Abstract
Currently, the establishment of synthetic microbial consortia with rational strategies has gained extensive attention, becoming one of the important frontiers of synthetic biology. Systems biology can offer insights into the design and construction of synthetic microbial consortia. Taking the high-efficiency production of 2-keto-l-gulonic acid (2-KLG) as an example, we constructed a synthetic microbial consortium “Saccharomyces cerevisiae-Ketogulonigenium vulgare” based on systems biology analysis. In the consortium, K. vulgare was the 2-KLG producing strain, and S. cerevisiae acted as the helper strain. Comparative transcriptomic analysis was performed on an engineered S. cerevisiae (VTC2) and a wild-type S. cerevisiae BY4741. The results showed that the up-regulated genes in VTC2, compared with BY4741, were mainly involved in glycolysis, TCA cycle, purine metabolism, and biosynthesis of amino acids, B vitamins, and antioxidant proteases, all of which play important roles in promoting the growth of K. vulgare. Furthermore, Vitamin C produced by VTC2 could further relieve the oxidative stress in the environment to increase the production of 2-KLG. Therefore, VTC2 would be of great advantage in working with K. vulgare. Thus, the synthetic microbial consortium "VTC2-K. vulgare" was constructed based on transcriptomics analyses, and the accumulation of 2-KLG was increased by 1.49-fold compared with that of mono-cultured K. vulgare, reaching 13.2 ± 0.52 g/L. In addition, the increased production of 2-KLG was accompanied by the up-regulated activities of superoxide dismutase and catalase in the medium and the up-regulated oxidative stress-related genes (sod, cat and gpd) in K. vulgare. The results indicated that the oxidative stress in the synthetic microbial consortium was efficiently reduced. Thus, systems analysis confirmed a favorable symbiotic relationship between microorganisms, providing guidance for further engineering synthetic consortia.
Keywords: 2-Keto-l-gulonic acid, S. cerevisiae, K. vulgare, Synthetic microbial consortia, Transcriptomic analysis, Synthetic biology
1. Introduction
Oxidative stress could lead to macromolecular damage and disruption of redox signaling [1]. The accumulation of reactive oxygen species (ROS) is one of the main causes of oxidative stress [2]. The production of ROS can be scavenged by the cellular antioxidant system, including enzymatic components (superoxide dismutase, catalase, peroxidase, etc.) and non-enzymatic components (glutathione, NADPH, thioredoxin, glutaredoxin, vitamin C, etc.) [3]. Vitamin C is a water-soluble vitamin with important physiological functions, which has been proven to inhibit the generation of free radicals and reduce the oxidative damage caused by ROS [4,5].
Currently, 2-keto-l-gulonic acid (2-KLG) is mainly produced through a two-step fermentation process developed in the 1980s [6]. The second step is to convert l-sorbose into 2-KLG by microbial co-cultivation, which is also the focus of microbial transformation research. 2-KLG-producing strain (Ketogulonigenium vulgare) and the helper strain (Bacillus sp.) are usually used as industrial strains for the synthesis of 2-KLG. Exploring the interaction between the two strains during co-culture has always been one of the hotspots in the field of 2-KLG fermentation. K. vulgare shows poor growth and low yield when cultured alone. Based on the research results of genomics, transcriptomics, proteomics, and metabolomics, scholars have done a lot of work in exploring the mechanism of interaction between the two strains from the perspective of amino acids, vitamins, and environmental stress [[7], [8], [9], [10]]. K. vulgare lacks key enzymes for the synthesis of amino acids such as histidine, threonine, leucine, and isoleucine [11], while helper strains can synthesize amino acids lacking in K. vulgare. Our lab reconstructed the threonine biosynthetic pathway in K. vulgare to improve the biomass and 2-KLG production [12]. The expressions of genes related to amino acids transport and metabolism were significantly up-regulated in K. vulgare [13]. Studies have shown that K. vulgare needs the helper strain to provide B vitamins and purines to maintain normal metabolism [14,15]. In addition, the helper strain could secrete proteases (glutaredoxin, catalase, etc.) and up-regulate the expression of the antioxidant enzymes (superoxide dismutase, thioredoxin, etc.) in K. vulgare to alleviate oxidative stress caused by ROS metabolism disorder [16].
The construction of the synthetic microbial consortium is of great significance for the production of 2-KLG by K. vulgare. The helper strains for the industrial production of 2-KLG mainly include Bacillus megaterium [17], Bacillus thuringiensis [18], and Bacillus cereus [19], all of which are prokaryotic microorganisms. However, taking eukaryotic microorganisms as helper strains were rarely reported. S. cerevisiae is a conventional and well-studied model organism for which advanced genetic tools for metabolic pathway manipulation are available [20]. The co-cultured relationships between cells in synthetic microbial consortia are dynamically balanced, leading to greater adaptability and stability to variable environments, thus providing an important new field for synthetic biology. There are mainly two strategies for designing and constructing synthetic microbial consortia [21]. The first one is to construct microbial consortia based on genetic elements, modules, and metabolic pathways. The other one is to de novo design microbial consortia based on multiple omics analyses. Transcriptome analyses can discover the dynamic expression and regulation mechanism of microbial genes, laying a foundation for exploring microbial community structure and ecological functions [22].
In this study, the synthetic microbial consortium was rationally designed based on system biology. Transcriptomic analyses were performed on the wild-type S. cerevisiae (BY4741) and the engineered S. cerevisiae (VTC2), which was a vitamin C producing strain constructed by our lab [4]. Taking advantage of VTC2, we constructed a synthetic microbial consortium system containing the K. vulgare and VTC2, which significantly increased the production of 2-KLG. This research could provide an effective reference for rational design of the synthetic microbial consortium to efficiently produce 2-KLG or other high value-added products.
2. Materials and methods
2.1. Strains
K. vulgare was an industrial strain, provided by LuWei Pharmaceutical Co. Ltd. S. cerevisiae BY4741 was a parent strain. S. cerevisiae VTC2 was a vitamin C-producing strain constructed by our laboratory [4]. Five exogenous modules from Arabidopsis were introduced into the chassis cells (BY4741) and the key gene vtc2 was overexpressed to obtain the strain VTC2 harboring VC biosynthesis (Table S1).
2.2. Culture media and conditions
K. vulgare was grown in sorbose-corn steep liquor (CSL) medium containing 2% l-sorbose, 0.3% CSL, 1% peptone, 0.3% yeast extract, 0.3% beef extract, 0.1% urea, 0.1% KH2PO4, 0.02% MgSO4 · 7H2O, and 0.1% CaCO3 with pH 7.0, while S. cerevisiae was grown in yeast extract-peptone-dextrose (YPD) media containing 20 g/L of glucose, 10 g/L of yeast extract, and 20 g/L of peptone with pH 6.5. The medium of the co-cultured system was composed of 20 g/L of glucose and the CSL medium.
The seed culture of K. vulgare or S. cerevisiae was firstly prepared in 250 mL flasks with 50 mL seed medium at 30 °C, 220 rpm for 48 and 24 h, respectively. Then, the seed culture of K. vulgare or S. cerevisiae was transferred into another fresh seed medium to culture, acting as the final seed for subsequent fermentation, respectively. For mono-culture of K. vulgare, S. cerevisiae, or co-culture of K. vulgare and S. cerevisiae, the strains were inoculated into 250 mL flasks with 50 mL fresh seed medium with the initial OD600 of 0.1 and were cultured in the same conditions (30 °C, 220 rpm) for 72 h. The fermentation experiments were conducted in triplicates and data were shown as the means ± S.D.
2.3. Biomass and metabolite analysis
Cell optical density (OD) was measured at 600 nm with TU-1810 spectrophotometer. The concentrations of extracellular l-sorbose and 2-KLG were detected by High Performance Liquid Chromatography (HPLC) (Waters, MA, USA), equipped with an Aminex HPX-87H column (Bio-Rad, CA, USA) and a refractive index detector. After fermentation, 2 mL of the fermentation broth was centrifuged at 12000 rpm for 10 min, and the supernatant was filtered through a 0.22 μm aqueous membrane for HPLC detection. 5 mM H2SO4 was used as the mobile phase with a flow rate of 0.6 mL/min at 65 °C.
2.4. Transcriptomic analysis
Fermentation broths of VTC2 and BY4741 collected separately at 72 h were rapidly frozen and stored at −80 °C. RNA of these samples were extracted and transcriptomic analyses were carried out on the BGISEQ-500 platform. The sequencing data was filtered with SOAPnuke (v1.5.2) [23] to remove sequencing adapter, low quality, and unknown reads. Then the clean reads obtained and stored in FASTQ format were mapped to the references genome using HISAT2 (v2.0.4) [24]. Bowtie 2 (v2.2.5) [25] was applied to align the clean reads to the reference coding gene set. The expression level of genes was calculated by RSEM (v1.2.12) [26]. The significant differentially expressed genes (DEGs) were detected using DESeq2(v1.4.5) [27] according to the criteria of the absolute value of log2 ratio ≥1 and Q value ≤ 0.05. The distribution of DEGs in metabolic pathway was performed based on the Kyoto encyclopedia of genes and genome (KEGG) database [28].
2.5. Analysis of superoxide dismutase activity and catalase activity
The activities of superoxide dismutase (SOD) and catalase (CAT) were measured using commercially available kits. The SOD activity assay kit was from Sino Best Biological Technology Co. (Shanghai, China). The CAT activity assay kit was from Beyotime Co. (Nantong, China). These experiments were performed under the instructions of manufactures.
2.6. Analysis of gene expression by quantitative RT-PCR
The relative expression levels of related genes in K. vulgare were measured by quantitative PCR (qPCR). 1 mL of the 72 h fermentation broth from the mono-cultured and co-cultured shake flasks, respectively, was centrifuged at 4 °C and 4500 rpm to collect the supernatant, which was snap-frozen in liquid nitrogen. RNA was extracted using the RNAprep pure Cell/Bacteria Kit (Tiangen). The extracted RNA was used for cDNA synthesis with the Transcriptor First Strand cDNA Synthesis Kit (Roche), following manufacturer's instructions. cDNA samples were diluted with ddH2O and were used for qPCR. qPCR analysis was performed on the q225-0265 qPCR system using TransStart Top Green qPCR SuperMix (Transgen). The primers used for qPCR to quantify relevant genes were listed in Supplementary Table S2. The 16s rRNA was chosen as the internal reference gene and each sample was replicated three times. The relative expression of the genes was calculated using the 2﹣ΔΔCt method [29].
3. Results
3.1. Transcriptomic analysis
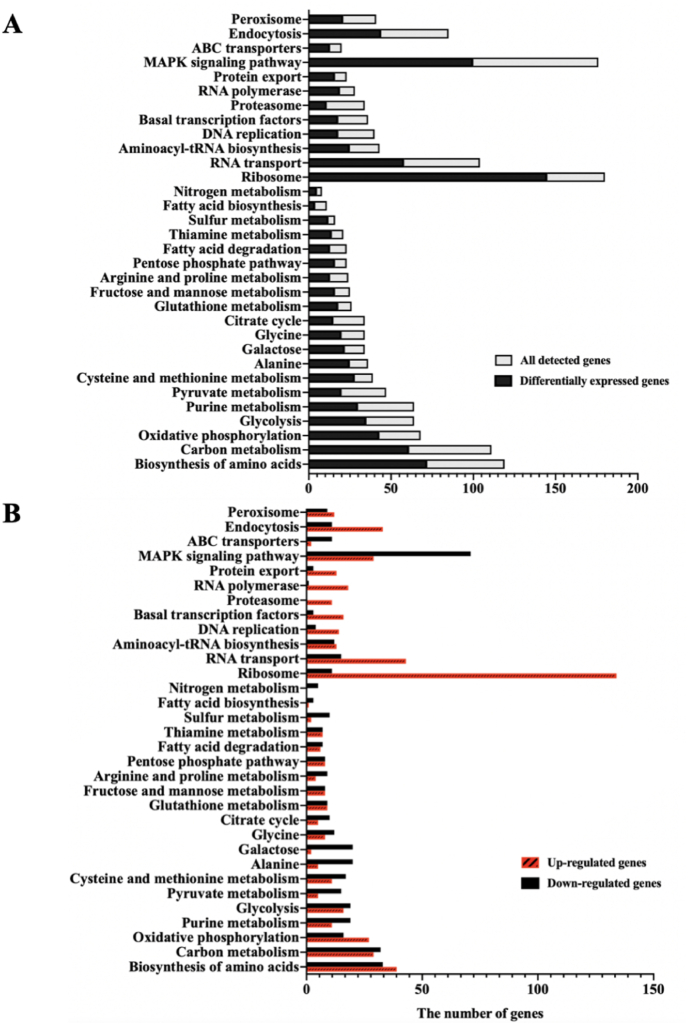
The vitamin C-producing strain, S. cerevisiae VTC2, was constructed by introducing heterologous vitamin C biosynthetic pathways [4]. To investigate changes in the transcriptional levels of genes in the engineered VTC2, the transcriptomes of VTC2 and BY4741 were studied using RNA-sequencing. According to the criteria for judging DEGs [27], it was found that vitamin C production resulted in a significantly different expression of 2990 genes, 1492 of which were up-regulated and 1498 were down-regulated. KEGG pathway enrichment analysis of DEGs can help biologists better understand the functional relevance of DEGs [30]. KEGG analysis of DEGs for S. cerevisiae VTC2 vs. BY4741 were presented in Fig. 1. Based on the results of KEGG analysis, we specifically analyzed the important metabolic pathways related to the production of 2-KLG.
Fig. 1.
KEGG pathway ontology enrichment analysis. (A) The numbers of all predicted genes and DEGs. (B) The numbers of up-regulated genes and down-regulated genes in DEGs.
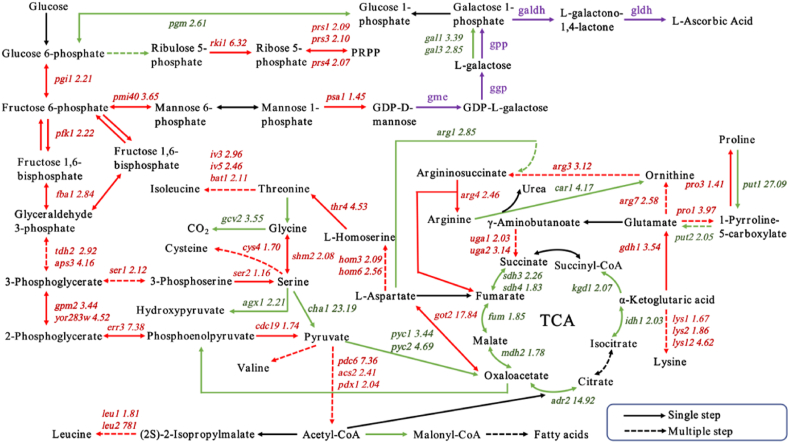
DEGs involved in the metabolic pathways were presented in Fig. 2 and Fig. 3. In the present study, the annotation of significant changes in transcript expression of genes related to glycolysis pathway and TCA cycle of VTC2 was shown in Fig. 2. By analyzing the gene expression profile of central carbon metabolism, it was found that most of the genes (pgi1, pfk1, fba1, aps3, tdh2, gpm2, yor283w, err3, cdc19, pdc6, acs2, and pdx1) involved in glycolysis were significantly up-regulated in VTC2 compared with that of BY4741. In addition, most of the genes (pyc1, pyc2, adr2, mdh2, idh1, kgd1, gdh1, and sdh3) throughout the TCA cycle were notably down-regulated. As shown in Fig. 2, several genes (rki1, prs1, prs3, and prs4) encoded by VTC2 in the pentose phosphate pathway (PPP) were significantly up-regulated, which enhanced the synthesis of ribulose-5-phosphate (Ru5P) and 5-phosphoribosyl 1-pyrophosphate (PRPP). In the metabolic pathway of fructose and mannose shown in Fig. 2, transcript levels of pmi40 and psa1 were observed to be up-regulated in VTC2, indicating that the carbon flux of the synthetic vitamin C precursor pathway was increased. However, the expressions of gal1 and gal3 were significantly down-regulated in the galactose metabolism pathway (Fig. 2), which might be related to the heterologous expression of the gene gpp, both of which catalyzed the conversion of l-galactose to galactose-1-phosphate. The step catalyzed by gpp was one of the rate-limiting steps in the synthesis of vitamin C [4]. Thus, overexpression of exogenous genes might result in decreased expression of endogenous genes.
Fig. 2.
Schematic diagram of DEGs involved in glycolysis, the TCA cycle, pyruvate metabolism, pentose phosphate pathway, amino acid biosynthesis, and vitamin C biosynthesis pathway. The numbers indicate the values of the ratios of the expression levels in VTC2 vs. BY4741. Red indicates significant up-regulation. Green indicates significant down-regulation. Black indicates non-significant change. Purple indicates the genes introduced exogenously.
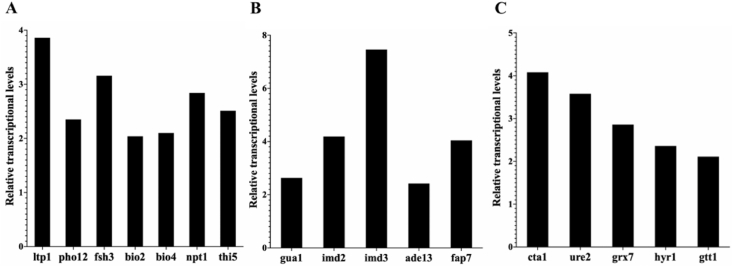
Fig. 3.
The relative transcriptional levels of genes involved in different metabolic pathways. (A) B vitamins biosynthesis. (B) Purines metabolism. (C) Antioxidant proteases. The numbers indicate the values of the ratios of the expression levels in VTC2 vs. BY4741. The error bars showed the standard deviations of three biological replicates.
Expression of genes related to amino acids synthesis pathway was up-regulated in VTC2 (Fig. 2), which mainly including cys4, got2, hom3, hom6, thr4, iv3, bat1, leu2, arg3, arg4, arg7, lys12, pro1, ser1, and shm2. In addition, several genes (gcv2, car1, put1, agx1, and cha1) related to the amino acid degradation were significantly down-regulated (Fig. 2). These genes were mainly involved in the synthesis of aspartate, threonine, isoleucine, leucine, glutamate, ornithine, arginine, valine, lysine, glycine, cysteine, proline, and serine. The gene ykl069w, involved in methionine synthesis, was up-regulated by 2.32-fold and catalyzed the reversible redox of the R-enantiomer of free methionine sulfoxide to methionine, which was involved in resistance to oxidative stress [31]. It was also found that the uga1 and uga2 genes were significantly up-regulated in the catabolic pathway of glutamate. γ-aminobutyric acid in the glutamate metabolic pathway of S. cerevisiae is further catabolized to succinate, producing the major intracellular antioxidant NAD(P)H, an essential contributor to the maintenance of intracellular antioxidant levels [32]. As shown in Fig. 3A, significantly up-regulated genes related to B vitamins were mainly concentrated in thi5, ltp1, pho12, fsh3, bio2, bio4 and npt1. The transcriptional levels of genes involved in purines metabolisms were up-regulated, such as gua1, imd2, imd3, ade13, and fap7 (Fig. 3B). As shown in Fig. 3C, the expression level of genes (cta1, ure2, grx7, hyr1, and gtt1) involved in the synthesis of antioxidant proteases were up-regulated in VTC2 compared with that of BY4741.
3.2. Design and construction of synthetic microbial consortium
The transcriptomic analyses showed that VTC2, compared with BY4741, had great advantages in the metabolism of carbon, amino acids, vitamins B, purines, and antioxidant proteases. Therefore, VTC2 might have the ability to promote the growth of K. vulgare and enhance 2-KLG production by providing metabolites and assisting in resisting environmental stress. From the perspective of rational design and construction, VTC2 and BY4741 were used as the helper strain of K. vulgare, respectively, to construct the synthetic microbial consortia.
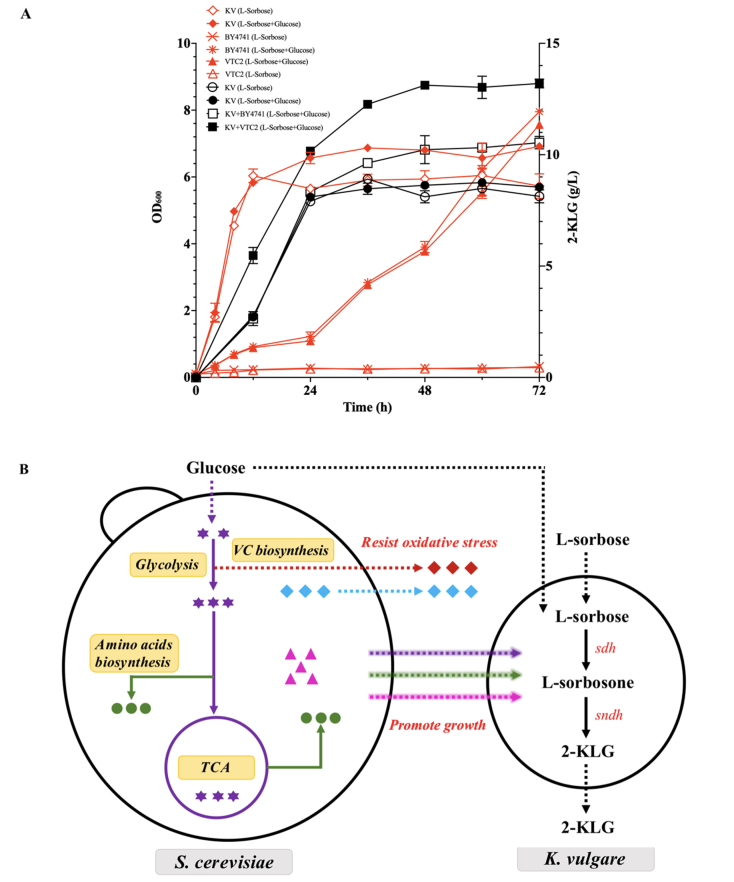
Firstly, the effects of different carbon sources on K. vulgare and S. cerevisiae were investigated. As shown in Fig. 4A, S. cerevisiae could not grow in the medium with l-sorbose as the only carbon source, i.e. S. cerevisiae did not consume l-sorbose. Besides, the growth of K. vulgare had better performance in the mixture of glucose and l-sorbose compared to the sole l-sorbose, which indicated that K. vulgare could also metabolize glucose to promote its growth (Fig. 4A). However, K. vulgare contributed almost the same yield of 2-KLG whether there was glucose in the CSL medium or not, i.e. glucose did not have an effect on the yield of 2-KLG (Fig. 4A).
Fig. 4.
The construction of the synthetic microbial consortium. (A) Time courses showed changes in cell concentration (red), and 2-KLG production (black). The content in brackets represents the type of carbon sources. KV: K. vulgare. The error bars showed the standard deviations of three biological replicates. (B) Schematic diagram of the interaction between S. cerevisiae VTC2 and K. vulgare. Purple stars: intermediate products of central carbon metabolism; Green circles: amino acids; Pink triangle: vitamins and purines; Blue rhombus: antioxidant proteases; Red rhombus: vitamin C.
Compared with the mono-cultured system, both VTC2 and BY4741 had the ability to increase the yield of 2-KLG. As shown in Fig. 4A, in the VTC2-K. vulgare microbial consortium, K. vulgare produced 13.2 ± 0.52 g/L of 2-KLG, which was 1.49 times higher than the mono-cultured fermentation (8.5 ± 0.21 g/L). A 20% increase in production of 2-KLG was also observed in BY4741–K. vulgare microbial consortium compared with that of mono-cultured system. Combined with the results of transcriptomic analyses, both VTC2 and BY4741, as helper strains, could provide an adaptive microenvironment through producing amino acids, vitamins, antioxidant proteases, and other substances to promote the cellular growth of K. vulgare (Fig. 4B). And this result could further promote the 2-KLG production of K. vulgare. However, in VTC2-K. vulgare microbial consortium, the yield of 2-KLG was 1.25 times higher than that of BY4741–K. vulgare. The higher yield appeared in VTC2-K. vulgare system might result from the higher transcriptional levels of key metabolic genes and the antioxidant effect of vitamin C.
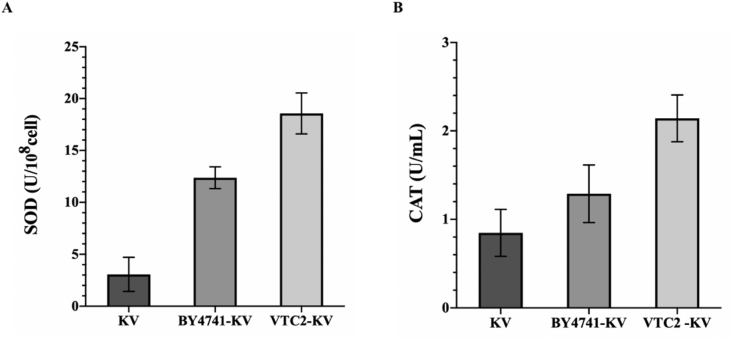
3.3. Analysis of SOD and CAT activities in fermentation system
Superoxide dismutase (SOD) is a protective enzyme of antioxidants that can scavenge ROS [33]. Determination of SOD activities helps to understand the ability of cells to clear superoxide anions. The total activity of superoxide dismutase was shown in Fig. 5A, telling that there were significant differences in SOD activities between mono-cultured systems and co-cultured systems. The SOD values of BY4741–K. vulgare and VTC2-K. vulgare microbial consortia were 4 times and 6 times higher than those of K. vulgare mono-cultured system, respectively. It was found that the cells of VTC2-K. vulgare microbial consortium had stronger superoxide anion scavenging abilities and could effectively protect Fe–S clusters from the attack of superoxide anion [34].
Fig. 5.
Determination of antioxidant activities in different fermentation systems. (A) SOD activities (B) CAT activities. KV: K. vulgare. The error bars showed the standard deviations of three biological replicates.
Catalase is an important antioxidant enzyme that can decompose hydrogen peroxide, a kind of ROS, into the water and molecular oxygen [35]. The CAT activities were also measured in this study to reflect the efficiency of removing H2O2 from the fermentation system. As shown in Fig. 5B, the CAT values of BY4741–K. vulgare and VTC2-K. vulgare microbial consortia were 1.5 times and 2.5 times higher than those of K. vulgare in the mono-cultured system, respectively.
The results of SOD and CAT activities showed that the total antioxidant capacity of microbial consortia was higher, which should be closely related to the antioxidant substances released by S. cerevisiae. In the presence of various antioxidant substances, ROS generated by cell metabolism and substrate conversion could be degraded and eliminated in time to alleviate the oxidative stress on the cells of K. vulgare. Since the vitamin C produced by VTC2 had great antioxidant effects, the total antioxidant capacity of the VTC2-K. vulgare system was stronger than that of BY4741–K. vulgare system.
3.4. Transcriptional analysis of K. vulgare in microbial consortium
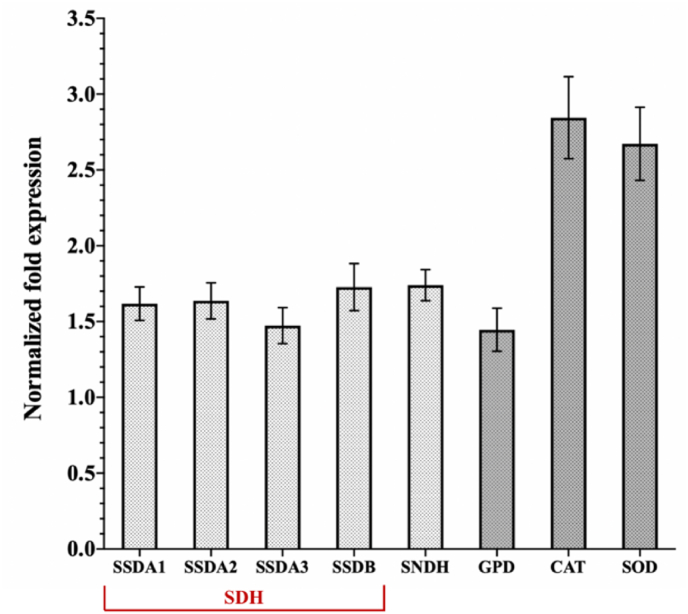
To further investigate the significant increase in the production of 2-KLG in VTC2-K. vulgare microbial consortium, the transcriptional levels of key genes in the 2-KLG synthesis pathway, and oxidative stress-related genes in K. vulgare were simultaneously investigated by qPCR. As shown in Fig. 6, genes involved in the metabolic process of reducing sorbose and sorbosone to 2-KLG, such as ssda1, ssda2, ssda3, ssdb, and sndh were up-regulated by 1.62, 1.64, 1.47, 1.73, and 1.74 times, respectively.
Fig. 6.
Transcriptional level of relevant genes in K. vulgare. sorbose dehydrogenase: ssda1, ssda2, ssda3, ssdb; sorbosone dehydrogenase: sndh; 6-phosphogluconate dehydrogenase: gpd; catalase: cat; superoxide dismutase: sod. The values in the graphs indicate the ratio of gene expression levels of K. vulgare in co-culture vs. mono-culture. The error bars showed the standard deviations of three biological replicates.
Studies have shown that the pathways of glycolysis and TCA circle in K. vulgare were incomplete, and the carbon metabolism mainly relied on the pentose phosphate pathway (PPP) [36]. The PPP is accompanied by the production of a large amount of NADPH, which can provide cells with more reducing power. And gpd is the rate-limiting enzyme of the PPP pathway. In addition, cat and sod are common antioxidant genes. The expression levels of gdp, cat, and sod genes in the co-cultured system were up-regulated by 1.45, 2.84, and 2.67 times (Fig. 6), respectively, significantly higher than those in the mono-cultured system. Therefore, VTC2 induced the up-regulated expression of antioxidant-related genes in K. vulgare to eliminate the ROS metabolism disorder and enhanced the ability of K. vulgare to produce 2-KLG.
3.5. Effect of vitamin C addition on the production of 2-KLG
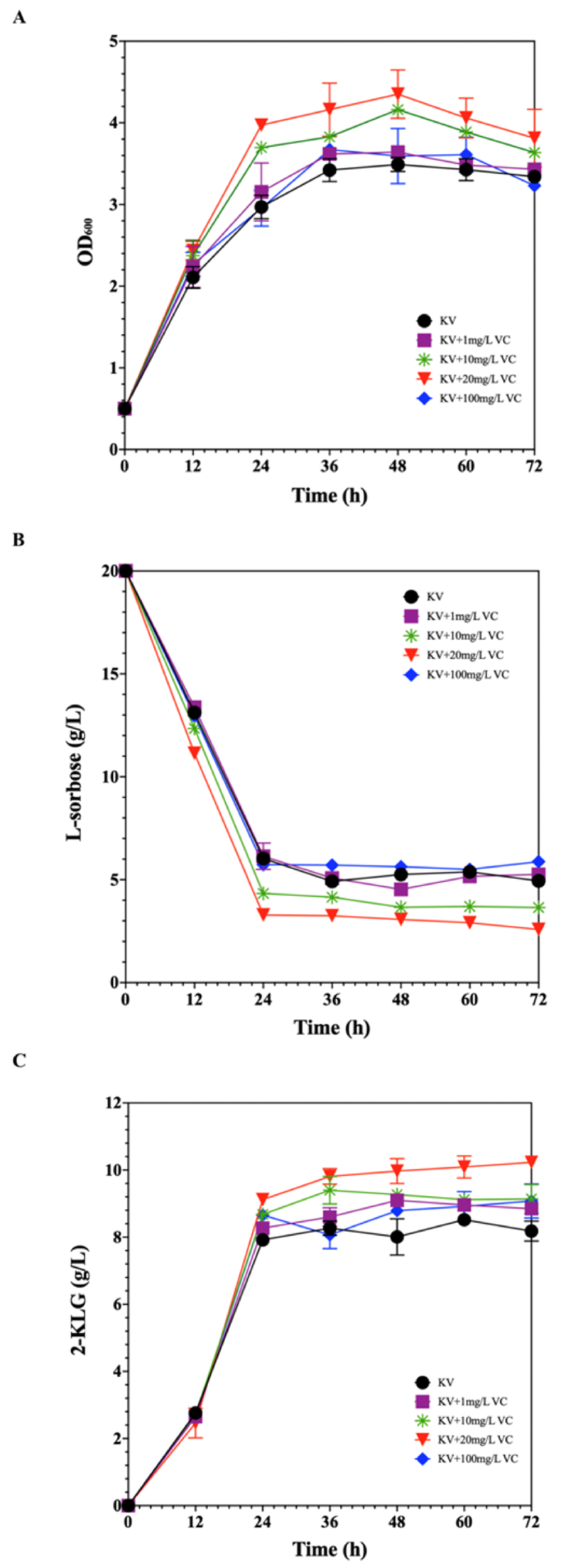
In order to investigate whether the oxidative stress on K. vulgare was relieved by vitamin C, a product of VTC2, different concentrations of vitamin C were exogenously added to the mono-cultured fermentation. The curves of the growth, the l-sorbose consumption, and the 2-KLG production of K. vulgare were indicated in Fig. 7. As shown in Fig. 7A and B, K. vulgare grew better and consumed l-sorbose more efficiently under exogenous addition of 20 mg/L vitamin C, and had the highest yield of 2-KLG (10.23 ± 0.15 g/L) (Fig. 7C). However, the yield of 2-KLG was not improved with the addition of 1 mg/L and 100 mg/L of vitamin C to the fermentation system. The antioxidant effect of low-concentration vitamin C was poor since Vitamin C is easily consumed by oxidation in the air [37]. However, the growth and 2-KLG production of K. vulgare were not significantly enhanced when a high concentration of vitamin C was added exogenously, indicating that a high concentration of vitamin C was not suitable to be added in the fermentation system.
Fig. 7.
Effect of vitamin C addition on K. vulgare. (A) Cell concentration, (B) l-sorbose consumption, (C) 2-KLG production. The error bars showed the standard deviations of three biological replicates.
It was found that exogenous vitamin C with a certain concentration significantly promoted the growth and 2-KLG production of K. vulgare. This result indirectly confirmed that vitamin C produced by VTC2 helped to alleviate the oxidative stress suffered by K. vulgare. Therefore, the construction of this synthetic microbial consortium had practical implications.
4. Discussion
Synthetic microbial consortia could perform complicated tasks and endure the changeable environment, thus providing an important new frontier for synthetic biology [38]. Recent advances in multi-omics and automation technology have enabled researchers to focus on engineering the microbiome's metabolic network and microbial interactions. The design principles for synthesizing microbial consortia are mainly based on the patterns of microbial interactions, including cell-cell communications, and exchange of metabolites and energy, etc. To achieve predictions of microbiome function at a systems level, measurements of the microbiome's in situ metabolic network structure and activity are essential [39]. Systems biology approaches enable the objective description of genetic and metabolic pathways in microbial consortia, which facilitates the design and optimization of synthetic consortia [40]. Systems biology analysis [21], particularly meta-omics techniques (e.g. meta-genomics [41,42], meta-proteomics [16], meta-transcriptomics [43], and meta-metabolomics [8]), can be combined with bioinformatic tools to enable the analysis of individual species in the microbiome and global measurements of proteins and metabolites. This provides insights into the design and construction of functional genes, functional modules, and entire synthetic consortia. In this study, comparative transcriptome analyses of VTC2 and BY4741 were performed to objectively characterize the genetic and metabolic pathways of VTC2, which facilitated the design and optimization of the synthetic consortium.
As described in previous studies, the glycolysis pathway and the TCA cycle were central metabolic pathways of glucose metabolism in S. cerevisiae [44]. The genes involved in glycolysis were significantly up-regulated in VTC2 compared with those of BY4741. Thus, VTC2 had higher glucose utilization efficiency compared to BY4741 and could provide more precursors for the synthesis of vitamin C. The transcription levels of most genes were significantly down-regulated in VTC2 throughout the TCA cycle compared with that of BY4741, indicating a decrease in the production of reducing substances and ATP, i.e. the strains were in a stable growth phase at 72 h. And 2-ketoglutarate decarboxylase (encoded by kgd1) catalyzed the oxidative decarboxylation of 2-ketoglutarate to succinyl coenzyme A, a key reaction of the TCA cycle [45]. The down-regulation of kgd1 transcription might lead carbon flux into the biosynthetic of glutamate, the source of 80% of cellular nitrogen [46]. The PPP is also a fundamental component of central carbon metabolism, maintaining carbon homeostasis. The PPP provided precursors for nucleotide and amino acid synthesis, and also provided reducing substances to eliminate oxidative stress [47,48]. The up-regulation of the transcription of key metabolic genes in the PPP pathway could lead to an increase in the synthesis of Ru5P and PRPP. Ru5P is a precursor for the synthesis of nucleotides. PRPP is an important precursor of de novo synthesis of purine nucleotide and pyrimidine nucleotide [49,50].
The establishment of symbiosis between strains via metabolites exchanges is a common mechanism in synthetic microbiota [51]. Modularity is introduced into the pathway design of microbial metabolite production, in which metabolic intermediates provided by one microorganism were transferred to the other one. This unidirectional interaction can stabilize the Escherichia coli-S. cerevisiae co-culture for high production of oxygenated taxanes [52]. In addition, the mutualism is enhanced by the exchange of metabolites between strains. It is considered that cross-feeding of metabolites in synthetic consortia would facilitate the heterologous production of flavonoids [53]. The expression of vitamin C heterologous biosynthetic pathway in VTC2 resulted in the transcriptional up-regulation of genes involved in the biosynthesis of amino acids, B vitamins, purines and antioxidant proteases compared with BY4741.
Amino acids play an important role in cell growth, metabolism, and cell survival in severe environments. Proline is an essential stress protector in yeast cells and also participates in numerous important intracellular physiological processes, such as maintaining protein and membrane stability and scavenging ROS [54]. Valine, leucine, glycine, and serine are derived from the glycolysis pathway. Higher intracellular levels of amino acids derived from the glycolysis pathway reflect the higher glycolytic capacity of the cell [55]. Cysteine can be used to synthesize glutathione, which is the most important intracellular antioxidant. And cysteine oxidation can be used to control protein function and cell signaling pathways [56]. Previous studies have shown that K. vulgare itself could not synthesize B vitamins [15,57], whereas S. cerevisiae contained the B vitamins synthesis pathway and supplied the synthesized B vitamins to K. vulgare to maintain its normal growth metabolism in our constructed consortium. The B vitamins comprised a group of water-soluble vitamins (thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, folic acid, biotin, etc.) that acted as cofactors, precursors, and substrates for biological processes [58]. Pyridoxine is a cofactor for more than 140 biochemical reactions in cells [59], most of which are related to amino acids biosynthesis. In addition, pyridoxine can also quench ROS [60]. Folic acid and its derivatives are also important factors for the growth of K. vulgare [61]. However, K. vulgare is unable to utilize folic acid for deficiency of folate reductase, while S. cerevisiae can metabolize folic acid to dihydrofolate and tetrahydrofolate that can be utilized by K. vulgare. In addition, adenine, guanine, xanthine, and hypoxanthine produced by helper strains could contribute to the resistance of the microflora to ROS [14]. Cellular redox systems included glutathione, thioredoxin, pyridine nucleotides, and NADPH [62]. Among them, antioxidant proteases mainly include superoxide dismutase, catalase, glutathione, and glutathione. In summary, the metabolites released by VTC2 could compensate for the defective growth of K. vulgare. In addition, the results that exogenous addition of vitamin C raised 2-KLG production also further confirmed that VTC2, producing high vitamin C, had better concomitant effects.
The consumption of the same carbon source by two strains can lead to competitive interactions and unstable symbiosis in microbial communities. Differences in nutrient metabolism in symbiotic systems can reduce or eliminate competitive exclusion, and make symbiosis possible. Therefore, avoiding carbon source competition is one of the strategies to achieve effective symbiosis. For example, a bacterial consortium consisting of a glucose-selective strain and a xylose-selective strain was constructed to achieve high yields of n-butanol [63]. Here, we used a dual carbon source of glucose and l-sorbose for fermentation. The results showed that S. cerevisiae did not compete with K. vulgare for l-sorbose, thus achieving optimal growth of K. vulgare. S. cerevisiae, as a model organism, is widely used in large-scale industrial production due to intensive research on its genetic and growth characteristics. The focus of this study was to explore the advantages of applying engineered S. cerevisiae to the microbial consortia. K. vulgare suffered from weak growth and low production due to oxidative stress and lack of nutrients required for growth. However, the results of the transcriptomic analysis indicated that VTC2 had the potential to promote the growth of K. vulgare. Therefore, this study took VTC2 as the helper strain with K. vulgare to form the synthetic microbial consortium, and successfully achieved a significant increase in the production of 2-KLG. In addition, ROS are harmful byproducts of aerobic metabolism. When ROS levels increase, oxidative stress will occur, destroying cellular biomolecules (DNA, proteins, and lipids) [2], limiting cellular growth and 2-KLG production. In order to explore the antioxidant capacity of different 2-KLG fermentation systems, the SOD and CAT activities were evaluated, respectively. The results showed that the synthetic microbial consortium had a stronger antioxidant capacity compared to the mono-cultured system, which was associated with the high vitamin C production of VTC2. It was also demonstrated that VTC2, as the helper strain, induced a significant up-regulation of 2-KLG producing and antioxidant-related genes in K. vulgare. These results further validated the advantages of the VTC2 as the helper strain in the synthetic microbial consortium. This microbial consortium also has implications for continued research and improvement. For example, engineered K. vulgare could work with VTC2 to form a more efficiently microbial consortium for 2-KLG production. And transcriptomic analyses of the microbial consortium are indeed an important direction to obtain more information of the microbial consortium. In addition, the constructed consortium could be expanded in controlled fermenters to achieve a higher yield of 2-KLG.
5. Conclusions
In this study, systems biology analysis was applied to construct a meaningful synthetic microbial consortium. The yield of 2-KLG was significantly improved in the co-cultured system compared with that of the mono-cultured system. To the best of our knowledge, this is the first report on increasing 2-KLG production via taking engineered S. cerevisiae as helper strain. This study provided a reference for subsequent rational design and the construction of synthetic microbial consortia.
CRediT authorship contribution statement
Yan Wang: Conceptualization, Methodology, Investigation, Writing – original draft. Hengchang Li: Investigation, Methodology. Yu Liu: Investigation, Methodology. Mengyu Zhou: Investigation, Methodology. Mingzhu Ding: Conceptualization, Supervision, Project administration, Writing – review & editing. Yingjin Yuan: Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Key Research and Development Program of China (2018YFA0902100), National Natural Science Foundation of China (21676190).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2021.12.001.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borisov V.B., Siletsky S.A., Nastasi M.R., Forte E. ROS defense systems and terminal oxidases in bacteria. Antioxidants-Basel. 2021;10:e839. doi: 10.3390/antiox10060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou M.Y., Bi Y.H., Ding M.Z., Yuan Y.J. One-step biosynthesis of vitamin C in Saccharomyces cerevisiae. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.643472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngo B., Van Riper J.M., Cantley L.C., Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. 2019;19:271–282. doi: 10.1038/s41568-019-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock R.D., Viola R. Biotechnological approaches for L-ascorbic acid production. Trends Biotechnol. 2002;20:299–305. doi: 10.1016/s0167-7799(02)01991-1. [DOI] [PubMed] [Google Scholar]
- 7.Ma Q., Zou Y., Lv Y., Song H., Yuan Y.-J. Comparative proteomic analysis of experimental evolution of the Bacillus cereus-Ketogulonicigenium vulgare co-culture. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du J., Zhou J., Xue J., Song H., Yuan Y.J. Metabolomic profiling elucidates community dynamics of the Ketogulonicigenium vulgare-Bacillus megaterium consortium. Metabolomics. 2012;8:960–973. doi: 10.1007/s11306-011-0392-2. [DOI] [Google Scholar]
- 9.Jia N., Ding M.Z., Gao F., Yuan Y.J. Comparative genomics analysis of the companion mechanisms of Bacillus thuringiensis Bc601 and Bacillus endophyticus Hbe603 in bacterial consortium. Sci Rep. 2016;6 doi: 10.1038/srep28794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou W., Liu L.M., Chen J. Structure, mechanism and regulation of an artificial microbial ecosystem for vitamin C production. Crit Rev Microbiol. 2013;39:247–255. doi: 10.3109/1040841x.2012.706250. [DOI] [PubMed] [Google Scholar]
- 11.Liu L.M., Chen K.J., Zhang J., Liu J., Chen J. Gelatin enhances 2-keto-L-gulonic acid production based on Ketogulonigenium vulgare genome annotation. J Biotechnol. 2011;156:182–187. doi: 10.1016/j.jbiotec.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Pan C.H., Wang E.X., Jia N., Dong X.T., Liu Y., Ding M.Z., et al. Reconstruction of amino acid biosynthetic pathways increases the productivity of 2-keto-l-gulonic acid in Ketogulonicigenium vulgare-Bacillus endophyticus consortium via genes screening. J Ind Microbiol Biotechnol. 2017;44:1031–1040. doi: 10.1007/s10295-017-1928-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J., Ma Q., Yi H., Wang L., Song H., Yuan Y.-J. Metabolome profiling reveals metabolic cooperation between Bacillus megaterium and Ketogulonicigenium vulgare during induced swarm motility. Appl Environ Microbiol. 2011;77:7023–7030. doi: 10.1128/aem.05123-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Q., Zhou J., Zhang W.W., Meng X.X., Sun J.W., Yuan Y.J. Integrated proteomic and metabolomic analysis of an artificial microbial community for two-step production of vitamin C. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Zhou J., Liu J., Chen K., Liu L., Chen J. Development of chemically defined media supporting high cell density growth of Ketogulonicigenium vulgare and Bacillus megaterium. Bioresour Technol. 2011;102:4807–4814. doi: 10.1016/j.biortech.2010.10.124. [DOI] [PubMed] [Google Scholar]
- 16.Ma Q., Zhang W.W., Zhang L., Qiao B., Pan C.S., Yi H., et al. Proteomic analysis of Ketogulonicigenium vulgare under glutathione reveals high demand for thiamin transport and antioxidant protection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Liu J., Shi Z.P., Liu L.M., Chen J. Manipulation of B-megaterium growth for efficient 2-KLG production by K-vulgare. Process Biochem. 2010;45:602–606. doi: 10.1016/j.procbio.2009.11.016. [DOI] [Google Scholar]
- 18.Jia N., Ding M.Z., Zou Y., Gao F., Yuan Y.J. Comparative genomics and metabolomics analyses of the adaptation mechanism in Ketogulonicigenium vulgare-Bacillus thuringiensis consortium. Sci Rep. 2017;7 doi: 10.1038/srep46759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding M.Z., Zou Y., Song H., Yuan Y.J. Metabolomic analysis of cooperative adaptation between co-cultured Bacillus cereus and Ketogulonicigenium vulgare. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lian J.Z., Mishra S., Zhao H.M. Recent advances in metabolic engineering of Saccharomyces cerevisiae: new tools and their applications. Metab Eng. 2018;50:85–108. doi: 10.1016/j.ymben.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Jia X.Q., Liu C., Song H., Ding M.Z., Du J., Ma Q., et al. Design, analysis and application of synthetic microbial consortia. Syn Syst Biotechno. 2016;1:109–117. doi: 10.1016/j.synbio.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M., Park B.G., Kim J., Kim J.Y., Kim B.G. Exploiting transcriptomic data for metabolic engineering: toward a systematic strain design. Curr Opin Biotechnol. 2018;54:26–32. doi: 10.1016/j.copbio.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Li R., Li Y., Kristiansen K., Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24:713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- 24.Kim D., Landmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:e323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15 doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:480–484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le D.T., Lee B.C., Marino S.M., Zhang Y., Fomenko D.E., Kaya A., et al. Functional analysis of free methionine-R-sulfoxide reductase from Saccharomyces cerevisiae. J Biol Chem. 2009;284:4354–4364. doi: 10.1074/jbc.M805891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo H.Z., Liu Z., Xie F., Bilal M., Liu L.N., Yang R.L., et al. Microbial production of gamma-aminobutyric acid: applications, state-of-the-art achievements, and future perspectives. Crit Rev Biotechnol. 2021;41:491–512. doi: 10.1080/07388551.2020.1869688. [DOI] [PubMed] [Google Scholar]
- 33.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Fuss J.O., Tsai C.-L., Ishida J.P., Tainer J.A. Emerging critical roles of Fe-S clusters in DNA replication and repair. BBA-Mol Cell Res. 2015;1853:1253–1271. doi: 10.1016/j.bbamcr.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glorieux C., Zamocky M., Sandoval J.M., Verrax J., Calderon P.B. Regulation of catalase expression in healthy and cancerous cells. Free Radical Biol Med. 2015;87:84–97. doi: 10.1016/j.freeradbiomed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Jia N., Ding M.Z., Du J., Pan C.H., Tian G., Lang J.D., et al. Insights into mutualism mechanism and versatile metabolism of Ketogulonicigenium vulgare Hbe602 based on comparative genomics and metabolomics studies. Sci Rep. 2016;6 doi: 10.1038/srep23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. BBA-Rev Cancer. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner K., You L.C., Arnold F.H. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008;26:483–489. doi: 10.1016/j.tibtech.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Lawson C.E., Harcombe W.R., Hatzenpichler R., Lindemann S.R., Loffler F.E., O'Malley M.A., et al. Common principles and best practices for engineering microbiomes. Nat Rev Microbiol. 2019;17:725–741. doi: 10.1038/s41579-019-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song H., Ding M.Z., Jia X.Q., Ma Q., Yuan Y.J. Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev. 2014;43:6954–6981. doi: 10.1039/c4cs00114a. [DOI] [PubMed] [Google Scholar]
- 41.Woyke T., Teeling H., Ivanova N.N., Huntemann M., Richter M., Gloeckner F.O., et al. Symbiosis insights through metagenomic analysis of a microbial consortium. Nature. 2006;443:950–955. doi: 10.1038/nature05192. [DOI] [PubMed] [Google Scholar]
- 42.Turnbaugh P.J., Gordon J.I. An invitation to the marriage of metagenomics and metabolomics. Cell. 2008;134:708–713. doi: 10.1016/j.cell.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 43.Stewart F.J., Ulloa O., DeLong E.F. Microbial metatranscriptomics in a permanent marine oxygen minimum zone. Environ Microbiol. 2012;14:23–40. doi: 10.1111/j.1462-2920.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 44.Chubukov V., Gerosa L., Kochanowski K., Sauer U. Coordination of microbial metabolism. Nat Rev Microbiol. 2014;12:327–340. doi: 10.1038/nrmicro3238. [DOI] [PubMed] [Google Scholar]
- 45.Repetto B., Tzagoloff A. Structure and regulation of KGD1, the structural gene for yeast alpha-ketoglutarate dehydrogenase. Mol Cell Biol. 1989;9:2695–2705. doi: 10.1128/mcb.9.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mara P., Fragiadakis G.S., Gkountromichos F., Alexandraki D. The pleiotropic effects of the glutamate dehydrogenase (GDH) pathway in Saccharomyces cerevisiae. Microb Cell Factories. 2018;17:e170. doi: 10.1186/s12934-018-1018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stincone A., Prigione A., Cramer T., Wamelink M.M.C., Campbell K., Cheung E., et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sosa V., Moline T., Somoza R., Paciucci R., Kondoh H., Lleonart M.E. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Ljungdahl P.O., Daignan-Fornier B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics. 2012;190:885–929. doi: 10.1534/genetics.111.133306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hove-Jensen B., Andersen K.R., Kilstrup M., Martinussen J., Switzer R.L., Willemoes M. Phosphoribosyl diphosphate (PRPP): biosynthesis, enzymology, utilization, and metabolic significance. Microbiol Mol Biol Rev. 2017;81 doi: 10.1128/mmbr.00040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding M.Z., Song H., Wang E.X., Liu Y., Yuan Y.J. Design and construction of synthetic microbial consortia in China. Syn Syst Biotechno. 2016;1:230–235. doi: 10.1016/j.synbio.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou K., Qiao K.J., Edgar S., Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol. 2015;33:377–383. doi: 10.1038/nbt.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X.N., Cheng J., Zhang G.H., Ding W.T., Duan L.J., Yang J., et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches. Nat Commun. 2018;9:e448. doi: 10.1038/s41467-018-02883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukai Y., Kamei Y., Liu X., Jiang S., Sugimoto Y., Nanyan N., et al. Proline metabolism regulates replicative lifespan in the yeast Saccharomyces cerevisiae. Microb Cell. 2019;6:482–490. doi: 10.15698/mic2019.10.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pears M.R., Codlin S., Haines R.L., White I.J., Mortishire-Smith R.J., Mole S.E., et al. Deletion of btn1, an orthologue of CLN3, increases glycolysis and perturbs amino acid metabolism in the fission yeast model of Batten disease. Mol Biosyst. 2010;6:1093–1102. doi: 10.1039/b915670d. [DOI] [PubMed] [Google Scholar]
- 56.Paulsen C.E., Carroll K.S. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev. 2013;113:4633–4679. doi: 10.1021/cr300163e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeidler J., Sayer B.G., Spenser I.D. Biosynthesis of vitamin B-1 in yeast. Derivation of the pyrimidine unit from pyridoxine and histidine. Intermediacy of urocanic acid. J Am Chem Soc. 2003;125:13094–13105. doi: 10.1021/ja030261j. [DOI] [PubMed] [Google Scholar]
- 58.Lyon P., Strippoli V., Fang B., Cimmino L. B vitamins and one-carbon metabolism: implications in human health and disease. Nutrients. 2020;12:e2867. doi: 10.3390/nu12092867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parra M., Stahl S., Hellmann H. Vitamin B-6 and its role in cell metabolism and physiology. Cells. 2018;7:e84. doi: 10.3390/cells7070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalto D.B., Matte J.-J. Pyridoxine (vitamin B-6) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrients. 2017;9:e189. doi: 10.3390/nu9030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leduc S., de Troostembergh J.C., Lebeault J.M. Folate requirements of the 2-keto-L-gulonic acid-producing strain Ketogulonigenium vulgare LMP P-20356 in L-sorbose/CSL medium. Appl Microbiol Biotechnol. 2004;65:163–167. doi: 10.1007/s00253-004-1562-1. [DOI] [PubMed] [Google Scholar]
- 62.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saini M., Lin L.J., Chiang C.J., Chao Y.P. Synthetic consortium of Escherichia coli for n-butanol production by fermentation of the glucose-xylose mixture. J Agric Food Chem. 2017;65:10040–10047. doi: 10.1021/acs.jafc.7b04275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.