Abstract
Plasmalogens are a class of phospholipids containing vinyl ether linked aliphatic groups at the sn-1 position. Plasmalogens are known to contain 16- and 18-carbon aliphatic groups at the sn-1 position. Here, we reveal that the human neutrophil plasmenylethanolamine pool uniquely includes molecular species with very long carbon chain (VLC) aliphatic groups, including 20-, 22- and 24-carbon vinyl ether linked aliphatic groups at the sn-1 position. We identified these novel VLC plasmalogen species by electrospray ionization mass spectrometry methods. VLC plasmalogens were only found in the neutrophil plasmenylethanolamine pool. During neutrophil activation, VLC plasmenylethanolamines undergo myeloperoxidase-dependent oxidation to produce VLC 2-chlorofatty aldehyde and its oxidation product, 2-chlorofatty acid (2-ClFA). Furthermore, plasma concentrations of VLC 2-ClFA are elevated in human sepsis. These studies demonstrate for the first time VLC plasmenylethanolamine molecular species, their myeloperoxidase-mediated chlorolipid products and the presence of these chlorolipids in human sepsis.
Keywords: Plasmalogens, Chlorolipids, Myeloperoxidase, Neutrophil activation, Sepsis
Abbreviations: 2-ClFA, 2-chlorofatty acid; 2-ClFALD, 2-chlorofatty aldehyde; ESI MS/MS, electrospray ionization tandem mass spectrometry; FA, fatty acid; FALD, fatty aldehyde; GC/MS, gas chromatography mass spectrometry; HCl, hydrochloric acid; HOCl, hypochlorous acid; LPE, lysophosphatidylethanolamine; MPO, myeloperoxidase; PC, choline glycerophospholipid; PE, ethanolamine glycerophospholipid; PMA, phorbol 12-myristate 13-acetate; TLC, thin layer chromatography; VLC, very long chain
Graphical abstract
Highlights
-
•
Novel very long chain (VLC) plasmenylethanolamine molecular species are discovered in human neutrophils.
-
•
VLC plasmenylethanolamines undergo myeloperoxidase dependent oxidation to produce new VLC chlorinated lipids.
-
•
VLC 2-chlorofatty acids are elevated in human sepsis.
1. Introduction
Plasmalogens are a subclass of glycerophospholipids characterized by a vinyl ether bond linking the aliphatic group at the sn-1 position. These lipids are present in cell membranes and lipid rafts of many cell types including neutrophils, monocytes, cardiac cells, endothelial cells, and smooth muscle cells [[1], [2], [3], [4], [5]]. The plasmalogens, plasmenylethanolamine and plasmenylcholine, are predominantly present in ethanolamine (PE) and choline (PC) glycerophospholipid pools, respectively. The sn-1 position of plasmalogens have been characterized to contain C16:0 (C indicates carbon and XX:Y indicates the # of carbons: # of double bonds), C18:0, and C18:1 aliphatic groups. Additionally, the LIPIDMAPS consortium include C20:0 plasmalogens in their mass spectral library and they have been shown in human red blood cells [6].
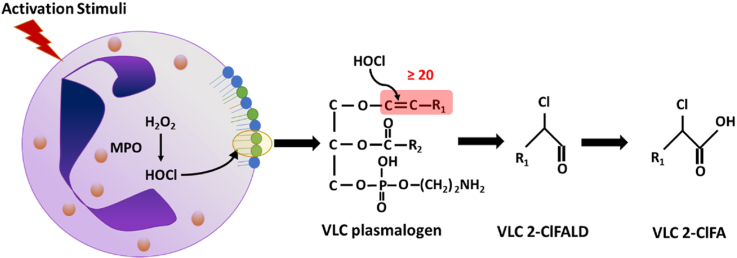
During neutrophil activation, myeloperoxidase (MPO)-derived HOCl targets the sn-1 vinyl ether bond of plasmalogens resulting in 2-chlorofatty aldehyde (2-ClFALD) release [7]. 2-ClFALDs are readily oxidized to 2-chlorofatty acids (2-ClFA), which are stable metabolites. The most common 2-ClFALDs and 2-ClFAs identified are C16:0 and C18:0 species and these chlorinated lipids have been associated with inflammatory conditions such as endotoxemia, atherosclerosis, and myocardial infarction [[8], [9], [10]]. Moreover, increased plasma 2-ClFA levels are associated with acute respiratory distress syndrome and 30-day mortality in sepsis patients [11]. Chlorinated lipids elicit diverse cellular effects including the induction of neutrophil chemotaxis and neutrophil extracellular trap formation [12,13], endothelial dysfunction [14,15], and activation of monocyte apoptosis [16]. Additionally, these lipids have antibacterial properties against E.coli [17]. Since chlorolipids and their precursor plasmalogens are important in the milieu of oxidative stress and sepsis, the present study was designed to determine the possibility of novel molecular species of chlorolipids and their precursor plasmalogens.
2. Materials and Methods
2.1. Neutrophil and monocyte preparations
Human neutrophils were isolated from healthy donors as previously described [17] and approved by St. Louis University IRB protocol 9952. Human monocytes, purchased through Gulf Coast Regional Blood Center, were isolated as previously described [18].
2.2. Human plasma specimens and analysis
Sepsis plasma samples were obtained from subjects admitted to the intensive care unit (ICU) with suspected infection and acute organ dysfunction (sepsis) on the day of ICU admission (day 0) and days 2 and 7 if subjects remained in the ICU. The cohort has been previously described [19]. The cohort study is approved by the University of Pennsylvania institutional review board (IRB protocol #808542) and all subjects or their proxies provided informed consent to participate. Control healthy plasma samples were obtained at Saint Louis University under IRB protocol 26646. Plasma concentrations were compared between healthy control subjects and sepsis subjects by Wilcoxon rank sum test. For visualization, we display each VLC species by quartile of 16:0 2-ClFA.
2.3. Lipid analysis
Neutrophil lipids were extracted by modified Bligh Dyer extraction using lipid class internal standards as previously described [[20], [21], [22]]. Lipid-specific mass spectrometry scan parameters are included in Table S1. 2-Chloro-[d4]-hexadecanal and 2-chloro-[d4]-hexadecanoic acid were used as internal standards for 2-ClFALD and 2-ClFA measurements, respectively [13,23]. 2-ClFALD, dried lipid extracts were incubated with Amplifex Keto reagent (SCIEX; catalog no. 4465962) as described with the reagent and quantified by LC-MS. 2-ClFA species in the lipid extracts and plasma were measured as previously described [11,24].
Lipidomics were performed using parallel reaction monitoring with a Q Exactive mass spectrometer equipped with a Vanquish UHPLC System (Thermo Scientific). Lipids were separated on an Accucore™ C18 column 2.1 × 150 mm (Thermo Scientific) with mobile phase A comprised of 60% acetonitrile, 40% water, 10 mM ammonium formate, and 0.1% formic acid and mobile phase B comprised of 90% isopropanol, 10% acetonitrile with 2 mM ammonium formate, and 0.02% formic acid. Initial conditions were 30% B with a discontinuous gradient to 100% B at a flow rate of 0.260 ml/min. Shotgun lipidomics were performed on both the total lipid extract to quantitate sphingomyelin, ceramide, and PC and fluorenylmethoxycarbonyl-Cl derivatized PE species, as described previously [25]. Samples were analyzed using ESI/MS/MS (TSQ Quantum Ultra, Thermo Scientific). FA from human neutrophil lipid extracts were determined as 2,3,4,5,6-pentafluorobenzoyl ester derivatives as previously described [24,26]. Fatty alcohol concentrations from neutrophil lipid extracts were determined via derivatization with 2,3,4,5,6-pentafluorobenzoyl chloride, as previously described [27].
3. Results and discussion
3.1. Identification of VLC plasmalogens
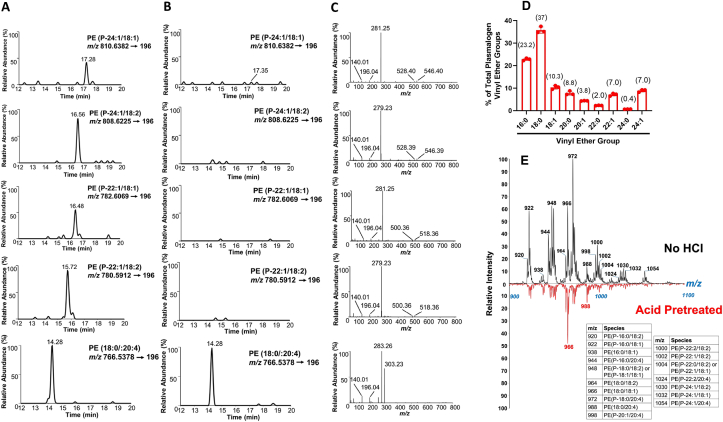
We investigated the presence of VLC plasmalogens in neutrophil lipid extracts using high resolution MS and shotgun lipidomics. Novel VLC molecular species of plasmenylethanolamine were detected using both lipid mass spectrometry methods (Fig. 1). High-resolution MS chromatograms for PE P-24:1/18:1 (P indicates plasmalogen and XX:Y/XX:Y indicate sn-1/sn-2 constituents), PE P-24:1/18:2, PE P-22:1/18:1, and PE P-22:1/18:2 species (Fig. 1A) and their representative fragments (Fig. 1C) confirm the presence of these VLC plasmenylethanolamine molecular species. The fragment ion for glycerol phosphoethanolamine (m/z 196) and the ethanolamine phosphate ion (m/z 140) confirm they are PE species, while loss of the sn-2 acyl chain ketene from [M − H]- (m/z 518 for 22:1 species and m/z 546 for 24:1 species), neutral loss of the sn-2 fatty acid group from [M − H]- (m/z 500 for 22:1 species and m/z 528 for 24:1 species), and the fatty acid ion confirm the respective sn-1 and sn-2 composition. Plasmalogens are acid-labile [28,29]. Chromatographic peaks from the novel molecular species of plasmenylethanolamines were not observed following HCl vapor exposure (Fig. 1B). Diacyl PE (18:0/20:4) was not altered by acid treatment. Additionally, thin layer chromatography (TLC)-purified neutrophil PE lipids and whole lipid extracts of neutrophils were acid treated and plasmalogen-derived FALD species were detected by mass spectrometry following Amplifex derivatization (Fig. 1D). TLC-purified plasmenylethanolamines consist of 6.8% and 6.6% 22:1 and 24:1 FALD, respectively. Interestingly, the percentages of FALD species liberated from TLC-purified plasmenylethanolamine and neutrophil whole lipid extracts were nearly identical, suggesting that the majority of VLC plasmalogens exist in the plasmenylethanolamine pool. Shotgun lipidomics was also employed as an alternative strategy to identify these novel plasmenylethanolamine molecular species. The spectra with respective m/z for fluorenylmethoxycarbonyl-derived plasmenylethanolamines before and after acid treatment are shown in Fig. 1E. Acid treatment led to the selective disappearance of plasmenylethanolamine molecular species. Plasmenylethanolamine molecular species in neutrophil lipid extracts were quantified using liquid chromatography with Q-Exactive MS/MS detection (Table 1). Similar quantitative results for these plasmenylethanolamine molecular species were determined using shotgun lipidomics (Table S2).
Fig. 1.
Identification of novel very long chain plasmenylethanolamine molecular species. Lipids were extracted from 2 x 106 neutrophils and analyzed for plasmenylethanolamine molecular species by PRM using QE MS/MS as described in “Materials and Methods”. Chromatograms of selected plasmenylethanolamine molecular species and respective MS/MS spectra of each plasmenylethanolamine is shown in A and C. B shows the chromatographs of the same sample as A following treatment with HCl vapors (90 s). D) TLC-purified neutrophil ethanolamine glycerophospholipids and whole lipid extracts of neutrophils from 3 different donors (red) were exposed to concentrated HCl vapors for 90 s. Amplifex derivatized FALD (the product of plasmalogen treatment with HCl vapors) was determined using PRM QE MS/MS. Percentage of each FALD molecular species is calculated compared to total FALD measured. Numbers within brackets indicate the % of FALD molecular species identified in TLC-purified ethanolamine glycerophospholipids. (E) Neutrophil ethanolamine glycerophospholipids were derivatized with fluorenylmethoxycarbonyl-Cl and subjected to shotgun lipidomics. Plasmenylethanolamine molecular species were identified by exposing lipid extracts to HCl vapor for 90 s. The PE molecular species with their respective m/z are indicated in the table. The x-axis is m/z. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Quantification of plasmalogens in neutrophils. Plasmenylethanolamine molecular species in human neutrophil lipid pool were determined by QE MS/MS using PRM as described in “Materials and Methods”. ND indicates not detected. Values are mean ± SEM for n=9.
| Molecular Species | ng/106 neutrophils |
|---|---|
| PE(P-16:0/18:1) | 291.59 ± 10.24 |
| PE(P-16:0/18:2) | 97.84 ± 4.34 |
| PE(P-16:0/20:4) | 240.29 ± 9.64 |
| PE(P-18:0/18:1) | 289.27 ± 9.59 |
| PE(P-18:0/18:2) | 316.24 ± 13.53 |
| PE(P-18:0/20:4) | 598.81 ± 24.37 |
| PE(P-18:1/18:1) | 315.06 ± 11.41 |
| PE(P-18:1/18:2) | 84.45 ± 6.54 |
| PE(P-18:1/20:4) | 128.58 ± 6.78 |
| PE(P-20:0/18:1) | 37.39 ± 3.74 |
| PE(P-20:0/18:2) | 42.47 ± 3.49 |
| PE(P-20:0/20:4) | 78.14 ± 3.51 |
| PE(P-22:0/18:1) | 8.63 ± 1.83 |
| PE(P-22:0/18:2) | ND |
| PE(P-22:1/18:1) | 38.02 ± 1.43 |
| PE(P-22:1/18:2) | 57.41 ± 1.43 |
| PE(P-22:1/20:4) | 64.88 ± 3.35 |
| PE(P-24:1/18:1) | 42.78 ± 4.92 |
| PE(P-24:1/18:2) | 64.73 ± 3.54 |
| PE(P-24:1/20:4) | 72.24 ± 4.61 |
Previous studies have shown that 66% of human neutrophil phospholipids consist of plasmenylethanolamines while only 9% consist of plasmenylcholines [30]. We did not detect VLC plasmenylcholine molecular species in human neutrophils. Our limit of detection for plasmenylethanolamine is ∼1 ng for each molecular species containing a 24:1 VLC as the vinyl ether group (Fig. S1). Under these conditions we did not detect VLC plasmenylethanolamine molecular species in any other tissues and cells tested including monocytes, endothelial cells, epithelial cells, and heart tissue. This finding may indicate distinct functions of VLC plasmenylethanolamines in neutrophils. It has long been known that plasmalogens have structural roles in membrane integrity [[31], [32], [33]]; therefore, it is possible that VLC plasmenylethanolamines may provide additional membrane stabilizing or destabilizing properties during phagocytosis that require further investigation. Lodhi et al. demonstrated that the inhibition of ether lipid synthesis resulted in neutrophil apoptosis and endoplasmic reticulum stress [34]. Moreover, blocking ether lipid synthesis in peroxisomes led to neutropenia in mice [34]. Thus, the presence of these VLC plasmenylethanolamines may also be associated with the viability of the neutrophils.
We also quantified ceramides, sphingomyelin, fatty acid, and fatty alcohols to determine the abundance of VLC aliphatic groups in the neutrophils and monocyte lipidomes (Table S3). 22:1 and 24:1 containing FA were found in low abundance compared to 16:0 and 18:0 FA. Neutrophil PE and PC diacyl molecular species containing 22:1 and 24:1 aliphatic groups were found at lower concentrations compared to levels of VLC plasmenylethanolamines (Table S3 and Table 1). 24:1 containing sphingomyelin and ceramide molecular species were detected in both monocytes and neutrophils (Table S3).
3.2. VLC chlorolipid production in activated neutrophils
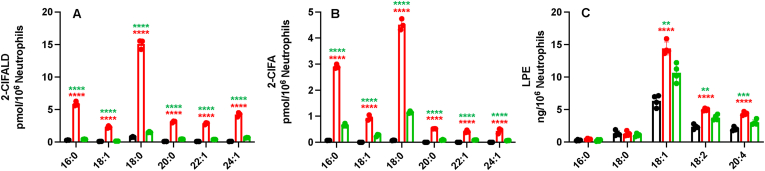
We have previously shown that the vinyl ether bonds of plasmalogen species are targeted by MPO-derived HOCl to produce chlorolipids [7]. Neutrophils stimulated with PMA produce 16:0 and 18:0 2-ClFALD and 2-ClFA [8,13]. We hypothesized that the novel VLC plasmenylethanolamine molecular species may also get oxidized to produce VLC 2-ClFALD and 2-ClFA during neutrophil activation. Indeed, PMA-activated neutrophils showed significant increases in 22:1 2-ClFALD, 24:1 2-ClFALD, 22:1 2-ClFA and 24:1 2-ClFA (Fig. 2A and B).
Fig. 2.
MPO-dependent chlorolipid production in PMA-activated neutrophils. 2 x 106/ml neutrophils were treated with (red) or without 200 nM PMA (black) for 30 min at 37 °C. Neutrophils were also treated with 10 mM 3-amino-1,2,4-triazole for 5 min prior to treatments with 200 nM PMA (green). 2-ClFALD (A), 2-ClFA (B) and LPE (C) molecular species were measured as described in “Materials and Methods”. ****p<0.0001 for comparisons between neutrophils treated with PMA to controls; **, ***, and ****p<0.01, 0.001, and 0.0001, respectively for comparisons between PMA treated and PMA treated with 3-amino-1,2,4-triazole pretreatment. Statistics were performed using ANOVA with Tukey multiple comparison test, error bars for ± SD, Data represents n=3 for A & B, and n=4 for C. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Additionally, both 2-ClFALD and 2-ClFA production were reduced by MPO inhibition by 3-amino-1,2,4-triazole. This result demonstrates that novel VLC chlorolipid production is MPO dependent. We examined plasmenylethanolamine molecular species levels following PMA activation of neutrophils. Concomitant with the appearance of VLC chlorolipids during neutrophil activation were increases in LPE molecular species containing predominantly C18:1, C18:2 and C20:4 fatty acids suggesting they are derived from VLC plasmenylethanolamine molecular species. Lysoplasmenylethanolamine molecular species were not detectable.
3.3. Elevation of plasma VLC 2-ClFA in human sepsis
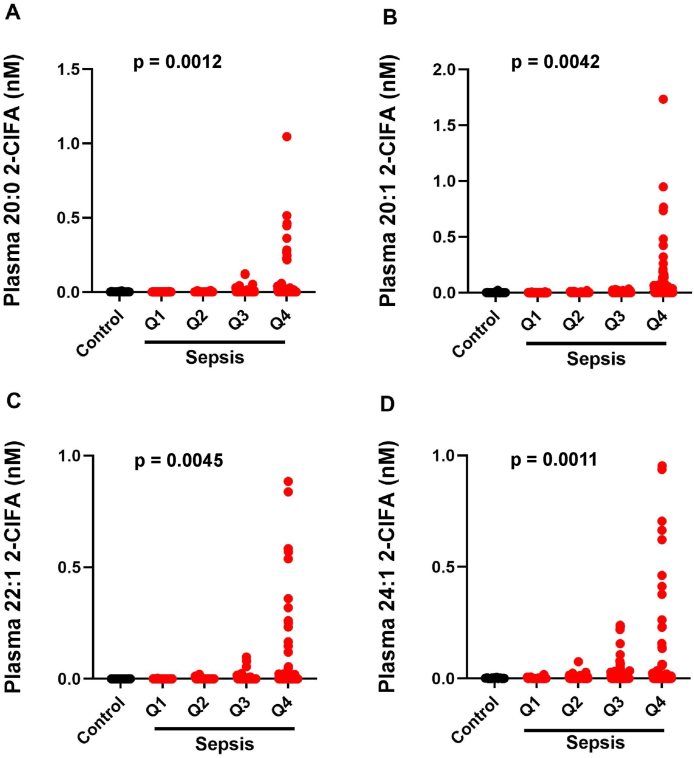
Human sepsis patients have previously been shown to have elevated plasma 16:0 and 18:0 2-ClFA concentrations compared to healthy, non-septic controls. Moreover, 2-ClFA levels were associated with acute respiratory distress syndrome of sepsis patients [11]. Accordingly, we examined the novel VLC 2-ClFA species in human sepsis plasma. Plasma concentrations of 24:1 2-ClFA, 20:1 2-ClFA, 20:0 2-ClFA and 22:1 2-ClFA were significantly elevated in sepsis patient plasma compared to healthy controls (Fig. 3). For visualization, we display the sepsis subjects by quartile of measured plasma 16:0 2-ClFA to emphasize the heterogeneity among the sepsis population. Between 21 and 26% of the sepsis population had detectable VLC species. Statistical comparisons are between the healthy controls and all four quartiles as an aggregate by Wilcoxon rank sum test.
Fig. 3.
VLC 2-ClFA in human sepsis. 2-ClFAs were analyzed from plasma of healthy controls (n=31) and septic patients (n=371) by LC/MS as described in “Material and Methods”. For sepsis plasma, quartiles were determined by 2-ClPA levels with Q1 (0-0.088 nM), Q2 (0.088-0.272 nM), Q3 (0.275-0.745 nM) and Q4 (0.763-6.297 nM). p values for comparisons between healthy control and sepsis levels by 2-sample Wilcoxon rank-sum Mann-Whitney test.
2-ClFALD and 2-ClFA have been shown to induce multiple effects on cells such as endothelial dysfunction, monocyte apoptosis, neutrophil chemotaxis, and NETosis [8,14,16]. Most of these effects are deleterious to host cells. However, they may also serve as antibacterial compounds, which is beneficial during infection [17]. We speculate that VLC 2-ClFALD and 2-ClFA species may also cause cellular effects that need further investigation. Furthermore, plasma VLC 2-ClFA levels in sepsis may associate with specific outcomes during sepsis compared to long chain 2-ClFA molecular species, which may be attributed to their precursors being specifically localized to the neutrophils.
4. Conclusions
We have discovered VLC plasmenylethanolamine molecular species with C22:1 and C24:1 at the sn-1 position. They are uniquely found in neutrophils. These VLC plasmenylethanolamine molecular species undergo MPO-dependent oxidation to produce VLC chlorolipids, which are significantly elevated in human sepsis patients. These observations warrant further study of VLC plasmenylethanolamine biosynthesis, their specific roles in neutrophils and the clinical importance of VLC chlorolipids in inflammation and sepsis.
Funding
This study was supported (in part) by research funding from the National Institutes of Health R01 GM-115553, R21 ES031562, and S10OD025246 to DAF. The human sepsis cohort was funded by NIH R01 HL-137006 and HL-137915 to NJM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.redox.2021.102208.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Gross R.W. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984;23:158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- 2.Chilton F.H., Connell T.R. 1-ether-linked phosphoglycerides. Major endogenous sources of arachidonate in the human neutrophil. J. Biol. Chem. 1988;263:5260–5265. [PubMed] [Google Scholar]
- 3.Ford D.A., Gross R.W. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3479–3483. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy E.J., Joseph L., Stephens R., Horrocks L.A. Phospholipid composition of cultured human endothelial cells. Lipids. 1992;27:150–153. doi: 10.1007/BF02535816. [DOI] [PubMed] [Google Scholar]
- 5.Zoeller R.A., Grazia T.J., LaCamera P., Park J., Gaposchkin D.P., Farber H.W. Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H671–H679. doi: 10.1152/ajpheart.00524.2001. [DOI] [PubMed] [Google Scholar]
- 6.Han X., Gross R.W. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert C.J., Crowley J.R., Hsu F.F., Thukkani A.K., Ford D.A. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J. Biol. Chem. 2001;276:23733–23741. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 8.Anbukumar D.S., Shornick L.P., Albert C.J., Steward M.M., Zoeller R.A., Neumann W.L., Ford D.A. Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal. J. Lipid Res. 2010;51:1085–1092. doi: 10.1194/jlr.M003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thukkani A.K., Martinson B.D., Albert C.J., Vogler G.A., Ford D.A. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2955–H2964. doi: 10.1152/ajpheart.00834.2004. [DOI] [PubMed] [Google Scholar]
- 10.Thukkani A.K., McHowat J., Hsu F.F., Brennan M.L., Hazen S.L., Ford D.A. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 11.Meyer N.J., Reilly J.P., Feng R., Christie J.D., Hazen S.L., Albert C.J., Franke J.D., Hartman C.L., McHowat J., Ford D.A. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI Insight. 2017;2 doi: 10.1172/jci.insight.96432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palladino E.N.D., Katunga L.A., Kolar G.R., Ford D.A. 2-Chlorofatty acids: lipid mediators of neutrophil extracellular trap formation. J. Lipid Res. 2018;59:1424–1432. doi: 10.1194/jlr.M084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thukkani A.K., Hsu F.F., Crowley J.R., Wysolmerski R.B., Albert C.J., Ford D.A. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 2002;277:3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 14.Hartman C.L., Duerr M.A., Albert C.J., Neumann W.L., McHowat J., Ford D.A. 2-Chlorofatty acids induce Weibel-Palade body mobilization. J. Lipid Res. 2018;59:113–122. doi: 10.1194/jlr.M080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHowat J., Shakya S., Ford D.A. 2-Chlorofatty aldehyde elicits endothelial cell activation. Front. Physiol. 2020;11:460. doi: 10.3389/fphys.2020.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W.Y., Albert C.J., Ford D.A. Alpha-chlorofatty acid accumulates in activated monocytes and causes apoptosis through reactive oxygen species production and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 2014;34:526–532. doi: 10.1161/ATVBAHA.113.302544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amunugama K., Kolar G.R., Ford D.A. Neutrophil myeloperoxidase derived chlorolipid production during bacteria exposure. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.701227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasi V., Wood D.C., Eickhoff C.S., Xia M., Pozzi N., Edwards R.L., Walch M., Bovenschen N., Hoft D.F. Granzyme A produced by gamma9delta2 T cells activates ER stress responses and ATP production, and protects against intracellular mycobacterial replication independent of enzymatic activity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.712678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilly J.P., Wang F., Jones T.K., Palakshappa J.A., Anderson B.J., Shashaty M.G.S., Dunn T.G., Johansson E.D., Riley T.R., Lim B., Abbott J., Ittner C.A.G., Cantu E., Lin X., Mikacenic C., Wurfel M.M., Christiani D.C., Calfee C.S., Matthay M.A., Christie J.D., Feng R., Meyer N.J. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018;44:1849–1858. doi: 10.1007/s00134-018-5328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.Han X., Gross R.W. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 22.Maner-Smith K.M., Goll J.B., Khadka M., Jensen T.L., Colucci J.K., Gelber C.E., Albert C.J., Bosinger S.E., Franke J.D., Natrajan M., Rouphael N., Johnson R.A., Sanz P., Anderson E.J., Hoft D.F., Mulligan M.J., Ford D.A., Ortlund E.A. Alterations in the human plasma lipidome in response to tularemia vaccination. Vaccines. 2020;8:414. doi: 10.3390/vaccines8030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wacker B.K., Albert C.J., Ford B.A., Ford D.A. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic. Biol. Med. 2013;59:92–99. doi: 10.1016/j.freeradbiomed.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pike D.P., Vogel M.J., McHowat J., Mikuzis P.A., Schulte K.A., Ford D.A. 2-Chlorofatty acids are biomarkers of sepsis mortality and mediators of barrier dysfunction in rats. J. Lipid Res. 2020;61:1115–1127. doi: 10.1194/jlr.RA120000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X., Yang K., Cheng H., Fikes K.N., Gross R.W. Shotgun lipidomics of phosphoethanolamine-containing lipids in biological samples after one-step in situ derivatization. J. Lipid Res. 2005;46:1548–1560. doi: 10.1194/jlr.D500007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quehenberger O., Armando A.M., Brown A.H., Milne S.B., Myers D.S., Merrill A.H., Bandyopadhyay S., Jones K.N., Kelly S., Shaner R.L., Sullards C.M., Wang E., Murphy R.C., Barkley R.M., Leiker T.J., Raetz C.R., Guan Z., Laird G.M., Six D.A., Russell D.W., McDonald J.G., Subramaniam S., Fahy E., Dennis E.A. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wildsmith K.R., Albert C.J., Anbukumar D.S., Ford D.A. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 2006;281:16849–16860. doi: 10.1074/jbc.M602505200. [DOI] [PubMed] [Google Scholar]
- 28.Murphy E.J., Stephens R., Jurkowitz-Alexander M., Horrocks L.A. Acidic hydrolysis of plasmalogens followed by high-performance liquid chromatography. Lipids. 1993;28:565–568. doi: 10.1007/BF02536090. [DOI] [PubMed] [Google Scholar]
- 29.Zemski Berry K.A., Murphy R.C. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J. Am. Soc. Mass Spectrom. 2004;15:1499–1508. doi: 10.1016/j.jasms.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Mueller H.W., O'Flaherty J.T., Greene D.G., Samuel M.P., Wykle R.L. 1-O-alkyl-linked glycerophospholipids of human neutrophils: distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. J. Lipid Res. 1984;25:383–388. [PubMed] [Google Scholar]
- 31.Glaser P.E., Gross R.W. Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry. 1994;33:5805–5812. doi: 10.1021/bi00185a019. [DOI] [PubMed] [Google Scholar]
- 32.Han X.L., Gross R.W. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry. 1990;29:4992–4996. doi: 10.1021/bi00472a032. [DOI] [PubMed] [Google Scholar]
- 33.Han X.L., Gross R.W. Proton nuclear magnetic resonance studies on the molecular dynamics of plasmenylcholine/cholesterol and phosphatidylcholine/cholesterol bilayers. Biochim. Biophys. Acta. 1991;1063:129–136. doi: 10.1016/0005-2736(91)90362-c. [DOI] [PubMed] [Google Scholar]
- 34.Lodhi Irfan J., Wei X., Yin L., Feng C., Adak S., Abou-Ezzi G., Hsu F.-F., Link Daniel C., Semenkovich Clay F. Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metabol. 2015;21:51–64. doi: 10.1016/j.cmet.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.