Abstract
Background
The science of stress exposure and health in humans has been hampered by differences in operational definitions of exposures and approaches to defining timing, leading to results that lack consistency and specificity. In the present study we aim to empirically derive variability in type, timing and chronicity of stress exposure for Black and White females using prospectively collected data in the Pittsburgh Girls Study (PGS).
Methods
The PGS is an ongoing 20-year longitudinal, community-based study. In this paper we focused on annual caregiver reports of three domains of stress: subsistence (e.g., resource strain, overcrowding); safety (e.g., community violence, inter-adult aggression), and caregiving (e.g., separation, maternal depression) from early childhood through adolescence. Z-scores were used to conduct a finite mixture model-based latent class trajectory analysis. Model fit was compared using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). We examined differences in timing and chronicity of stress exposure between Black and White girls.
Results
Distinct trajectory groups characterized by differential timing and chronicity of stress exposure were observed across all stress domains. Six trajectories characterized subsistence and safety stress, and five characterized caregiving stress. Variability in initial level, chronicity, and magnitude and timing of change was observed within and across domains of stressors. Race differences also varied across the domains: race differences in timing and chronicity were most pronounced for the subsistence and safety domains, whereas Black and White girls had similar levels of exposure to caregiving stress.
Conclusions
Substantial variability in timing and chronicity was observed within and across stress domains. Modeling specific domains and dimensions of stress exposure is likely important in testing associations between exposure and health; such specificity may lead to more effective deployment of preventive interventions based on stress exposure.
Keywords: Stress, Exposure, Timing, Chronicity, Development, PGS
Highlights
-
•
Distinct trajectories in exposure were observed for subsistence, safety, and caregiving stress domains.
-
•
Race differences in timing and chronicity of exposure were pronounced for subsistence and safety domains.
-
•
Patterns of exposure (e.g., level, timing) are likely critical for understanding the impact on stress exposure on health.
1. Capturing the dynamic nature of stress exposure in the Pittsburgh Girls Study
The impact of acute and chronic stress exposure on systems that maintain health is well documented in experimental animal and human studies (Calcagni & Elenkov, 2006; Cavigelli & Caruso, 2015; Karlén et al., 2015; Matthews & Gallo, 2011). Although the results from correlational studies in humans on stress exposure and outcomes are relatively robust, there remains substantial heterogeneity and lack of specificity in methodology across studies. One reason for this is the overreliance on cumulative risk indices, often conceptualized as contributing to "allostatic load." McEwen and Stellar (1993) defined allostatic load as, "The strain on the body produced by repeated ups and downs of physiologic response, as well as by the elevated activity of physiologic systems under challenge, and the changes in metabolism and the impact of wear and tear on a number of organs and tissues, can predispose the organism to disease." The goal of introducing this conceptualization was to reveal the cost of stress exposure to the body over time. Although the authors referred to the "ups and downs of physiologic response," in the vast majority of human research chronic and cumulative stress exposure are measured via ordinal scales of events without regard for type, timing, or chronicity of exposure. These elements may be critical in accurately determining the mechanisms by which stress exposure influences health outcomes over time.
Indeed, Schwartz, Bellinger, and Glass (2011) argued that the science on the effects of environmental risk to health in humans has been hampered by a lack of rigor in the conceptualization and measurement of exposures, and enumerated five erroneous assumptions regarding underlying risk: 1) exposures are independent of one another; 2) the distribution of exposures among subpopulations is random and thus can be averaged; 3) risk from exposure is non-transferrable to subsequent generations; 4) the risk from exposure is constant across development and independent of dose of exposure; and 5) the cumulative burden of a specific exposure is independent of the distribution of other risk factors. As a result of these assumptions, many of the existing paradigms used to test associations between exposure and health have failed to capture differential vulnerability and susceptibility to environmental risk factors (Schwatrz, Bellinger & Glass, 2011). The authors further explain that when assessments of risk rely on snapshots of exposure based on one point in time, or on lifetime exposure, they fail to capture data relevant to critical windows, rate of exposure, and patterns of change across the entire life course. These dimensions of exposure are critical for ultimately understanding vulnerable periods as well as levels of exposure that would indicate more rigorous assessments of a health domain. Moreover, the authors argue that failing to test for differential risk exposure among subgroups can lead to masking inequalities in exposure and perpetuating racial and ethnic disparities in health. A recently published scoping review on measurement of adult retrospective report of adverse childhood experiences (ACES) yielded similar conclusions about the state of the science of stress exposure on health (Krinner, Warren-Findlow, Bowling, Issel, & Reeve, 2021). The authors reported little consistency in measurement, a lack of conceptual grounding, and insufficient rigor in assessing timing and chronicity, in part due to the reliance on retrospective recall.
Research using animal models supports the contention that the impact of stress exposure on health and behavior varies significantly as a function of timing and type. There are several reviews on this topic based on studies conducted in rodents (Boersma et al., 2014) and non-human primates (Parker & Maestripieri, 2011). A few illustrative examples include a study by Badache et al. (2017), in which pregnant rats were exposed to noise, restraint, or a combination of noise and restraint stress. Adolescent offspring of the three groups of animals differed in terms of locomotion and exploration. The offspring of dams exposed to both stressors displayed increased anxiety-like behavior but no changes in balance and locomotion, whereas the offspring of dams exposed only to noise stress evidenced significant impairments in balance and locomotion. Veru, Laplante, Luheshi, and King (2014) proposed the bifurcation of stress exposure into neurogenic (e.g., shock and restraint) and psychogenic (e.g., overcrowding) based on differential outcomes in offspring immune functioning. Timing of exposure also leads to differential impact on offspring. Twenty-four hours of maternal separation from pups at post-natal days 3–4 led to an enhanced stress response later in development (post-natal day 20), whereas the same duration of maternal separation at post-natal days 11–12 resulted in an attenuated response in comparison to control animals (VanOers, DeKloet, & Levine, 1998). Similarly, a nutrition stressor (i.e., high fat/high sucrose) administered at 1 week prior to conception, but not at 3 weeks prior to conception, resulted in a syndrome similar to that of gestational diabetes including glucose intolerance and impaired insulin secretion (Pennington, van der Walt, Pollock, Talton, & Schulz, 2017).
The study of type and timing of stress exposure in humans is clearly more complex, given the lack of experimental control and a less developed approach to a functional taxonomy compared to animal studies (Grant, Compas, Thurm, McMahon, & Gipson, 2004). Studies of the effects of the timing of exposure in humans often rely on retrospective reports. One domain of stress exposure that has been commonly modeled is timing of abuse. For example, in comparing recall of abuse during early, middle, or late childhood, sexual abuse during early childhood (5 years of age and younger) and physical abuse during late childhood (13 years and older) were stronger predictors of adult distress symptoms than abuse that occurred at other developmental periods (Capretto, 2020). Teicher and colleagues have reported effects of timing of abuse, assessed via retrospective recall, on several health outcomes including neural connectivity and HPA axis functioning (Kaiser et al., 2018), brain morphology (Teicher et al., 2018), and mental health (Khan et al., 2015; Schalinski et al., 2016). Khoury, BosquetEnlow, Plamondon, and Lyons-Ruth (2019) conducted a meta-analysis to characterize type and timing of adverse experiences on level of cortisol assayed from hair samples. Both type and timing were associated with cortisol levels, but type and timing of adversity tended to be confounded, and lack of variability in duration prevented tests of the effect of chronicity of adversity on cortisol levels. Similarly, a study of maltreatment on neurocognitive function revealed associations between timing and chronicity, with timing (e.g., infancy) and chronicity of exposure associated with poorer inhibitory control and working memory in childhood; but disentangling the two was not possible (Cowell, Cicchetti, Rogosch, & Toth, 2015).
Data from a few prospective studies have also revealed effects of timing of stress exposure on health outcomes. In a follow up to the National Child Development Study, a cohort study of children born in Britain during one week in 1958 and followed up from middle childhood into early adulthood (Atherton, Fuller, Shepherd, Strachan, & Power, 2008), female participants were asked about birth outcomes (e.g., gestational age, birth weight). Exposures to financial, parenting, family, and community stressors most strongly influenced birth outcomes if they occurred during adolescence (Harville, Boynton-Jarrett, Power, & Hyppönen, 2010). In contrast, Dunn et al. (2018) compared three exposure models (most recent, accumulation, and sensitive periods) on childhood functioning at age 8 using prospectively collected data from the Avon Longitudinal Study of Parents and Children. The authors reported that recency and accumulation of stress explained later variance in behavioral and emotional functioning, although there was some evidence that financial stress during infancy, as opposed to other developmental periods, was associated with girls’ later compromised functioning.
Recalling the criticisms of Schwartz et al. (2011), we propose that the science of stress exposure-health outcomes in humans would benefit from work directed at characterization of stress before attempting to link these exposures to health outcomes for several reasons. First, associations, or lack thereof, between exposure trajectories and a given health index does not address evidence of the pattern of exposure, but rather suggests that the health index is or is not impacted by such exposure. As such, the selection of a single validation may lead to the false conclusion that the pattern of exposure is not "meaningful" for health broadly speaking. Second, inconsistency in tests of associations between exposure and health to date appear to be in part attributed to use of different operational definitions of exposure. For example, studies in which associations between pubertal development and emotional health were tested have yielded highly inconsistent findings (e.g., Galvao et al., 2014). This may be due to a true lack of association, but also may be due to the use of different definitions of pubertal development such as timing of menarche, tanner staging at a given point in time, or changes in secondary sex characteristics over time, all of which likely oversimplify the complexity of timing and temp of gonadal and adrenal axis maturation (Conley, Bernstein, & Nguyen, 2012). Finally, developmental timing (childhood versus adolescence) and patterns (chronic versus episodic) may be associated with different patterns of response of biological systems (e.g., attenuating versus intensifying reactivity), which may further vary as a function of sex and race (e.g., Johnson et al., 2020). Our goal in the present paper is to provide an example of an approach to generating stress exposure phenotypes that considers dimensions of exposure and whether patterns of exposure vary between subgroups.
Our proposed stress domains extend from a substantial literature based on animal models (see reviews by Boersma et al., 2014; Parker & Maestripieri, 2011) and on some recent work in humans. For example, McLaughlin, Sheridan, and Lambert (2014) categorized stress as falling into one of two categories: threat or deprivation, with threat including observing community violence, witnessing domestic violence, and being the victim of chronic physical abuse, and deprivation including poverty, neglect, and institutionalization. Their conceptualization was based on a selective review of literature on stress exposure and neural development, and they aimed to provide a framework for further research conducted across species. We build on their work in several ways. First, as noted by the authors, caregiver "deprivation" may be an important stressor that uniquely impacts health above and beyond deprivation related to resources. Thus, we separate caregiving stress from resource related stress. Caregiving should not be assumed to be compromised for families living in low resourced environments. Second, within each domain of stress exposure we include both less and more commonly occurring indices that do not necessarily co-occur as well as indices that are experienced by subgroups. For example, neighborhood level threats to safety are likely to be experienced by racially marginalized subgroups, whereas family level threats to safety (e.g., inter-adult conflict, child sexual abuse) are likely to be experienced across subgroups. Relying on public assistance may reflect resource stress for some families, whereas credit card debt may be a source of subsistence stress for those not receiving public assistance. Finally, we aim to generate approaches to measuring stress that are generally conserved across species, such as overcrowding as an index of subsistence stress, and separation as an index of caregiver stress.
In the present study, we aim to characterize longitudinal patterns in severity, timing, and chronicity of stress exposures using prospectively and annually collected data from the Pittsburgh Girls Study (PGS), an ongoing longitudinal, community-based study of girls. We focus on three domains of stress: subsistence (e.g., resource strain, overcrowdings), safety (e.g., community violence, inter-adult aggression), and caregiving (e.g., separation, maternal depression). In addition, we examine the impact of Black or White race on stressor type, timing and chronicity, with an eye towards overcoming the assumption that exposures among subpopulations are random. As described by Schwartz et al. (2011), "Disparities in health arise from inequities in the distributions of resources and risks." Racial disparities in multiple health domains in the U.S. are largely attributed to differential exposures to psychosocial stressors including those embedded in structural and systemic racism such as discrimination, poverty, and victimization (Ford et al., 2021; Paradies et al., 2015; Williams, Lawrence, Davis, & Vu, 2019). Recent work on measuring racial discrimination in studies of health calls for measurement approaches that account for differential stress exposure across domains and time (Cuevas & Boen, 2021). We therefore explore whether differential exposure to stressor types, timing and chronicity are observed for Black and White girls.
2. Methods
2.1. Sampling
Participants in the PGS were identified in 1999–2000 via random household sampling, with over-sampling of households in low resourced neighborhoods. Our team enumerated 103,238 Pittsburgh households to locate girls between the ages of 5 and 8 years (Hipwell et al., 2002; Keenan et al., 2010). Neighborhoods in the City of Pittsburgh in which at least 25% of the families were living at or below the poverty level were fully enumerated (i.e., all homes were contacted to determine if the household contained an eligible girl), and a random selection of 50% of households in all other city neighborhoods were enumerated. The enumeration identified 3118 separate households in which an eligible girl resided. From these households, families who moved out of state and families in which the girl would be age ineligible by the start of the study were excluded. When two age-eligible girls were enumerated in a single household, one girl was randomly selected for participation. Of the 2992 eligible families, 2875 (96%) were successfully re-contacted to determine their willingness to participate in the longitudinal study. Of those families, 85% agreed to participate, resulting in a total sample size of 2450.
Approximately half of the girls in the PGS sample identify as Black (52%, n = 1296); 41% identify as White (n = 1009), and the remaining 6.8% girls (n = 145) are multiracial or represent another race. In wave 1, 22% of families had an annual income that was below the poverty threshold (≈$17,500/year in 2000), 33% received public assistance, 17% of parents had completed less than 12 years of education, and 44% of caretakers were single. Nearly all the primary caregivers were biological mothers (92%).
2.2. Procedures
Approval for all study procedures was obtained from the University of Pittsburgh Institutional Review Board. Written informed consent from the caregiver and verbal assent from the child were obtained prior to data collection. Annual interviews were conducted separately for the parent and child in the home by trained interviewers. The PGS uses an accelerated longitudinal design with 588 5-, 630 6-, 611 7-, and 621 8-year-old girls enrolled in the study at wave 1. Data are then aligned by age for longitudinal analyses from ages 7–17 years. Analyses were conducted with weighted data to correct for the over-sampling of the low-income neighborhoods.
2.3. Measures
Data for the present study were derived from annual interviews with caregivers for each of the three domains. Items used to measure each domain and scoring criteria for presence (1), or absence (0) are detailed in Table 1. Within each domain, the binary items were summed to yield a total score.
Table 1.
Stress Domains: Items and scoring.
| Stress Domain | Subdomain | Items | Criteria for Scoring as 1 (vs. 0) |
|---|---|---|---|
| Subsistence |
Resources |
|
|
| Housing |
|
|
|
| Safety |
Neighborhood |
|
|
| Domestic |
|
|
|
| Caregiving | Disruptions |
|
|
| Strain |
|
|
2.3.1. Subsistence stress
This domain includes indices of resource and housing stress. Resource stress was based on caregivers’ reports (yes/no) of receipt of public assistance (e.g., Nutrition Program for Women, Infants, and Children, food stamps, welfare, Medicaid) on the Demographic Questionnaire (DEMO; developed for the PGS), trouble with credit rating, and long-term debts (other than a mortgage) as reported by caregivers (yes/no) on the Difficult Life Circumstances measure (DLC; Barnard et al., 1989). Housing stress included overcrowding, defined as more than 2 people per bedroom as assessed on the DEMO, and suboptimal housing based maternal report of no suitable or affordable place to live (yes/no) and having trouble with the landlord (yes/no) on the DLC. Binary items measuring resource and housing stress were summed to yield a total score for subsistence stress.
2.3.2. Safety stress
Neighborhood safety was assessed by caregiver report on the extent of illegal activities and neighborhood crime (e.g., vandalism, organized crime, drug-dealing, prostitution) using the Your Neighborhood Questionnaire (YN; Loeber et al., 1998). For each item, participants reported whether each item was "not a problem", "somewhat of a problem" or a "big problem" (alpha coefficients range from 0.94 at age 7 years to 0.95 at age 17 years). Scores falling in the upper quartile indicated neighborhood safety stress. Caregivers also indicated whether they had witnessed and/or were victimized by violent crime (e.g., homicide, assault, rape) (yes/no) on the Police Contacts (PC; Loeber, Farrington, Stouthamer-Loeber, & VanKammen, 1998) questionnaire developed for the PGS. Finally, caregivers reported on lack of safety on neighborhood streets on the Community Survey (COMS; Gorman-Smith, Tolan, & Henry, 2000), defined as endorsing "disagree" or "strongly disagree" with the statement "I feel safe on the streets in my neighborhood."
Domestic safety was assessed using items from the DLC including whether any child was being emotionally, sexually or physically abused by anyone, and whether the caregiver's partner had physically abused her. In addition, caregivers reported on inter-adult aggression on the revised Conflict Tactics Scale (CTS2; Straus, Hamby, Boney-McCoy, & Sugarman, 1996; alpha coefficients range from 0.80 at age 7 years to 0.77 at age 17 years). Threatening to hit, throwing objects at the other, or slapping/hitting the other was coded as domestic violence. Binary items measuring neighborhood and domestic safety were summed to yield a total score for safety stress.
2.3.3. Caregiving stress
Disruptions in caregiving were based on reports of child separation/out-of-home care (e.g., foster home, special facility) for more than 1 month within a 12-month period (yes/no), and change in primary caregivers (yes/no) assessed using the DEMO. Caregiving strain was measured by low maternal warmth using six items form the Parent/Child Relationship Scale (PCRS; Hipwell et al., 2008). (e.g., "How often have you thought she was a difficult child?"). Alpha coefficients ranged from 0.70 at age 7 years to 0.81 at age 17 years. Items were summed and the upper quartile defined low maternal warmth. The Beck Depression Inventory-II (BDI-II; Beck, Brown, & Steer, 1996) was administered in each year to assess maternal report of depression (alpha coefficients range from 0.91 at age 7 years to 0.93 at age 17 years). A score of 11 or higher on the BDI was used to assess any level of depression severity. The Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983) was used to measure caregiver stress (alpha coefficients = .87 at ages 7 and 17 years). Fourteen items (e.g., "Have you been upset because of something that happened unexpectedly?", "Have you been coping well?’) were rated on a three-point scale (1 = almost never, 2 = sometimes, 3 = never) and summed, and the upper quartile defined maternal stress. The six binary items measuring caregiver stress were summed to yield a total score.
2.4. Analytic approach
As described above, each domain was measured by the sum of binary indicators of risk at each age of the child. Z-scores were then computed for each of the three stressor domains (subsistence, safety, and caregiving) across age and assessment year for the trajectory analyses. We included all cases for which data on at least 5 of the 6 items within a domain were available.
We used SAS TRAJ procedure to model latent group-based growth curve trajectories for each stress domain. This procedure adopts the mixture model approach, which allows the model to be a mixture of different probability density functions and thus account for the heterogeneity in the population level (Jones, Nagin, & Roeder, 2001). Assuming denotes the longitudinal observation for a subject with the time period from 1 to T. Then the probability density function of using the concept of mixture model can be expressed as:
Here is the probability of the individual belongs to the group j and denotes the probability density function of given parameters . can be estimated using the multinomial logit function:
where are risk factors that are used to estimate the group membership and are relevant parameters.
We used an iterative approach to compare models that differed in the number of groups and shapes of trajectories (e.g., linear, quadratic). Model fit was compared using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), with lower AIC and BIC values indicating better fit. We also considered the magnitude of decrease in AIC and BIC fit indices with each increase in number of groups to ensure parsimony. We then tested whether Black or White race was associated with trajectory group based on modal class assignment using chi-square tests of significance; a p level of less than 0.01 was considered statistically significant.
We describe groups in terms of initial level (i.e., low, average, moderate and high), with low defined as scores falling below −0.5, average as scores falling with −0.5 and 0.5, moderate as scores falling above 0.5 but below 1.0, and high scores as those falling at or above 1.0. Change in level of exposure over time was defined using a change of greater than 0.5 SD. Thus, consistent exposure was defined by scores that remained within 0.5 SD, and increasing and decreasing exposure by changes of at least 0.5 SD.
3. Results
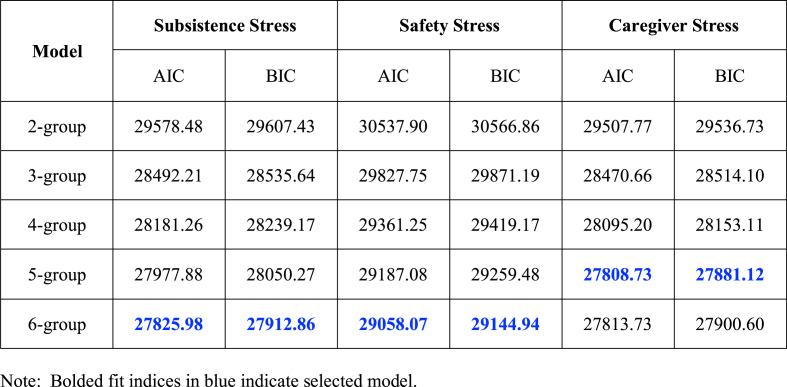
Fit indices for models with 1 through 6 groups for each domain are presented in Table 2. Model selection and characterization of trajectories within each domain are described below.
Table 2.
Fit Indices for model testing within each stress domain.
3.1. Subsistence stress
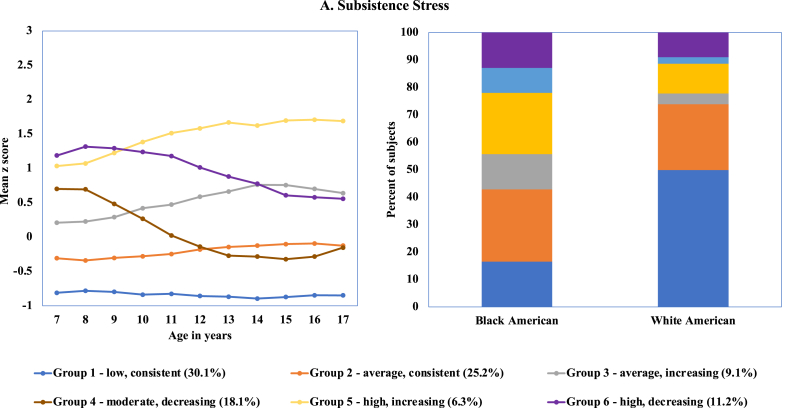
The 6-group model showed the best fit for the Subsistence domain; two groups had fairly consistent levels of exposure over time and four groups showed changes in exposure. As shown in Fig. 1, approximately half of the sample had consistent levels of exposure to subsistence stress from age 7 to 17: Group 1 (30.1%) had consistently low levels over time, and Group 2 (25.2%) had consistent, average levels of exposure over time. Four trajectory groups were characterized by changing levels of subsistence stress. Subsistence stress increased over time for girls in Group 3 (9.1%), with levels largely remaining within the average range. For girls in Group 4 (18.1%), exposure levels started out in the moderate range, but then decreased across childhood and remained at average levels throughout adolescence. Girls in Groups 5 (6.3%) and 6 (11.2%) both started out with high level of exposure from ages 7–10 years, and then began to diverge with Group 5 continuing to experience increases in exposure during adolescence and Group 6 experiencing a steady decrease in exposure.
Fig. 1.
Best fitting model for exposure to subsistence stress based on latent class trajectory.
Test of race difference in group membership for the Subsistence domain revealed statistically significant differences for the overall distribution (chi-square = 367.55, p < .0001) (see Table 3 and Fig. 1). Significant race differences were observed in level and pattern of exposures: close to 50% of White girls had consistently low exposure to subsistence stress (Group 1), compared to 16.5% of Black girls (chi-square = 313.83, p < .0001). No differences were observed for membership in the average, consistent group (Group 2). The percent of Black girls experiencing an increase in subsistence stress over time was about three time the rate compared to White girls (Groups 3 and 5) (see Fig. 1).
Table 3.
Subsistence stress: Race differences in group membership.
| Latent Groups | White |
Black |
chi-square | p level | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| 1 (low, consistent) | 504 | 49.9 | 238 | 16.5 | 313.82 | <0.0001 |
| 2 (average, consistent) | 242 | 24.0 | 379 | 26.3 | 1.71 | 0.1911 |
| 3 (average, increasing) | 39 | 3.9 | 186 | 12.9 | 58.26 | <0.0001 |
| 4 (moderate, decreasing) | 109 | 10.8 | 338 | 23.5 | 63.82 | <0.0001 |
| 5 (moderate, increasing) | 24 | 2.4 | 131 | 9.1 | 45.17 | <0.0001 |
| 6 (moderate, decreasing) | 91 | 9.0 | 185 | 12.9 | 8.70 | 0.0032 |
Overall difference: chi-square = 367.55, p < .0001.
3.2. Safety stress
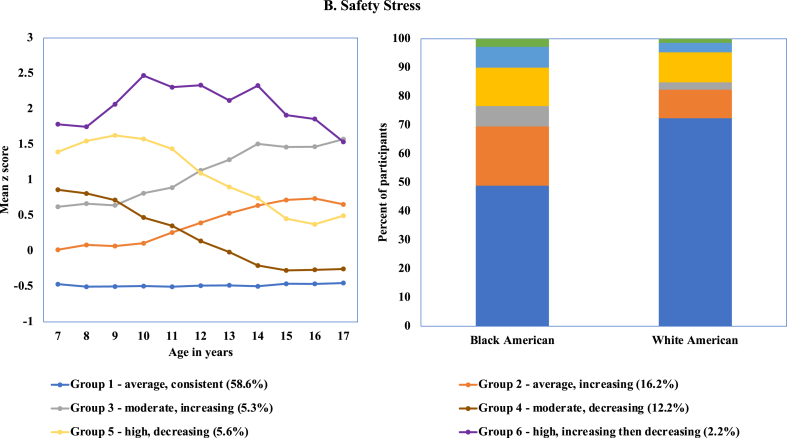
The 6-group model demonstrated the best fit for the Safety Domain. The modal trajectory group, Group 1 (58.6%), experienced consistently low exposure to safety stress (Fig. 2); all other groups evidenced change over time. The next most common pattern was described by average levels of safety stress that increased over time but remained within average levels (Group 2). Groups 5 and 6 had high levels of exposure to safety stress initially, with Group 5 declining to average levels by age 15 and Group 6 experiencing their highest levels of exposure from 10 to 14 years, followed by decreasing levels of exposure.
Fig. 2.
Best fitting model for exposure to safety stress based on latent class trajectory.
There were statistically significant differences in safety stress group membership for Black and White girls (overall chi-square = 147.50, p < .001) (Table 4 and Fig. 2). The majority of White girls (72.4%) had consistently low exposure to safety stress; a significantly smaller percentage of Black girls had consistently low exposure (48.9%) (chi-square = 135.73, p < .0001). The percentage of Black girls with average and moderate levels of safety stress that increased over time (Groups 2 and 3) was about twice as high as the percentage of White girls. No significant race differences were observed for membership in Groups 4 and 6, both of which experienced decreases in exposure to safety stress later in adolescence.
Table 4.
Safety stress: Race differences in group membership.
| Latent Groups | White |
Black |
chi-square | p level | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| 1 (average, consistent) | 731 | 72.4 | 704 | 48.9 | 135.73 | <0.0001 |
| 2 (average, increasing) | 100 | 9.9 | 296 | 20.6 | 49.59 | <0.0001 |
| 3 (moderate, increasing) | 26 | 2.6 | 103 | 7.1 | 24.90 | <0.0001 |
| 4 (moderate, decreasing) | 105 | 10.4 | 193 | 13.4 | 4.98 | 0.0256 |
| 5 (high, decreasing) | 33 | 3.3 | 104 | 7.2 | 17.54 | <0.0001 |
| 6 (high, increasing, then decreasing) | 14 | 1.4 | 40 | 2.8 | 5.32 | 0.0211 |
Overall difference: chi-square = 147.50, p < .0001.
3.3. Caregiving stress
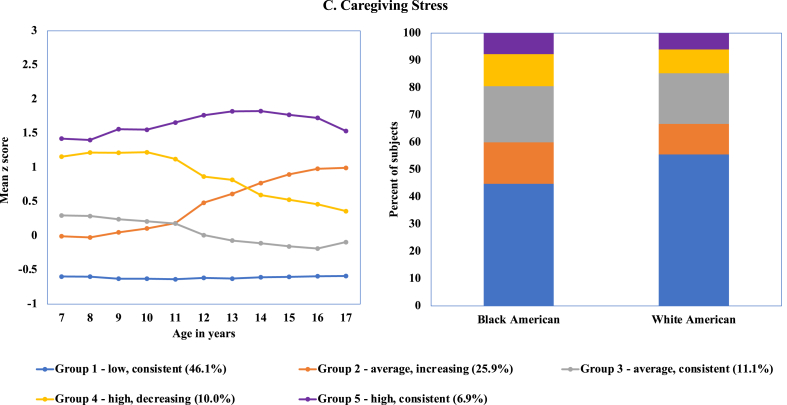
The 5-group model was the best-fitting model for the Caregiving Domain (Fig. 3). Most (46.1%) participants experienced low levels of caregiving stress from ages 5–17 years. The smallest group (Group 5; 6.9%) comprised participants with consistent, high levels of exposure over time. Groups 2 (25.9%) and 3 (11.1%) had average levels of caregiving stress exposure from ages 7–11 years, with the former experiencing moderate increases in exposure to caregiving stress over time. Groups 4 (10.0%) and 5 (6.9%) also had relatively similar levels of caregiving stress at age 7 years, with the former group showing a decrease to average levels by age 17 years.
Fig. 3.
Best fitting model for exposure to caregiving stress based on latent class trajectory For ease of explication, group numbers are assigned in ascending order of level of exposure at baseline (age 7 years).
In contrast to the other two stress domains, there were few race differences in caregiving stress trajectories (Table 5 and Fig. 3). The consistently low exposure to caregiving stress trajectory (Group 1) had a larger proportion of White girls than Black girls (55.5% versus 44.8%, chi-square = 26.87, p < .0001). A higher percentage of Black girls had average, increasing levels of caregiving stress (Group 3) than White girls (15.2% versus 11.2%, chi-square = 8.14, p = .0043). No statistically significant race differences were observed for the other three groups.
Table 5.
Caregiving Stress: Race differences in group membership.
| Latent Groups | White |
Black |
chi-square | p level | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| 1 (low, consistent) | 560 | 55.5 | 646 | 44.8 | 26.87 | <0.0001 |
| 2 (average, increasing) | 113 | 11.2 | 219 | 15.2 | 8.14 | .0043 |
| 3 (average, consistent) | 188 | 18.6 | 296 | 20.6 | 1.38 | 0.2394 |
| 4 (moderate, decreasing) | 88 | 8.7 | 168 | 11.7 | 5.50 | 0.0190 |
| 5 (moderate, consistent) | 60 | 6.0 | 111 | 7.7 | 2.84 | 0.0922 |
Overall difference: chi-square = 29.34, p < .0001.
4. Discussion
The aim of the present study was to use annual assessments of stress exposure from childhood to adolescence to explore an approach to capturing the dynamic nature of exposure over time. In contrast to collapsing exposure into cumulative indices and assuming equivalence of stressors, we sought to delineate patterns of stress exposure in three domains: subsistence, safety, and caregiving stress. Findings indicated variability in initial level of severity, chronicity, and timing of shifts in exposure level within and across domains. Moreover, group membership differed in terms of racial composition, especially in the domains of subsistence and safety stress.
Six trajectories were observed for exposure to subsistence stress (i.e., resource and housing stress), including two relatively stable groups of low and average levels of exposure and four groups with varying levels of exposure over time comprising about half of the sample. Close to one fifth of the sample experienced a change in level of exposure over time of greater than 1 SD. Two other groups of girls had comparable, high levels of exposure from ages 5 though 10 years, at which point they diverged such that the two groups differed by a full SD by age 17 years. Thus, for some girls, initial levels of exposure were comparable, followed by comparable changes in magnitude but in opposite directions. The six trajectories observed for safety stress included a large group of girls (58.6%) experience average, consistent levels of safety stress that hovered at one half a SD below the mean at all ages. Change over time in level of exposure was the pattern for all other groups, including a small group (n = 54) of girls who had high levels of exposure that increased during ages 10–14, followed by a decrease over the following three years; one of the few quadratic patterns of exposure observed. Caregiving stress exposure demonstrated the least variability of the three domains, with 46.1% of the sample with consistently low exposure, and nearly two-thirds with consistent exposure (at low, average, and high levels) over time. All 5 groups experienced consistent levels of exposure from ages 7–11 years, with only two groups experiencing changes in level of exposure from 12 to 17 years.
Regarding race differences in group membership, Black American girls were generally exposed to higher levels of stressors over time than White American girls, but important differences in specific domains are noted. Close to half of the White girls had consistently low levels of subsistence stress compared to 16.5% of Black girls. The groups had equivalent representation (about 25%) in the group characterized by average, consistent exposure, but twice as many Black girls as White girls experienced changes (both increases and decreases) in subsistence stress over time. Similar findings were observed for exposure to safety stress. Average level of exposure to safety stress was by far the normative experience for Black and White girls (70% and 82%, respectively), but greater variability in level of exposure over time was more common for Black girls compared to White girls. Therefore, in tests of moderation of stress exposure-health associations, race may be confounded with greater variability in exposures. In contrast to subsistence and safety stress, few statistically significant effects of race on exposure to caregiving stress were observed. This finding not only speaks to the importance of measuring exposure within and across domains, but also highlights the role of resilience among Black American families for whom the provision of caregiving is protected even in the context of high exposure to subsistence and safety stress. In fact, there is evidence that caregiving attenuates the impact of exposure to subsistence and safety stress on health outcomes for Black American youth (Scott, Wallander, & Cameron, 2015).
Level, consistency, and timing and magnitude of change in exposure to environmental stressors are likely critical factors for understanding the impact on health systems, which also evidence dynamic patterns of maturation. Changes in brain morphology and neural connectivity, diurnal patterns within the endocrine system, and the activity of gonadal and adrenal axes, as a few examples, occur in part in response to environmental inputs. Such systems also may have different periods of plasticity during which exposure to stressors lead to lasting alterations in function. Based on the results of the present study, it is plausible and likely prudent to compare different health outcomes for youth who have comparable levels of exposure on one dimension, such as initial severity, but differ on another dimension, such as chronicity. Moreover, interactions between severity level and timing of change is likely important. For example, does consistent exposure at average levels allow for adaptation and greater protection of health than increases in exposure from low to average levels, and is the risk conferred by an increase in exposure dependent on the timing of the increase (e.g., childhood or adolescence) and the measured health system/outcome (e.g., obesity or neural function).
In addition, dimensions of exposure may also be specific to patterns of alterations in health systems. For example, stress exposure early in development has been associated with both blunted and heighted responsiveness of the HPA axis later in development. A review of studies using rodent models suggest that stressors involving caregiving yield hyper-responsiveness, whereas social deprivation results in hypo-responsiveness, with each pattern involving distinct neural/endocrine alterations (van Bodegom, Homberg &, Henckens, 2017). Thus, refining our tests of exposure-health associations across development and domain may help explain heterogeneity in health outcomes, which in turn will yield improved capacity to identify mechanisms.
This study's findings should be interpreted in light of limitations. A primary limitation of the present study is the exclusion of males. Moderation effects of sex on stress exposure–health associations are often observed in experimental animal and human studies and correlational studies in humans (Niknazar et al., 2016; Ruttle, Shirtcliff, Armstrong, Klein, & Essex, 2015; Stephens, Mahon, McCaul, & Wand, 2016). An important component to understanding such differences is testing whether exposure trajectories and sensitive periods of exposure vary as a function of sex. Observed sex differences are in part due to differences in sex steroids, concentrations of which change over the course of development (Schulz & Sisk, 2016). Thus, it is possible the timing and chronicity of exposure may influence whether sex effects on exposure-health associations are observed. A second limitation is the lack of information on exposure prior to age 7 years. There are likely several sensitive periods of development for different biological systems in humans. Periods of rapid maturation are often hypothesized as particularly sensitive to stress exposure. The first 6 years of life is an incredibly dynamic period of growth in cognition, emotion and behavior, and there is substantial evidence for the impact of stress exposure on multiple systems including neural development (Hackman & Farah, 2009), immune function (Krebs, Lozoff, & Georgieff, 2017) and social emotional development (Humphreys & Zeanah, 2015) during this period. Extending the characterization of exposure trajectories to include the first six years of life will be critical to this effort. Third, we note that our tests of race differences in trajectory group membership in each domain was based on modal assignment to groups and as such may be impacted by errors in assignment. Finally, we note that best statistical practices for measuring domains of stress exposures that include multiple forms that vary in base rate are needed. Chronbach's alpha, a measure of internal consistency, suffers from major limitations (Sijtsma, 2009), and is not appropriate for testing small numbers of binary items that are not normally distributed (McNeish, 2018). As described in a recent commentary on measurement of adverse childhood experiences, neglect takes many forms and as such inherently suffers from low internal consistency because components of neglect do not represent an internally consistent construct (Widom, 2020).
5. Conclusion
Variability in initial level, chronicity, and magnitude and timing of change was observed within and across domains of stressors. Modeling these dimensions of stress exposure is likely important in testing associations between exposure and health, may improve specificity, and lead to more effective deployment of preventive interventions based on stress exposure. The current study was limited by the exclusion of males, and the reliance on caregiver reports. Despite these limitations, the results are compelling and call for further efforts to increase the rigor of measurement of stress exposure and explore approaches to characterizing type, timing, and chronicity. The goal of the present study was to provide an example of this effort, and to stimulate additional work to capture the dynamic nature of stress exposure in studies of human health and disease.
Ethics statement
These data come from a study with full IRB approval from the University of Pittsburgh. There is not a full report under consideration for publication elsewhere, and there is no overlap with other papers published from the Pittsburgh Girls Study (PGS). The authors report no conflicts of interest.
Author statement
Kate Keenan: Data curation, Conceptualization, Analytic plan, Writing. Haoyi Fu: Data Analysis, Writing. Irene Tung: Reviewing and editing. Johnny Berona: Reviewing and editing. Kristen Carpio: Data Analysis, Reviewing and Editing. Robert T. Krafty: Conceptualization, Analytic plan, Reviewing and editing. Stephanie Stepp: Data curation, Reviewing and editing. Alison Hipwell: Data curation, Conceptualization, Reviewing and editing.
Acknowledgment
Received support from NIH grants: R01 MH56630, R01 HL137246, UH3 OD023244.
References
- Atherton K., Fuller E., Shepherd P., Strachan D.P., Power C. Loss and representativeness in a biomedical survey at age 45 years: 1958 British birth cohort. Journal of Epidemiology & Community Health. 2008;62:216–223. doi: 10.1136/jech.2006.058966. [DOI] [PubMed] [Google Scholar]
- Badache S., Bouslama S., Brahmia O., Baïri A.M., Tahraoui A.K., Ladjama A. Prenatal noise and restraint stress interact to alter exploratory behavior and balance in juvenile rats, and mixed stress reverses these effects. Stress: The International Journal on the Biology of Stress. 2017;20:320–328. doi: 10.1080/10253890.2017.1307962. [DOI] [PubMed] [Google Scholar]
- Barnard K.E., Johnson S., Booth C.L., Bee H. NCAST; Seattle, WA: 1989. Difficult life Circumstances. [Google Scholar]
- Beck A.T., Brown G., Steer R.A. Psychological Corporation; San Antonio, TX: 1996. Beck depression inventory-II manual. [Google Scholar]
- van Bodegom M., Homberg J.R., Henckens M.J.A.G. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Frontiers in Cellular Neuroscience. 2017;11:87. doi: 10.3389/fncel.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma G.J., Bale T.L., Casanello P., Lara H.E., Lucion A.B., Suchecki D., et al. Long-term impact of early life events on physiology and behaviour. Journal of Neuroendocrinology. 2014;26:587–602. doi: 10.1111/jne.12153. [DOI] [PubMed] [Google Scholar]
- Calcagni E., Elenkov I. Stress system Activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- Capretto J.J. Developmental timing of childhood physical and sexual maltreatment predicts adult depression and post-traumatic stress symptoms. Journal of Interpersonal Violence. 2020;35:13–14. doi: 10.1177/0886260517704963. [DOI] [PubMed] [Google Scholar]
- Cavigelli S.A., Caruso M.J. Sex, social status and physiological stress in primates: The importance of social and glucocorticoid dynamics. Philosophical Transactions of the Royal Society B. 2015;26:370. doi: 10.1098/rstb.2014.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Conley A.J., Bernstein R.M., Nguyen A.D. Adrenarche in nonhuman primates: The evidence for it and the need to redefine it. Journal of Endocrinology. 2012;214:121–131. doi: 10.1530/JOE-11-0467. [DOI] [PubMed] [Google Scholar]
- Cowell R.A., Cicchetti D., Rogosch F.A., Toth S.L. Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Development and Psychopathology. 2015;27:521–533. doi: 10.1017/S0954579415000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas A.G., Boen C. 2021. Tip of the iceberg: Measuring racial discrimination in studies of health. Stress Health. Epub ahead of print. PMID: 33739613; PMCID: PMC8449795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn E.C., Soare T.W., Raffeld M.R., Busso D.S., Crawford K.M., Davis K.A., et al. What life course theoretical models best explain the relationship between exposure to childhood adversity and psychopathology symptoms: Recency, accumulation, or sensitive periods? Psychological Medicine. 2018;48:2562–2572. doi: 10.1017/S0033291718000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.L., Browning C.R., Boch S.J., Kertes D.A., Tarrence J., Way B.M., et al. Racial and economic adversity differences in stress markers and immune function among urban adolescents. Nursing Research. Advance online publication. 2021 doi: 10.1097/NNR.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao T.F., Silva M.T., Zimmermann I.R., Souza K.M., Martins S.S., Pereira M.G. Pubertal timing in girls and depression: A systematic review. Journal of Affective Disorders. 2014;155:13–19. doi: 10.1016/j.jad.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Gorman-Smith D., Tolan P.H., Henry D.B. A Developmental-ecological model of the relation of family functioning to patterns of delinquency. Journal of Quantitative Criminology. 2000;16:169–198. [Google Scholar]
- Grant K.E., Compas B.E., Thurm A.E., McMahon S.D., Gipson P.Y. Stressors and child and adolescent psychopathology: Measurement issues and prospective effects. Journal of Clinical Child and Adolescent Psychology. 2004;33:412–425. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville E.W., Boynton-Jarrett R., Power C., Hyppönen E. Childhood hardship, maternal smoking, and birth outcomes: A prospective cohort study. Archives of Pediatrics and Adolescent Medicine. 2010;164:533–539. doi: 10.1001/archpediatrics.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell A., Keenan K., Kasza K., Loeber R., Stouthamer-Loeber M., Bean T. Reciprocal influences between girls' conduct problems and depression, and parental punishment and warmth: A six-year prospective analysis. Journal of Abnormal Child Psychology. 2008;36:663–677. doi: 10.1007/s10802-007-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell A.E., Loeber R., Stouthamer-Loeber M., Keenan K., White H.R., Kroneman L. Characteristics of girls with early onset disruptive and antisocial behaviour. Criminal Behaviour and Mental Health. 2002;12:99–118. doi: 10.1002/cbm.489. [DOI] [PubMed] [Google Scholar]
- Humphreys K.L., Zeanah C.H. Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology. 2015;40:154–170. doi: 10.1038/npp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Chaudieu I., Ritchie K., Scali J., Ancelin M.L., Ryan J. The extent to which childhood adversity and recent stress influence all-cause mortality risk in older adults. Psychoneuroendocrinology. 2020;111 doi: 10.1016/j.psyneuen.2019.104492. [DOI] [PubMed] [Google Scholar]
- Jones B.L., Nagin D.S., Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29:374–393. [Google Scholar]
- Kaiser R.H., Clegg R., Goer F., Pechtel P., Beltzer M., Vitaliano G., et al. Childhood stress, grown-up brain networks: Corticolimbic correlates of threat-related early life stress and adult stress response. Psychological Medicine. 2018;48:1157–1166. doi: 10.1017/S0033291717002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlén J., Ludvigsson J., Hedmark M., Faresjö Å., Theodorsson E., Faresjö T. Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics. 2015;135:1450–1457. doi: 10.1542/peds.2014-2561. [DOI] [PubMed] [Google Scholar]
- Keenan K., Hipwell A., Chung T., et al. The Pittsburgh girls study: Overview and initial findings. Journal of Clinical Child and Adolescent Psychology. 2010;39:506–521. doi: 10.1080/15374416.2010.486320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., McCormack H.C., Bolger E.A., McGreenery C.E., Vitaliano G., Polcari A., et al. Childhood maltreatment, depression, and suicidal ideation: Critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Frontiers in Psychiatry. 2015;6:42. doi: 10.3389/fpsyt.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury J.E., Bosquet Enlow M., Plamondon A., Lyons-Ruth K. The association between adversity and hair cortisol levels in humans: A meta-analysis. Psychoneuroendocrinology. 2019;103:104–117. doi: 10.1016/j.psyneuen.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs N.F., Lozoff B., Georgieff M.K. Neurodevelopment: The Impact of nutrition and inflammation during infancy in low-resource settings. Pediatrics. 2017;139:S50–S58. doi: 10.1542/peds.2016-2828G. [DOI] [PubMed] [Google Scholar]
- Krinner L.M., Warren-Findlow J., Bowling J., Issel L.M., Reeve C.L. The dimensionality of adverse childhood experiences: A scoping review of ACE dimensions measurement. Child Abuse & Neglect. 2021;121 doi: 10.1016/j.chiabu.2021.105270. [DOI] [PubMed] [Google Scholar]
- Loeber R., Farrington D.P., Stouthamer-Loeber M., Van Kammen W.B. Lawrence Erlbaum Associates; Manwah, NJ: 1998. Antisocial behavior and mental health problems: Explanatory factors in childhood and adolescence. [Google Scholar]
- Matthews K.A., Gallo L.C. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish D. Thanks coefficient alpha, we'll take it from here. Psychological Methods. 2018;23:412–433. doi: 10.1037/met0000144. [DOI] [PubMed] [Google Scholar]
- Niknazar S., Nahavandi A., Peyvandi A.A., Peyvandi H., Akhtari A.S., Karimi M. Comparison of the adulthood chronic stress effect on hippocampal BDNF signaling in male and female rats. Molecular Neurobiology. 2016;53:4026–4033. doi: 10.1007/s12035-015-9345-5. [DOI] [PubMed] [Google Scholar]
- Paradies Y., Ben J., Denson N., Elias A., Priest N., Pieterse A., et al. Racism as a determinant of health: A systematic review and meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K.J., Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience Biobehaviroal Reviews. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington K.A., van der Walt N., Pollock K.E., Talton O.O., Schulz L.C. Effects of acute exposure to a high-fat, high-sucrose diet on gestational glucose tolerance and subsequent maternal health in mice. Biology of Reproduction. 2017;96:435–445. doi: 10.1095/biolreprod.116.144543. [DOI] [PubMed] [Google Scholar]
- Ruttle P.L., Shirtcliff E.A., Armstrong J.M., Klein M.H., Essex M.J. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology. 2015;57:688–704. doi: 10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalinski I., Teicher M.H., Nischk D., Hinderer E., Müller O., Rockstroh B. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:295. doi: 10.1186/s12888-016-1004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K.M., Sisk C.L. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience & Biobehavioral Reviews. 2016;70:148–158. doi: 10.1016/j.neubiorev.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Bellinger D., Glass T. Expanding the scope of environmental risk assessment to better include differential vulnerability and susceptibility. American Journal of Public Health. 2011;101:S88–S93. doi: 10.2105/AJPH.2011.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S.M., Wallander J.L., Cameron L. Protective mechanisms for depression among racial/ethnic minority youth: Empirical findings, issues, and recommendations. Clinical Child and Family Psychology Review. 2015;18:346–369. doi: 10.1007/s10567-015-0188-4. [DOI] [PubMed] [Google Scholar]
- Sijtsma K. On the use, the misuse, and the very limited usefulness of Cronbach's alpha. Psychometrika. 2009;74:107–120. doi: 10.1007/s11336-008-9101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M.A., Mahon P.B., McCaul M.E., Wand G.S. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus M.A., Hamby S.L., Boney-McCoy S., Sugarman D.B. The revised conflict Tactics scales (CTS2) Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- Teicher M.H., Anderson C.M., Ohashi K., Khan A., McGreenery C.E., Bolger E.A., et al. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. NeuroImage. 2018;169:443–452. doi: 10.1016/j.neuroimage.2017.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oers H.J.J., De Kloet R.R., Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Developmental Brain Research. 1998;111:245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- Veru F., Laplante D.P., Luheshi G., King S. Prenatal maternal stress exposure and immune function in the offspring. Stress: The International Journal on the Biology of Stress. 2014;17:133–148. doi: 10.3109/10253890.2013.876404. [DOI] [PubMed] [Google Scholar]
- Widom C.S. Commentary: A challenge for a higher bar in research on childhood trauma - reflections on Danese (2020) The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2020;61:251–254. doi: 10.1111/jcpp.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Lawrence J.A., Davis B.A., Vu C. Understanding how discrimination can affect health. Health Services Research. 2019;54:1374–1388. doi: 10.1111/1475-6773.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]