Abstract
Background
Neurological injury and mortality remain high in comatose patients resuscitated from out-of-hospital cardiac arrest (OHCA). Hypotension and hypoxia during post-resuscitation care have been associated with poor outcome, but the optimal oxygenation- and blood pressure-targets are unknown. The impact of different doses of norepinephrine on advanced hemodynamic after OHCA and the impact of different oxygenation-targets on pulmonary circulation and resistance (PVR), are unknown. The aims of this substudy of the “Blood pressure and oxygenations targets after out-of-hospital cardiac arrest (BOX)”-trial are to investigate the effect of two different MAP- and oxygenation-targets on advanced systemic and pulmonary hemodynamics measured by pulmonary artery catheters (PAC).
Methods
The BOX-trial is an investigator-initiated, randomized, controlled study comparing targeted MAP of 63 mmHg vs 77 mmHg (double-blinded intervention) and 9–10 kPa versus PaO2 of 13–14 kPa oxygenation-targets (open-label). Per protocol, all patients will be monitored systematically with PACs. The primary endpoint of the hemodynamic-substudy is cardiac output for the MAP-intervention, and PVR for the oxygenation-intervention. For both endpoints, the difference within 48 h between groups are assessed. Secondary endpoints are pulmonary capillary wedge pressure and pulmonary arterial pressure and association between advanced hemodynamic variables and mortality and biomarkers of inflammation and brain injury.
Discussion
In the BOX-trial, patients will be randomly allocated to two levels of MAP and oxygenation, which are central parts of post-resuscitation care and where evidence is sparse. The advanced-hemodynamic substudy will give valuable knowledge of the hemodynamic consequences of changing blood pressure and oxygen-levels of the critical cardiac patient. It will be one of the largest clinical, prospective trials of advanced hemodynamics measured by serial PACs in consecutive comatose patients, resuscitated after OHCA. The randomized and placebo-controlled trialdesign of the MAP-intervention minimizes risk of selection bias and confounders. Furthermore, hemodynamic characteristics and associations with outcome will be investigated.
Trial registration
ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT03141099). Registered March 30, 2017.
Keywords: Out-of-hospital cardiac arrest, Neuroprotection, Blood pressure, Hemodynamics, Pulmonary artery catheter, Cardiac output
Introduction
Out-of-hospital cardiac arrest (OHCA) is a major cause of mortality and disability. In Europe, approximately 40–80 patients per 100,000 inhabitants per year suffer an OHCA,1., 2., 3. and the in-hospital mortality of comatose survivors remain at 30–50%.4., 5. In comatose survivors of OHCA, hypoxic ischemic brain injury is the primary cause of mortality and long-term neurologic disability in survivors.6 The primary anoxic insult occurs during the arrest, while secondary injury takes place in the hours and days following the initial insult, due to compromised oxygen-delivery to the brain. Factors contributing to secondary injury include among others systemic hypotension, low cardiac output, impaired cerebral autoregulation, hypoxemia and/or hyperoxia.7., 8., 9., 10., 11.
Cardiovascular function during Post Cardiac Arrest Syndrome
Hemodynamic dysfunction including reduced systolic myocardial function, vasoplegia and low cardiac filling pressures are frequent findings in comatose survivors of OHCA.12., 13. Clinically, the patients have low blood pressure, requirements of vasopressors, low cardiac output and reduced left-ventricular ejection fraction, which contributes to organ hypoperfusion and dysfunction.7., 14. Early studies of septic shock improved prognosis significantly with bundles of care including vasopressors to maintain a mean arterial pressure (MAP) of at least 65 mmHg,15 and still, the “Surviving Sepsis Campaign” recommends to target a MAP of 65 mmHg in patients requiring vasopressors (strong recommendation; moderate quality of evidence).16 In OHCA-patients, observational studies have reported associations between low blood pressure after OHCA and poor outcome10., 17., 18., 19., 20., 21., 22. and currently, the European Resuscitation Council Guidelines for post-resuscitation care recommends avoiding hypotension (<65 mmHg, weak recommendation, low-quality evidence),3 but an adequately powered trial evaluating clinically relevant endpoints is needed to investigate whether a higher MAP-target infers a clinical benefit. The Blood pressure and Oxygenation Targets after OHCA (BOX-trial, clinicaltrials.gov NCT03141099) is an investigator-initiated, randomized, controlled, double-blinded multi-center study comparing targeted MAP of 63 mmHg (MAP63-group) versus 77 mmHg (MAP77-group) commencing at the time of randomization (prior to or immediately after ICU-admission) and continued for as long as the patients needed invasive blood pressure measurements during ICU stay. Based on a pilot-trial, the intervention is expected to significantly increase vasopressor-doses in the MAP77-group.23., 24.
Hemodynamic and cardiovascular responses to different doses of norepinephrine
Higher vasopressor-doses may pose significant hemodynamic changes in the patients and additional challenges to the treating physicians. Current knowledge of hemodynamic implications of higher vasopressor-doses is limited because few human studies have investigated the effects of different doses of norepinephrine, and these few studies are observational, small11., 13., 24. or used noninvasive methods for hemodynamic evaluation.25., 26., 27., 28. More knowledge is needed, since norepinephrine is the primary vasopressor used to increase blood pressure in cardiac intensive care.3 Norepinephrine increases blood pressure primarily due to vasoconstriction and consequently increasing systemic vascular resistance (SVR). However, this effect may have unpredictable effects on cardiac output through a complex interaction with the myocardium including increased left ventricular afterload, but a higher coronary perfusion pressure. We plan a prospective substudy within the BOX-trial focusing on advanced hemodynamics measured by serial right heart catheterizations.
Hemodynamic and cardiovascular responses to different doses of oxygen
Another central clinical challenge during post-resuscitation care after OHCA is adequate oxygenation. International guidelines recommend a targeted oxygen saturation of 94–98% and/or an oxygenation-target of 10–13 kPa (75–100 mmHg).3 The BOX-trial will in another intervention, randomly allocate patients to low-normal paO2 of 9–10 kPa (restrictive) and high-normal PaO2 of 13–14 kPa (liberal). Higher oxygenation will theoretically decrease pulmonary vascular resistance, and the impact of different oxygenation-targets may impact pulmonary arterial pressures.
Aims
The coprimary aim of this substudy is to investigate the impact of a targeted MAP of 63 mmHg versus 77 mmHg on cardiac output, pulmonary capillary wedge pressure (PCWP), pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR). The second coprimary aim is to investigate the impact of a restrictive) versus a liberal oxygenation-strategy on the PVR and PAP.
Specifically, we aim to:
-
1.
Investigate the impact of a targeted MAP of 63 mmHg versus 77 mmHg on cardiac output, SVR and PCWP.
-
2.
Investigate the impact of a liberal versus restrictive oxygenation-target on PVR and PAP.
-
3.Investigate the impact of a targeted MAP of 63 mmHg versus 77 mmHg on
-
a.Arterial-Venous (A-V) oxygen difference, cardiac power output, lactate-clearance, mixed venous oxygen saturation.
-
b.PAP, PVR, PCWP/RAP-ratio, central venous pressure, and pulmonary artery pulsatility index.
-
c.Fraction of time with pulmonary hypertension (mPAP > 25 mmHg) out of total time with vasopressor infusion.
-
d.Right ventricular failure. Defined as cardiac index (CI) < 2.0 and central venous pressure (CVP) > 18 mmHg.
-
e.Ventriculoarterial coupling (Ea/EES ratio).
-
a.
-
4.To investigate the impact of a liberal versus restrictive oxygenation-target on
-
a.Arterial-Venous (A-V) oxygen difference, cardiac power output, lactate-clearance, mixed venous oxygen saturation.
-
b.PAP, PVR, PCWP/RAP-ratio, central venous pressure, and pulmonary artery pulsatility index.
-
c.Fraction of time with pulmonary hypertension (mPAP > 25 mmHg) out of total time with vasopressor infusion.
-
d.Right ventricular failure. Defined as cardiac index (CI) < 2.0 and central venous pressure (CVP) > 18 mmHg.
-
e.Ventriculoarterial coupling (Ea/EES ratio).
-
a.
-
5.
To investigate associations between inflammatory biomarkers and advanced hemodynamics.
-
6.
To investigate associations between advanced hemodynamics (CO, PVR, SVR, PCWP) and clinical outcome (mortality, renal function, length of ICU-stay, long-term cognitive function, and biomarkers of brain injury).
-
7.
To identify hemodynamic phenotypes during post-resuscitation intensive care after OHCA.
All hemodynamic variables will be reported together with their corresponding indexed values (related to body-surface area).
Our hypotheses are:
-
1.
Randomly allocated higher versus lower dose of vasopressors will increase SVR and PCWP without affecting cardiac output.
-
2.
Liberal compared to restrictive oxygenation will decrease PVR and PAP.
-
3.
Randomly allocated higher versus lower doses of vasopressors will have an impact on advanced hemodynamic parameters (pulmonary vascular resistance, A-V oxygen difference, cardiac power output, SVR, lactate-clearance, pulmonary vascular resistance, PCWP/RAP ratio, pulmonary artery pulsatility index and ventriculoarterial coupling (Ea/EES ratio)).
-
4.
Randomly allocated liberal versus restrictive oxygenation will have an impact on advanced hemodynamic parameters (pulmonary vascular resistance, A-V oxygen difference, cardiac power output, SVR, lactate-clearance, pulmonary vascular resistance, PCWP/RAP ratio, pulmonary artery pulsatility index and ventriculoarterial coupling (Ea/EES ratio)).
-
5.
Cardiac output, during post-resuscitation care are associated with long-term cognitive function.
-
6.
Biomarkers of inflammation will be associated with increased advanced hemodynamic variables.
-
7.
Specific hemodynamic phenotypes of can be identified with differences in hemodynamic profile and prognosis.
Methods
Study design, setting and population
The BOX-trial is an investigator-initiated, randomized, controlled multi-center study comparing targeted MAP of 63 mmHg vs 77 mmHg (double-blinded intervention) and liberal vs restrictive oxygenation-targets (open-label). The trial will include 800 adult comatose OHCA-survivors of a presumed cardiac origin in two Danish tertiary heart centers, namely Odense University Hospital (primary hospital for highly specialized cardiac care for 1.3 million citizens) and Copenhagen University Hospital, Rigshospitalet (primary hospital for highly specialized cardiac care for 2.7 million citizens). Per protocol, we include all patients in this sub-study. Inclusion and exclusion criteria for the BOX-trial is described in Table 1. Additional exclusion criteria for this sub-study are death prior to PAC-insertion, complications related to PAC-procedure making the PAC-insertion impossible such as ventricular arrythmias or severe hemodynamic instability. This protocol is written in accordance with the SPIRIT.29
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Age of at least 18 years | 1. Conscious patient (GCS score of at least 8) |
| 2. Out-of-hospital cardiac arrest (OHCA) of presumed cardiac cause | 2. Female of child-bearing potential, unless a negative human chorionic gonadotropin (hCG) test can rule out pregnancy within the inclusion window |
| 3. Sustained return of spontaneous circulation (ROSC), defined as ROSC when chest compressions have not been required for 20 consecutive minutes and signs of circulation persist | 3. In-hospital cardiac arrest (IHCA) |
| 4. Unconsciousness (Glasgow Coma Scale (GCS) score of less than 8) after sustained ROSC | 4. OHCA of presumed non-cardiac cause, such as after trauma, dissection/rupture of major artery or arrest caused by hypoxia (i.e., drowning, hanging, etc.) |
| 5. Target temperature management (TTM) is indicated. | 5. Known bleeding diathesis (medically induced coagulopathy does not exclude patient) |
| 6. Suspected or confirmed acute intracranial bleeding | |
| 7. Suspected or confirmed acute ischemic stroke | |
| 8. Unwitnessed asystole | |
| 9. Known limitations in therapy and do-not-resuscitate order | |
| 10. Known disease making 180-day survival unlikely | |
| 11. Known pre-arrest cerebral performance category (CPC) score of 3 or 4 | |
| 12. More than 4 h (240 min) from ROSC to randomization | |
| 13. Systolic blood pressure of less than 80 mm Hg in spite of fluid loading/vasopressor and/or inotropic medication and/or mechanical circulatory support* | |
| 14. Temperature of less than 30 °C on admission | |
| 15. Uncorrected blood glucose of less than 2.5 mmol/L at admission |
If systolic blood pressure is recovering during the inclusion window the patient can be included.
Study procedure
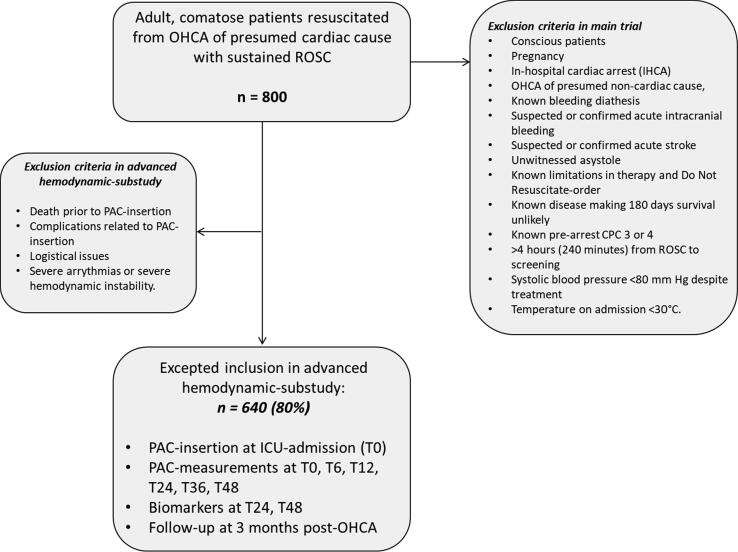
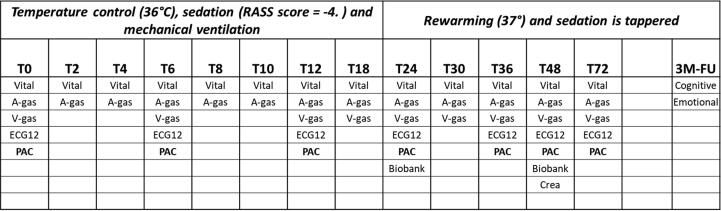
Inclusion and randomization are performed online at a dedicated website. Upon inclusion, the patient will be randomly allocated to treatment goals in a 1:1:1:1 manner using varying block sizes of 4–16. The oxygenation-allocation is kept concealed until time of randomization, whereas the MAP-intervention remained blinded (trial personal, trial participants, care providers, outcome assessors) until end-of-follow-up. As part of the hemodynamic monitoring, a PAC is inserted via the jugular or subclavian vein under ultrasound guidance. This sub-study will collect data on serial hemodynamic assessment including cardiac output measurements by thermodilution performed at insertion of PAC, after 6 h, 12 h, 24 h, 36 h, and 48 h. Assessment after 72 h is also performed if the PAC is still in the patient for clinical indications (Figs. 1 and 2). Assessment of myocardial function from serial echocardiographies recorded during the daily work-up is also collected.
Fig. 1.
Flowchart summarizing patient enrollment and main study procedures. OHCA: out-of-hospital cardiac arrest; ROSC: return of spontaneous circulation; IHCA: In-hospital cardiac arrest; CPC: cerebral performance category; PAC: Pulmonary artery catheter; T0: time at ICU-admission; ICU: intensive care unit.
Fig. 2.
Detailed overview of study-related procedures. RASS: Richmond Agitation-Sedation Scale; T0: time at ICU-admission; 3 M−FU: 3 months follow-up; Vital: vital signs; A-gas: Arterial blood gas; V-gas: central venous blood gas; ECG12: electrocardiogram; PAC: measurements from pulmonary artery catheter; Crea: creatinine-clearence.
Postcardiac arrest care and hemodynamic measurements
All patients are admitted to the intensive care unit (ICU) and sedated, intubated, and mechanically ventilated with propofol and fentanyl titrated to achieve a Richmond Agitation-Sedation Scale score of −4; neuromuscular blocking drugs are administered to reduce shivering if needed. All patients are actively cooled with an invasive cooling device and active cooling is initiated immediately after ICU-admission with a 4-hours induction period to achieve the target temperature, followed by a period of 24 hours with stable temperature at 36 °C and hereafter rewarming of 0.5 °C per hour. Acute coronary angiography, defined as prior or immediately after admission to ICU, is performed in all patients presenting with ST-segment elevations or at the discretion of the treating physician. Isotonic crystalloids are administered in all patients with general treatment goals of central venous pressure (CVP) of 10 to 15 mm Hg to optimize right ventricular filling pressure, and urine output > 1.5 mL/kg per hour to secure adequate organ perfusion. As part of the intervention in the BOX-trial, patients are randomly allocated to a MAP-target of 63 or 77 mmHg. Allocation is blinded to caregivers by manipulation of the blood pressure modules (HP/Philips M1006B Invasive Pressure module).23 Norepinephrine (μg/kg/min solution) is increased in incremental doses until the required blood pressure target is reached. Dopamine (μg/kg/min solution) will be used in addition to norepinephrine if deemed needed by the attending physician.
Monitoring
Hemodynamics are monitored as soon as possible after intensive care unit admission with an arterial pressure catheter in the radial or brachial artery and insertion of PAC is inserted via the internal jugular or subclavian vein under ultrasound guidance with CVP values obtained from the proximal port. At the site in Copenhagen, Rigshospitalet a 7.5F triple lumen Swan-Ganz thermistor and balloon-tipped catheter (Edwards Lifesciences, Irvine, CA) is used. In the Odense University Hospital-site, advanced hemodynamics are measured continuously by PAC (Continuous Cardiac Output (CCOmbo) PAC®, Edwards Lifesciences, Irvine, CA, USA) linked to the correct monitor (Vigilance II®, Edwards Lifesciences). These data are transferred electronically to a computer with a 2-second time interval. Pulmonary capillary wedge pressure, CVP, systolic pulmonary artery pressure, diastolic pulmonary artery pressure, mean pulmonary artery pressure, and cardiac output are measured at prespecified time points. If cardiac output is low, the attending physician can choose the following inotropic agents if deemed necessary: (a) milrinone (0.375–0.75 μg/kg per min), (b) dobutamine (2–20 mcg/kg/min), and (c) levosimendan (0.1 μg/kg per min). If an inotropic agent at maximal dose combined with vasopressors cannot maintain cardiac output, mechanical circulatory support may be considered. In Copenhagen, cardiac output is estimated using the thermodilution technique with infusion of chilled isotonic glucose. Cardiac output was measured as the average of 3 measurements with ≤10% variance.30 Our group has previously investigated interobserver variation: Cardiac output measurements in 13 patients showed low bias (0.42%; mean difference of 0.02 ± 0.52 L/min; P = 0.57), standard error: 0.26 L/min and good reproducibility with a coefficient of variation of 3%.13 In Odense, cardiac output was measured continuously through intermittent heating of blood in the right ventricle and the resultant signal is measured by thermistor near the tip of the catheter. Average value of CO measured over time is displayed on the monitor and registered at prespecified timepoints.31 The different methods of measures cardiac output has previously showed excellent correlation, accuracy, and precision.32 Cardiac power index is calculated as MAP*cardiac index (CI)/451 W/m2.33 Central mixed venous blood and arterial blood are drawn at the previous mentioned prespecified time points and analyzed for mixed venous oxygen saturation (SVO2), lactate, pH, paO2 and paCO2.
Outcome measures
Table 2 gives an overview of outcomes in this study. The different hypothesis of this substudy have different outcomes. For the two co-primary aims related to the MAP- and oxygenation-interventions, the primary analyses will be a comparison of advanced hemodynamic variables, primarily cardiac output and secondarily PCWP and PAP for the MAP-intervention, and primarily PVR and secondarily PAP for the oxygenation-intervention, during 48 h between allocated intervention-groups. Other outcomes are MAP-group difference in cardiac power index, pulmonary vascular pressures, systemic and pulmonary vascular resistances, systolic myocardial function, Tricuspid annular plane systolic excursion, SVO2 < 48 hours, lactate, lactate-clearance (defined as the difference between admission-lactate and 12-hour lactate) and NT-proBNP 48 hours after randomization.
Table 2.
Outcomes.
| Outcomes related to the blood pressure and oxygenation-interventions | Outcomes associations with central hemodynamics |
|---|---|
| Cardiac output* | All-cause mortality until day 180 |
| Cardiac power index (MAP*cardiac index (CI)/451 W/m2) | Renal replacement therapy |
| Pulmonary vascular pressures (mean, systolic, diastolic) | Neuron-Specific Enolase (NSE) level at 48 hours |
| Central venous pressure (mean) | Montreal Cognitive Assessment (MoCA) score at three months (lowest score allocated to patients not available for follow-up) |
| Pulmonary capillary wedge pressure | Modified Ranking Scale (mRS) at 3 months |
| Systemic vascular resistance* | NT-pro-BNP at 48 hours |
| Pulmonary vascular resistance* | LVEF at last available measurement |
| Mixed venous oxygen saturation and arterial-venous oxygen difference | Cumulated vasopressor requirement |
| Tricuspid annular plane systolic excursion (TAPSE) | Creatinine-clearance at 48 hours |
| Left Ventricular Ejection Fraction | Urinary output per day |
| Pulse-pressure (MAP-CVP) | Interleukin-6 at 48 hours |
| Arterial elastance | lactate |
| Lactate clearance |
and corresponding indexed values (related to body-surface area).
To investigate our secondary hypotheses, we aim to correlate advanced hemodynamic measures with clinical outcomes: Primary outcome is all-cause mortality until day 90. Secondary end-points are need for renal replacement therapy, neuron-Specific Enolase (NSE) level at 48 hours, Montreal Cognitive Assessment (MoCA) score at three months (lowest score allocated to patients not available for follow-up), Modified Ranking Scale (mRS) at 3 months, NT-pro-BNP at 48 hours, creatinine-clearance at 48 hours, eGFR at 48 hours and LVEF at last available measurement with 3 month of follow-up and cumulated vasopressor requirement during first 48 hours of ICU stay.
Subgroups
Prespecified subgroups are 1. Age, 2. Sex (male/female), 3. Time to from OHCA to ROSC in minutes, 4. Initial rhythm (shockable/non-shockable), 5. Shock an admission (Shock on admission was defined as a systolic blood pressure of less than 90 mm Hg for more than 30 minutes or end-organ hypoperfusion (cool arms and legs, urine output < 30 ml per hour, and heart rate < 60 beats per minute), 6. LVEF, 7. BMI, 8. Presence of atrial fibrillation, 9. STEMI at admission, 10. Preexisting hypertension.
Ethics
The BOX-trial and this sub-study were approved by Regional Ethics Committee at the Capital Region of Denmark. Written informed consent to participate in the study including the use of PACs for research purposes is obtained from relatives and a legal guardian as soon as possible and subsequently from patients when they regain consciousness. Risks and discomforts are few, since the PAC is often used as part of the routine monitoring and thus the excess risk associated with this sub-study is considered to be minimal. The repeated monitoring is associated with minimal risk and the volume of isotonic glucose infused to measure cardiac output is approximately 50 ml per assessment and is not associated with risk of hypervolemia in adults. Echocardiography is part if the routine non-invasive assessment of the patients and is not associated with risks or discomfort to the patients. The study is enrolling at two tertiary heart centers with experience in conducting clinical trials of OHCA-patients. Good clinical practice (GCP) is followed and the study is monitored by trained GCP-staff.
Information about potential and enrolled participants will be collected and maintained under confidentiality. Data will be stored in REDcap,34 a secure, electronic case report form, with access restricted to selected study-researchers. All access to the eCRF will be electronically logged and requires a two-factor authentication process.
Sample size
MAP-intervention: The sample size estimate was based on 12-hour cardiac index findings from patients undergoing TTM at 33 °C compared with 36 °C.13 With a mean cardiac index of 2.1 L/min per m2 (standard deviation of 0.7), α = 0.05, and β = 0.90, a sample size of 514 patients allowed for detecting of a 0.2 L/min per m2 between groups, which we considered the minimal clinically important difference. The main trial plans inclusion of 800 patients, and previous trials of right ventricular catheterization in OHCA-patients have showed about 80 % inclusion rate with successful measurements from PACs,14 thus expected inclusion is 640 patients in this substudy (Fig. 1). The analyses will be performed in all patients with at least one successful PAC-measurement.
Oxygenation-intervention: The sample size estimate was based on 12-hour mPAP from patients undergoing TTM at 33 °C compared with 36 °C.13 With a mPAP of 23 mmHg (standard deviation of 5), α = 0.05, and β = 0.90, a sample size of 262 (131 in each group) patients allowed for detecting of a 2 mmHg difference between groups, which we considered the minimal clinically important difference.
Statistical analysis plan
Descriptive information and patient characteristics will be presented, with potential differences between the two groups described with an appropriate significance test. Analyses for the coprimary hypotheses, the difference in advanced hemodynamics between allocated intervention-groups, will be evaluated with repeated-measures mixed models with an unstructured covariance structure with blood pressure-group, time point, and the interaction term of blood pressure-group with time, if significant, as fixed effects. Differences at each time point will be derived from the mixed models including the interaction term of intervention-group with time if significant or by standard t test and Wilcoxon rank-sum test as appropriate.
The hypotheses relating associations between advanced hemodynamic variables and outcome will be assessed with chi-squared test and Cox- and logistic-regression. The multivariate regression models will be adjusted for age, sex, time to ROSC, witnessed arrest, cardiopulmonary resuscitation by bystander, shockable primary rhythm, lactate level at admission, randomization group (blood pressure and oxygenation). Survivors and non-survivors will be compared as regards to their cardiac output during post-resuscitation care with repeated measures mixed models. Missing data will be reported. In case of more than 5% missing data in outcome variables, multiple imputation with creation of 50 imputed datasets will be analyzed individually and aggregated into an estimate of the intervention’s effect on the outcome.35 For non-fatal events, competing risk of events will be accounted for.
Discussion
Despite intensive research, the mortality of patients remaining comatose after resuscitation from OHCA have remained around 50% during the last two decades. In the search for improving outcome and optimizing ICU-treatment, many clinical studies have been performed, but so far limited evidence for treating post-OHCA patients exist.3 A central challenge in post-resuscitation care is the dysfunctional cardiovascular system. Post-resuscitation myocardial dysfunction and low cardiac output are often present in post-cardiac arrest patients12., 14., 36., 37. thus vasopressors and inotropic drugs are frequently used to “normalize” hemodynamic variables.3., 38., 39. The use of these drugs has been sparsely investigated in clinical studies, and so far no certain evidence supports the hypothesis, that these drugs actually improves clinical relevant outcomes.3., 14. Cerebral oxygen-delivery is central post-OHCA and due to impaired cerebral autoregulation,9., 11. systemic MAP and oxygenation may be especially important compared to other ICU-patient-groups. A MAP between 87 and 101 mmHg during post-resuscitation intensive care was associated with better cerebral oxygenation values in one study40 and the hypothesis has been proposed, that a higher MAP-target during post-resuscitation care can improve organ perfusion and ultimately outcome.41 Two pilot-studies of higher MAP-targets after OHCA did not show signs of benefit on surrogate outcomes,27., 28. however a pooled post-hoc subgroup analysis from the studies, found lower biomarkers of myocardial injury42 and another post-hoc analysis suggested lower levels of neurofilament light chain, which is a marker of brain injury.43 A recent experimental study compared a MAP-target of 60 mmHg with 90 mmHg in 41 post-OHCA pigs.44 The higher target increased brain-microperfusion and cleared more brain carbon dioxide. However, microdialysis findings were similar between the groups, and this suggest no alleviation of cerebral ischemia.45
In the BOX-trial, the allocated higher MAP-group will receive larger doses of norepinephrine compared to the lower MAP-group and accordingly this study will be the first large clinical trial of different vasopressor doses after cardiac arrest. Theoretically, norepinephrine increases blood pressure in multiple ways46 affecting heart rate, preload, contractility and afterload. As norepinephrine minimally affects heart rate, most of the effect on cardiac output is related to its effects on stroke volume, which is attributed to increased right ventricular preload through venoconstriction by stimulating alpha-1 receptors and beta-2 receptors.47 However, this effect seem only present in presence of preload dependency such as hypovolemia.47 Secondly, norepinephrine increases cardiac contractility through a direct stimulating effect on the beta-1 receptor in the cardiomyocytes.48 This lead to increased ventricular ejection fraction and stroke volume but also increased myocardial-oxygen consumption.49 Third, alpha-1 receptors in arterioles are stimulated resulting in arterial vasoconstriction and a direct increase in SVR and blood pressure.46 Intuitively, increased left-ventricular afterload will decrease cardiac output, and thus the effect of norepinephrine on cardiac output may be unpredictable and dependent of preload and left ventricular ejection fraction. Most likely, subgroups of OHCA-patients with differences in their hemodynamic phenotype at baseline, may respond differently. Previous studies of central hemodynamics after OHCA have been small and thus subgroup analysis difficult.14 With the current substudy, we hope to have a large sample with possibilities of defining clinically meaningful subgroups. Adequate cardiovascular function is characterized not only by adequate blood pressure, but also adequate blood flow, in where cardiac output may be a potential target for improving outcomes. Whilst hypotension has been associated with poor outcome in several observational studies, the association of low cardiac output and outcome has been investigated in few studies.14., 50. A significant limitation to these few studies is the monitoring of cardiac output in selected patients from large registers and retrospective study designs.12., 36., 51. Since the main cause of death in comatose OHCA-patients is brain injury, measures to improve brain perfusion after OHCA is a hypothetical way of improving outcome. At least one other substudy of the BOX-trial is planned and protocol is published.52
Conclusion
This study will be one of the largest clinical, prospective studies of advanced hemodynamics measured by serial right heart catheterizations in consecutive comatose patients, resuscitated after OHCA. It will address the effect of a higher MAP-target and consequently higher vasopressor-doses on advanced hemodynamics in comatose patients resuscitated from OHCA. The randomized and placebo-controlled trial design minimizes risk of selection bias and confounders. Also, the impact of two oxygenations-targets on the pulmonary circulation will be investigated and addition to.
Funding
This work is supported by grants from the research fund “Gangstedfonden” and the Research fund of Rigshospitalet has supported this study with unrestricted salary in Dr. Grand’s PhD project. The Novo Nordisk Foundation supported the main trial: NNF17OC0028706.
Role of the sponsor
The sponsors had no involvement in the study design, in the collection of data, or in the forthcoming analyses and interpretation of data, writings of manuscript of manuscripts or in the decisions to submit manuscripts for publication. The sponsors are all funds with the purpose to promote research and do not have any commercial interests.
Name and Contact information for the trial sponsor
Jesper Kjærgaard, consultant, MD, PhD, DMSc Department of Cardiology, The Heart Centre, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark. Jesper.kjaergaard.05@regionh.dk.
Access to data and data sharing
The steering group and a selected number of researchers appointed by the steering group will have access to the final trial dataset. On request and based on approval from relevant authorities, any data required to support the protocol can be supplied. At the time of protocol-publication, there are no plans on making patient-level data publicly available.
Dissemination
All results, positive or neutral, will be published in international, peer-reviewed journals and presented at international congresses. Coauthorship will be granted in accordance with the Vancouver guidelines.
CRediT authorship contribution statement
Johannes Grand: Conceptualization, Methodology, Writing – review & editing, Visualization. Christian Hassager: Conceptualization, Methodology, Writing – review & editing, Visualization. Henrik Schmidt: Conceptualization, Methodology, Writing – review & editing, Visualization. Jacob E. Møller: Conceptualization, Methodology, Writing – review & editing, Visualization. Simon Mølstrøm: Conceptualization, Methodology, Writing – review & editing, Visualization. Benjamin Nyholm: Conceptualization, Methodology, Writing – review & editing, Visualization. Jesper Kjaergaard: Conceptualization, Methodology, Writing – review & editing, Visualization.
Declaration of Competing Interest
CH: Lecture honorarium from Abiomed. Research grands: Lundbeck Foundation (R186-2015-2132) and Novo Nordisk Foundation (NNF20OC0064043).
References
- 1.Atwood C., Eisenberg M.S., Herlitz J., Rea T.D. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation. 2005;67:75–80. doi: 10.1016/j.resuscitation.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Gräsner J.-T., Lefering R., Koster R.W., et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: A prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188–195. doi: 10.1016/j.resuscitation.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Nolan J.P., Sandroni C., Böttiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation. 2021;161:220–269. doi: 10.1016/j.resuscitation.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen N., Wetterslev J., Cronberg T., et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 5.Carr B.G., Kahn J.M., Merchant R.M., Kramer A.A., Neumar R.W. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80:30–34. doi: 10.1016/j.resuscitation.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Laver S., Farrow C., Turner D., Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 7.Nolan J.P., Neumar R.W., Adrie C., et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Bro-Jeppesen J., Johansson P.I., Hassager C., et al. Endothelial activation/injury and associations with severity of post-cardiac arrest syndrome and mortality after out-of-hospital cardiac arrest. Resuscitation. 2016;107:71–79. doi: 10.1016/j.resuscitation.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Sundgreen C., Larsen F.S., Herzog T.M., Knudsen G.M., Boesgaard S., Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128–132. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 10.Grand J., Lilja G., Kjaergaard J., et al. Arterial blood pressure during targeted temperature management after out-of-hospital cardiac arrest and association with brain injury and long-term cognitive function. Eur Heart J Acute Cardiovasc Care. 2019 doi: 10.1177/2048872619860804. [DOI] [PubMed] [Google Scholar]
- 11.Grand J., Wiberg S., Kjaergaard J., Wanscher M., Hassager C. Increasing mean arterial pressure or cardiac output in comatose out-of-hospital cardiac arrest patients undergoing targeted temperature management: Effects on cerebral tissue oxygenation and systemic hemodynamics. Resuscitation. 2021 doi: 10.1016/j.resuscitation.2021.08.037. S0300-9572(21)00338-5. [DOI] [PubMed] [Google Scholar]
- 12.Laurent I., Monchi M., Chiche J.-D., et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 13.Bro-Jeppesen J., Hassager C., Wanscher M., et al. Targeted temperature management at 33°C versus 36°C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: a sub-study of the Target Temperature Management Trial. Circ Cardiovasc Interv. 2014;7:663–672. doi: 10.1161/CIRCINTERVENTIONS.114.001556. [DOI] [PubMed] [Google Scholar]
- 14.Grand J., Kjaergaard J., Bro-Jeppesen J., et al. Cardiac output, heart rate and stroke volume during targeted temperature management after out-of-hospital cardiac arrest: Association with mortality and cause of death. Resuscitation. 2019;142:136–143. doi: 10.1016/j.resuscitation.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Rivers E., Nguyen B., Havstad S., et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 16.Howell M.D., Davis A.M. Management of Sepsis and Septic Shock. JAMA. 2017;317:847–848. doi: 10.1001/jama.2017.0131. [DOI] [PubMed] [Google Scholar]
- 17.Russo J.J., Di Santo P., Simard T., et al. Optimal mean arterial pressure in comatose survivors of out-of-hospital cardiac arrest: An analysis of area below blood pressure thresholds. Resuscitation. 2018;128:175–180. doi: 10.1016/j.resuscitation.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Grand J., Hassager C., Winther-Jensen M., et al. Mean arterial pressure during targeted temperature management and renal function after out-of-hospital cardiac arrest. J Crit Care. 2019;50:234–241. doi: 10.1016/j.jcrc.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Grand J., Hassager C., Skrifvars M.B., et al. Haemodynamics and vasopressor support during prolonged targeted temperature management for 48 hours after out-of-hospital cardiac arrest: a post hoc substudy of a randomised clinical trial. Eur Heart J Acute Cardiovasc Care. 2020 doi: 10.1177/2048872620934305. [DOI] [PubMed] [Google Scholar]
- 20.Bhate T.D., McDonald B., Sekhon M.S., Griesdale D.E.G. Association between blood pressure and outcomes in patients after cardiac arrest: A systematic review. Resuscitation. 2015;97:1–6. doi: 10.1016/j.resuscitation.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Laurikkala J., Wilkman E., Pettilä V., et al. Mean arterial pressure and vasopressor load after out-of-hospital cardiac arrest: Associations with one-year neurologic outcome. Resuscitation. 2016;105:116–122. doi: 10.1016/j.resuscitation.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Kilgannon J.H., Roberts B.W., Jones A.E., et al. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest*. Crit Care Med. 2014;42:2083–2091. doi: 10.1097/CCM.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 23.Grand J., Meyer A.S.P., Hassager C., Schmidt H., Møller J.E., Kjaergaard J. Validation and Clinical Evaluation of a Method for Double-Blinded Blood Pressure Target Investigation in Intensive Care Medicine. Crit Care Med. 2018;46:1626–1633. doi: 10.1097/CCM.0000000000003289. [DOI] [PubMed] [Google Scholar]
- 24.Grand J., Meyer A.S., Kjaergaard J., et al. A randomised double-blind pilot trial comparing a mean arterial pressure target of 65 mm Hg versus 72 mm Hg after out-of-hospital cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2020 doi: 10.1177/2048872619900095. [DOI] [PubMed] [Google Scholar]
- 25.Bergman R., Braber A., Adriaanse M.A., van Vugt R., Tjan D.H.T., van Zanten A.R.H. Haemodynamic consequences of mild therapeutic hypothermia after cardiac arrest. Eur J Anaesthesiol. 2010;27:383–387. doi: 10.1097/EJA.0b013e3283333a7d. [DOI] [PubMed] [Google Scholar]
- 26.Nordmark J., Johansson J., Sandberg D., et al. Assessment of intravascular volume by transthoracic echocardiography during therapeutic hypothermia and rewarming in cardiac arrest survivors. Resuscitation. 2009;80:1234–1239. doi: 10.1016/j.resuscitation.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Jakkula P., Pettilä V., Skrifvars M.B., et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: a randomised pilot trial. Intensive Care Med. 2018;44:2091–2101. doi: 10.1007/s00134-018-5446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ameloot K., De Deyne C., Ferdinande B., et al. Mean arterial pressure of 65 mm Hg versus 85–100 mm Hg in comatose survivors after cardiac arrest: Rationale and study design of the Neuroprotect post-cardiac arrest trial. Am Heart J. 2017;191:91–98. doi: 10.1016/j.ahj.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Chan A.-W., Tetzlaff J.M., Altman D.G., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monnet X., Persichini R., Ktari M., Jozwiak M., Richard C., Teboul J.-L. Precision of the transpulmonary thermodilution measurements. Crit Care Lond Engl. 2011;15:R204. doi: 10.1186/cc10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta Y., Arora D. Newer methods of cardiac output monitoring. World J Cardiol. 2014;6:1022–1029. doi: 10.4330/wjc.v6.i9.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A., Juneja R., Mehta Y., Trehan N. Comparison of continuous, stat, and intermittent cardiac output measurements in patients undergoing minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2002;16:186–190. doi: 10.1053/jcan.2002.31063. [DOI] [PubMed] [Google Scholar]
- 33.Fincke R., Hochman J.S., Lowe A.M., et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44:340–348. doi: 10.1016/j.jacc.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 34.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donders A.R.T., van der Heijden G.J.M.G., Stijnen T., Moons K.G.M. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Oksanen T., Skrifvars M., Wilkman E., Tierala I., Pettilä V., Varpula T. Postresuscitation hemodynamics during therapeutic hypothermia after out-of-hospital cardiac arrest with ventricular fibrillation: a retrospective study. Resuscitation. 2014;85:1018–1024. doi: 10.1016/j.resuscitation.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Bernard S.A., Gray T.W., Buist M.D., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 38.Bro-Jeppesen J., Annborn M., Hassager C., et al. Hemodynamics and vasopressor support during targeted temperature management at 33°C Versus 36°C after out-of-hospital cardiac arrest: a post hoc study of the target temperature management trial*. Crit Care Med. 2015;43:318–327. doi: 10.1097/CCM.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 39.Bro-Jeppesen J., Kjaergaard J., Wanscher M., et al. Systemic Inflammatory Response and Potential Prognostic Implications After Out-of-Hospital Cardiac Arrest: A Substudy of the Target Temperature Management Trial. Crit Care Med. 2015;43:1223–1232. doi: 10.1097/CCM.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 40.Ameloot K., Meex I., Genbrugge C., et al. Hemodynamic targets during therapeutic hypothermia after cardiac arrest: A prospective observational study. Resuscitation. 2015;91:56–62. doi: 10.1016/j.resuscitation.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Sekhon M.S., Griesdale D.E. Individualized perfusion targets in hypoxic ischemic brain injury after cardiac arrest. Crit Care Lond Engl. 2017;21:259. doi: 10.1186/s13054-017-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ameloot K., Jakkula P., Hästbacka J., et al. Optimum Blood Pressure in Patients With Shock After Acute Myocardial Infarction and Cardiac Arrest. J Am Coll Cardiol. 2020;76:812–824. doi: 10.1016/j.jacc.2020.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Wihersaari L., Ashton N.J., Reinikainen M., et al. Neurofilament light as an outcome predictor after cardiac arrest: a post hoc analysis of the COMACARE trial. Intensive Care Med. 2021;47:39–48. doi: 10.1007/s00134-020-06218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skåre C., Karlsen H., Strand-Amundsen R.J., et al. Cerebral perfusion and metabolism with mean arterial pressure 90 vs. 60 mmHg in a porcine post cardiac arrest model with and without targeted temperature management. Resuscitation. 2021;S0300–9572(21):00234–243. doi: 10.1016/j.resuscitation.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Mölström S., Nielsen T.H., Nordström C.H., et al. Bedside microdialysis for detection of early brain injury after out-of-hospital cardiac arrest. Sci Rep. 2021;11:15871. doi: 10.1038/s41598-021-95405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foulon P., De Backer D. The hemodynamic effects of norepinephrine: far more than an increase in blood pressure! Ann Transl Med. 2018;6(S25) doi: 10.21037/atm.2018.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monnet X., Jabot J., Maizel J., Richard C., Teboul J.-L. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med. 2011;39:689–694. doi: 10.1097/CCM.0b013e318206d2a3. [DOI] [PubMed] [Google Scholar]
- 48.Alhayek S., Preuss C.V. StatPearls Publishing; StatPearls, Treasure Island (FL): 2021. Beta 1 Receptors. [PubMed] [Google Scholar]
- 49.Hamzaoui O., Jozwiak M., Geffriaud T., et al. Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br J Anaesth. 2018;120:517–524. doi: 10.1016/j.bja.2017.11.065. [DOI] [PubMed] [Google Scholar]
- 50.Grand J., Bro-Jeppesen J., Hassager C., et al. Cardiac output during targeted temperature management and renal function after out-of-hospital cardiac arrest. J Crit Care. 2019;54:65–73. doi: 10.1016/j.jcrc.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Huang C.-H., Tsai M.-S., Ong H.N., et al. Association of hemodynamic variables with in-hospital mortality and favorable neurological outcomes in post-cardiac arrest care with targeted temperature management. Resuscitation. 2017;120:146–152. doi: 10.1016/j.resuscitation.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Mölström S., Nielsen T.H., Nordström C.H., et al. Design paper of the “Blood pressure targets in post-resuscitation care and bedside monitoring of cerebral energy state: a randomized clinical trial”. Trials. 2019;20:344. doi: 10.1186/s13063-019-3397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]