Abstract
The mucin2 (MUC2) mucus barrier acts as the first barrier that prevents direct contact between intestinal bacteria and colonic epithelial cells. Bacterial factors related to the MUC2 mucus barrier play important roles in the response to changes in dietary patterns, MUC2 mucus barrier dysfunction, contact stimulation with colonic epithelial cells, and mucosal and submucosal inflammation during the occurrence and development of ulcerative colitis (UC). In this review, these underlying mechanisms are summarized and updated, and related interventions for treating UC, such as dietary adjustment, exogenous repair of the mucus barrier, microbiota transplantation and targeted elimination of pathogenic bacteria, are suggested. Such interventions are likely to induce and maintain a long and stable remission period and reduce or even avoid the recurrence of UC. A better mechanistic understanding of the MUC2 mucus barrier and its related bacterial factors may help researchers and clinicians to develop novel approaches for treating UC.
Keywords: MUC2, Mucus barrier, Dysbiosis, Microbiota transplantation, Ulcerative colitis
Abbreviations: CAC, Colitis-associated cancer; CK, Cysteine knot; DSS, Dextransodiumsulfate; ER, Endoplasmic reticulum; FMT, Fecal microbiota transplantation; GF, Germ-free; IBD, Inflammatory bowel disease; MUC2, Mucin2; NHE3, Na+/H+ exchanger 3; ROS, Reactive oxygen species; SCFAs, Short-chain fatty acids; TLR, Toll-like receptor; TNBS, 2,4,6-trinitrobenzenesulfonic acid; UC, Ulcerative colitis; VWD, Von Willebrand D
1. Introduction
Ulcerative colitis (UC), a type of inflammatory bowel disease (IBD), is a chronic non-specific inflammatory disease that primarily involves the distal colonic mucosa and submucosa [1]. UC is characterized by alternating active and remission periods, and patients with persistent or repeatedly recurring UC are particularly at risk of colitis-associated cancer (CAC) [2]. At present, the main goal of UC treatment involves induction and maintenance of a long and stable remission, to decrease the incidence of relapses and avoid the development of CAC.
The pathogenesis of UC is multifactorial, involving genetic abnormalities, altered dietary patterns, intestinal barrier dysfunction, microbiota dysbiosis, and abnormal host immune reactions [3]. Currently, clinical treatment of UC mainly targets the immune response and proinflammatory factors, to induce remission [4,5]. Exploring effective interventions for other factors may facilitate researchers in developing new treatment methods. This review focuses on summarizing and analyzing the functions and molecular mechanisms of the mucin2 (MUC2) mucus barrier and its related bacterial factors during the occurrence and development of UC, to provide an overview of their interactions and explore more suitable intervention options for the treatment of UC.
2. Intestinal mucus barrier dominated by MUC2
The intestinal barrier includes the chemical barrier of the mucus layer, the mechanical barrier of the epithelial cell layer, and the immune barrier of the lamina propria [6]. In the colon, the mucus layer, which acts as the first barrier on the surface of the gastrointestinal tract, can effectively separate the intestinal epithelial cells from the intestinal lumen, to protect the epithelial cells from being contacted and stimulated by intestinal bacteria, their metabolites, or food antigens. The intestinal mucus layer consists of about 30 core proteins, including mucins, antimicrobial peptides, and secreted immunoglobulin A. Among them, MUC2, which is synthesized by intestinal goblet cells in the epithelial cell layer, is the most important component [7,8].
MUC2 is a gel-forming mucin that contains multiple domains, including N-terminal von Willebrand D1 (VWD1), VWD2, VWD'D3, PTS domains interspersed with CysD domains, and C-terminal VWD4, followed by VWB, VWC, and a cysteine-knot (CK) domain [9,10]. After synthesis, MUC2 dimerizes in the endoplasmic reticulum (ER) via disulfide bonds in the CK domain. The dimers then localize to the Golgi apparatus, where the PTS domains become O-glycosylated, and these dimers further polymerize via the N terminus by disulfide-bonded trimerization [10], [11], [12]. Next, the polymers are packed into MUC2 secretory granules [11]. Along the secretory pathway, the pH decreases gradually from 7•2 in the ER to 6•0 and 5•2 in the trans-Golgi network and secretory granules, respectively, and it has been revealed that low pH can promote the N-terminal aggregation of MUC2, contributing to the dense packing of MUC2 in secretory granules [10,12]. In addition, Ca2+-dependent cross-linking of negatively charged glycans present on the PTS domains can further stabilize the folding and packing of MUC2 polymers [12]. MUC2 secretory granules can be secreted via basic secretion and regulatory secretion [12]. Basic secretion involves continuous low-dose secretion of secretory granules, depending on the movement of the cytoskeleton, while regulatory secretion involves the compound exocytosis of MUC2 granules, which are stimulated by some active factors, such as microorganisms and microbial products [13,14].
After secretion, with rapid increase in pH and the removal of Ca2+, the MUC2 N- and C-terminal ends are unfolded, and MUC2 expands and forms a stratified mucus gel. At the surface of epithelial cells, Na+/H+ exchanger 3 (NHE3) contributes to an acidic mucosal milieu, and the acidic pH may play an important role in forming a compact inner mucus layer by maintaining the tight structure of the MUC2 N-terminal aggregates [15]. At the luminal side of the inner mucus layer, where the acidic milieu does not exist, the mucus is then converted to a more voluminous loose outer mucus layer [16]. Analysis by in situ hybridization using a general 16S rRNA probe and PCR of bacterial 16S rRNA genes demonstrated that bacteria are absent in the inner stratified mucus layer [14]. In a study conducted to determine the reason for the absence of bacteria, bacterium-sized 1μm beads could not penetrate the inner mucus layer under physiological conditions, suggesting that the inner stratified mucus layer, devoid of bacteria, acts as a physical barrier with minute pore sizes that physically block the entry of bacteria [14]. In addition, phosphatidylcholine, which is secreted in the ileum, can bind to MUC2 to help the latter serve as a hydrophobic barrier to repel bacteria in the aqueous lumen from the mucosal surface [17]. Moreover, to maintain homeostasis, MUC2 mucus is constantly renewed by secreted and expanded mucins to add layers underneath the existing mucus layer [16]. Because the mucus is renewed from the surface of the intestinal epithelial layer toward the outer mucus layer, the turnover and renewal of the inner mucus can further expel bacterial intruders from the epithelial cells to create a bacteria-restricted or bacteria-free region of inner dense mucus at the surface of the colonic epithelium [18].
Although the inner MUC2 mucus layer is extensively sterilized, all regions of this layer are not uniform in terms of permeability, and they are secreted by distinct goblet cell subtypes. Intestinal crypt-resident goblet cells secrete plume mucus to provide a protective barrier that can even restrict beads sized 0.2μm. The spatial regions between mucus plumes are filled with relatively penetrable mucus produced by the intercrypt goblet cells located on the colonic epithelial layer [19]. The intercrypt and crypt plume mucus together form a mixed, net-like structure with selective permeability, which allows the MUC2 mucus barrier to filter intestinal substances. Small nutrient molecules, such as ions and other compounds, can penetrate through the intercrypt mucus barrier and are absorbed by colonic epithelial cells, while the bacterial intruders are effectively blocked by both the intercrypt and crypt plume mucus [19].
3. The interaction between MUC2 mucin barrier and intestinal bacteria
Though the MUC2 mucus barrier acts as an important barrier that prevents intestinal bacteria from contacting colonic epithelial cells [14], it is now suggested that certain bacteria or their metabolites are needed for the establishment of complete MUC2 mucus structure and function [20], [21], [22]. The mucus barrier in the colon of sterile (germ-free, GF) mice is thin, and even absent locally [20,21]. Moreover, the mucus layer is penetrable to bacteria-sized beads [20]. In addition, in GF mice, the MUC2 glycans are also shorter than that of wild-type mice [22]. When GF mice are colonized with complex microbial communities, the mucus barrier of the colon can become impenetrable after about 5 weeks [20,21]. Furthermore, the composition of microbiota can also affect the characteristics of the mucus. For example, among mice with the same breeding conditions colonized with different microbiota, one colonizing microbiota induced a normal and impenetrable colon mucus layer, while the mucus induced by another colonizing microbiota was penetrable [23].
Intestinal bacteria and their metabolites may participate in the regulation of the intestinal mucus barrier by affecting the synthesis and secretion of MUC2 or regulating its glycosylation and other post-translational modifications [24], [25], [26]. In a previous study, during the colonization of GF mice with cecal microbiota from mice with well-developed impenetrable mucus, MUC2 glycosylation patterns changed, even before a change in inner mucus penetrability could be observed [20]. Moreover, upon colonization, the relative levels of glycosyltransferases involved in O-glycan formation changed considerably and were consistent with the levels of altered glycans [20]. Compared with that in GF mice, the levels of some of the glycosyltransferases involved in O-glycan elongation in conventionally raised mice were increased, and O-glycosyltransferase abundance markedly correlated with Muc2 O-glycan levels [22]. Elucidating the mechanisms through which bacteria promote a change in colonic mucus may facilitate their utilization to promote colonic mucus barrier recovery. In addition, it has been shown that in the colon of mice, MUC2 mucus near the intestinal cavity or in fecal pellets is mainly derived from the proximal colon, and the intestinal microbiota mainly induces the formation of mucus secreted by proximal colon goblet cells [27]. Thus, future studies should focus on the above-mentioned aspects.
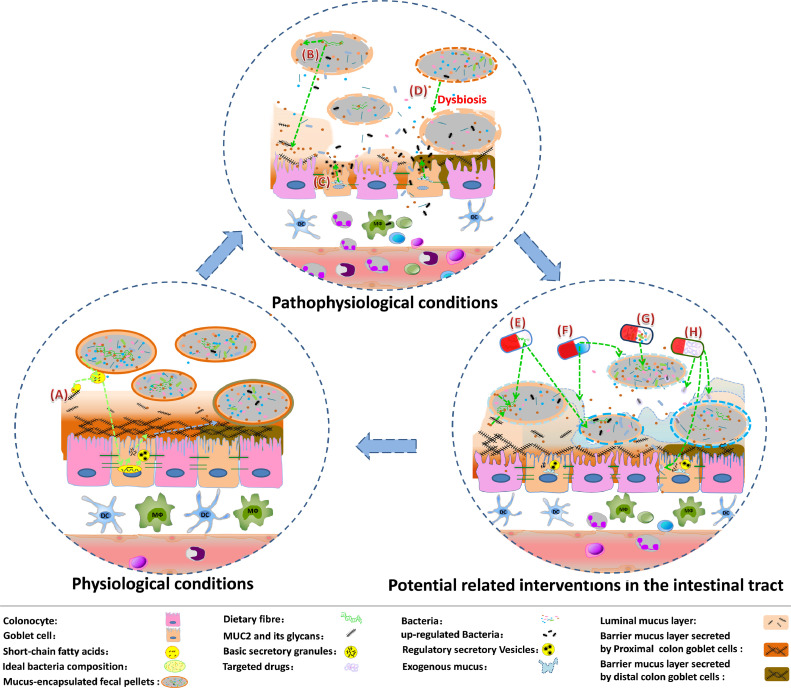
Intestinal bacteria not only take part in promoting and improving the MUC2 mucus barrier but are also closely related to its metabolic degradation. The glycan repertoire of MUC2 is a nutrient source that can be degraded by distinct mucosa-associated bacteria, such as Akkermansia muciniphila [28]. Under physiological conditions, the commensal intestinal microbiota is limited to the expanded MUC2 luminal mucus layer, where the constituent bacteria can enter and thrive by adhering and utilizing the MUC2 glycans as an energy source with the help of lectin-type adhesions and glycan-degrading enzymes [25,29]. The degradation of MUC2 glycans also exposes the MUC2 protein core and allows proteases to further degrade MUC2, to further promote the conversion of the inner mucus to the loose outer mucus [30]. Meanwhile, bacterial metabolites, such as short-chain fatty acids (SCFAs) [31], can in turn induce MUC2 transcription by binding with AP-1 or increasing the histone acetylation and methylation of the MUC2 promoter, to form a compensatory response [32]. In addition, SCFAs also regulate the expression of NHE3, which aids the formation of a dense inner mucus layer at the surface of epithelial cells by maintaining the acidic mucosal milieu [15]. Therefore, under physiological conditions, the metabolic degradation of MUC2 mucus by intestinal bacteria, the conversion of the inner mucus layer to the loose outer mucus layer and the continuous secretion of MUC2 mucin can form a dynamic balance, contributing to the maintenance and renewal of the MUC2 mucus barrier. Meanwhile, the two MUC2 mucus sub-layers, which comprise a dense sterile inner mucus layer at the surface of the colonic epithelium (barrier layer) and a less organized, soluble outer mucus layer (luminal mucus layer), are formed [14,20,27] (Fig. 1A).
Fig. 1.
MUC2 mucus barrier and its related bacterial factors under physiological conditions, pathophysiological conditions, and potential interventions in the intestinal tract for treating ulcerative colitis.
Recently, the understanding of the colonic mucus system has expanded. The inner mucus layer is recognized to be also in flux, and the mucus secreted by proximal colon goblet cells contributes extensively to forming a protective barrier in the distal colon [27]. In mice, the secreted mucus also detaches gradually and continuously encapsulates the passing fecal pellets [27,33]. Mucus encapsulation can even create numerous “bacteria-sparse” zones to further reduce the possibility of bacteria in the colon contacting the intestinal epithelium [27,34]. In addition, mucus encapsulation of fecal pellets provides lubrication, promotes the unhindered excretion of feces, and reduces contact between intestinal bacteria and the intestinal epithelium [35].
4. Excessive degradation of MUC2 polysaccharides by intestinal bacteria impairs the integrity of the MUC2 mucus barrier, and limited refilling of MUC2 granules induced by bacterial stimulation further aggravates MUC2 mucus barrier dysfunction
Under pathophysiological conditions, such as a long-term lack of food-derived polysaccharide substrates that can cause intestinal microbiota to increasingly degrade MUC2 polysaccharides (Fig. 1B), intestinal bacteria may consume an excess of MUC2 glycans, including those present in fecal pellet-encapsulating mucus and luminal mucus, and then increase the metabolic degradation of MUC2 mucus. Moreover, the inner mucus may be increasingly converted to the loose outer mucus layer. If the expression and secretion of MUC2 is not enough to compensate for its consumption, the inner layer of MUC2 mucus barrier would be gradually thinner and become penetrable [36], [37], [38]. At this time, intestinal bacteria can invade the mucus layer and even get in contact with the colonic epithelial cell layer.
At the opening of the colonic crypt, several bacterial components and their metabolites are endocytosed by the special goblet cells, known as “sentinel” goblet cells, which can bind to Toll-like receptor (TLR) 2/1, TLR4, and TLR5 ligands and activate TLR-/myeloid differentiation primary response 88-dependent Nox/Duox reactive oxygen species (ROS) synthesis, triggering the formation of the NLRP6 inflammasome and a Ca2+-dependent compound exocytosis of MUC2-containing granules [39,40]. Furthermore, the sentinel goblet cells coordinate and transmit the instruction of mucus secretion activation to adjacent goblet cells via intercellular gap junction Ca2+-dependent signaling [41]. The increased regulatory secretion results in a large amount of MUC2 being secreted, to help restore the mucus barrier and detach the bacteria from colonic epithelial cells [40,42].
However, under repeated or continuous bacterial stimulation, MUC2 granules are continuously secreted, following which the goblet cells are gradually emptied, forming small, thin goblet cells that are not easily identified [41,43]. There are not enough secretory particles stored in the goblet cells, and the regulatory secretion of sentinel goblet cells will no longer be able to remove the invading bacteria, causing the epithelial cells to be continuously stimulated by the bacteria. At this time, MUC2 expression in goblet cells will be continually upregulated, accompanied with partially synthesized or misfolded MUC2 accumulating in the ER, to induce ER stress and initiate the unfolded protein response [44]. The unfolded protein response can physiologically reduce the input of newly synthesized proteins into the ER, promote the correct folding of proteins, and degrade misfolded proteins, thereby relieving ER stress and protecting cells from damage [45,46]. Therefore, prolonged unfolded protein response stimulated by MUC2 upregulation eventually reduces the production of MUC2 granules (Fig. 1C), and the limited refilling of MUC2 granules in goblet cells further decreases the secretion of MUC2, aggravates MUC2 mucus barrier dysfunction, and increases the exposure of colonic epithelial cells to intestinal bacteria, triggering a vicious cycle and further disrupting the MUC2 mucus barrier.
5. Intestinal bacteria are important factors for the development of UC and dysbiosis further increases susceptibility to UC
When the colonic MUC2 mucus barrier is severely impaired, even normal intestinal bacteria can also stimulate the colonic epithelial cells to induce colitis and even CAC. It has been demonstrated that in the absence of other stimuli, the MUC2-deficient mice, whose colonic epithelium is no longer covered by a bacterium-free mucus layer, would gradually develop colitis over 7 weeks and colon cancer after 6–12 months [47], [48], [49]. In addition, it is currently suggested that intestinal bacteria are necessary for the development of UC. In multiple UC models, including chemically induced (dextran sodium sulfate (DSS), 2,4,6-trinitrobenzenesulfonic acid (TNBS)), lymphocyte transfer, and genetic (TCR-alpha-/-, IL-10-/-, IL-2-/-) models, intestinal inflammation is induced weakly or not at all in GF conditions [50], [51], [52], [53], [54], [55]. In addition, in the antibiotic-sterilized pseudo-GF mice, the DSS-induced inflammation is also clearly reduced [56]. These experiments have gradually demonstrated the role of gut microbiota in the occurrence and progression of the inflammation during UC, independent of genetic factors. Further analysis of these bacterial factors can potentially provide new ideas for exploring effective methods to treat the UC.
During the occurrence and development of UC, in responses to changes in dietary patterns, MUC2 mucus barrier dysfunction, contact stimulation with colonic epithelial cells, and mucosal and submucosal inflammation, the bacteria in the intestines are also constantly changing in the density, composition, and diversity, causing microbiota dysbiosis (Fig. 1D). Long-term dietary changes alter the composition of intestinal bacteria, while the change in diet-related flora is closely related to the intestinal mucus barrier. Only special subsets of intestinal microbiota species, such as Bacteroides thetaiotaomicron, Bifidobacterium bifidum, Ruminococcus gnavus, Ruminococcus torques and Akkermansia muciniphila, can utilize the glycans in the MUC2 mucus barrier as a nutrient source [57], [58], [59]. Chronic and intermittent dietary fiber deficiency will cause the mucin-utilizing bacteria to increasingly degrade the MUC2 mucus barrier. Meanwhile, the ability to degrade mucus as a substitute for nutrients also increases the competitive advantage of these bacteria. Transcriptomic analysis has shown that in mice fed a fiber-free diet, the transcription of genes encoding mucus-targeting enzymes increases, further suggesting an increase in the proportion of mucus-degrading bacteria [60,61]. The increase of mucus-degrading bacteria can further accelerate the degradation of MUC2 mucus, and trigger contact between bacteria and intestinal epithelial cells, inducing inflammation. In addition, related products of MUC2 mucus degradation can further assist the growth of some mucosa-associated bacteria, which alone have negligible mucin-degrading activity. Meanwhile, with the increased growth of mucin-degrading bacteria and their mucosa-associated bacterial consortium, some species of mucus-degrading bacteria such as Akkermansia muciniphila would eventually decrease in numbers, perhaps caused by mutual inhibition, further aggravating microbiota dysbiosis [62].
Besides the altered proportions of bacteria that are related to the degradation of MUC2 mucus barrier, the composition of intestinal bacteria also gets affected by other factors, such as the host inflammatory response [63]. Inflammation will increase the production of ROS or other oxidation by-products, which serve as electron acceptors to support the growth of facultative anaerobic bacteria by anaerobic respiration [64]. The resulting outgrowth of facultative anaerobic bacteria, accompanied with the relative decrease of exclusively anaerobic bacteria, further contribute to the microbiota dysbiosis.
Intestinal bacterial composition is also regulated by many other factors, such as mental stress, dietary salt and food additives [65], [66], [67], and the underlying mechanisms are being gradually revealed. Moreover, intestinal bacteria can also significantly affect susceptibility to colitis, possibly by changing the properties of the colonic mucus layer [23]. Diet-induced alteration of both luminal and mucosal microbiota communities in the distal colon occurs in parallel or even precedes the increase in mucus penetrability. Notably, a previous study has revealed that intestinal microbiota transplantation can significantly alleviate or prevent inner mucus layer dysfunction, further demonstrating a causal role of intestinal bacterial composition in MUC2 mucus barrier dysfunction [68]. Suitably modifying gut microbiota composition may help ameliorate mucus barrier dysfunction. Fecal microbiota transplantation (FMT) is a simple and effective procedure for modifying the gut microbiota. It has been demonstrated that compared with spontaneous recovery without intervention treatment, FMT can help in the rapid recovery of mucosal permeability caused by dysbiosis [69]. In addition, for experimentally induced UC, FMT has been shown to lead to improved intestinal barrier integrity and reduction in colonic inflammation [70]. Therefore, FMT can be used to alleviate, treat, or prevent the occurrence and development of UC. The ability to improve intestinal barrier integrity may be an important factor for effective FMT treatment [71]. In the future, detecting and analyzing a suitable treatment time and frequency of FMT or exploring more targeted treatments for dysbiosis may help to better guide the treatment of UC.
6. Detection of MUC2 mucus barrier dysfunction, intestinal bacterial invasion, and dysbiosis during UC development clinically and manipulation of the intestinal microbiota in the treatment of UC
The examination of colon specimens from UC patients indicated that MUC2 expression significantly decreased compared to that from healthy individuals [72]. The mucus layer was also revealed to become thinner and even absent in some parts, independent of local inflammation [73,74], and the inner mucus layer of patients with active UC is reported to be more penetrable [74,75]. Further analysis of UC patients’ mucus layer revealed that the proportion of polymerized mucin was significantly reduced and the mucin structure was also changed, manifested by reduced glycosylation, shortened oligosaccharide side chains, and reduced sulfation [76], which is likely to lead to mucus barrier dysfunction and consequently increase susceptibility to inflammation. These results demonstrate that the MUC2 mucus barrier shows abnormalities during the development of clinical UC. In addition, electron microscopy of the specimens further revealed that partially synthesized or misfolded MUC2 mucin accumulates in the ER, suggesting the increased ER stress of goblet cells in the clinical specimens [77]. Increased ER stress may lead to reduce the production of MUC2 granules and limit the refilling of MUC2 granules in goblet cells. In addition, in the tissue specimens obtained from active UC patients, a reduction in the detectable number of sentinel and intercrypt goblet cells has been reported. Further, a decrease in the number of intercrypt goblet cells has also been detected in the biopsied tissue samples obtained from UC patients in remission [19,74]. These goblet cells should be stimulated to almost empty their MUC2 particles, and have not be refilled. These changes of the goblet cells may also be an important factor for the decreased secretion of MUC2 and MUC2 mucus barrier dysfunction in the colons of UC patients, especially that in the active period.
MUC2 mucus barrier dysfunction permits intestinal bacteria to invade the mucosa. In normal mucosal tissues, negligible numbers of bacteria are present. However, the presence of mucosal bacteria was found in 83% of colonic specimens from UC patients [78], suggesting that bacterial invasion of the mucosa is involved in the occurrence or progression of UC. The bacteria that enter the intestinal mucosa are affected by the composition of bacteria in the intestinal tract. The analysis of clinical specimens from patients with UC also confirmed that qualitative and quantitative changes occurred in the composition of intestinal bacteria [79]. Research shows that fecal bacteria from UC patients can cause a stronger inflammatory response than those from healthy controls [80]. Therefore, changing the composition of bacteria in the intestinal tract may also help to regulate inflammation. In the clinical treatment of UC, manipulation of the intestinal microbiota has already been applied.
FMT is a relatively simple intervention procedure that involves the transfer of the full spectrum of enteric microbiota from a healthy donor into a recipient's intestine. In recent years, FMT has been increasingly used for the treatment of clinical UC, and various studies have attempted to evaluate the efficacy and safety of FMT, mostly for treating active UC [81]. Until now, five randomized clinical trials of FMT have been reported (Table 1) [82], [83], [84], [85], [86]. For active UC, although the stool suspensions for patients in different trials were prepared differently and administered at different doses and frequencies, the therapeutic effect of FMT, when administered via the lower gastrointestinal tract, was mostly promising, with a similar remission rate of approximately 30% [87]. Pilot studies suggest that the predictors of responses to FMT might include younger age, shorter disease duration, smaller extent of disease, smaller endoscopic Mayo score, some concomitant treatments, and more suitable donors [83,[88], [89], [90]]. It was revealed that, after FMT treatment, the microbiota composition of responders usually shifted and became more similar to that of healthy donors [82,83]. During the FMT-induced remission period, fecal microbial composition was found to be unstable, and over a year, the similarity to the original composition of fecal microbiota would steadily decrease [85,91]. Therefore, appropriate additional FMT at suitable time points may be helpful for maintaining clinical remission of UC [85,86]. In a study by Sood et al. [86], patients with UC in clinical remission were treated by multi-session FMT, and it was found that this type of FMT can help to sustain or further enhance clinical, endoscopic, and histological remission in UC patients, with fewer relapses [86].
Table 1.
Outcome of fecal microbial transplantation for ulcerative colitis in randomised controlled trials.
| Refs. | No. of cases (FMT: Controls) | Periods of UC patients | Route of administration and duration of treatment | Clinical remission | Endoscopic remission |
|---|---|---|---|---|---|
| Rossen et al. [82] | 23 versus 25 | Active | Nasoduodenal infusions twice (at start and 3 weeks later) | 26•1% versus 32•0% (6 weeks) | – |
| Moayyedi et al. [83] | 38 versus 37 | Active | Enemas once weekly for 6 weeks | 24% versus 5% (7 weeks) | 24% versus 5% (7 weeks) |
| Paramsothy et al. [84] | 41 versus 40 | Active | Colonoscopic infusion followed by enemas 5 days per week for 8 weeks | 44% versus 20% (8 weeks) | 12% versus 8% (8 weeks) |
| Costello et al. [85] | 38 versus 35 | Active | Colonoscopic infusion followed by 2 enemas over 7 days | 47% versus 17% (8 weeks) | 11% versus 0% (8 weeks) |
| Sood et al. [86] | 31 versus 30 | Remission | Colonoscopic infusion every 8 weeks for 48 weeks | 87•1% versus 66•7% (48 weeks) | 58•1% versus 26•7% (48 weeks) |
FMT can induce clinical remission and even endoscopic improvement, possibly because the transplantation of fecal microbiota derived from a healthy donor restores some functions of normal intestinal microbiota, such as Akkermansia muciniphila and Bacteroides thetaiotaomicron [90,92]. These bacteria may help in the recovery of MUC2 mucus barrier integrity by inducing the expression and secretion of MUC2 [27]. However, the rate of remission following FMT remains unsatisfactory. In addition, patients treated with FMT may experience serious adverse events, such as worsening colitis, septic shock, toxic megacolon, and mortality [85,93]. These events may be related to increased bacterial loads after FMT, especially in patients with active UC, whose mucus barrier function is disrupted. Additional studies are needed to identify beneficial bacteria, and microbiota transplantation with low bacterial loads can further help expand the application of intestinal bacterial treatments [68,90].
7. Conclusion
Dysfunction of MUC2 mucus barrier and its related bacteria factors play important roles during the occurrence and development of UC. Suitable interventions in the active and remission periods of UC may become conventional, effective treatments in the future, to induce and maintain a long and stable remission period and reduce or even avoid the recurrence of UC.
8. Outstanding questions
The prevalence of UC is particularly high in industrialized countries. In newly-industrialized regions where people's diet has gradually changed to a Western-style diet, the incidence of UC is also steadily increasing. At present, many well-designed researches have gradually demonstrated that the MUC2 mucus barrier and its related bacteria factors play important roles during the occurrence and development of UC. Based on the analysis of the related mechanisms, some potential therapies can be attended or maybe further improved, including:
-
1
A lack of food-derived polysaccharides from dietary fiber is an important factor that increases the consumption of the MUC2 mucus barrier by intestinal bacteria, and the diet-induced consumption of the MUC2 mucus barrier will contribute to increased susceptibility of the occurrence or recurrence of UC. Thus, in the remission period, increased intake of dietary fiber is likely to reduce consumption of the MUC2 mucus barrier by the microbiota, thus promoting and improving the recovery of intestinal mucosal barrier function and prolonging remission (Fig. 1E). However, in active UC, some interventions may have adverse impacts because the effects of dietary fiber on the MUC2 mucus barrier vary according to the degree of microbiota dysbiosis. Future studies may further reveal intestinal bacteria that consume specific dietary fibers as well as the associated metabolic enzymes. For the treatment of UC, highly purified dietary fibers may be used.
-
2
In the active period of UC, goblet cells are mostly stimulated to secrete excessive MUC2, accompanied with increased ER pressure. Further stimulation of intestinal cells to increase the transcription, expression, and secretion of MUC2, is likely to increase the ER pressure of goblet cells. At this time, exogenous mucin with suitable glycosylation, which contributes to the formation of loose mucus, can be tentatively applied. Exogenous mucus can appropriately increase the luminal mucus barrier of MUC2, to reduce the degradation of endogenous MUC2 mucus on the intestinal mucosal surface, and promote the recovery of intestinal goblet cells (Fig. 1F). Although the exogenous mucus might trap bacteria, it can also help encapsulate fecal pellets, reduce the contact of bacteria in fecal pellets with the colonic epithelial cells, and create some “bacteria-sparse” zones. Moreover, with the recovery of the MUC2 mucus barrier, the directed renewal and transport of secreted MUC2 will further eliminate bacteria near the intestinal epithelial mucosa. In the remission period of UC, when the MUC2 mucus barrier has gradually recovered, the application of mucus-associated proteins, such as trefoil factor 3, can also be attempted. These proteins can help increase the stability of the intestinal mucus barrier. In addition, topical application of phosphatidylcholine, which can be loaded onto MUC2, could also be helpful in protecting epithelial cells from being contacted and stimulated by intestinal bacteria and their metabolites. In the future, such biosynthetic materials may be extensively used in the clinical treatment of UC.
-
3
Microbiota transplantation can promote changes in intestinal bacterial composition, recovering the ideal composition (Fig. 1G). Active UC patients with mild clinical manifestations or a low endoscopic Mayo score are more sensitive to FMT than other active UC patients, possibly due to the presence of more goblet cells with MUC2 particles, better MUC2 mucus barrier function, and healthier gut bacterial composition, which could contribute to rapid recovery. For patients in the remission period, when the MUC2 mucus barrier is restored or partially restored, microbiota transplantation may be more suitable to maintain a prolonged and stable remission period or even avoid recurrence. More basic and clinical research is still needed to identify which bacteria are beneficial, and treatments with more beneficial bacteria would further reduce the related treatment risks, expanding the application of intestinal bacterial treatment. In addition, when intestinal bacterial treatment is performed, the bacterial flora whose abundance is increased during dysbiosis could be simultaneously targeted for inhibition (Fig. 1H) to help restore an ideal bacterial composition.
-
4
The above-mentioned treatments all aim at the intestinal tract, and the oral capsule-delivered shells that target colonic release have also been well developed. In the future, treatment with commercially available oral capsules containing optimal contents may become conventional, effective treatments for UC.
9. Search strategy and selection criteria
References for this review were identified through searches of PubMed to identify relevant English-language papers published between Jan 1, 1980 and Jan 31, 2021. The search term “MUC2” was used in combination with the “AND” operator for the terms “mucus barrier”, “microbiota”, “dysbiosis”, and “ulcerative colitis”. The final reference list was generated on the basis of originality and relevance to the broad scope of this review, with a focus on the most recently published papers.
Contributors
YD, DW, DM and WS designed the overall study; YD, DW, DM and DC collected information, and prepared figures; YD and DW performed a systematic literature search, and drafted manuscript; YD, DW, DM, DC and WS revised the manuscript critically and approved final version of manuscript.
The funders had no role in paper design, data collection, data analysis, interpretation or writing of the paper.
Mucosa-associated bacteria degrade the MUC2 mucus, while short-chain fatty acids (SCFAs) are simultaneously produced. SCFAs can in turn induce the transcription of MUC2. The metabolic degradation of MUC2 mucus and the secretion of MUC2 form a dynamic balance, contributing to the formation of MUC2 mucus barrier sub-layers, luminal mucus layer and barrier layer. The mucus barrier layer secreted by proximal and distal colon goblet cells is not the same, with different O-glycosylation. The mucus secreted by proximal colon goblet cells can also spread (blue arrow), and contribute to the formation of the protective barrier in the distal colon. The secreted mucus can also be detached and encapsulate the fecal pellets, to create numbers of “bacteria-sparse” zones. (B) Lack of food-derived polysaccharide substrates in the intestinal tract will increase the bacterial degradation of MUC2 luminal and barrier mucus, and even damage the MUC2 mucus barrier. (C) Bacterial components and their metabolites trigger massive regulatory secretion of MUC2 granules, and increased endoplasmic reticulum stress can further reduce the production of the MUC2 granules. (D) Altered density, composition and diversity of intestinal bacteria result in microbiota dysbiosis. (E) Increased dietary fiber could reduce the consumption of the MUC2 mucus barrier by mucin-degrading bacteria and their mucosa-associated bacterial consortium. (F) Exogenous mucus may be helpful in resealing the barrier function and reducing the stimulation of intestinal epithelial cells by intestinal bacteria. (G) Microbiota transplantation can promote the change of intestinal bacteria, to recover the ideal composition of intestinal microbiota. (H) Highly pathogenic bacteria should be treated as soon as possible.
Declaration of Competing Interest
The authors confirm that there are no conflicts of interest.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81400659), the Scientific Research Funding Project of Liaoning Educational Committee (QN2019017), and the Shenyang Youth Science and Technology Project (RC190495).
References
- 1.Ungaro R., Mehandru S., Allen P.B., Peyrin-Biroulet L., Colombel J.F. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olén O., Erichsen R., Sachs M.C., et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123–131. doi: 10.1016/S0140-6736(19)32545-0. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T., Siegmund B., Le Berre C., et al. Ulcerative colitis. Nat Rev Dis Prim. 2020;6:74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka K., Kobayashi T., Ueno F., et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353. doi: 10.1007/s00535-018-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese S., Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez de Medina F., Romero-Calvo I., Mascaraque C., Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis. 2014;20:2394–2404. doi: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 7.Vancamelbeke M., Vanuytsel T., Farré R., et al. Genetic and transcriptomic bases of intestinal epithelial barrier dysfunction in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1718–1729. doi: 10.1097/MIB.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergstrom K., Liu X., Zhao Y., et al. Defective intestinal mucin-type o-glycosylation causes spontaneous colitis-associated cancer in mice. Gastroenterology. 2016;151:152–164. doi: 10.1053/j.gastro.2016.03.039. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gum J.R., Hicks J.W., Toribara N.W., et al. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 10.Javitt G., Khmelnitsky L., Albert L., et al. Assembly mechanism of mucin and von Willebrand factor polymers. Cell. 2020;183:717–729. doi: 10.1016/j.cell.2020.09.021. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson M.E., Ambort D., Pelaseyed T., et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambort D., Johansson M.E., Gustafsson J.K., et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson M.E., Sjövall H., Hansson G.C. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison C.A., Laubitz D., Ohland C.L., et al. Microbial dysbiosis associated with impaired intestinal Na+/H+ exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol. 2018;11:1329–1341. doi: 10.1038/s41385-018-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson M.E. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS ONE. 2012;7:e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stremmel W., Vural H., Evliyaoglu O., Weiskirchen R. Delayed-release phosphatidylcholine is effective for treatment of ulcerative colitis: a meta-analysis. Dig Dis. 2021;39:508–515. doi: 10.1159/000514355. [DOI] [PubMed] [Google Scholar]
- 18.Johansson M.E., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyström E.E.L., Martinez-Abad B., Arike L., et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science. 2021;372:eabb1590. doi: 10.1126/science.abb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson M.E., Jakobsson H.E., Holmén-Larsson J., et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18:582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersson J., Schreiber O., Hansson G.C., et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arike L., Holmén-Larsson J., Hansson G.C. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology. 2017;27:318–328. doi: 10.1093/glycob/cww134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakobsson H.E., Rodríguez-Piñeiro A.M., Schütte A., et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16:164–177. doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J., Li Y., Wan Y., et al. A novel postbiotic from lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol. 2019;10:477. doi: 10.3389/fmicb.2019.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder B.O. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep. 2019;7:3–12. doi: 10.1093/gastro/goy052. Oxf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrzosek L., Miquel S., Noordine M.L., et al. Bacteroides thetaiotaomicron and faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergstrom K., Shan X., Casero D., et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science. 2020;370:467–472. doi: 10.1126/science.aay7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derrien M., Vaughan E.E., Plugge C.M., de Vos W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 29.Juge N. Microbial adhesins to gastrointestinal mucus. Trends Microbiol. 2012;20:30–39. doi: 10.1016/j.tim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Li H., Limenitakis J.P., Fuhrer T., et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J., Bjursell M.K., Himrod J., et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 32.Burger-van Paassen N., Vincent A., Puiman P.J., et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 33.Kamphuis J.B.J., Mercier-Bonin M., Eutamène H., Theodorou V. Mucus organisation is shaped by colonic content; a new view. Sci Rep. 2017;7:8527. doi: 10.1038/s41598-017-08938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birchenough G.M.H., Johansson M.E.V. Forming a mucus barrier along the colon. Science. 2020;370:402–403. doi: 10.1126/science.abe7194. [DOI] [PubMed] [Google Scholar]
- 35.Swidsinski A., Loening-Baucke V., Verstraelen H., Osowska S., Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568–579. doi: 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Fu J., Wei B., Wen T., et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia L. Core 3-derived O-glycans are essential for intestinal mucus barrier function. Methods Enzymol. 2010;479:123–141. doi: 10.1016/S0076-6879(10)79007-8. [DOI] [PubMed] [Google Scholar]
- 38.Bergstrom K., Fu J., Johansson M.E., et al. Core 1- and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol. 2017;10:91–103. doi: 10.1038/mi.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wlodarska M., Thaiss C.A., Nowarski R., et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birchenough G.M., Nyström E.E., Johansson M.E., Hansson G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352:1535–1542. doi: 10.1126/science.aaf7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Specian R.D., Neutra M.R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol. 1980;85:626–640. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornick S., Kumar M., Moreau F., Gaisano H., Chadee K. VAMP8-mediated MUC2 mucin exocytosis from colonic goblet cells maintains innate intestinal homeostasis. Nat Commun. 2019;10:4306. doi: 10.1038/s41467-019-11811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson M.E. Mucus layers in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2124–2131. doi: 10.1097/MIB.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 44.Tawiah A., Cornick S., Moreau F., et al. High MUC2 mucin expression and misfolding induce cellular stress, reactive oxygen production, and apoptosis in goblet cells. Am J Pathol. 2018;188:1354–1373. doi: 10.1016/j.ajpath.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Satpute-Krishnan P., Ajinkya M., Bhat S., Itakura E., Hegde R.S., Lippincott-Schwartz J. ER stress-induced clearance of misfolded GPI-anchored proteins via the secretory pathway. Cell. 2014;158:522–533. doi: 10.1016/j.cell.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denic V., Quan E.M., Weissman J.S. A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell. 2006;126:349–359. doi: 10.1016/j.cell.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 47.Wenzel U.A., Magnusson M.K., Rydström A., et al. Spontaneous colitis in Muc2-deficient mice reflects clinical and cellular features of active ulcerative colitis. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van der Sluis M., De Koning B.A., De Bruijn A.C., et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Wu M., Wu Y., Li J., Bao Y., Guo Y., Yang W. The dynamic changes of gut microbiota in Muc2 deficient mice. Int J Mol Sci. 2018;19:2809. doi: 10.3390/ijms19092809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Q., Wang L., Wang F., Li Q. Role of gut microbiota in a zebrafish model with chemically induced enterocolitis involving toll-like receptor signaling pathways. Zebrafish. 2014;11:255–264. doi: 10.1089/zeb.2013.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemoto Y., Kanai T., Kameyama K., et al. Long-lived colitogenic CD4+ memory T cells residing outside the intestine participate in the perpetuation of chronic colitis. J Immunol. 2009;183:5059–5068. doi: 10.4049/jimmunol.0803684. [DOI] [PubMed] [Google Scholar]
- 52.Veltkamp C., Tonkonogy S.L., De Jong Y.P., et al. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg(epsilon26) mice. Gastroenterology. 2001;120:900–913. doi: 10.1053/gast.2001.22547. [DOI] [PubMed] [Google Scholar]
- 53.Dianda L., Hanby A.M., Wright N.A., Sebesteny A., Hayday A.C., Owen M.J. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 54.Sellon R.K., Tonkonogy S., Schultz M., et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waidmann M., Allemand Y., Lehmann J., et al. Microflora reactive IL-10 producing regulatory T cells are present in the colon of IL-2 deficient mice but lack efficacious inhibition of IFN-gamma and TNF-alpha production. Gut. 2002;50:170–179. doi: 10.1136/gut.50.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernández-Chirlaque C., Aranda C.J., Ocón B., et al. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J Crohns Colitis. 2016;10:1324–1335. doi: 10.1093/ecco-jcc/jjw096. [DOI] [PubMed] [Google Scholar]
- 57.Sonnenburg J.L., Xu J., Leip D.D., et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 58.Hoskins L.C., Agustines M., McKee W.B., Boulding E.T., Kriaris M., Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985;75:944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derrien M., Van Baarlen P., Hooiveld G., Norin E., Müller M., de Vos W.M. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader akkermansia muciniphila. Front Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desai M.S., Seekatz A.M., Koropatkin N.M., et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gamage H.K.A.H., Chong R.W.W., Bucio-Noble D., et al. Changes in dietary fiber intake in mice reveal associations between colonic mucin O-glycosylation and specific gut bacteria. Gut Microbes. 2020;12 doi: 10.1080/19490976.2020.1802209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Png C.W., Lindén S.K., Gilshenan K.S., et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 63.Albenberg L., Esipova T.V., Judge C.P., et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063. doi: 10.1053/j.gastro.2014.07.020. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winter S.E., Lopez C.A., Bäumler A.J. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013;14:319–327. doi: 10.1038/embor.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao X., Cao Q., Cheng Y., et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci USA. 2018;115:E2960–E2969. doi: 10.1073/pnas.1720696115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miranda P.M., De Palma G., Serkis V., et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome. 2018;6:57. doi: 10.1186/s40168-018-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chassaing B., Koren O., Goodrich J.K., et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schroeder B.O., Birchenough G.M.H., Ståhlman M., et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23:27–40. doi: 10.1016/j.chom.2017.11.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li M., Liang P., Li Z., et al. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front Microbiol. 2015;6:692. doi: 10.3389/fmicb.2015.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L., Ma X., Liu P., et al. Treatment and mechanism of fecal microbiota transplantation in mice with experimentally induced ulcerative colitis. Exp Biol Med (Maywood) 2021;246:1563–1575. doi: 10.1177/15353702211006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Y., Li X., Yu H., et al. The functional role of fecal microbiota transplantation on dextran sulfate sodium-induced colitis in mice. Front Cell Infect Microbiol. 2019;9:393. doi: 10.3389/fcimb.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wibowo A.A., Pardjianto B., Sumitro S.B., Kania N., Handono K. Decreased expression of MUC2 due to a decrease in the expression of lectins and apoptotic defects in colitis patients. Biochem Biophys Rep. 2019;19 doi: 10.1016/j.bbrep.2019.100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swidsinski A., Loening-Baucke V., Theissig F., et al. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Post S., Jabbar K.S., Birchenough G., et al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68:2142–2151. doi: 10.1136/gutjnl-2018-317571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansson M.E., Gustafsson J.K., Holmén-Larsson J., et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsson J.M., Karlsson H., Crespo J.G., et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis. 2011;17:2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- 77.Heazlewood C.K., Cook M.C., Eri R., et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kleessen B., Kroesen A.J., Buhr H.J., Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034–1041. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
- 79.Andoh A., Imaeda H., Aomatsu T., et al. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn's disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol. 2011;46:479–486. doi: 10.1007/s00535-010-0368-4. [DOI] [PubMed] [Google Scholar]
- 80.Wang S., Yao L., Liu Y. Fecal microbiome from patients with ulcerative colitis is potent to induce inflammatory responses. Int Immunopharmacol. 2018;59:361–368. doi: 10.1016/j.intimp.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 81.Liu X., Li Y., Wu K., Shi Y., Chen M. Fecal microbiota transplantation as therapy for treatment of active ulcerative colitis: a systematic review and meta-analysis. Gastroenterol Res Pract. 2021;2021 doi: 10.1155/2021/6612970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossen N.G., Fuentes S., van der Spek M.J., et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118. doi: 10.1053/j.gastro.2015.03.045. e4. [DOI] [PubMed] [Google Scholar]
- 83.Moayyedi P., Surette M.G., Kim P.T., et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. e6. [DOI] [PubMed] [Google Scholar]
- 84.Paramsothy S., Kamm M.A., Kaakoush N.O., et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 85.Costello S.P., Hughes P.A., Waters O., et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sood A., Mahajan R., Singh A., et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. 2019;13:1311–1317. doi: 10.1093/ecco-jcc/jjz060. [DOI] [PubMed] [Google Scholar]
- 87.Fuentes S., Rossen N.G., van der Spek M.J., et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017;11:1877–1889. doi: 10.1038/ismej.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sood A., Singh A., Mahajan R., et al. Clinical predictors of response to faecal microbiota transplantation in patients with active ulcerative colitis. J Crohns Colitis. 2020:jjaa163. doi: 10.1093/ecco-jcc/jjaa163. Epub ahead of print. PMID: 32772093. [DOI] [PubMed] [Google Scholar]
- 89.Vermeire S., Joossens M., Verbeke K., et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis. 2016;10:387–394. doi: 10.1093/ecco-jcc/jjv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kump P., Wurm P., Gröchenig H.P., et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol Ther. 2018;47:67–77. doi: 10.1111/apt.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez C., Antolin M., Santos J., et al. Unstable composition of the fecal microbiota in ulcerative colitis during clinical remission. Am J Gastroenterol. 2008;103:643–648. doi: 10.1111/j.1572-0241.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 92.Nomura K., Ishikawa D., Okahara K., et al. Bacteroidetes species are correlated with disease activity in ulcerative colitis. J Clin Med. 2021;10:1749. doi: 10.3390/jcm10081749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Imdad A., Nicholson M.R., Tanner-Smith E.E., et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11 doi: 10.1002/14651858.CD012774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]