Abstract
The mitochondrial genome is continuously subject to attack by reactive oxygen species generated through aerobic metabolism. This leads to the formation of a variety of highly genotoxic DNA lesions, including abasic sites. Yeast Apn1p is localized to the nucleus, where it functions to cleave abasic sites, and apn1 Δ mutants are hypersensitive to agents such as methyl methanesulfonate (MMS) that induce abasic sites. Here we demonstrate for the first time that yeast Apn1p is also localized to the mitochondria. We found that Pir1p, initially isolated as a cell wall constituent of unknown function, interacts with the C-terminal end of Apn1p, which bears a bipartite nuclear localization signal. Further analysis revealed that Pir1p is required to cause Apn1p mitochondrial localization, presumably by competing with the nuclear transport machinery. pir1Δ mutants displayed a striking (∼3-fold) increase of Apn1p in the nucleus, which coincided with drastically reduced levels in the mitochondria. To explore the functional consequences of the Apn1p-Pir1p interaction, we measured the rate of mitochondrial mutations in the wild type and pir1Δ and apn1Δ mutants. pir1Δ and apn1Δ mutants exposed to MMS exhibited 3.6- and 5.8-fold increases, respectively, in the rate of mitochondrial mutations, underscoring the importance of Apn1p in repair of the mitochondrial genome. We conclude that Pir1p interacts with Apn1p, at the level of either the cytoplasm or nucleus, and facilitates Apn1p transport into the mitochondria to repair damaged DNA.
The yeast Saccharomyces cerevisiae possesses a 40.5-kDa DNA repair enzyme, Apn1p, that is localized to the nucleus (29, 30). Apn1p, a key enzyme in the base excision repair pathway, functions to hydrolyze apurinic/apyrimidinic (AP) sites produced either spontaneously or upon removal of damaged bases by DNA glycosylases (17, 30). This enzyme also possesses a 3′-diesterase activity that removes blocked 3′ ends from single-strand DNA breaks. Cells lacking Apn1p are hypersensitive to the alkylating agent methyl methanesulfonate (MMS) due to defective repair of MMS-induced AP sites (30). apn1Δ mutants also display a 10- to 15-fold increase in the rate of spontaneous single-base-pair mutations arising mainly as a result of unrepaired AP sites (9, 20, 21). Recent studies have shown that Apn1p homologues also exist in the fission yeast Schizosaccharomyces pombe, as well as in the nematode Caenorhabditis elegans, underscoring the importance of Apn1p family members in the repair of damaged DNA (27). To date, no homologue of Apn1p has been found in human cells (27).
Deletion analysis and immunofluorescence studies established that the extreme C-terminal end of Apn1p possesses a bipartite nuclear localization signal (NLS), which is characterized by two interdependent positively charge clusters separated by a spacer of 10 to 12 amino acid residues (28, 29). Removal of a portion of the NLS, i.e., the last 12 amino acid residues (SQMTKKRKTKKE), from the C terminus of Apn1p had no effect on the enzymatic activities of the resultant protein (Apn355) but abolished the nuclear localization of Apn1p, leading to extranuclear accumulation (29). Consequently, the Apn355 variant is unable to restore MMS resistance to an apn1Δ mutant. Addition of the simian virus 40 nuclear localization signal to Apn355 failed to target this protein to the nucleus, suggesting that the Apn1p nuclear transport mechanism is highly specific (29). Proteins with a classical monopartite NLS, such as that of simian virus 40, and bipartite NLS, such as nucleoplasmin, are imported in the nucleus by a heterodimer consisting of karyopherin α (Karαp; also called importin α) and karyopherin β (Karβp, or importin β). Karαp recognizes the classical NLS, and Karβp binds to Karαp, thus attaching the heterotrimeric complex to the nuclear pore complex for subsequent nuclear transport (2, 11, 44). Whether Apn1p is imported into the nucleus through the Karαp/Kar-βp complex, or directly by one of the several Karβ-like proteins that bind to NLS (14, 15, 42), is not known.
The N-terminal region of Apn1p possesses a putative mitochondrial transit sequence as revealed by the PSORT (http: //psort.nibb.ac.jp/) program. This raises the possibility that Apn1p could also be targeted to the mitochondria and involved in the repair of mitochondrial DNA. Consistent with this notion, many DNA repair enzymes are targeted to both the nucleus and the mitochondria. These include (i) S. cerevisiae Ntg1p, which repairs oxidative DNA lesions such as thymine glycol (51); (ii) S. pombe Uvdep, which incises a variety of DNA lesions including pyrimidine dimers and AP sites (49); and (iii) several human DNA glycosylases, e.g., uracil DNA glycosylase, OGG1, hNTH1, and MTH1 (4, 25, 32, 40). Additional DNA repair enzymes belonging to the base excision repair pathway such as DNA polymerase and DNA ligase also exist in the mitochondria. Recent studies show that the entire base excision repair pathway can be reconstituted from purified enzymes derived from Xenopus laevis mitochondria, and complete repair of uracil opposite guanine has been demonstrated with rat liver mitochondrial extract (26, 39). The mitochondrial DNA is proximate to the electron transport chain, which produces, as by-products, reactive oxygen species. Reactive oxygen species are known to generate a variety of DNA lesions including AP sites (12, 31); it is therefore logical that DNA repair enzymes would exist in the mitochondria to maintain stability of the genome. For example, yeast mutants lacking the mitochondrial mismatch repair protein Msh1p exhibit a high rate of mitochondrial mutation (38). Thus, defects in mitochondrial DNA repair could contribute to human diseases (36, 46). In fact, a multitude of mitochondrial mutations have been identified, and some have been associated with a variety of human disorders including Parkinson's disease, Alzheimer's disease, and some forms of diabetes mellitus (1, 6, 33).
We initially set out to identify the karyopherin that recognizes the bipartite NLS of Apn1p for subsequent translocation into the nucleus by using the Apn1p C-terminal end as bait in a yeast two-hybrid screen. However, we report the unexpected identification of Pir1p, a previously isolated cell wall protein, which interacts with the Apn1p C-terminal end. We show that this interaction mediates Apn1p translocation into the mitochondria. Deletion of the PIR1 gene from two different parental backgrounds did not hamper Apn1p translocation into the nucleus but instead decreased cytoplasmic and mitochondrial Apn1p levels. Our findings support a model where Pir1p binds to Apn1p, in either the cytoplasm or nucleus, and facilitates its entry into the mitochondria to prevent genetic instability.
MATERIALS AND METHODS
Strains, media, genetic analysis, and transformation.
The S. cerevisiae strains used in this study were PJ69-4A (MATa trp-901 leu2-3, 112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ), SEY6210 (APN1+, laboratory stock), DRY377 (apn1Δ::HIS3), YAT1530 (PIR1+), and YAT1529 (pir1Δ pir2Δ) (kindly provided by A. Toh-E, Tokyo, Japan). The pir1Δ::KanMX mutant strains RVY1 and RVY2 were derived from SEY6210 and YAT1530, respectively, by one-step gene targeting using the KanMX gene module (45). Yeast cells were grown in either complete yeast peptone dextrose (YPD) or minimal synthetic complete medium, to which nutritional supplements were added at 20 μg/ml (37). Standard genetic analysis and transformation were carried out as described previously (10, 13). The Escherchia coli strain used for plasmid maintenance was DH5α.
Construction of the bait plasmid pGBD-APN1-CT.
Plasmid YEpAPN1, which contains the entire S. cerevisiae APN1 gene with its transcriptional termination sequence (29), was used as the template to amplify by PCR (34) the 3′ end of the APN1 gene (bp +780 to +1435). The primers used were APN1-1 (5′-+780GCGCACTCTGAATTCCTGCAGGG+803-3′) and DR2 (5′-+1435CCAGCGGTCGACCATTACAAGTA+1413-3′) bearing restriction sites (underlined) for EcoRI and SalI, respectively. This yielded a 600-bp fragment containing basic clusters 1 and 2 of the APN1 gene, which was digested with EcoRI and SalI and then subcloned next to the Gal4 DNA binding domain in the S. cerevisiae expression vector pGBDUC2 to produce pGBD-APN-CT (16).
Yeast two-hybrid screening.
Yeast two-hybrid screening was performed as described by James et al. (16). The bait plasmid pGBD-APN-CT was introduced in the two-hybrid strain PJ69 (Ura3− Leu2− Ade− His−), which bears three reporter genes, ADE, HIS, and lacZ. This strain cannot grow in the absence of adenine and histidine unless the reporter genes are activated upon association of the Gal4 DNA binding and activation domains (16). A genomic library consisting of yeast DNA fragments fused to the Gal4 activation domain (GAD) bearing the selective marker LEU2 was subsequently introduced into strain PJ69 already carrying plasmid pGBD-APN-CT. The transformed cells were allowed to grow in 100 ml of selective SD medium (lacking leucine and uracil) overnights at 30°C to allow expression of both plasmids. The cells were resuspended in 3 ml of H2O, and 100 μl of this suspension was plated on SD medium lacking leucine, adenine, and uracil. The resulting transformants (60,000 Ura3+ Leu2+ colonies) were screened for growth in the absence of adenine. At least 20 Ade+ colonies were obtained, and only one (PJ69/pGBD-APN-CT/pGAD-RV2) strongly expressed the two additional reporter genes HIS3 and lacZ (data not shown). In these experiments, the IMP2 gene, encoding a transcriptional coactivator that interacts with the product of the YLR368w gene, was used as a control (43). Plasmid pGAD-RV2 was extracted from the positive colony and subjected to DNA sequence analysis.
DNA sequence analysis.
The primers 2H-GADUP (5′-TTCGATGATGAAGATACC-3′) and 2H-GADDOWN (5′-TGAAGTGAACTTGCGGGG-3′) were used to sequence the 5′ and 3′ ends, respectively, of the insert. The insert was sequenced completely by the dideoxy-chain termination method (35).
Construction of full-length PIR1.
Yeast genomic DNA was used as template to amplify the full-length PIR1 gene (bp −23 to +1208) using primers PIR1-UP(B) (5′-−23CCCCTATAGTGAATTCAAGAAAATGC+4-3′) and PIR1-DO (5′-+1208GTGAAGAACTGTCGACCTCATTTCATAGG+1180-3′) bearing restriction sites (underlined) for EcoRI and SalI, respectively. The PCR-amplified 1.2-kb fragment contains the entire coding region of the PIR1 gene. After digestion with EcoRI and SalI, the fragment was subcloned to the GAD in the yeast plasmid pGADC3 (16).
Protein extracts.
Total extracts were prepared as previously described (21) in yeast extraction buffer (50 mM Tris-HCl [pH 8.0], 50 mM NaCl, 5% glycerol, 1 mM each EDTA, phenylmethylsulfonyl fluoride, and dithiotheritol, 0.5 μg each of pepstatin, aprotinin, and leupeptin per ml).
Preparation of green fluorescent protein (GFP) fusions.
Plasmids pGBD-APN1-CT, pGAD-pRV2, and pGAD-PIR1, and pNlexA-IMP2 (22) were digested with EcoRI and SalI to release the DNA fragments encoding the Apn1 C-terminal basic clusters (Apn-Ct), 87% of PIR1, and the full-length PIR1 and IMP2 genes, respectively. Each fragment was then subcloned adjacent to the GFP gene, which was under the GAL1 promoter in the yeast expression vector pYES2.0, to produce pGFP-APN-CT, pGFP-pRV2, pGFP-PIR1, and pGFP-IMP2. Plasmid pGFP-APN1 was created by digesting pGBDU-APN1 with EcoRI, and the resulting APN1 fragment was inserted next to GFP in the pYES2.0 vector.
Phenyl-agarose column.
For the preparation of phenyl-agarose columns, 1 ml of the phenyl-agarose slurry (Pharmacia) was added to an Eppendorf tube and centrifuged to pellet the beads, which were then washed twice with 2 M (NH4)SO4 in yeast extraction buffer. The equilibrated matrix was transferred to a 2-ml column (Bio-Rad). Crude extracts (500 μg) derived from yeast strains expressing the indicated GFP fusion protein was adjusted to 1.7 M (NH4)SO4 and allowed to bind to the column over a 20-min period at room temperature. The column was washed with extraction buffer until the optical density at 280 nm (OD280) was baseline. The (GST) glutathione S-transferase (GST)–Apn-Ct fusion protein (200 ng) or total extract (500 μg) derived from strain SEY6210 carrying the Apn1p-overproducing plasmid (YEpAPN1) was slowly applied onto the columns at 4°C over a period of 8 h. The column was washed with 1× phosphate-buffered saline to remove nonspecific interactions and directly analyzed by Western blotting.
Preparation of subcellular fractions from yeast.
Cells were grown overnight in 1 liter of −ura selective medium with 2% raffinose at 30°C. The next day, the cultures were induced, where indicated, with 0.5% galactose for 0, 2, and 4 h. Samples of 300 ml were taken at each time point for nuclear and cytoplasmic extractions. The cell pellets were weighed, washed with H2O, resuspended (0.3 g/ml) in 100 mM Tris-SO4 (pH 9.3) buffer containing 10 mM dithiothereitol, incubated with gentle shaking at 30°C for 10 min, and centrifuged 3,000 × g for 5 min at room temperature. Following centrifugation, the pellets were washed once with buffer B (1.2 M d-sorbitol, 20 mM KPB [pH 7.4]), and zymolyase at 2.5 mg/g of cell pellet in 0.1 g of cell pellet/ml of buffer B was added. The pellets were incubated for 60 min at 30°C with gentle shaking until the cell wall was completely digested. The spheroplasts were collected at 3,000 × g for 5 min at room temperature and washed three times (1 g/10 ml of buffer B). From this point, all manipulations were performed at 4°C. The spheroplasts were suspended at 1 g/2 ml of MIB (0.6 M d-mannitol, 20 mM HEPES-KOH [pH 7.4], 0.5 mM phenylmethylsulfonyl fluoride) and broken in a Dounce homogenizer with 15 strokes using the pestle (glass and Teflon). The homogenate was then diluted 2-fold in MIB and centrifuged at 3,000 × g for 5 min. The pellet contained crude nuclei, and the supernatant represents the crude cytoplasm and mitochondria. The supernatant was spun at 12,000 × g; the resulting pellet contained the crude mitochondria, and the supernatant is termed the cytoplasmic fraction.
To obtain purified mitochondria, the crude mitochondria were diluted in 200 μl of MIB and layered on a two-step Nycodenz (Sigma) gradient made in a 14- by 89-mm Ultra-Clear centrifuge tube. The bottom layer of the gradient contained 5 ml of 18% and the top layer contained 5 ml of 14% Nycodenz in MIB. The tubes were spun at 40,000 × g in an SW41 rotor for 30 min, and the purified mitochondria were recovered between the two layers as a light brown band. The purified mitochondria were diluted fivefold in MIB and centrifuged at 12,000 × g for 10 min. The resulting mitochondrial pellet was lysed by addition of 200 μl of yeast extraction buffer to produce the mitochondrial fraction. Protease K treatment was carried out before and after lysis of the mitochondria at 37°C for 30 min, using 1 μg of protease K per ml. Succinate dehydrogenase was used to normalize the amount of mitochondrial protein extract.
To obtain purified nuclei, the crude nuclei were washed three times with MIB and each time spun at 3,000 × g for 5 min. The final washed pellet was resuspended in MIB and loaded onto a 30 to 50% Ficoll 400 (Pharmacia) step gradient in Ultra-Clear tubes (7). The gradient was spun in a Beckman SW28 rotor at 18,000 rpm for 60 min at 2°C. The nuclei band in the 40% layer was collected using a 20-ml syringe with a 16-gauge needle.
Immunoblotting.
The antibodies used in this study were monoclonal anti-GFP, monoclonal anti-GST, and polyclonal anti-Apn1p, which were raised against purified Apn1p (29). For Western analysis, the antibodies were used at dilutions of 1:5,000, 1:5,000, and 1:2,500, respectively, in 10 mM Tris-HCl (pH 7.5)–150 mM NaCl–5% powdered milk (8). Ten milliliters of this mixture was used to probe nitrocellulose blots (8 by 10 cm) overnight at 4°C. The secondary antibodies were anti-mouse for the GFP monoclonal antibodies and anti-rabbit for the GST and Apn1p polyclonal antibodies. Anti-mouse and anti-rabbit were used at dilutions of 1:2,500 and 1:5,000, respectively, and detected by enhanced chemiluminescence (Dupont, NEN).
Gradient plate assays.
Gradient plate assays were performed as previously described (23).
Enzyme assays.
β-Galactosidase activity was determined from crude extracts as previously described (22). AP endonuclease activity was assayed using an AP site substrate prepared by incorporation of [α-32P]dUTP, derived by deamination of [α-32P]dCTP (48). The AP sites were produced by removal of the uracil by uracil DNA glycosylase. A typical assay consists of 1 pmol of substrate in 25 μl of reaction mix containing 50 mM HEPES-KOH (pH 7.6), 50 mM KCl, 1 mM EDTA, 100 μg of bovine serum albumin, and the indicated concentration of protein extracts or purified protein. One unit of enzyme cleaves 1 pmol of the substrate/min under the standard reaction condition (48).
Mutation rate assay.
The frequency of erythromycin-resistant (Eryr) colonies was determined from 15 to 20 independent cultures and used to compute the rates (18). Cells were cultured to log phase (OD600 = 0.8 to 1.0), treated or not with MMS for 1 h, washed, and plated onto YPEG plates containing erythromycin (2 mg/ml). Eryr colonies were scored after 10 days of incubation at 30°C.
RESULTS
Apn-Ct interacts with Pir1.
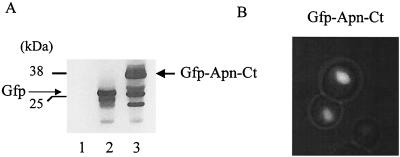
The APN1 C-terminal DNA segment (APN-CT), encoding the last 103 amino acid residues encompassing the entire bipartite NLS of Apn1p, was selected as bait for a yeast two-hybrid screen. To verify that the bait retained nuclear localization, the APN-CT fragment was fused to the C-terminal end of the GFP gene and placed under the control of the galactose-inducible GAL1 promoter. The resulting plasmid, pGFP-APN-CT, was introduced into strain PJ69 and examined for production of the GFP–Apn-Ct fusion protein using monoclonal anti-GFP antibody. Western blot analysis revealed that plasmid pGFP-APN-CT directed the expression of a polypeptide that corresponded to the expected size (38 kDa) of the GFP–Apn-Ct fusion protein (Fig. 1A, lane 3); the pGFP vector produced the native (∼26-kDa) GFP (lane 2). Immunofluorescence analysis revealed that GFP–Apn-Ct was localized to the nucleus as expected, whereas GFP alone was distributed throughout the cell (Fig. 1B and data not shown). These data clearly indicate that Apn-Ct harbors a functional NLS.
FIG. 1.
Expression and cellular location of GFP–Apn-Ct in yeast. (A) Total extracts were derived from the two-hybrid strain PJ69 bearing the pYES2.0 vector (lane 1) and plasmids pGFP (lane 2) and pGFP-APN-CT (lane 3) after 2 h of induction (see below). Each lane contained 50 μg of total extract, and the blot was probed with monoclonal anti-GFP antibody. Molecular weight standards are shown on the left. (B) Immunofluorescence analysis of strain PJ69 expressing GFP–Apn-Ct. Cells were grown in selective medium with 2% raffinose as the carbon source followed by induction with 0.5% galactose for 2 h.
The APN-CT DNA fragment was used in the yeast two-hybrid screen, and one positive clone was identified. DNA sequence analysis revealed that this clone contained 87% of the PIR1 gene, encoding a protein with internal repeats (Fig. 2) (24, 41). This partial PIR1 gene, designated PIR1Δ-44, lacked the sequence that corresponded to the N-terminal 44 amino acid residues (Fig. 2). The native PIR1 gene was predicted to encode a polypeptide with 341 amino acid residues and a calculated molecular mass of 34.6 kDa. The PIR1 gene product was previously identified as a cell wall protein with no known biological function (24, 41). The observed interaction between Apn-Ct and Pir1-p was not restricted to truncated forms of the two proteins, as the full-length genes APN1 and PIR1 also produced a strong interaction in the two-hybrid assay (see below). The control gene used throughout these experiments was IMP2, which encodes a transcriptional coactivator that interacts with the product of the YLR368w gene (22, 43). The above data indicate that the C-terminal end of Apn1p may directly or indirectly interact with Pir1p in vivo and that this interaction is specific for the combination of Apn1p and Pir1p.
FIG. 2.
Deduced amino acid sequence of Pir1p. The seven tandemly repeated sequences are shown in bold. The N-terminal 44 amino acid residues (underlined) are missing in plasmid pGAD-RV2, and the remaining portion is designated PIR1Δ-44.
Pir1p interacts with Apn1p in vitro.
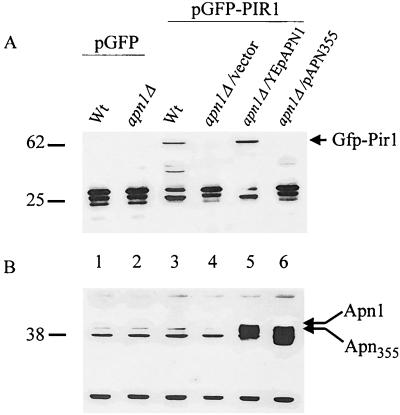
To test if Pir1p is associated with Apn1p in vitro, two independent expression plasmids, pGFP-PIR1 and pGST-APN-CT, were designed to produce different tagged forms of the proteins, GFP-Pir1 (∼62 kDa) and GST–Apn-Ct (∼38 kDa), in wild-type yeast and in E. coli, respectively (Fig. 3A, lane 3; Fig. 3B, lane 1). The strongly hydrophobic nature of GFP was exploited to couple the GFP-Pir1 fusion protein onto phenyl-Sepharose columns to serve as an affinity step to bind GST–Apn-Ct (see Materials and Methods). Binding of GFP-Pir1 or the control GFP-Imp2 fusion protein (∼63 kDa) onto the column was detected by directly analyzing the column matrix by Western analysis using monoclonal anti-GFP antibody (Fig. 3C, lanes 2 and 1, respectively). To assess whether GST–Apn-Ct can bind to the GFP-Pir1 column, a fixed amount of GST–Apn-Ct (200 ng) was loaded onto both columns, the columns were extensively washed, and the matrices were directly subjected to Western analysis using monoclonal anti-GST antibodies (Fig. 3D). While GST–Apn-Ct was bound to the GFP-Pir1 column (Fig. 3D, lane 2), it showed no detectable binding to the control GFP-Imp2 column (Fig. 3D, lane 1). In the case of the control GFP-Imp2 column, the GST–Apn1-Ct was recovered in the flowthrough washed fractions (data not shown). The GFP-Pir1 column (Fig. 3E, lane 2) but not the GFP-Imp2 column (lane 1) also specifically retained the native Apn1p when total yeast extract derived from an Apn1p-overproducing strain SEY6210/YEpAPN1 was applied. In this latter experiment, the two additional polypeptides detected by the anti-Apn1 antibodies were nonspecific and were retained by both columns (Fig. 3E). Collectively, the data suggest that Pir1p interacts with Apn1p and that the interaction involves the C-terminal end of Apn1p. Consistent with this observation, Apn1p and GFP-Pir1 were found to copurify on two separate columns, DEAE-Sepharose and single-stranded DNA–agarose (data not shown).
FIG. 3.
Expression of GFP-Pir1 and GST–Apn-Ct and interaction detected by affinity column. (A) Total extracts were derived from strain SEY6210 harboring either the vector pYES2.0 (lane 1) or plasmid pGFP (lane 2) or pGFP-PIR1 (lane 3) and probed with anti-GFP monoclonal antibody. (B) Total extracts derived from bacteria carrying plasmid pGST-APN-CT (lane 1) or pGST (lane 2) were subjected to purification on a GST affinity column and stained with Ponceau red. (C) Binding of the control protein GFP-Imp2 (lane 1) or GFP-Pir1 (lane 2) to the phenyl-agarose column. The matrices were probe by Western analysis using anti-GFP antibody. (D) Equal amounts of GST–Apn-Ct (200 ng) were separately loaded onto GFP-Imp2 (lane 1) and GFP-Pir1 (lane 2) phenyl-agarose columns, followed by extensive washing and direct analysis of the column matrix by Western blotting with anti-GST antibody as the probe. (E) Same as panel D except that total extract (500 μg) derived from strain SEY6210 overproducing Apn1p was used and the column matrix was probed by Western blotting using anti-Apn1p polyclonal antibodies. In all panels, sizes are indicated in kilodaltons.
Detection of full-length GFP-Pir1 depends on the presence of native Apn1p.
We then tested if GFP-Pir1 fusion protein levels are dependent on the presence of Apn1p. Freshly prepared crude extracts derived from the parent strain SEY6210 (i.e., harboring pGFP-PIR1) expressed the full-length GFP-Pir1 protein (Fig. 4A, lane 3), but extracts derived from an apn1Δ mutant carrying pGFP-PIR1 did not (lane 4). However, full-length GFP-Pir1 was detected in the apn1Δ mutant upon reintroduction of the native APN1 gene on a multicopy plasmid YEpAPN1 (Fig. 4A and B, lane 5) or the bait plasmid pGBD-APN-CT (data not shown). The full-length GFP-Pir1 was not seen in the apn1Δ mutant carrying the multicopy plasmid pAPN355, which expresses the Apn355 protein lacking the extreme C-terminal 12 amino acid residues (Fig. 4A and B, lane 6). The data are consistent with the notion that Pir1p and native Apn1p can form a complex in vivo. In the case of the apn1Δ mutant, it is not clear whether the GFP-Pir1 protein is degraded or translocated to the cell wall (see Discussion).
FIG. 4.
Detection of full-length GFP-Pir1 depends on the presence of native Apn1p. Western blots of freshly prepared total extracts derived from strains SEY6210/pGFP (wild type [wt]; lane 1), apn1Δ/pGFP (lane 2), SEY6210/pGFP-PIR1 (lane 3), and apn1Δ harboring pGPF-PIR1 (lanes 4 to 6) and either the vector Yep352 (lane 4) or the multicopy plasmids YEpAPN1 (lane 5) and pAPN355 (lane 6) were probed with anti-GFP monoclonal (A) and anti-Apn1p polyclonal (B) antibodies. In crude extracts, the anti-Apn1p polyclonal antibodies recognize several nonspecific polypeptides (B, lane 2) and detect Apn1p only when the latter is overproduced (B, lanes 5 and 6). Each lane contained 50 μg of total protein extracts.
pir1Δ mutants are not sensitive to MMS.
To explore the possible biological role of Apn1p-Pir1p interaction, we first tested if pir1Δ mutants were similarly hypersensitive as the apn1Δ mutants to MMS. The PIR1 gene was deleted from two different parental strains, SEY6210 and YAT1530, to produce the pir1Δ strains RVY1 and RVY2, respectively. Interestingly, the pir1Δ mutants showed parental resistance to MMS compared to the apn1Δ mutant (data not shown) (30). Moreover, an apn1Δ pir1Δ double mutant was no more sensitive than the single apn1Δ mutant (data not shown). It would thus appear that Pir1p is not absolutely required for Apn1p function in the repair of MMS-induced DNA lesions.
Pir1p deficiency causes Apn1p to accumulate in the nucleus.
Since Pir1p does not directly influence Apn1p-modulated DNA repair, we examined if it affects the distribution of Apn1p in the cell. In this experiment, plasmid pGFP-APN1 was designed to express a N-terminal GFP-Apn1 functional fusion protein under the control of the GAL1 promoter. Introduction of the pGFP-APN1 plasmid into either the parent strain SEY6210 or the pir1Δ mutant, following induction, revealed that the GFP-Apn1 fusion protein was present in the nucleus (Fig. 5A and B). Strikingly, however, the GFP-Apn1 protein was visually at least three times more intense in the nucleus of the pir1Δ mutant compared to the parent strain. In contrast, the control protein GFP-Imp2 showed both cytoplasmic and nuclear localization; most important, the pattern of staining did not differ between the parent (Fig. 5C) and the pir1Δ mutant (Fig. 5D). It appears that GFP-Apn1 accumulated in the nucleus of the pir1Δ mutant.
FIG. 5.
Increased staining intensity of GFP-Apn1 in the nucleus of the pir1Δ mutant. Strains SEY6210 (parent)/pGFP-APN1 (A), RVY1 (pir1Δ)/pGFP-APN1 (B), SEY6210/pGFP-IMP2 (C), and RVY1/pGFP-IMP2 (D) were grown in selective medium with 2% raffinose and induced with 0.5% galactose for 2 h before being photographed at a magnification of ×100 with a CoolSnap camera attached to a Leitz immunofluorescence microscrope. Final magnification, ×50.
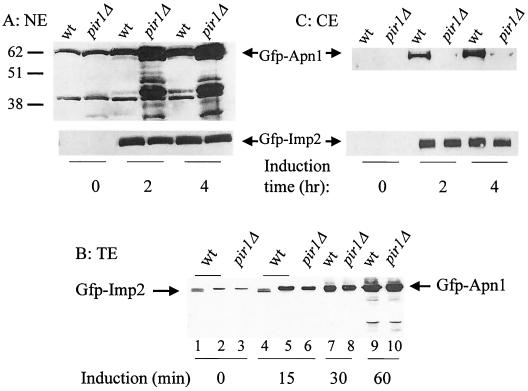
To further support the above finding, nuclear extracts were prepared from purified nuclei derived from the four strains as in Fig. 5 after various time of induction, and the levels of GFP-Apn1 and GFP-Imp2 were quantified by Western blot analysis using anti-GFP antibody. In the absence of induction, i.e., at time zero, a basal level of GFP-Apn1 expression was detected in both the parent and pir1Δ mutant due to leaky expression from the GAL1 promoter (Fig. 6A) (29). After 2 h of induction, at least fourfold more GFP-Apn1 accumulated in the nucleus of the pir1Δ mutant compared to the parent (Fig. 6A). The level of accumulated GFP-Apn1 in the pir1Δ nucleus is likely higher than fourfold, as a considerable amount of the protein existed as truncated forms, presumably due to nuclear degradation (Fig. 6A). The difference in the nuclear amount of GFP-Apn1 between the two strains cannot be due to the protein being more readily lost from the parent during preparation of the nuclei, as the same phenomenon was observed in living cells (Fig. 5A and B). Moreover, parallel experiments conducted with the parent and pir1Δ mutant showed no difference in the nuclear amount of the control protein GFP-Imp2 (Fig. 6A, bottom panel).
FIG. 6.
Altered distribution of GFP-Apn1 in the nucleus and cytoplasm of the pir1Δ mutant. (A) Nuclear extract (NE) derived from strains SEY6210 (wild type [wt]) and RVY1 (pir1Δ) bearing either plasmid pGFP-APN1 (top) or plasmid pGFP-IMP2 (bottom). (B) Total extract (TE) prepared from strains SEY6210 and RVY1 carrying plasmid pGFP-APN1 (lanes 2, 3, and 5 to 10). Lanes 1 and 4, total extracts from SEY6210 harboring pGFP-IMP2. Extracts were derived from the strains following the indicated times of induction. (C) Cytoplasmic extract (CE) derived from strains SEY6210 and RVY1 bearing either plasmid pGFP-APN1 (top) or plasmid pGFP-IMP2 (bottom). The extracts were prepared from cells after the indicated times of induction with galactose, as in Fig. 5, and all panels were probed with anti-GFP monoclonal antibodies. The amounts of nuclear, total, and cytoplasmic extracts assessed by Western blot analysis were 30, 50, and 150 μg, respectively. Sizes are indicated in kilodaltons.
In a separate experiment, the level of AP endonuclease activity was measured in the nuclear extracts using a synthetic substrate containing AP sites (48). After 2 h of induction, AP endonuclease levels in the extracts derived from purified nuclei isolated from the four strains SEY6210/pGFP-APN1, RVY1/pGFP-APN1, SEY6210/pGFP-IMP2, and RVY1/pGFP-IMP2 were 169.1, 1,015.6, 42.3, and 189.2 U per mg of protein, respectively. Induction had no effect on the levels of AP endonuclease in the two latter strains bearing plasmid pGFP-IMP2 and in nuclear extracts from an apn1Δ mutant that expressed extremely low level of AP endonuclease, <1.0 U per mg of protein. The data are consistent with an increased level of GFP-Apn1 in the pir1Δ nucleus. Moreover, it indicates that endogenous levels of Apn1p, i.e., in the absence of the overproducing plasmid pGFP-APN1, were also accumulating in the pir1Δ nucleus. Since the accumulated Apn1p in the pir1Δ nucleus is also fully active, Pir1p cannot play a role in modulating Apn1p activity. Despite the higher level of Apn1p in the pir1Δ nucleus, this mutant was no more resistant to MMS than the parent. This finding was not unexpected, as Apn1p is not a limiting enzyme in parent strains (30).
To exclude the possibility that the accumulation of GFP-Apn1 in the nucleus of the pir1Δ mutant did not reflect either increased protein expression or decreased turnover, the level of GFP-Apn1 was determined in total cell extracts derived from the parent and pir1Δ mutant. Western analysis revealed that the amount of GFP-Apn1 expressed was nearly the same in both strains within a linear range of induction (Fig. 6B). In addition, the extent of degradation of GFP-Apn1 was no different between the two strains after 1 h of induction (Fig. 6B, lanes 9 and 10). Therefore, the accumulated level of GFP-Apn1 within the nucleus of pir1Δ mutant must reflect an imbalance of Apn1p level from another organelle.
The pir1Δ mutant has reduced levels of cytoplasmic and mitochondrial Apn1p.
Since Apn1p is synthesized in the cytoplasm and translocated to the nucleus, we examined the amount of GFP-Apn1 in the cytoplasm in both the parent and pir1Δ strains harboring pGFP-APN1 after various time of induction. In the absence of induction, no GFP-Apn1 was detected in the cytoplasm of either strain (Fig. 6C). However, after 2 h of induction, GFP-Apn1 was detected in the cytoplasm of the parent but not the pir1Δ mutant (Fig. 6C). In contrast, the control GFP-Imp2 protein was detected at the same level in the cytoplasm of the parent and pir1Δ mutant-bearing plasmid pGFP-IMP2 (Fig. 6C, bottom panel). These data indicate that Pir1p is not required to target Apn1p into the nucleus per se but rather regulates its distribution in the cell.
We therefore considered the possibility that Pir1p may target Apn1p to the mitochondria. Extracts were prepared from purified mitochondria isolated from the parent, pir1Δ, and apn1Δ strains, and Apn1p polypeptide was analyzed by Western blotting using anti-Apn1p polyclonal antibodies (Fig. 7). Mitochondria derived from the pir1Δ mutant contained at least fivefold less Apn1p cross-reactive polypeptide compared to the parent (Fig. 7A, lane 3 versus lane 1). No Apn1p-reactive polypeptide was detected in mitochondria derived from the apn1Δ mutant (Fig. 7A, lane 5). Plasmid pDR6, overproducing Apn1p, increased the level of the protein in the mitochondria and bypassed the need for Pir1p (Fig. 7, lane 4). Thus, in normal cells, Pir1p is required to ensure that Apn1p is efficiently distributed to the mitochondria, but this function can be compensated for by overproduction of Apn1p. It is noteworthy that overproduction of Pir1p did not increase Apn1p levels in the mitochondria.
FIG. 7.
Diminished level of Apn1p in mitochondria derived from the pir1Δ mutant. (A and B) Western blot analysis of mitochondrial extracts. For panel A, mitochondrial extracts were prepared from the parent strain SEY6210 (wild type[wt]), the pir1Δ mutant, and the apn1Δ mutant bearing the indicated vector or plasmid. Plasmid pDR6 contained the entire coding region of Apn1p placed under the control of the GAL1 promoter in vector pYES2.0 (29). For panel B, intact or disrupted mitochondria were untreated and treated with proteinase K. Each lane contained 200 μg of mitochondrial extract, and the blot was probed with anti-Apn1p antibodies. (C) Levels of AP endonuclease activity in extracts derived from purified mitochondria obtained from the indicated strains. Prot., protein.
To ascertain that Apn1p is indeed in the mitochondria and not attached to the outer membranes, intact mitochondria derived from strain SEY6210/pDR6 were either untreated or treated with proteinase K and extensively washed before extract preparation (Fig. 7B). The proteinase K pretreatment of intact mitochondria did not alter the amount of detectable Apn1p polypeptide, strongly indicating that Apn1p is present in the mitochondria (Fig. 7B, lane 2). However, if the mitochondria were first disrupted and pretreated with proteinase K, the amount of Apn1p polypeptide detected was much less than with no pretreatment (Fig. 7B, lane 4 versus lane 3).
To assess if the level of Apn1p polypeptide detected in the mitochondrial extracts corresponded to the AP endonuclease activity, the extracts were quantified for AP endonuclease using the synthetic AP site substrate. Mitochondrial extracts derived from the pir1Δ mutant contained substantially (∼10%) lower levels of AP endonuclease activity compared to the parent (Fig. 7C). No AP endonuclease activity was detected in the mitochondrial extract derived from the apn1Δ mutant (Fig. 7C). Introduction of a plasmid (pPIR) carrying the PIR1 gene into the pir1Δ mutant restored the level of AP endonuclease in the mitochondria to nearly parental levels (Fig. 7C). These data strongly indicate that Apn1p level in the mitochondria is dependent on Pir1p function.
The pir1Δ mutant exhibits increased level of mitochondrial mutations, which can be prevented by Apn1p overproduction.
Since Apn1p is essential to maintain the stability of the nuclear genome, we tested if Apn1p plays a similar role in the mitochondria. The rate of mitochondrial mutations was determined in the parent, pir1Δ, and apn1Δ mutants by scoring independent colonies for Eryr. Eryr colonies can occur as a result of mutation in the mitochondrial gene encoding the large (21S)rRNA (5). Interestingly, the spontaneous mutation rates of Eryr colonies were similar for the parent, pir1Δ, and apn1Δ strains, suggesting that these mutations originate independently of Apn1p function (Table 1). However, if the cells were exposed to a low dose of MMS (0.05% for 30 min), levels of Eryr mutations increased 3.6- and 5.8-fold in the pir1Δ and apn1Δ mutants, respectively, compared to the parent (Table 1). Overproduction of Apn1p or introduction of the PIR1 gene into the pir1Δ mutant prevented the MMS-induced mutations (Table 1). Collectively, the data strongly indicate that Pir1p facilitates Apn1p translocation into the mitochondria, where it can act to process excess AP sites in the mitochondrial genome.
TABLE 1.
MMS-induced Eryr colonies
| Strain genotype | Rate of induction (no. [108]/cell division)a
|
|
|---|---|---|
| 0% MMS | 0.05% MMS | |
| APN1 PIR1 SEY6210 [parent] | 6.1 ± 0.5 | 6.3 ± 0.4 |
| apn1Δ | 7.3 ± 0.3 | 42.3 ± 3.2 |
| apn1Δ/pPIR1 | 6.4 ± 0.4 | 38.8 ± 2.3 |
| pir1Δ | 7.7 ± 0.5 | 26.6 ± 1.2 |
| pir1Δ/YEpAPN1 | 6.3 ± 0.6 | 7.2 ± 0.2 |
| pir1Δ/pPIR1 | 6.8 ± 0.3 | 8.3 ± 0.4 |
Calculated from results for 10 to 15 independent cultures (see Materials and Methods).
DISCUSSION
We demonstrate that the C-terminal end of the DNA repair enzyme Apn1p, comprising a bipartite NLS, interacts either directly or indirectly with Pir1 protein, a cell wall constituent of previously unknown function. We believe that this interaction is required to facilitate Apn1p translocation into the mitochondria. This is supported by our findings that Pir1p-deficient cells manifest an imbalance in the subcellular distribution of Apn1p, i.e., an accumulation in the nucleus and concomitant reduction in the mitochondria. An apparently dire consequence of Pir1p deficiency is reflected by the elevated levels of MMS-induced mitochondrial mutations due to reduced levels of mitochondrial Apn1p. We conclude that Apn1p exists among the plethora of DNA repair enzymes that maintain genome stability in the mitochondria. Apn1p may serve in the elimination of excess AP sites and/or to repair specific oxidative DNA lesions (30), as well as to remove 3′-blocking lesions that are created after the action of AP lyases such as Ntg1p (30, 50).
The involvement of Pir1p in the subcellular distribution of Apn1p is unexpected and suggests that Pir1p is subjected to intracellular localization in compartments other than the cell wall (24, 41). Pir1p bears no obvious organelle targeting signal sequence, and no extensive cell fractionation experiments have been performed to examine its cellular location. It is noteworthy that a significant portion of the GFP-Pir1 fusion protein used in this study is cleaved to generate GFP, and thus this fusion cannot be used to examine Pir1p proper cellular location. Accordingly we are currently engineering a much smaller epitope tag (hemagglutinin) next to Pir1p to precisely determine how the cellular distribution of this protein is affected by Apn1p.
A major challenge is to determine the mechanism by which Pir1p facilitates Apn1p distribution into the mitochondria. At least two models can be envisaged. In one model, Pir1p would compete with Karαp for binding to Apn1p bipartite NLS; alternatively, it would directly bind to the NLS and sequester a fraction of the Apn1p to be translocated into mitochondria. Pir1p contains seven tandem repeats of a stretch of 18 to 19 amino acid residues (AAAVSQIGDGQIQATTKTT/K), constituting 38% of the protein. These repeats may engage in the interaction with the basic amino acid residues of Apn1p NLS, so as to prevent Karαp from accessing the NLS. Karαp itself consists of 10 tandemly repeated modules termed armadillo (ARM) motifs that form an α-helical structure which creates several contacts with the basic amino acid residues of monopartite and bipartite NLS (2). Although Pir1p repeats are not identical to those of Karαp, three short amino acid stretches, VSO, QIQA, and KTK, belonging to the Pir1 repeats are present in ARM 9 (VSQ and QIQA) and ARM 10 (KTK) of Karαp. These short amino acid stretches of Pir1p could compete for the NLS of Apn1p.
In a second model, Pir1p could be viewed as a transport receptor or exportin like the Karβp superfamily that shuttles between the nucleus and cytoplasm (14, 15, 42). In such a case, Pir1p could mediate the export of Apn1p through the nuclear pore complexes, thus facilitating Apn1p uptake into the mitochondria. This model predicts that a pir1Δ mutant would also favor a nuclear distribution of Apn1p, and concomitant depletion in the mitochondria, as observed here. This model is supported by a recent study showing that overproduction of either Pse1p/Karβ121p or Karβ123p, two members of the Karβp family, facilitates the translocation of hydrophobic proteins into the mitochondria (3). These proteins include the mitochondrial ABC transporter Atm1p and a reporter protein fused to the transmembrane domains of apocytochrome p (3). Based on this model, it would be expected that overproduction of Pir1p would deplete the nuclear level of Apn1p and display a DNA repair-deficient phenotype. However, this was not observed, as yeast cells do not appear to sustain higher levels of Pir1p expression from the GAL1 control promoter. The second model further predicts that Pir1p would be recycled into the nucleus, thereby exhibiting a nucleocytoplasmic distribution. In fact, many exportins, e.g., Crm1p and Msn5p which export Yap1p and Pho4p, respectively, are known to cycle between the cytoplasm and nucleus (19, 47).
One caveat associated with the second model is that it is energetically less feasible, since it would require that Apn1p cross the nuclear membrane twice before entry into the mitochondria, as opposed to the first model, where Apn1p would enter directly from the cytoplasm. However, considering the high rate at which spontaneous mutagenic AP sites are generated in the nuclear genome, the cell may opt to first import Apn1p into the nucleus followed by its distribution to the mitochondria. This seems feasible, as yeast cells retain at least 50 mitochondria under normal growth conditions and may be more tolerant of mitochondrial than of nuclear DNA damage. To distinguish between the above two models, it is imperative to define a single amino acid substitution (perhaps involving the unique proline in the spacer region of the bipartite NLS) that blocks Apn1p entry into the nucleus, while still allowing it to interact with Pir1p. According to the first model, the modified Apn1p should be able to enter the mitochondria, unless Apn1p nuclear entry precedes mitochondrial translocation.
Although Apn1p has a putative N-terminal presequence, this is apparently not sufficient to target the protein to the mitochondria. Such a notion is supported by the observation that Apn355, which lacks a portion of the bipartite NLS, was predominantly localized to the cytoplasm, as determined by indirect immunofluorescence microscopy and by GFP-tagged Apn355 (29) (R. Vongsamphanh and D. Ramotar, unpublished data). In any case, the putative N-terminal presequence is not likely to be a strong signal for targeting Apn1p to the mitochondria. An independent study demonstrated that attachment of the presequence of S. pombe, but not of S. cerevisiae Apn1p to GFP was sufficient to target the protein to the mitochondria of HeLa cells (J. Connolly, R. Perez-Jannotti, and D. Bogenhagen, personal communication). Since S. pombe lacks Pir1p, this organism may have evolved to effectively use the N-terminal presequence. Thus, any experimental design to demonstrate that the Apn1p N-terminal presequence is capable of targeting a heterologous protein into the mitochondria will work only if the fusion protein also bears the stretch of amino acids residues that promote interaction with Pir1p.
ACKNOWLEDGMENTS
We sincerely thank Eric Alani, Dan Bogenhagen, Brian Burke, Michael Weinfeld, and Elliot Drobetsky for critical comments on the manuscript. We also thank Andrea Shatilla and Xiaoming Yang for technical assistance in preparation of the AP site substrate, Jocelyn David for constructing the pGFP plasmid, and Anick Leduc for sequencing the fusion constructs.
This work was supported by research grant OGP0138503 to D.R. from the Natural Science and Engineering Research Council of Canada. D.R. is a career Scientist of the National Cancer Institute of Canada.
REFERENCES
- 1.Bandmann O, Sweeney M G, Daniel S E, Marsden C D, Wood N W. Mitochondrial DNA polymorphisms in pathologically proven Parkinson's disease. J Neurol. 1997;244:262–265. doi: 10.1007/s004150050082. [DOI] [PubMed] [Google Scholar]
- 2.Conti E, Uy M, Leighton L, Blobel G, Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell. 1998;94:193–204. doi: 10.1016/s0092-8674(00)81419-1. [DOI] [PubMed] [Google Scholar]
- 3.Corral-Debrinski M, Belgareh N, Blugeon C, Claros M G, Doye V, Jacq C. Overexpression of yeast karyopherin Pse1p/Kap121p stimulates the mitochondrial import of hydrophobic proteins in vivo. Mol Microbiol. 1999;31:1499–1511. doi: 10.1046/j.1365-2958.1999.01295.x. [DOI] [PubMed] [Google Scholar]
- 4.Croteau D L, ap Rhys C M, Hudson E K, Dianov G L, Hansford R G, Bohr V A. An oxidative damage-specific endonuclease from rat liver mitochondria. J Biol Chem. 1997;272:27338–27344. doi: 10.1074/jbc.272.43.27338. [DOI] [PubMed] [Google Scholar]
- 5.Cui Z, Mason T L. A single nucleotide substitution at the rib2 locus of the yeast mitochondrial gene for 21S rRNA confers resistance to erythromycin and cold-sensitive ribosome assembly. Curr Genet. 1989;16:273–279. doi: 10.1007/BF00422114. [DOI] [PubMed] [Google Scholar]
- 6.Davis R E, Miller S, Herrnstadt C, Ghosh S S, Fahy E, Shinobu L A, Galasko D, Thal L J, Beal M F, Howell N, Parker W D., Jr Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:4526–4531. doi: 10.1073/pnas.94.9.4526. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Dove J E, Brockenbrough J S, Aris J P. Isolation of nuclei and nucleoli from the yeast Saccharomyces cerevisiae. Methods Cell Biol. 1998;53:33–46. doi: 10.1016/s0091-679x(08)60873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershoni J M, Palade G E. Protein blotting: principles and applications. Anal Biochem. 1983;131:1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs P E, Lawrence C W. Novel mutagenic properties of abasic sites in Saccharomyces cerevisiae. J Mol Biol. 1995;251:229–236. doi: 10.1006/jmbi.1995.0430. [DOI] [PubMed] [Google Scholar]
- 10.Gietz R D, Schiestl R H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast. 1991;7:253–263. doi: 10.1002/yea.320070307. [DOI] [PubMed] [Google Scholar]
- 11.Gorlich D, Henklein P, Laskey R A, Hartmann E. A 41 amino acid motif in importin-alpha confers binding to importin-beta and hence transit into the nucleus. EMBO J. 1996;15:1810–1807. [PMC free article] [PubMed] [Google Scholar]
- 12.Grishko V I, Druzhyna N, LeDoux S P, Wilson G L. Nitric oxide-induced damage to mtDNA and its subsequent repair. Nucleic Acids Res. 1999;27:4510–4516. doi: 10.1093/nar/27.22.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:3–37. [PubMed] [Google Scholar]
- 14.Henderson B R, Percipalle P. Interactions between HIV Rev and nuclear import and export factors: the Rev nuclear localisation signal mediates specific binding to human importin-beta. J Mol Biol. 1997;274:693–707. doi: 10.1006/jmbi.1997.1420. [DOI] [PubMed] [Google Scholar]
- 15.Jakel S, Gorlich D. Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–4502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James P, Halladay J, Craig E A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson A W, Demple B. Yeast DNA 3′-repair diesterase is the major cellular apurinic/apyrimidinic endonuclease: substrate specificity and kinetics. J Biol Chem. 1988;263:18017–18022. [PubMed] [Google Scholar]
- 18.Jones M E. An improved estimator of spontaneous mutation rates in Luria-Delbruck fluctuation experiments. Mutat Res. 1993;292:191–198. doi: 10.1016/0165-1161(93)90147-r. [DOI] [PubMed] [Google Scholar]
- 19.Kaffman A, Rank N M, O'Neill E M, Huang L S, O'Shea E K. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature. 1998;396:482–486. doi: 10.1038/24898. [DOI] [PubMed] [Google Scholar]
- 20.Kunz B A, Henson E S, Roche H, Ramotar D, Nunoshiba T, Demple B. Specificity of the mutator caused by deletion of the yeast structural gene (APN1) for the major apurinic endonuclease. Proc Natl Acad Sci USA. 1994;91:8165–8169. doi: 10.1073/pnas.91.17.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masson J Y, Ramotar D. Normal processing of AP sites in Apn1-deficient Saccharomyces cerevisiae is restored by Escherichia coli genes expressing either exonuclease III or endonuclease III. Mol Microbiol. 1997;24:711–721. doi: 10.1046/j.1365-2958.1997.3841748.x. [DOI] [PubMed] [Google Scholar]
- 22.Masson J Y, Ramotar D. The Saccharomyces cerevisiae IMP2 gene encodes a transcriptional activator that mediates protection against DNA damage caused by bleomycin and other oxidants. Mol Cell Biol. 1996;16:2091–2100. doi: 10.1128/mcb.16.5.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masson J Y, Ramotar D. The transcriptional activator Imp2p maintains ion homeostasis in Saccharomyces cerevisiae. Genetics. 1998;149:893–901. doi: 10.1093/genetics/149.2.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mrsa V, Tanner W. Role of NaOH-extractable cell wall proteins Ccw5p, Ccw6p, Ccw7p and Ccw8p (members of the Pir protein family) in stability of the Saccharomyces cerevisiae cell wall. Yeast. 1999;15:813–820. doi: 10.1002/(SICI)1097-0061(199907)15:10A<813::AID-YEA421>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Weeks S, Mastran B, Caradonna S. The nuclear isoform of the highly conserved human uracil-DNA glycosylase is an Mr 36,000 phosphoprotein. J Biol Chem. 1998;273:21909–21917. doi: 10.1074/jbc.273.34.21909. [DOI] [PubMed] [Google Scholar]
- 26.Pinz K G, Bogenhagen D F. Efficient repair of abasic sites in DNA by mitochondrial enzymes. Mol Cell Biol. 1998;18:1257–1265. doi: 10.1128/mcb.18.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramotar D. The apurinic-apyrimidinic endonuclease IV family of DNA repair enzymes. Biochem Cell Biol. 1997;75:327–336. [PubMed] [Google Scholar]
- 28.Ramotar D, Demple B. Functional expression of Escherichia coli endonuclease IV in apurinic endonuclease-deficient yeast. J Biol Chem. 1996;271:7368–7374. doi: 10.1074/jbc.271.13.7368. [DOI] [PubMed] [Google Scholar]
- 29.Ramotar D, Kim C, Lillis R, Demple B. Intracellular localization of the Apn1 DNA repair enzyme of Saccharomyces cerevisiae. Nuclear transport signals and biological role. J Biol Chem. 1993;268:20533–20539. [PubMed] [Google Scholar]
- 30.Ramotar D, Popoff S C, Gralla E B, Demple B. Cellular role of yeast Apn1 apurinic endonuclease/3′-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol Cell Biol. 1991;11:4537–4544. doi: 10.1128/mcb.11.9.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter C. Reactive oxygen and DNA damage in mitochondria. Mutat Res. 1992;275:249–255. doi: 10.1016/0921-8734(92)90029-o. [DOI] [PubMed] [Google Scholar]
- 32.Rosenquist T A, Zharkov D O, Grollman A P. Cloning and characterization of a mammalian 8-oxoguanine DNA glycosylase. Proc Natl Acad Sci USA. 1997;94:7429–7434. doi: 10.1073/pnas.94.14.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotig A, Bonnefont J P, Munnich A. Mitochondrial diabetes mellitus. Diabetes Metab. 1996;22:291–298. [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 35.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schapira A H. Mitochondrial disorders. Biochim Biophys Acta. 1999;1410:99–102. doi: 10.1016/s0005-2728(98)00160-1. [DOI] [PubMed] [Google Scholar]
- 37.Sherman F, Fink G, Hicks J, editors. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1983. [Google Scholar]
- 38.Sia E A, Butler C A, Dominska M, Greenwell P, Fox T D, Petes T D. Analysis of microsatellite mutations in the mitochondrial DNA of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2000;97:250–255. doi: 10.1073/pnas.97.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stierum R H, Dianov G L, Bohr V A. Single-nucleotide patch base excision repair of uracil in DNA by mitochondrial protein extracts. Nucleic Acids Res. 1999;27:3712–3719. doi: 10.1093/nar/27.18.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takao M, Aburatani H, Kobayashi K, Yasui A. Mitochondrial targeting of human DNA glycosylases for repair of oxidative DNA damage. Nucleic Acids Res. 1998;26:2917–2922. doi: 10.1093/nar/26.12.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toh-e A, Yasunaga S, Nisogi H, Tanaka K, Oguchi T, Matsui Y. Three yeast genes, PIR1, PIR2 and PIR3, containing internal tandem repeats, are related to each other, and PIR1 and PIR2 are required for tolerance to heat shock. Yeast. 1993;9:481–494. doi: 10.1002/yea.320090504. [DOI] [PubMed] [Google Scholar]
- 42.Truant R, Cullen B R. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uetz P, Giot L, Cagney G, Mansfield T A, Judson R S, Knight J R, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg J M. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 44.Vetter I R, Arndt A, Kutay U, Gorlich D, Wittinghofer A. Structural view of the Ran-importin beta interaction at 2.3 ° resolution. Cell. 1999;97:635–646. doi: 10.1016/s0092-8674(00)80774-6. [DOI] [PubMed] [Google Scholar]
- 45.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 46.Wallace D C. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 47.Yan C, Lee L H, Davis L I. Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO J. 1998;17:7416–7429. doi: 10.1093/emboj/17.24.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X, Tellier P, Masson J Y, Vu T, Ramotar D. Characterization of amino acid substitutions that severely alter the DNA repair functions of Escherichia coli endonuclease IV. Biochemistry. 1999;38:3615–3623. doi: 10.1021/bi9824083. [DOI] [PubMed] [Google Scholar]
- 49.Yasuhira S, Yasui A. Alternative excision repair pathway of UV-damaged DNA in Schizosaccharomyces pombe operates both in nucleus and in mitochondria. J Biol Chem. 2000;275:11824–11828. doi: 10.1074/jbc.275.16.11824. [DOI] [PubMed] [Google Scholar]
- 50.You H J, Swanson R L, Doetsch P W. Saccharomyces cerevisiae possesses two functional homologues of Escherichia coli endonuclease III. Biochemistry. 1998;37:6033–6040. doi: 10.1021/bi973042h. [DOI] [PubMed] [Google Scholar]
- 51.You H J, Swanson R L, Harrington C, Corbett A H, Jinks-Robertson S, Senturker S, Wallace S S, Boiteux S, Dizdaroglu M, Doetsch P W. Saccharomyces cerevisiae Ntg1p and Ntg2p: broad specificity N-glycosylases for the repair of oxidative DNA damage in the nucleus and mitochondria. Biochemistry. 1999;38:11298–11306. doi: 10.1021/bi991121i. [DOI] [PubMed] [Google Scholar]