Abstract
Purpose
This case report demonstrates the effectiveness of a combined unique soft contact lens design and hypertonic saline at reducing corneal edema symptoms. In addition, this case shows that using tomographic data is invaluable for detecting and monitoring of these presentations.
Observations
A 61 year old patient diagnosed with Fuchs endothelial corneal dystrophy (FECD) presented with complaints over the past year of intermittent blurry, foggy vision upon awakening and glare while driving. Slit lamp examination showed no signs of corneal edema. Data acquired from the Scheimpflug tomographer revealed subclinical signs, including increased corneal thickness, displacement of the thinnest point of the cornea, focal posterior depression, elevated densinometry, "camel's back” sign, irregular isopachs, and a plane slope of pachymetry progression in both eyes. The patient was fit with Therapeutic Hyper-CL™ soft contact lenses for eight days extended wear and instilled 5% sodium chloride six times a day. Visual acuity improved in the right and left eye from 0.5(-2) and 0.5(+1) to 0.4(+2) and 0.3(-1), respectively. Corneal thickness at the thinnest point decreased from 650μm to 632μm–632μm and 604μm in the right and left eye respectively and a significant decrease in total densinometry was noted from 34.7 to 33.8 standardized grayscale units (GSU) to 23.1 and 24 GSU, in the right and left eye respectively. The patient reported a decrease in symptoms and his 8-item Contact Lens Dry Eye Questionnaire (CLDEQ-8) score was 19 after treatment.
Conclusions and importance
Treatment for one week with Therapeutic Hyper-CL™ soft contact lenses combined with 5% sodium chloride decreased corneal edema signs and symptoms. Tomographic data facilitated diagnosis and monitoring of improvement.
Keywords: Fuch's dystrophy, Therapeutic contact lens, Corneal edema
1. Introduction
Corneal edema's clinical causes are numerous and can be largely divided into congenital, degenerative inflammatory, a complication of surgery and scarring. It is a result of excess fluid in the tissue categorized either as stromal or epithelial edema which can present either in isolation or concurrently.1, 2, 3 While each has unique pathophysiology, both affect the transparency of the cornea and vision. The physiological sources of corneal edema are primarily changes in the intra ocular pressure or endothelial decompensation which can originate from external causes such as hypoxia due to an ill-fitting contact lens to internal causes such as post cataract surgery complications or glaucoma.1,4
One type of degeneration is known as Fuchs endothelial corneal dystrophy (FECD), or corneal guttata. It is a bilateral (though commonly asymmetric), degenerative, inherited disease that commonly progresses over the span of a few decades from asymptomatic to an occasionally very painful condition.2,5 FECD is the most common corneal dystrophy to affect the endothelium worldwide.2 Estimated incidence varies greatly, probably due to different clinical definitions of guttata, but it is suggested to have a higher prevalence in Europe than other areas of the world and presents in an estimated 4% of the population in the USA.1,2,4,5 Individuals above the age of 40 and women have a higher risk of developing FECD.1,2,4, 5, 6 Smoking, UV exposure and diabetes seem to affect the disease's severity.7 FECD can be divided into two categories defined by the age of diagnosis, known as early-onset (approximately 30 years old) and late-onset (approximately 50 years old).1,4, 5, 6 It is characterized by corneal endothelial decompensation resulting in corneal guttae, a thickening of Descemet's membrane, a decline in corneal endothelial density and ion transport function, edema, corneal haze and decreased vision.8,9 As the disease progresses it can cause secondary anterior cornea degradation, such as abnormal sub-epithelial cells, which cause anterior corneal backscatter and eventually, corneal vascularization and scarring.10
Independent of etiology or length of time of edema, management approaches are to delay penetrating keratoplasty. Treatments include phototherapeutic keratectomy, amniotic membrane transplantation, epithelial and bowman puncture, and collagen cross-linking.4,5 Less invasive therapy for mild to moderate cases are topical steroids if there is an inflammatory component, reducing intraocular pressure, manual drying of the eye with a hairdryer, hypertonic agents in drop or cream formulations and bandage contact lenses.11
Hypertonic agents have a higher osmotic pressure than the eye.4 Therefore water from the eye is drawn towards it. It is an accessible, non-invasive, convenient treatment that effectively extracts fluid from the stroma though its' effect is limited due to a short retention time on the ocular surface, a result of their elimination via reflex tears and blinking.4
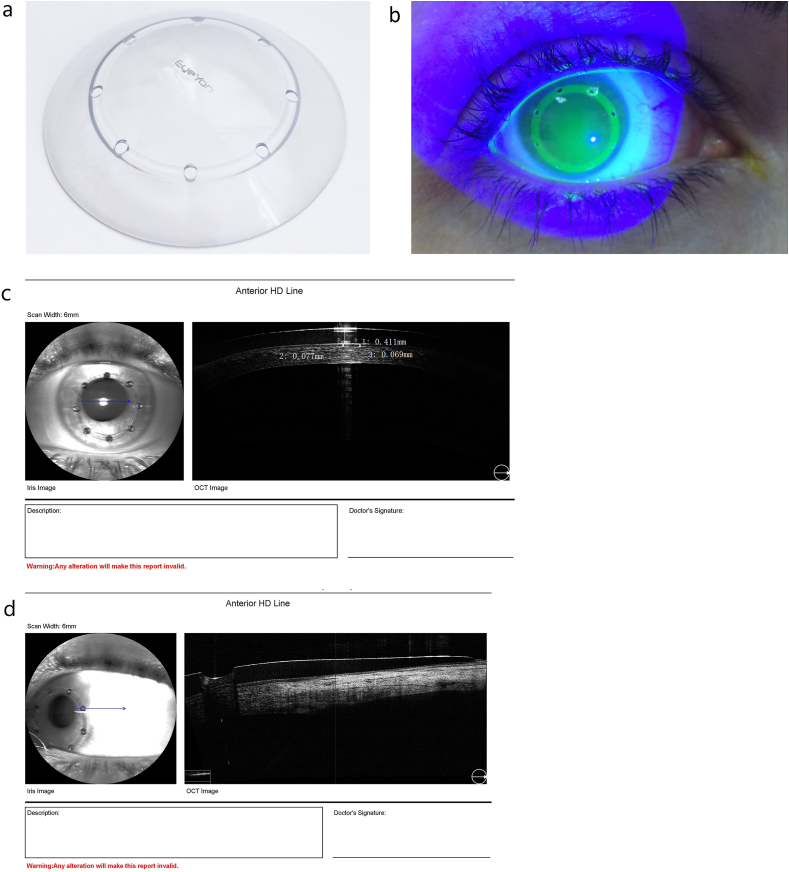
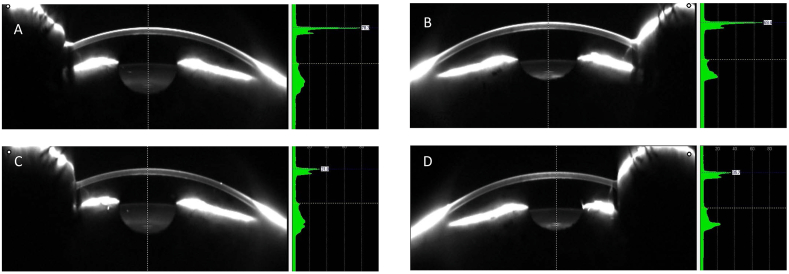
Therapeutic Hyper-CL™ (EyeYon Medical, Ness Tziona, Israel) is a contact lens designed to increase eye drops' contact time on the corneal surface. The unique design forms a cavity between the lens and the cornea, in which instilled eye drops become trapped, extending their contact time with the cornea (Fig. 1 A,B,C and D).
Fig. 1.
The Therapeutic Hyper-CL™ lens (A) and on the eye through a blue filter and high molecule Flourescein(B). OCT image exhibiting a liquid reservoir between lens and cornea (C) and showing a fenestration and the draping of the lens on the conjunctiva (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Presented here is a case showing the ability of Pentacam (Oculus GmBH, Wetzlar, Germany) Scheimpflug tomographer to identify subclinical corneal edema as well as the efficacy of this contact lens in combination with hypertonic agents in management of corneal edema and reduced visual acuity as a result of FECD.
2. Case report
The patient was a 61-year-old Caucasian professional tour guide. When presenting to the clinic, his chief complaint was suffering throughout the past year from blurry vision primarily upon awakening, describing it as “haloes and foggy vision”. The patient also complained of intermittent glare, particularly while driving. The patient had been diagnosed three years earlier with FECD. There was no known family history of this disease.
The patient wore rigid gas permeable lenses for approximately four years at age twenty but had not worn any lenses since. The patient reported general health, did not take any topical or systemic medication and had undergone no ocular surgeries or ocular trauma. The patient had been diagnosed two years earlier with nuclear sclerosis 2+ in both eyes, no surgery was recommended and he remained under observation.
Refraction was measured using the early treatment diabetic retinopathy study (ETDRS) chart at 100% contrast and was found to be −2.50/-0.50x160 VA 0.5(−2) in the right eye and −3.25/-0.50x175 VA 0.5(+1) in the left eye.
Intra ocular pressure (IOP) was 17 mmHg in both eyes measured using the ICare (Tiolat Oy, Helsinki, Finland) non-contact tonometer.
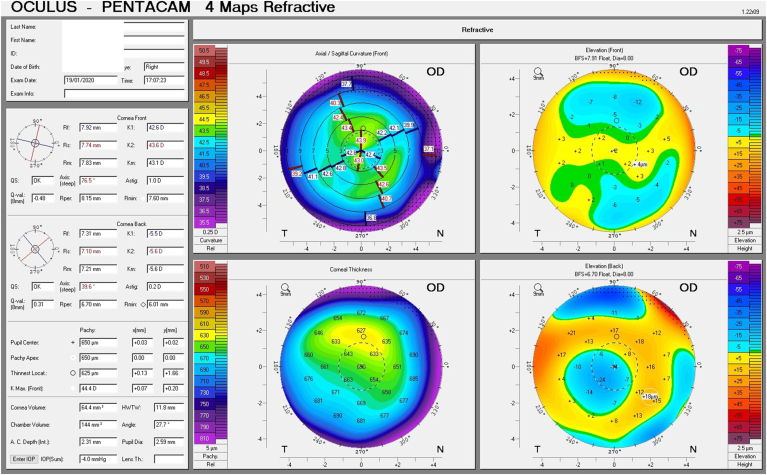
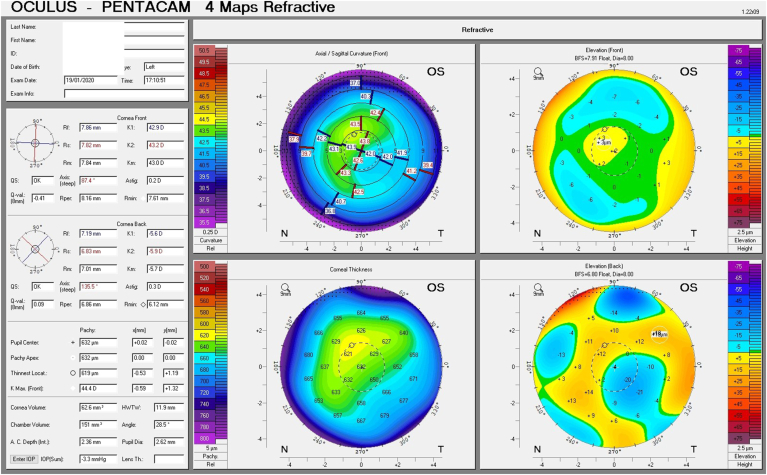
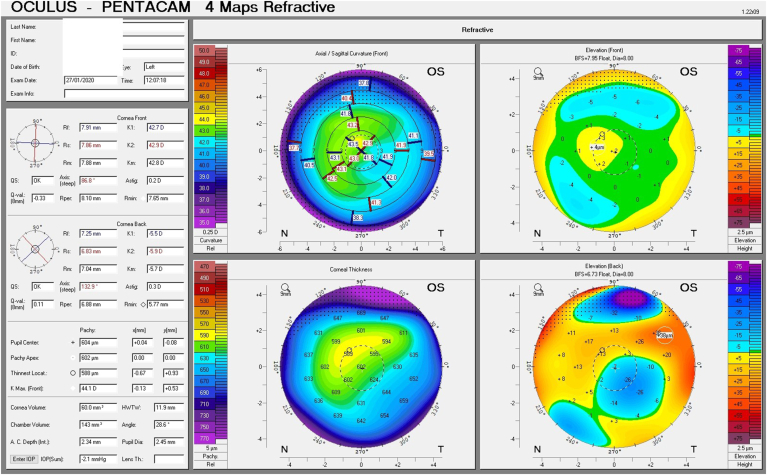
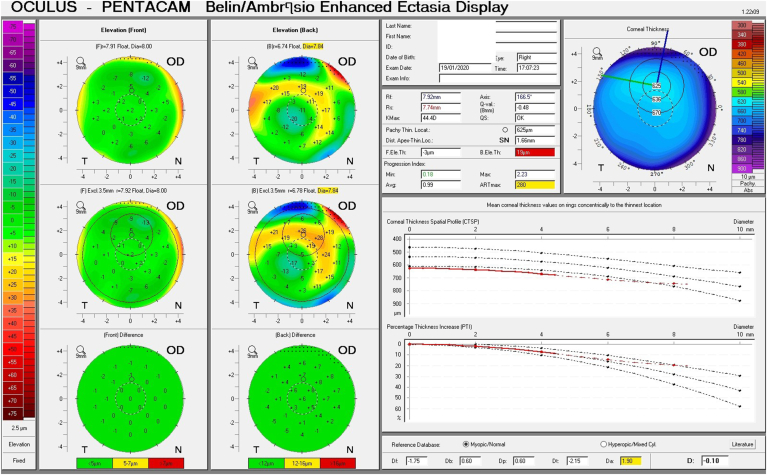
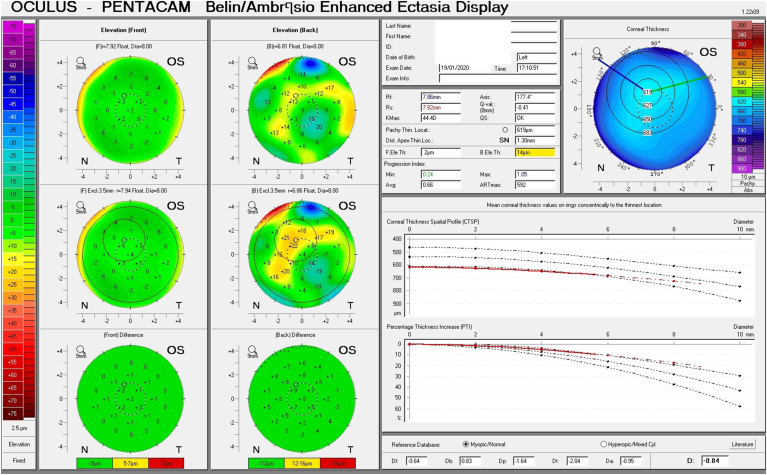
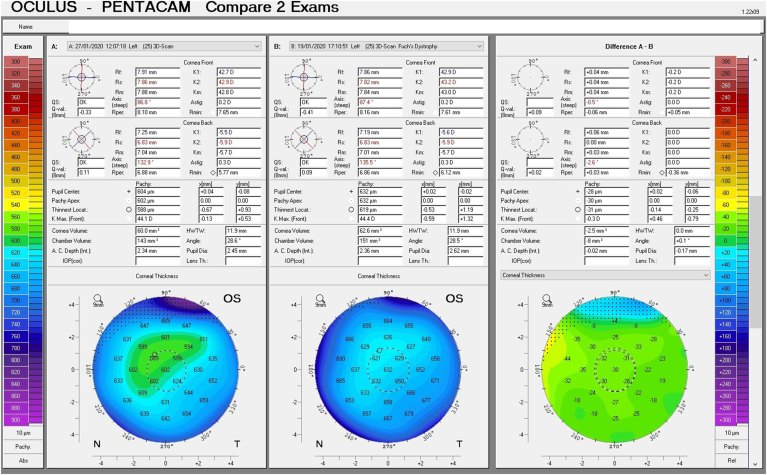
Given his symptoms, medical history along with evidence of guttata noted during slit lamp examination, and the tomographic pachymetry and posterior elevation maps derived from the Pentacam exhibiting focal posterior corneal surface depression, displacement of the thinnest point of the cornea, irregular isopachs, elevated densinometry and a plane corneal thickness spatial profile (CTSP), edema was suspected even without exhibiting clinical signs of edema on slit-lamp examination (Fig. 2 A and B, Fig. 3 A and B).
Fig. 2.
Tomographic maps before treatment exhibiting in the right eye focal posterior corneal surface depression, displacement of the thinnest point of the cornea, irregular isopachs in the right and left eye(A and B).Tomographic maps after eight days of treatment exhibiting in significant decrease in corneal thickness in the right and left eye(C and D).
Fig. 3.
Belin Ambrosio Display shows the corneal thickness spatial profile (CTSP) in the right and left eye (A and B). Note the almost completely level slope depicting the central thickening of the cornea.
A Therapeutic Hyper-CL™ 15.5 diameter, base curve 8.2mm, Rx Plano was placed on each eye. They were well centered and moved 0.5 mm upon blink. This lens was one of the two standard options available (one diameter and two base curves, 8.2 and 8.6) and no further customization was necessary. The patient wore the lenses consecutively for seven days and was instructed to administer 5% sodium chloride six times a day.
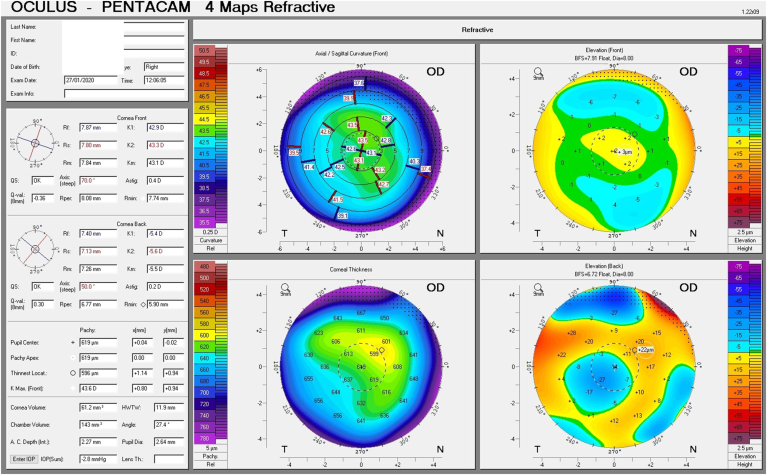
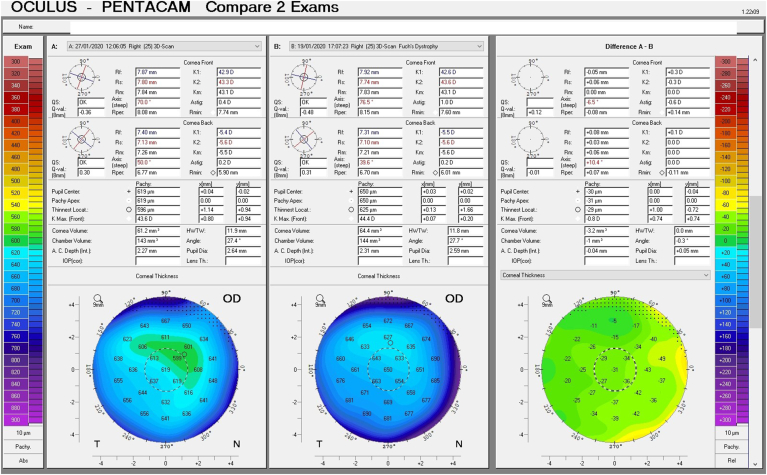
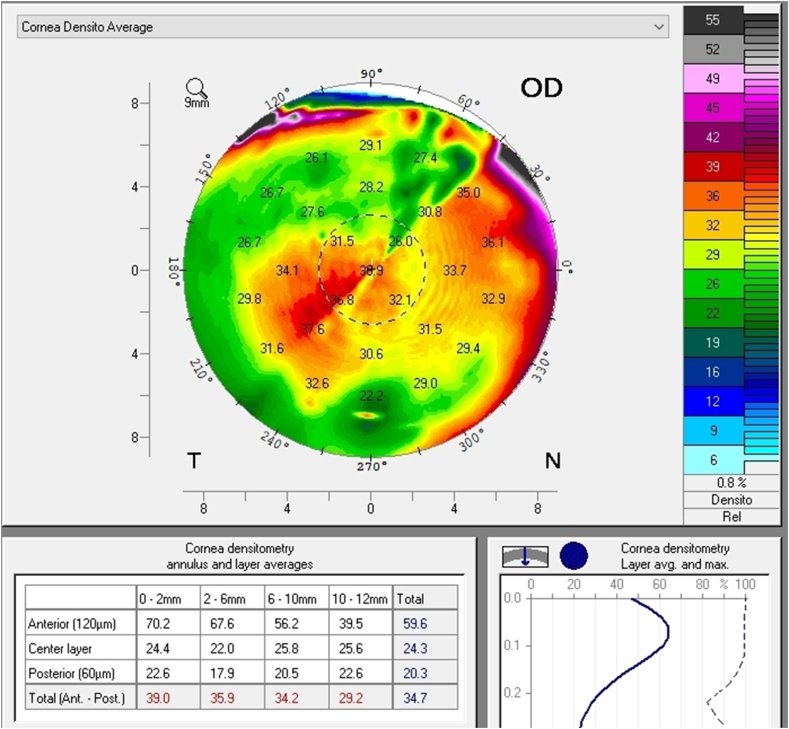
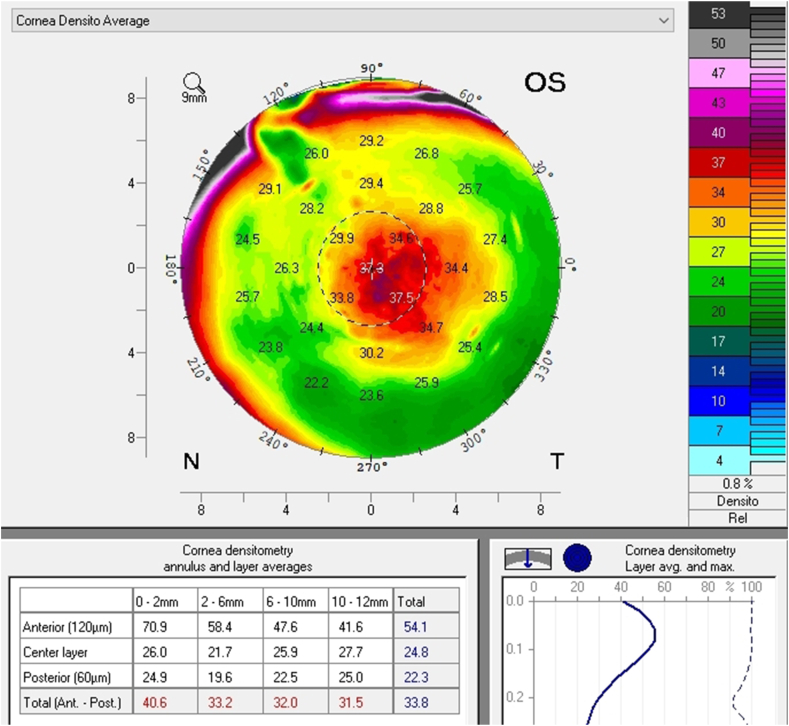
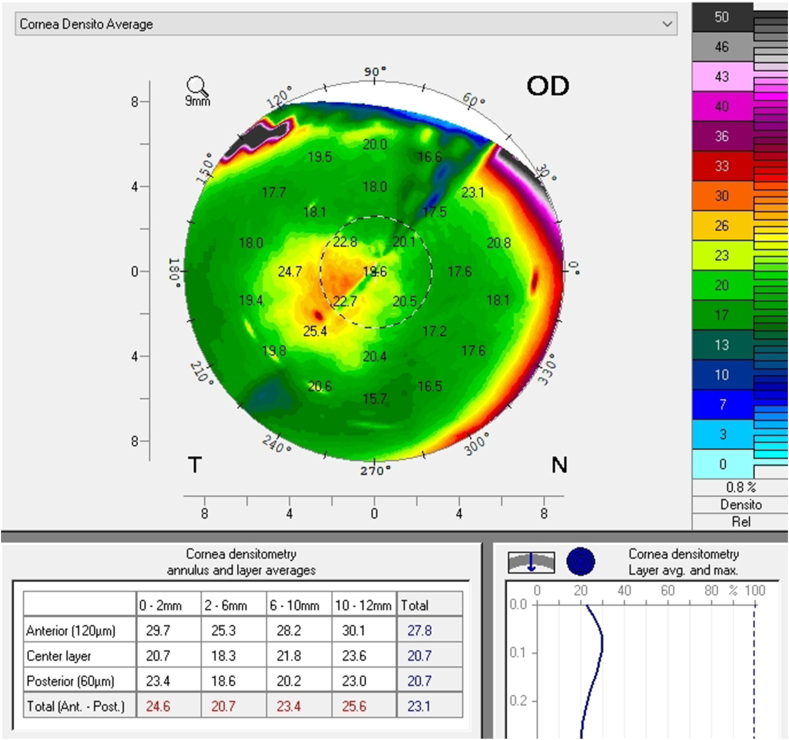
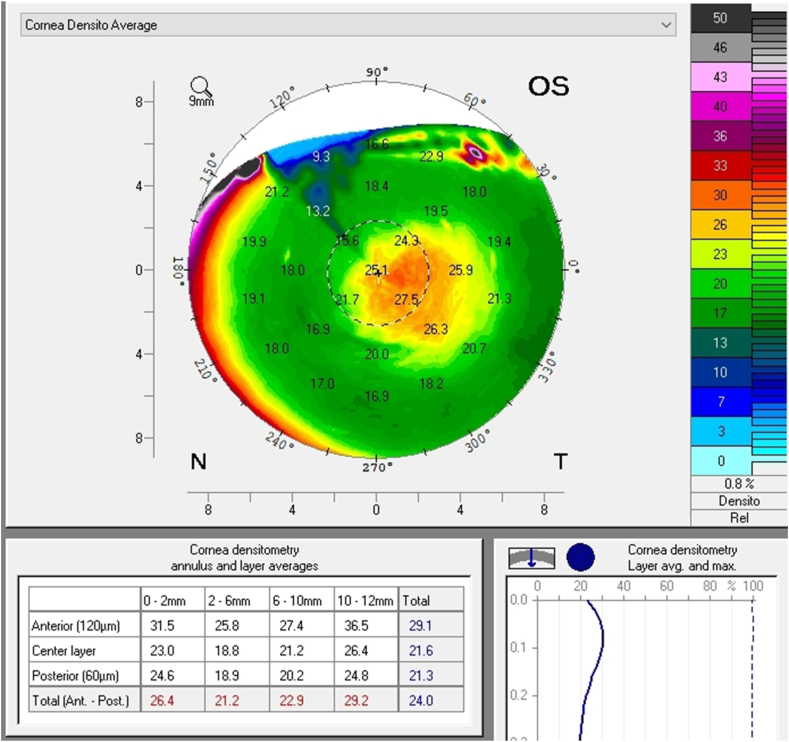
A follow up evaluation was then conducted. There was a significant reduction in central corneal thickness (CCT) noted comparing measurements taken at the initial visit and at the follow-up visit from 650 μm to 619 μm and from 632 μm to 604 μm in the right and left eye respectively (Fig. 4 A and B, Fig. 5). The Pentacam densinometry maps showed a significant decrease in central and total densinometry from 39 to 34.7 grayscale units (GSU) to 24.6 and 23.1GSU in the right eye and from 40.6 to 33.8 GSU to 26.4 and 24.0 GSU in the left eye (Fig. 6 A, B, C and D) as well as a significant decrease in both eyes of the "camel's back” sign (Fig. 7 A, B, C and D).
Fig. 4.
Difference maps of pachymetry before and after treatment in the right and left eye (A and B).
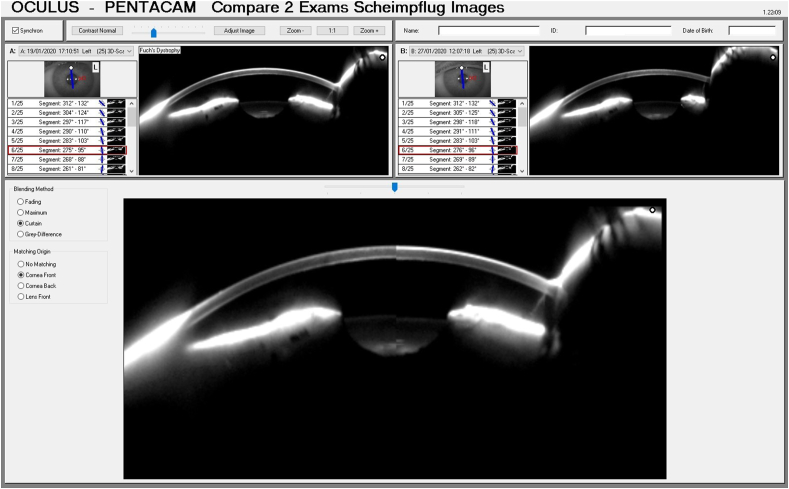
Fig. 5.
Scheimpflug image split to compare the corneal thickness before treatment (top left image and bottom right half of image) to the corneal thickness after treatment (top right image and bottom left half of image).
Fig. 6.
Densinometry of the right eye and left eye before treatment (A and B). Densinometry decreased in the right and left eye after treatment (C and D).
Fig. 7.
Scheimpflug image of the right and left eye before treatment. Note the high density depicted in the green scale on the right of the image as well as the "Camel's Back"(A and B). The images after treatment of the right and left eye (C and D) Note the decrease in density of the anterior cornea and a decrease, though not elimination, of the posterior “hump". (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The visual acuity improved to 0.4(+2) in the right eye and 0.3(−1) in the left eye with no change in the refraction. IOP was 17 mmHg in each eye.
The anterior ocular surface was examined with fluorescein through a yellow filter and lissamine green through a red filter that ruled out corneal erosions or abrasions and staining on the conjunctiva. The tarsal conjunctiva exhibited no signs of giant papillary conjunctivitis. The contact lens did not have significant mucin, lipid, or protein deposits.
The patient reported an absence of haloes in the morning and less glare while driving. His CLDEQ-8 score at that visit was 19.
3. Discussion
In this case presentation visual symptoms included intermittent haloes glare and foggy vision, especially upon wakening and while driving. Corneal edema was determined to be the cause as shown in the data derived from Pentacam measurements. These visual symptoms rapidly decreased when treated with the Therapeutic Hyper-CL™ contact lens and 5% sodium chloride.
The cornea's sponge-like stromal layer constitutes approximately 90% of the corneal tissue features and consists of collagen fibers surrounded by negatively-charged inter-fibrillar glycosaminoglycans, which deflect each other, driving imbibition of fluid.1, 2, 3, 4, 5 An additional vector responsible for the influx of fluid from the anterior chamber is the intra-ocular pressure.1, 2, 3
The cornea's mechanisms to prevent excessive hydration include externally, by the epithelium and the tear film and internally, by the endothelium.1, 2, 3 The epithelium forms a passive barrier to the flow of water and electrolytes into the cornea.1, 2, 3 Water evaporation via the tear film on an open eye yields a slightly hypertonic solution at the eye surface, which draws fluid out of the cornea through osmosis.1, 2, 3, 4 The endothelium maintains cornea dehydration by actively pumping water into the anterior chamber.1, 2, 3, 4
Clinically, significant edema above the normal 4–5% of swell during prolonged eye closure9 with subsequent separation and disorder of the collagen fibers can affect visual acuity, contrast sensitivity and cause additional symptoms as previously mentioned. Studies have exhibited a correlation between the extent of edema and a decrease in visual acuity.4,9,12 Yet, corneal edema's diurnal variation often compels clinicians to rely on patient reports of symptoms and subclinical signs as symptoms and clinical presentations often dissipate before arriving for an examination.12, 13, 14, 15
These subclinical signs are effectively monitored by instruments such as the Pentacam. They are independent of increased corneal thickness and are more sensitive and subtle, appearing at very early stages of emerging edema, allowing for early detection and treatment. They include a focal posterior corneal surface depression towards the anterior chamber as a result of endothelial damage, displacement of the thinnest point of the cornea (usually but not exclusively nasally) and irregular (non-parallel) isopachs within the central 4mm of the cornea.16,17 An additional parameter is elevated central densinometry. While densinometry elevates as a function of age, in this patient, it was well above the expected norms of his age category of a total corneal densinometry 21.9 ± 3.87 GSU.18, 19, 20 An alternative Pentacam display of this characteristic is the “hanging hammock” or "camel's back” sign. It signifies a higher reflectivity of Descemet's membrane and presents as a second peak in the density graph of the cornea. These can be observed in very mild FECD and can cause significant backscatter and resulting glare, as in this patient.8 The normal cornea pachymetry is thinner in the center and gradually becomes thicker towards the limbus, expressed in the Pentacam as a descending slope. When corneal edema is present, the central cornea thickens, thereby becoming more equal to the periphery and eliminating this slope. The Pentacam CTSP factor will exhibit the corneal thickness progression from the center to the periphery as a plateau or plane shape. All these signs were present in this patient, supporting the implementation of therapy.
The Therapeutic Hyper-CL™ Soft Contact Lens contains 59% water, with a Dk/t of 26.21 There are multiple options available to custom design the diameter, base curve and prescription parameters, including correction for astigmatism. The base curve of the lens is dynamic, where the central base curve is steeper than the peripheral curve, which results in an elevation of the lens at the center of the cornea. This elevation forms a gap between the lens and the cornea, creating a fluid reservoir. The lens lands on the limbal area with the reservoir enabling prolonged retention of any applied eye drops. Fenestrations in the mid-periphery improve drug accessibility to the corneal surface, as well as oxygen supply to the cornea. Preclinical and in vitro studies yet unpublished, have shown that the reservoir under this lens can sustain eye drop solutions on the corneal surface for a period of at least 10 minutes, compared to 20–30 seconds without the lens.
The preferred protocol is extended wear for seven days, after which the lens should be removed to be cleaned and could be reapplied for additional seven days if the condition warrants. After resolution of the edema the Hyper-CL™ should be removed.
The effectiveness of this lens has been compared to standard bandage contact lens in a prospective, randomized, crossover study and found superior.21 As mentioned, the Hyper-CL™ enhances the contact time of topical drops in the central part of the cornea for an extended period, thus increases their efficiency. This lens potentially can assist in treating corneal infections, edema, and other medical conditions requiring heavy doses of drops to enhance corneal healing. It is common practice to concurrently prescribe prophylactic antibiotics with a bandage contact lens, in this case they were not prescribed as per the clinical judgement of the prescriber as the corneal epithelial was intact.
These Pentacam identifying markers have been shown to assist in deciding when to perform endothelial keratoplasty in FECD. While this case presentation also demonstrates the successful application on a patient with FECD, the principals apply in conditions without guttata as well. Indeed it has been suggested that the appearance or lack thereof of these tomographic signs are not correlated directly with the presence of guttae.13 Here a patient presented with symptoms consistent with corneal edema but only tomographic subclinical signs. Treatment of one week of extended wear of the Therapeutic Hyper-CL™ contact lens and 5% sodium chloride achieved a rapid decrease in signs and symptoms of the corneal edema.
Patient consent
This report does not contain any personal information that could lead to the identification of the patient.
Funding support
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases).
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Contact with the editorial office
The Corresponding Author declared on the title page of the manuscript is: Naomi London.
This author submitted this manuscript using his/her account in EVISE.
We understand that this Corresponding Author is the sole contact for the Editorial process (including EVISE and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
We confirm that the email address shown below is accessible by the Corresponding Author, is the address to which Corresponding Author's EVISE account is linked, and has been configured to accept email from the editorial office of American Journal of Ophthalmology Case Reports: imnl4u@gmail.com.
Someone other than the Corresponding Author declared above submitted this manuscript from his/her account in EVISE:
We understand that this author is the sole contact for the Editorial process (including EVISE and direct communications with the office). He/she is responsible for communicating with the other authors, including the Corresponding Author, about progress, submissions of revisions and final approval of proofs.
Declaration of competing interest
Dr. Nir Erdinest is a consultant for EyeYon Medical.
Acknowledgements
None.
References
- 1.Jeang L., Margo C., Espana E. Diseases of the corneal endothelium. Exp Eye Res. 2021 Feb 14 doi: 10.1016/j.exer.2021.108495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tone S.O., Kocaba V., Böhm M., Wylegala A., White T.L., Jurkunas U.V. Fuchs endothelial corneal dystrophy: the vicious cycle of Fuchs pathogenesis. Prog Retin Eye Res. 2021;80 doi: 10.1016/j.preteyeres.2020.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voss K., Nguyen A., Heur M. Non-infectious and non-hereditary diseases of the corneal epithelium. Exp Eye Res. 2021;202 doi: 10.1016/j.exer.2020.108316. [DOI] [PubMed] [Google Scholar]
- 4.Chow S.-C., Chan J. Review on the use of topical ocular hypertonic saline in corneal edema. Cornea. 2021;40(4):533–539. doi: 10.1097/ICO.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 5.Soh Y., Kocaba V., Pinto M., Mehta J.S. Fuchs endothelial corneal dystrophy and corneal endothelial diseases: east meets West. Eye. 2020;34(3):427–441. doi: 10.1038/s41433-019-0497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthaei M., Hribek A., Clahsen T., Bachmann B., Cursiefen C., Jun A.S. Fuchs endothelial corneal dystrophy: clinical, genetic, pathophysiologic, and therapeutic aspects. Ann Rev Vis Sci. 2019;5:151–175. doi: 10.1146/annurev-vision-091718-014852. [DOI] [PubMed] [Google Scholar]
- 7.Moshirfar M., Somani A.N., Vaidyanathan U., Patel B.C. StatPearls Publishing; 2019. Fuchs Endothelial Dystrophy (FED). StatPearls [Internet] [PubMed] [Google Scholar]
- 8.Cui Z., Zeng Q., Guo Y., et al. Pathological molecular mechanism of symptomatic late-onset Fuchs endothelial corneal dystrophy by bioinformatic analysis. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0197750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wacker K., McLaren J.W., Kane K.M., Patel S.V. Corneal optical changes associated with induced edema in Fuchs endothelial corneal dystrophy. Cornea. 2018;37(3):313. doi: 10.1097/ICO.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin S.R., Baratz K.H., McLaren J.W., Patel S.V. Corneal abnormalities early in the course of Fuchs' endothelial dystrophy. Ophthalmology. 2014;121(12):2325–2333. doi: 10.1016/j.ophtha.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vedana G., Villarreal G., Jr., Jun A.S. Fuchs endothelial corneal dystrophy: current perspectives. Clin Ophthalmol. 2016;10:321. doi: 10.2147/OPTH.S83467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carney L.G., Jacobs R.J. Mechanisms of visual loss in corneal edema. Arch Ophthalmol. 1984;102(7):1068–1071. doi: 10.1001/archopht.1984.01040030862034. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S., Oie Y., Fujimoto H., et al. Relationship between corneal guttae and quality of vision in patients with mild Fuchs' endothelial corneal dystrophy. Ophthalmology. 2015;122(10):2103–2109. doi: 10.1016/j.ophtha.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Kobashi H., Kamiya K., Shimizu K. Factors influencing visual acuity in Fuchs' endothelial corneal dystrophy. Optom Vis Sci. 2018;95(1):21–26. doi: 10.1097/OPX.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 15.Fritz M., Grewing V., Maier P., et al. Diurnal variation in corneal edema in Fuchs endothelial corneal dystrophy. Am J Ophthalmol. 2019;207:351–355. doi: 10.1016/j.ajo.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Sun S.Y., Wacker K., Baratz K.H., Patel S.V. Determining subclinical edema in Fuchs endothelial corneal dystrophy: revised classification using Scheimpflug tomography for preoperative assessment. Ophthalmology. 2019;126(2):195–204. doi: 10.1016/j.ophtha.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Patel S.V., Hodge D.O., Treichel E.J., Spiegel M.R., Baratz K.H. Predicting the prognosis of Fuchs endothelial corneal dystrophy by using Scheimpflug tomography. Ophthalmology. 2020;127(3):315–323. doi: 10.1016/j.ophtha.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Dhubhghaill S.N., Rozema J.J., Jongenelen S., Hidalgo I.R., Zakaria N., Tassignon M.-J. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Investig Ophthalmol Vis Sci. 2014;55(1):162–168. doi: 10.1167/iovs.13-13236. [DOI] [PubMed] [Google Scholar]
- 19.Asrar A., Ikram B., Khan H., Asrar M. Normal values of corneal optical densitometry using Pentacam Scheimpflug camera. Adv Ophthalmol Vis Syst. 2016;5(1) [Google Scholar]
- 20.Ishikawa S., Kato N., Takeuchi M. Quantitative evaluation of corneal epithelial edema after cataract surgery using corneal densitometry: a prospective study. BMC Ophthalmol. 2018;18(1):1–7. doi: 10.1186/s12886-018-0998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daphna O., Mimouni M., Keshet Y., et al. Therapeutic HL-contact lens versus standard bandage contact lens for corneal edema: a prospective, multicenter, randomized, crossover study. J Ophthalmol. 2020:2020. doi: 10.1155/2020/8410920. [DOI] [PMC free article] [PubMed] [Google Scholar]