syndrome; AEH, atypical endometrial hyperplasia; CR, complete remission.
Metabolic Features Were Independent Risk Factors for a Recurrent Event
To investigate the relationship between metabolic features and recurrence, multivariate analysis was conducted. Different models adjusting for different confounding factors were built to see whether IR and MetS were independent risk factors for the recurrence of fertility-sparing patients. Model I adjusted for baseline information including patient age, BMI, gestation, and parity. Model II added all significant risk factors in univariate analysis based on Model I, such as diabetes, histological type, and maintenance treatment. In the recurrence group, IR was found to be significantly associated with recurrence, whether confounding factors were adjusted for or not (Model I: OR = 13.3, 95% CI: 4.0–43.9, P <0.001; Model II: OR = 12.6, 95% CI: 3.7–43.3. P <0.001, Table 3 ), and MetS was also an independent risk factor for recurrence (Model I: OR = 5.5, 95% CI: 1.5–19.9, P = 0.009; Model II: OR = 5.8, 95% CI: 1.5–22.7, P = 0.012). In the RCR group ( Table 4 ), both IR (Model I: OR = 2.9, 95% CI: 1.2–4.6, P = 0.002; Model II: OR = 2.6, 95% CI: 1.8–4.4, P = 0.005) and MetS (Model I: OR = 6.9, 95% CI: 4.7–9.0, P <0.001; Model II: OR = 6.8, 95% CI: 4.7–8.9, P = 0.005) were found to increase the risk of lengthened CR time after recurrence. IR and MetS were two of the major metabolic features for patients with both diagnoses, and patients with both diagnoses were more likely to have recurrence after CR (Model I: OR = 29.6, 95% CI: 17.5–388.0, P <0.001; Model II: OR = 45.6, 95% CI: 21.5–595.2, P <0.001), as well as a longer RCR time (Model I: OR = 8.2, 95% CI: 15.8–10.7, P <0.001; Model II: OR = 7.9, 95% CI: 5.4–10.3, P <0.001). Above all, IR and MetS were identified as two independent risk factors for both recurrence and RCR time in fertility-preserving patients, with the combination of both diagnoses intensively increasing risk.
Table 3.
Logistic regression models evaluating the relationship between metabolic features and recurrence .
| Exposure | Non-adjusted | Model I (OR, 95%CI) P | Model II (OR, 95%CI) P |
|---|---|---|---|
| IR | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 9.5 (3.3, 27.0) <0.001 | 13.3 (4.0, 43.9) <0.001 | 12.6 (3.7, 43.3) <0.001 |
| MetS | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 4.9 (1.5, 15.5) 0.008 | 5.5 (1.5, 19.9) 0.009 | 5.8 (1.5, 22.7) 0.012 |
| IR and MetS | |||
| IR- and MetS- | 1.0 | 1.0 | 1.0 |
| IR+ or MetS+ | 7.2 (2.4,21.1) <0.001 | 16.1 (3.4,76.1) <0.001 | 19.1 (3.2, 115.4) <0.001 |
| IR+ and MetS+ | 21.0 (4.8.92.7) <0.001 | 29.6 (17.5, 388.0) <0.001 | 45.6 (21.5,595.2)<0.001 |
Non-adjusted model adjusted model for none; Model I adjusted for: age, BMI; Model II adjusted for: age, BMI; IR; MetS; histological type; maintenance treatment.
IR, insulin resistance; MetS, metabolic syndrome; OR, odds ratio; CI, confidence interval.
Table 4.
Logistic regression models evaluating the relationship between metabolic features and RCR time.
| Exposure | Non-adjusted | Model I (OR, 95%CI) P | Model II (OR, 95%CI) P | |
|---|---|---|---|---|
| IR | ||||
| No | 0 | 0 | 0 | |
| Yes | 3.0 (1.5, 4.5) <0.001 | 2.9 (1.2, 4.6) 0.002 | 2.6 (1.8, 4.4) 0.005 | |
| MS | ||||
| No | 0 | 0 | 0 | |
| Yes | 6.2 (4.2, 8.2) <0.001 | 6.9 (4.7, 9.0) <0.001 | 6.8 (4.7, 8.9) <0.001 | |
| IR and MetS | ||||
| IR- and MetS- | 0 | 0 | 0 | |
| IR+ or MetS+ | 1.6 (0.2, 3.0) 0.029 | 1.5 (-0.1, 3.2) 0.062 | 1.2 (-0.5, 2.8) 0.167 | |
| IR+ and MetS+ | 7.6 (5.5, 9.8) <0.001 | 8.2 (5.8, 10.7) <0.001 | 7.9 (5.4, 10.3) <0.001 | |
Non-adjusted model adjusted model for none; Model I adjusted for: age, BMI; Model II adjusted for: age, BMI; IR; MetS; diatebes; histological type; maintenance treatment.
IR, insulin resistance; MetS, metabolic syndrome; OR, odds ratio; CI, confidence interval.
IR and MetS Increase the Diagnostic Accuracy for Recurrence in AEH and G1EA Patients
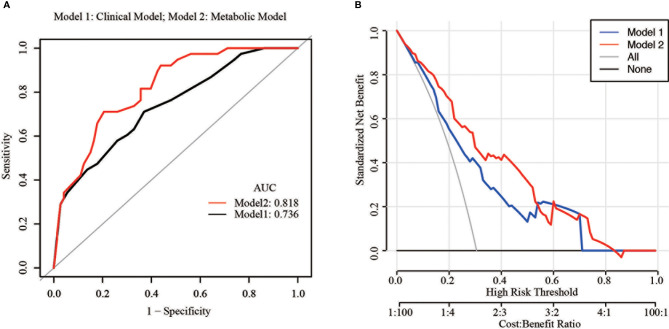
In order to estimate the accuracy of metabolic features in predicting the recurrence in conservative EC patients, a ROC curve for different combinations of risk factors was built. The area under the ROC curve (AUC) ranges from 0 to 1 and a model is considered to have a poor, fair, or good performance if the AUC lies between 0.5–0.6, 0.6–0.7, and >0.7, respectively. As shown in Figure 2A , the AUC of the combination of three risk factors (clinical model) namely, BMI, histological type, and maintenance time was 0.74 (95% CI: 0.66–0.83). However, after adding in the two metabolic features, IR, and MetS (metabolic model), the AUC reached to 0.82 (95% CI: 0.70–0.89), a significant improvement over the clinical model (P = 0.034). The recurrence rates were 15.9 and 20.0% in metabolic model and clinical model, respectively ( Table S1 ). The decision curve analysis (DCA) resulted for the clinical model and metabolic model are shown in Figure 2B . For predicted probability thresholds between 0% and nearly 60%, the metabolic model showed a positive net benefit for both the conservative treatment patients. Therefore, these results suggested that metabolic features significantly increased the accuracy in predicting recurrence of the fertility-sparing treatment.
Figure 2.
Predictive accuracy of different models. (A) Area under the receiver operating characteristic curves (AUCs) of models 1 and 2 for the prediction of recurrence in fertility-sparing treatment patients; (B) Decision curve analysis of the models.
Prognostic Value of the IR and MetS for Relapse
To evaluate and test whether the metabolic features affected RFS in AEH and G1EA patients, K–M recurrence curve of different IR and MetS groups was conducted. Log-rank test analysis revealed that there was a significant difference in recurrence rate between IR group and the combination group (IR+ and MetS+ group). The median time for patients with or without IR was 20.9 and 46.2 months, respectively. K–M recurrence curve is shown in Figure S1 and log-rank analysis revealed that the recurrence rate of women with IR was significantly lower than that of those without IR (P <0.05, Figure S1A ). Patients were then divided into three groups according to their metabolic characteristics: IR− and MetS−, IR+ or MetS+, and IR+ and MetS+. K–M recurrence curve demonstrated that the recurrence time frame of women with IR+ and MetS+ was significantly lower than that in the other two groups (P <0.05, Figure S1C ). However, no difference in recurrence speed was found between MetS+ and MetS− groups (P >0.05, Figure S1B ). These K–M curves revealed that IR, especially combined with MetS, has a great influence on recurrence in fertility-sparing treatment patients.
Discussion
Considering the rising incidence of endometrial cancer (EC) in reproductive age women and their relatively good prognosis, it is imperative to provide them with an effective fertility-preserving treatment option. Especially in China, where such work could actively address the country’s aging trend by facilitating the universal two-child policy. However, limited studies are available on risk factors for recurrence of EC in these patients. In this study, we elucidated risk factors for recurrence. Chief among them, being two metabolic features discovered to be independent risk factors. The two metabolic characteristics, insulin resistance (IR) and metabolic syndrome (MetS), substantially increased the accuracy of predicting recurrence after initial complete remission (CR).
The recurrence rate of conservative treatment varies across different studies. Chen etal. (13) reviewed 53 patients diagnosed with atypical hyperplasia or endometrioid adenocarcinoma and reported a recurrence rate of 26% in 2016. Wang etal. (14) reported that the recurrence rate ranged from 21.1 to 36.4% for patients whose treatment duration lasted from less than 6 months to more than 9 months.
Our study demonstrated that BMI, maintenance treatment, and histological type were all risk factors for recurrence and CR time after recurrence (RCR time). What’s more, we also found that diabetes was related to the length of RCR time. Body mass index (BMI), a measure of obesity and early-life obesity, has been associated with a moderately increased risk of EC later in life (15). In a randomized controlled trial, intervention weight loss for obese women could improve the survival of many cancers, including EC (16). However, the exact relationship between obesity and recurrence still remained elusive for AEH patients. Our findings were in according with this former research, in that a higher BMI correlated with worse therapeutic effects and a higher recurrence in AEH and EC patients (17). The underlying mechanisms linking obesity to relapse are still a matter of debate, but metabolic syndrome and insulin resistance/modification levels of adipocytokines appear to be of great importance.
One study found that maintenance treatment was a protective factor against relapse and RCR time. However, another study revealed that the treatment duration had no relationship with the recurrence rate (3). We concluded that maintenance treatment matters but not treatment duration for the recurrence of conservative therapy in AEH or G1EA patients.
The relationship between endometrial histological type and the prognosis of EC patients is well studied, and some of the studies stratified the patients according to the histological type (18, 19). One study concentrated on the prognostic factors for CR and found that atypical hyperplasia was easier to achieve CR (20). AEH or a lower grade was also a positive factor for a successful pregnancy and preventing recurrence (21). There are many cellular biomarkers in essential roles could be the potential mechanism underlying recurrence and lengthened RCR time, standouts include p53, p16, DNA mismatch repair proteins, PTEN, and ARID1A (22). AEH and early EC patients with diabetes, compared to those without, had worse patient characteristics, such as higher FIGO stage, similar recurrence rates, and worse overall survival (23). Our study supported this conclusion. In our analysis, patients with diabetes were more likely to experience longer CR time after recurrence.
Studies had demonstrated that IR played a vital role in the genesis and progression of many types of cancer, especially EC (24). IR and being overweight were also associated with longer therapeutic duration in EAH patients undergoing fertility-preserving treatment (25). However, the role of IR in the recurrence of AEH and early EC in patients undergoing fertility-preserving treatment remained elusive. Our study demonstrated that IR was an independent risk factor for recurrence in AEH and G1EA patients. Patients with IR needed a longer time to achieve CR after relapse compared with those without. IR was an essential risk factor for EC and may even be a possible mechanism involved in the development of EC (26). The level of insulin in patients with IR is higher than that in normal patients. It is plausible that extra insulin could bind to insulin receptors in endometrial cells promoting cancer cell proliferation, inhibiting apoptosis, and inducing angiogenesis, which in turn leads to the occurrence of EC (27). Additionally, insulin is involved in tumor development by directly or indirectly affecting endogenous estrogen metabolism and promoting the expression of endometrial estrogen receptor (ER), which in turn enhances the function of estrogen in the tumorigenesis of EC (28). It has been demonstrated that continued insulin increases the proliferation of endometrial cells under the effects of estrogen, thereby increasing the incidence of EC (28).
Another vital independent risk factor for recurrence and extended RCR time was metabolic syndrome. MetS had become one of the major worldwide public-health challenges and has been highlighted as a risk factor in several tumors, especially in EC (29). In recent years, epidemiological and clinical studies have found that MetS associated with metabolic diseases was closely related to the incidence of EC. Though there were few studies concentrating on MetS and fertility-sparing treatment in AEH and early EC patients, a great many investigations have focused on the use of metformin, an anti-MetS drug, for AEH or early EC patients with conservative therapy. Most young patients with AEH and EC who undergo fertility-sparing treatment have a background of obesity, IR and abnormal glucose tolerance complicated with polycystic ovary syndrome. Metformin had been used to counteract with these metabolic syndromes and has been attracting more attention in the field of cancer research (30). Metformin is an insulin sensitizer and has been widely investigated to treat various malignant diseases adjunctively. It has been demonstrated that metformin is an effective fertility-sparing treatment, as seen through reduction in the relapse rate after MPA therapy, particularly in obese patients (31). In the treatment of AEH and early EC, if metformin was combined with MPA, a higher early CR rate was induced compared with MPA alone.
The fact that both IR and MetS had been negatively associated with recurrence and the following CR time in progestin-based fertility-sparing treatment duration in AEH or early EC patients, indicating that IR and MetS could play synergistic roles in counteracting progestin function and compromise its therapeutic effects. Patients with both complications had the worst prognosis of fertility-preserving patients.
We hypothesized that since both IR and MetS patients have higher levels of insulin, this might induce excessive production of circulative estrogen (32). MetS is often characterized by IR, which some have suggested as a major underpinning link between physical inactivity and MetS. A possible mechanism of synergy could be that estrogen and insulin-like growth factor-1 (IGF-1) can synergistically promote the development of tumors by activating the MAPK and the AKT signaling pathways (33). Other studies have found that estrogen can bind to IGF-1R and exert non-genetic transcriptional effects through the Ras/MAPK signaling pathway and that the Ras/MAPK pathway could lead to the cancer recurrence (34, 35).
To our knowledge, this is the first study to explore the association between metabolic features and fertility-sparing treatment for AEH and early EC patients. However, there are limitations in this study. First of all, this is a single-center retrospective analysis and the number of the patients is relatively small. Moreover, endometrial tissue is collected by D&C, and this method is not as accurate as diagnostic hysteroscopy. Besides, another possible treatment of AHE or early EC also consisted of hysteroscopic removal and subsequent medical therapy. However, fertility-preserving treatment is limited by age, cancer stage, and patient desire, so it is difficult to include a large number of patients from a single institution. Therefore, a multi-center and large-population study will be needed to prove our conclusions.
Conclusion
In conclusion, this study demonstrates that the risk factors for the recurrence and RCR time includes BMI, IR, MetS, maintenance duration, and histological type in AEH and early EC patients. Among these factors, IR and MetS are two independent risk factors which could significantly increase the accuracy of predicting recurrence in patients undergoing fertility-sparing treatment. IR and MetS may play a synergistic role in counteracting progestin function during treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Ethics Committees of Peking University People’s Hospital (No. 2020PHB063-01).
Author Contributions
XL and YF conceived and designed the experiments. RZ and YW collected the data. LT and JiaqW analyzed the data. XL wrote the paper. JianW and YF reviewed the draft. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (Grant No. BMU2021PYB012), the National Natural Science Foundation of China (Grant No. 81874108), and National Key Technology R&D Program of China (Grant No. 2019YFC1005200 and 2019YFC1005201).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We especially appreciate Jiayang Jin of the Peking University People’s Hospital Rheumatology department for study design consultations and editing the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.744689/full#supplementary-material
Recurrence-free survival in different (A) insulin resistance (IR); (B) metabolic syndrome (MetS); (C) different IR and MetS groups.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics 2019. CA Cancer J Clin (2019) 69:7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3. Park JY, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, et al. Long-Term Oncologic Outcomes After Fertility-Sparing Management Using Oral Progestin for Young Women With Endometrial Cancer (KGOG 2002). Eur J Cancer (2013) 49:868–74. doi: 10.1016/j.ejca.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 4. Park JY, Nam JH. Progestins in the Fertility-Sparing Treatment and Retreatment of Patients With Primary and Recurrent Endometrial Cancer. Oncologist (2015) 20:270–8. doi: 10.1634/theoncologist.2013-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corzo C, Barrientos Santillan N, Westin SN, Ramirez PT. Updates on Conservative Management of Endometrial Cancer. J Minim Invasive Gynecol (2018) 25:308–13. doi: 10.1016/j.jmig.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 6. Koskas M, Uzan J, Luton D, Rouzier R, Darai E. Prognostic Factors of Oncologic and Reproductive Outcomes in Fertility-Sparing Management of Endometrial Atypical Hyperplasia and Adenocarcinoma: Systematic Review and Meta-Analysis. Fertil Steril (2014) 101:785–94. doi: 10.1016/j.fertnstert.2013.11.028 [DOI] [PubMed] [Google Scholar]
- 7. Guillon S, Popescu N, Phelippeau J, Koskas M. A Systematic Review and Meta-Analysis of Prognostic Factors for Remission in Fertility-Sparing Management of Endometrial Atypical Hyperplasia and Adenocarcinoma. Int J Gynaecol Obstet (2019) 146:277–88. doi: 10.1002/ijgo.12882 [DOI] [PubMed] [Google Scholar]
- 8. Onstad MA, Schmandt RE, Lu KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J Clin Oncol (2016) 34:4225–30. doi: 10.1200/JCO.2016.69.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qu HQ, Li Q, Rentfro AR, Fisher-Hoch SP, Mccormick JB. The Definition of Insulin Resistance Using HOMA-IR for Americans of Mexican Descent Using Machine Learning. PLoS One (2011) 6:e21041. doi: 10.1371/journal.pone.0021041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shan W, Ning C, Luo X, Zhou Q, Gu C, Zhang Z, et al. Hyperinsulinemia Is Associated With Endometrial Hyperplasia and Disordered Proliferative Endometrium: A Prospective Cross-Sectional Study. Gynecol Oncol (2014) 132:606–10. doi: 10.1016/j.ygyno.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 11. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic Syndrome and Risk of Cancer: A Systematic Review and Meta-Analysis. Diabetes Care (2012) 35:2402–11. doi: 10.2337/dc12-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Wang J. The Role of Metabolic Syndrome in Endometrial Cancer: A Review. Front Oncol (2019) 9:744. doi: 10.3389/fonc.2019.00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen M, Jin Y, Li Y, Bi Y, Shan Y, Pan L. Oncologic and Reproductive Outcomes After Fertility-Sparing Management With Oral Progestin for Women With Complex Endometrial Hyperplasia and Endometrial Cancer. Int J Gynaecol Obstet (2016) 132:34–8. doi: 10.1016/j.ijgo.2015.06.046 [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Zhou R, Wang H, Liu H, Wang J. Impact of Treatment Duration in Fertility-Preserving Management of Endometrial Cancer or Atypical Endometrial Hyperplasia. Int J Gynecol Cancer (2019) 29:699–704. doi: 10.1136/ijgc-2018-000081 [DOI] [PubMed] [Google Scholar]
- 15. Shaw E, Farris M, Mcneil J, Friedenreich C. Obesity and Endometrial Cancer. Recent Results Cancer Res (2016) 208:107–36. doi: 10.1007/978-3-319-42542-9_7 [DOI] [PubMed] [Google Scholar]
- 16. Kitson S, Ryan N, Mackintosh ML, Edmondson R, Duffy JM, Crosbie EJ. Interventions for Weight Reduction in Obesity to Improve Survival in Women With Endometrial Cancer. Cochrane Database Syst Rev (2018) 2:CD012513. doi: 10.1002/14651858.CD012513.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang YF, Liao YY, Liu XL, Su SG, Li LZ, Peng NF. Prognostic Factors of Regression and Relapse of Complex Atypical Hyperplasia and Well-Differentiated Endometrioid Carcinoma With Conservative Treatment. Gynecol Oncol (2015) 139:419–23. doi: 10.1016/j.ygyno.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 18. Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and Reproductive Outcomes With Progestin Therapy in Women With Endometrial Hyperplasia and Grade 1 Adenocarcinoma: A Systematic Review. Gynecol Oncol (2012) 125:477–82. doi: 10.1016/j.ygyno.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 19. Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, Relapse, and Live Birth Rates With Fertility-Sparing Therapy for Endometrial Cancer and Atypical Complex Endometrial Hyperplasia: A Systematic Review and Metaanalysis. Am J Obstet Gynecol (2012) 207:266 e261–212. doi: 10.1016/j.ajog.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 20. Acosta-Torres S, Murdock T, Matsuno R, Beavis AL, Stone RL, Wethington SL, et al. The Addition of Metformin to Progestin Therapy in the Fertility-Sparing Treatment of Women With Atypical Hyperplasia/Endometrial Intraepithelial Neoplasia or Endometrial Cancer: Little Impact on Response and Low Live-Birth Rates. Gynecol Oncol (2020) 157:348–56. doi: 10.1016/j.ygyno.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 21. Chae SH, Shim SH, Lee SJ, Lee JY, Kim SN, Kang SB. Pregnancy and Oncologic Outcomes After Fertility-Sparing Management for Early Stage Endometrioid Endometrial Cancer. Int J Gynecol Cancer (2019) 29:77–85. doi: 10.1136/ijgc-2018-000036 [DOI] [PubMed] [Google Scholar]
- 22. Murali R, Davidson B, Fadare O, Carlson JA, Crum CP, Gilks CB, et al. High-Grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int J Gynecol Pathol (2019) 38 Suppl 1:S40–63. doi: 10.1097/PGP.0000000000000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zanders MM, Boll D, Van Steenbergen LN, Van De Poll-Franse LV, Haak HR. Effect of Diabetes on Endometrial Cancer Recurrence and Survival. Maturitas (2013) 74:37–43. doi: 10.1016/j.maturitas.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 24. Hernandez AV, Pasupuleti V, Benites-Zapata VA, Thota P, Deshpande A, Perez-Lopez FR. Insulin Resistance and Endometrial Cancer Risk: A Systematic Review and Meta-Analysis. Eur J Cancer (2015) 51:2747–58. doi: 10.1016/j.ejca.2015.08.031 [DOI] [PubMed] [Google Scholar]
- 25. Yang B, Xie L, Zhang H, Zhu Q, Du Y, Luo X, et al. Insulin Resistance and Overweight Prolonged Fertility-Sparing Treatment Duration in Endometrial Atypical Hyperplasia Patients. J Gynecol Oncol (2018) 29:e35. doi: 10.3802/jgo.2018.29.e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lai Y, Sun C. Association of Abnormal Glucose Metabolism and Insulin Resistance in Patients With Atypical and Typical Endometrial Cancer. Oncol Lett (2018) 15:2173–8. doi: 10.3892/ol.2017.7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol (2016) 34:4270–6. doi: 10.1200/JCO.2016.67.4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tian W, Teng F, Zhao J, Gao J, Gao C, Sun D, et al. Estrogen and Insulin Synergistically Promote Type 1 Endometrial Cancer Progression. Cancer Biol Ther (2017) 18:1000–10. doi: 10.1080/15384047.2017.1394547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uzunlulu M, Telci Caklili O, Oguz A. Association Between Metabolic Syndrome and Cancer. Ann Nutr Metab (2016) 68:173–9. doi: 10.1159/000443743 [DOI] [PubMed] [Google Scholar]
- 30. Mitsuhashi A, Shozu M. New Therapeutic Approaches for the Fertility-Sparing Treatment of Endometrial Cancer. J Obstet Gynaecol Res (2020) 46:215–22. doi: 10.1111/jog.14155 [DOI] [PubMed] [Google Scholar]
- 31. Mitsuhashi A, Habu Y, Kobayashi T, Kawarai Y, Ishikawa H, Usui H, et al. Long-Term Outcomes of Progestin Plus Metformin as a Fertility-Sparing Treatment for Atypical Endometrial Hyperplasia and Endometrial Cancer Patients. J Gynecol Oncol (2019) 30:e90. doi: 10.3802/jgo.2019.30.e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun W, Lu J, Wu S, Bi Y, Mu Y, Zhao J, et al. Association of Insulin Resistance With Breast, Ovarian, Endometrial and Cervical Cancers in non-Diabetic Women. Am J Cancer Res (2016) 6:2334–44. doi: 10.1002/ijc.28262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang H, Liao Y, Xu L, Zhang C, Liu Z, Deng Y, et al. Estrogen and Insulin-Like Growth Factor 1 Synergistically Promote the Development of Lung Adenocarcinoma in Mice. Int J Cancer (2013) 133:2473–82. doi: 10.1002/ijc.28262 [DOI] [PubMed] [Google Scholar]
- 34. Eleveld TF, Schild L, Koster J, Zwijnenburg DA, Alles LK, Ebus ME, et al. RAS-MAPK Pathway-Driven Tumor Progression Is Associated With Loss of CIC and Other Genomic Aberrations in Neuroblastoma. Cancer Res (2018) 78:6297–307. doi: 10.1158/0008-5472.CAN-18-1045 [DOI] [PubMed] [Google Scholar]
- 35. Eleveld TF, Oldridge DA, Bernard V, Koster J, Colmet Daage L, Diskin SJ, et al. Relapsed Neuroblastomas Show Frequent RAS-MAPK Pathway Mutations. Nat Genet (2015) 47:864–71. doi: 10.1038/ng.3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recurrence-free survival in different (A) insulin resistance (IR); (B) metabolic syndrome (MetS); (C) different IR and MetS groups.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.